Abstract
Objective Cyclin D1 is an important nuclear protein required for progression of cells through the G1 phase of the cell cycle. The proliferative potential of meningiomas has been studied using various proliferative markers. However, there have been only few published studies evaluating Cyclin D1 immunoreactivity in meningiomas. Purpose of the study The aim of our study was to analyze the Cyclin D1 expression in meningiomas and correlate it both with proliferation markers Ki67 and PCNA, and with meningiomas of WHO grade. Material and methods We evaluated immunoreactivity for proliferative markers (Cyclin D1, Ki-67, and PCNA) in a consecutive series of 64 meningioma samples obtained from patients who underwent surgical resection because of cerebral or spinal meningiomas. Immunohistochemical staining with Ki-67, PCNA, and Cyclin D1 was performed using the microwave processing procedure and LSAB+ methodology. The number of positive cells for each antibody has been determined and shown in percentage in relation to 1000 counted cells. Results All meningioma samples showed immunostaining for Ki-67, PCNA, and Cyclin D1 antibodies. The Cyclin D1 scores exhibited a close correlation with Ki-67 and PCNA immunostaining (P < 0.01). Some meningiomas (15 cases) showed a combination of nuclear and cytoplasmatic (fine granular) Cyclin D1 immunoreactivity. All proliferative indexes have been in positive correlation with meningioma grade. Conclusion Our comparative study of proliferative markers in meningiomas demonstrated Cyclin D1 as a very useful proliferative marker in meningiomas.
Keywords: Meningiomas, Proliferative indices, Cyclin D1, Ki67, PCNA, Immunohistochemistry
Introduction
Meningiomas are one of the most interesting groups of central nervous system tumors, due to a frequent discordance between histology and biology. Surgical treatment is a therapy of choice for meningiomas, but the recurrence rate even in totally resected tumors (Simpson grades 1–3) (Simpson 1957) is approximately 10–20% within the first 5 years, 20–30% within the first 10 years, and approximately 50% after 20 years, according to long-term studies (Simpson 1957; Jaaskelainen 1986; Marks et al. 1986).
Routine pathological examination cannot precisely predict the clinical course of meningiomas. However, it is well established that proliferative activity has a prognostic significance in a large variety of human solid tumors (Daidone et al. 2001) and cell cycle kinetics play an important role in tumor behavior. Thus, quantifying meningioma’s proliferative potential may help to predict differences in biological behavior of tumors with comparable histology. Among methods used to assess cell proliferation, the most popular one is the immunohistochemical detection of cell cycle proteins (PCNA, Ki67/MIB, cyclin A) by visual inspection or image quantification (Torp et al. 2001). Yet, the search for the most relevant and accurate proliferation marker, or their combination, for meningiomas is not coming even close to end.
During normal cell cycle, progression through various cell cycle stages is regulated by tightly controlled actions of cyclin dependent kinases (Cdks) and cyclins (Clurman and Roberts 1995). Cyclin D1 is an important nuclear protein required for progression of cells through the G1 phase of the cell cycle (Sherr 1995). The role of cyclin D1 in the development, progression, and prognosis of meningiomas is not well established. Only few molecular genetic studies have investigated checkpoint protein cyclin D1 as prognostic indicators in meningiomas, and present results reveal that increased expression of cyclin D1 is significantly associated with higher proliferative activity, as assessed by PCNA levels and Ki 67 percentage of labeled cells (PLC) (Sato et al. 1996; Diehl et al. 1998; Motokura et al. 1992; Alama et al. 2007). The aim of our study was to explore the usefulness of cyclin D1 expression as a proliferation marker in meningiomas for the routine immunohistochemistry of archival tumor tissue in relation to PCNA and Ki-67 and WHO grade of tumors.
