Abstract
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection in infants, young children, and high-risk adults. Currently, there is no vaccine for the prevention of RSV infection, and available therapeutics are of limited utility. Peptide conjugated phosphorodiamidate morpholino oligomers (PPMO) are a class of antisense agents that can enter cells readily and interfere with viral protein expression through steric blocking of complementary RNA. Two antisense PPMO, designed to target sequence that includes the 5′ terminal- and translation start site-regions of RSV L mRNA, were tested for anti-RSV activity in cultures of two human airway cell lines. Both PPMO showed minimal cytotoxicity, and one of them (AUG-2), reduced viral titers by more than 2.0 log10. Intranasal treatment of BALB/c mice with AUG-2 PPMO prior to RSV inoculation produced a reduction in viral titer of 1.2 log10 in lung tissue at day 5 post-infection, and attenuated pulmonary inflammation at day 7 post-infection. These data show that the AUG-2 PPMO possessed potent anti-RSV activity and is worthy of further investigation as a candidate for therapeutic development.
Keywords: RSV, morpholino oligomers, antisense, PPMO, antiviral agents
INTRODUCTION
Respiratory syncytial virus (RSV) is the single most important viral respiratory pathogen in young children worldwide. RSV-associated lower respiratory conditions, such as bronchiolitis and pneumonia, often require hospitalization in children less than 1-year-old. RSV infection is also an important illness in high-risk adults, and it is the second–most commonly identified cause of viral pneumonia in the US elderly population. [1] No vaccine is currently available for the prevention of RSV infection. Ribavirin is the only licensed antiviral drug for treating RSV infection, and its use is limited to high-risk or severely-ill infants. The utility of Ribavirin has been limited by its cost, variable efficacy, and tendency to generate resistant viruses. [2–5] Postinfection treatment of RSV with anti-RSV immune globulin shows no benefit, [6] and the current need for additional effective anti-RSV agents is well-acknowledged. [7]
RSV is a member of subfamily Pneumovirinae in the family Paramyxoviridae, which are non-segmented viruses with genomes composed of a single molecule of RNA in negative-sense orientation. After an RSV virion enters a cell, the genomic RNA is transcribed into 5′ capped and 3′ polyadenylated mRNAs that each code for one of the 11 individual RSV proteins [8,9]. The RSV genome is replicated through the production of a complementary positive-sense full-length replicative intermediate (the antigenome). The viral ‘L’ protein is a major component of the RNA-dependent RNA polymerase complex responsible for all viral RNA synthesis, [10–12] and likely also plays roles in the capping [12,13] and polyadenylation of viral mRNA. [12] Antisense agents are typically short, synthetic oligonucleotides that are designed to hybridize with complementary sequence in specific mRNA, and thereby produce inhibition of gene expression. There have been several reports over the past few years describing studies in which RSV infections have been diminished through antisense-mediated approaches. These studies utilized phosphorothionate oligonucleotides (ODN), [14] 2′, 5′-oligoadenylates (2-5A), [15–18] or short interfering double-stranded RNA (siRNA). [19,20] All of these antisense-mediated strategies, however, will likely yet require considerable improvements in order to achieve clinical usefulness. Reduced toxicity, cost, and off-target effects, along with increased in-vivo stability, expression level and delivery into relevant cell populations may be necessary, variously, for the development of any candidate compounds from these technologies into effective therapeutic agents.
Phosphorodiamidate morpholino oligomers (PMO) have the same nucleobases as DNA, but differ in backbone chemistry, with a morpholine ring replacing the deoxyribose sugar, and a phosphorodiamidate linkage replacing the phosphodiester. [21,22] A PMO duplexed with complementary RNA sequence can produce an antisense effect through steric blockage of the RNA, [23] and therefore differs in mechanism of action from antisense agents based on DNA chemistry, which induce RNase H-mediated cleavage of RNA. [24,25] Each PMO used in this study was 5′-end conjugated to an arginine-rich cell-penetrating peptide (CPP) (Figure 1a). CPP-conjugated-PMO (PPMO) have been shown to achieve efficient entry into cells in cell culture systems [26–28] and in-vivo. [28] PPMO compounds have recently been reported to produce potent antiviral activity against several RNA viruses in cell cultures [26,28–33] and in-vivo. [28,29,34,35]
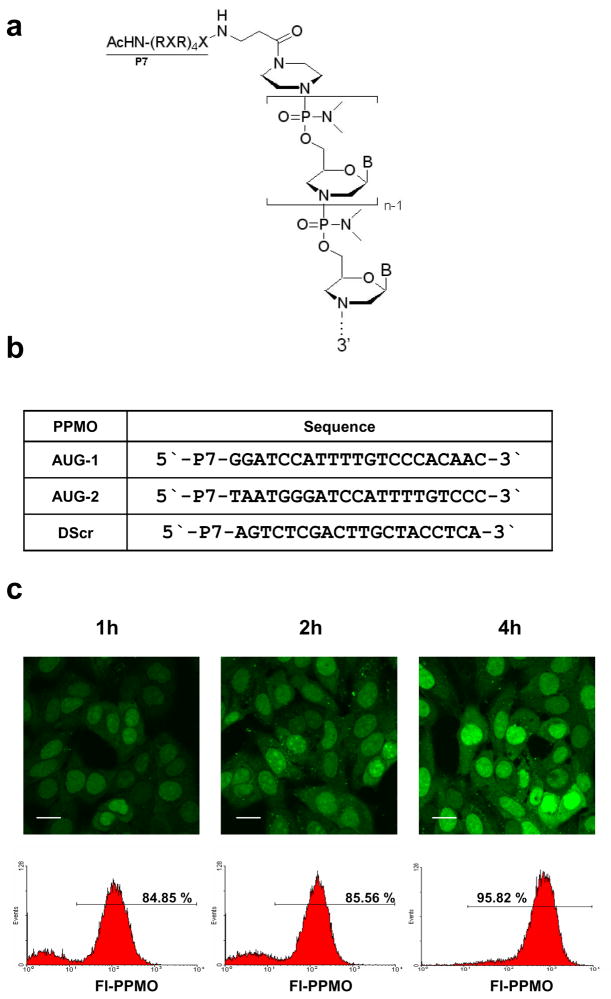
Figure 1. Structure, sequence, and cellular uptake of PPMO.
(a) The deoxyribose ring and phosphodiester linkage of DNA are replaced by a morpholine ring and phosphorodiamidate linkage in PMO. The arginine-rich peptide (RXR)4XB (see Materials and Methods), designated as P7, is covalently conjugated to the 5′ end of each PMO. “B” in the structure diagram represents the DNA bases A, C, G, or T. (b) PPMO (AUG-1 and AUG-2) were designed against a region of sequence that includes the AUG translation initiation codon of RSV L mRNA. DScr has a random PMO base sequence conjugated to a peptide identical to that of the other PPMO, to serve as a negative control. (c) HEp-2 cells were incubated with 20 μM of fluorescein-labeled DScr PPMO (Fl-PPMO) for 1h, 2h, and 4h. F1-PPMO entry was evaluated by confocal microscopy (top row) and flow cytometry (bottom row). Scale bar = 100 μm.
In this study, a 21mer PPMO designed to target sequence spanning the 5′ terminal- and translation start site-regions of RSV L mRNA was found to generate potent inhibition of RSV replication in cell cultures and in mice.
