Abstract
Arsenic, cadmium, chromium, lead, manganese, mercury and selenium were analyzed in the feathers of Black-legged Kittiwakes (Rissa tridactyla) from Shoup Bay in Prince William Sound, Alaska to determine if there were age-related differences in metal levels, and in Black Oystercatchers (Haematopus bachmani)) from the same region to determine if there were differences in oiled and unoiled birds. Except for mercury, there were no age-related differences in metals levels in the feathers of kittiwakes. Kittiwakes over 13 years of age had the highest levels of mercury. There were no differences in levels of metals in the feathers of oystercatchers from oiled and unoiled regions of Prince William Sound. Except for mercury, the feathers of oystercatchers had significantly higher levels of all metals than those of kittiwakes. Levels of mercury in kittiwake feathers (mean of 2910 ng/g [ppb]) were within the range of many species of seabirds reported for other studies, and were generally below adverse effects levels.
Keywords: Black-legged kittiwake, Black oystercatcher, Age-related, Oil, Arsenic, Cadmium, Chromium, Lead, Manganese, Mercury, Prince William Sound, Alaska, Metals
1. Introduction
Chemical contamination of the coastal and marine environment may pose a threat to some species or to some individuals within populations. Even in sparsely occupied, non-industrialized oceanic and polar regions, animals are exposed to pollutants through atmospheric transport and ocean currents (Macdonald et al., 2005). Despite declines in contaminants in some regions of the world, some species have not shown declines in metals (Braune, 2007), and for some species, levels of contaminants are higher at higher latitudes (Braune et al., 2002).
Some metals that are persistent in nature readily accumulate in organisms, particularly those that are long-lived, high on the food chain, or live in industrialized areas exposed to anthropogenic sources (Fowler, 1990; Burger, 2002; Burger and Gochfeld, 1999, 2002). Because some pollutants undergo biomagnification up the food chain, and can accumulate with age, concentrations are generally higher in top trophic level birds and mammals (Lewis and Furness, 1991; Ohlenhdorf, 1993; Bargagli et al., 1998; Scheifler et al., 2005). Factors that affect uptake, accumulation, and biomagnification of metals in birds include exposure pathways, species of the metal, and bioavailability, as well as a number of host factors, such as trophic status, location, foraging behavior, nutrition, body condition, gender, size, genetic variability, and age (Stewart et al., 1997; Debacker et al., 2001; Burger et al., 2003). Marine birds are exposed to a wide range of chemicals because they occupy a wide range of trophic levels, and those at the top of the food chain are susceptible to bioaccumulation of pollutants (Furness and Rainbow, 1990; Lewis and Furness, 1991; Burger and Gochfeld, 2002; Nygard et al., 2001).
In this paper we examine two questions about metal concentrations in feathers using black-legged kittiwakes (Rissa tridactyla) and black oystercatchers (Haematopus bachmani): 1) Are there age-related differences in metal levels in kittiwakes of known ages, and 2) Are there differences in metals levels in black oystercatchers from an area that was heavily oiled during the Exxon Valdez oil spill and an area that was not. We examined concentrations of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in the breast feathers of both species from Prince William Sound, Alaska. We expected that older kittiwakes would have higher levels of metals than younger birds, based on the principle of bioaccumulation with age, although Monteiro and Furness (1995) noted that mercury in body feathers did not accumulate with adult age in their studies. Burger and Gochfeld (1994) found age-related differences for chromium, manganese and selenium, but not mercury, cadmium and lead for known-aged common terns (Sterna hirundo). In birds, age-related differences have generally been examined between young of the year and adults, mainly because these age classes are easily distinguished by plumage patterns, especially in seabirds (Burger, 1993). Once birds reach adulthood, there are no age-related differences in plumage, making it impossible to age unless they were marked at hatching. In some cases oil carries some metals, including mercury (Woodle and Chandler, 1952; USEPA, 2007), predicting that metal levels might be higher in the feathers of oystercatchers from oiled compared to unoiled regions of the bay, although the oil spill occurred 15 years prior to the present study. Further, since some oil persists in the region from the oil spill, we wanted to ascertain whether metal levels were higher in birds that were oiled.
