Abstract
Butyrate-degrading bacteria in four methanogenic sludges were studied by RNA-based stable isotope probing. Bacterial populations in the 13C-labeled rRNA fractions were distinct from unlabeled fractions, and Syntrophaceae species, Tepidanaerobacter sp., and Clostridium spp. dominated. These results suggest that diverse microbes were active in butyrate degradation under methanogenic conditions.
Butyrate is one of the important intermediates in the degradation of organic matter under methanogenic conditions (14, 16). Under these conditions, butyrate degradation is carried out by a syntrophic association of butyrate-oxidizing bacteria and hydrogenotrophic methanogens, because of thermodynamic constraints (17). Due to the fastidious nature of this syntrophic metabolism, isolation of butyrate-degrading syntrophs has been difficult and thus information on butyrate-degrading bacteria is based on some isolates belonging to the family Syntrophomonadaceae. Due to this lack of knowledge and the lack of appropriate molecular markers, culture-independent studies have focused only on species of the family Syntrophomonadaceae (5, 15, 25). Consequently, the natural diversity of syntrophic butyrate-degrading bacteria has not been studied in any detail.
The recent development of stable isotope probing (SIP) enables metabolic function and taxonomic identity to be examined concurrently (3). Only one study on methanogenic butyrate degradation has been reported with this technique (1), but not within waste/wastewater-treated methanogenic sludges. SIP provides a potentially fruitful tool for identifying potential butyrate-degraders in a methanogenic environment. In this study, therefore we used RNA-based SIP (RNA-SIP) with [13C4]butyrate as a substrate to explore the microorganisms involved in butyrate degradation in four methanogenic sludges.
Four methanogenic sludges were used in this study. Mesophilic granular sludge MP and thermophilic granular sludge TP were taken from two lab-scale multistage upflow anaerobic sludge blanket reactors treating palm oil mill effluent. Mesophilic anaerobic digester sludge treating palm oil mill effluent (sludge MBF) and thermophilic digester sludge treating municipal solid waste (sludge JET) were taken from commercial plants. Detailed properties of these sludges were described in our previous report (7). Incubation was carried out anaerobically at 37°C (for mesophilic sludges) or 55°C (for thermophilic sludges). The granular sludges TP and MP were preincubated with 5 mM butyrate because of prolonged storage at 4°C for over 2 years. Degradation of butyrate was monitored by measuring methane production using gas chromatography as described previously (6). Preincubation was conducted for 14 days, added butyrate was completely converted to methane, and then the sludge was sampled as an unlabeled control microbial consortium. Digester sludges MBF and JET were used immediately after sampling, and RNA extracted from unincubated sludges was used as an unlabeled control RNA. Incubation of stable isotope-labeled substrate was performed by a previously described method (7), using [13C4]butyrate (Isotec, Miamisburg, OH) at a concentration of 5 mM (0.12 mmol). During incubation, over 95% of [13C4]butyrate was converted to methane in 3 and 7 days for sludges TP and MP, respectively. For sludges MBF and JET, [13C4]butyrate was mostly (over 80%) converted to methane within 5 and 2 days, respectively. After the incubation, the sludge (∼5 ml) was sampled. This [13C4]butyrate incubation step was repeated to obtain the second incubated sludge. The incubation experiment was repeated twice at almost the same methanogenesis rate.
Total RNA extraction from the collected sludge samples and RNA purification were conducted by the method described previously (21). RNA was separated by equilibrium density gradient centrifugation and fractionated. The bacterial rRNA content in each fraction was quantified by quantitative reverse transcription-PCR (RT-PCR) with bacterial universal primers (6). The unlabeled control rRNA gradient showed peaks at a buoyant density (BD) around 1.77 g·ml−1, which is characteristic of unlabeled bacterial rRNAs in cesium trifluoroacetate (see Fig. S1 in the supplemental material) (12). In sludge MP, the rRNA gradient profile for the first incubation sludge had an increase in heavier (>1.78 g·ml−1) rRNA, but the peak BD was almost the same as in control rRNA (see Fig. S1A in the supplemental material). In sludges JET, TP, and MBF, after the first incubation with [13C4]butyrate, the rRNA profile gradually shifted toward the heavier BDs and a tailing of 13C-enriched (>1.8 g·ml−1) rRNA was detected (see Fig. S1B, C, and D in the supplemental material). After the second incubation, the entire rRNA BDs shifted toward the heavier fraction in all sludges (see Fig. S1 in the supplemental material). We used the RNAs extracted from the first [13C4]butyrate incubation sludges for further analysis, because all sludges could be effectively labeled by the first [13C4]butyrate incubation. This pulse sampling could avoid extended incubation and minimize carbon cross-feeding (13).