RESULTS
Design of PPMO
Previous studies have shown that the translation-initiator AUG codon region of mRNA can be a productive target region for PPMO, in both positive-strand [26,32,33] and negative-strand [29,30] RNA viruses. The L gene of RSV codes for a critical component of the viral RNA dependent RNA polymerase complex. [12] Both antisense PPMO were designed against sequence spanning the AUG translation start-site codon of the RSV L gene mRNA (Figure 1b). The AUG-2 PPMO is complementary to sequence from the ‘gene-start’ sequence (GS) present at the 5′ terminus of the mRNA to 13 nt into the coding sequence. [36] As the mature RSV-A2 L mRNA begins 8 nt before the AUG start codon, the AUG-1 PPMO was designed such that the 5 bases at the 3′ end of the oligomer have no complement in L mRNA. The AUG-1 PPMO is, however, fully complementary to contiguous sequence in the positive-sense antigenomic replicative intermediate RNA, corresponding to that from 13 nt upstream to 5 nt downstream of the L AUG translation start codon. As the M2 gene overlaps L by 68 nucleotides, [37] both L-AUG-targeting PPMO are also complementary to sequence shared between M2 and L in antigenomic RNA, and to sequence in the 3′ end of M2 mRNA.
PPMO enters cells effectively
PMO conjugated to the CPP P7 have been shown to achieve increased entry into various types of cultured cells compared to non-conjugated PMO. [26,28] To investigate the efficiency of P7-PMO uptake by HEp-2 cells under our culturing conditions, we incubated cells with 20 μM fluorescein-labeled DScr PPMO (Fl-PPMO) for various lengths of time. Confocal microscopy analysis showed that the majority of treated cells were fluorescence positive 30 min after the initiation of treatment (data not shown). The intensity of intracellular fluorescence increased in parallel with increasing incubation period (Figure 1c). Fluorescent signal was observed throughout all regions of the cells and appeared concentrated in the nucleus. Focal points of concentrated signal were clearly present in the cytosol, and may correspond to trapping of Fl-PPMO in the endosomal compartment. [38]
Flow cytometry analysis of single-cell suspensions of Fl-PPMO treated monolayers was used to quantify the percent of cells containing Fl-PPMO after a 1 or 4 h incubation period. After a 1 or 4 h incubation period, 85% or 96% of cells, respectively, were fluorescence-positive (Figure 1c). Based on this result, and results from other studies employing similar compounds, [26,28] we used a 4 h PPMO incubation period for the viral cell culture experiments described below.
PPMO cause little cytotoxicity
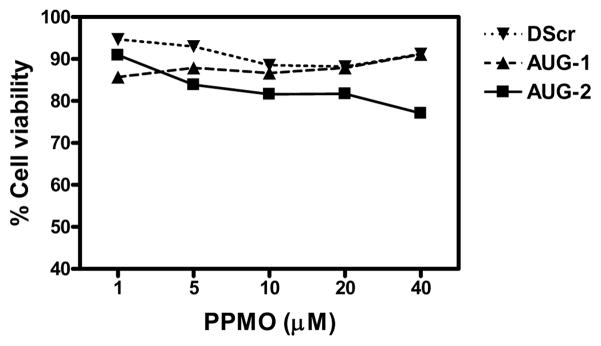
To evaluate whether PPMO treatment may result in cytotoxicity, we treated HEp-2 or A549 cells with various doses of PPMO, under the same culture conditions used in the antiviral experiments reported below. Cells were treated with PPMO for 4 h, then incubated in the absence of PPMO for another 24 h and assayed with a quantitative cell viability assay. HEp-2 cells incubated with AUG-1 or DScr PPMO showed minimal loss of viability at all concentrations tested, including 40 μM. Cells treated with 20 μM or 40 μM displayed approximately 10% to 15% loss in viability (Figure 2). AUG-2 PPMO generated slightly higher cytotoxicity of ~20 % at 20 μM and 25 % at 40 μM (Figure 2). PPMO caused somewhat higher cytotoxicity to A549 cells than to HEp-2 cells; however, even at a concentration of 40 μM PPMO A549 cell viability was greater than 70% (data not shown). The low cytotoxicity observed in the cell-viability assays described above strongly suggests that the inhibition of viral replication described below was not due to PPMO-associated cytotoxicity.
Figure 2. Evaluation of PPMO effect on cell viability.
HEp-2 cells were treated with different concentrations of AUG-1, AUG-2 or DScr PPMO for 4 hours. The PPMO-containing media was then removed and the cells incubated in fresh media for an additional 24h before cell viability was evaluated by using the MTT assay. Data are expressed as percentage of viable cells compared to mock-treated cells (set at 100%). (See details in Materials and Methods).
Anti-RSV effects of PPMO in cell culture assays
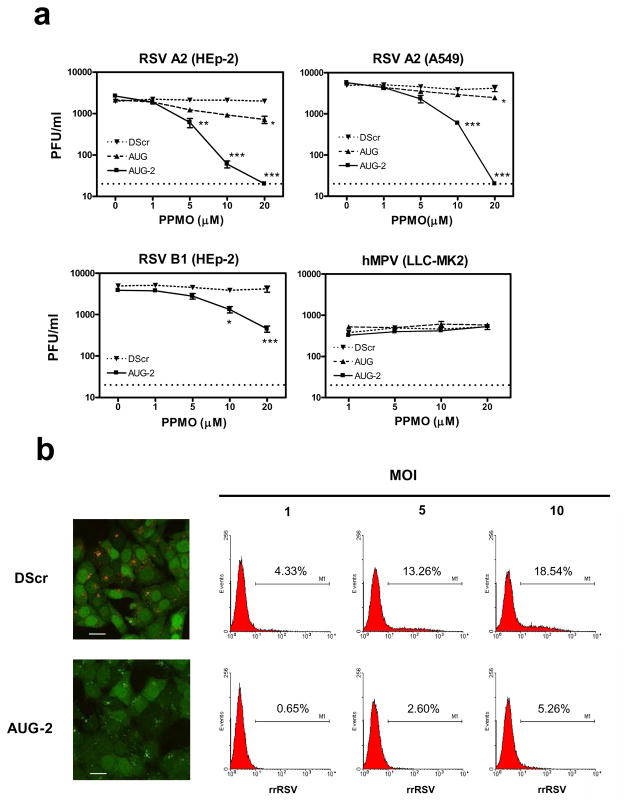
HRP plaque assays [39] were used to evaluate the impact of PPMO treatment on RSV-replication in HEp-2 and A549 cells. As shown in Figure 3a, there was no difference in viral titer between mock- and DScr-treated cells, indicating that treatment with a PPMO compound had little generic impact on the cellular processes necessary for virus replication. In HEp-2 cells, AUG-1 PPMO produced only moderate antiviral activity (~ 0.5 log10 reduction of titer when present at 20 μM). In contrast, AUG-2 PPMO generated approximately 0.6, 1.6, and greater than 2.0 log10 reductions in viral titer at concentrations of 5, 10, and 20 μM, respectively. These reductions take on greater significance when the relatively low peak titer of between 103 and 104 PFU per ml in the control samples is considered. In A549 cells, AUG-2 PPMO generated 0.8 and at least 2.3 log10 reductions of viral titer when used at 10 or 20 μM, respectively. Treatment of either HEp-2 or A549 cells with 20 μM AUG-2 PPMO suppressed viral production to below the level of RSV detection of the plaque assays (10 PFU/ml). In addition, we investigated the effect of AUG-2 on the replication of RSV B1 (a strain of RSV subgroup B). As shown in Figure 3a, a dose of 10 or 20 μM AUG-2 PPMO significantly reduced the titer of RSV B1 titer in HEp-2 cells, compared to the effect of DScr PPMO. To further evaluate the sequence-specificity of the PPMO, we examined their effect on the propagation of hMPV under similar experimental conditions to those used in the above RSV antiviral experiments. hMPV belongs to the same subfamily, Pneumovirinae, as RSV, but differs widely in sequence from RSV at the L AUG-2 PPMO target site. As above, cells were treated with PPMO for 4 h, infected with hMPV (MOI of 0.01) and evaluated for viral titer at 24 h post-infection (p.i.). The HRP plaque assays showed that hMPV replication was unaffected by any of the three PPMO used in this study, at all concentrations tested (up to 20 μM). This result provided further confirmation that the anti-RSV activity of AUG-2 PPMO was due to sequence-specific interaction with RSV RNA, as non-sequence specific interference would likely have resulted in titer reduction of hMPV, which is biologically very similar to RSV.