Materials and Methods
Samples
Paraffin-embedded meningioma tissue samples were obtained from 62 patients who were diagnosed and operated consecutively at the Department of Neurosurgery, Clinical Hospital Center Zemun-Belgrade, during the period January to December 2005. The study was performed according to all law regulations and with agreement of the Institutional Review Board. Fifteen patients were male and 47 were female. We analyzed 64 meningioma samples because three samples were taken from one female patient (Milenkovic et al. 2005). The mean age of the patients was 61.62 years (61.96 for female and 62.5 for male patients). The tumors were most frequently located basally in the frontal or middle cranial fossa (24 tumors), followed by location in the convexity (21 tumors), spinal canal (11 tumors), the posterior fossa (5 tumors) and the ventriculi cerebri (3 tumors). The tumors ranged from 1.20 to 78.40 g (median, 20.65 g). We used the Simpson (1957) system to record the extent of tumor removal. The extent of resection was deduced from the recorded description of the operation and confirmed by inspecting postoperative computed tomography (CT) and/or magnetic resonance (MR) images in the patients’ medical records.
Tissue samples were fixed in 4% formaldehyde and paraffin-embedded. Sections (5-μm thick) were routinely stained with hematoxylin and eosin (H&E). Histopathological diagnosis of all meningiomas (64 samples) was done according to the actual published WHO classification of tumors of the nervous system, and tumors were grouped as benign, atypical, and anaplastic meningiomas (Louis et al. 2000).
Immunohistochemistry
Immunohistochemical reactions were carried out on formalin-fixed, paraffin-embedded four-micron sections of each tumor specimen using the streptavidin-biotin-peroxidase method (LSAB-2/HRP system, Dako Corporation, Glostrup, Denmark) with 3,3′-diaminobenzidine-tetrahydrochloride (DakoCytomation K3468) as the chromogen. The antibodies used were specific for Ki-67 antigen (MIB-1 DAKO Cytomation No. N1633), PCNA (DAKO Cytomation N1529), and Cyclin D1 (DAKO Cytomation No M7155 anti-cyclin D1 [dilution 1:20]). Negative controls for each tissue section were made by substituting the primary antibody with the respective preimmune serum for cyclin D1, and by leaving out the primary antibody for Ki 67 and PCNA. Mantle cell lymphoma tissue was used as a positive control. All samples were processed under the same conditions. The percentages of cells expressing Ki67, PCNA, and Cyclin D1 were determined by counting 1000 cells per slide in areas of highest density of staining (“hot spot”) over a minimum of 10 high power fields. Slides were analyzed using a Leica DM1000® light microscope. For each case, the quantification of immunostaining was made by semiquantitative methods. Percentage of labeled cells (PLC) was determined, according to the following equation: PLC (%) = number of labeled cells/total counted cells X100.
Statistical Analysis
Interrelations among the proliferation markers PLC for PCNA, Ki-67, and Cyclin D1 were analyzed with Pearson’s correlation coefficient with significance at the 0,01 level (statistical significance was attributed P-values < 0.01). The relationships between the meningioma WHO grade and immunoexpression Cyclin D1 and Ki 67 were analyzed by Multiple Comparison-Dependent Variable test, and P-values <0.05 were considered as significant.
Results
To assess the suitability of cyclin D1 as a proliferation marker in meningiomas, we analyzed cyclin D1 expression in 64 bioptic samples and compared it with expression of Ki67 and PCNA. Out of the 64 analyzed meningiomas 57 (89.06%) were benign, 5 (7.81%) were atypical, and 2 (3.13%) were anaplastic. Benign meningiomas included meningothelial (23 cases, 35.94%), transitional (15 cases, 23.43%), psammomatous (9 cases, 14.06%), fibrous (7 cases, 10.94%), and angiomatosus (3 cases, 4.69%) types.
Expression of cyclin D1, Ki67, and PCNA was observed in all 64 samples. The mean PLC for PCNA (2.20%) was greater than the mean Ki-67 PLC (1.64%) and Cyclin D1 PLC (1.36%) (Table 1).
Table 1.
Immunoexpression of all proliferative markers in 64 samples of meningioma tissue
| Markers | Range | Mean ± SD |
|---|---|---|
| Ki 67 | 0.10–5.90 | 1.64 ± 1.54 |
| PCNA | 0.00–6.00 | 2.20 ± 1.33 |
| Cyclin D1 | 0.00–5.60 | 1.36 ± 1.22 |
There was a significant correlation between Ki-67 and Cyclin D1 as continuous variables, as well as between Cyclin D1 and PCNA (Pearson’s correlation coefficient: P < 0.01) (Table 2).
Table 2.