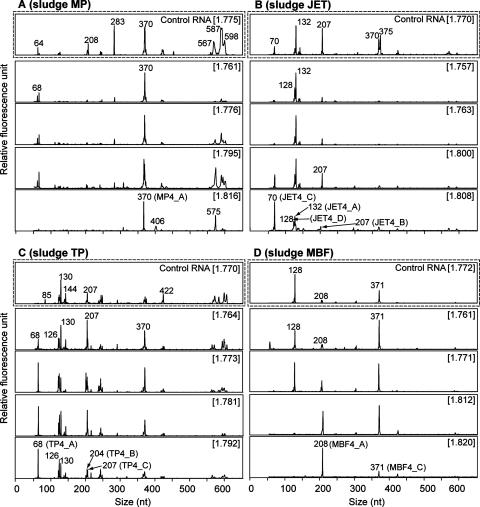
The representative bacterial terminal restriction fragment length polymorphism (T-RFLP) fingerprint profiles of 13C-labeled and unlabeled fractions from [13C4]butyrate incubation sludges and control RNA are shown in Fig. 1. We constructed clone libraries from the heaviest RNA fractions and analyzed 50 clones for each library as described previously (6). The phylogenetic affiliations of all analyzed bacterial clones are summarized in Table 1, and the positions of selected phylotypes, represented by at least four clones, are shown in Fig. 2.
FIG. 1.
Bacterial T-RFLP fingerprinting of density-resolved RNA from representative 13C-labeled and unlabeled fractions of sludges MP (A), JET (B), TP (C), and MBF (D). Electropherograms were generated from gradient fractions of RNA from the first [13C4]butyrate incubation as a template. Dashed boxes show T-RFLP fingerprints of density-resolved unlabeled control RNA. Cesium trifluoroacetate BDs (g·ml−1) of gradient fractions are given in brackets. The numbers at the T-RF peaks indicate the T-RF lengths. Representing phylotypes of T-RFs identified by clone analysis are indicated in parentheses.
TABLE 1.
Phylogenetic affiliation and numbers of bacterial 16S rRNA clones retrieved from clone libraries generated from 13C-labeled RNA fractions
| Phylogenetic groupa | No. of clones
|
T-RF length of clones (bp)b | |||
|---|---|---|---|---|---|
| MP | JET | TP | MBF | ||
| Bacteroidetes | 1 | 9 | 5 | 6 | 207, 370, 371 |
| Clostridium | 46 | 14 | 2 | 68, 70, 369, 371 | |
| Syntrophomonas | 1 | 299 | |||
| Syntrophothermus | 2 | 372 | |||
| Tepidanaerobacter | 7 | 19 | 68 | ||
| Desulfotomaculum | 1 | 1 | 132, 244 | ||
| Syntrophaceae | 31 | 208 | |||
| Clone MST group | 5 | 248 | |||
| Petrobacter | 3 | 313 | |||
| Synergistes | 2 | 130 | |||
| Spirochaetes | 4 | 129 | |||
| Coprothermobacter | 12 | 132 | |||
| Anaerobaculum | 6 | 2 | 128 | ||
| EM3 | 8 | 204 | |||
| OP7 | 1 | 130 | |||
| OP5 | 3 | 72, 132 | |||
| OP10 | 3 | 127 | |||
| Marine group A | 3 | 207 | |||
| WWE1 | 1 | 305 | |||
| NKB19 | 1 | 210 | |||
| Unidentified | 1 | 73 | |||
| Total | 50 | 50 | 50 | 50 | |
Phylogenetic groups with cultivated representatives are named according to the taxonomic outline of Bergey's Manual of Systematic Bacteriology (4). Candidate phyla are named based on the review by Hugenholtz (9).