Figure 3. Antiviral effect of PPMO in vitro.
(a) HEp-2 and A549 cells treated with different concentrations of AUG-1, AUG-2 or DScr PPMO for 4h followed by infection with RSV A2 strain at an MOI of 0.01. Viral replication was determined 24h post-infection by HRP-staining plaque assay (see Methods). The activity of AUG-2 PPMO was also tested against the RSV B1 strain. hMPV-infected LL-MK2 cells were also challenged with PPMO, under the same conditions as above, as a control for RSV-specific PPMO activity. Vehicle-only treated cells produced titers that were almost identical to DScr-treated cells throughout these experiments. The limit of detection for this assay is 20 PFU/ml, indicated by the dotted line. *P< 0.05, **P< 0.01, ***P< 0.001 when comparing AUG-2- with DScr-treated cells. (b) HEp-2- cells were treated with 20 μM AUG-2 as described above and infected with rrRSV at different MOIs. Confocal images taken at 45 h p.i. show rrRSV-infected cells treated with DScr (top left) and AUG-2 (bottom left). Bar scale = 100 μm. RSV positive cells were determined by flow cytometry analysis. Numbers in each histogram represent percent of positive cells.
The ability of AUG-2 PPMO to reduce RSV infections was further investigated with the use of a recombinant red fluorescent protein (RFP)-expressing RSV (rrRSV). The level of viral replication in rrRSV-infected HEp-2 cells was determined by FACS analysis of RFP expression. Cells were treated with PPMO for 4 h, and then exposed to rrRSV for 45 h at different MOI’s (1, 5, and 10). At an MOI of 1, 20 μM AUG-2 PPMO caused a greater than 85% reduction in the number of cells expressing RFP, compared to DScr-treated control cells. We observed an increasing number of RFP-positive cells as the rrRSV MOI increased from 1 (4.33% positive cells) to 10 (18.54% positive cells) in DScr-treated cells. AUG-2 PPMO produced 85%, 80% and 72% reductions of RFP-positive cells at MOI’s of 1, 5 and 10, respectively (Figure 3b). These data indicate that AUG-2 PPMO was effectively anti-viral over a range of rrRSV inoculum levels.
Post-infection treatment with PPMO reduces viral replication
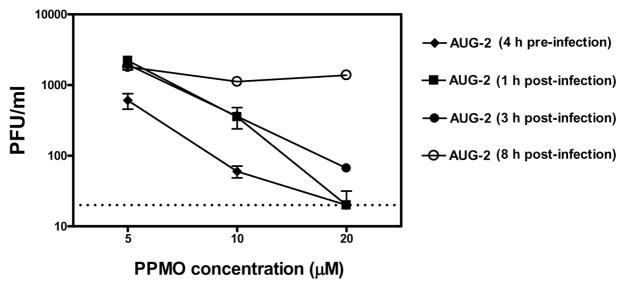
The above experiments demonstrated that AUG-2 PPMO treatment prior to infection produced potent anti-RSV activity in cultured cells. We then tested the ability of the AUG-2 PPMO to reduce the replication of an ongoing infection. Treatment of HEp-2 with 20 μM AUG-2 PPMO at 1 or 3 h after infection was effective in reducing viral replication, although slightly less-so than that produced by pre-infection treatment (Figure 4). When cells were treated with AUG-2 PPMO at 8 h after infection, RSV replication was unaffected at all PPMO concentrations tested. These results show that treatment of cells with PPMO prior to RSV infection was more effective in blocking viral replication than treatment after infection, but that treatment starting as late as 3 h after infection still resulted in considerable anti-viral activity.
Figure 4. Pre- and post-infection treatment of cell cultures with PPMO.
HEp-2 cells were infected with RSV at an MOI of 0.01. Different concentrations of PPMO were added at indicated time-points either before or after RSV infection. Viral replication was determined 24h after infection by HRP-staining plaque assay. The limit of detection for this assay was 20 PFU/ml, indicated by the dotted line.
AUG-2 PPMO suppresses viral protein expression
To further characterize the antiviral activity of AUG-2 PPMO, we assessed the expression of RSV proteins by Western blot. HEp-2 cells were treated with different concentrations of PPMO and infected with RSV. At 24 h after infection, cells extracts were prepared, and RSV proteins then detected with a polyclonal antibody. In RSV-infected cells, viral proteins including G, N, P, and M were expressed at comparable levels in untreated and DScr-treated cells, whereas minimal or no expression of RSV proteins was detected in infected cells that were treated with AUG-2 PPMO (Figure 5). Although the level of L protein was too low to detect by this antibody in any of the samples, the blotting results provided further confirmation of the sequence-specific anti-RSV activity of the AUG-2 PPMO, and indicated that the mechanism of action of this compound involves prevention of viral protein expression.
Figure 5. Inhibition of RSV protein expression by PPMO treatment.
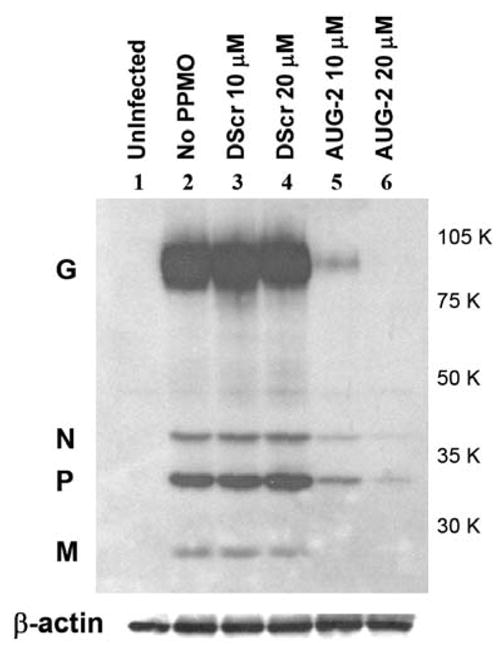
HEp-2 cells were treated with PPMO for 4h followed by infection with RSV at an MOI of 3. Cells were lysed 24 h after infection and RSV proteins were detected by western blot using an anti-RSV polyclonal antibody. RSV proteins and molecular weight markers are denoted on the left and right sides respectively. The blots were also probed for β-actin as a control; shown below the viral proteins.
