Abstract
The onset of leaf senescence is regulated by a complex mechanism involving positive and negative regulators. Among positive regulators, jasmonic acid (JA) accumulates in senescing leaves and the JA-insensitive coi1-1 mutant displays delayed leaf senescence in Arabidopsis. A strong activated expression of the gene coding for the JA-biosynthetic β-oxidation enzyme 3-ketoacyl-CoA thiolase 2 (KAT2) in natural and dark-induced senescing leaves of Arabidopsis thaliana is reported here. By using KAT2::GUS and KAT2::LUC transgenic plants, it was observed that dark-induced KAT2 activation occurred both in excised leaves as well as in whole darkened plants. The KAT2 activation associated with dark-induced senescence occurred soon after a move to darkness, and it preceded the detection of symptoms and the expression of senescence-associated gene (SAG) markers. Transgenic plants with reduced expression of the KAT2 gene showed a significant delayed senescence both in natural and dark-induced processes. The rapid induction of the KAT2 gene in senescence-promoting conditions as well as the delayed senescence phenotype and the reduced SAG expression in KAT2 antisense transgenic plants, point to KAT2 as an essential component for the timely onset of leaf senescence in Arabidopsis.
Keywords: Arabidopsis, β-oxidation, jasmonic acid, KAT2, leaf senescence
Introduction
The development of plants comprises a succession of phases and the corresponding transitions. The beginning and the end of each phase as well as the timing of the transition are regulated by environmental and endogenous factors, the latter reflecting the age and the metabolic status of the plant. Some of these developmental phases and transitions are specific for organs or structures of the plant. The transition from vegetative development to reproductive phase occurs in a reduced number of cells at the shoot apical meristem. However, other developmental phases affect all kinds of plant organs and structures, although the timing and location of the corresponding processes may vary. Plant structures that have fulfilled their functions initiate a developmental programme of senescence characterized by the degradation of cells. This process allows the plant to mobilize nutrients and metabolites from source to sink organs, maximizing material and energy resources, which results in optimized growth. As a result of the senescence programme, the abscission of the senescing organ or structure occurs (Lewis et al., 2006).
Senescence is a complex process involving physiological, biochemical, and gene expression changes that are regulated by endogenous and exogenous factors (Lim et al., 2007). Hormones are endogenous components that mediate the effect of environmental factors to regulate senescence. Some hormones such as ethylene, abscisic acid (ABA), jasmonic acid (JA), and salicylic acid (SA) act as inducers of senescence, whereas cytokinins and gibberellins play a role in its suppression (Lim and Nam, 2005). Recent data point to auxins as positive regulators of senescence (Ellis et al., 2005). However, the role of auxins in leaf senescence remains controversial (Lim and Nam, 2005). Senescence in leaves has been studied in the model plant Arabidopsis thaliana by a combination of genetic approaches, analysis of senescence-associated gene (SAG) expression, and functional genomics. Molecular events participating in or regulating leaf senescence have been recently identified and characterized (Buchanan-Wollaston et al., 2003, 2005; Lin and Wu, 2004). Leaf senescence is a natural consequence of plant ageing but it may also be induced in young plants or in excised leaves by stress factors. The incubation of excised leaves under darkness causes a rapid and efficient induced senescence that correlates with enhanced SAG gene expression and chlorosis (Weaver and Amasino, 2001). Natural and dark-induced senescence share some symptoms and molecular components. However, recent transcriptome analysis of leaves from plants undergoing different types of senescence revealed significant differences in gene expression profiles and signalling pathways. The JA/ethylene pathway seems to participate in natural and dark-induced senescence while the SA pathway plays a role only in natural senescence (Buchanan-Wollaston et al., 2005). The function of JA in leaf senescence in Arabidopsis is supported by the delayed senescence in the JA-insensitive coi1-1 mutant and the strong accumulation of JA in senescing leaves (He et al., 2002). JA is synthesized in plants through the octadecanoid pathway, a complex sequence of enzyme reactions in different subcellular locations, which requires three rounds of β-oxidation reactions in the peroxisome (Schaller, 2001). The last reaction in the pathway is catalysed by 3-ketoacyl-CoA thiolase, an enzyme that is encoded by three genes in the Arabidopsis genome. It has recently been reported that 3-ketoacyl-CoA thiolase 2 (KAT2) is responsible for the majority of JA biosynthesis in Arabidopsis (Castillo et al., 2004; Afitlhile et al., 2005), and also that KAT2 expression was up-regulated under senescence-promoting conditions (Charlton et al., 2005).
This work presents data supporting an essential role for β-oxidation in the onset of natural and dark-induced senescence in Arabidopsis. The rapid and strong activation of KAT2 expression that preceded the symptoms of natural and dark-induced leaf senescence, as well as the delayed senescence phenotype of leaves from KAT2 antisense transgenic plants, suggest that KAT2 participates not only in the catabolism associated to senescence but also in the early events required for the onset of senescence.