Breast feathers were selected because they are considered to be more representative of exposure to metals (Furness et al., 1986; Burger, 1993). Further, Thompson et al. (1991) showed that essentially 100% of the mercury in feathers and muscle is methylmercury. Metals enter feathers during the 2–3 weeks it takes for them to grow, then the blood supply atrophies and there is no further uptake of metals (Burger, 1993; Thompson et al., 1998). Thus, feathers are an archive of metal exposure during feather formation weeks or months earlier.
2. Study area and methods
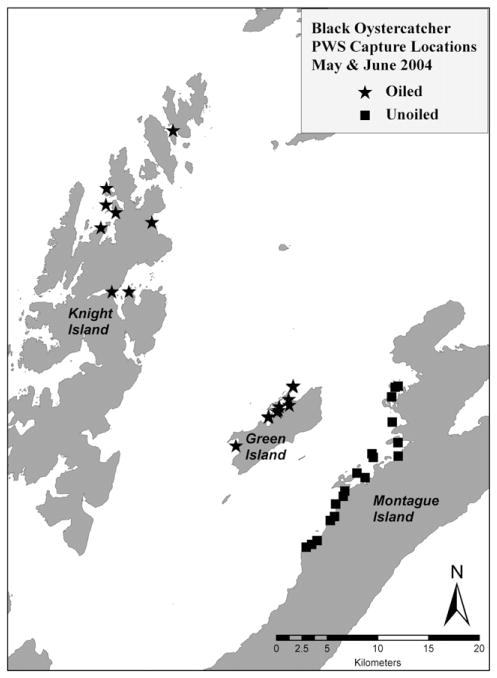
Under appropriate state and federal collecting permits feathers from 61 kittiwakes were collected from Shoup Bay in Prince William Sound of Alaska in 2004, and feathers from 44 black oystercatchers were collected from Knight, Green and Montague Islands in Prince William Sound (Figs. 1 and 2). Feathers were used because the birds were part of an on-going study of marked birds; thus we did not want to sacrifice birds for the collection of internal tissues; breast feathers were collected because they are considered a better indicator of body burden. The species were selected because they were part of a long-term study to examine the health of these species as bioindicators of ecosystem recovery.
Fig. 1.
Map showing locations where black-legged kittiwake (Rissa tridactyla) and black oystercatcher (Haematopus bachmani) from Prince William Sound were captured for feather collection.
Fig. 2.
Capture area of black oystercatcher in Western Prince William Sound.
Birds were captured as part of a continuing effort to monitor ecological conditions in Prince William Sound following the 1989 Exxon Valdez oil spill (Peterson et al., 2003). Kittiwakes and most oystercatchers were trapped at their nests (during the breeding season) using a string noose placed around the nest bowl with a pull line; some oystercatchers were also caught in mist nets. After setting the trap, laying out the pull line, and selecting a well-camouflaged hiding spot, one team member would hide while the other made an obvious departure. The incubating bird generally returned to the nest within 5 min, the string was pulled, catching the bird (which was removed immediately). Breast feathers (10–20) were plucked and the placed in an envelope, marked with bird band number and location, and the bird was released. Feathers were shipped to the Rutgers University for analysis. We targeted known-aged kittiwakes that had been marked as chicks at the Shoup Bay colony.
