Abstract
Dilated cardiomyopathy (DCM), the most common form of cardiomyopathy, often leads to heart failure and sudden death. While a substantial proportion of DCMs are inherited, mutations responsible for the majority of DCMs remain unidentified. A genome-wide linkage study was performed to identify the locus responsible for an autosomal recessive inherited form of juvenile DCM (JDCM) in Portuguese water dogs using 16 families segregating the disease. Results link the JDCM locus to canine chromosome 8 with two-point and multipoint LOD scores of 10.8 and 14, respectively. The locus maps to a 3.9 Mb region, with complete syntenic homology to human chromosome 14, that contains no genes or loci known to be involved in the development of any type of cardiomyopathy. This discovery of a DCM locus with a previously unknown etiology will provide a new gene to examine in human DCM patients and a model for testing therapeutic approaches for heart failure.
Keywords: Cardiomyopathy, primary, dilated; Death, sudden; Canine; Genetic Linkage
Introduction
Cardiomyopathies are disorders of the heart muscle that are associated with significant morbidity and mortality due to heart failure. Heart failure has been described as the emerging health epidemic of the 21st century [1, 2, 3, 4]. The mortality rate due to cardiomyopathies in the United States is greater than 10,000 deaths per year. Approximately 14% of children affected with dilated cardiomyopathy (DCM) die at age 2 [5] and the median age of children listed for heart transplantation is 4 years [2]. Cardiomyopathies are a heterogeneous group of diseases. DCM is the most common form of the primary cardiomyopathies and is characterized by ventricular dilation and impaired systolic function with normal left ventricle (LV) wall thickness [6]. Inherited forms are common and may account for up 35% of the cases [7, 8, 9], most of which show an autosomal dominant mode of inheritance. Autosomal recessive inheritance accounts for 24% of infantile forms of DCM [3, 10]. There is evidence of incomplete and age-dependent penetrance of DCM in humans, which together with the considerable mortality, has limited genetic studies. To date, 29 chromosomal loci that give rise to DCM are known in humans [9, 11] with genes and mutations known for 27 of these. Nevertheless, there are still many aspects of familial DCM that remain poorly understood and it is anticipated that additional genes causative of familial DCM will be discovered [7]. Identification of these genes and knowledge of their roles in cardiac function and in pathogenesis of DCM are major goals of ongoing research.
Dogs provide advantages for the study of genetic disease due to the many isolated populations that exist as individual dog breeds and the high degree of medical scrutiny to which individual animals are subjected. Particularly, recessive and complex disorders are more likely to be recognized as inherited diseases than in human populations, and genetic heterogeneity is likely to be reduced, at least within breeds. Large sibships and the ability to perform specific matings are additional advantages, and increase the capacity for naturally-occurring dog models to accelerate the discovery, characterization, and development of treatment modalities for heart disease.
As in humans, DCM is the most prevalent form of cardiomyopathy in dogs, with increased risk in large and giant breed dogs and with advancing age [12]. Breed predisposition is apparent [13], and while the notion of genetic predisposition is widely accepted, there are no reports of specific gene mutations associated with canine DCM. We have been investigating a juvenile form of canine DCM (JDCM) that is unique in Portuguese water dog (PWD) families and is inherited as a fully penetrant autosomal recessive trait [14, 15, 16].
JDCM in PWD was first reported in a retrospective analysis of postmortem and biopsy case records of 12 related PWDs [14]. This was followed by a prospective study of 124 PWD puppies, ten of which were affected [15]. The clinical course of JDCM ranges from sudden unexpected collapse and death with no preceding clinical signs (most common) to presence of clinical signs of depression and reduced appetite for up to 5 days before collapse and fulminant congestive heart failure. Affected dogs died between 2 and 32 weeks of age. Echocardiography allows the earliest detection of JDCM, beginning 1–4 weeks before the onset of clinical signs. Extensive biochemical analysis has not revealed any significant differences between affected pups and normal age-matched controls [15]. Morphological and histological changes of the affected hearts have been described [14]. Based on the availability of DNA from families with JDCM affected pups and the simple mode of inheritance, we initiated a whole genome scan to map the canine JDCM locus.
