Abstract
Six rhesus monkeys responding under a three-component multiple schedule were administered haloperidol to determine its effects on cocaine self-administration and on cocaine's disruptive effects on the repeated acquisition and performance of response chains. In the absence of haloperidol, 0.0032–0.032 mg/kg/infusion of cocaine increased response rate and the number of infusions in the self-administration component when compared to saline administration, whereas 0.1–0.32 mg/kg/infusion decreased response rate and the number of infusions. When compared to saline administration, the two lowest infusion doses of cocaine had little or no effect on responding in the acquisition and performance components; however, higher infusion doses of cocaine dose-dependently decreased response rate in these components. In addition, the higher doses of cocaine also increased the percentage of errors in the acquisition and performance components. Pretreatment with haloperidol (0.0032 or 0.01 mg/kg, i.m.) antagonized the effects of low doses of cocaine on the number of infusions in the self-administration component, whereas only the 0.01-mg/kg dose antagonized the effects of high doses of cocaine on the number of infusions. Neither dose of haloperidol antagonized the rate-decreasing effects of cocaine on responding in the acquisition and performance components significantly; the highest dose of haloperidol alone decreased rates of responding in each component. Antagonism of cocaine's error-increasing effects by haloperidol was only evident at one dose of cocaine (0.032 mg/kg/infusion), and was more complete in the performance components than in the acquisition components. Together, these data show the limited suitability of haloperidol for selectively antagonizing cocaine self-administration in the context of a multiple schedule involving transition behavior, and show the lack of uniform antagonism across operant behaviors.
Keywords: self-administration, repeated acquisition, multiple schedule, cocaine, haloperidol, key press, rhesus monkeys
As cocaine remains one of the most widely-used illicit substances in the United States (Substance Abuse and Mental Health Services Administration (SAMHSA), 2003), there is a continuing need for the development of effective pharmacotherapies for cocaine abusers (Vocci, Acri, & Elkashef, 2005). Much of the research to date has focused on drugs that bind to either the dopamine transporter or to D1-like and D2-like dopamine receptors, but none have proven successful clinically for a variety of reasons (Howell & Wilcox, 2001; Negus, Mello, Lamas, & Mendelson, 1996; Platt, Rowlett, & Spealman, 2002). For example, drugs that selectively block the dopamine transporter such as GBR 12909 can also be self-administered (Stafford, LeSage, Rice, & Glowa, 2001; Wojnicki & Glowa, 1996), and many of the D2-like dopamine receptor antagonists such as chlorpromazine or flupenthixol can increase cocaine self-administration (Glowa & Wojnicki, 1996; Roberts & Vickers, 1984; Wilson & Schuster, 1973), decrease it (Negus et al., 1996; Woolverton, 1986; Woolverton & Virus, 1989) or have other behavioral effects that are not well tolerated such as drowsiness or extrapyramidal effects (Magliozzi, Gillespie, Lombrozo, & Hollister, 1985). Antagonizing the behavioral effects of cocaine might also be difficult with dopamine antagonists because both norepinephrine and serotonin have been shown to play a role in many of cocaine's behavioral effects (Hadfield, Mott, & Ismay, 1980; Ross & Renyi, 1969). Therefore, there is the possibility that the most effective pharmacotherapies for cocaine abuse should target multiple receptor systems, just as the development of “selectively nonselective” central nervous system (CNS) drugs has become a popular and successful approach for treating depression and schizophrenia (Roth, Sheffler, & Kroeze, 2004). This approach may also be valuable for treating drug abuse because, similar to these disorders, drug abuse is more than likely a polygenic disorder with substantial environmental components (e.g., see Nestler, 2004).
Another area where the search for an effective pharmacotherapy for cocaine abuse seems to have fallen short is in the development of behavioral baselines that can assess the effects of potential treatments on more than one behavior during a single session. Furthermore, the primary dependent measure in most animal studies has been the quantity (rate) of behavior, which may or may not be as important as other dependent measures such as the quality (accuracy) of behavior. Although new procedures have evolved for examining the variables that control drug self-administration and for examining the effects of pharmacotherapies on those variables (e.g., Dworkin, Mirkis, & Smith, 1990; Nader, Sinnott, Mach, & Morgan, 2002; Paronis, Gasior, & Bergman, 2002), studying a greater variety of behaviors along with self-administration may be important in helping to uncover additional variables that might affect drug self-administration. For example, if the behavior that precedes or follows drug self-administration is a transitional behavior such as “learning” (cf. Thompson & Moerschbaecher, 1978), then many of the variables that affect transitional behavior might also affect self-administration. A more careful examination of the interaction between self-administration behavior and transitional behavior would also seem appropriate because: (1) most organisms frequently engage in transitional behavior; (2) transitional behavior may engage different neuronal circuitry and neurotransmitters than steady-state behavior (Myhrer, 2003); and (3) transitional behavior can be more sensitive than steady-state behavior (Thompson & Moerschbaecher, 1979), and this could make behavior that occurs with transition behavior more or less sensitive to disruption by a drug. Finally, a behavioral analysis of the potential interaction between self-administration behavior and transitional behavior may be important because the most effective pharmacotherapies may well be those that can selectively alter one behavior while leaving others intact (Howell & Wilcox, 2001).
Recently, a multiple schedule with components of cocaine self-administration, repeated acquisition of response chains, and performance of response chains was used to compare the reinforcing effects of cocaine with its disruptive effects on food-maintained responding in the acquisition and performance components. In that study, Winsauer, Silvester, Moerschbaecher and France (2000) demonstrated that the reinforcing effects of cocaine (under the schedule parameters chosen) had similar potency to its disruptive effects on food-maintained responding in the acquisition and performance components. More specifically, varying doses of cocaine maintained responding in the self-administration component while also producing both rate-decreasing and error-increasing effects on responding in the acquisition and performance components. Consistent with much of the previous literature involving repeated acquisition (e.g., Thompson & Moerschbaecher, 1979), responding in the acquisition components was more susceptible than responding in the performance components to the rate-decreasing and error-increasing effects of cocaine. Another important aspect of this study was the finding that the fixed-ratio (FR) schedule in the self-administration component, which affected both the total dose of cocaine that was obtained and the maximum number of infusions per component, served as a controlling variable for responding in the self-administration component and as a contextual variable for responding in the acquisition and performance components of the multiple schedule. More specifically, both the rate-decreasing and error-increasing effects of cocaine tended to be smaller in the acquisition and performance components when the ratio in the self-administration component was increased from 30 to 90. This type of contextual effect, which resulted from altering the response requirement for cocaine, was reminiscent of a finding by Nader and Woolverton (1992), who found that increasing the FR for cocaine affected responding for food in another component in a discrete-trials choice procedure.
The same three-component multiple schedule of self-administration, repeated acquisition and performance was used with monkeys in a second study to investigate whether there were quantitatively and qualitatively similar effects produced by contingent (response dependent) and noncontingent (response independent) administration of cocaine (Winsauer, Moerschbaecher, Molina, & Roussell, 2003). Although results from the first study suggested that there may only be small differences between experimenter-delivered and self-administered cocaine in terms of its disruptive effects, data from the literature involving both rats and monkeys had reported that the effects of contingently or noncontingently administered cocaine could differ in important ways (cf. Winsauer et al., 2003). Therefore, subjects in the second study were allowed to self-administer cocaine under a FR-60 schedule, and the rate-decreasing and error-increasing effects in the acquisition and performance components were characterized. Following the establishment of dose-effect curves for cocaine in all the subjects, the FR schedule in the self-administration component was replaced with a response-independent variable-time (VT) schedule and the individual infusion patterns obtained for each infusion dose were “replayed” to the subject in order to observe the effects of these response-independent infusions on responding in the acquisition and performance components. After testing all of the infusion doses under the VT schedule, the results indicated that both contingently and noncontingently administered cocaine produced comparable disruptions of repeated acquisition and performance behavior.
The purpose of the present experiment was to use the same multiple schedule to determine the effectiveness of an antagonist in antagonizing the reinforcing effects of cocaine and its disruptive effects on learning and performance. If, for example, an antagonist could effectively antagonize the reinforcing effects of cocaine, the responding of subjects in the self-administration component might decrease along with the number of infusions, and thereby decrease the disruptive effects on responding in the acquisition and performance components. If, however, the antagonist was ineffective or only partially antagonized the reinforcing effects of cocaine, responding in the self-administration component might not change or possibly increase, and thereby increase the disruptive effects on responding in the acquisition and performance components.
The antagonist that was chosen for this study was the dopamine type-2 receptor antagonist haloperidol. Although there are studies in both rats and monkeys to suggest that haloperidol has limited effectiveness for antagonizing cocaine self-administration (de la Garza & Johanson, 1982; Richardson, Smith, & Roberts, 1994; Roberts & Vickers, 1987), evaluating a known drug was important in order to determine if its effects in the present task were similar to those reported previously in other studies. Haloperidol was also chosen because it is a drug that has high affinity for a number of dopamine receptors (D2 > D1 = D3 = D4), alpha receptors (α1B > α1A) and a serotonin receptor (5-HT2A) (Roth et al., 2004). Finally, haloperidol has been shown to be rapidly absorbed and to produce a sustained pharmacological effect (Magliozzi et al., 1985) that could be examined over lengthy experimental sessions.