Materials and methods
Plant material and growth conditions
Seeds of Arabidopsis Col (Lehle Seed, Tucson, AZ) were either sown in moistened soil and grown under photoperiod cycles of 16/ 8 h day/night (long days, at 22 °C and 20 °C, respectively) or 8/16 h day/night (short days, at 22 °C and 20 °C, respectively) under 150 μE m−2 s−1 cool-white fluorescent lamps and 60% relative humidity, or surface-sterilized and germinated in agar-supplemented Murashige and Skoog (MS) medium (Duchefa, Haarlem, The Netherlands). Seeds from the JA-insensitive Arabidopsis coi1-1 mutant (Feys et al., 1994), kindly supplied by John Turner, were sown on MS-agar plates supplemented with 1% (w/v) sucrose and 20 μM JA (Duchefa, Haarlem, The Netherlands), and selected 8 d post-germination for those showing normal root growth. Antisense transgenic lines for KAT2 were previously generated and characterized (Castillo et al., 2004). To generate transgenic lines overexpressing KAT2, the full-length cDNA was subcloned in sense orientation in the BinA7 binary vector and plants were transformed and selected as described previously for antisense lines (Castillo et al., 2004).
Transgenic plants carrying a 2.2 kb promoter sequence of the KAT2 gene fused to β-glucuronidase (GUS) and luciferase (LUC) reporter genes were generated by cloning the promoter sequence in the binary pBI101 (Jefferson et al., 1987) and pPZPXomegaL+ vectors (kindly donated by Dr Steve Kay, The Scripps Research Institute, La Jolla, CA), respectively. Several independent homozygous transgenic lines for every construct were generated by the floral dipping transformation method with Agrobacterium (Clough and Bent, 1998). Homozygous transgenic lines with a single transgene insertion were selected for further analysis.
RNA isolation, northern-blot, and RT-PCR analysis
Total RNA was isolated, separated, and analysed by northern blot as previously described by Castillo et al. (2004). The KAT2 and KAT5 probes (described in Castillo et al., 2004) were labelled with α [32P]dCTP Redivue and the Rediprime labelling kit (Amersham Pharmacia Biotech, Uppsala, Sweden). Ethidum bromide-stained rRNA was used as loading control in northern experiments. KAT2, SAG12, CAB, JR2, and MSG2/IAA19 transcripts were quantified by quantitative RT-PCR using the specific primers qKAT2-2F, 5′ TCG AAT AAA GCA CAC AAC CAA TG 3′; qKAT2-2R 5′ CCC CTT CAC TTA AAA AAA ATA CTA AAC AC 3′; qSAG12-2F 5′ GGA AAA CAA TCG CTA CGT TGT G 3′ and qSAG12-2R 5′ TCC GGC AGG AAT GCT ATT TAA 3′; qCAB-F 5′ TCA GAA TGG CTG CTC ACT GG 3′ and qCAB-R 5′ CAA GGT AAG CTG GTC GTG GC 3′; qJR2-F 5′ TGC GAT CCT CAT GGC AAA C 3′ and qJR2-R 5′ CTC CAG GAT CTC ATT TCG TGG 3′; qMSG2-F 5′ CTC GGG CTT GAG ATA ACG GA 3′ and qMSG2-R 5′ CCA CAT CTC TCC CCG GAA G 3′, and the values were normalized with the endogenous ACTIN2/8 (ACT2/8) transcript content by using the specific primers qACT2-F 5′ TTG TTC CAG CCC TCG TTT GT 3′ and qACT2-R 5′ TGT CTC GTG GAT TCC AGC AG 3′.
Natural and dark-induced senescence assays
The natural leaf senescence was analysed by quantifying the total chlorophyll content in every rosette leaf from plants of the different genotypes grown under long day photoperiodic conditions. Dark-induced leaf senescence was assayed with excised leaves from 6-week-old plants grown under short day photoperiodic conditions, because the longer vegetative phase allows leaves of a homogenous size and appearance to be used, and also the potential interference of the transition to the reproductive phase of growth was avoided. Nevertheless, some of the senescence experiments in darkness were also repeated with leaves from long-day-grown plants with similar results. Leaves were laid onto wet filter paper inside Petri dishes and incubated in the dark. At different times after incubation in the dark, excised darkened leaves were harvested, photographed, used for chlorophyll quantification or stained for GUS activity, as indicated.
Analysis of chlorophyll content, GUS staining, and LUC activity
The chlorophyll content in leaves was determined by the original method of Arnon (1949) with modifications. The reproducibility of the presented data was assessed by repeating each experiment at least twice with similar results. β-glucuronidase (GUS) activity was assayed in situ after harvesting samples in ice-cold 90% (v/v) acetone. After extensive washing with staining buffer (50 mM phosphate buffer, pH 7.2, containing 0.2% (v/v) Triton X-100 and 2 mM potassium ferrocyanide and ferricyanide) the leaves were vacuum-infiltrated for 20 min with staining buffer supplemented with 2 mM 5-bromo-4-chloro-3-indolyl glucuronide (X-gluc). Infiltrated samples were incubated at 37 °C for 10–16 h. Staining buffer was removed by ethanol series of 20% (v/v), 35% (v/v), and 50% (v/v) at room temperature for 30 min. Stained samples were then treated for 30 min at room temperature with fixation solution containing 50% (v/v) ethanol, 10% (v/v) acetic acid, and 5% (v/v) formaldehyde. Finally, stained samples were washed thoroughly with 70% (v/v) ethanol with continuous and gentle shaking. GUS-stained samples were scanned with a high resolution scanner. Plants of two independent pKAT2::LUC transgenic lines, kept under darkness in a high humidity environment, were sprayed with 0.1 mM luciferin 4 h before the detection of luminescence with a low-light Hamamatsu digital camera DC2400. The images were recorded and processed with Argus-20 software (Hamamatsu, Japan).
