Abstract
Aims It is a huge challenge to understand the blood–brain barrier (BBB), which is a key element in neuroinflammation associated with many brain diseases. The BBB also regulates the passage of xenobiotics into the central nervous system (CNS), and therefore influences drug efficacy. This may be due to the presence of ATP binding cassette transporters such as P-glycoprotein (Pgp) on the BBB, which are efflux pumps known to transport many drugs. The peptide endothelin 1 (ET-1) is involved in different kinds of CNS diseases and neuroinflammation, and is known to modulate Pgp transport activity. Although there are data from animal models, data from human models are scarce. We evaluated Pgp expression and transport activity in adult human brain microvascular endothelial cells (HBMECs) when exposing an adult human in vitro BBB model to ET-1. Methods Adult HBMECs were cocultured with human adult glial cells on a TranswellsR to mimic blood and CNS compartments. These human in vitro BBBs were exposed for 24 h to 100 nM and 10 nM ET-1. Pgp expression was assessed by flow cytometry and its transport activity by measuring radiolabelled digoxin passage. Results After exposure to ET-1, flow cytometry showed no shift of fluorescence intensity for a Pgp specific antibody. The passage of digoxin increased with a significant decrease of Q ratio for 10 nM ET-1. Conclusion Our results show that ET-1 has no effect on Pgp expression of adult HBMECs, but does modulate Pgp transport activity.
Keywords: Blood–brain barrier (BBB), Endothelin 1 (ET-1), P-glycoprotein (Pgp)
Introduction
In the central nervous system (CNS), there are various barriers that isolate neurons from blood or cerebrospinal fluid to maintain an optimal chemical environment. The blood–brain barrier (BBB) is composed of several layers, starting on the blood side with capillary endothelial cells, then a basement membrane, pericytes and astrocytes. The endothelial cells at the BBB have a large number of tight junctions to inhibit paracellular movements, a low rate of transcytosis and high expression of certain multispecific ATP-driven xenobiotic efflux pumps (Hawkins et al. 2006; Kim et al. 2006; Loscher and Potschka 2005a).
Specialised transporters at the BBB govern some of the molecular efflux. One of the best studied transporters is P-glycoprotein (Pgp) since it has an affinity for a large number of therapeutic drugs (Rautio et al. 2006). Pgp is an ATP Binding Cassette (ABC) transporter localised on the apical side of capillary endothelial brain cells (Cordon-Cardo et al. 1989; Loscher and Potschka 2005a; Tatsuta et al. 1992).
Therefore, the BBB limits the penetration into the brain of most drug candidates. The latest are a huge challenge to find nowadays, considering the growing age of the population and the CNS diseases that are following it (Pardridge 2007). Accumulated evidence suggests that neurodegenerative diseases are associated with a local inflammatory response (Schwartz et al. 2006). For instance, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease and AIDS dementia complex are associated with neuroinflammation (de Vries et al. 1997; Tansey et al. 2008). BBB permeability is often increased by neuroinflammation in brain diseases (Ballabh et al. 2004; de Vries et al. 1997).
Inflammation also has an effect on Pgp expression and activity in brain cells. In vitro, Pgp expression and transport activity decrease in rat astrocytes exposed to pro-inflammatory cytokines (Ronaldson and Bendayan 2006). In rat brain capillary endothelial cells exposed to a pro-inflammatory cytokine, the mRNA expression of Pgp genes is increased while Pgp activity is decreased (Theron et al. 2003). In vivo, inflammation localised in the rat CNS downregulates mdr1 mRNA expression (Goralski et al. 2003).
