Abstract
MHC class I molecules are important components of immune surveillance.
There are no available methods to directly visualize and determine the quantity and distribution of MHC/peptide complexes on individual cells or to detect such complexes on antigen presenting cells in tissues. MHC-restricted recombinant antibodies with the same specificity of T-cell receptors may become a valuable tool to address these questions. They may also serve as valuable targeting molecules that mimic the specificity of cytotoxic T cells.
We isolated by phage display a panel of human recombinant Fab antibodies with peptide-specific, MHC-restricted TCR-like reactivity directed toward HLA-A2-restricted T-cell epitope derived from a novel antigen termed TCRγ Alternative Reading frame Protein (TARP) which is expressed on prostate and breast cancer cells. We have characterized one of these recombinant antibodies and demonstrated its capacity to directly detect specific HLA-A2/TARP T-cell epitopes on antigen presenting cells that have complexes formed by naturally occurring active intracellular processing of the antigen as well as on the surface of tumor cells.
Moreover, by genetic fusion we armed the TCR-like antibody with a potent toxin and demonstrated that it can serve as a targeting moiety killing tumor cells in a peptide-specific, MHC-restricted manner similar to cytotoxic T-cell Lymphocytes.
1. Introduction
Most patients with metastatic prostate and breast cancers are provided with the limited benefits from standard chemo and hormone-based therapies. During the recent years, much effort has been invested in developing new approaches, such as immunotherapy, to improve therapeutic abilities, by combining the tumor-specificity of cell-mediated immunity with the freedom from toxic chemotherapies.
Recent immunotherapy approaches employ the principle that CD8+ CTL's recognize and kill tumor cells which display peptides from tumor-associated antigens presented by MHC class I molecules. Several tumor antigens and HLA allele-specific peptides from prostate cancer-associated antigens have been identified as CD8+ T cell epitopes, including HLA-A2-binding peptides derived from prostate specific antigen (PSA)[1-2], prostate-specific membrane antigen (PSMA)[3], prostate stem cell antigen (PSCA)[4-5], and prostate acid phosphatase6, that are all now components of current vaccine trials [5-11].
Identification of new tumor specific antigens is an essential step for successful development of immunotherapy approaches. As a result of this understanding, new genes specifically expressed in human breast and prostate cancer have been identified recently by utilizing new gene discovery tools [12-14]. One of these, TARP(T-cell receptor gamma alternate reading frame protein), is expressed on breast and prostate cancer cells [15,16]. It was shown that TARP was expressed on >90% of cancer specimens examined [9,15]. In order to define new breast and prostate CD8+ T cell tumor antigens, two wild-type HLA-A2 epitopes from TARP were identified [17]. The wild-type sequences were also improved by sequence modifications to produce epitope-enhanced peptides. Both wild-type and enhanced epitopes induced peptide-specific CD8+ T cell responses in A2Kb transgenic mice. In vitro restimulation of human CD8+ T cells from a prostate cancer patient resulted in CD8+ T cells reactive to the peptide epitopes that could lyse HLA-A2+ human breast cancer cells (MCF-7) expressing TARP. Epitope-specific human CD8+ T cells were also enumerated in patients' peripheral blood by tetramer staining. These data suggest that HLA-A2-binding TARP epitopes could be incorporated into a potential vaccine for both breast and prostate cancer.
One of the major problems with class I MHC-peptide complexes is the lack of molecular tools to directly study, quantitate, and visualize their expression on tumor cells, antigen-presenting cells, and other lymphoid tissues. Soluble T cell receptors have been proven difficult to engineer [18] and their inherent low affinity for their target limit their use as a molecular tool to study the ligand [19,20]. An alternative approach is to mimic the unique specificity of the TCR by generating high-affinity soluble antibody molecules that bind the T cell epitope with a peptide-specific, MHC-restricted manner. Using antibody phage display approaches, we have recently generated such unique molecules, coined TCR-like antibodies, against a variety of tumor and viral epitopes restricted to human HLA-A2, the most frequent MHC allele in humans. These antibodies were utilized to detect, visualize, and quantitate the number of specific tumor and viral associated HLA-A2/peptide complexes on tumor cells, APCs, and virus-infected cells [21-26].
Another possible application of TCR-like antibodies is their use as targeting molecules to efficiently eliminate tumor cells due to the fact that they can mimic the unique fine specificity of T-cells. This can achieved by using the antibodies or their fragments as targeting moieties and arming them with effector modalities such as potent toxins, chemotherapeutic drugs, isotopes, or biological entities such as cytokines [27-33].
In this study, we isolated human recombinant TCR-like antibodies directed against the novel TARP T cell epitopes expressed in complex with MHC class I HLA-A2 molecules. These molecules have been used to directly detect, by flow cytometry, the specific HLA-A2/TARP epitopes on antigen presenting cells as well as on breast and prostate cancer cells. Moreover, the TARP/HLA-A2-specific TCR-like antibody gene was fused to a truncated form of Pseudomonas exotoxin A to form a recombinant antibody-toxin fusion molecule [34]. The fusion molecule exhibited specific cytotoxic activity on breast and prostate cancer cells that correlated with their TARP and HLA expression pattern. The fusion was able to inhibit the growth of human breast tumor cell in nude mice. These results demonstrate the power of our approach to transform the unique specificity but low affinity properties of TCRs into high affinity and specific antibodies that may be used as a new molecular tool to study antigen (MHC-peptide) expression on diseased cells and APCs as well as generating novel targeting molecules to specifically eliminate tumor cells with the unique specificity observed in cytotoxic CD8+ T-cells.