Method
Subjects
Six male rhesus monkeys (Macaca mulatta) ranging in age from 5 to 16 years old were implanted with vascular access ports and served as subjects. Each subject had a history of responding under repeated-acquisition procedures and had a long history of experimenter-delivered drug administration while responding under these complex operant schedules. During the past several years, 4 of these subjects (79, LU, TR and JO) had an extensive history of responding under the three-component multiple schedule used in this study. All of the monkeys were housed individually in stainless-steel cages and were moderately food restricted (approximately 95% of their free-feeding weight) by limiting their postsession feeding. The mean weight for the group was 10.4 kg and the weights ranged from 9.9 to 11.7 kg. Their diet consisted of banana-flavored food pellets (Research Diets, Inc., New Brunswick, NJ), monkey chow (Harlan Teklad, Madison, WI), fresh fruit and vitamins. The pellets were earned during the experimental session whereas the remainder of the diet was fed to each subject several hours after their daily session. Water was available ad lib in the home cage. For behavioral testing, each subject was removed from the colony-room cage and transported via a macaque restrainer (Primate Products, Inc., Redwood City, CA) to experimental stations located in another room. These studies were carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center, and guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institute of Health.
Apparatus
Six experimental stations were used to test the subjects. Each monkey was seated in a restrainer for the duration of the experimental session and was secured in position at approximately arms-length from a response panel that was mounted to the wall of each experimental station. As shown in Figure 1, each aluminum response panel (measuring 54 cm × 35.5 cm) contained three translucent response keys aligned horizontally (8 cm apart, center to center at eye level) and a single response lever located 7 cm above the center key. An in-line stimulus projector, mounted behind each key, projected colors and geometric forms onto the key. In addition to these stimuli, there was a single tricolored stimulus located 7 cm above the response lever and a single response key that could be illuminated with white light located above the food pellet aperture. Response keys required a minimum force of 0.15 N for activation and each correct response on the keys produced an audible click of a feedback relay. A response on the lever also produced a click of the feedback relay. Recessed incandescent lights located in each chamber provided overhead lighting. All six experimental stations and their associated infusion pumps (Razel, model A; Stamford, CT) and cumulative recorders (Gerbrands Corp., Arlington, MA) were connected via an interface to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc. St. Albans, VT).
Fig 1.
Drawing of the response panel used to test the subjects under the three-component multiple schedule of self-administration, repeated acquisition and performance.
Surgical Procedure for Catheter Implantation
All 6 monkeys were instrumented with subcutaneous vascular access ports (SIMS Deltec, Inc., St. Paul, MN). Both the vascular access port system and the method used to surgically implant the catheters and ports have been described previously (Winsauer et al., 2000). The monkeys were catheterized in the internal jugular vein, the external jugular vein or the femoral veins.
Behavioral Procedure
Three-component Multiple Schedule of Self-administration, Repeated Acquisition and Performance
During the self-administration component, the light above the response lever was illuminated yellow and responding in this component was maintained under a fixed-ratio (FR) 60 schedule of cocaine presentation. During cocaine infusions, the stimulus light above the lever changed to red and an infusion pump was activated. The training dose was 0.032 mg/kg/injection. A 60-s timeout followed each cocaine infusion. During each timeout the stimulus light above the lever remained off and responses had no programmed consequence.
During the acquisition component, each of the three response keys was illuminated simultaneously with one of five geometric symbols on a black background. The geometric symbols were squares, horizontal bars, triangles, vertical bars or circles. The task for each subject was to respond (key press) on the correct key in the presence of each sequentially illuminated set of geometric symbols (e.g., keys with squares–center correct, keys with horizontal bars–left correct, keys with triangles–center correct, keys with vertical bars–right correct, keys with circles–left correct). This type of sequential responding is procedurally considered to be a chained schedule (i.e., [chain FR 5(FR1):S]) and defined as a “chain” because each response except the last produced discriminative stimuli controlling the response that followed (Kelleher, 1966). After the completion of the fifth correct response, the keylights turned off and the response key over the food pellet aperture was illuminated. A press on this key reset the sequence. The same response chain (in this case, center–left–center–right–left or CLCRL) was repeated in the acquisition components throughout a given session and was maintained by food presentation under a second-order FR-4 schedule; that is, every fourth response on the “food key” following the completion of the five-response chain produced a 500-mg food pellet. When the subject pressed an incorrect key (in the example, the left or right key when the square symbols were presented), the error was followed by a 5-s timeout in which the keylights were extinguished and responding had no programmed consequence. Errors did not reset the response sequence or the second-order FR schedule (i.e., the stimuli and the position of the correct response were the same before and after a timeout).
To establish a steady state of repeated acquisition (Thompson & Moerschbaecher, 1978), the five-response sequence was changed from session to session. An example of a typical set of six sequences (for six different sessions) was as follows: LRCRC, CLRLR, LRLCL, RCRLC, CLCRL, RCLCR, with the order of the geometric symbols always squares, horizontal bars, triangles, vertical bars and circles. The sequences were carefully selected to be equivalent in several ways and there were restrictions on their ordering across sessions. Briefly, 34 five-response sequences were arranged in a specific order and presented to each monkey one after another until the list was completed. In this list, each of the 34 sequences occurred twice and adhered to the other restrictions on their ordering (i.e., adjacent positions within a sequence were different from day to day and no position within a sequence was duplicated across days). This eliminated sequence presentations such as right–right–center–left–right (RRCLR) and sequence ordering such as RLCRL followed by CLRCR where the second response in the chain would be “left”, across days. Once the list was completed, the same list of sequences was presented again. Thus, each subject was exposed to a given five-response sequence twice every 68 test sessions.
During the performance components of the multiple schedule, the geometric symbols that identified each response in the five-response sequence were projected on a green background. The green background on each key served as discriminative stimuli for this component. Unlike the acquisition component, the five-response sequence in this component remained the same from session to session (i.e., LCLRL), and was never used as an acquisition sequence. In all other aspects (second-order FR-4 schedule of food presentation, timeout duration of 5 s, etc.), the performance component was identical to the acquisition component.
Each session began with a self-administration component and was followed immediately (i.e., no timeouts occurred between components) by either a repeated-acquisition component or a performance component depending on the day. That is, the component that followed the self-administration component alternated across days yielding two different possible cycles of the three-component multiple schedule (self-administration – repeated acquisition – performance or self-administration – performance – repeated acquisition). Each self-administration and repeated-acquisition component was 10 min in duration, whereas each performance component was 5 min in duration. The shorter component duration was chosen for the performance components because it was sufficient to provide an appropriate sample of behavior while not unduly lengthening the session duration. Exactly four cycles of the multiple schedule comprised each session and sessions were generally conducted 7 days per week.
Drug Administration
Self-administration under the FR-60 schedule was always instituted with a 0.032 mg/kg/infusion dose of cocaine hydrochloride (National Institute on Drug Abuse, Research Technical Branch, Rockville, MD) and followed by saline substitution. After this initial substitution with saline, other logarithmic infusion doses of cocaine (i.e., 0.0032, 0.01, 0.1 and 0.32 mg/kg) were substituted in a mixed order to obtain a dose-effect curve for cocaine. Each infusion dose was also administered at least twice in order to establish the dose-effect curve. Whether an infusion dose of cocaine was administered alone, or with haloperidol subsequent to establishing the dose-effect curve, saline was substituted between each of the infusion doses of cocaine to minimize carry-over effects between dosing conditions. Both saline and each infusion dose of cocaine, whether they were administered alone or in combination with haloperidol, were studied until one of three stability criteria was met: (1) the variation in the number of injections did not exceed ± 20% for 3 (saline) or 4 (cocaine) consecutive daily sessions, (2) the number of infusions did not exceed eight for 3 (saline) or 4 (cocaine) consecutive days, or (3) a maximum of 10 consecutive days. If a condition lasted for 10 days, however, only the last 3 (saline) or 4 (cocaine) days of the 10 were used in the data analysis to maintain a consistent comparison between conditions. The cocaine was dissolved in sterile saline (0.9%) and the administration volumes for both saline and cocaine ranged from 0.39 to 0.59 ml/infusion depending on the subject. The administration volumes were kept constant for individual subjects by adjusting the concentration (mg/ml), and the solutions of each dose were prepared fresh every day. The infusion rate was 2.385 ml/min. Haloperidol (McNeil Laboratories, Inc., Fort Washington, PA) was dissolved in a vehicle composed of 60% propylene glycol, 20% ethanol and 20% saline. The doses of haloperidol were administered intramuscularly 30 min prior to the session and the injection volume was always 0.05 ml/kg.
Data Analyses
The data for each session were analyzed in terms of: (1) the overall response rate (total responses/s, excluding timeouts) in each of the three components; (2) the number of infusions/session, and (3) the overall accuracy in the repeated-acquisition and performance components, expressed as a percentage of errors [(incorrect responses/total responses) × 100]. In general, individual and grouped subject data were analyzed by comparing the mean drug sessions to criterion with the mean control sessions to criterion, which were sessions where saline was substituted for cocaine in the absence and presence of haloperidol. Thus, if the effects of a given infusion dose of cocaine were determined twice and both determinations met the criterion specifying less than 20% variability over four sessions, then eight sessions comprised the mean for that infusion dose. Given that saline was determined between each infusion dose, and the vast majority of these determinations met the criterion specifying less than 20% variability over three sessions, more than 30 sessions (i.e., 10 determinations) may have comprised the control mean. The grouped data for response rate and the number of infusions were tabulated by averaging the mean data for each subject and then analyzed by a two-way analysis of variance (ANOVA) with repeated measures for haloperidol treatment and dosage. A one-way ANOVA was used to isolate the effects if a significant interaction occurred. The percentage of errors for an individual subject was not included in the data analyses when response rate was less than 0.083 responses/s (i.e., 5 responses/min) because of the small number of responses emitted. Due to the elimination of an error term for individual subjects following very low response rates, a two-way repeated-measures ANOVA could not be conducted on the error data. Therefore, the statistical analysis of the effects on the percentage of errors was limited to the data obtained with infusion dosages of 0.032 mg/kg/infusion or less, and was conducted only on the data for cocaine alone and cocaine in the presence of 0.0032 mg/kg of haloperidol using a one-way repeated-measures ANOVA. A one-way ANOVA was also conducted on the percent error data for the 0.032-mg/kg infusion dose to determine if there was an effect of haloperidol treatment.