Results
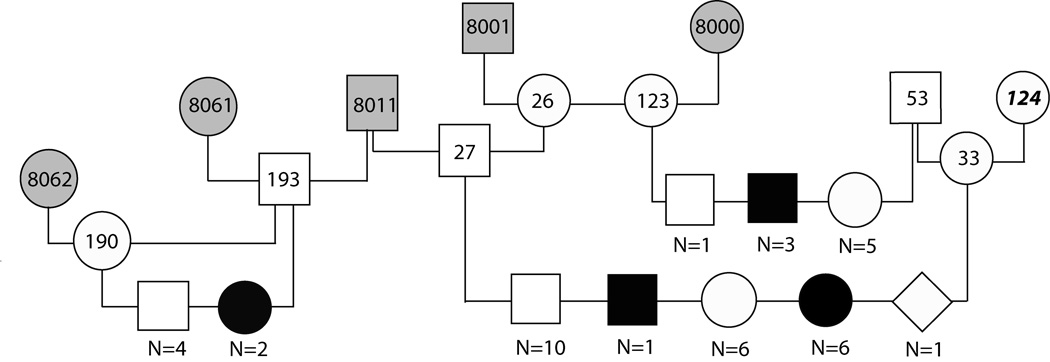
One of the more statistically powerful pedigrees included in this study was produced in part in our breeding colony at the University of Pennsylvania (Fig. 1). Three matings between the same carrier dogs, a purebred PWD and a mixed breed dog (dogs 27 and 33, respectively, in Fig. 1) produced 7 affected puppies out of 24 offspring, consistent with autosomal recessive inheritance of a single disease causing allele.
Figure 1.
Pedigree drawing of a PWD family segregating JDCM. No DNA was available for dogs represented by grey shaded symbols. Black boxes or circles indicate affected male and female dogs, respectively; unfilled and gray symbols represent unaffected animals, the diamond shaped symbol represents a puppy born dead. All dogs were purebred PWDs with the exception of dog 124, an unrelated mixed breed dog, and her descendents. N = number of dogs of the indicated sex and phenotype, normal or affected.
A whole genome scan was initiated using animals from 16 families consisting of 119 dogs of which 40 were affected with JDCM. We initially analyzed 120 markers, distributed throughout the whole genome, for linkage to JDCM. Based on the linkage results, an additional 22 markers were added to CFA3, CFA9 and CFA33 to evaluate regions that contained markers with a LOD score value greater than 1. None of these additional markers generated LOD scores greater than one. Having excluded these regions we continued to add an additional 29 polymorphic markers throughout the genome in order to fill in the largest gaps in the original linkage scan. One of these markers, FH3316 on CFA8, produced a LOD score of 4.77 with a recombination fraction of 0.05.
We continued to analyze markers on CFA8 using SNP, SSR (simple sequence repeat), and SINE markers (insertion/deletion polymorphisms, see reference [17]) that were developed based on publicly available information, finding 12 markers that were polymorphic in the JDCM families. These markers were spread throughout a 28 Mb region, with an average inter-marker distance of 1.6 Mb. Also, an additional 31 dogs, including 8 affected dogs, were added to the analysis. Two-point LOD scores greater than 3.5 were found for all markers in the region between 38.9 Mb and 50.95 Mb on CFA8. A maximum LOD score of 10.78 at a recombination fraction of 0.035 was calculated for marker FH3316.