2. Results
Selection of TCR-like, peptide-specific recombinant Fab fragments toward HLA-A2/TARP29-37
Recombinant peptide-HLA-A2 complexes that present the TARP 29-37-derived peptide were generated using a single chain MHC (scMHC) construct that was described previously [35]. In this construct, the extracellular domains of HLA-A2 are connected into a single-chain molecule with β2m using a 15-aa flexible linker. The scMHC-peptide complexes were produced by in vitro refolding of inclusion bodies in the presence of the TARP 29-37-derived peptide. The refolded scHLA-A2/TARP complexes were found to be highly purified, homogenous, and monomeric by SDS-PAGE (data not shown). Recombinant scMHC-peptide complexes generated by this strategy had been previously characterized in detail for their biochemical, bio-physical, and biological properties, and were found to be correctly folded and functional [35,36].
We have previously used the scHLA-A2/TARP complexes in the form of tetramers in order to detect TARP-specific CTLs from patient's PBMCs[17].
To select TCR-like antibodies, we used a large naïve Fab antibody phage library consisting of a repertoire of 3.7 × 1010 independent Fab clones [37]. In our selection strategy (see Materials and Methods), we first depleted the library of streptavidin binders and subsequently applied the library to panning in solution on soluble recombinant scHLA-A2-peptide complexes containing the native TARP 29-37 peptide. A two-fold enrichment in phage output was observed after the third round of panning using an assay of phage titration before and after each round of selection. The specificity of the selected phage clones was determined by a differential ELISA assay on streptavidin-coated wells incubated with biotinylated scHLA-A2 complexes containing either the TARP 29-37 peptide or a control complex containing a gp100 melanoma differentiation antigen-derived peptide G9-209. Phage clones analyzed after the third round of selection exhibited two types of binding pattern toward the scHLA-A2-peptide complex: one class of Abs consisted of pan-MHC binders that cannot differentiate between the various MHC-peptide complexes; the second type consisted of Abs that bound the MHC-peptide complex in a peptide-specific manner. The ELISA screen revealed that 46 of 94 randomly selected clones screened (49%) from the third round of panning bound HLA-A2/ peptide complex. Thirty-two clones of the 94 screened (34%), were peptide specific and bound only to the scHLA-A2/TARP complex used in the selection.
A representative analysis of five TCR-like Fab clones that reacted only with the scHLA-A2/TARP complex and not with control scHLA-A2/peptide complexes is shown in Figure 1A. The diversity of peptide-specific clones from the third round of panning was examined by DNA fingerprint analysis and revealed 14 different restriction patterns, indicating the selection of several different Abs with TCR-like specificity.
Figure 1. Isolation and characterization of TARP/HLA-A2 specific TCR-like antibodies.

- ELISA reactivity assay of Fab antibody clones with recombinant purified TARP/HLA-A2 and control complexes. Biotinylated scHLA-A2-peptide complexes were immobilized through BSA-streptavidin. Detection of Fab binding was with Peroxidase-labeld anti-human Fab.
- Expression and purification of TCR-like D2 Fab. SDS-PAGE analysis of purified D2 Fab. Fab clone was expressed in E.coli and purified by metal affinity chromatography as described. M: Molecular weight markers, NR: Non-reduced SDS-PAGE.
- ELISA assay for D2 Fab reactivity with TRAP/HLA-A2 and control recombinant purified complexes. Detection was with Peroxidase-labeled anti-human Fab.
- Binding of D2 Fab by flow cytometry to peptide-loaded APCs. JY EBV-transformed HLA-A2 positive B cells were loaded with the TARP29-37 and control HLA-A2-restricted peptides and the reactivity with purified D2 Fab (10-50μg/ml) was tested. Detection of binding was with PE-labeled anti-human Fab. Control peptides were: MART1, hTERT (540), gp 100-derived peptides G9-209, G9-280, Human T cell lymphotropic virus type 1(HTLV-1) derived peptide TAX and Cytomegalovirus (CMV)- derived peptide. Data are representative of 5 experiments.
Characterization of scHLA-A2/TARP-specific TCR-like Abs
Based on the specificity assays performed with the phage clones (Fig. 1A) we have selected clone D2 for further analysis. Fab D2 was sequenced, its heavy chain belongs to subgroup III of VH and the light chain belongs to the human Kappa subgroup I family. The sequence of D2 was deposited in Genebank under accession number: heavy chain-bankit691458 and light chain-bankit691544.
Fab D2 was expressed in E. coli BL21 or TG1 cells and soluble Fab that is tagged with a hexahistidine sequence fused to the CH1 domain was purified by metal affinity chromatography from the periplasmic fraction. SDS-PAGE analysis (Figure 1B) of the affinity-purified material revealed homogenous, pure Fab with the expected molecular weight. Approximately 2 mg of purified Fab could be obtained from 1 L of bacterial shake flask culture.