Changes in the subject's sensitivity to cocaine, in the presence and absence of haloperidol, were quantified by comparing ED50 values (as defined below) across conditions (i.e., the ED50 values were calculated for cocaine alone and these points on the curve were compared to the same ordinal position on the curves obtained with each dose of haloperidol). ED50 values for individual subjects were determined by linear regression using two or more data points reflecting the slopes of either the ascending or descending portions of the curve for cocaine alone. For response rate, the ED50 represented the estimated dose of cocaine that decreased response rates to 50% of their maximum in the absence of haloperidol. For infusions, which produced an inverted U-shaped curve, the ED50 for the ascending limb represented the dose that increased the mean number of infusions by 50% over the values obtained for the lowest dose of cocaine, whereas the ED50 for the descending limb represented the dose that decreased the mean number infusions to 50% of their maximum. Lastly, in order to characterize any downward shifts in the cocaine dose-effect curve for infusions, the area under the curve was determined using a transform provided by SigmaPlot (SPSS Inc. Chicago, IL). The transform provided was an algorithm that integrates under curves using the trapezoidal rule, which can be used for equal or unequally spaced x values. Changes in the ED50 values for infusions or response rates were considered to be significant when the ED50 values obtained for cocaine after the haloperidol pretreatments were outside of the 95% confidence intervals obtained for cocaine self-administration alone. An area-under-the-curve (AUC) was also determined and compared statistically using a 95% confidence interval.
Results
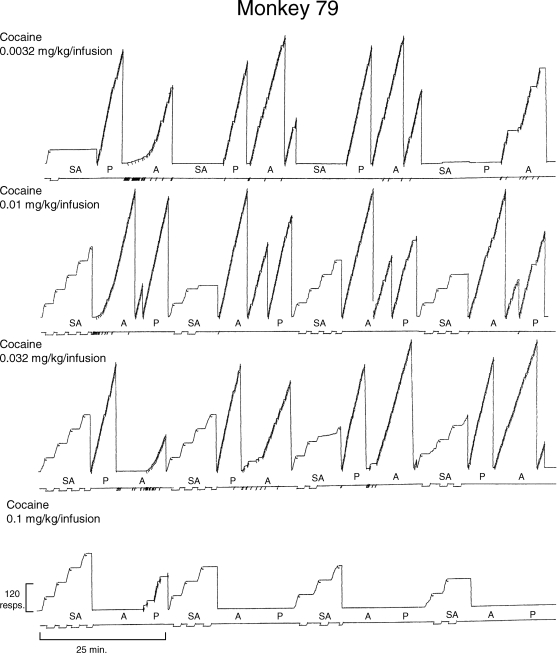
The pattern of within-session effects obtained after increasing infusion doses of cocaine is shown in the cumulative records for a representative subject in Figure 2. As indicated by the record in the top row, the 0.0032-mg/kg infusion dose of cocaine engendered very little responding under the FR-60 schedule in the self-administration components. The subject essentially completed one ratio and then ceased responding in this component for the remainder of the session. Unlike responding in the self-administration component, food-maintained responding in the acquisition and performance components was more consistent throughout the session with the exception of the last cycle of the multiple schedule where no responding occurred in the performance component and small pauses in responding occurred in the acquisition component. Despite these decreases in responding, which were not observed across all the determinations for this infusion dose, response rates for this subject during this session averaged 1.29 responses/s in the acquisition component and 1.43 responses/s in the performance component. Moreover, these rates compared favorably with the mean rates for all the sessions for this infusion dose in this subject, which were 1.1 and 1.54 responses/s, respectively. Acquisition of the response chain also occurred shortly after the start of the first acquisition component and it was characterized by a marked increase in the number of consecutive correct responses and a decrease in the overall error rate. Following acquisition of the response sequence, the pattern of responding in the repeated-acquisition components was similar to that obtained in the performance components. Error levels were also consistently lower in the performance component than the acquisition component throughout the session, because the response sequence in this component did not change across sessions.
Fig 2.
Cumulative records from Monkey 79 showing the within-session effects of increasing infusion doses of cocaine in the self-administration component of the three-component multiple schedule. Each session began with a self-administration (SA) component and was followed by either a repeated-acquisition (A) or a performance (P) component depending on the session. In the self-administration components, the response pen stepped upward with every response and it was deflected downward for the duration of each cocaine infusion. In the acquisition and performance components, the response pen stepped upward with each correct response and it was deflected downward each time the five-response sequence was completed; every fourth downward deflection of the stepping pen a food pellet was delivered. The response pen reset at the edge of the paper or at the completion of each component. Downward deflections of the event pen (below each record) indicate timeouts in the self-administration components or incorrect responses in the acquisition and performance components. Each session terminated after four cycles of the multiple schedule.
As shown by the records in the second and third rows of this figure, responding under the FR-60 schedule in the self-administration component was maintained by the 0.01- and 0.032-mg/kg infusion doses of cocaine. In general, between two and four infusions were obtained in each component after both infusion doses, and the response pattern was characteristic of responding under FR schedules (i.e., responding prior to completing the ratio occurred at a high constant rate and a pause preceded the initiation of responding for each subsequent ratio). Disruptions in the running rate were, however, evident for this subject in the third and fourth self-administration components when the infusion dose was 0.032 mg/kg. For the session in which 0.01 mg/kg/infusion was available to this subject in the self-administration component, the overall rate, running rate, and average preratio pause were 0.81 responses/s, 0.97 responses/s and 10.5 s, respectively. For the session in which 0.032 mg/kg/infusion was available, the overall rate in the self-administration component was 1.06 responses/s, the running rate was 1.24 responses/s, and the average preratio pause was 7.48 s.
Responding was also maintained in the acquisition and performance components when the 0.01- and 0.032-mg/kg infusion doses were available; however, there were small disruptions of responding in the acquisition component when the 0.032-mg/kg infusion dose was available. For example, compared to sessions where saline or small infusion doses of cocaine were available, acquisition of the response sequence was delayed because there were fewer correct responses at the beginning of the component and there were pauses in responding totaling several minutes. These pauses decreased the overall rate in the acquisition component to 0.93 responses/s, whereas the overall rate in the performance component for this session was 2.05 responses/s. In addition, there was an increase in the number of errors at the beginning of the second acquisition component that was not observed when saline or lower infusion doses of cocaine were available.
When 0.1 mg/kg/infusion was available in the self-administration component, this subject continued to obtain between two and four infusions in each component and the pattern of responding remained typical of that observed under FR schedules. For this infusion dose the running rate was 1.08 responses/s, whereas the overall response rate decreased to 0.58 responses/s and the average preratio pause increased to 40.5 s. In contrast to responding in the self-administration component, there were marked disruptions in responding in both the acquisition and performance components. More specifically, responding only occurred in one performance component and was virtually eliminated in the acquisition component. The overall rate for the performance component for this session was 0.13 responses/s.
Figure 3 shows the grouped data for self-administered cocaine, both in the absence and presence of haloperidol (0.0032 or 0.01 mg/kg). Similar to the individual subject data, the grouped data for infusions was represented by an inverted U-shaped curve (i.e., compared to saline presentations or saline presentations after each dose of haloperidol, the average number of infusions increased from 0.0032 to 0.032 mg/kg/infusion, and then decreased from 0.1 to 0.56 mg/kg/infusion). This pattern of cocaine self-administration in both the absence and presence of haloperidol was also verified by a two-way ANOVA, which indicated that there was a significant effect of dose (F(5,48) = 9.62, p < 0.001), and an absence of an effect for each haloperidol pretreatment (F(2,48) = 0.18, p > 0.1); however, there was a significant interaction of the haloperidol pretreatment with the dose of self-administered cocaine, F(10,48) = 2.35, p < 0.05. In this case, the significant interaction was an indication that the dose-effect curves for cocaine in the absence and presence of haloperidol were shifted with respect to each other. The shifts in the dose-effect curves were also apparent after an analysis of the mean ED50 values for both the ascending and descending limbs of the inverted U-shaped curve (Table 1). With regard to the ascending limb of the curve for infusions, both the 0.0032- and 0.01-mg/kg doses of haloperidol shifted the curve significantly to the right approximately twofold, whereas only the 0.01-mg/kg dose shifted the infusion curve significantly to the right for the descending limb, and this shift was less than twofold. As indicated by the individual subject data presented in Table 1, the shift of the ascending limb after 0.01 mg/kg of haloperidol was smaller than the shift after 0.0032 mg/kg of haloperidol and a rightward shift was only evident in 2 subjects (79 and LU).
Fig 3.