Results
Activated expression of KAT2 gene in senescing leaves
During leaf senescence, the plant undergoes multiple changes that involve gene expression, biochemical alterations, and differential accumulation of metabolites. This complex sequence of events is finely regulated by a network that integrates endogenous cues as well as environmental factors. Many of the components of the regulatory network are not specific for senescence but participate in signalling pathways related to other developmental or stress-related processes. Among them, JA plays a central role in leaf senescence. Leaf senescence correlated with the increased synthesis and accumulation of JA and required its perception (He et al., 2002). Our previous data pointed to KAT2 as an essential component for the wound-induced synthesis of JA in Arabidopsis (Castillo et al., 2004). It has been explored whether KAT2 is also functionally connected to leaf senescence. Leaves undergoing natural senescence accumulated 4-fold KAT2 transcript levels above those detected in non-senescing leaves of the rosette (Fig. 1). The senescence-induced expression was specific for KAT2 among KAT genes since no changes in the transcript levels of the functional analogue KAT5 were detected in senescing leaves (Fig. 1A) and no expression of KAT1 was detected in senescent leaves (data not shown). Increased KAT2 transcript accumulation correlated with the accumulation of the senescence-associated SAG12 gene transcript and reduced levels of the CAB gene coding for chlorophyll a/b binding protein, both considered as up-regulated and down-regulated markers in senescing leaves (Buchanan-Wollaston, 1994; Lohman et al., 1994; Gombert et al., 2006).
Fig. 1.
Transcript accumulation in non-senescing (lane 1, no chlorotic symptoms) and senescing (lane 2; around 30% of the surface of each leaf showing chlorotic symptoms) rosette leaves of Arabidopsis thaliana plants. (A) KAT2 and KAT5 transcript levels were analysed by northern blot with 10 μg of total RNA samples. Ethidium bromide-stained ribosomal RNAs (rRNA) were included as loading control. (B) After hybridization with specific probes for KAT2 and KAT5 genes, the corresponding transcript levels were quantified by PhosphorImager analysis, the values normalized to the endogenous content of 18S ribosomal RNA and expressed as relative values to non-senescing leaves (mean values ±standard error from three replicate experiments). The CAB and SAG12 transcript levels were quantified by qRT-PCR. Values represent the mean ±standard error of three replicates and were normalized by the endogenous content of ACT2/8 transcript.
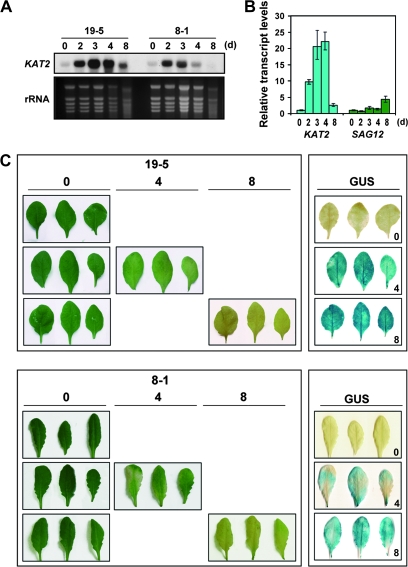
To investigate further the role of the KAT2 gene in leaf senescence, transgenic lines expressing the β-glucuronidase (GUS) reporter gene were generated under the control of the KAT2 promoter. Figure 2 shows that the expression of the KAT2 gene was also activated in experiments of dark-induced senescence in excised leaves. The accumulation of KAT2 transcript was detected as early as 2 d after dark-incubation of excised leaves from two independent KAT2::GUS transgenic lines (Fig. 2A). The accumulation of KAT2 transcript was detected significantly earlier than that of the senescence marker SAG12 gene (Fig. 2B). By the fourth day under darkness, when yellowing symptoms started to show up, leaves displayed strong GUS staining that persisted at least for 8 d (Fig. 2C), probably due to the stability of the GUS protein since a decay in transcript accumulation was already detected at that time (Fig. 2A). GUS staining in the dark-incubated leaves of the transgenic line 8-1 was weaker than that observed in the line 19-5 (Fig. 2C), in agreement with the levels of KAT2 transcript detected by northern blot (Fig. 2A). Line 8-1 allowed the observation that KAT2-directed GUS staining was markedly associated to leaf areas undergoing yellowing symptoms (Fig. 2C).
Fig. 2.
Dark-induced senescence in excised leaves of pKAT2::GUS transgenic plants. (A) The levels of KAT2 transcript were analysed by northern blot from total RNAs (10 μg) isolated from excised leaves of two independent pKAT2::GUS transgenic lines that were incubated in the dark for the indicated times in days after incubation in darkness. Ethidium bromide-stained ribosomal RNAs (rRNA) were included as loading control. (B) Quantification of KAT2 and SAG12 transcript levels by qRT-PCR at the indicated times in days after incubation in darkness. Values represent the mean ±standard error of three replicates and were normalized by the endogenous content of ACT2/8 transcript. (C) Excised leaves from both pKAT2::GUS transgenic lines were harvested at 0, 4, and 8 d after incubation in the dark, photographed, and then stained for GUS activity. Yellowing symptoms as a result of chlorophyll degradation correlated with GUS-stained leaves.