All feathers were analyzed in the Elemental Analysis Laboratory of the Environmental and Occupational Health Sciences Institute in Piscataway. Feathers were washed three times with acetone, and then digested individually in warm nitric acid mixed with the addition of 30% hydrogen peroxide, and subsequently diluted with deionized water. Mercury was analyzed by cold vapor technique, and the other elements were analyzed by graphite furnace atomic absorption spectrometry (Burger and Gochfeld, 1991). All concentrations are expressed in ng/g (ppb) on a dry weight basis using weights obtained from air-dried specimens. Detection limit ranges were: 0.2 ng/g for arsenic, 0.02 ng/g for cadmium, 0.08 ng/g for chromium, 0.15 ng/g for lead, 0.09 ng/g for manganese, 0.2 ng/g for mercury, and 0.7 ng/g for selenium. All specimens were analyzed in batches with known standards, calibration standards, and spiked specimens. Recoveries ranged from 86% to 105%. Batches with recoveries of less than 85% would be reanalyzed. The coefficient of variation on replicate, spiked samples ranged up to 10%.
Non-parametric Kruskal–Wallis chi square test was used to examine age-related differences, because of small sample sizes (SAS, 1995). Both arithmetic and geometric means are given to facilitate comparisons with other studies. We used the Kendall tau to examine relationships between metal levels and age. We accept a probability level of 0.05 for significance, but present all values below 0.10 to allow the reader to assess the significance themselves.
3. Results
Herein we examine age-related differences in kittiwakes, oil-related differences in oystercatchers, and then compare metals levels in the feathers of kittiwakes and oystercatchers.
There were statistically significant age-related differences in the levels of mercury in the feathers of kittiwakes, but not for the other metals (Table 1). For mercury, birds over 13 years of age had the highest mean mercury levels. These oldest birds also had the highest cadmium and selenium levels and lowest chromium, lead, and manganese levels, although none of these were statistically significant. There were no differences in metal levels in oystercatchers from oiled and unoiled nesting areas in Prince William Sound (Table 2).
Table 1.
Metal levels (ng/g=ppb, dry weight) in feathers of black-legged kittiwakes collected from Shoup Bay, Alaska in 2004
| All feathers | Age
|
χ2(p) | Correlation with age | ||||
|---|---|---|---|---|---|---|---|
| <7 | 7–9 | 10–13 | >13 | ||||
| n=61 | n=14 | n=19 | n=15 | n=13 | |||
| Arsenic | 166±18.6 38.2 |
171±40.5 52.5 |
145±28.5 17.9 |
183±46.7 55.1 |
169±36.9 54.2 |
0.3 (NS) | 0.0 (NS) |
| Cadmium | 32.8±3.2 16.0 |
32.4±6.04 25.4 |
27.1±5.11 7.64 |
26.3±4.78 12.5 |
49.1±8.93 37.5 |
5.4 (NS) | 0.1 (NS) |
| Chromium | 954±129 699 |
1010±216 683 |
1140±347 719 |
931±196 776 |
656±76.1 608 |
2.2 (NS) | −0.1 (NS) |
| Lead | 707±131 455 |
1024±433 597 |
537±139 359 |
828±289 585 |
476±111 360 |
4.3 (NS) | −0.1 (NS) |
| Manganese | 751±73.8 607 |
960±277 609 |
705±91.8 600 |
710±77.8 646 |
638±79.2 571 |
0.5 (NS) | 0.0 (NS) |
| Mercury | 2910±186 2440 |
3070±450 2430 |
2360±272 1960 |
2570±302 2230 |
3910±400 3710 |
9.5 (NS) | 0.2 (0.09) |
| Selenium | 2420±112 2120 |
2560±266 2110 |
2190±197 1820 |
2300±176 2170 |
2730±257 2590 |
2.6 (NS) | 0.0 (NS) |
Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis Chi Square values and p values in parentheses. NS=p>0.10.
Table 2.