Mean effects of haloperidol pretreatment on the average number of cocaine infusions obtained by 6 monkeys during the self-administration component of the multiple schedule. Each infusion dose of cocaine was tested alone before and after their combination with the 0.0032 or 0.01 mg/kg doses of haloperidol. Infusion doses of cocaine, both alone and in combination with a pretreatment of haloperidol, were available until the criterion was met (cf. Methods). The points and vertical lines above S indicate the mean and standard error of the mean (SEM) for the number of infusions to criterion during saline substitution or saline substitution after a pretreatment with each dose of haloperidol. The points and vertical lines above each unit dose of cocaine indicate the mean and SEM for the number of infusions to criterion after cocaine alone (open bars) or cocaine after a pretreatment with haloperidol (squares and triangles for 0.0032 and 0.01 mg/kg of haloperidol, respectively). Crosses (†) indicate a significant difference from the effects of cocaine alone as determined by a one-way ANOVA with repeated measures followed by Dunnetts post hoc tests, which were conducted after the two-way ANOVA identified main effects or a significant interaction (p < 0.05). The numbers in parentheses indicate the number of subjects represented by that data point when the number was less than 6.
Table 1.
Individual and mean ED50 values for the ascending and descending limbs of the cocaine dose-effect curve for infusions in mg/kg/infusion, in the presence and absence of two doses of haloperidol. The right most columns contain the respective areas under each curve (AUC).
| ED50: Infusions |
AUC |
||||||||
| Ascending Limb |
Descending Limb |
||||||||
| Subject | Coc. Alone | Halo. 0.0032 | Halo. 0.01 | Coc. Alone | Halo. 0.0032 | Halo. 0.01 | Coc. Alone | Halo. 0.0032 | Halo. 0.01 |
| 79 | 0.006 | 0.012 | 0.009 | 0.18 | 0.148 | 0.226 | 47.25 | 39.34 | 45.31 |
| TR | 0.011 | 0.051 | 0.005 | 0.107 | 0.119 | 0.226 | 42.49 | 34.69 | 58.81 |
| LU | 0.008 | 0.018 | 0.034 | 0.104 | 0.193 | 0.248 | 51.83 | 48.87 | 41.75 |
| HA | 0.006 | 0.003 | - | 0.101 | 0.115 | - | 29.04 | 36.5 | 14.75 |
| SQ | - | - | - | - | - | - | 25.13 | 21.13 | 49.5 |
| JO | 0.006 | 0.008 | 0.006 | 0.18 | 0.199 | 0.234 | 61.11 | 59.88 | 62.88 |
| Mean | 0.007 | 0.018* | 0.014* | 0.134 | 0.155 | 0.234* | 42.81 | 40.07 | 45.5 |
| 95% CI | 0.005–0.01 | 0.082–0.186 | 28.43–57.18 | ||||||
Coc. = cocaine. Halo. = haloperidol.
indicate values outside of the 95% confidence interval.
(-) indicates ED50 values that could not be ascertained without extrapolation.
The individual subject data indicating a rightward shift for the descending limb can be seen in Table 2. For example, when 0.32 mg/kg/infusion was available in the self-administration component, both the mean number of infusions and the mean cumulative dose increased as the dose of haloperidol increased. However, despite these changes in the mean number of infusions, the overall shape of the dose-effect curve for infusions was similar before and after haloperidol, and this was indicated by the similarity in the values for the areas under the dose-effect curves (AUC, Table 1), which were within the 95% confidence interval established for cocaine alone.
Table 2.
Individual subject data for the mean number of infusions obtained during the availability of each unit dose of cocaine in the absence or presence of two doses of haloperidol. The data in the rightmost columns show the mean cumulative dose of cocaine obtained during the availability of each unit dose tested.
| Infusions |
|||||||||||||
| Self-Administration Component |
Cumulative Dose (mg/kg) |
||||||||||||
| Subject | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | 0.56 | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | 0.56 |
| 79 | 4.87 | 0.75 | 14.5 | 14.88 | 14 | 7 | 0.002 | 0.13 | 0.48 | 1.4 | 2.24 | ||
| TR | 5.86 | 2.25 | 8.17 | 17.5 | 12.88 | 6.25 | 0.007 | 0.08 | 0.56 | 1.29 | 2 | ||
| LU | 4.92 | 6.5 | 15.63 | 17.75 | 12.38 | 5.67 | 0.021 | 0.16 | 0.57 | 1.24 | 1.81 | ||
| HA | 8.93 | 1.75 | 9.13 | 10.92 | 6.88 | 2.5 | 0.006 | 0.09 | 0.35 | 0.69 | 0.8 | ||
| SQ | 7.67 | 6.5 | 9 | 6 | 5 | 3.75 | 0.021 | 0.09 | 0.19 | 0.5 | 1.2 | ||
| JO | 6.94 | 8.63 | 19 | 18.55 | 15.88 | 6.75 | 0.028 | 0.19 | 0.59 | 1.59 | 2.16 | ||
| Mean | 6.53 | 4.4 | 12.57 | 14.27 | 11.17 | 5.32 | 0.014 | 0.12 | 0.46 | 1.12 | 1.7 | ||
| Haloperidol 0.0032 mg/kg | |||||||||||||
| 79 | 4 | 0.25 | 7.5 | 13 | 12.25 | 7.13 | 4.5 | 0.001 | 0.08 | 0.42 | 1.23 | 2.28 | 2.52 |
| TR | 4 | 5.5 | 4.75 | 7.5 | 12.5 | 8 | 4.75 | 0.018 | 0.05 | 0.24 | 1.25 | 2.56 | 2.66 |
| LU | 3.75 | 1.25 | 10.75 | 14.25 | 16.25 | 7.75 | 4.75 | 0.004 | 0.11 | 0.46 | 1.63 | 2.48 | 2.66 |
| HA | 12.25 | 6.25 | 13.75 | 10 | 8 | 3.25 | 0.02 | 0.14 | 0.32 | 0.8 | 1.04 | ||
| SQ | 3.25 | 9.75 | 5.75 | 8.25 | 4.5 | nt | 0.031 | 0.06 | 0.26 | 0.45 | |||
| JO | 3 | 10.5 | 13.75 | 21.25 | 15.38 | 8.5 | 0.034 | 0.14 | 0.68 | 1.54 | 2.72 | ||
| Mean | 5.04 | 5.58 | 9.38 | 12.38 | 11.48 | 6.93 | 4.67 | 0.018 | 0.09 | 0.4 | 1.15 | 2.22 | 2.61 |
| Haloperidol 0.01 mg/kg | |||||||||||||
| 79 | 3.25 | 2.75 | 5 | 16 | 14.75 | 9 | 5.75 | 0.009 | 0.05 | 0.51 | 1.48 | 2.88 | 3.22 |
| TR | 4 | 7.5 | 13.75 | 15.75 | 17.25 | 8.75 | 7 | 0.024 | 0.14 | 0.5 | 1.73 | 2.8 | 3.92 |
| LU | 2 | nt | 2.5 | 12.5 | 20 | 9 | 5 | nt | 0.03 | 0.4 | 2 | 2.88 | 2.8 |
| HA | 1.75 | 1.5 | 5.25 | 2.75 | 4.5 | 3 | 0.005 | 0.05 | 0.09 | 0.45 | 0.96 | ||
| SQ | 3 | 13.25 | 15 | 13.25 | 9.75 | 9.75 | 0.042 | 0.15 | 0.42 | 0.98 | 3.12 | ||
| JO | 2.5 | 9.5 | 18.5 | 17.5 | 17.5 | 9.25 | 0.03 | 0.19 | 0.56 | 1.75 | 2.96 | ||
| Mean | 2.75 | 6.9 | 10 | 12.96 | 13.96 | 8.13 | 5.92 | 0.022 | 0.1 | 0.42 | 1.4 | 2.6 | 3.31 |
(nt) indicates doses or dose combinations that were not tested.
Figure 4 shows the interaction of each haloperidol pretreatment with increasing unit doses of self-administered cocaine on the overall rate of responding and the percentage of errors in the acquisition and performance components of the multiple schedule. In both the acquisition (F(5,25) = 15.05, p < 0.001) and performance (F(5,25) = 21.96, p < 0.001) components, self-administered cocaine alone dose-dependently decreased response rate when compared to control conditions in which saline was available. More specifically, the mean rate of responding under control conditions in acquisition was 1.45 responses/s and it decreased to a mean of 0.14 responses/s after administration of 0.32 mg/kg/infusion, whereas the mean rate of responding under control conditions in performance was 2.17 responses/s and it decreased to a mean of 0.27 responses/s after administration of 0.32 mg/kg/infusion. When haloperidol was administered prior to the session, the rate-decreasing effects of cocaine in each component were dependent on the dosage of haloperidol (acquisition, F(10,48) = 6.33, p < 0.001; performance, F(10,48) = 9.04, p < 0.001). This was largely due to the fact that 0.01 mg/kg of haloperidol alone significantly (p < 0.05) decreased the overall response rate in both components (see data above S). In the acquisition component, 0.01 mg/kg of haloperidol alone decreased the mean response rate from 1.45 under control conditions to 0.35 responses/s, and in the performance component, from 2.17 to 0.67 responses/s. Interestingly, the rate-decreasing effects of this dose of haloperidol were significantly reduced in the performance component by the self-administration of 0.01 mg/kg/infusion of cocaine (i.e., following a pretreatment with 0.01 mg/kg of haloperidol and self-administration of 0.01 mg/kg/infusion the mean rate in this component increased from 0.67 to 1.71 responses/s). As indicated by the ED50 values for response rate in these components, haloperidol did not significantly change the sensitivity of cocaine's rate-decreasing effects in acquisition or performance by shifting the dose-effect curves (cf. Appendixes A and B).
Fig 4.