For in planta analysis, transgenic lines expressing the luciferase (LUC) reporter gene have been generated under the control of the KAT2 promoter. These plants allowed the activated expression of KAT2 to be monitored in whole darkened plants during the dark incubation period. Figure 3 shows the emitted luminescence of plants corresponding to two independent transgenic KAT2::LUC lines at different times up to 10 d after the shift from light to dark conditions. LUC-dependent luminescence was detected by the third day, peaked between the fifth and sixth days, and declined thereafter (Fig. 3).
Fig. 3.
Luciferase-mediated luminescence in darkened pKAT2::LUC transgenic plants. Plants from two independent pKAT2::LUC transgenic lines, 1A in the top row and 12C in the bottom row as described in the scheme, were grown under short day photoperiodic conditions for 8 weeks and then moved to darkness. At the indicated times after the move plants were sprayed in the dark with 0.1 mM luciferin and the luminescence was monitored 4 h later by a low-light video CCD camera Hamamatsu C2400. The recorded luminescence images at the indicated times up to 10 d after the move to darkness are displayed in clock-wise sense. The colour code is an indication of luminescence intensity, blue being the lowest and red the highest level. The panel in the centre of the top row displays a clear field image of the plants before incubation in the dark.
KAT2 is required for timely triggering natural and dark-induced leaf senescence
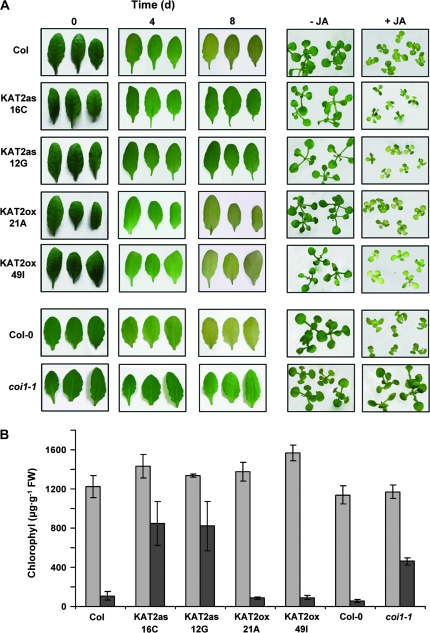
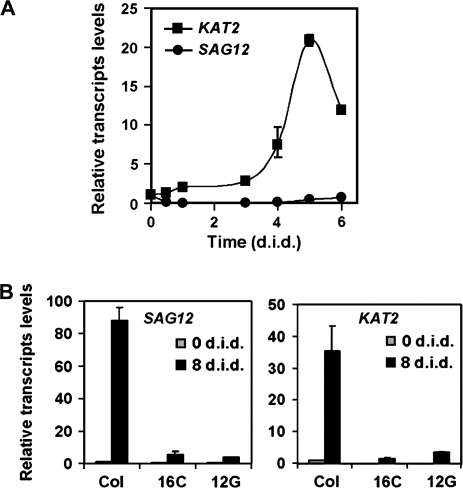
The activated expression of the KAT2 gene in senescing leaves may be a consequence of senescence-triggered catabolism of lipid-containing cell structures or may be, in turn, a component of the process triggering senescence. To test whether KAT2 is required for triggering leaf senescence in Arabidopsis, transgenic lines have been generated overexpressing the KAT2 cDNA in sense (KAT2ox) and antisense (KAT2as) orientation (Castillo et al., 2004; this work). Based on a qRT-PCR analysis of the different transgenic lines, the selected overexpressing lines contained transcript levels at least 100-fold higher than that detected in wild-type plants, whereas antisense transgenic lines contained less than 10% of the KAT2 transcript levels detected in untransformed plants. Experiments of dark-induced senescence in excised leaves from plants corresponding to two independent lines for each construct were conducted. After 7 d in darkness, the yellowing symptoms that were detected in wild-type Col leaves were strongly attenuated in KAT2as leaves (Fig. 4A). The delayed senescence phenotype of KAT2as plants was even more pronounced than that detected in the JA-insensitive coi1-1 mutant (Fig. 4A). By contrast, KAT2ox displayed senescence symptoms similar to those detected in the wild-type plants. The transcript levels of the wound- and JA-responsive JR2 gene were measured (Titarenko et al., 1997; Rojo et al., 1998), and no basal or wound-induced levels of the transcript in transgenic plants overexpressing the KAT2 gene were found higher than those detected in wild-type plants (see Supplementary Fig. S2 at JXB online), suggesting that no increased synthesis of JA occurred in KAT2ox plants. This is probably due to the fact that the KAT2-catalysed reaction is not the rate-limiting step in JA biosynthesis. The analysis of the chlorophyll content in darkened leaves of the different transgenic and wild-type plants allowed the symptoms of senescence to be quantified. Figure 4B shows that, by the eighth day under darkness, leaves from wild-type and KAT2ox plants lost around 90% of their chlorophyll content. However, darkened leaves from KAT2as plants reduced their chlorophyll content by only 35–40%, and those from the coi1-1 mutant lost around 60% of the initial chlorophyll content (Fig. 4B). As demonstrated by experiments of induced senescence in seedlings grown on media supplemented with JA, chlorotic symptoms of senescence were observed in seedlings from all transgenic and wild-type plants but not in JA-insensitive coi1-1 plants (Fig. 4A). The delayed senescence in KAT2as leaves is thus presumably due to the defect of these plants in raising JA content through its activated biosynthesis. The possibility cannot be ruled out that KAT2 participates in the biosynthesis or metabolism of other components different from JA and involved in triggering senescence. Anyway, the requirement for KAT2 function to trigger leaf senescence should correlate with an early enough response in senescence-induced KAT2 expression. The kinetics of senescence-induced KAT2 and SAG12 transcript accumulation were compared in darkened excised leaves. Figure 5A shows that a significant accumulation of KAT2 transcript was detected as early as one day after dark incubation and, by the fourth day, the transcript levels were 8-fold higher that that detected in the light-grown leaves. By contrast, no significant SAG12 transcript accumulation could be detected during the first 5 d under darkness, and the induction of the SAG12 gene was only detected at day eight after dark incubation (Fig. 5B). In fact, SAG12 transcript accumulation was severely reduced in KAT2 antisense lines correlating with the strongly reduced expression of the KAT2 gene (Fig. 5B) and with the attenuated chlorosis symptoms of senescence in excised leaves from KAT2 antisense plants (Fig. 4). This effect on SAG12 is probably not due to the reduced synthesis of JA in KAT2 antisense lines, because the expression of the SAG12 gene was not regulated by jasmonates (from microarray data deposited in Genevestigator and eFP browsers).