Phylotypes that are represented by at least three clones and whose sequence length matches the lengths of specific T-RFs are highlighted in boldface, and others had T-RF lengths predicted using sequence data. Note that measured T-RFs are typically 0 to 3 bases shorter than the predicted T-RFs (24).
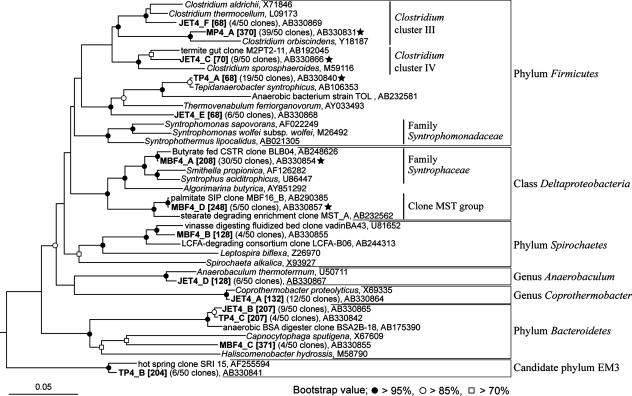
FIG. 2.
Phylogenetic placement of representative bacterial 16S rRNA phylotypes from 13C-labeled RNA fractions. The phylogenetic tree was constructed by the neighbor-joining method. The measured T-RF lengths of phylotypes digested with MspI are shown in brackets. The scale bar represents the number of changes of nucleotides per sequence position. The symbols at each branch point show the bootstrap values obtained with 1,000-resampling analysis. Stars indicate putative butyrate degraders inferred from our results.
The 370-bp terminal restriction fragment (T-RF) was dominant throughout the fractions of sludge MP (Fig. 1A). The T-RF corresponds to phylotype MP4_A (39 clones), and the most closely related isolate was Clostridium orbiscindens (96% of 16S rRNA gene sequence similarity) (Fig. 2). The 575-bp T-RF also dominated the 13C-labeled fraction (Fig. 1A) but was not detected in clones that indicate the 575-bp T-RF. Since we used different primer sets for T-RFLP analysis and construction of clone library, it might be influence these results. Within sludge JET, the 132-bp T-RF was dominant in fingerprints generated from unlabeled control RNA (Fig. 1B), but not after [13C4]butyrate incubation. The 70-bp T-RF increased in relative abundance, specifically in the 13C-labeled fraction, corresponding to the second-most-abundant phylotype, JET4_C, belonging to Clostridium cluster IV (Fig. 2). Other dominant phylotypes JET4_A (12 clones) and JET4_D (6 clones) were closely related to Coprothermobacter spp. and Anaerobaculum spp., respectively, but intensities of corresponding T-RFs were reduced toward heavier fractions (Fig. 1B). Phylotype JET4_B (nine clones) was deeply branched within phylum Bacteroidetes, with T-RFs corresponding to 207 bp (Fig. 2). No known butyrate-oxidizing bacteria were sequenced from the sludges MP and JET. Based on these results, we speculate that Clostridium spp. of phylotypes MP4_A and JET4_C may be closely linked to the degradation of butyrate in sludges MP and JET, respectively (Fig. 2). Chauhan and Ogram also reported the Clostridium sequence, derived from their DNA-SIP study using butyrate, might be represent a novel butyrate-oxidizing bacterium (1).
Diverse T-RFs were detected from sludge TP, and the major T-RFs of 130, 207, and 370 bp in the unlabeled light fractions were markedly reduced in abundance in the 13C-labeled fractions (Fig. 1C). The 68-bp T-RF that dominated the 13C-labeled fraction (Fig. 1C), corresponding to the most abundant phylotype, TP4_A (19 clones), was closely related to Tepidanaerobacter syntrophicus and an anaerobic strain, TOL (Fig. 2). Strain TOL is likely involved in palmitate degradation, as previously indicated (6), and thus phylotype TP4_A might also be involved in butyrate degradation.