Suppression of RSV replication and attenuation of inflammation in lungs of AUG-2 PPMO-treated mice
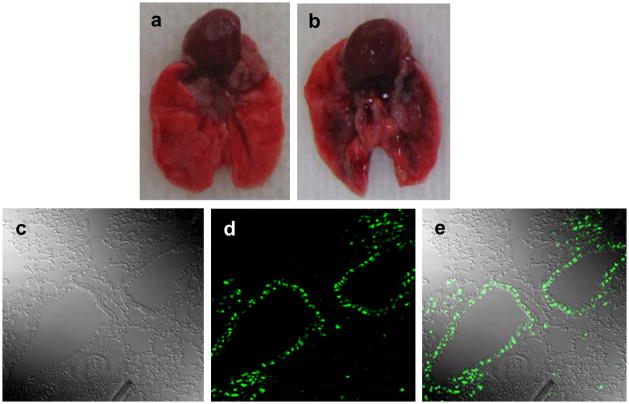
Based on the high antiviral efficiency of AUG-2 PPMO against RSV in cell cultures, we sought to evaluate PPMO activity in a mouse model of RSV infection. To evaluate the possible toxicity of a prospective PPMO dosing regimen, a single dose of PBS or PBS containing 45 or 90 μg of AUG-2 PPMO was administered intranasally to non-infected mice. The mice were observed for abnormal appearance or behavior, and all animal body weights were determined daily, to monitor for drug-associated illness. No significant ill-effects or differences in body weight between PBS- and PPMO-treated mice were observed over the 7-day observation period. Furthermore, histopathology of lung tissue revealed no obvious inflammation or structure abnormalities in PPMO-treated mice (data not shown, and Figure 8). After establishing that the AUG-2 PPMO was subtoxic at the above doses, we investigated PPMO distribution in the lung after intransal (i.n.) administration. As shown in Figure 6a, b, i.n. administration of 50 μl of a solution of PPMO and trypan blue (to allow visualization of PPMO location) to anesthetized mice resulted in rapid distribution of PPMO to the bronchial tree. In other experiments, we administered Fl-PPMO i.n. to mice and 4 h later removed and sectioned the lungs for analysis by confocal microscopy. As shown in Figure 6c–e, green fluorescence was distributed mostly in alveolar and bronchiolar epithelial cells. Scattered distribution of fluorescence was also observed in other unidentified interstitial cells of the lung.
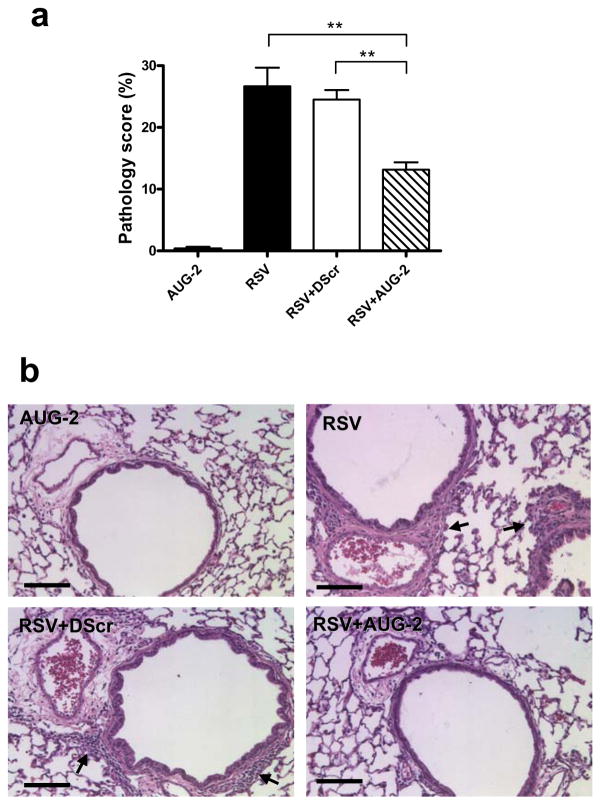
Figure 8. AUG-2 PPMO treatment reduces lung inflammation of RSV-infected mice.
BALB/c mice were treated with 90 μg PPMO 4 h before RSV infection (5 × 106 PFU). Lungs were harvested at day 7 after RSV infection, fixed for slide preparation and H&E stained. (a) Pathology score of prepared slides. Bar graphs represent mean ± SEM. n = 5 mice, with 6 slides/mouse. **P< 0.01. (b) Representative stained lung tissue sections from the indicated treatment. Arrows indicate cells infiltrating the perivascular and peribronchial spaces, (scored as described in Material and Methods). Scale bar = 200 μm.
Figure 6. Biodistribution of Fl-PPMO in mouse lung.
PPMO mixed 1:2 with trypan blue was administered i.n. to non-infected mice. Lungs were removed 2 hours later and opened along the sagittal plane. Panels a and b show lungs from a nontreated and PPMO-trypan blue-treated mouse, respectively. In a separate experiment, mice received DScr Fl-PPMO via i.n. administration, the lungs were removed 4h later and fixed. Lung section slides were examined by confocal microscopy as shown by (c) interference contrast, (d) fluorescent light, and (e) dual phase image.
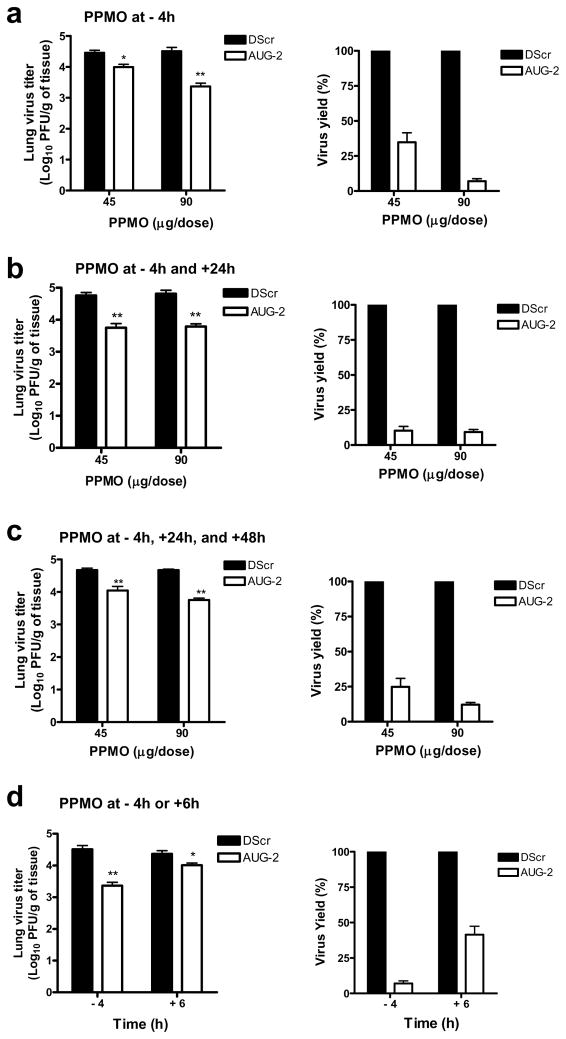
To investigate the antiviral effect of PPMO against RSV infection in vivo, mice were administered a single dose of DScr or AUG-2 PPMO i.n. and 4 h later infected with RSV. Five days after infection, lungs were collected and their viral titers determined by plaque assays. Doses of 45 μg or 90 μg of AUG-2 PPMO produced approximately 0.5 and 1.2 log10 reduction in viral titer, respectively, compared to DScr-treated mice (Figure 7a).