Pearson’s analysis of the correlation between immunoexpression of all proliferative markers, Cyclin D1, Ki 67, and PCNA
| KI 67 | PCNA | Cyclin D1 | ||
|---|---|---|---|---|
| KI 67 | Correl. coefficient | 1.000 | 0.544* | 0.969* |
| Sig. (2-tailed) | 0.000 | 0.000 | ||
| N | 64 | 64 | 64 | |
| PCNA | Correl. coefficient | 0.544* | 1.000 | 0.563* |
| Sig. (2-tailed) | 0.000 | 0.000 | ||
| N | 64 | 64 | 64 | |
| Cyclin D1 | Correl. coefficient | 0.969* | 0.563* | 1.000 |
| Sig. (2-tailed) | 0.000 | 0.000 | ||
| N | 64 | 64 | 64 |
* The mean difference is significant at the .01 level (2-tailed)
The relationship between the WHO grade meningioma and Cyclin D1 and Ki 67 immunoexpression was analyzed by Multiple Comparison-Dependent Variable test, and P-values of <0.05 were considered as significant (Table 3). The expression of Ki67 and Cyclin D1 was elevated in meningioma grades II and III in comparison with grade I (P-values of <0.05) (2.85 units for WHO grade II and 4.41 for WHO grade III compared to WHO grade I). Results for Cyclin D1 were virtually identical (2.59 units for grade II and 4.22 for units for grade III compared to WHO grade I). Difference between grades II and III was 1.56 for Ki67 and 1.63 units for Cyclin D1.
Table 3.
Relationship between the meningioma grade and immmunoexpression Cyclin D1 and Ki 67
| Grades | Grades | Difference | Significance | |
|---|---|---|---|---|
| Ki 67 | I | II | −2.85930* | 0.000 |
| III | −4.41930* | 0.000 | ||
| II | III | −1.56000* | 0.032 | |
| Cyclin D1 | I | II | −2.59193* | 0.000 |
| III | −4.22193* | 0.000 | ||
| II | III | −1.63000* | 0.010 |
* The mean difference is significant at the .05 level
We made an interesting observation while analyzing the subcellular expression pattern of these 3 markers. Immunostains of Ki67 were nuclear in all 64 samples (Fig. 1a); however, expression of PCNA and, even more, cyclin D1 appeared to be more complex. In the majority of cases 42/64 stains for cyclin D1 and PCNA were nuclear (Fig. 1b, c). But, in 15 cases cyclin D1 was clearly present both in the nucleus and in the cytoplasm of tumor cells (picture 3). This pattern was observed both in the benign as well in 1 malignant sample (picture 3). In those 15 samples Ki 67 and PCNA expression remain nuclear. Finally, beside of nuclear staining in 7 samples from the patients with benign meningioma, staining for both cyclin D1 and PCNA appeared in the form of a ring juxtaposed to nuclear membrane (Figs. 1b, 2). It is not clear if this staining pattern is specific for certain meningioma types and WHO grades, as the number of malignant samples was rather small. To repeat, Ki67 stains were nuclear in all samples, speaking against the possibility that the observed staining pattern represents a technical artifact. As the number of malignant samples analyzed in this study was rather small, it is not possible to conclude whether there is a difference between benign and malignant meningiomas regarding the expression pattern for Cyclin D1 and PCNA (Fig. 3).
Fig. 1.