To determine the specificity of the soluble TCR-like Fab molecule, we performed ELISA assays on biotinylated scHLA-A2/peptide complexes that were immobilized to BSA-streptavidin-coated wells. The BSA-streptavidin-biotin spacer enables the correct folding of the complexes, which may be distorted by direct binding to plastic. To determine the correct folding of the bound complexes and their stability during the binding assays, we monitored their ability to react with the conformation-specific mAb, w6/32, that recognizes HLA complexes only when folded correctly and when containing peptide (not shown). Figure 1C shows a representative analysis of the reactivity of Fab D2 with the HLA-A2/ TARP 29-37 and other control HLA-A2/peptide complexes. As shown, D2 reacted specifically with the TARP-containing HLA-A2 complex, but not with control HLA-A2/peptide complexes displaying various tumor or viral-derived T cell epitopes (peptides). Thus, the peptide, MHC-specific, Fab D2 exhibited binding characteristics and fine specificity of a TCR-like molecule.
The binding affinity of the TCR-like soluble purified D2 antibody was assessed using a saturation ELISA assay in which biotinylated complexes were bound to BSA-biotin-streptavidine-coated plates to which increasing amounts of D2 Fab were added. The binding of Fab D2 to the specific HLA-A2/TARP29-37 complex was dose-dependent and saturable (not shown). Extrapolating the 50% binding signal revealed that this antibody possessed an apparent binding affinity of 120 nM.
Detection of HLA-A2/TARP complexes on peptide-pulsed APCs
To demonstrate that the isolated soluble Fab D2 can bind the specific MHC-peptide complex not only in the recombinant soluble form, but also in the native form, as expressed on the cell surface, we used the TAP+ EBV-transformed B-lymphoblast HLA-A2+ JY cells as APCs. They have normal TAP; consequently, peptide loading is facilitated by the exchange of endogenously derived peptides with HLA-A2-restricted peptides supplied externally by incubation of the cells with the desired peptides. We incubated these cells first with the TARP 29-37 peptide and control HLA-A2-restricted peptides, then washed the cells and incubated them with Fab D2. As shown in Figure 1D, Fab D2 recognized only JY cells pulsed with the specific TARP peptide but not with control HLA-A2-restricted peptides derived from tumor or viral antigens. These results demonstrate the ability of the TCR-like D2 antibody to detect the specific TARP29-37 MHC-peptide complex on the surface of cells.
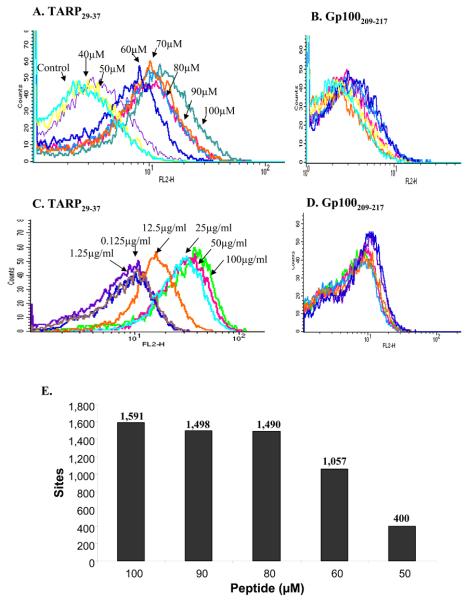
To determine the minimum number of TARP29-37-HLA-A2 complexes detected by the D2Fab we performed titrations experiments in which the Fab and TARP peptide concentrations were titrated on JY cells and the binding of the D2 Fab was monitored by flow cytometry. As shown in Figure 2, the binding of the D2 Fab was dependent on peptide (Fig. 2A) and antibody (Fig. 2C) concentration. Titrations on cells loaded with control peptide confirmed specificity (Fig. 2B and 2D). D2 Fab at 25-50 μg/ml and TARP peptide concentration of ∼60 μM were observed as saturating concentrations for maximal binding to JY cells. In order to translate the mean fluorescence intensity (MFI) obtained by flow cytometry on JY cells to number of sites (pMHC) recognized by the Fab we used a calibration curve constructed from fluorescence intensities of calibration beads with known numbers of PE molecules per bead (QuantiBRITE PE beads; BD Biosciences), thus providing a mean of quantifying PE-stained cells using a flow cytometry. This yielded a detection threshold of ∼400 pMHC complexes per cell as the minimal number of complexes detected by the D2 Fab antibody. Similar sensitivities were obtained with other TCR-like antibodies targeting tumor or viral complexes isolated and characterized by us using the same antibody phage library used to select the D2 Fab [22,23].
Figure 2. Sensitivity of D2 Fab in the detection of TARP/HLA-A2 complexes.
Titration of D2 Fab (A) and TARP29-37 peptide (C) by flow cytometry on JY cells pulsed with the TARP29-37 peptide. Controls are JY cells pulsed with the melanoma differentiation-derived HLA-A2-restricted peptide 207-217 derived from gp100.
To quantify the minimal number of TARP-HLA-A2 complexes recognized by the D2 Fab, The level of fluorescence intensity on stained cells in A was compared with the fluorescence intensities of calibration beads with known numbers of Phycoerythrin (PE) molecules per bead (QuantiBRITE PE beads; BD Biosciences), thus providing a mean of quantifying PE-stained cells using a flow cytometry. The calculated numbers of complexes/cell at various concentrations of peptide are indicated.