ET-1, an in vivo and in vitro artery vasoconstrictive and vasodilator peptide (Wright and Fozard 1988; Yanagisawa et al. 1988), is released in several CNS disorders (Barone et al. 1995; Hebert et al. 2004; Jiang et al. 2006; Nakajima et al. 1994; Nie and Olsson 1996; Rolinski et al. 1999; Sasaki et al. 1997; Schinelli 2006; Speciale et al. 2000; Suzuki et al. 1992; Ziv et al. 1992). ET-1 has various biological roles (Mangahas et al. 2005; Takuwa et al. 1989; Bagnato et al. 2004). Members of the endothelin family are released by various cell types in brain, including endothelial cells and some glial cells, and in the case of inflammation (Chauhan et al. 2007; Didier et al. 2002, 2003; Ehrenreich et al. 1993; Yoshimoto et al. 1990).
ET-1 regulates Pgp transport activity in rat brain capillaries (Bauer et al. 2007; Hartz et al. 2004). Although there are data from animal models, human models have been studied much less. Therefore, we studied Pgp transport activity in a human in vitro model of BBB exposed to ET-1.
Methods
In vitro Model of Human BBB
Cells were isolated from human patient brain samples after 1.5-h digestion with collagenase/dispase (1 mg/ml, Roche, France) containing DNAse I (20 units/ml, Roche, France), followed by a 20% BSA gradient. The model, previously described by Josserand et al. (2006), Megard et al. (2002), consisted of a monolayer of primary adult human brain microvascular endothelial cells (HBMECs) with primary adult human glial cells (GCs) from the same individual.
BBB Integrity Assessment
Before use, BBB integrity was checked on 12% (2 wells out of 12 generated) of monolayers by sucrose (Amersham Buckinghamshire, UK) passage. TranswellsR with HBMEC monolayers were transferred to new plates. T buffer (150 mM NaCl, 5.2 mM KCl, 2.2 mM CaCl2, 0.2 mM MgCl2, 6 mM NaHCO3, 2.8 mM glucose and 5 mM Hepes) was added: 1.5 ml to the lower chamber (B) and 0.5 ml to the upper (A) one containing 0.37 1010 Bq/ml of [14C]-labelled sucrose. After 30 min of incubation at 37°C, A and B compartment supernatants were collected and the amount of tracer that passed through the endothelial monolayer was determined by scintillation counting. Monolayers were validated for a sucrose clearance A to B (CLAB) below 0.5 μl/min (8.10−6 cm/sec), as described by Megard et al. (2002).
Pgp Expression Evaluation
Flow cytometry analysis of cells was performed on a FACS-Calibur cytometer (Becton-Dickinson Biosciences, San Diego, CA, USA). For cytoplasmic staining, cells were washed with PBS, then scraped, permeabilised and fixed with Cytofix/Cytoperm solution (Becton-Dickinson Biosciences, San Diego, CA, USA). For membrane staining, cells were washed with PBS, then scraped and just fixed without permeabilisation with CellFIX solution (Becton-Dickinson Biosciences, San Diego, CA, USA) for 20 min at 4°C. The cells were washed and resuspended in 70 μl of PermWash (Becton-Dickinson Biosciences, San Diego, CA, USA) when permeabilised or in PBS when unpermeabilised and then labelled for 30 min at 4°C with a phycoerythrin-conjugated antibody specific to Pgp (Immunotech, Beckman Coulter, Marseille, France). After two washing steps, cells were analysed by flow cytometry using Cell Quest on FACScan. Negative control analyses were performed using isotype-matched irrelevant antibodies conjugated to phycoerythrin.
Pgp Transport Activity Test
The specific Pgp substrate, [3H]-radiolabelled digoxin (Perkin-Elmer, France), was used. TranswellsR with HBMEC monolayers were transferred to new plates with 1.5 ml of T buffer (see above) in the B chamber and 0.5 ml in the A one. Digoxin solution was added either to the upper or lower chamber at a concentration of 0.1 μM. After 60 min of incubation at 37°C, A and B supernatants were collected and the amount of radiolabelled digoxin that passed through the endothelial monolayer was determined by scintillation counting. Clearance A to B and B to A was calculated, and then the ratio Q = CLBA/CLAB, as described previously (Josserand et al. 2006; Megard et al. 2002).