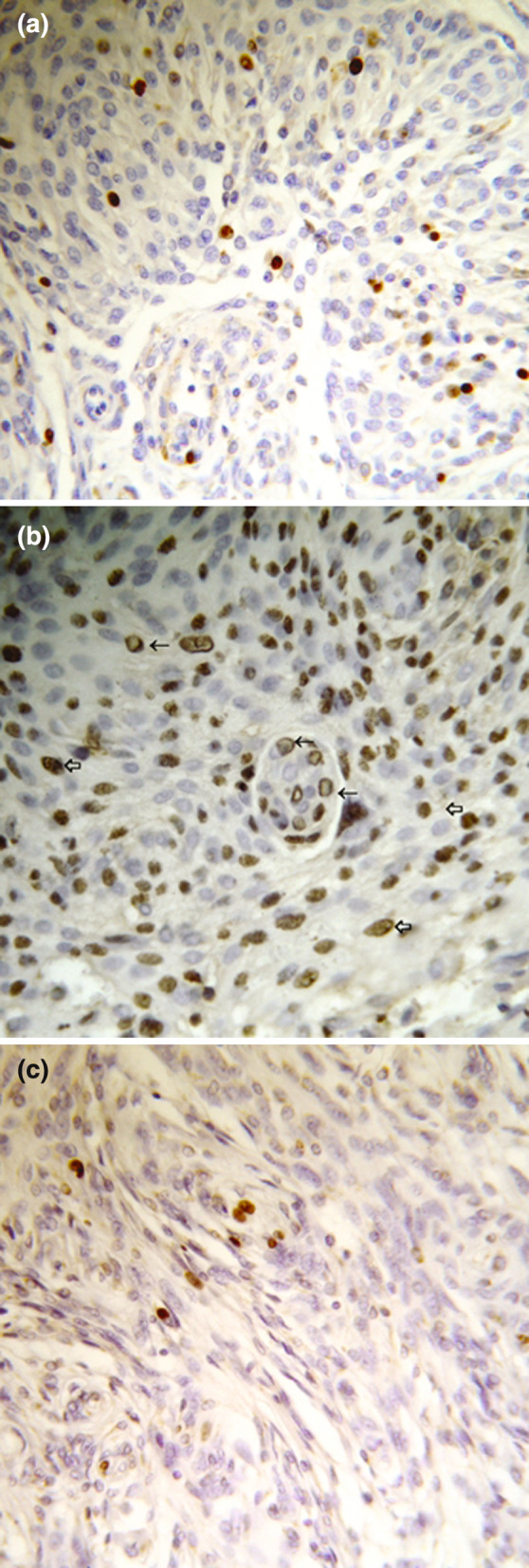
(a) Nuclear anti-Ki67 positive staining in meningothelial meningioma (Immunoperoxidase 40×). (b) Nuclear anti-PCNA (⇦) and “ring” shape (←) positive staining in meningothelial meningioma (Immunoperoxidase 40×). (c) Nuclear anti-cyclin D1 positive staining in meningothelial meningioma (Immunoperoxidase 40×)
Fig. 2.

Nuclear (←) and “ring” (⇦) anti-cyclin D1 positive staining in meningothelial meningioma (Immunoperoxidase 40×)
Fig. 3.

Nuclear and cytoplasmatic granular (⇦) anti-cyclin D1 positive staining in malignant meningioma (Immunoperoxidase 40×)
Discussion
Quantification of the meningioma proliferative capacity, expressed as tumor growth fraction, appears to be the most significant parameter for disease prognosis. Ki-67/MIB-1 is widely accepted as a proliferation marker and a strong correlation between MIB-1, PCNA proliferative indices, and the histologic grade of the meningiomas is established (Takeuchi et al. 1997; Madsen and Schroeder 1997; Hsu et al. 1998; Karamitopoulou et al. 1998; Perry et al. 1998; Abramovich and Prayson 1998; Nakaguchi et al. 1999; Lanzafame et al. 2000; Amatya et al. 2001; Takahashi et al. 2004).
However, immunostaining with Ki-67/MIB-1 has some serious deficiencies: Ki-67 antigen is not yet identified and the possibility of false-positive results cannot be excluded. Importantly, Ki-67 immunoreactivity is highly dependent on fixation time, specimen storage protocols, and antigen retrieval technique (Korshuno et al. 2002). In the present study, PCNA immunostaining was more prominent than Ki-67 in all tumor samples and this feature may be the result of the long half-life of PCNA which also stains the resting cells. Thus, a search for a reliable proliferation marker is still open.
Our finding indicates cyclin D1 as a good candidate for the quantitative assessment of proliferation by immunohistochemistry of meningioma tumor tissue. Correlation between Ki-67 and cyclin D1 as well as between cyclin D1 and PCNA, which also stains the resting cells, as continuous variables further strengthens this conclusion. Thus, a search for a reliable proliferation marker is still open.
Significance of cyclin D1 expression in meningiomas was already postulated by Alama et al. (2007). However, in their study the expression of cyclin D1 is evaluated by Western blot. Beside the fact that Western blot analysis does not give an insight into cellular localization of the marker, it is not highly suitable for the routine use.