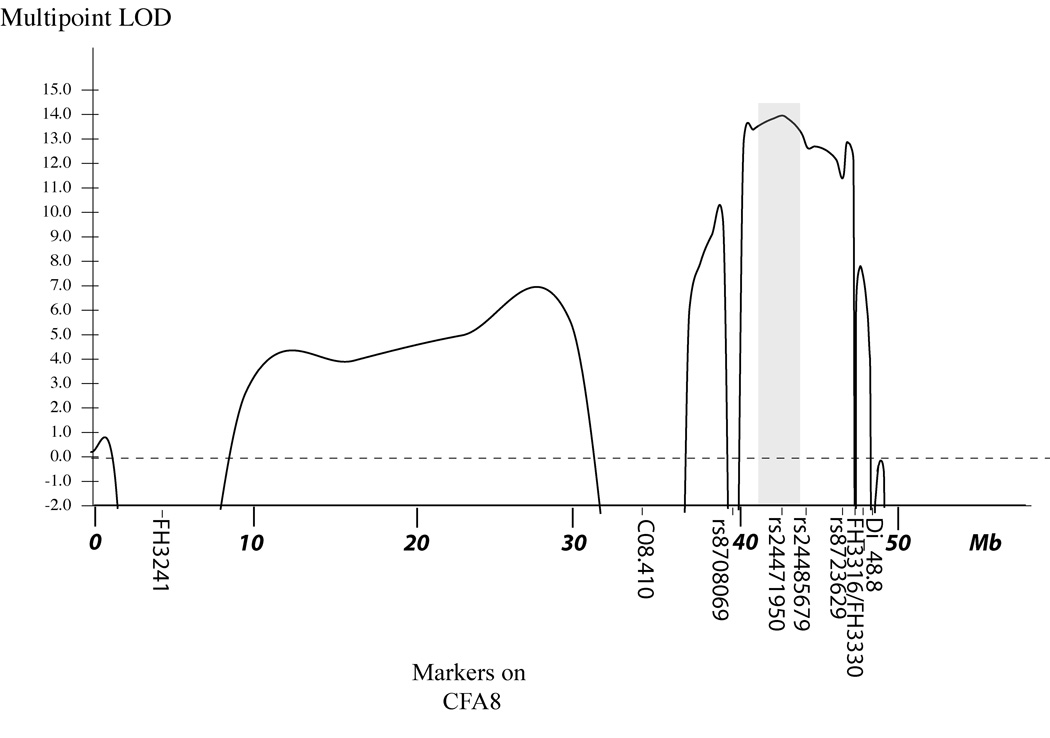
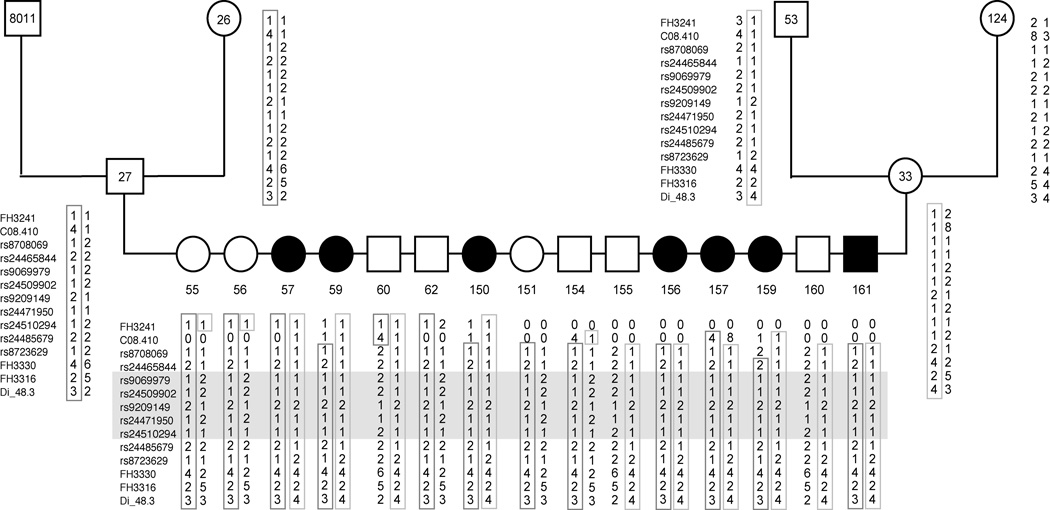
Multipoint linkage analysis with markers on CFA8 was performed using GENEHUNTER 2.1 (Fig. 2). A maximum multipoint LOD score of 14.0 was found for SNP marker rs24471950 at 42.4 Mb. We added 5 additional SNP markers surrounding this marker to further define the region of interest. Haplotype analysis defined the JDCM critical region to the interval from 40.5 Mb to 44.4 Mb. The haplotypes at markers across the region, for selected offspring of the large 24-member sibship shown in Fig. 1, are shown in Fig. 3. This figure illustrates that markers rs24465844 (at 40.5 Mb) and rs24485679 (at 44.4 Mb) differ in the two haplotypes carrying the mutant gene in dog 27 and 33, and therefore identify ancestral recombination events surrounding the JDCM locus that delimit the JDCM critical interval.