Mean effects of haloperidol pretreatment on the overall response rate (top) and percentage of errors (bottom) of 6 monkeys responding under the three-component multiple schedule of self-administration, repeated acquisition and performance. The left-hand panels depict the effects obtained during the repeated-acquisition components, whereas the right-hand panels depict the effects obtained during the performance components. In both panels, the data points with vertical lines above S indicate mean and SEM for the sessions to criterion when saline was substituted for cocaine (circles), and the mean and SEM for saline substitution sessions that were preceded by a pretreatment of either 0.0032 (squares) or 0.01 (triangles) mg/kg of haloperidol. Data points with vertical lines in the dose-effect curves indicate the mean and SEM for the respective infusion dose of cocaine with or without haloperidol. Note that the percentage of errors was not included in the data analyses when response rate was less than 5 responses/min (0.083 responses/s). Asterisks (*) indicate a significant difference from the respective control data and crosses (†) indicate a significant difference from the effects of cocaine alone as determined by a one-way ANOVA with repeated measures followed by Dunnetts post hoc tests, which were conducted after the two-way ANOVA identified main effects or a significant interaction (p < 0.05). The numbers in parentheses indicate the number of subjects represented by that data point when the number was less than 6.
The bottom panels of Figure 4 show the mean effects of self-administered cocaine on the percentage of errors in the acquisition and performance components in the absence and presence of haloperidol. In the absence of a pretreatment with haloperidol, 0.0032- and 0.01-mg/kg/infusion of cocaine did not significantly increase the percentage of errors in either component when compared with the control data obtained after saline administration. The mean percentage of errors for the group was 1.81 for responding in the acquisition components and 0.31 for responding in the performance components. When the unit dose of cocaine was increased to 0.032 mg/kg, there was a significant effect on the percentage of errors and the effect was evident in both components (acquisition, F(3,15) = 5.55, p < 0.01; performance, F(3,15) = 5.26, p < 0.05). In the acquisition component, the percentage of errors increased from 1.81 to 16.17, whereas in the performance component the percentage of errors increased from 0.31 to 2.41. Although error-increasing effects were still evident in some subjects after larger unit doses of cocaine alone (cf. Appendix C), these error-increasing effects could not be analyzed statistically as a repeated measure for the group due to the large rate-decreasing effects that occurred in many subjects.
When subjects were pretreated with the 0.0032-mg/kg dose of haloperidol, self-administration of the 0.032-mg/kg infusion dose increased the mean percentage of errors in the acquisition component from 1.81 to 3.29 and in the performance component from 0.31 to 0.33; however, these changes were not significant for either the acquisition (p > 0.1) or performance (p > 0.1) components. Therefore, 0.0032 mg/kg of haloperidol reduced the error-increasing effects of the 0.032-mg/kg infusion dose of cocaine. The reduction of cocaine's error-increasing effects at this infusion dose is also evident upon examination of the individual subject data (see Appendix C). For example, in 4 of the 6 subjects that had a substantial increase in acquisition errors after self-administering the 0.032-mg/kg infusion dose of cocaine alone, only one of those subjects (JO) had a notable increase in acquisition errors over control levels after pretreatment with the 0.0032-mg/kg dose of haloperidol.
Although the 0.01-mg/kg dose of haloperidol also reduced the percentage of errors produced by the 0.032-mg/kg infusion dose of cocaine from 16.17 to 9.33 in the acquisition components and from 2.41 to 0.62 in the performance components, a post-hoc analysis of these data indicated the reduction was only significant for responding in the performance components (p < 0.05 compared to cocaine alone). In other words, the reduction of cocaine's error-increasing effects after pretreatment with the larger dose of haloperidol was not as complete as it was for the smaller dose, which produced a significant reduction in both schedule components (p < 0.05 for both treatment doses compared to cocaine alone). Moreover, as shown in Appendix C, self-administration of the 0.01- and 0.032-mg/kg infusion doses still produced large error-increasing effects in 3 subjects (TR, LU and JO) after pretreatment with 0.01 mg/kg of haloperidol.
The within-session pattern of effects obtained after each dose of haloperidol alone is shown in Figure 5. The record in the top row depicts responding obtained when saline was available in the self-administration component and there was no pretreatment with haloperidol. Briefly, little or no responding occurred under the FR schedule in the self-administration component after the first component, whereas responding in the food-maintained acquisition and performance components was emitted at a rate of 1.22 and 1.54 responses/s, respectively, despite the small pauses that were evident in the final performance component. In addition, acquisition of the response sequence occurred in the first acquisition component and it was characterized by a marked reduction in errors and an increase in consecutive correct responses. The record in the second row of Figure 5 shows that 0.0032 mg/kg of haloperidol had little or no effect on the pattern of responding present when saline was available in the self-administration component. When comparing this record to that for saline administration alone the overall patterns of responding were comparable, although the rates in each component were somewhat higher at 1.54 and 2.04 responses/s, respectively. Thus, even though a relatively long pause was evident in the final acquisition component, responding in the acquisition components up to that point had occurred at a consistent, high rate (e.g., compare responding in the initial acquisition component of the top record to responding in the initial acquisition component of the middle record).
Fig 5.
Cumulative records from Monkey 79 showing the within-session effects of a pretreatment of haloperidol, either 0.032 or 0.01 mg/kg, when saline was available in the self-administration component. The record in the top row represents the pattern of responding that occurred in the absence of a haloperidol pretreatment when saline was available in the self-administration component. For additional details, see legend for Fig. 1.
Compared to the pattern of responding obtained after 0.0032 mg/kg of haloperidol, the pattern of responding obtained after 0.01 mg/kg of haloperidol was substantially altered. For example, responding was virtually eliminated in all of the acquisition components with the exception of a few errors at the start of the initial acquisition component, and responding in the performance components was limited to the first two cycles of the multiple schedule. For these reasons, the overall rate of responding for this session was 0.01 responses/s for the acquisition components and 0.23 responses/s for the performance components.
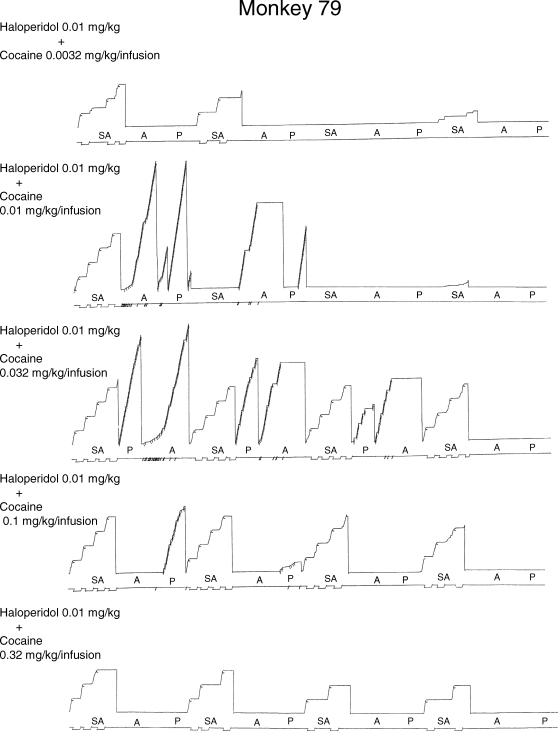
Figure 6 shows the interaction of increasing infusion doses of cocaine with the 0.01-mg/kg dose of haloperidol on the within-session pattern of responding under the multiple schedule for Monkey 79. When the smallest infusion dose of cocaine was available in the self-administration component (i.e., 0.0032 mg/kg/infusion), the overall pattern of responding was comparable to that when saline administration followed this dose of haloperidol (see Fig. 5). That is, some responding occurred in the first two self-administration components, and little or no responding occurred in the acquisition and performance components. Interestingly, as the infusion dose of cocaine was increased from 0.0032 mg/kg to 0.01 and 0.032 mg/kg, there was an increase in overall responding in the acquisition and performance components after self-administration of each dose and then an increase in responding in all three components after self-administration of the 0.032-mg/kg dose. Thus, when 0.032 mg/kg/infusion was available, this subject consistently obtained four infusions in the self-administration components, acquired the response chain during the first acquisition component, and responded errorlessly throughout three performance components. Disruptions in responding were evident, though, in the acquisition and performance components as long pauses occurred in several acquisition components after acquisition of the response sequence and responding was eliminated during the final acquisition and performance components.
Fig 6.
Cumulative records from Monkey 79 showing the within-session effects of 0.01 mg/kg of haloperidol when varying infusion doses of cocaine were available in the self-administration component. For additional details, see legend for Fig. 1.
When 0.1- and 0.32-mg/kg/infusion of cocaine were available in the self-administration components, this subject obtained a consistent number of cocaine infusions in each cycle of the multiple schedule (i.e., three to four for the 0.1-mg/kg dose and two to three for the 0.32-mg/kg dose); however, there was a substantial decrease in responding in the acquisition and performance components. For example, after self-administering 0.1 mg/kg/infusion of cocaine, Monkey 79 did not respond in the acquisition components and only responded during two of the performance components. After self-administering 0.32 mg/kg/infusion, there was no responding in either the acquisition or performance components.
Discussion
Similar to the data from two previous studies (Winsauer et al., 2003; Winsauer et al., 2000), data from the present study demonstrated that responding under a three-component multiple schedule of self-administration, repeated acquisition and performance could be reliably maintained in rhesus monkeys at specific infusion doses. Unlike the previous studies, which largely focused on the disruptive effects of cocaine within the context of this multiple schedule, the current study focused on the capacity of a drug to antagonize cocaine-maintained responding as well as the disruptive effects of cocaine on food-maintained responding in the acquisition and performance components. Part of the reason for this focus was also to determine if the baseline could serve as an appropriate tool for testing drugs with therapeutic potential for treating cocaine abusers as the promise of most pharmacotherapies depend, in part, on their profile of adverse effects (i.e., their capacity to reduce self-administration selectively while preserving other behaviors; cf. Howell & Wilcox, 2001).