Within sludge MBF, phylotype MBF4_A (30 clones), representing a 208-bp T-RF, clustered with the family Syntrophaseae and was closely related to clone BLB04, with 99% 16S rRNA gene sequence similarity. Clone BLB04 was the most abundant clone retrieved from a butyrate-fed methanogenic chemostat at a low dilution rate (23), and cultured related species of Smithella propionica and Syntrophus aciditrophicus were known to degrade butyrate (10, 11). The second-most-abundant phylotype was MBF4_D (five clones), but its corresponding T-RF of phylotype MBF4_D of 248 bp was not detected (Fig. 1D). Phylotype MBF4_D was clustered with the clone MST group of the class Deltaproteobacteria, which might be considered a novel fatty acid-degrading bacterial group (6, 7). Thus, based on our results and previous findings (6, 7, 10, 11, 23), microbes detected as phylotypes MBF4_A and MBF4_D likely degrade butyrate in sludge MBF.
The community analysis of 13C-labeled RNA showed that different species were retrieved from different sludges. We have detected only three clones (two clones from sludge TP and one clone from sludge MBF) related to Syntrophomonadaceae species that were typically shown to be fatty acid-degrading microorganisms under the methanogenic conditions in this study (Table 1). Previously, Zellner et al. reported that organisms related to Syntrophomonadaceae species were not abundant enough to contribute to the observed butyrate turnover rate in the bioreactors (25). They concluded that the main syntrophic butyrate-oxidizing bacteria in the reactors might be an as-yet-unknown species. Recently, Tang et al. also showed that non-Syntrophomonadaceae species were dominant in a butyrate-fed methanogenic chemostat (23). Given the previous reports and our results, non-Syntrophomonas bacteria may also have a role in butyrate degradation in methanogenic environments. However, our result might be affected by longer periods of sludge storage, especially for sludge MP, resulting in survival of spore-forming bacteria. Furthermore, we need unlabeled substrate control experiments for comments on the impact of community change upon incubation, which is a potential limitation of this study.
During the β-oxidation of butyrate, cross-feeding of intermediately formed [13C]acetate could label the non-butyrate degrader. Some clostridial species could degrade acetate in syntrophic association with hydrogenotrophic methanogens (8, 19), and Syntrophus spp. have also been reported to utilize acetate (2). However, acetate is converted to methane by aceticlastic methanogens in methanogenic sludges immediately (22) and [13C]acetate might be assimilated by methanogenic archaea, because archaeal rRNAs also became heavier (see Fig. S1 in the supplemental material). Furthermore, syntrophic acetate oxidation tends to occur under certain conditions, such as high ammonium or volatile fatty acid concentrations (18, 20). Therefore, the dominant sequences identified here were considered to be labeled as a result of utilization of [13C4]butyrate. Application of SIP with [13C]acetate could elucidate the flow of acetate in the methanogenic sludges.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequence data obtained in this study have been deposited in the GenBank/EMBL/DDBJ database under accession no. AB330831 to AB330871.
Supplementary Material
Acknowledgments
We thank Eiji Masai at Nagaoka University of Technology for use of the ultracentrifuge.
This study was financially supported by the New Energy and Industrial Technology Development Organization; Japan Society for the Promotion of Science; and the 21st Century COE program “Global Renaissance by Green Energy Revolution,” subsidized by the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Footnotes
Published ahead of print on 11 April 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Chauhan, A., and A. Ogram. 2006. Fatty acid-oxidizing consortia along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:2400-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan, A., and A. Ogram. 2006. Phylogeny of acetate-utilizing microorganisms in soils along a nutrient gradient in the Florida Everglades. Appl. Environ. Microbiol. 72:6837-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumont, M. G., and J. C. Murrell. 2005. Stable isotope probing—linking microbial identity to function. Nat. Rev. Microbiol. 3:499-504. [DOI] [PubMed] [Google Scholar]
- 4.Garrity, G. M., J. A. Bell, and T. G. Lilburn. 2004. Taxonomic outline of the prokaryotes. Bergey's manual of systematic bacteriology, 2nd ed., release 5.0. Springer-Verlag, New York, NY. doi: 10.1007/bergeysoutline200405. [DOI]