Figure 2.
The multipoint LOD score curve calculated for CFA8, LOD score indicated on the Y axis, and the relative position based on Mb location on CFA8 indicated on the X axis. The shaded region indicates the JDCM critical region defined by haplotype analysis (see Fig. 3). Markers are named below the X axis.
Figure 3.
Pedigree and haplotypes for the informative animals from the single largest sibship used in this study, which is also part of Fig. 1. No DNA was available for dogs represented by shaded symbols. Black boxes or circles indicate affected male and female dogs, respectively; unfilled symbols represent unaffected animals. All dogs were purebred PWDs with the exception of dog 124, an unrelated mixed breed dog. The marker order is from the centromere. The haplotypes from the mutant allele baring chromosomes are boxed. The JDCM critical interval is indicated by gray shading.
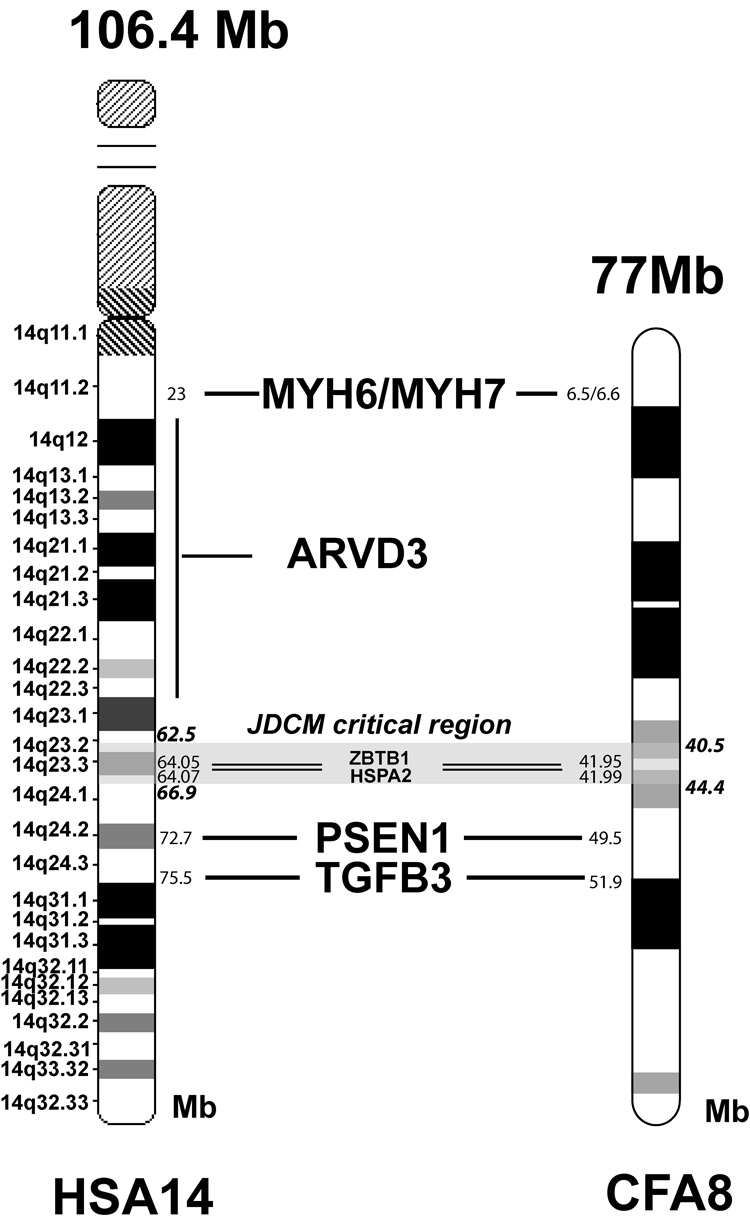
CFA8 is orthologous to the long arm of human chromosome 14 (HSA14q), with nearly perfect synteny, with the exception of a small segment near the telomere in human that is displaced to the centromere in dog [18]. This segment is outside of the JDCM critical region, which is completely syntenic to a region on HSA14. Four genes and one locus located on HSA14 have been identified to cause cardiomyopathies in humans (see Fig. 4) but none of these loci, are located within the identified interval on CFA8. Of these DCM loci, MYH6 [19] and MYH7 [20] are located on CFA8 at 6.6 Mb and 6.5 Mb, respectively, PSEN1 [21] at 49.5 Mb and TGFB3 at 51.9Mb [22]. The orthologous region of HSA14q12–22 given for an ARVD3 locus [23] is also located outside the JDCM region. Because of the initial nearness of PSEN1 to the originally identified JDCM linked marker, and because mutations in the human PSEN1 gene were associated with DCM [21], we sequenced and, consequently, excluded the canine PSEN1 gene as a candidate gene for JDCM in the PWDs by failing to find coding region or splice site mutations. Two additional genes that were initially chosen as candidate genes were HSPA2 (heat shock protein 2), due to the involvement of HSPs in dilated cardiomyopathy [24] and ZBTB1 (zinc finger and BTB domain containing protein 1) due to a high number of canine ESTs expressed in heart. Sequencing of coding regions from these potential candidate genes from affected dogs did not reveal any sequence differences.