Metal levels (ng/g=ppb, dry weight) in feathers of oiled and non-oiled black oystercatchers collected from Prince William Sound in 2004
| Feathers
|
χ2(p) | ||
|---|---|---|---|
| Oiled | Non-oiled | ||
| n=24 | n=20 | ||
| Arsenic | 343±45.0 253 |
293±43.6 248 |
0.7 (NS) |
| Cadmium | 91.9±9.58 82.4 |
90.6±10.7 74.0 |
0.0 (NS) |
| Chromium | 2070±566 1380 |
1990±701 1140 |
1.7 (NS) |
| Lead | 1100±87.8 1010 |
1420±191 1220 |
0.8 (NS) |
| Manganese | 1060±121 924 |
1150±146 1000 |
0.2 (NS) |
| Mercury | 1130±141 924 |
1380±140 1250 |
2.2 (NS) |
| Selenium | 8670±828 7770 |
7200±490 6730 |
1.0 (NS) |
Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis Chi Square values and p values in parentheses. NS=p>0.10.
Because there were neither age-related differences for most metals in feathers of kittiwakes, nor between oiled and unoiled oystercatcher nesting areas, we then compared metals levels in kittiwakes and oystercatchers (Table 3). Black oystercatchers had significantly higher levels of all metals (except mercury) in their feathers compared to kittiwakes.
Table 3.
Metal levels (ng/g=ppb, dry weight) in feathers of black oystercatchers and black-legged kittiwakes collected from Prince William Sound in 2004
| Feathers
|
χ2(p) | ||
|---|---|---|---|
| Black oystercatchers | Black-legged kittiwakes | ||
| n=44 | n=61 | ||
| Arsenic | 321±31.5 251 |
166±18.6 38.2 |
17.0 (<0.0001) |
| Cadmium | 91.3±7.05 78.5 |
32.8±3.20 16.0 |
48.4 (<0.0001) |
| Chromium | 2030±438 1270 |
954±129 699 |
14.4 (0.0002) |
| Lead | 1250±101 1102 |
707±131 455 |
36.9 (<0.0001) |
| Manganese | 1100±93 959 |
751±74 607 |
13.9 (0.0002) |
| Mercury | 1240±100 1060 |
2910±186 2440 |
37.2 (<0.0001) |
| Selenium | 8000±511 7270 |
2420±112 2120 |
64.3 (<0.0001) |
Given are arithmetic means±SE (geometric means below) with Kruskal–Wallis Chi Square values and p values in parentheses. NS=p>0.10.
4. Discussion
4.1. Age-related differences
Age-related differences in metals have generally compared young birds with adults because of the ease of distinguishing adults from young by plumage patterns. In a review of metal levels in feathers, Burger (1993) reported that adults had significantly higher levels than young of the year for cadmium (3 of 5 studies), lead (4 of 7), manganese (5 of 5), mercury (20 of 21), and selenium (3 of 3), with chromium showing less of a pattern (1 of 4 studies). Since that paper was published, differences, differences between adults and young have been reported for other species and metals (Stewart et al., 1997; DeBacker et al., 2001; and reviewed in Burger and Gochfeld, 2002). The differences between adults and young likely reflect differences due to exposure in different places. The levels in the feathers of young birds come from the female (exposure from the egg) and from their food, which is derived locally. In contrast, the metal levels in feathers of adults come from exposure during feather formation, which occurs during the molt period (which likely occurs away from the breeding colonies).
To truly understand metal levels as a function of age, however, it is necessary to have known-aged birds, which requires long-term banding studies. Several studies have examined metals levels in marked known-aged adult birds. Monteiro and Furness (1995) noted that mercury in body feathers did not accumulate with adult age in these studies. Since then, Burger and Gochfeld (1994) found age-related differences in the feathers of known-aged common terns (S. hirundo) for chromium, manganese and selenium, but not in mercury, cadmium and lead. Donaldson et al. (1997) reported age-related increased in cadmium, but no significant differences for lead, mercury and selenium in breast muscle of thick-billed murres (Uria lomvia).
In this study, we did not find age-related differences in the feathers of kittiwakes, except for mercury. Kittwakes from 7–9 years of age had the lowest levels, and those over 13 years of age had the highest levels (refer back to Table 1). The levels in breeding kittiwakes under 7 years, and from 10–13 years were not significantly different. The lack of an age-related difference for most metals, and the relatively small difference in mercury was surprising since such age-related differences have often been found (see above).