Data Analysis
Discrimination of aberrant values was assessed with a Dixon test. Concerning clearances, a two-tailed paired Student’s t-test was performed. Concerning ratio Q, because of the highly variable basal expression of the assayed molecules among patients, data were expressed as a percentage of Q modulation after exposure to ET-1 compared with control (%Q = QET-1 * 100/Qcontrol). A two-tailed Student’s t-test with unequal variances was performed on the percentages (Porcheray et al. 2005).
Results
Experiments were performed on three different patients, each time in quintuplicate. After 24 h of exposure to either 10 or 100 nM ET-1 (Bachem, France), no modulation of total (intracellular and membrane bound when cells were permeabilised) nor membrane (unpermeabilised cells) Pgp expression was observed (Fig. 1).
Fig. 1.
Pgp expression in adult HBMECs of an adult human in vitro BBB model exposed to ET-1. An adult human autologous in vitro BBB model was exposed to either 10 or 100 nM ET-1 for 24 h in the apical (luminal) compartment. Then, HBMECs were scraped, permeabilised or not and fixed in order to be stained with a specific phycoerythrin-conjugated Pgp antibody (or isotypic control). Pgp expression was then assessed by flow cytometry (in each case, between 5,000 and 10,000 cells were counted). (a) Total Pgp expression in adult permabilised HBMECs of an adult human in vitro BBB model exposed to 100 nM ET-1 for 24 h. (b) Total Pgp expression in adult permabilised HBMECs of an adult human in vitro BBB model exposed to 10 nM ET-1 for 24 h. (c) Pgp expression in adult unpermabilised HBMECs of an adult human in vitro BBB model exposed to either 100 or 10 nM ET-1 for 24 h
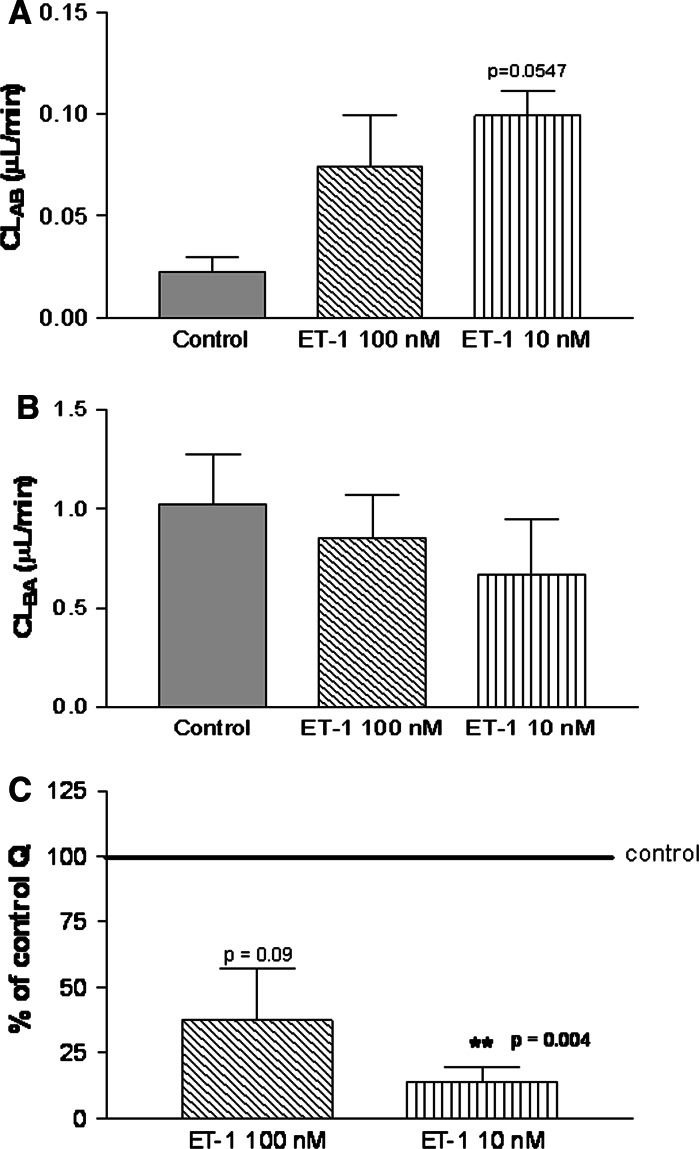
Clearance CLAB (luminal to abluminal) for 10 nM ET-1 (Fig. 2a) increased 4-fold compared with the control, and CLBA (abluminal to luminal) for the same concentration (Fig. 2b) decreased 0.35-fold compared with the control.