Fine specificity of Fab D2 to modified TARP-derived peptides
When TARP-derived HLA-A2 –restricted peptides were identified and characterized TARP-29-37 peptide exhibited moderate binding affinity to HLA-A2. The affinity of peptide to MHC class I molecules is a major factor determining the immunogenicity of peptide epitopes. To enhance the binding affinity of wild-type epitopes, TARP-29-37 amino acids in the peptides were substituted with others. For TARP-29-37, Arg at position 3 and Leu at position 9 were substituted with Ala (TARP-29-37-3A) and Val (TARP-29-37-9V), respectively. These substitutions were shown to increase the affinity of the peptide to HLA-A2 [17]. To determine the fine specificity of the D2 Fab TCR-like antibody, tests were conducted in order to assess its reactivity with the modified peptides. As shown in Figure 3A, Fab D2 recognized the wild type TARP 29-37 and the modified TARP 29-37-9V peptides but did not recognize the TARP 29-37-3A peptide. The inability of Fab D2 to recognize the TARP 29-37-3A complex is not due to complex instability since, as shown in Figure 3A, the reactivity of the three complexes with MAb W6/32, which recognize properly folded peptide-containing HLA molecules, was similar. The same pattern of reactivity was observed on peptide-pulsed RMAS-HHD cells which are TAP-mutant APCs stably transfected with HLA-A2. The stabilization/expression of the HLA-A2 complexes on the surface of peptide-pulsed RMAS-HHD cells was determined by anti-HLA-A2 monoclonal antibody BB7.2. As shown in Figure 3B, the presentation level of the wild-type TARP 29-37, modified TARP 29-37-3A, TARP 29-37-9V and other control peptides were very similar. However, Fab D2 recognized only wild-type TARP 29-37 and TARP 29-37-9V peptides on the cell surface of peptide-pulsed RMAS-HHD cells (Fig. 3C).
Figure 3. Fine specificity of D2 Fab to TARP enhanced epitopes.

- ELISA binding assay of D2 Fab to recombinant purified HLA-A2 complexes generated with wild-type and epitope enhanced TARP peptides. scHLA-A2/peptide complexes were generated as described in materials and methods. The stability of the wild-type and modified complexes was monitored by reactivity with MAb W6/32 which recognizes properly folded complexes that contain peptide. Reactivity was monitored with Peroxidase-labeled anti-human Fab.
- Stabilization of class I MHC carrying wild type and epitope enhanced TARP peptides on the cell surface. Flow cytometric analysis of RMAS-HHD cells which are TAP-mutant APCs stably transfected with HLA-A2. Cells were loaded with wild type TARP29-37, TARP29-37-9V, TARP29-37-3A and control Tax peptides. The stabilization of complexes on the cell surface was monitored by the reactivity of BB7.2, a MAb to HLA-A2. Detection was with FITC-labeled anti-mouse IgG.
- Reactivity of D2 Fab with wild-type and epitope enhanced TARP peptides on the surface of cells. Flow cytometry analysis of RMAS-HHD cells that were loaded with TARP29-37, TARP29-37-9V, TARP29-37-3A and control Tax peptides and the binding of D2 Fab was monitored with PE-labeled anti-human Fab.
Detection of TARP complexes formed by active intracellular processing
To examine the ability of the TCR-like D2 Fab Ab to detect HLA-A2/TARP complexes produced by physiological active intracellular antigen processing, we transfected the full length TARP gene into the EBV-transformed B cell HLA-A2-positive Ag-presenting JY cells. Twenty-four hours after transfection, we tested the reactivity of the HLA-A2/TARP-specific Ab D2 (in the form of a tetramer) with the transfected cells by flow cytometry. As shown in Figure 4A, significant and specific reactivity was observed with HLA-A2-positive JY cells 24 hrs after transfection with the TARP gene but not with the melanoma differentiation antigen MART gene or with vector alone. Forty eight hours after transfection the reactivity of Fab D2 was tested again and as shown in Figure 4B, the number of HLA-A2/TARP complexes (fluorescence intensity) on the surface of the transfected cells increased but the total number of stained cells (events) was decreased probably due to a decrease in cell viability. Control EBV-transformed APD B cells which are HLA-A2 negative, HLA-A1 positive were also used as controls and when transfected with the TARP gene no reactivity with the D2 Fab tetramer was observed (Figure 4C). The efficiency of TARP gene transduction into JY cells was high as monitored by transfection with a GFP construct (Figure 4D). These results demonstrate that Fab D2 can detect the authentic HLA-A2/TARP29-37 complex after naturally occurring intracellular processing of the gene.
Figure 4. Binding of D2 Fab to TARP/HLA-A2 complexes generated by active intracellular processing.