Cyclin D1 was originally described as a nuclear protein (Sherr 1995). In this study we demonstrated nuclear staining with cyclin D1 in 42 (69.5%) tumors; however, a significant portion of tumor samples (15/64) showed a cytoplasmic reaction alone or in combination with nuclear staining. This finding cannot be attributed to the leakage of antigen into cytoplasm due to tissue manipulation as in the same sample expression of Ki67, and this is not the first description of the cyclin D1 presence in the cytoplasm. In several other studies cytoplasmic cyclin D1 expression was demonstrated. Similar to our result, combination of cytoplasmatic and nuclear cyclin D1 stains of varying intensities was observed. Beside, exclusive cytoplasmic localization of cyclin D1 has also been reported (Holland et al. 2001; Gijtenbeek et al. 2005). The presence of cyclin D1 in the cytoplasm was observed in esophageal, gastric, pancreatic, and colonic carcinomas as well as in hemangioblastomas (Holland et al. 2001; Gijtenbeek et al. 2005; Arber et al. 1999; Lebe et al. 2004); however, this is the first report of cytoplasmatic cyclin D1 immunostaining in meningiomas. This finding indicates cyclin D1 as a more subtle diagnostic marker compared with Ki 67.
The obvious question is the patho-physiological role of cytoplasmatic cyclin D1 in meningiomas. According to Lebe et al., the presence of cyclin D1 in the cytoplasm is a consequence of binding and inactivation of cyclin D1 or its related cyclin-dependent kinases by the substrata, which leads to translocation of cyclin D1 to cytoplasm (Lebe et al. 2004). This translocation is believed to take place upon completing G1 stage of cell cycle. The G1 phase is important for integration of the signals which regulate exit from the cell division cycle to differentiation and the reactivation of cell proliferation. A large body of evidence implies cyclin D1 as a key regulator of this phase. Thus, the presence of cyclin D1 in the cytoplasm of meningioma cells might suggest that these cells completed G1 stage. There is also a possibility that cytoplasmatic cyclin D1 is a consequence of inactivation of the genes that are described as responsible for the nuclear localization of cyclin D1 (vaf-1 or p21, for example) (Tut et al. 2001; Alt et al. 2001).
The molecular mechanisms responsible for the cyclin D1 localization in meningioma tumor cells remain to be elucidated. Moreover, it remains to be elucidated whether cyclin D1 localization can add a distinct value to the use of this cellular marker for the prognosis of meningioma behavior. This also holds true for the third kind of cyclin D1 immunostaining, the “ring” appearance. Again, quite regular nuclear staining of Ki67 in the 7 meningioma samples that showed “ring” staining pattern for cyclin D1 speaks against technical artifact. Interestingly, while there were no obvious differences in PCNA staining pattern in samples with nuclear and cytoplasmatic cyclin D1 staining, in samples that showed “ring” staining for cyclin D1, PCNA also showed ring-like image. The interaction between cyclin D1 and PCNA is already described. In vitro study showed that these two proteins co-precipitate in a complex that also includes 21-kDa protein (Cip1) and Cdks (Xiong et al. 1992, 1993). Pagano et al. suggested that cyclin D1 and PCNA interaction inhibits PCNA-dependent DNA replication (Pagano et al. 1994).
There are also reports that expression of both cyclin D1 and PCNA may be regulated by the same protein, ZONAB (Sourisseau et al. 2006). Although additional analysis revealed that cyclin D1 and PCNA interact directly (Kato et al. 1994) our results suggest more complex relationship between these two proteins. Obviously, in the vast majority of cases analyzed in this study both cyclin D1 and PCNA proteins were present, yet, the “ring” structures were observed only in 7 cases. The methodology applied in this study cannot answer whether cyclin D1 and PCNA form complexes in the cells that do not show “ring” staining, nor if “rings” indeed represent directly interacting proteins. We may speculate that these interesting “structures” reflect the impaired (or overwhelmed) mechanisms responsible for the normal localization of cyclin D1 and PCNA during cell cycle.
Again, not only the mechanism responsible for the accumulation of cyclin D1 and PCNA just below nuclear membrane has to be revealed, but also patho-physiological meaning of this finding should be determined. Determination of the role for “rings” and cytoplasmatic cyclin D1 staining for meningioma biology might extend the use of this proliferation marker for diagnostic purposes.