Fig. 4.
Dark-induced senescence in excised leaves from wild-type and transgenic plants overexpressing KAT2 in sense (KAT2ox) or antisense (KAT2as) orientations as well as from the JA-insensitive coi1-1 mutant. (A) Plants from the indicated genotypes were grown under short day photoperiodic conditions for 6 weeks. Then, leaves were excised from the plants and incubated in the dark for 0, 4, and 8 d. At the indicated times leaves were photographed and subsequently processed for chlorophyll quantification. In the right panel, the figure shows 10-d-old seedlings of the different genotypes that were grown on MS-agar medium supplemented (+JA) or not (–JA) with 20 μM JA. (B) Quantification of chlorophyll a+b content of leaves at 0 d and 8 d after the move to darkness. Values are the mean of six independent replicates of 100 mg fresh weight of leaves. Error bars represent standard error. The experiment was repeated twice with similar results.
Fig. 5.
Kinetics of KAT2 and SAG12 transcript accumulation in dark-induced senescing excised leaves from wild-type Col and KAT2as transgenic plants. (A) The KAT2 (filled squares) and SAG12 (filled circles) transcript levels were quantified, by qRT-PCR as described before, in leaf samples excised from wild-type Col plants exposed to darkness for the indicated times (days in darkness; d.i.d). (B) SAG12 and KAT2 transcripts were quantified at 0 d.i.d (grey bars) and 8 d.i.d (black bars) in excised darkened leaves from wild-type Col and the KAT2as transgenic lines 16C and 12G. All values for transcript levels were normalized by the endogenous content of ACT2/8 transcript and expressed relative to the levels quantified for Col plants at day 0. The experiment was repeated three times with similar results.
The delayed senescence phenotype observed in KAT2as leaves incubated in darkness was also observed in leaves from plants under natural senescence conditions. Figure 6 shows the relative chlorophyll content in every rosette leaf from Col and KAT2as 12G and 16C plants versus leaf position (from bottom older to top younger leaves which represent the unit in relative chlorophyll content). Wild-type Col leaves showed a progressive yellowish appearance (data not shown) when going from the top to the bottom leaves to leaf number 4 and that correlated with a linear decrease in the chlorophyll content (Fig. 6). From leaves 1 to 4 no changes in chlorophyll content were observed (Fig. 6). The loss of chlorophyll in leaves from KAT2as plants was attenuated when compared to wild-type plants. Every rosette leaf but the oldest one contained chlorophyll levels above those detected in the corresponding wild-type leaves (Fig. 6). The relative chlorophyll content dropped to less than 35% in the fourth oldest senescing Col leave but only to around 60% in KAT2as leaves (Fig. 6). The retarded kinetic of chlorophyll loss in leaves from KAT2as plants supports the requirement for KAT2 during the onset and development of natural leaf senescence in Arabidopsis.
Fig. 6.
Natural leaf senescence in wild-type and KAT2as transgenic plants. Every leaf from the rosette of wild-type Col and transgenic KAT2as lines 12G and 16C plants grown under long day photoperiodic conditions for 8 weeks were excised and the chlorophyll (a+b) contents quantified and plotted versus leaf position. Values are the mean of four independent replicate samples (three leaves per sample) and the error bars corresponded to the standard error. Values are expressed relative to the chlorophyll content of the youngest leaf in each genotype.