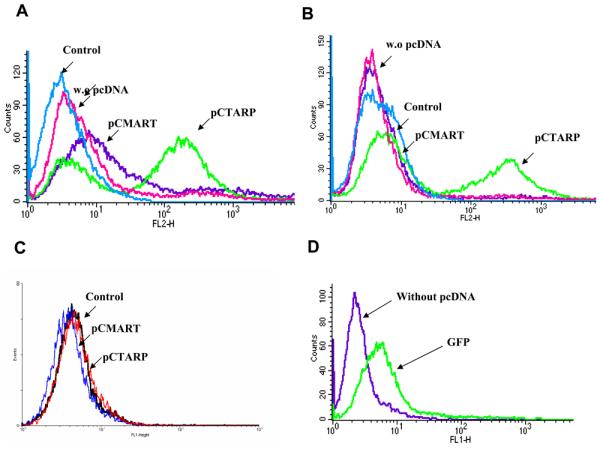
JY HLA-A2+ or APD HLA-A2− APCs were transfected with pCDNA containing the intact full length TARP gene (pCTARP), full length melanoma differentiation antigen MART1 (pCMART) or with empty pCDNA control vector. 24 (A) or 48 (B) hrs after transfection cells were stained by flow cytometry using the HLA-A2/TARP-specific D2 Fab or a control CLA12 TCR-like Fab specific for melanoma differentiation antigen MART1 epitope. In C, control APD cells which are HLA-A2−/HLA-A1+ were transfected with pCTARP and pCMART and reacted with the D2 Fab. The efficiency of TARP gene transduction into JY cells was monitored by transfection of the pCDNA vector carrying the GFP gene (D).
Detection of TARP complexes on tumor cells
To explore whether the TCR-like D2 Fab antibody is capable of binding endogenously derived MHC-peptide complexes and therefore may eventually be used to visualize these complexes on the surface of tumor cells, we performed flow cytometry analysis on tumor cell lines that express TARP and HLA-A2. These cells better simulate the native state in which MHC-peptide complexes are expected to be present at a lower cell surface density when compared to peptide-loaded cells or to cells transfected with the antigen gene. Clear and reproducible reactivity was observed with Fab D2 on TARP expressing breast carcinoma MCF7 cells (Fig. 5A) which also express moderate levels of HLA-A2 (Fig. 5A) as detected by anti-HLA-A2 MAb BB7.2. TARP-positive prostate carcinoma LNCaP cells (Fig. 5C) exhibited a very weak but reproducible signal, however, their HLA-A2 expression is low (Fig. 5C). Fab D2 did not react with HLA-A2-, TARP- prostate carcinoma PC3 cells (Fig. 5B) [16] or with HLA-A2- TARP- human epidermal carcinoma A431 cells (Fig. 5D). D2 Fab did not react with HEK293 cells which are TARP negative and HLA-A2 positive (Fig. 5E), neither with HLA-A2 negative PC3 cells that were stably transfected with the TARP gene (Fig. 5F). The mean fluorescence intensity (MFI) of antigen positive MCF7 and LNCaP cells was 6- and 3-fold higher, respectively compared to the mean MFI results of the antigen negative controls PC3,A431, HEK294, and PC3-TARP (a representative summary from 8 experiments summarized in Table 1). Titration experiments with the D2 Fab presented in Figure 2 suggest that the minimum numbers of antigenic complexes that are still detectable by the D2 Fab in flow cytometry on JY APCs are ∼400 complexes/cell. Thus, this information may indicate the relative number of antigenic complexes present in antigen and HLA-A2 positive cells such as MCF7 which are weakly recognized by the D2 Fab using the same flow cytometry assay.
Figure 5. Binding of D2 Fab to tumor cells.

Flow cytometry analysis of the binding of D2 Fab to tumor cells. TARP and HLA-A2 positive MCF7 and LNCaP cells or TARP and HLA-A2 negative cells were stained with D2 Fab. HLA-A2 expression was monitored by MAb BB7.2. Detection was with FITC-labeled anti-human Fab (for D2 Fab) or FITC-labeled anti-mouse IgG (for BB7.2).
Table 1.
Mean Fluorescence intensity of binding of Fab D2 to tumor cells
| Cell line | Control | Specific | Δ |
|---|---|---|---|
| MCF7 | 3.76 | 6.58 | 2.82 |
| LnCap | 4.04 | 5.30 | 1.26 |
| PC3 | 3.93 | 3.53 | - |
| A431 | 2.69 | 2.92 | 0.23 |
| HEK293 | 3.74 | 3.55 | - |
| PC3-TARP | 3.90 | 4.05 | 0.15 |
The data are MFI of a representative experiments out of 8 experiments performed. When control MFI is above specific the Δ is marked –
The D2 Fab reactivity with MCF7 cells appears to be bimodal which may suggest that the distribution of the peptide-MHC complexes on cells is not homogeneous which may result in a population of cells with very low expression which is below the detection limit of the Fab. Ideally, separation of the two subpopulations using the Fab will help to address this question.
These results demonstrate that the D2 Fab TCR-like antibody is capable to detect and visualize in a specific manner TARP-derived MHC-peptide complexes on the surface of tumor cells. Hence, these TCR-like antibodies can bind to cells that express the specific MHC-peptide complex at a density most likely to be found on TARP-expressing tumor cells and antigen-presenting cells.