Conclusion
Our study indicates cyclin D1 as an attractive target for the quantitative assessment of cell proliferation by immunohistochemistry in routine archival tumor tissue. We are also for the first time describing an unusual staining pattern for cyclin D1 whose role in tumor biology remains to be determined.
Acknowledgments
The authors wish to thank Prof. Dr. Dubravka Cvetkovic-Dozic and Prof. Milica Skender-Gazibara and Prof. Slobodan Dozic for useful suggestions and helpful discussion of the manuscript. We also thank Milan Gajic for the crucial help with statistical analysis of data.
References
- Abramovich CM, Prayson RA (1998) MIB-1 labeling indices in benign, aggressive, and malignant meningiomas: a study of 90 tumors. Hum Pathol 29:1420–1427 [DOI] [PubMed] [Google Scholar]
- Alama A, Barbieri B, Spaziante R, Bruzzo C, Dadati P, Dorcaratto A (2007) Significance of cyclin D1 expression in meningiomas. A preliminary study. J Clin Neurosci 14:355–358 [DOI] [PubMed] [Google Scholar]
- Alt JR, Gladden AB, Diehl JA (2001) p21cip1 promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J Biol Chem 277:8517–8523 [DOI] [PubMed] [Google Scholar]
- Amatya JV, Takeshima Y, Sugiyama K, Kurisu K, Nishisaka T, Fukuhara T, Inai K (2001) Immunohistochemical study of Ki-67 (MIB-1), p53 Protein, p21WAF1, and p27KIP1 expression in Benign, atypical, and anaplastic meningiomas. Hum Pathol 32(9):970–975 [DOI] [PubMed] [Google Scholar]
- Arber N, Gammon MD, Hibshoosh H, Britton JA, Zhang Y, Schonberg JB, Roterdam H, Fabian I, Holt PR, Weinstein B (1999) Overexpression of cyclin D1 occurs in both squamous carcinomas and adenocarcinomas of the esophagus and in adenocarcinomas of the stomach. Hum Pathol 30:1087–1092 [DOI] [PubMed] [Google Scholar]
- Clurman BE, Roberts JM (1995) Cell cycle and cancer. J Natl Cancer Inst 87:1499–1501 [DOI] [PubMed] [Google Scholar]
- Daidone MG, Costa A, Silvestrini R (2001) Cell proliferation markers in human solid tumors: assessing their impact in clinical oncology. Methods Cell Biol 64:359–384 [DOI] [PubMed] [Google Scholar]
- Diehl JA, ChengM, Roussel MF, Sherr CJ (1998) Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijtenbeek MMJ, Boots-Sprenger HES, Franke B, Wesseling P, Jeuken WMJ (2005) Cyclin D1 genotype and expression in sporadic hemangioblastomas. J Neurooncol 74:261–266 [DOI] [PubMed] [Google Scholar]
- Holland TA, Elder J, McCloud JM, Hall C, Deakin M, Fryer AA et al (2001) Subcellar localization of cyclin D1 protein in colorectal tumours is associated with p21WAF1/CIP1265 expression and correlates with patient survival. Int J Cancer (Pred Oncol) 95:302–306 [DOI] [PubMed] [Google Scholar]
- Hsu DW, Efird JT, Headley-White ET (1998) MIB-1 (Ki-67) index and transforming growth factor-alpha (TGF alpha) immunoreactivity are significant prognostic predictors for meningiomas. Neuropathol Appl Neurobiol 24:441–452 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J (1986) Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 26:461–469 [DOI] [PubMed] [Google Scholar]
- Karamitopoulou E, Perentes E, Tolnay M, Probst A (1998) Prognostic significance of MIB-1, p53, and bcl-2 immunoreactivity in meningiomas. Hum Pathol 29:140–145 [DOI] [PubMed] [Google Scholar]
- Kato JY, Matsuoka M, Strom DK, Sherr CJ (1994) Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol 14(4):2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshuno A, Shishkina L, Golanov A (2002) DNA Topoisomerase II-a and Cyclin A. Immunoexpression in meningiomas and its prognostic significance an analysis of 263 cases. Arch Pathol Lab Med 126:1079–1086 [DOI] [PubMed] [Google Scholar]