Figure 4.
Ideogram drawings of HSA14 and CFA8 showing the location of the genes known to be involved in cardiomyopathies or ARVD1. The JDCM critical region, corresponding to parts of HSA14q23–q24, is shaded.
Discussion
Our data indicate that we have discovered a previously unrecognized DCM locus on CFA8. The availability of a high quality 7.6X canine genome sequence [18] makes comparative mapping possible, and indicates complete synteny between the canine and human genomes in the JDCM critical regions. No genes known to be involved in the development of any type of cardiomyopathy or arrhythmogenic right ventricular dysplasia (ARVD) are located within this region in any species. One gene, PSEN1, located nearby but outside of this region, was associated with DCM in humans, but was also excluded as causative of JDCM by sequencing. Two additional candidate genes located within the critical region were excluded as well. Genes with DCM-causing alleles can by found in multiple different pathways. DCM-causing mutations have been found in proteins of the myocyte cytoskeleton, the dystrophin-associated complex, proteins involved in cytoarchitecture, the nuclear envelope, sarcomere, and the sarcoplasmic reticulum, thus implicating calcium regulation. Perturbations in metabolic processes can also cause DCM, including mitochondrial disease [see 25 for review]. There are 28 annotated genes remaining within the identified critical region for JDCM, some of which can be considered very good candidate genes based on known function and tissue expression patterns. Among the most intriguing candidate genes is Nesprin 2 (SYNE2), which codes for a giant protein that connects the nucleus and the actin cytoskeleton and has been shown to interact with ACTC1 (actin, alpha, cardiac muscle 1), mutations in which have been associated with idiopathic DCM cardiomyopathy and familial hypertrophic cardiomyopathy [26]. Sequence variations in SYNE1 and SYNE2 were found in families segregating Emery–Dreifuss muscular dystrophy, a genetically heterogeneous neuromuscular disorder. Some of the patients studied suffered from severe DCM [27].