The breast feathers of adults collected at breeding colonies represent exposure during feather formation, thus differences due to molting cycle were not expected. Black-legged kittiwakes breeding at Shoup bay in Prince William Sound generally do not remain in the Sound during the winter, but move offshore in Alaska (DI, based on surveys) and down along the Pacific Coast. The exposure of kittiwakes during the winter is likely from oceanic resources, but there is no reason to believe that different-aged adults winter in different regions. Thus, age-related differences in metal levels should reflect accumulation with age, rather than exposure differences. And there appears to be relatively little accumulation with age in mercury of kittiwakes.
4.2. Oil-related differences
We found no differences in metals levels in feathers from oystercatchers collected from nesting areas in oiled and unoiled regions of Prince William Sound. This could result from two factors: 1) the oil released during the Exxon Valdez oil spill did not contain metals, and/or 2) little oil remains in the foods acquired by the oystercatchers, and is thus not passed up the food chain.
4.3. Species differences
Our results indicate that the feathers of oystercatchers had higher levels of metals than those of kittiwakes, except for mercury. Mercury levels, known to accumulate in fish, were higher in kittiwakes than in oystercatchers, reflecting a trophic level relationship. However, we did not predict that the other metals would be higher in oystercatchers than kittiwakes. Kittiwakes eat mainly fish (Baird, 1994), and black oystercatchers eat mainly invertebrates (shellfish, Andres and Falxa, 1995); fish are higher on the food chain than invertebrates. Since oystercatchers are foraging mainly in the intertidal, however, the higher levels of other metals may reflect higher contamination in the intertidal environment compared to the more open oceans where kittiwakes feed.
Moreover, levels of metals in feathers reflect exposure during the time of feather formation. Breast feathers collected during incubation were largely grown during the winter, when kittiwakes are foraging offshore in the open ocean, and oystercatchers continue to feed in the intertidal. However, data on wintering locations are not available.
4.4. Geographical comparisons
Although not the primary focus of this study, it is still instructive to compare the overall levels of metals, particularly mercury, in the kittiwakes and oystercatchers from Prince William Sound with those from elsewhere. In comparison to other seabirds, Braune et al. (2005) found that kittiwakes have similar mercury levels to murres (Uria lomvia) and guillemots (Cepphus grylle), and significantly lower levels than glaucous gulls (Larus glaucescens). Although Braune (2007) did not examine mercury levels in feathers, she did report that mercury levels in kittiwake eggs did not change significantly from 1875 to 2003 in colonies in the Canadian Arctic. In the northeastern Atlantic (Iceland and Scotland), Thompson et al. (1992) reported mercury levels from several seabird colonies of kittiwakes that ranged from 2900 ng/g to 5500 ng/g. Braune (1987) reported mercury levels of 1200–5900 ng/g in kittiwake breast feathers from the Bay of Fundy (Canada) from 1978–1984. Compared to gulls generally, mercury in kittiwake feathers from Prince William Sound (mean of 2900 ng/g) were somewhat higher than the median for 73 studies (median of 1700 ng/g; Burger and Gochfeld, 2002).
There are few data on metal levels in oystercatchers. However, Stock et al. (1989) reported that cadmium levels in Eurasian oystercatcher (Haematopus ostralegus) from the German Wadden Sea had levels of cadmium of 180 ng/g, compared to 91 ng/g in the present study. Selenium in the flight feathers of Eurasian oystercatcher averaged from 10,000 to 30,000 ng/g (Goede, 1991), compared to 8,000 ng/g for the oystercatchers from Prince William Sound. Thus for the data available, the levels of metals in kittiwakes and oystercatchers from Prince William Sound are similar to those reported for other regions.