Discussion
Leaves function as a source organ throughout the young and adult phases of development. Their photosynthetic capacity is sufficient to generate chemical energy for the maintenance of tissue and cell structure and also to export photosynthates to other parts of the plant. The onset of senescence represents the end of the functional life of the leaves and the entrance into a catabolic phase that ends with cell death and degradation. It is noteworthy that senescence and germination share some metabolic processes that play an essential role in the mobilization of nutrients and the subsequent energy generation. Among these processes, β-oxidation catalyses the shortening of long-chain fatty acids from reserve lipids during germination and early post-germination (Footitt et al., 2006). In addition, β-oxidation mediates the processing of lipid-derived metabolites during the degradation of membranes in senescing plant structures (Graham and Eastmond, 2002). From this perspective, the activation of β-oxidation would result from the cellular requirements of active catabolic machineries with the capacity to degrade complex molecules. In this work, it was addressed whether β-oxidation is the cause or consequence of leaf senescence. The last step catalysed by KAT2 was chosen because, among three genes coding for KATs in the Arabidopsis genome, only the KAT2 gene was activated in senescent leaves (He et al., 2002; Charlton et al., 2005; Van der Graaff et al., 2006) [see also the Arabidopsis Electronic Fluorescent Protein (eFP) Browser http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi, which compiles data from microarray experiments of Gene Expression Map of Arabidopsis Development from Schmid et al., 2005]. Moreover, the KAT2 gene was activated by stress and showed higher levels of expression than their analogues KAT1 and KAT5 (Castillo et al., 2004; Afitlhile et al., 2005). It has been confirmed that KAT2 expression was strongly activated in leaves undergoing natural as well as dark-induced senescence. The KAT2-directed expression of the GUS reporter gene was detected as early as 2 d after the incubation of excised leaves from KAT2::GUS plants grown in darkness, and it persisted for at least eight days. The fact that the KAT2 gene was expressed before the appearance of yellowing symptoms and the activation of senescence-associated genes in darkened leaves suggest that early KAT2 activation may not be a consequence of induced senescence. The expression persisted far beyond the onset of senescence, which is an indication that KAT2 also plays a role in the responses triggered in the leaves when senescence is already set up and progressing. It has previously been reported that dark-induced leaf senescence only occurred in individually darkened leaves and, in fact, was inhibited in the whole darkened plant (Weaver and Amasino, 2001). However, KAT2-directed expression of the LUC reporter gene has been detected in darkened whole KAT2::LUC plants 3 d after dark incubation when no significant chlorosis was observed in the leaves of darkened plants. The increased LUC-mediated luminescence in the expanded leaves was accompanied by a strong luminescence in the young leaves of the shoot apex. However, a similar pattern of luminescence has also been shown in non-senescing leaves from other independently generated KAT2::LUC transgenic lines (Charlton et al., 2005), suggesting that this pattern is dependent mainly on the vegetative developmental stage. By 5–6 d, plants displayed a peak in LUC-mediated luminescence. These data suggest that the activation of KAT2 expression preceded the onset of dark-induced senescence in excised leaves as well as in those attached to the darkened plant. The participation of KAT2 in the synthesis of JA in Arabidopsis has been reported previously (Castillo et al., 2004; Afitlhile et al., 2005), and also that JA synthesis, perception, and signalling play a central role in triggering leaf senescence (He et al., 2002; Buchanan-Wollaston et al., 2005). In agreement with these data, it has been observed that leaf senescence was strongly delayed in antisense KAT2 transgenic plants that are impaired in the stress-activated biosynthesis of JA. Of note, the reduced leaf senescence in KAT2as plants was even more marked than that detected in the JA-insensitive coi1-1 mutant plant, which was previously characterized as defective in JA-triggered leaf senescence (He et al., 2002). Since KAT2as plants are not defective in JA perception (Castillo et al., 2004,) and it was checked that KAT2as seedlings were responsive to JA-activated senescence, the reduced leaf senescence in KAT2as plants may be due to defective induced JA synthesis. Regarding this, KAT2as plants are defective in the wound-induced synthesis of JA but they have basal levels similar to wild-type plants (Castillo et al., 2004) suggesting that JA would have to be accumulated above a threshold level to trigger leaf senescence. However, the levels of the JA-responsive JR2 transcript have been analysed, which is strictly dependent on JA biosynthesis and perception to be activated (Titarenko et al., 1997; Rojo et al., 1998), during the first 4 d after darkness. Supplementary Fig. S1A at JXB online shows that no JR2 transcript accumulation was detected at days 2 or 4 after dark incubation, when KAT2 expression was already activated. Moreover, from data of the transcriptome analysis of dark-induced leaf senescence (Lin and Wu, 2004) we have searched for the transcript levels of biosynthetic and JA-responsive genes in dark-induced senescence. Supplementary Fig. S1B at JXB online shows that, among biosynthetic genes, only LOX1 and the β-oxidation (ACX1, AIM1, MFP2, and KAT2) genes were up-regulated, and none of the JA-responsive JR2, PDF1.2 or VSP1 genes were activated under dark-induced senescence conditions. These data suggest that no increase in JA content occurred early in dark-induced senescence, and also that KAT2 function may be related to the participation of β-oxidation in the synthesis of a different signal molecule potentially regulating senescence.