Having ruled out all known DCM genes by exclusion from the JDCM interval, further studies will therefore lead to the identification of a heretofore unknown DCM locus and will help unravel a pathway which leads to a rapid decline of myocardial function resulting in heart failure. The precedence that the molecular pathways involved in human heart failure can be elucidated by the study of animal models is well-established. Rodent DCM models have proven extremely applicable to human inherited cardiac disease [see for example 28, 29]. Given the greater sequence homology between dog and human than between human and rodents, the larger size and longer lifespan of dogs compared to mice, and the physiological differences in heart function between mouse and man, the discovery of the pathogenesis of this JDCM in this dog model has significant potential to yield new knowledge that will have an impact on human medicine and advance our understanding of heart disease in general. Despite improvements in medical treatment of heart failure in recent years, the clinical outcome in human patients diagnosed with DCM has not changed. A better understanding of the causes and pathogenesis of DCM, as well as new therapies, are needed in order to achieve better care and outcome in children and adults [9, 10]. Once the mutation is identified this knowledge will provide valuable new information about the causes and pathogenesis of DCM, but it will also provide a relevant, well-characterized model to evaluate therapeutic approaches for dilated cardiomyopathy and for other disease mechanisms that result in heart failure.
Materials and Methods
Blood samples, clinical and pedigree data for affected and normal PWDs were collected from client owned animals and members from a family of dogs maintained at the University of Pennsylvania (Fig. 1). Breeders and private owners also contributed samples and data for members of PWD families segregating JDCM, with informed consent. Diagnosis for privately owned animals was usually based on post mortem examination of heart and lungs, combined with an increased heart weight/total body weight ratio, as described in [14]. Some litters were evaluated weekly by echocardiography for signs of failing heart function as described earlier [15], so that an ante mortem diagnosis was possible for some dogs. Affected animals were euthanized before the development of overt congestive heart failure after definite and sustained deterioration of systolic function had been observed over several serial examinations. Diagnoses of JDCM were confirmed post mortem. Kidney tissue or whole blood was collected for DNA isolation. All matings, specimen collection, determination of clinical phenotype, and euthanasia were performed according to protocols approved by the Institutional Animal Care and Use Committee, and conforming to National Institutes of Health guidelines. Genomic DNA was isolated using either a standard phenol/chloroform extraction protocol or, for small amounts of blood, using a PUREGENE® DNA Purification kit (Gentra Systems Inc., Minneapolis, MN). PWDs surviving past 8 months of age were considered normal.
A total of 119 dogs from 16 families, including 40 affected dogs, were included in this study. While some of the families shared common ancestors, large extended families with more then 10 offspring were divided into single families to allow linkage analysis with GENEHUNTER. The same pedigree structure was used for the analysis with FASTLINK. Initially, 120 markers were analyzed with an additional 56 markers added later to increase marker density in specific regions. Most of the markers used were described previously [30, 31] and their amplification followed either published conditions or conditions previously described [32, 33]. Unlabelled PCR products were analyzed on 6.4% acrylamide gels, whereas, fluorescently labeled products were electrophoresed on an ABI Prism 377 Sequencer (Applied Biosystems, Foster City, CA) or on an NEN® Global IR2 4200 Sequencer System (Licor Biosciences, Lincoln Nebraska). Allele sizes and genotypes were determined using GeneScan® Analysis and Genotyper® Software (Applied Biosystems) or SAGAGT (Licor Biosciences, Lincoln, Nebraska). Two-point linkage analysis was carried out using the computer program FASTLINK 4.1P [34] and GENEHUNTER 2.1 [35] was used for multipoint analysis using a model of fully penetrant autosomal recessive inheritance and a disease allele frequency of 0.001. Marker allele frequencies were assumed equal for the whole genome linkage analysis. For the analysis on CFA8, the marker allele frequencies were calculated by counting all alleles within the analyzed pedigree. The differences in LOD scores values using the different frequencies were minor.