Unfortunately, as shown in several other studies involving monkeys and rats (de la Garza & Johanson, 1982; Roberts & Vickers, 1987), the dopamine receptor antagonist haloperidol did not decrease cocaine-maintained behavior by shifting the cocaine dose-effect curve downward or producing an extinction-like pattern of responding. In the present study, haloperidol only changed the sensitivity of the subjects to the reinforcing effects of cocaine by shifting the cocaine dose-effect curve for infusions modestly (i.e., twofold or less) to the right, which resulted in higher intake on a mg/kg basis at larger infusion doses (cf. Table 2). One important finding from the present study, however, was the fact that neither a behaviorally ineffective (0.0032 mg/kg) nor an effective (0.01 mg/kg) dose of haloperidol was able to antagonize the rate-decreasing effects produced by cocaine in the acquisition and performance components, and that there was only limited antagonism of its error-increasing effects. The differential antagonism observed with haloperidol was also somewhat unexpected given the similarity in potency demonstrated for the reinforcing effects of cocaine in the self-administration component, and for its disruptive effects on food-maintained responding in the acquisition and performance components. Similar to the data from the initial study using this baseline (Winsauer et al., 2000), the 0.032-mg/kg infusion dose in this study produced the most robust responding in the self-administration component, while also producing significant rate-decreasing and error-increasing effects in the acquisition and performance components. Thus, the antagonism of cocaine's reinforcing effects might also have led to the antagonism of cocaine's rate-decreasing and error-increasing effects, especially if the two were directly related.
With regard to the differential antagonism that was obtained, the present study contrasts with many self-administration studies involving a dopamine receptor antagonist as they have shown that effective doses of the antagonist generally decreased responding for both cocaine and food presentation (e.g., Glowa & Wojnicki, 1996; Herling & Woods, 1980; Negus et al., 1996; Woolverton & Virus, 1989), or decreased responding for cocaine presentation while not affecting responding for food presentation (e.g., Winsauer & Thompson, 1991). In the present study, haloperidol antagonized the reinforcing effects of cocaine while having little or no antagonistic effect on cocaine's error-increasing and rate-decreasing effects up to doses that disrupted responding for food presentation. For instance, the 0.01-mg/kg dose of haloperidol produced large rate-decreasing effects in the acquisition and performance components when small infusion doses of cocaine were available, which is consistent with what has been reported previously for the effects of haloperidol alone in monkeys responding under a repeated-acquisition procedure (Thompson & Moerschbaecher, 1978). In that study, 0.01 mg/kg of haloperidol produced both rate-decreasing and error-increasing effects; however, most of the error-increasing effects occurred after a pause of approximately 115 min. A pause of this duration could explain why haloperidol alone may not have produced large error-increasing effects in the subjects from the present study where the session duration was only 100 min.
When the 0.01-mg/kg dose of haloperidol preceded intermediate infusion doses of cocaine, responding in the acquisition and performance components was also disrupted, but not as completely because these infusion doses of cocaine were able to antagonize the rate-decreasing effects of this dose of haloperidol. This type of reciprocal effect has been reported in several other antagonist studies involving FR schedules of food-maintained behavior (e.g., Glowa & Wojnicki, 1996; Herling & Woods, 1980; Woolverton & Virus, 1989). The reciprocal antagonism that occurred between haloperidol and cocaine would also seem to argue against the possibility of an additive effect on food intake. For example, haloperidol has been shown to lower breakpoint values in rats responding under progressive-ratio schedules for sucrose reinforcers, which suggests that it can reduce the effectiveness of sucrose and other gustatory stimuli such as food as a reinforcer (Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000; Reilly, 1999). CNS stimulants can also decrease food intake in nonhuman primates (e.g., Foltin, 2005), which suggests the possibility that the combination of the two drugs might work additively to eliminate food-maintained responding in the acquisition and performance components entirely. Yet, haloperidol's effects on food-maintained responding were attenuated or antagonized in the acquisition and performance components, respectively, when intermediate infusion doses of cocaine were self-administered (e.g., see Figure 6).
Finally, when large infusion doses of cocaine were available in either the presence or absence of haloperidol, responding was largely eliminated in the food-maintained components while responding in the self-administration components was maintained. Together, these data suggest that haloperidol's capacity for altering the reinforcing effects of cocaine is quite dose specific and that there might be important differences in the pharmacological mechanisms underlying cocaine's various behavioral effects. If nothing else, this finding is suggestive of the differential participation of type-2 dopamine receptors in the particular behaviors assayed under this multiple schedule, because their blockade by the same dose of haloperidol did not produce the same degree of antagonism across behaviors. Although the explanation for this is unknown, differential antagonistic effects have been described for the neuroleptics in humans who abuse cocaine (e.g., Gawin, 1986).
Two behavioral variables that have been shown to play key roles in maintaining self-administration, and that could have contributed to the differential antagonism observed, are the schedule of reinforcement and the reinforcer. For example, there is the possibility that responding under the simple FR schedule in the self-administration component was less sensitive to disruption than responding under the second-order schedule that was operating in the acquisition and performance components. This would be entirely consistent with a study by Winsauer, Thompson and Moerschbaecher (1985) who found that phencyclidine, pentobarbital and d-amphetamine produced greater disruption of a chain schedule than a FR schedule when both were combined under a multiple schedule. More specifically, responding on a single key under a simple FR-20 schedule was compared with completing the same four-response chain on three keys under a second-order FR-5 schedule. Thus, 20 responses were required for reinforcement under each schedule, but responding under the chain-performance schedule required responding on multiple keys in the presence of multiple stimuli and incorrect responses resulted in a 5-s timeout. When each drug was administered, the disruption of responding under the chain schedule was found to be greater than the disruption of responding under the FR schedule as indicated by slower returns to control rates and patterns of responding. In that study, the control rates of responding and reinforcement may have contributed to the overall selectivity of the effects of each drug because they differed across components (i.e., the FR-20 schedule had higher rates of responding and reinforcement under control conditions); however, in the present study, both the rate of responding and rate of reinforcement tended to be higher in the acquisition and performance components even at unit doses of cocaine that maintained the highest response rates in the self-administration component (cf. Table 4 for individual response rate data). In any case, evidence from the present study seems to indicate that cocaine's reinforcing and disruptive effects (as established under the three-component multiple schedule) are differentially sensitive to the antagonistic effects of haloperidol.
Another possible explanation for the limited effectiveness of haloperidol in the self-administration component may be that the change in the reinforcing effects was not large enough to prevent cocaine from acting as a positive reinforcer under the FR-60 schedule. Stated differently, haloperidol may not be able to decrease the magnitude of the reinforcer sufficiently to extinguish responding under this schedule. This notion is consistent with a study by Roberts, Loh and Vickers (1989), who found that haloperidol decreased the break point in rats responding under a progressive-ratio schedule, but only by an average of 35%. In this study, the effective dose also increased the injection rate under small ratio values. Together, these results are a reminder of the overall problem of dissociating cocaine's reinforcing effects and its effects on response rate under typical FR schedules of cocaine reinforcement (Winger, 1988).
In summary, the present data demonstrate the utility of preclinical models that consist of more than one type of operant behavior, as few behaviors occur in complete isolation, including human drug self-administration. The present study also demonstrates the likely need to examine the effects of pharmacotherapies for cocaine abuse on more than one type of behavior, in order to fully establish their potential for clinical use. From a practical standpoint, preclinical models involving multiple behaviors might also reduce the number of subjects and the time necessary to accomplish the evaluations of potential treatments, particularly if these drug evaluations would generally be conducted separately anyway. Finally, the use of multiple schedules of behavior in preclinical models will provide investigators with an opportunity to more carefully examine an understudied source of behavioral control or interaction in self-administration studies, namely, the context or environment in which this behavior is emitted.
Appendix A
Individual and mean ED50 values in mg/kg/infusion for decreases in response rate in the acquisition and performance components in the absence and presence of two doses of haloperidol.
| ED50: Response Rate |
||||||
| Acquisition Component |
Performance Component |
|||||
| Subject | Coc. Alone | Halo. 0.0032 | Halo. 0.01 | Coc. Alone | Halo. 0.0032 | Halo. 0.01 |
| 79 | 0.026 | 0.037 | - | 0.028 | 0.043 | 0.023 |
| TR | 0.026 | 0.045 | - | 0.029 | 0.041 | 0.018 |
| LU | 0.027 | 0.029 | - | 0.031 | 0.045 | 0.043 |
| HA | 0.18 | 0.159 | 0.227 | 0.149 | 0.066 | - |
| SQ | 0.175 | 0.06 | - | 0.18 | 0.089 | - |
| JO | 0.018 | 0.02 | 0.02 | 0.034 | 0.042 | 0.034 |
| Mean | 0.075 | 0.058 | 0.124 | 0.075 | 0.054 | 0.03 |
| 95% CI | −0.008–0.158 | 0.002–0.149 | ||||
Coc. = cocaine. Halo. = haloperidol.
(-) indicates ED50 values that could not be ascertained due to decreases in response rate.