Figure 7. PPMO treatment of RSV-infected mice.
BALB/c mice were infected with 5 × 106 PFU RSV and treated with indicated PPMO as follows: (a) one dose, at 4h before infection. (b) two doses, one at 4h before and one at 24h after infection. (c) three doses, at 4h before and at 24 h and 48 h after infection. (d) A single dose of 90 μg was administered 4 h before or 6 h after infection. Viral replication was quantified by HRP staining at day 5 after infection. Vehicle-only treated cells produced titers that were almost identical to DScr-treated cells throughout these experiments. The virus yields of AUG-2 PPMO were calculated by setting DScr PPMO values as 100%. Bar graphs represent mean ± SEM. n = 5 mice. *P< 0.05, **P< 0.01 when comparing AUG-2- with DScr-treated mice.
To evaluate whether multiple PPMO doses (of 45 μg or 90 μg), could further improve antiviral outcome, we treated mice with two doses (4 h before and 24 h after infection) (Figure 7b) or three doses (4 h before, 24 h and 48 h after infection) (Figure 7c) of AUG-2 PPMO or DScr. Two or three doses of 90 μg AUG-2 PPMO produced no further reduction in viral titer over that which was observed with a single 90 μg pre-infection treatment. (compare Figures 7b and c with figure 7a). Figure 7b shows that two doses of 45 μg AUG-2 PPMO provided approximately equivalent protection to a single dose of 90 μg. Treatment with three doses of AUG-2 PPMO at 45 μg (Figure 7c) had an effect that was slightly better than a single dose but not as effective as two doses. These results show that a single dose of 90 μg of AUG-2 PPMO given 4 h prior to RSV inoculation or two doses of 45 μg given at 4 h prior to and 24 h p.i. produced a highly significant reduction of RSV replication in this mouse model.
To investigate the antiviral effect of AUG-2 PPMO administered after infection (i.e. therapeutic administration), a single 90 μg dose of AUG-2 PPMO or DScr was administered 6 h or 10 h after infection and compared to a single dose administered 4 h prior to infection. As shown in Figure 7d, treatment with AUG-2 PPMO 6 h p.i. resulted in 65% reduction in peak viral titer compared to the over-90% reduction observed with the pre-infection treatment. Although the treatment before infection was superior to treatment after infection, the 6 h p.i. treatment produced a statistically significant reduction in titer compared to treatment with DScr. AUG-2 PPMO treatment administered at 10 h p.i. did not produce a significant reduction in viral titer compared to mock- and DScr-treated control groups (data not shown). Therefore, the timing of PPMO treatment relative to RSV inoculation was a major factor in determining antiviral efficacy of AUG-2 PPMO in this experimental mouse model.
Finally, to investigate whether the antiviral effect of AUG-2 PPMO could also provide some degree of protection from RSV-induced lung inflammation, we determined lung histopathology in RSV-infected mice. Lung tissue sections were scored for the severity of perivascular and peribronchial inflammation, the hallmark of RSV-induced histopathology, at day 7 (peak of lung inflammation) after PPMO treatment and RSV infection. As mentioned above, non-infected mice treated with AUG-2 PPMO had no pulmonary inflammation (Figure 8). In RSV-infected mice, inflammation in perivascular and peribronchial areas was greatly attenuated in the AUG-2 PPMO-treated group compared to DScr-treated mice or mice that did not receive any treatment.
DISCUSSION
This study analyzed the antiviral efficacy and specificity of PPMO designed to interfere with the expression of the L gene of RSV, in cell cultures and in vivo. The RSV L gene codes for a major component of the viral RNA dependent RNA polymerase, and its mRNA has a 5′ UTR of only 8 nucleotides. The mRNA of L provides a rational target for PPMO technology, as two RNA regions previously established as productive PPMO targets, the 5′-terminal and AUG-regions of mRNA, could be targeted with a single PPMO. The AUG-2 PPMO was highly effective in two types of cultured human cells, and in a murine model of RSV infection. The entire target sequences of both AUG-1 and AUG-2 PPMO are present in RSV antigenomic RNA; however, only AUG-2 has its entire complement present in L mRNA. AUG-1 can bind to a maximum of only 16 contiguous bases of L mRNA. This difference in oligomer design, along with the superior inhibition displayed by AUG-2, makes it possible to suggest that at least part of the efficacy produced by AUG-2 is due to its ability to interfere with translation. As AUG-2 is designed to hybridize to sequence from the 5′ terminus through the AUG start codon of L mRNA it is possible to speculate that this PPMO is interfering in one or more of the events of mRNA capping, pre-initiation and/or initiation of translation. The Western blot of Figure 5 provides a clear indication that AUG-2 is interfering with the production of viral proteins. It is not possible at this time to infer if either AUG-1 or AUG-2 PPMO interfered with the production of genomic RNA from antigenomic template. Of note, comparative sequence analysis indicates that the target sequence of AUG-2 PPMO is perfectly conserved in various strains of the two major RSV subtypes, A2 and B1, available through GenBank (data not shown). Indeed, the AUG-2 PPMO was effective against representative strains from both major subtypes (Figure 3a).
PPMO showed rapid and effective uptake into cells in culture (Figure 1c) and into lung tissue (Figure 6). The unassisted uptake and subsequent antiviral activity of AUG-2, with low associated toxicity, raises the prospect that this compound could represent a potentially useful therapeutic. Confocal microscopy images (Figure 1c) indicate that PPMO becomes distributed throughout the cell, and although the compound appears to concentrate in the nucleus and in cytoplasmic foci (probably endosomes/lysosomes), [38] there is clearly a diffuse cytoplasmic distribution as well. This pattern is meaningful, as the life cycle of RSV, like most RNA viruses, transpires in the cytosol.
The favorable antiviral profile exhibited by AUG-2 PPMO in the A549 cell line (Figures 2 and 3) is notable, in that A549 is a pneuomocyte cell line, and is similar in architecture to the type of cells in which RSV replicates during in-vivo infections. The low cytotoxicity of AUG-2 at concentrations capable of reducing virus to below detectable limits was an encouraging result. Inhibition of the replication of RSV, but not of hMPV, provided further confirmation of the sequence-specific effect of AUG2 PPMO on RSV replication (Figure 3a). The results we obtained using PPMO against wild-type RSV and rrRSV (Figures 3a, b) were consistent with each other. The presence of a reporter-protein feature raises the possibility of using rrRSV as an in vitro drug-screening reagent, and our results provide validation of that application.