The importance of the co-ordination of β-oxidation with cytosolic, chloroplastic, and mitochondrial metabolism for the development and stress-activated signalling in plants has been highlighted (Baker et al., 2006). The reduced KAT2 function may not only lead to defective biosynthesis of JA inside the peroxisome but also to the concomitant accumulation of some of its chloroplastic precursors. This metabolite might exert feedback inhibition of chloroplastic photosynthetic activity, thus causing a reduced oxidative metabolism rate and, as a consequence, delayed senescence. This delayed natural senescence phenotype has previously been reported for mutants in the photosynthetic machinery such as ore4-1 in Arabidopsis (Woo et al., 2002), or transgenic tobacco with reduced Rubisco expression (Miller et al., 2000). It is also possible that the accumulation of an intermediate in the JA biosynthetic pathway may act as a loop inhibitor of upstream components of the pathway. A lipase-catalysed hydrolysis of lipids from membranes is one of the earliest steps in the biosynthesis of jasmonates and other structurally related oxylipins. It has been reported that transgenic plants with reduced expression of either a senescence-inducible lipase or phospholipase D showed delayed senescence (Thompson et al., 2000; Fan et al., 1997). Moreover, despite the essential role of β-oxidation in JA synthesis, this metabolic pathway also participates in one of the auxin biosynthetic routes, transforming indole-3-butyric acid (IBA) into the active auxin indole-3-acetic acid (IAA) (Woodward and Bartel, 2005). Mutants of auxin synthesis and/or action have been reported to display delayed leaf senescence and organ abscission (Vandenbussche et al., 2003; Ellis et al., 2005; Pineau et al., 2005). The conversion of IBA to IAA required by the KAT2 function, as demonstrated by the IBA-resistant phenotype in the root elongation assays of ped1/kat2 mutant seedlings (Zolman et al., 2000). Whether the β-oxidation-mediated conversion of IBA to IAA is functionally connected to the regulation of senescence is still not clear. Levels of IAA in senescing leaves are higher than in non-senescing leaves of the plant (Quirino et al., 1999). However, IAA did not induce the expression of the senescence marker SAG12 gene (Noh and Amasino, 1999). More recently, published data related to the function of auxin response factors (ARFs) suggest that auxins regulate the senescence and abscission of different organs in Arabidopsis (Ellis et al., 2005; Okushima et al., 2005). To assess whether the KAT2 function in dark-induced senescence may be related to altered production of IAA, the levels of the IAA-responsive MSG2/IAA19 gene which have been reported to be strongly and readily activated by IAA (Zhao et al., 2002) were checked. The levels of MSG2/IAA19 transcript at 2 d and 4 d after darkness were analysed by quantitative RT-PCR and no increase was detected (see Supplementary Fig. S1A at JXB online), suggesting that no increase in IAA levels occurred during the early stage of dark-induced leaf senescence. Moreover, there is a search for the levels of the transcripts corresponding to auxin biosynthesis and response genes in the transcriptome analysis by Lin and Wu (2004). It was found that despite of the up-regulation detected in nitrilase (NIT) and indole-3-acetaldehyde oxidase (AAO) biosynthetic genes of the Trp-dependent pathway, no significant increase in the accumulation of different IAA-responsive genes, including MSG2/IAA19 gene, was detected (see Supplementary Fig. S1C at JXB online). Our findings support a dual role for KAT2-mediated β-oxidation as an early regulator triggering leaf senescence, probably through its participation in the synthesis of a signal molecule different from JA or IAA, and as a late component of the metabolic machinery devoted to cellular and tissue degradation of lipid components. Nevertheless, these data do not rule out the possibility that the regulatory function of KAT2 may be exerted at later stages of leaf senescence through involvement in the balanced synthesis of both jasmonates and some other senescing inducer molecules that may well be active auxins. In fact, the analysis of the transcriptome regulated in senescing leaves reflected that there was a substantial co-regulation of JA and IAA biosynthetic genes (van der Graaff et al., 2006), suggesting that the progression of leaf senescence may require changes in both JA and auxins. More work will be needed to clarify the functional relations between JA and auxins in promoting senescence, and also to identify other β-oxidation-derived molecules with potential regulatory function in the onset of leaf senescence.
In conclusion, our data support KAT2 as an important factor in controlling either the onset or the early steps of progression of leaf senescence, and also that this function may be related to the production of a possible lipid-related and senescence-promoting signal, which is presumably different from jasmonates or auxins. In turn, these hormones might be important regulators of the later phases of leaf senescence as KAT2 is also probably involved in these late processes.
Supplementary data
Supplementary figures are at JXB available online. These non-essential data provide additional information for data interpretation as depicted in the text.
Supplementary Material
Acknowledgments
We thank MD Gómez for the technical assistance with luciferase-mediated luminescence detection. This work was supported by grants BIO2002-03533 and BIO2005-00222 from MEC, Plan Nacional de Biotecnología, Spain to (JL). MCC was supported by a fellowship from Generalitat Valenciana.