Fig. 2.
Digoxin passage through a human in vitro BBB model exposed to ET-1. In vitro human adult autologous BBBs were exposed to either 10 nM or 100 nM ET-1 in compartment A for 24 h. [14C]-Radiolabelled digoxin passage from A (upper chamber) to B (lower chamber) and from B to A compartment was determined in order to calculate the clearance of this substrate. (a) Digoxin clearance from compartment A to B (CLAB, luminal to abluminal) in a human adult in vitro BBB model exposed to ET-1 for 24 h. (b) Digoxin clearance from compartment B to A (CLBA, abluminal to luminal) in a human adult in vitro BBB model exposed to ET-1 for 24 h. (c) Digoxin Q ratio compared with control in adult human in vitro BBB after 24-h exposure to ET-1. The ratio Q equals CLBA/CLAB
The Q ratio for digoxin passage (Fig. 2c) decreased significantly (QET-1 10 nM = 14% of control ± SEM 4.4%, i.e., 85% decrease compared with control, P value = 0.004) after 24 h of exposure to 10 nM ET-1. The results were significant only at this concentration, but there was still a tendency for Q to decrease at 100 nM ET-1 (QET-1 100 nM = 37% of control ± SEM 16%, i.e., 63% decrease compared with control, P = 0.09).
Discussion
Digoxin is a typical Pgp substrate (Fernandez et al. 2004; Goralski et al. 2003). Even though it has been shown to be taken in charge by some transporters of the OATP family in rodents and in the human kidney, no data are available concerning human BBB (Hagenbuch et al. 2002; Kodawara et al. 2002; Mikkaichi et al. 2004). Besides, digoxin is a recommended probe to study Pgp in humans (Rautio et al. 2006). Therefore, ET-1 leads to a reduction in Pgp transport activity in adult HBMECs of a BBB in vitro model. This activity modulation is mainly due to the increase in CLAB, i.e., passage from blood to CNS mimicking compartments, and to a slight reduction in CLBA (Fig. 2). As the transporter activity drops, it can no longer efflux as much digoxin. These results are in partial agreement with those of Bauer et al. (2007), who reported a decrease in Pgp activity in rat capillaries exposed to ET-1 for 1–3 h using a cyclosporin A derivative. However, in the rat, a longer exposure (6 h) to ET-1 led to an increase in Pgp activity. Our findings were recorded in a human model exposed to ET-1 for 24 h. To understand the discrepancies between our observations and those in the rat, we have to keep in mind that the species used are different and so too are the model and Pgp substrate. However, we can still hypothesise that human cells may behave differently and may take longer to increase Pgp activity.
Another interesting finding in the human model is that the decrease in Pgp transport activity is much greater with 10 nM ET-1 than with 100 nM ET-1, and ET-1 is known to have different effects at different concentrations (Shirakami et al. 1993). Another pathway might be involved in the case of 100 nM ET-1, or a regulation event might be triggered faster in order to recover control Pgp activity. Different times of exposure to ET-1 could discriminate these hypotheses.