Acknowledgments
Feathers were collected under appropriate state and federal permits. Our studies were approved by the Rutgers University Animal Review Board. Technical help was provided by T. Shukla, C. Jeitner, M. Donio, and L. McKnight. This research was partly funded by CRESP through the Department of Energy (DE-FC01-86EW07053; DE-FC01-06EW07053), the Division of Life Sciences of Rutgers University, Wildlife Trust, NIEHS (P30ES005022) and the Exxon Valdez Oil Spill Council (# 0774). The results, conclusions, and interpretations reported herein are the sole responsibility of the authors, and should not in any way be interpreted as representing the views of the funding agencies.
References
- Andres BA, Falxa GA. Black oystercatcher (Haematopus bachmani) Birds N Am. 1995;155:1–20. [Google Scholar]
- Baird PH. Black-legged kittiwake (Rissa tridactyla) Birds N Am. 1994;92:1–28. [Google Scholar]
- Bargagli R, Monaci R, Sanchez-Hernandez JC, Cateni D. Biomagnification of mercury in an Antarctic marine coast food web. Mar Ecol Prog Ser. 1998;169:65–76. [Google Scholar]
- Braune BM. Comparison of total mercury levels in relation to diet and molt for nine species of marine birds. Arch Environ Contam Toxicol. 1987;16:217–24. [Google Scholar]
- Braune BM. Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975–2003. Environ Pollut. 2007;XX:1–15. doi: 10.1016/j.envpol.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Braune BM, Donaldson GM, Hobson KA. Contaminant residues in seabird eggs from the Canadian Arctic. II. Spatial trends and evidence from stable isotopes for intercolony differences. Environ Pollut. 2002;117:133–45. doi: 10.1016/s0269-7491(01)00186-5. [DOI] [PubMed] [Google Scholar]
- Braune BM, Outridge PM, Fisk AT, Muir DC, Helm PA, Hobbs K, Hoekstra PF, Kuzyk ZA, Kwan M, Letcher RJ, Lockhart WL, Norstrom RJ, Stern GA, Stirling I. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: an overview of spatial and temporal trends. Sci Total Environ. 2005;351/352:4–56. doi: 10.1016/j.scitotenv.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Burger J. Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol. 1993;5:203–311. [Google Scholar]
- Burger J. Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ Res. 2002;90:33–9. doi: 10.1006/enrs.2002.4381. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Cadmium and lead in common terns (Aves: Sterna hirundo): Relationship between levels in parents and eggs. Environ Monit Assess. 1991;16:253–8. doi: 10.1007/BF00397612. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metal and selenium levels in feathers of known-aged common terns (Sterna hirundo) Arch Environ Contam Toxicol. 1994;26:351–5. doi: 10.1007/BF00203562. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Heavy metals in Franklin’s gull tissues: age and tissue differences. Environ Toxicol Chem. 1999;18:673–8. [Google Scholar]
- Burger J, Gochfeld . Effects of chemicals and pollution on seabirds. In: Schreiber EA, Burger J, editors. Biology of marine birds. Boca Raton: CRC Press; 2002. pp. 485–525. [Google Scholar]
- Burger J, Diaz-Barriga F, Marafante E, Pounds J, Robson M. Methodologies to examine the importance of host factors in bioavailability of metals. Ecotoxicol Environ Saf. 2003;56:20–31. doi: 10.1016/s0147-6513(03)00047-2. [DOI] [PubMed] [Google Scholar]
- DeBacker V, Schiettecatte LS, Jauniaux T, Bouquergneau JM. Influence of age, sex and body condition on zinc, copper, cadmium and metallothioneins in common guillemots (Uriia aalge) stranded at the Belgian coast. Mar Environ Res. 2001;52:427–44. doi: 10.1016/s0141-1136(01)00096-4. [DOI] [PubMed] [Google Scholar]