The results of Figure 4 indicate that, in cell culture, AUG-2 PPMO treatment starting at 3 h after infection can reduce RSV titer (measured at 24 h after infection). However, treatment starting at 8 h after infection had little effect, as apparently RSV L protein is produced in sufficient quantity by that time to allow virus production to proceed in a manner unaffected by the presence of AUG-2 PPMO. Likewise, AUG-2 produced a substantial reduction of lung viral titer in vivo if it was administered before, before and after, or only 6 h after infection (Figures 7a–d); however, if initial treatment was delayed until 10 h after infection little reduction in titer was observed (data not shown). Apparently, PPMO must be present in the cell quite soon after initial infection to have an impact on virus production. Together, these results imply that intervention early in infection may be necessary for successful application of this type of technology in a therapeutic setting.
We employed this mouse model in an initial attempt to address the feasibility of applying PPMO technology against RSV infection in vivo. Many aspects of RSV infection differ between the mouse model and humans, and we are reluctant to speculate on the predictive power of our results towards treating human infections. However, considering that a typical RSV infection spreads into and throughout the lungs over a period of several days, [40] it is likely that a drug which reduces viral replication could be useful to limit the severity of an infection. The impact of reduced viral replication on pathogenesis of RSV disease has been demonstrated in previous studies on vaccine development. [41–43] Our results show that AUG-2 PPMO treatment could produce an approximately 14-fold reduction in virus level (Figure 7) and a marked reduction in the severity of tissue inflammation in the lungs in-vivo (Figure 8). The lack of interferon (IFN-α) induction in mice treated with either AUG-2 or DScr PPMO indicates that interferon-mediated host responses did not contribute to the AUG-2 PPMO-mediated inhibition of RSV replication, (Supplementary Figure S1). These results suggest that this technology may be able to offer considerable clinical benefit, assuming, of course, that the anti-viral effect can be achieved without attendant toxicity.
Intranasal instillation was an effective route of PPMO administration to mice in this study, as indicated by PPMO distribution into bronchial epithelial cells (Figure 6), the major target of RSV infection. It is unclear at this time whether PPMO administered by other routes would likewise produce anti-RSV efficacy. Our results suggest that development of a respirable form of PPMO merits exploration.
A variety of nucleic-acid based approaches are showing promise in protection against RSV, [14–20,44], notably siRNA, and it is apparent that sequence-specific intervention in the RSV life cycle represents a productive avenue for anti-RSV drug development. Future investigation will include comparison of the relative merits of PPMO to other antisense-mediated approaches to a potential RSV therapeutic, with particular attention to the issues of, in vivo and ex-vivo stability, effective dose levels in vivo, toxicity, generation of resistant virus and cost. Overall, considering the impact on public health caused by RSV, and the results of the present study, PPMO-technology against RSV appears to warrant further development.
MATERIALS AND METHODS
Cells and viruses
Human A549 pulmonary type II epithelial cells (A549) (American Type Culture Collection (ATCC), Manassas, VA) were grown in F12K medium with 10% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and glutamine (10mM) at 37°C in a 5% CO2 incubator. RSV A2 was grown in HEp-2 cells (ATCC, Manassas, VA) and purified by sucrose-gradient as previously described. [45] RSV B1 strain (kindly provided by Dr. RM Chanock, NIAID) was grown in Vero cells and purified as described for RSV A2. The construction of recombinant red fluorescent protein (RFP)-expressing RSV (rrRSV) was described in detail previously. [39] Briefly, rrRSV was generated from MP224, a full-length RSV plasmid containing GFP, [46] by replacing the GFP codong sequence with that of RFP obtained from pDsRed (Clontech, PaloAlto, CA). As with GFP in MP224, RFP is located within the coding sequence of NS1 in rrRSV. The virustiters of RSV A2 and rrRSV were both determined by methylcellulose plaque assay. [45,47]The MPV strain CAN97–83 was obtained from the Centersfor Disease Control (CDC, Atlanta, GA), with kind permission from Dr. Guy Boivin (Laval University, Quebec City, Canada), and was propagated in LLC-MK2 cells (ATCC), as previously described. [47,48]
Synthesis of PPMO
PMO were synthesized by methods previously described. [21] The CPP (RXR)4XB (designated ‘P7’) (where R=arginine, X=6-aminohexanoic acid, and B=beta-alanine) was covalently conjugated to the 5′ end of each PMO (Figure 1a). P7-PMO (usually abbreviated PPMO in this report) compounds were synthesized, purified and analyzed at AVI BioPharma, Inc. (Corvallis, OR) by methods described previously. [38] Two PPMO of 21 bases in length, each targeting sequence in the 5′ end of RSV L gene positive-polarity RNA were designed based on GenBank Accession No. M74568. In addition, a 20mer random sequence of 50% G/C content was prepared for use as a negative control (named ‘DScr’ PPMO) (Figure 1b). The lyophilized PPMO were dissolved to a concentration of 2 mM in sterile distilled water and stored at 4°C. Additionally, 3′-fluoresceinated PPMO (Fl-PPMO) were synthesized for evaluation of cell-entry efficiency and antiviral effect using confocal microscopy and flow cytometry.
Antiviral assays in the presence of PPMO
HEp-2, A549, or LLC-MK2cells were seeded into 48-well plates at 4.3 to 4.7 log10 cells per well. After the cells had grown to monolayer confluence, growth medium was removed and the cells were treated with PPMO suspended in serum-free MEM (or mock-treated in MEM alone) in triplicate wells for 4 h, unless otherwise noted. After aspirating PPMO-containing medium and rinsing twice with MEM, the cells were infected with RSV or hMPV at a multiplicity of infection (MOI) of 0.01 PFU/cell. Following adsorption of virus for 1 h at 37°C and 5% CO2, viral inocula were aspirated, the cells washed three times with MEM, then MEM with 2% FBS added. The mock- or PPMO-treated and infected cells were then incubated at 37°C and 5% CO2 for 24 h. For the experiment evaluating post-infection treatment, PPMO was added directly into the medium at the indicated time point and left there until the titering procedure. Titers of both RSV and hMPV were determined by using polyclonal antibodies and an HRP staining method, as previously described. [47] Briefly, cells were fixed with absolute methanol and 2% H2O2 before incubation with anti-hMPV polyclonal antibody (MedImmune, Inc., Gaithersburg, MD) or anti-RSV polyclonal antibody (Biogenesis, Kingston, New Hampshire) followed by HRP-conjugated anti–guinea pig secondary antibody (Zymed, San Francisco, CA). Plaques were visualized by the addition of 3-amino-9-ethyl-carbazole (AEC) substrate and enumerated by light microscopy.
MTT cytotoxicity assay
Cell viability assays were carried out using the same basic protocol as above, but in the absence of virus. Briefly, confluent monolayers of HEp-2 or A549 cells grown in 96-well plates were treated in MEM containing PPMO at various concentrations or MEM alone, in triplicate, for 4 h. After removal of the treatment and rinsing twice, cells were incubated for 24 h at 37°C and 5% CO2. A quantitative colorimetric MTT assay (Sigma, St. Louis, Mo) was then used to quantify cell viability. Briefly, 10 μl of MTT reagent was added to each well and cells were incubated for 3 h until purple formazan crystal formation. After removing the MTT containing media, 100 μl of fomazan solubilization solution was added to each well. Following 20 min incubation plates were read at 570 nm with an automatic plate reader. Cell viability was quantitated as the percentage of absorbance of the PPMO-treated samples relative to that of mock-treated samples.