Construction and characterization of a toxin armed recombinant D2 Fab fusion
To further characterize the binding specificity and the biological properties of the selected D2 Fab TCR-like antibody and to test its ability to serve as a specific targeting molecule we armed the D2 Fab with a potent truncated bacterial toxin. We fused the D2 Fab Fd gene to a truncated form of Pseudomonas Exotoxin A (PE38) to generate a Fab-immunotoxin [38]. This truncated form of PE contains the translocation and ADP-ribosylation domains of whole PE but lacks the cell-binding domain, which is replaced by the Fab fragment fused at the N-terminus of the truncated toxin. The D2 light chain and heavy chain-PE38 fusion were cloned separately into a T7-promotor pET-based expression vector and were expressed in E. coli BL21 cells as intracellular inclusion bodies. Using established renaturation protocols, a D2 Fab-PE38 fusion molecule was refolded and purified (see materials and methods). A highly purified D2 Fab-PE38 fusion protein with the expected molecule weight of 88 kDa was obtained as analyzed by SDS-PAGE under non reducing conditions (not shown). The D2 Fab-immunotoxin fusion was generated with two forms of PE38, one containing a native C-terminal sequence which serves as an ER retention signal (REDLK) and one with a modified C-terminal sequence (KDEL), which is the common ER retention signal.
Binding of D2 Fab-PE38 to APCs displaying the TARP29-37 epitope and to tumor cells
To demonstrate that purified D2 Fab-PE38 can bind the specific MHC-peptide complex as expressed on the cell surface, we utilized the EBV-transformed B lymphoblast JY cells, which express HLA-A2. These cells were incubated with the TARP29-37 or control peptides and the binding of the fusion protein was tested by monitoring the reactivity of anti-PE38 antibodies.
As shown in Figure 6A, the D2 Fab-toxin fusion exhibited specific binding to JY cells loaded with the TARP29-37 peptide but not to cells that were loaded with control tumor or viral-derived epitopes. These results demonstrate that the Fab-fusion can specifically recognize its corresponding native HLA-A2 complex on the surface of cells and that the reactivity was not altered as a result of this fusion.
Figure 6. Binding of D2 Fab-PE38 fusion molecule to tumor cells.
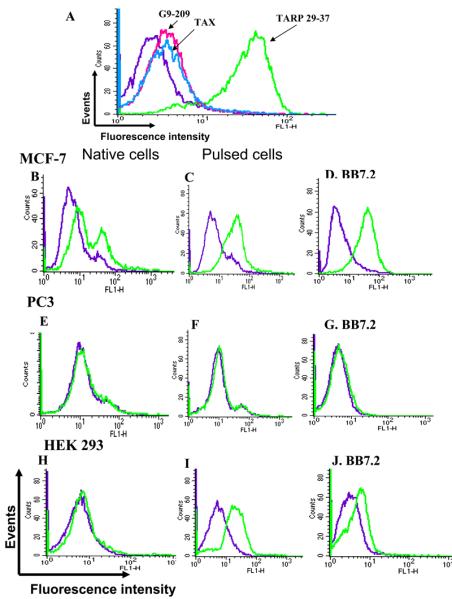
- A. Flow cytometry binding analysis of D2 Fab-PE38 fusion to JY APCs. JY cells were loaded with TARP and control Tax and G9-209 peptides and the reactivity of the D2 Fab-PE38 fusion molecule was determined by using rabbit anti-PE38 antibodies. Detection was with FITC-anti rabbit IgG.
- B-J. Flow cytometry binding analysis of D2 Fb-PE38 fusion to tumor cells. Binding of D2 Fab-PE38 to TARP positive and negative tumor cells was monitored by rabbit anti-PE38 antibodies. In B,E, and H binding to tumor cells was tested. In C,F, and I binding was to tumor cells pulsed with the TARP peptide. Monitoring of HLA-A2 expression was with BB7.2 (D,G,and J). Binding of PE38 fusion was detected with FITC-labeled anti rabbit IgG. Binding of BB7.2 was with FITC-labeled anti-mouse IgG.
Next, the reactivity of the D2-Fab fusion was tested on TARP-expressing tumor cells. HLA-A2 was monitored with MAb BB7.2 and the binding of the fusion molecule with anti-PE38 antibodies. As shown in Figure 6B, the D2 Fab-immunotoxin reacted specifically with HLA-A2 positive (Fig. 6D) and TARP positive MCF7 carcinoma cells, but not with PC3 prostate carcinoma cells which neither express HLA-A2 nor TARP (Fig. 6E) Human embryo kidney 293 (HEK293) cells which express HLA-A2 (Fig. 6J) but are TARP negative [16] did not react with the D2 Fab-fusion molecule (Fig. 6H). As a control, we loaded these tumor cells with the TARP29-37 peptide and monitored the binding of the D2 Fab-fuison molecule. As shown in Figure 6C, F, and I, the D2 Fab-fusion reacted with TARP 29-37 pulsed MCF7 and HEK293 HLA-A2 positive but not PC3 HLA-A2 negative cells.
These results demonstrate that the D2 Fab-PE38 retains its specificity to TARP/HLA-A2 peptide complexes expressed on the surface of cells.