- Donaldson GM, Braune BM, Gaston AJ, Noble DG. Organochlorine and heavy metal residues in breast muscle of known-age thick-billed murres (Uria lomvia) from the Canadian Arctic. Environ Contam Toxicol. 1997;33:430–5. doi: 10.1007/s002449900273. [DOI] [PubMed] [Google Scholar]
- Fowler SW. Critical reviews of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar Environ Res. 1990;29:1–64. [Google Scholar]
- Furness RW, Rainbow PS, editors. Heavy metals in the marine environment. Boca Raton, Florida: CRC Press; 1990. [Google Scholar]
- Furness RW, Muirhead SJ, Woodburn M. Using bird feathers to measure mercury in the environment: relationship between mercury content and moult. Mar Pollut Bull. 1986;17:27–37. [Google Scholar]
- Goede AA. The variability and significance of selenium concentrations in shorebird feathers. Environ Monit Assess. 1991;18:203–10. doi: 10.1007/BF00398699. [DOI] [PubMed] [Google Scholar]
- Lewis SA. Furness Mercury accumulation and excretion by laboratory reared black-headed Gulls (Larus ridibundus) chicks. Arch Environ Contam Toxicol. 1991;21:316–20. [Google Scholar]
- Macdonald RW, Harner T, Fyfe J. Recent climate changes in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ. 2005;342:5–86. doi: 10.1016/j.scitotenv.2004.12.059. [DOI] [PubMed] [Google Scholar]
- Monteiro LR, Furness RW. Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut. 1995;80:831–70. [Google Scholar]
- Nygard TE, Lie NR, Steinnes E. Metal dynamics in an Antarctic food chain. Mar Pollut Bull. 2001;42:598–602. doi: 10.1016/s0025-326x(00)00206-x. [DOI] [PubMed] [Google Scholar]
- Ohlenhdorf HM. In: Marine birds and trace elements in the temperate North Pacific: the status, ecology and conservation of marine birds of the North Pacific. Vermeer K, Briggs KT, Morgan KH, Siegel-Causey D, editors. Ottawa, Canada: Can Wildl Serv Spec Publ; 1993. [Google Scholar]
- Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, Ballachey BE, Irons DB. Long-term ecosystem response to the Exxon Valdez oil spill. Science. 2003;302:2082–6. doi: 10.1126/science.1084282. [DOI] [PubMed] [Google Scholar]
- SAS (Statistical Analysis System) SAS user’s guide. Cary, North Carolina: SAS Institute; 1995. [Google Scholar]
- Scheifler R, Gauthier-Clerc M, Bohec CL, Crine N, Coeurdassier M, Badot PM, et al. Mercury concentrations in King Penguin (Aptenodytes patagonicus) feathers at Crozet Islands (sub-antarctica): temporal trend between 1966–1974 and 2000–2001. Environ Toxicol Chem. 2005;24:125–8. doi: 10.1897/03-446.1. [DOI] [PubMed] [Google Scholar]
- Stewart FM, Phillips RA, Catry P, Furness RW. Influence of species, age and diet on mercury concentrations in Shetland seabirds. Mar Ecol Progr Ser. 1997;151:237–44. [Google Scholar]
- Stock M, Herber RFM, Geron HMA. Cadmium levels in oystercatcher Haematopus ostralegus from the German Wadden Sea. Mar Ecol Progr Ser. 1989;53:227–34. [Google Scholar]
- Thompson DR, Hamer KC, Furness RW. Comparison of the levels of total and organic mercury in seabird feathers. Mar Pollut Bull. 1991;20:577–9. [Google Scholar]
- Thompson DR, Bearhop S, Speakman JR, Furness RW. Feathers as a means of monitoring mercury in seabirds: insights from stable isotope analysis. Environ Pollut. 1998;101:193–200. doi: 10.1016/s0269-7491(98)00078-5. [DOI] [PubMed] [Google Scholar]
- USEPA (Environmental Protection Agency) [Accessed July 21, 2007];Mercury in petroleum and natural gas. 2007 www.epa.gov/nmrl/pubs/600r01066/600r01066.htm.
- Woodle RA, Chandler WB., Jr Mechanism of occurrence of metals in petroleum distillates. Indust Engineer Chem. 1952;11:2591. [Google Scholar]