FACS analysis of cells inoculated with rrRSV
HEp-2 cells at a density of 5.3 log10 cells/well were treated with PPMO for 4 h, as described above, in 12-well plates. After rinsing, cells were infected with rrRSV at a MOI of between 1 and 10. A 45-h-incubation produced a single-cell suspension suitable for analysis with a FACS can flow cytometer equipped with CellQuest software (both from Becton Dickinson Immunocytometry Systems, San Jose, CA). Data analysis was performed with WinMDI software(Scripps, La Jolla, CA).
Western blot analysis
Prior to inoculation with RSV at MOI of 3.0 PFU/cell, HEp-2 cells were treated with PPMO or MEM for 4 h, as described above. Twenty-four h post-inoculation, cells were lysed with ice-cold lysis buffer, [49] and centrifuged at 14,000 × g for 15 min at 4°C. Samples of cell lysates were adjusted to contain the same amount of total protein(40 μg), and then electrophoresed at 4°C on a sodiumdodecyl sulfate (SDS)-10% polyacrylamide gel. After transfer to a polyvinylidene difluride membrane, the membranes were blocked with 5% fat-free milk in TBST (10 mM Tris–HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20) for1 h at RT. After a single rinse in TBST, the membranes were incubated with a 1:250 dilution of the goat anti-RSV polyclonal antibody described above. Proteins were visualized using ECL (Amersham Pharmacia Biotech) according to the manufacturer’s protocol. The same membranes were washed with Restore™ Western blot stripping buffer (Pierce, Rockford, IL) and then reprobed with anti-β-actin antibody (Sigma, St. Louis, Mo).
Mice and animal infection protocol
Female, 8- to 10-wk-old BALB/c mice were purchased from HarlanInc. (Houston, TX) and housed under pathogen-free conditions in theanimal research facility of the University of Texas Medical Branch, Galveston, TX, in accordance with the National Institutes of Health and University of Texas Medical Branch institutional guidelines for animal care. Under light anesthesia, mice were treated i.n. with 50 μl of phosphate-buffered saline (PBS) or PBS containing various amounts of PPMO at indicated times in relation to i.n. inoculation with 5 × 106 PFU purified RSV diluted in 50 μl PBS. Five days after inoculation and prior to opening the thoracic cavity, mice were euthanized with an intraperitoneal injection of a mixture of ketamine and xylazine. Lungs were removed and stored at −80°C for subsequent virus titration.
RSV titration of lung tissue
Lung virus titration was performed as previously described. [47] Briefly, homogenized lung samples were centrifuged and serially diluted before added to HEp-2 cells for methylcellulose plaque assay. Virus titer was reported as PFU per gram of lung tissue.
Biodistribution of PPMO and pulmonary histopathology
The distribution of i.n. instilled PPMO in mouse lung was investigated with PPMO diluted 1:2 in trypan blue (Sigma) (total volume of 50 μl). Lungs were removed en bloc 2 h after administration of the PPMO-trypan blue solution and opened along the sagittal plane to visually assess the pulmonary distribution of the PPMO. In a separate procedure, mice were administered 90μg of DScr Fl-PPMO i.n. and sacrificed 4 h later. Before removal, the lungs were lavaged 4 times with PBS to wash residual Fl-PPMO from airways. After removal, the lungs were perfused and fixed overnight in 10% buffered formalin, and stored in 70% ethanol at 4°C until sectioning. The tissue sections were examined by confocal microscopy (Zeiss LSM 510).
For histopathological analysis, lungs were collected on day 7 after infection and processed as previously described. [47] Briefly, lung tissue was fixed in 10% buffered formalin, subsequently embedded in paraffin and tissue sections were stained with hematoxylin and eosin (H&E). Slides were analyzed by two independent pathologists, in a blinded fashion, as previously described. [50]
Statistical analysis
Statistical analyses were performed with the InStat 3.05 biostatistics package (GraphPad, San Diego, CA) using a two-tailed, unpaired Student’s t test. Unless otherwise indicated, mean ±standard error of the mean is shown.
Supplementary Material
Figure S1. PPMO does not induce IFN-α production in mice. IFN-α production was determined by ELISA of BAL samples obtained from mice at 24 h after treatment with AUG-2, DScr PPMO or ODN-CpG (as positive control). The bar graph represents mean ± standard error of the mean (n = 3). ***, P < 0.001.
Acknowledgments
We thank Dr. Mark Peeples (Ohio State University, Columbus, Ohio) for providing rrRSV, the AVI BioPharma Chemistry Group for their expert production of all PPMO used in this study, and Dr. Thomas Albrecht at the Infectious Disease and Toxicology Optical Imaging Core, UTMB, for his help with confocal microscopy imaging. We are grateful to Cynthia Tribble for helping with the manuscript submission process. The work was supported in part by NIAID (P01 AI062885 and N01 AI30039). Shen-Hao Lai was supported by a training grant from Chang Gung University. The authors David Stein and Patrick Iversen work for the company (AVI BioPharma, Inc), which manufactures the PMO compounds used in this study.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 2.Marquardt ED. Cost of ribavirin therapy for respiratory syncytial virus infection. J Pediatr. 1995;126:847. doi: 10.1016/s0022-3476(95)70434-5. [DOI] [PubMed] [Google Scholar]
- 3.Crotty S, Andino R. Implications of high RNA virus mutation rates: lethal mutagenesis and the antiviral drug ribavirin. Microbes Infect. 2002;4:1301–1307. doi: 10.1016/s1286-4579(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S, Cameron C, Andino R. Ribavirin’s antiviral mechanism of action: lethal mutagenesis? J Mol Med. 2002;80:86–95. doi: 10.1007/s00109-001-0308-0. [DOI] [PubMed] [Google Scholar]
- 5.Prince GA. An update on respiratory syncytial virus antiviral agents. Expert Opin Investig Drugs. 2001;10:297–308. doi: 10.1517/13543784.10.2.297. [DOI] [PubMed] [Google Scholar]
- 6.Peebles RS, Jr, Moore ML. A mechanistic advance in understanding RSV pathogenesis, but still a long way from therapy. Am J Respir Cell Mol Biol. 2007;37:375–377. doi: 10.1165/rcmb.2007-0003ED. [DOI] [PubMed] [Google Scholar]
- 7.Torrence PF, Powell LD. The quest for an efficacious antiviral for respiratory syncytial virus. Antivir Chem Chemother. 2002;13:325–344. doi: 10.1177/095632020201300601. [DOI] [PubMed] [Google Scholar]
- 8.Collins PL, Dickens LE, Buckler-White A, Olmsted RA, Spriggs MK, Camargo E, et al. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci USA. 1986;83:4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowton VM, McGivern DR, Fearns R. Unravelling the complexities of respiratory syncytial virus RNA synthesis. J Gen Virol. 2006;87:1805–1821. doi: 10.1099/vir.0.81786-0. [DOI] [PubMed] [Google Scholar]
- 10.Stec DS, Hill MG, III, Collins PL. Sequence analysis of the polymerase L gene of human respiratory syncytial virus and predicted phylogeny of nonsegmented negative-strand viruses. Virology. 1991;183:273–287. doi: 10.1016/0042-6822(91)90140-7. [DOI] [PubMed] [Google Scholar]
- 11.Collins PL, McIntosh K, Chanock RM. Respiratory Syncytial Virus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3. Lippincott-Raven; Philadelphia: 1996. pp. 1313–1351. [Google Scholar]
- 12.Lamb RA, Parks GD. Paramyxoviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams, and Wilkins; Philadephia: 2007. pp. 1449–1496. [Google Scholar]