Cytotoxicity of D2 Fab-PE38 towards tumor cells displaying the TARP derived epitopes
The ability of the D2 (Fab)-PE38 to inhibit protein synthesis was used as a measure to test the specificity and biological activity of the TCR-like D2 Fab fusion molecules. Because the cell binding domain in the toxin was deleted, cytotoxicity induced by internalization of the Fab-Toxin fusion reflects specific antigen binding. To test this activity we incubated HLA-A2 and TARP positive MCF7 and LNCaP cells with increasing concentrations of the D2 Fab-fusion protein and tested protein synthesis by measuring incorporation of [3H]-leucine into cellular proteins as a well established measure to the cytotoxic activity of immunotoxins [30]. As controls we used HLA-A2 and TARP negative PC3 cells. As shown in Figure 7A, the D2 Fab-PE38 fusion inhibited in a dose dependent manner protein synthesis and was cytotoxic to MCF-7 and LNCaP cells but not to control PC3 cells. The cytotoxic activity correlated the reactivity of the D2 TCR-like Fab; MCF7 cells which react well with the antibody were killed more efficiently (IC50 of 150 ng/ml) compared to LNCaP cells (IC50 of 400 ng/ml) which exhibited low but specific reactivity with the D2 Fab. Control PC3 cells were not affected also at high concentrations of fusion protein as high as 10μg/ml.
Figure 7. Cytotoxic activity of D2 Fab-PE38 fusion.
Inhibition of protein synthesis in tumor cells by D2 Fab-PE38 fusions. TARP positive and control tumor cells were incubated for 20 hrs with increasing concentrations of D2 Fab-PE38 (A) or D2 Fab-PE38KDEL (B). Protein synthesis was determined by incorporation of 3H-Leucine into cellular proteins for 4 hrs.
We also tested a version of PE38 in which the C-terminal wild-type sequence REDLK was modified to KDEL, the most common ER retention sequence. This modification was shown previously to increase the cytotoxic activity of PE38-based fusion molecules [39]. As shown in Figure 7B, the cytotoxicity of the D2 Fab-PE38KDEL molecule was improved with an IC50 of 70 ng/ml on MCF7 cells and minor background on antigen negative PC3 and DU145 cells. Another measure for specificity of the D2Fab-PE38 fusion can be observed from the lack of cytotoxic activity on PC3-TARP cells which are stably transfected with the TARP gene but are HLA-A2 negative (Fig. 7B).
These results further demonstrate the fine and unique specificity of the D2 Fab antibody and its ability to serve as a targeting moiety to deliver a cytotoxic effector molecule with antigen (peptide)-specific, MHC-restricted specificity of T cells directed toward a human tumor T-cell epitope.
In vivo analysis of D2 Fab-PE38 in nude mice implanted with MCF-7
To establish the ability of the D2 Fab-PE38 to inhibit tumor growth in vivo, we implanted nude mice with MCF-7 tumor cells. MCF-7 tumor cells were mixed with either D2 Fab-PE38 or with buffer, and were injected s.c. As a control we used an immunotoxin generated from the TA2 Fab which is a TCR-like antibody directed to HLA-A2 in complex with peptide Tyr369-377 derived from the Tyrosinase melanoma differentiation antigen. This Fab was characterized in detail as was show to exhibit similar binding affinity to the D2 Fab and a characteristic TCR-like property against HLA-A2/ Tyrosinase369-377 complexes (data not shown). As shown in Figure 8, tumors began to appear in the buffer control-treated mice as well as the TA2-PE38 immunotoxin control mice between 18 and 23 days (week 4) after implantation, while none were evident in any of the mice treated with D2 Fab-PE38. Tumors continued to appear and expand in the control mice until day 33 (week 4 tumor volume = 80 mm3; week 10 tumor volume = 650mm3). Final scoring was evaluated on day 33, 20 days after the appearance of the last tumor in the control mice group. At day 33, all (7/7 in each group) mice in the buffer-treated and the TA2-PE38 immunotoxin control groups had developed tumors, while none of the eight mice in the group treated with the D2 Fab-PE38 showed evidence of tumor growth.
Figure 8. D2 Fab-PE38 prevents tumor growth in athymic nude mice.
Athymic mice were injected subcutaneously with 8 × 106 MCF-7 cells diluted in 0.2 ml PBS containing 1μg D2 Fab-PE38. Control athymic mice were injected subcutaneously with 8 × 106 MCF-7 cells diluted in 0.2 ml PBS only or with 1μg TA2 Fab-PE38 which targets Tyrosinase/HLA-A2 pMHC complexes. Tumor growth was observed in mice treated with PBS (◇) or control immunotoxin TA2 Fab-PE38 (▲) at wk 4, the tumor volume had increased >8-fold. In contrast, no tumor growth was observed in mice treated with the D2 Fab-PE38 (■). Tumors were monitored, and final scoring was evaluated at 33 days after implant.
3. Discussion
Identification and characterization of antigen-specific T lymphocytes during the course of an immune response was tedious and indirect until the advent in the application of recombinant class I MHC-peptide complexes and their tetrameric arrays, which enable detection of rare populations of antigen specific T cells [40,41]. Until recently, there was no tool that enabled phenotypic analysis of antigen (MHC-peptide) presentation, the other player in the cognate interaction between TCRs and their ligands. Using such tools fundamental questions in tumor immunology regarding antigen presentation could be addressed. One way to generate such molecules is by making TCR-like antibodies. In contrast to the inherent low affinity and stability of recombinant soluble TCRs, these molecules display the high affinity binding characteristics of antibodies, while retaining TCR specificity. Such antibodies are, owing to their soluble nature and their high affinity and stability, well suited for detecting and directly visualizing the presence of specific T-cell epitopes or MHC-peptide complexes by standard methods of flow cytometry and therefore should be very useful for the study and analysis of antigen presentation in cancer by determining the expression of specific tumor-related MHC-peptide complexes on the surface of tumor cells, metastasis, antigen-presenting cells, and lymphoid cells [42-45].