Once linkage was established, we used single nucleotide polymorphisms (SNPs) and bimorphic SINE [31] (short interspersed nuclear element) markers for mapping in the region of interest on CFA8, spanning a region from 38.7 Mb to 62.2 Mb, using publicly available information [17, 31]. SNP information for CFA8 was downloaded from ftp://ncbi.nlm.nih.gov/snp/organisms/dog_9615 and markers were chosen based on their physical location and the alteration of a restriction enzyme cleavage site for analysis purposes. To identify the modification of a restriction enzyme recognition site, the list of potential SNP markers was analyzed using the on-line tool WatCut (http://watcut.uwaterloo.ca/watcut/watcut/template.php). PCR primers flanking the SNP location were chosen using the computer program PrimerSelect from the Lasergene suite from DNASTAR, Inc (Madison, WI). Information regarding primer sequences and restriction enzymes used is available on request.
The positional candidate genes PSEN1, and HSPA2 were sequenced from genomic DNA from 2 to 3 affected dogs whereas ZBTB1 (Genbank accession numbers: EU5416225 and EU541626) was sequenced from cDNA that was reverse transcribed from mRNA from left ventricular muscle tissue of 2 affected 12 week old dogs that had been snap frozen in LN2 immediately after euthanasia. mRNA was prepared using the Fast Track 2.0 mRNA isolation kit and reverse transcribed into cDNA by the SuperScript III RTS First-Strand cDNA Synthesis kit, both from Invitrogen™ (Carlsbad, CA). Resulting sequences were compared to the published genome sequence or to sequence that was generated from a normal mixed breed dog (no PWD dog mix, no history of DCM).
Acknowledgments
We are extremely grateful for the hard work and dedication of many Portuguese water dog owners and breeders, in particular the past and present members of the Cardio Committee of the Portuguese Water Dog Club of America. Our appreciation is also extended to Hong Truong for laboratory assistance, and to Patricia O’Donnell, CVT, Carolyn Bryan and veterinary students for compassionate animal care.
This work was supported by grants from the National Institutes of Health (RR02512 Referral Center—Animal Models of Human Genetic Disease, and HL018848), Bethesda, MD and the Portuguese Water Dog Foundation, Parker Ford, PA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest
References
- 1.Miller LW, Missov ED. Epidemiology of heart failure. Cardiol Clin. 2001;19:547–555. doi: 10.1016/s0733-8651(05)70242-3. [DOI] [PubMed] [Google Scholar]
- 2.Towbin JA, Bowles NE. Dilated cardiomyopathy: a tale of cytoskeletal proteins and beyond. J Cardiovasc Electrophysiol. 2006;17:919–926. doi: 10.1111/j.1540-8167.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 3.Fatkin D, Graham RM. Molecular mechanisms of inherited cardiomyopathies. Physiol Rev. 2002;82:945–980. doi: 10.1152/physrev.00012.2002. [DOI] [PubMed] [Google Scholar]
- 4.Abelmann WH, Lorell BH. The challenge of cardiomyopathy. J Am Coll Cardiol. 1989;13:1219–1239. doi: 10.1016/0735-1097(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal D, et al. International Society for Heart and Lung Transplantation: Practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 7.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2005;45:969–981. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 8.Grunig E, et al. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol. 1998;31:186–194. doi: 10.1016/s0735-1097(97)00434-8. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MR, Carniel E, Mestroni L. Cardiomyopathy, familial dilated. Orphanet J Rare Dis. 2006;1:27. doi: 10.1186/1750-1172-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Towbin JA, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. Jama. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 12.Sisson D, O'Grady MR, Calvert CA. Myocardial diseases of dogs. In: Fox PR, Sisson D, Moise NS, editors. Textbook of Canine and Feline Cardiology. Philadelphia: WB Saunders; 1999. pp. 581–619. [Google Scholar]
- 13.Tidholm A, Haggstrom J, Borgarelli M, Tarducci A. Canine idiopathic dilated cardiomyopathy. Part I: Aetiology, clinical characteristics, epidemiology and pathology. Vet J. 2001;162:92–107. doi: 10.1053/tvjl.2001.0571. [DOI] [PubMed] [Google Scholar]