- 13.Liuzzi M, Mason SW, Cartier M, Lawetz C, McCollum RS, Dansereau N, et al. Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase. J Virol. 2005;79:13105–13115. doi: 10.1128/JVI.79.20.13105-13115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jairath S, Vargas PB, Hamlin HA, Field AK, Kilkuskie RE. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antiviral Res. 1997;33:201–213. doi: 10.1016/s0166-3542(96)01015-7. [DOI] [PubMed] [Google Scholar]
- 15.Barnard DL, Sidwell RW, Xiao W, Player MR, Adah SA, Torrence PF. 2-5A-DNA conjugate inhibition of respiratory syncytial virus replication: effects of oligonucleotide structure modifications and RNA target site selection. Antiviral Res. 1999;41:119–134. doi: 10.1016/s0166-3542(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 16.Cramer H, Okicki JR, Kuang M, Xu Z. Targeted therapy of respiratory syncytial virus by 2-5A antisense. Nucleosides Nucleotides Nucleic Acids. 2005;24:497–501. doi: 10.1081/ncn-200061780. [DOI] [PubMed] [Google Scholar]
- 17.Leaman DW. 2-5A antisense treatment of respiratory syncytial virus. Curr Opin Pharmacol. 2005;5:502–507. doi: 10.1016/j.coph.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Torrence PF. 2–5A-antisense chimeras: inhibitors of respiratory syncytial virus infection. Curr Opin Mol Ther. 1999;1:307–315. [PubMed] [Google Scholar]
- 19.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Yang H, Kong X, Mohapatra S, Juan-Vergara HS, Hellermann G, et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nat Med. 2005;11:56–62. doi: 10.1038/nm1174. [DOI] [PubMed] [Google Scholar]
- 21.Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 22.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 23.Stein D, Foster E, Huang SB, Weller D, Summerton J. A specificity comparison of four antisense types: morpholino, 2′-O-methyl RNA, DNA, and phosphorothioate DNA. Antisense Nucleic Acid Drug Dev. 1997;7:151–157. doi: 10.1089/oli.1.1997.7.151. [DOI] [PubMed] [Google Scholar]
- 24.Dash P, Lotan I, Knapp M, Kandel ER, Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walder RY, Walder JA. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988;85:5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deas TS, Binduga-Gajewska I, Tilgner M, Ren P, Stein DA, Moulton HM, et al. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J Virol. 2005;79:4599–4609. doi: 10.1128/JVI.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moulton HM, Nelson MH, Hatlevig SA, Reddy MT, Iversen PL. Cellular uptake of antisense morpholino oligomers conjugated to arginine-rich peptides. Bioconjug Chem. 2004;15:290–299. doi: 10.1021/bc034221g. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Stein DA, Lim T, Qiu D, Coughlin S, Liu Z, et al. Inhibition of coxsackievirus B3 in cell cultures and in mice by peptide-conjugated morpholino oligomers targeting the internal ribosome entry site. J Virol. 2006;80:11510–11519. doi: 10.1128/JVI.00900-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enterlein S, Warfield KL, Swenson DL, Stein DA, Smith JL, Gamble CS, et al. VP35 knockdown inhibits Ebola virus amplification and protects against lethal infection in mice. Antimicrob Agents Chemother. 2006;50:984–993. doi: 10.1128/AAC.50.3.984-993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Q, Pastey M, Kobasa D, Puthavathana P, Lupfer C, Bestwick RK, et al. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob Agents Chemother. 2006;50:3724–3733. doi: 10.1128/AAC.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinney RM, Huang CY, Rose BC, Kroeker AD, Dreher TW, Iversen PL, et al. Inhibition of dengue virus serotypes 1 to 4 in vero cell cultures with morpholino oligomers. J Virol. 2005;79:5116–5128. doi: 10.1128/JVI.79.8.5116-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuman BW, Stein DA, Kroeker AD, Churchill MJ, Kim AM, Kuhn P, et al. Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers. J Virol. 2005;79:9665–9676. doi: 10.1128/JVI.79.15.9665-9676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vagnozzi A, Stein DA, Iversen PL, Rieder E. Inhibition of foot-and-mouth disease virus infections in cell cultures with antisense morpholino oligomers. J Virol. 2007;81:11669–11680. doi: 10.1128/JVI.00557-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrer R, Neuman BW, Ting JP, Stein DA, Moulton HM, Iversen PL, et al. Antiviral effects of antisense morpholino oligomers in murine coronavirus infection models. J Virol. 2007;81:5637–5648. doi: 10.1128/JVI.02360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deas TS, Bennett CJ, Jones SA, Tilgner M, Ren P, Behr MJ, et al. In vitro resistance selection and in vivo efficacy of morpholino oligomers against West Nile virus. Antimicrob Agents Chemother. 2007;51:2470–2482. doi: 10.1128/AAC.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fearns R, Collins PL. Model for polymerase access to the overlapped L gene of respiratory syncytial virus. J Virol. 1999;73:388–397. doi: 10.1128/jvi.73.1.388-397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins PL, Olmsted RA, Spriggs MK, Johnson PR, Buckler-White AJ. Gene overlap and site-specific attenuation of transcription of the viral polymerase L gene of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987;84:5134–5138. doi: 10.1073/pnas.84.15.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abes S, Moulton HM, Clair P, Prevot P, Youngblood DS, Wu RP, et al. Vectorization of morpholino oligomers by the (R-Ahx-R)4 peptide allows efficient splicing correction in the absence of endosomolytic agents. J Control Release. 2006;116:304–313. doi: 10.1016/j.jconrel.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, et al. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman SJ, Laham FR, Polack FP. Mechanisms of illness during respiratory syncytial virus infection: the lungs, the virus and the immune response. Microbes Infect. 2004;6:767–772. doi: 10.1016/j.micinf.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt AC, Wenzke DR, McAuliffe JM, St CM, Elkins WR, Murphy BR, et al. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J Virol. 2002;76:1089–1099. doi: 10.1128/JVI.76.3.1089-1099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teng MN, Whitehead SS, Bermingham A, St CM, Elkins WR, Murphy BR, et al. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J Virol. 2000;74:9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright PF, Karron RA, Belshe RB, Thompson J, Crowe JE, Jr, Boyce TG, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 44.DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I, et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe SE, Mei F, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1alpha by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallak LK, Spillmann D, Collins PL, Peeples ME. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J Virol. 2000;74:10508–10513. doi: 10.1128/jvi.74.22.10508-10513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005;79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Zaman W, Kaphalia BS, Ansari GA, Garofalo RP, Casola A. RSV-induced prostaglandin E2 production occurs via cPLA2 activation: role in viral replication. Virology. 2005;343:12–24. doi: 10.1016/j.virol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PPMO does not induce IFN-α production in mice. IFN-α production was determined by ELISA of BAL samples obtained from mice at 24 h after treatment with AUG-2, DScr PPMO or ODN-CpG (as positive control). The bar graph represents mean ± standard error of the mean (n = 3). ***, P < 0.001.