- Lanzafame S, Torrisi A, Barbagallo G, Emmanuele C, Alberto N, Albanese V (2000) Correlation between histological grade, MIB-1, p53 and recurrence in 69 completely resected primary intracranial meningiomas. Pathol Res Pract 196:483–488 [DOI] [PubMed] [Google Scholar]
- Lebe B, Sağol Ö, Ulukus Ç, Çoker A, Sedat Karademir S, Astarcioglu H, Küpelioğlu A, Astarcioğlu I, Obuz F (2004) The importance of cyclin D1 and Ki67 expression on the biological behavior of pancreatic adenocarcinomas. Pathol Res Pract 200:389–396 [DOI] [PubMed] [Google Scholar]
- Louis DN, Scheithauer BW, Budka H, von Deimling A, Kepes JJ (2000) Meningiomas: pathology and genetics of tumours of the nervous system. In: Kleihues P, Cavenee WK (eds) World Health Organization classification of tumours. IARC Press, Lyon, pp 176–184 [Google Scholar]
- Madsen C, Schroeder HD (1997) Ki-67 immunoreactivity in meningiomas. Clin Neuropathol 16:137–142 [PubMed] [Google Scholar]
- Marks SM, Whitewell HL, Lye RH (1986) Recurrence of meningiomas after operation. Surg Neurol 25:436–440 [DOI] [PubMed] [Google Scholar]
- Milenkovic S, Berisavac I, Bojović V, Orlić M, Stanković D, Simić A (2005) Multiple spinal meningiomas with positive PR-case report. Mater Med 21(2):105–109 [Google Scholar]
- Motokura T, Keyomarsi K, Kronenberg HM, Arnold A (1992) Cloning and characterization of human Cyclin D3, a cDNA closely related in sequence to the PRADI/cyclin D1-proto-oncogene. J Biol Chem 267(28):20412–20415 [PubMed] [Google Scholar]
- Nakaguchi H, Fujimaki T, Matsuno A, Matsuura R, Asai A, Suzuki I et al (1999) Postoperative residual tumor growth of meningioma can be predicted by MIB-1 immunohistochemistry. Cancer 85:2249–2254 [PubMed] [Google Scholar]
- Pagano M, Theodoras AM, Tam SW, Draetta GF (1994) Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev 8:1627–1639 [DOI] [PubMed] [Google Scholar]
- Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM (1998) The prognostic significance of MIB-1, p53 and flow-cytometry in completely resected primary meningiomas. Cancer 82:2262–2269 [PubMed] [Google Scholar]
- Sato K, Schauble B, Kleihues P, Ohgaki H (1996) Infrequent altera-tions of the p15, p16, CDK4 and cyclin D1 genes in non-astrocytic human brain tumors. Int J Cancer 66(3):305–308 [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1995) D-type cyclins. Trends Biochem Sci 20:187–190 [DOI] [PubMed] [Google Scholar]
- Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatr 20:22–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda SM (2006) Regulation of PCNA and Cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol 26(6):2387–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi AJ, Ueba T, Hashimoto N, Nakashima Y, Katsuki N (2004) The combination of mitotic and Ki-67 indices as a useful method for predicting short-term recurrence of meningiomas. Surg Neurol 61:149–156 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kubota T, Kabuto M, Kitai R, Nozaki J, Yamashita J (1997) Prediction in recurrence of histologically benign meningiomas: PCNA and Ki-67 immunohistochemical study. Surg Neurol 48:501–506 [DOI] [PubMed] [Google Scholar]
- Torp SH, Lindboe CF, Granli US, Moen TM, Nordtomme T (2001) Comparative investigation of proliferation markers and their prognostic relevance in human meningiomas. Clin Neuropathol 20:190–195 [PubMed] [Google Scholar]
- Tut VM, Braithwaite KL, Angus B, Neal DE, Lunec J, Mellon JK (2001) Cyclin D1 expression in transitional cell carcinoma of the bladder: correlation with p53 wafl pRb and Ki67. Br J Cancer 84:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D (1992) D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71:505–514 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D (1993) Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev 7:1572–1583 [DOI] [PubMed] [Google Scholar]