- 5.Hansen, K. H., B. K. Ahring, and L. Raskin. 1999. Quantification of syntrophic fatty acid-β-oxidizing bacteria in a mesophilic biogas reactor by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 65:4767-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatamoto, M., H. Imachi, A. Ohashi, and H. Harada. 2007. Identification and cultivation of anaerobic, syntrophic long-chain fatty acid-degrading microbes from mesophilic and thermophilic methanogenic sludges. Appl. Environ. Microbiol. 73:1332-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatamoto, M., H. Imachi, Y. Yashiro, A. Ohashi, and H. Harada. 2007. Diversity of anaerobic microorganisms involved in long-chain fatty acid degradation in methanogenic sludges as revealed by RNA-based stable isotope probing. Appl. Environ. Microbiol. 73:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattori, S., Y. Kamagata, S. Hanada, and H. Shoun. 2000. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int. J. Syst. Evol. Microbiol. 50:1601-1609. [DOI] [PubMed] [Google Scholar]
- 9.Hugenholtz, P. 2002. Exploring prokaryotic diversity in the genomic era. Genome Biol. 3:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson, B. E., V. K. Bhupathiraju, R. S. Tanner, C. R. Woese, and M. J. McInerney. 1999. Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms. Arch. Microbiol. 171:107-114. [DOI] [PubMed] [Google Scholar]
- 11.Liu, Y., D. L. Balkwill, H. C. Aldrich, G. R. Drake, and D. R. Boone. 1999. Characterization of the anaerobic propionate-degrading syntrophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolinii. Int. J. Syst. Bacteriol. 49:545-556. [DOI] [PubMed] [Google Scholar]
- 12.Lueders, T., M. Manefield, and M. W. Friedrich. 2004. Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6:73-78. [DOI] [PubMed] [Google Scholar]
- 13.Lueders, T., B. Pommerenke, and M. W. Friedrich. 2004. Stable-isotope probing of microorganisms thriving at thermodynamic limits: syntrophic propionate oxidation in flooded soil. Appl. Environ. Microbiol. 70:5778-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackie, R. I., and M. P. Bryant. 1981. Metabolic activity of fatty acid-oxidizing bacteria and the contribution of acetate, propionate, butyrate, and CO2 to methanogenesis in cattle waste at 40 and 60°C. Appl. Environ. Microbiol. 41:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menes, R. J., and D. Travers. 2006. Detection of fatty acid beta-oxidizing syntrophic bacteria by fluorescence in situ hybridization. Water Sci. Technol. 54:33-39. [DOI] [PubMed] [Google Scholar]
- 16.Parkin, G. F., and W. F. Owen. 1986. Fundamentals of anaerobic digestion of wastewater sludge. J. Environ. Eng. 112:867-920. [Google Scholar]
- 17.Schink, B. 1997. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61:262-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnürer, A., F. P. Houwen, and B. H. Svensson. 1994. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch. Microbiol. 162:70-74. [Google Scholar]
- 19.Schnürer, A., B. Schink, and B. H. Svensson. 1996. Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium. Int. J. Syst. Bacteriol. 46:1145-1152. [DOI] [PubMed] [Google Scholar]
- 20.Schnürer, A., G. Zellner, and B. H. Svensson. 1999. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. FEMS Microbiol. Ecol. 29:249-261. [Google Scholar]
- 21.Sekiguchi, Y., Y. Uyeno, A. Sunaga, H. Yoshida, and Y. Kamagata. 2005. Sequence-specific cleavage of 16S rRNA for rapid and quantitative detection of particular groups of anaerobes in bioreactors. Water Sci. Technol. 52:107-113. [PubMed] [Google Scholar]
- 22.Speece, R. E. 1996. Anaerobic biotechnology for industrial wastewaters. Archae Press, Nashville, TN.
- 23.Tang, Y.-Q., T. Shigematsu, S. Morimura, and K. Kida. 2007. Effect of dilution rate on the microbial structure of a mesophilic butyrate-degrading methanogenic community during continuous cultivation. Appl. Microbiol. Biotechnol. 75:451-465. [DOI] [PubMed] [Google Scholar]
- 24.Yu, C.-P., R. Ahuja, G. Sayler, and K.-H. Chu. 2005. Quantitative molecular assay for fingerprinting microbial communities of wastewater and estrogen-degrading consortia. Appl. Environ. Microbiol. 71:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zellner, G., A. J. L. Macario, and E. Conway de Macario. 1997. A study of three anaerobic methanogenic bioreactors reveals that syntrophs are diverse and different from reference organisms. FEMS Microbiol. Ecol. 22:295-301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.