The rat and human models give different results in terms of Pgp expression, possibly because two genes encode Pgp in the rat but only one does in humans (Loscher and Potschka 2005b), and so their regulation and protein expression could differ. There are two receptor subtypes for the endothelin family -ETA and ETB- and ET-1 is able to bind both (Arai et al. 1990; Sakurai et al. 1990). Therefore, it would be interesting to determine which endothelin receptor is involved in such modulation of Pgp activity by ET-1, and subsequently to determine the mechanism of the effect (such as intracellular signalling events) and compare it with that highlighted in the rat by Bauer et al. (2007).
The effect of ET-1 on Pgp has a reality as, for instance, some HIV drugs increase ET-1 release from endothelial cells (Hebert et al. 2004; Jiang et al. 2006), and some HIV molecules themselves up-regulate ET-1 (Chauhan et al. 2007). Neuroinflammation can also modulate Pgp function (Morgan et al. 2008; Theron et al. 2003). The modulation of Pgp activity at the human BBB is of great interest as this transporter limits the entry of a large spectrum of therapeutical drugs (Rautio et al. 2006). Among Pgp substrates are some drugs used against Parkinson’s disease (Uhr et al. 2005), AIDS (Polli et al. 1999), amyotrophic lateral sclerosis (Milane et al. 2007) or Alzheimer’s disease (Saengkhae et al. 2007). However, this Pgp activity lowering can also counteract a natural brain defence as shown in the case of Alzheimer’s disease, for instance, where Pgp has an influence on limiting amyloid-β brain accumulation (Cirrito et al. 2005). Therefore, ET-1 could have a therapeutical potential by limiting Pgp activity and then enhancing drug delivery, but all the other effects of the transporter have to be considered.
In conclusion, ET-1 modulates the transport activity of Pgp in adult HBMECs after 24 h of exposure. This could be of relevance for diseases whose treatment requires the passage of drugs through the BBB.
Acknowledgements
We would like to thank the ANRS (Agence Nationale de Recherche sur le SIDA) for financial support. We would also like to thank Gabriel Gras (CEA Fontenay-aux-Roses, France) and Jean-François Heilier (Université Catholique de Louvain, Belgium) for their advice in statistics.
References
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348:730–732 [DOI] [PubMed] [Google Scholar]
- Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG (2004) Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res 64:1436–1443 [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13 [DOI] [PubMed] [Google Scholar]
- Barone FC, Willette RN, Yue TL, Feurestein G (1995) Therapeutic effects of endothelin receptor antagonists in stroke. Neurol Res 17:259–264 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Miller DS (2007) Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol 71:667–675 [DOI] [PubMed] [Google Scholar]
- Chauhan A, Hahn S, Gartner S, Pardo CA, Netesan SK, McArthur J, Nath A (2007) Molecular programming of endothelin-1 in HIV-infected brain: role of Tat in up-regulation of ET-1 and its inhibition by statins. FASEB J 21:777–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM (2005) P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest 115:3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR (1989) Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA 86:695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries HE, Kuiper J, de Boer AG, Van Berkel TJ, Breimer DD (1997) The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev 49:143–155 [PubMed] [Google Scholar]
- Didier N, Banks WA, Creminon C, Reuddre-Bosquet N, Mabondzo A (2002) HIV-1-induced production of endothelin-1 in an in vitro model of the human blood-brain barrier. Neuroreport 13:1179–1183 [DOI] [PubMed] [Google Scholar]
- Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A (2003) Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86:246–254 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Costa T, Clouse KA, Pluta RM, Ogino Y, Coligan JE, Burd PR (1993) Thrombin is a regulator of astrocytic endothelin-1. Brain Res 600:201–207 [DOI] [PubMed] [Google Scholar]