- 14.Dambach DM, Lannon A, Sleeper MM, Buchanan J. Familial dilated cardiomyopathy of young Portuguese water dogs. J Vet Intern Med. 1999;13:65–71. [PubMed] [Google Scholar]
- 15.Sleeper MM, et al. Dilated cardiomyopathy in juvenile Portuguese Water Dogs. J Vet Intern Med. 2002;16:52–62. doi: 10.1111/j.1939-1676.2002.tb01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alroy J, et al. Inherited infantile dilated cardiomyopathy in dogs: genetic, clinical, biochemical, and morphologic findings. Am J Med Genet. 2000;95:57–66. [PubMed] [Google Scholar]
- 17.Wang W, Kirkness EF. Short interspersed elements (SINEs) are a major source of canine genomic diversity. Genome Res. 2005;15:1798–1808. doi: 10.1101/gr.3765505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindblad-Toh K, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 19.Carniel E, et al. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112:54–59. doi: 10.1161/CIRCULATIONAHA.104.507699. [DOI] [PubMed] [Google Scholar]
- 20.Daehmlow S. Novel mutations in sarcomeric protein genes in dilated cardiomyopathy. Biochem Biophys Res Commun. 2002;298:116–120. doi: 10.1016/s0006-291x(02)02374-4. [DOI] [PubMed] [Google Scholar]
- 21.Li D, et al. Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet. 2006;79:1030–1039. doi: 10.1086/509900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rampazzo A, et al. Arrhythmogenic right ventricular cardiomyopathy type 1 (ARVD1): confirmation of locus assignment and mutation screening of four candidate genes. Eur J Hum Genet. 2003;11:69–76. doi: 10.1038/sj.ejhg.5200914. [DOI] [PubMed] [Google Scholar]
- 23.Severini GM, et al. A New Locus for Arrhythmogenic Right Ventricular Dysplasia on the Long Arm of Chromosome 14. Genomics. 1996;31:193–200. doi: 10.1006/geno.1996.0031. [DOI] [PubMed] [Google Scholar]
- 24.Sidorik L, et al. Molecular chaperone, HSP60, and cytochrome P450 2E1 co-expression in dilated cardiomyopathy. Cell Biol Int. 2005;29:51–55. doi: 10.1016/j.cellbi.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Brega A, Narula J, Arbustini E. Functional, structural, and genetic mitochondrial abnormalities in myocardial diseases. J Nucl Cardiol. 2001;8:89–97. doi: 10.1067/mnc.2001.112755. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, et al. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss Muscular Dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 28.Knoll R, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 29.Nigro V, et al. Identification of the Syrian hamster cardiomyopathy gene. Hum Mol Genet. 1997;6:601–607. doi: 10.1093/hmg/6.4.601. [DOI] [PubMed] [Google Scholar]
- 30.Richman M. Characterization of a minimal screening set of 172 microsatellite markers for genome-wide screens of the canine genome. Journal of Biochemical & Biophysical Methods. 2001;47:137–149. doi: 10.1016/s0165-022x(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 31.He Q, et al. Canine Imerslund-Grasbeck syndrome maps to a region orthologous to HSA14q. Mamm Genome. 2003;14:758–764. doi: 10.1007/s00335-003-2280-1. [DOI] [PubMed] [Google Scholar]
- 32.Werner P, et al. Anchoring of canine linkage groups with chromosome-specific markers. Mammalian Genome. 1999;10:814–823. doi: 10.1007/s003359901096. [DOI] [PubMed] [Google Scholar]
- 33.Werner P, Raducha MG, Prociuk U, Henthorn PS, Patterson DF. A comparative approach to physical and linkage mapping of genes on canine chromosomes using gene-associated simple sequence repeat polymorphisms illustrated by studies of dog chromosome 9. Journal of Heredity. 1999;90:39–42. doi: 10.1093/jhered/90.1.39. [DOI] [PubMed] [Google Scholar]
- 34.Schäffer AA. Faster linkage analysis computations for pedigrees with loops or unused alleles. Human Heredity. 1996;46:226–235. doi: 10.1159/000154358. [DOI] [PubMed] [Google Scholar]
- 35.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES. Parametric and nonparametric linkage analysis: a unified multipoint approach. American Journal of Human Genetics. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]