References
- Afitlhile MM, Fukushige H, Nishimura M, Hildebrand DF. A defect in glyoxysomal fatty acid beta-oxidation reduces jasmonic acid accumulation in Arabidopsis. Plant Physiology and Biochemistry. 2005;43:603–609. doi: 10.1016/j.plaphy.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Graham IA, Holdsworth M, Smith SM, Theodoulou FL. Chewing the fat: beta-oxidation in signalling and development. Trends in Plant Science. 2006;11:124–32. doi: 10.1016/j.tplants.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. Isolation of cDNA clones for genes that are expressed during leaf senescence in Brassica napus: identification of a gene encoding a senescence-specific metallothionein-like protein. Plant Physiology. 1994;105:839–846. doi: 10.1104/pp.105.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D. The molecular analysis of leaf senescence: a genomics approach. Plant Biotechnology Journal. 2003;1:3–22. doi: 10.1046/j.1467-7652.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. The Plant Journal. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- Castillo MC, Martínez C, Buchala A, Métraux J-P, León J. Gene-specific involvement of β-oxidation in wound-activated responses in Arabidopsis. Plant Physiology. 2004;135:85–94. doi: 10.1104/pp.104.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton WL, Johnson B, Graham IA, Baker A. Non-coordinate expression of peroxisome biogenesis, β-oxidation and glyoxylate cycle genes in mature Arabidopsis plants. Plant Cell Reports. 2005;23:647–653. doi: 10.1007/s00299-004-0879-7. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development. 2005;132:4563–4574. doi: 10.1242/dev.02012. [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase D alpha retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. The Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. The Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Marquez J, Schmuths H, Baker A, Theodoulou FL, Holdsworth M. Analysis of the role of COMATOSE and peroxisomal beta-oxidation in the determination of germination potential in Arabidopsis. Journal of Experimental Botany. 2006;57:2805–2814. doi: 10.1093/jxb/erl045. [DOI] [PubMed] [Google Scholar]
- Gombert J, Etienne P, Ourry A, Le Dily F. The expression patterns of SAG12/Cab genes reveal the spatial and temporal progression of leaf senescence in Brassica napus L. with sensitivity to the environment. Journal of Experimental Botany. 2006;57:1949–1956. doi: 10.1093/jxb/erj142. [DOI] [PubMed] [Google Scholar]
- Graham IA, Eastmond PJ. Pathways of straight and branched chain fatty acid catabolism in higher plants. Progress in Lipid Research. 2002;41:156–181. doi: 10.1016/s0163-7827(01)00022-4. [DOI] [PubMed] [Google Scholar]
- He Y, Fukushige H, Hildebrand DF, Gan S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiology. 2002;128:876–884. doi: 10.1104/pp.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanaugh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MW, Leslie ME, Liljegren SJ. Plant separation: 50 ways to leave your mother. Current Opinion in Plant Biology. 2006;9:59–65. doi: 10.1016/j.pbi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG. Leaf senescence. Annual Review of Plant Biology. 2007;58:115–136. doi: 10.1146/annurev.arplant.57.032905.105316. [DOI] [PubMed] [Google Scholar]
- Lim PO, Nam HG. The molecular and genetic control of leaf senescence and longevity in Arabidopsis. Current Topics on Developmental Biology. 2005;67:49–83. doi: 10.1016/S0070-2153(05)67002-0. [DOI] [PubMed] [Google Scholar]
- Lin JF, Wu SH. Molecular events in senescing Arabidopsis leaves. The Plant Journal. 2004;39:612–628. doi: 10.1111/j.1365-313X.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- Lohman KN, Gan S, John MC, Amasino RM. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiologia Plantarum. 1994;92:322–328. [Google Scholar]
- Miller A, Schlagnhaufer C, Spalding M, Rodermel S. Carbohydrate regulation of leaf development: prolongation of leaf senescence in Rubisco antisense mutants of tobacco. Photosynthesis Research. 2000;63:1–8. doi: 10.1023/A:1006367719639. [DOI] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Molecular Biology. 1999;41:181–194. doi: 10.1023/a:1006342412688. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Mitina I, Quach HL, Theologis A. AUXIN RESPONSE FACTOR 2 (ARF2): a pleiotropic developmental regulator. The Plant Journal. 2005;43:29–46. doi: 10.1111/j.1365-313X.2005.02426.x. [DOI] [PubMed] [Google Scholar]
- Pineau C, Freydier A, Ranocha P, et al. hca: an Arabidopsis mutant exhibiting unusual cambial activity and altered vascular patterning. The Plant Journal. 2005;44:271–289. doi: 10.1111/j.1365-313X.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- Quirino BF, Normanly J, Amasino RM. Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Molecular Biology. 1999;40:267–278. doi: 10.1023/a:1006199932265. [DOI] [PubMed] [Google Scholar]
- Rojo E, Titarenko E, León J, Berger S, Vancanneyt G, Sánchez-Serrano JJ. Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in Arabidopsis thaliana. The Plant Journal. 1998;13:153–165. doi: 10.1046/j.1365-313x.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- Schaller F. Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. Journal of Experimental Botany. 2001;52:11–23. [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nature Genetics. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- Thompson J, Taylor C, Wang TW. Altered membrane lipase expression delays leaf senescence. Biochemical Society Transactions. 2000;28:775–777. [PubMed] [Google Scholar]
- Titarenko E, Rojo E, León J, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiology. 1997;115:817–826. doi: 10.1104/pp.115.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge UI, Kunze R. Transcription analysis of arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiology. 2006;141:776–792. doi: 10.1104/pp.106.079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Smalle J, Le J, et al. The Arabidopsis mutant alh1 illustrates a cross talk between ethylene and auxin. Plant Physiology. 2003;131:1228–1238. doi: 10.1104/pp.010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiology. 2001;127:876–886. [PMC free article] [PubMed] [Google Scholar]
- Woo HR, Goh CH, Park JH, Teyssendier B, Kim JH, Park IY, Nam HG. Extended leaf longevity in the ore4-1 mutant of Arabidopsis with a reduced expression of a plastid ribosomal protein gene. The Plant Journal. 2002;31:331–340. doi: 10.1046/j.1365-313x.2002.01355.x. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes and Development. 2002;16:3100–3112. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.