- Fernandez C, Buyse M, German-Fattal M, Gimenez F (2004) Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci 7:359–371 [PubMed] [Google Scholar]
- Goralski KB, Hartmann G, Piquette-Miller M, Renton KW (2003) Downregulation of mdr1a expression in the brain and liver during CNS inflammation alters the in vivo disposition of digoxin. Br J Pharmacol 139:35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gao B, Meier PJ (2002) Transport of xenobiotics across the blood-brain barrier. News Physiol Sci 17:231–234 [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS (2004) Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol 66:387–394 [DOI] [PubMed] [Google Scholar]
- Hawkins RA, O’Kane RL, Simpson IA, Vina JR (2006) Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 136:218S–226S [DOI] [PubMed] [Google Scholar]
- Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR (2004) Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc Toxicol 4:117–131 [DOI] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Zavecz JH, Dugas TR (2006) Antiretrovirals induce direct endothelial dysfunction in vivo. J Acquir Immune Defic Syndr 42:391–395 [DOI] [PubMed] [Google Scholar]
- Josserand V, Pelerin H, de Bruin B, Jego B, Kuhnast B, Hinnen F, Duconge F, Boisgard R, Beuvon F, Chassoux F, umas-Duport C, Ezan E, Dolle F, Mabondzo A, Tavitian B (2006) Evaluation of drug penetration into the brain: a double study by in vivo imaging with positron emission tomography and using an in vitro model of the human blood-brain barrier. J Pharmacol Exp Ther 316:79–86 [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Park JA, Lee SW, Kim WJ, Yu YS, Kim KW (2006) Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol 39:339–345 [DOI] [PubMed] [Google Scholar]
- Kodawara T, Masuda S, Wakasugi H, Uwai Y, Futami T, Saito H, Abe T, Inu K (2002) Organic anion transporter oatp2-mediated interaction between digoxin and amiodarone in the rat liver. Pharm Res 19:738–743 [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H (2005a) Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci 6:591–602 [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H (2005b) Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76:22–76 [DOI] [PubMed] [Google Scholar]
- Mangahas CR, la Cruz GV, Friedman-Jimenez G, Jamal S (2005) Endothelin-1 induces CXCL1 and CXCL8 secretion in human melanoma cells. J Invest Dermatol 125:307–311 [DOI] [PubMed] [Google Scholar]
- Megard I, Garrigues A, Orlowski S, Jorajuria S, Clayette P, Ezan E, Mabondzo A (2002) A co-culture-based model of human blood-brain barrier: application to active transport of indinavir and in vivo-in vitro correlation. Brain Res 927:153–167 [DOI] [PubMed] [Google Scholar]
- Mikkaichi T, Suzuki T, Onogawa T, Tanemoto M, Mizutamari H, Okada M, Chaki T, Masuda S, Tokui T, Eto N, Abe M, Satoh F, Unno M, Hishinuma T, Inui K, Ito S, Goto J, Abe T (2004) Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc Natl Acad Sci USA 101:3569–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R (2007) Minocycline and riluzole brain disposition: interactions with p-glycoprotein at the blood-brain barrier. J Neurochem 103:164–173 [DOI] [PubMed] [Google Scholar]
- Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, Charles KA, Clarke SJ, Kacevska M, Liddle C, Richardson TA, Sharma R, Sinal CJ (2008) Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos 36:205–216 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Morimoto S, Takamoto S, Kitano S, Fukuo K, Onishi T, Ogihara T (1994) Endothelin-1 in cerebrospinal fluid in elderly patients with hypertension and dementia. Hypertension 24:97–100 [DOI] [PubMed] [Google Scholar]
- Nie XJ, Olsson Y (1996) Endothelin peptides in brain diseases. Rev Neurosci 7:177–186 [DOI] [PubMed] [Google Scholar]
- Pardridge WM (2007) Blood-brain barrier delivery. Drug Discov Today 12:54–61 [DOI] [PubMed] [Google Scholar]
- Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR, Woolley JL (1999) Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res 16:1206–1212 [DOI] [PubMed] [Google Scholar]
- Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Reuddre-Bosquet N, Dormont D, Gras G (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio J, Humphreys JE, Webster LO, Balakrishnan A, Keogh JP, Kunta JR, Serabjit-Singh CJ, Polli JW (2006) In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab Dispos 34:786–792 [DOI] [PubMed] [Google Scholar]
- Rolinski B, Heigermoser A, Lederer E, Bogner JR, Loch O, Goebel FD (1999) Endothelin-1 is elevated in the cerebrospinal fluid of HIV-infected patients with encephalopathy. Infection 27:244–247 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R (2006) HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol 70:1087–1098 [DOI] [PubMed] [Google Scholar]
- Saengkhae C, Salerno M, Ades D, Siove A, Le Moyeca L, Migonney V, Garnier-Suillerot A (2007) Ability of carbazole salts, inhibitors of Alzheimer beta-amyloid fibril formation, to cross cellular membranes. Eur J Pharmacol 559:124–131 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T (1990) Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348:732–735 [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Takimoto M, Oda K, Fruh T, Takai M, Okada T, Hori S (1997) Endothelin evokes efflux of glutamate in cultures of rat astrocytes. J Neurochem 68:2194–2200 [DOI] [PubMed] [Google Scholar]
- Schinelli S (2006) Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem 13:627–638 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Kipnis J (2006) Does inflammation in an autoimmune disease differ from inflammation in neurodegenerative diseases? Possible implications for therapy. J Neuroimmune Pharmacol 1:4–10 [DOI] [PubMed] [Google Scholar]
- Shirakami G, Nakao K, Saito Y, Magaribuchi T, Mukoyama M, Arai H, Hosoda K, Suga S, Mori K, Imura H (1993) Low doses of endothelin-1 inhibit atrial natriuretic peptide secretion. Endocrinology 132:1905–1912 [DOI] [PubMed] [Google Scholar]
- Speciale L, Sarasella M, Ruzzante S, Caputo D, Mancuso R, Calvo MG, Guerini FR, Ferrante P (2000) Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurovirol 6(Suppl 2):S62–S66 [PubMed] [Google Scholar]
- Suzuki R, Masaoka H, Hirata Y, Marumo F, Isotani E, Hirakawa K (1992) The role of endothelin-1 in the origin of cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg 77:96–100 [DOI] [PubMed] [Google Scholar]
- Takuwa Y, Yanagisawa M, Takuwa N, Masaki T (1989) Endothelin, its diverse biological activities and mechanisms of action. Prog Growth Factor Res 1:195–206 [DOI] [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, Ruhn KA, Tran TA (2008) Neuroinflammation in Parkinson’s disease: is there sufficient evidence for mechanism-based interventional therapy? Front Biosci 13:709–717 [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T (1992) Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 267:20383–20391 [PubMed] [Google Scholar]
- Theron D, de Barraud LS, Tardivel S, Pelerin H, Demeuse P, Mercier C, Mabondzo A, Farinotti R, Lacour B, Roux F, Gimenez F (2003) Influence of tumor necrosis factor-alpha on the expression and function of P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochem Pharmacol 66:579–587 [DOI] [PubMed] [Google Scholar]
- Uhr M, Ebinger M, Rosenhagen MC, Grauer MT (2005) The anti-Parkinson drug budipine is exported actively out of the brain by P-glycoprotein in mice. Neurosci Lett 383:73–76 [DOI] [PubMed] [Google Scholar]
- Wright CE, Fozard JR (1988) Regional vasodilation is a prominent feature of the haemodynamic response to endothelin in anaesthetized, spontaneously hypertensive rats. Eur J Pharmacol 155:201–203 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415 [DOI] [PubMed] [Google Scholar]
- Yoshimoto S, Ishizaki Y, Kurihara H, Sasaki T, Yoshizumi M, Yanagisawa M, Yazaki Y, Masaki T, Takakura K, Murota S (1990) Cerebral microvessel endothelium is producing endothelin. Brain Res 508:283–285 [DOI] [PubMed] [Google Scholar]
- Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M (1992) Increased plasma endothelin-1 in acute ischemic stroke. Stroke 23:1014–1016 [DOI] [PubMed] [Google Scholar]