Appendix B
Overall response rate for each subject in each component of the multiple schedule after saline or increasing unit doses of cocaine, both in the absence and presence of two doses of haloperidol.
| Response Rate (R/s) |
|||||||||||||||||||||
| Acquisition Component |
Performance Component |
Self-Administration Component |
|||||||||||||||||||
| Subject | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | 0.56 | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | 0.56 | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | 0.56 |
| 79 | 1.47 | 1.10 | 1.58 | 0.36 | 0.0 | 0.0 | 1.95 | 1.54 | 1.89 | 0.65 | 0.04 | 0.0 | 0.19 | 0.04 | 1.02 | 1.04 | 0.86 | 0.26 | |||
| TR | 1.98 | 2.04 | 2.35 | 0.65 | 0.17 | 0.02 | 2.49 | 2.65 | 2.85 | 1.39 | 0.55 | 0.14 | 0.22 | 0.09 | 0.38 | 1.68 | 0.74 | 0.21 | |||
| LU | 1.10 | 0.8 | 1.45 | 0.46 | 0.04 | 0.02 | 2.32 | 2.08 | 2.61 | 1.36 | 0.09 | 0.01 | 0.17 | 0.33 | 1.24 | 1.59 | 0.62 | 0.19 | |||
| HA | 1.35 | 1.25 | 1.37 | 1.32 | 1.22 | 0.74 | 2.54 | 2.57 | 2.73 | 2.41 | 2.24 | 1.28 | 0.47 | 0.07 | 0.49 | 0.64 | 0.29 | 0.11 | |||
| SQ | 1.03 | 1.06 | 1.09 | 1 | 0.93 | 0.06 | 1.22 | 0.95 | 1.28 | 1.35 | 1.07 | 0.19 | 0.34 | 0.27 | 0.38 | 0.21 | 0.17 | 0.12 | |||
| JO | 1.76 | 1.29 | 1.49 | 0.39 | 0.03 | 0.01 | 2.47 | 1.87 | 2.48 | 1.55 | 0.23 | 0.02 | 0.3 | 0.44 | 2.67 | 2.39 | 1.42 | 0.25 | |||
| Mean | 1.45 | 1.26 | 1.55 | 0.7 | 0.4 | 0.14 | 2.17 | 1.94 | 2.31 | 1.45 | 0.7 | 0.27 | 0.28 | 0.2 | 1.03 | 1.26 | 0.68 | 0.19 | |||
| Haloperidol 0.0032 mg/kg | |||||||||||||||||||||
| 79 | 1.36 | 0.87 | 1.6 | 0.98 | 0.08 | 0.0 | 0.0 | 1.60 | 1.35 | 2.24 | 1.44 | 0.11 | 0.0 | 0.0 | 0.14 | 0.01 | 0.33 | 0.8 | 0.68 | 0.26 | 0.14 |
| TR | 1.64 | 1.9 | 1.14 | 1.77 | 0.06 | 0.02 | 0.02 | 1.64 | 2.54 | 1.56 | 1.74 | 0.11 | 0.02 | 0.03 | 0.14 | 0.18 | 0.16 | 0.29 | 0.69 | 0.29 | 0.15 |
| LU | 0.81 | 0.6 | 0.8 | 0.69 | 0.05 | 0.04 | 0.01 | 1.81 | 1.95 | 2.19 | 1.94 | 0.08 | 0.06 | 0.06 | 0.12 | 0.05 | 0.46 | 0.86 | 1.12 | 0.28 | 0.15 |
| HA | 1.42 | 1.49 | 1.42 | 1.55 | 1.27 | 0.55 | 2.51 | 2.41 | 2.17 | 2.43 | 1.38 | 1.19 | 0.61 | 0.34 | 0.89 | 0.45 | 0.33 | 0.11 | |||
| SQ | 1.01 | 1.1 | 0.97 | 1.06 | 0.06 | nt | 0.97 | 1.48 | 1.12 | 1.3 | 0.54 | nt | 0.12 | 0.47 | 0.21 | 0.33 | 0.14 | nt | |||
| JO | 1.73 | 1.52 | 2.06 | 0.17 | 0.02 | 0.16 | 2.5 | 2.09 | 2.99 | 2.01 | 0.12 | 0.36 | 0.1 | 0.5 | 0.78 | 3.25 | 1.16 | 0.36 | |||
| Mean | 1.33 | 1.25 | 1.33 | 1.03 | 0.26 | 0.15 | 0.01 | 1.84 | 1.97 | 2.04 | 1.81 | 0.39 | 0.32 | 0.03 | 0.2 | 0.26 | 0.47 | 1 | 0.69 | 0.26 | 0.15 |
| Haloperidol 0.01 mg/kg | |||||||||||||||||||||
| 79 | 0.0 | 0.04 | 0.66 | 0.62 | 0.0 | 0.0 | 0.0 | 0.17 | 0.17 | 1.46 | 0.79 | 0.15 | 0.0 | 0.0 | 0.11 | 0.11 | 0.44 | 1.24 | 0.93 | 0.36 | 0.2 |
| TR | 0.95 | 0.61 | 0.47 | 0.46 | 0.04 | 0.03 | 0.03 | 1.11 | 1.28 | 1.72 | 1.22 | 0.25 | 0.03 | 0.04 | 0.12 | 0.28 | 0.84 | 1.19 | 1.33 | 0.35 | 0.24 |
| LU | 0.13 | nt | 0.25 | 0.33 | 0.08 | 0.15 | 0.03 | 0.68 | nt | 1.18 | 1.81 | 0.16 | 0.2 | 0.05 | 0.06 | nt | 0.08 | 0.83 | 2.42 | 0.36 | 0.16 |
| HA | 0.8 | 0.21 | 1.32 | 1.11 | 1.51 | 0.78 | 1.68 | 0.55 | 2.48 | 2.18 | 2.69 | 2.3 | 0.06 | 0.06 | 0.19 | 0.11 | 0.16 | 0.11 | |||
| SQ | 0.22 | 0.31 | 0.15 | 0.4 | 0.09 | 0.0 | 0.34 | 0.48 | 0.75 | 0.63 | 0.14 | 0.0 | 0.12 | 0.77 | 0.96 | 0.83 | 0.45 | 0.47 | |||
| JO | 0.0 | 0.04 | 2.01 | 0.25 | 0.04 | 0.01 | 0.0 | 0.35 | 2.7 | 1.57 | 0.04 | 0.02 | 0.09 | 0.45 | 2.57 | 2.15 | 2.28 | 0.39 | |||
| Mean | 0.35 | 0.24 | 0.81 | 0.53 | 0.29 | 0.16 | 0.02 | 0.67 | 0.57 | 1.71 | 1.37 | 0.57 | 0.43 | 0.03 | 0.09 | 0.33 | 0.85 | 1.06 | 1.26 | 0.34 | 0.2 |
Appendix C
Overall percentage of errors for each subject in the acquisition and performance components in the absence or presence of two doses of haloperidol.
| Acquisition Component |
Performance Component |
|||||||||||
| Subject | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 | Saline | 0.0032 | 0.01 | 0.032 | 0.1 | 0.32 |
| Cocaine Alone | ||||||||||||
| 79 | 1.28 | 2.30 | 0.92 | 6.74 | - | - | 0.06 | 0.05 | 0.02 | 2.73 | - | - |
| TR | 1.44 | 1.06 | 1.26 | 20.31 | 61.86 | - | 0.18 | 0.22 | 0.33 | 1.33 | 12.11 | 7.26 |
| LU | 4.07 | 7.35 | 3.17 | 30.87 | - | - | 1.18 | 1.35 | 0.81 | 4.46 | 69.65 | - |
| HA | 1.23 | 2.07 | 1.08 | 1.08 | 1.3 | 2 .45 | 0.14 | 0.08 | 0.12 | 0.04 | 0.09 | 0.4 |
| SQ | 0.97 | 0.64 | 0.66 | 0.81 | 0.62 | - | 0.16 | 0.1 | 0.11 | 0.04 | 0.12 | 0.0 |
| JO | 1.88 | 3.45 | 4.54 | 37.23 | - | - | 0.16 | 0.35 | 0.1 | 5.85 | 15.93 | - |
| Mean | 1.81 | 2.81 | 1.94 | 16.17 | 21.26 | 0.31 | 0.36 | 0.25 | 2.41 | 16.32 | 2.55 | |
| Haloperidol 0.0032 mg/kg + Cocaine | ||||||||||||
| 79 | 1.2 | 1.99 | 1.12 | 0.85 | - | - | 0.14 | 0.14 | 0.02 | 0.07 | 0.0 | - |
| TR | 1.43 | 0.98 | 1.74 | 1.46 | - | - | 0.21 | 0.13 | 0.34 | 0.21 | 29.8 | - |
| LU | 5.14 | 6.26 | 6.05 | 7.11 | - | - | 0.95 | 1.52 | 0.43 | 0.82 | - | - |
| HA | 1.18 | 1.4 | 1.27 | 1.14 | 2.52 | 3.19 | 0.03 | 0.07 | 0.13 | 0.3 | 0.03 | 0.08 |
| SQ | 0.73 | 0.39 | 0.84 | 0.85 | - | nt | 0.05 | 0.03 | 0.0 | 0.04 | 0.0 | |
| JO | 2.03 | 2.25 | 1.73 | 8.33 | - | 7.18 | 0.22 | 0.13 | 0.04 | 0.57 | - | 1.62 |
| Mean | 1.95 | 2.21 | 2.12 | 3.29 | 5.18 | 0.27 | 0.34 | 0.16 | 0.33 | 7.46 | 0.85 | |
| Haloperidol 0.01 mg/kg + Cocaine | ||||||||||||
| 79 | - | - | 1.33 | 2.95 | - | - | 1.08 | 0.15 | 0.01 | 0.09 | 0.46 | - |
| TR | 2.69 | 11.44 | 24.28 | 3.08 | - | - | 0.67 | 0.66 | 0.32 | 0.26 | 4.53 | - |
| LU | 8.8 | nt | 8.38 | 18.15 | - | 98.6 | 1.32 | nt | 1.13 | 1.74 | 71.1 | 100 |
| HA | 3.08 | 6.76 | 1.56 | 1.4 | 1.48 | 3.59 | 1.36 | 1.1 | 0.17 | 0.05 | 0.07 | 0.28 |
| SQ | 2.03 | 0.36 | 2.3 | 1.49 | 0.95 | - | 0.57 | 0.39 | 0.3 | 0.08 | 0.0 | - |
| JO | - | - | 0.96 | 28.93 | - | - | - | 0.97 | 0.09 | 1.5 | - | - |
| Mean | 4.15 | 6.19 | 6.47 | 9.33 | 1.21 | 51.09 | 1.0 | 0.65 | 0.34 | 0.62 | 15.23 | 50.14 |
(-) indicates error values that could not be ascertained due to decreases in response rate.
(nt) indicates doses or dose combinations that were not tested.
References
- de la Garza R, Johanson C.E. Effects of haloperidol and physostigmine on self-administration of local anesthetics. Pharmacology, Biochemistry and. Behavior. 1982;17:1295–1299. doi: 10.1016/0091-3057(82)90138-1. [DOI] [PubMed] [Google Scholar]
- Dworkin S.I, Mirkis S, Smith J.E. Reinforcer interactions under concurrent schedules of food, water, and intravenous cocaine. Behavioural Pharmacology. 1990;1:327–338. doi: 10.1097/00008877-199000140-00006. [DOI] [PubMed] [Google Scholar]
- Foltin R.W. Effects of dietary and pharmacological manipulations on appetitive and consummatory aspects of feeding in non-human primates. Appetite. 2005;45:110–120. doi: 10.1016/j.appet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Gawin F.H. Neuroleptic reduction of cocaine-induced paranoia but not euphoria? Psychopharmacology (Berl.) 1986;90:142–143. doi: 10.1007/BF00172886. [DOI] [PubMed] [Google Scholar]
- Glowa J.R, Wojnicki F.H. Effects of drugs on food- and cocaine-maintained responding, III: Dopaminergic antagonists. Psychopharmacology (Berl.) 1996;1996.Dec., 128:351–358. doi: 10.1007/s002130050144. [DOI] [PubMed] [Google Scholar]
- Hadfield M.G, Mott D.E, Ismay J.A. Cocaine: effect of in vivo administration on synaptosomal uptake of norepinephrine. Biochemical Pharmacology. 1980;29:1861–1863. doi: 10.1016/0006-2952(80)90154-9. [DOI] [PubMed] [Google Scholar]
- Herling S, Woods J.H. Chlorpromazine effects on cocaine-reinforced responding in rhesus monkeys: reciprocal modification of rate-altering effects of the drugs. The Journal of Pharmacology and Experimental Therapeutics. 1980;214:354–361. [PubMed] [Google Scholar]
- Howell L.L, Wilcox K.M. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. The Journal of Pharmacology and Experimental Therapeutics. 2001;298:1–6. [PubMed] [Google Scholar]
- Kelleher R.T. Chaining and conditioned reinforcement. In: Honig W.K, editor. Operant behavior: Areas of research and application. New York: Appleton-Century-Crofts; 1966. pp. 160–212. [Google Scholar]
- Magliozzi J.R, Gillespie H, Lombrozo L, Hollister L.E. Mood alteration following oral and intravenous haloperidol and relationship to drug concentration in normal subjects. The Journal of Clinical Pharmacology. 1985;25:285–290. doi: 10.1002/j.1552-4604.1985.tb02840.x. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T.J, Ho M.Y, Bradshaw C.M, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl.) 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Research Reviews. 2003;41:268–287. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Nader M.A, Sinnott R.S, Mach R.H, Morgan D. Cocaine- and food-maintained responding under a multiple schedule in rhesus monkeys: environmental context and the effects of a dopamine antagonist. Psychopharmacology (Berl.) 2002;163:292–301. doi: 10.1007/s00213-002-1202-3. [DOI] [PubMed] [Google Scholar]
- Nader M.A, Woolverton W.L. Effects of increasing response requirement on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl.) 1992;108:295–300. doi: 10.1007/BF02245115. [DOI] [PubMed] [Google Scholar]
- Negus S.S, Mello N.K, Lamas X, Mendelson J.H. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1996;278:879–890. [PubMed] [Google Scholar]
- Nestler E.J. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. TRENDS in Pharmacological Sciences. 2004;25:210–218. doi: 10.1016/j.tips.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Paronis C.A, Gasior M, Bergman J. Effects of cocaine under concurrent fixed ratio schedules of food and IV drug availability: a novel choice procedure in monkeys. Psychopharmacology (Berl.) 2002;163:283–291. doi: 10.1007/s00213-002-1180-5. [DOI] [PubMed] [Google Scholar]
- Platt D.M, Rowlett J.K, Spealman R.D. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl.) 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Reilly S. Reinforcement value of gustatory stimuli determined by progressive ratio performance. Pharmacology, Biochemistry and Behavior. 1999;63:301–311. doi: 10.1016/s0091-3057(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Richardson N.R, Smith A.M, Roberts D.C. A single injection of either flupenthixol decanoate or haloperidol decanoate produces long-term changes in cocaine self-administration in rats. Drug and Alcohol Dependence. 1994;36:23–25. doi: 10.1016/0376-8716(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Roberts D.C, Loh E.A, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl.) 1989;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts D.C, Vickers G. Atypical neuroleptics increase self-administration of cocaine: an evaluation of a behavioural screen for antipsychotic activity. Psychopharmacology (Berl.) 1984;82:135–139. doi: 10.1007/BF00426397. [DOI] [PubMed] [Google Scholar]
- Roberts D.C, Vickers G. The effect of haloperidol on cocaine self-administration is augmented with repeated administrations. Psychopharmacology (Berl.) 1987;93:526–528. doi: 10.1007/BF00207247. [DOI] [PubMed] [Google Scholar]
- Ross S.B, Renyi A.L. Inhibition of the uptake of tritiated 5-hydroxytryptamine in brain tissue. European Journal of Pharmacology. 1969;7:270–277. doi: 10.1016/0014-2999(69)90091-0. [DOI] [PubMed] [Google Scholar]
- Roth B.L, Sheffler D.J, Kroeze W.K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature Reviews Drug Discovery. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage M.G, Rice K.C, Glowa J.R. A comparison of cocaine, GBR 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug and Alcohol Dependence. 2001;62:41–47. doi: 10.1016/s0376-8716(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Annual survey on drug use and health (Rep. No. SMA 03-3836) Rockville, MD: National Clearinghouse for Alcohol and Drug Information; 2003. [Google Scholar]
- Thompson D.M, Moerschbaecher J.M. Operant methodology in the study of learning. Environmental Health Perspectives. 1978;26:77–87. doi: 10.1289/ehp.782677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.M, Moerschbaecher J.M. An experimental analysis of the effects of d-amphetamine and cocaine on the acquisition and performance of response chains in monkeys. The Journal of the Experimental Analysis of Behavior. 1979;32:433–444. doi: 10.1901/jeab.1979.32-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci F.J, Acri J, Elkashef A. Medication development for addictive disorders: The state of the science. American Journal of Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Wilson M.C, Schuster C.R. The effects of stimulants and depressants on cocaine self-administration behavior in the rhesus monkey. Psychopharmacologia. 1973;31:291–304. doi: 10.1007/BF00421274. [DOI] [PubMed] [Google Scholar]
- Winger G. Pharmacological modifications of cocaine and opioid self-administration. NIDA Research Monograph. 1988;88:125–136. [PubMed] [Google Scholar]
- Winsauer P.J, Moerschbaecher J.M, Molina P.E, Roussell A.M. Contingent and noncontingent cocaine administration in rhesus monkeys: a comparison of the effects on the acquisition and performance of response sequences. Behavioural Pharmacology. 2003;14:295–306. doi: 10.1097/01.fbp.0000081785.35927.08. [DOI] [PubMed] [Google Scholar]
- Winsauer P.J, Silvester K.R, Moerschbaecher J.M, France C.P. Cocaine self-administration in monkeys: effects on the acquisition and performance of response sequences. Drug and Alcohol Dependence. 2000;59:51–61. doi: 10.1016/s0376-8716(99)00105-2. [DOI] [PubMed] [Google Scholar]
- Winsauer P.J, Thompson D.M. Cocaine self-administration in pigeons. Pharmacology, Biochemistry and Behavior. 1991;40:41–52. doi: 10.1016/0091-3057(91)90318-v. [DOI] [PubMed] [Google Scholar]
- Winsauer P.J, Thompson D.M, Moerschbaecher J.M. Comparison of drug effects on fixed-ratio performance and chain performance maintained under a second-order fixed-ratio schedule. The Journal of the Experimental Analysis of Behavior. 1985;44:367–376. doi: 10.1901/jeab.1985.44-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki F.H, Glowa J.R. Effects of drug history on the acquisition of responding maintained by GBR 12909 in rhesus monkeys. Psychopharmacology (Berl.) 1996;123:34–41. doi: 10.1007/BF02246278. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L. Effects of a D1 and a D2 dopamine antagonist on the self-administration of cocaine and piribedil by rhesus monkeys. Pharmacology, Biochemistry and Behavior. 1986;24:531–535. doi: 10.1016/0091-3057(86)90553-8. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L, Virus R.M. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacology, Biochemistry and Behavior. 1989;32:691–69. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]