Moreover, antibodies which are specific for peptides presented in complex with MHC class I molecules can be conjugated or genetically fused to toxins or drugs and used to mimic T-cell responses eradicating tumor or virus-infected cells where T cells had failed to do so. Alternatively, anti-peptide-MHC antibodies may block inappropriate immune T-cell responses such as those leading to autoimmunity [46].
Antibodies to MHC-peptide complexes were very difficult to isolate because they require B cell immune response mounted toward a self antigen. There were several instances in which such molecules were isolated by hybridoma technology to murine MHC-peptide complexes [46-50] but not against human complexes.
However, in the last 2-3 years we and others utilized the selection power of the phage display approach [51-54] together with the availability of very large human naïve antibody libraries. These enabled us to screen these large libraries of such vast diversity and size in order to isolate these rare and unique TCR-like recombinant antibodies targeting human tumor and viral epitopes [21,22,24-26].
In this study we isolated TCR-like antibodies directed toward the TCRγ; alternative reading frame protein (TARP). TARP is a newly found protein expressed in breast and prostate cancers [16,55]. It was reported that human CD8 T cells from a prostate cancer patient specific for peptides derived from TARP, kill a human breast cancer cell line [17].
With the aim of mimicking this T-cell specificity by generating recombinant antibodies to TARP/HLA-A2 complexes we generated a new tool to study antigen presentation on TARP positive tumor cells as well as on APCs. We have shown that the TCR-like antibody Fab D2 can specifically bind purified TARP/HLA-A2 complexes but not other control complexes presenting tumor or viral-derived HLA-A2 restricted peptides. D2 was able to recognize specifically TARP/HLA-A2 complexes on the surface of peptide-pulsed cells as well as APCs that were transfected with the TARP gene and are making surface TARP/HLA-A2 complexes by naturally occurring active intracellular processing. Moreover, D2 was used to detect the TARP/HLA-A2 complex on the surface of tumor cells.
Interestingly, the TCR-like D2 Fab exhibited a very specific reactivity pattern with enhanced epitopes generated from the wild-type TARP29-37 peptide. Positions 2 and 9 in MHC binding peptides, termed anchor sites, react with specific sites in the groove of HLA. These positions play a major role in determining the binding of the peptide to the MHC molecule. [56-63]
Modification of amino acids which are different from the consensus residues in anchor positions is a known strategy to improve the affinity of tumor-derived T-cell epitopes to MHC. In many cases, T-cells generated to enhanced modified epitopes recognize the wild type peptide as well, but this does not exclude the possibility that alteration in the MHC-peptide conformation may occur as a result of such modifications.
We have shown in this study that the TCR-like antibody selected toward the TARP 29-37 peptide was able to bind only the HLA-A2 complexes displaying the wild type TARP 29-37 or the modified TARP 29-37 -9V peptide but not the TARP 29-37-3A modified peptide. These results are similar to our previous studies in which TCR-like antibodies selected toward melanoma gp100-derived peptides and their enhanced epitopes did not exhibit the same reactivity pattern [63]. These results indicate that modification in anchor positions or positions that affect peptide binding stability such as position 3, which is not considered a TCR contact residue, can influence the conformation of HLA-A2-peptide complex. Thus, substitutions in anchor residues or side anchor position can alter the overall conformation of the peptide-MHC complex and thereby the recognition by the cellular immune system.
An interesting question is how modifications in anchor or side anchor positions can influence reactivity of T cells. T cells stimulated using altered peptide ligands are not necessarily the same population of T cells that efficiently recognize the native complex expressed on the cell surface. As shown in Oh et al [17] , human CD8+ CTL raised with TARP 29-37 peptide could recognize and lyse target cells pulsed with the wild type as well as the two enhanced epitopes (TARP 29-37-3A and TARP 29-37-9V), while our D2 Fab Ab could only recognize the TARP 29-37-9V /MHC complex but not the TARP 29-37 3A/MHC complex. In the TARP29-37 modified peptide position 9 was changed from Leucine to Valine, both of them are aliphatic amino acids which have similar structure, however, position 3 was modified from Arginine which is a hydrophilic amino acid to Alanine, a hydrophobic amino acid. These types of alteration may explain the different features in the reactivity of the D2 Fab antibody with relation to the structure generated in the MHC-peptide complex.
Interestingly, recent studies demonstrated changes in the fine specificity of gp100-derived 209-reactive T cells in some patients, following their vaccination with the 209M-modified peptide [64]. Moreover, it was shown that some PBMC-derived cloids isolated from these patients recognize only the G9-209M-modified peptide but not the native one [64]. These results suggested that a modification at a MHC anchor position may influence the overall conformation of the MHC-peptide complex groove and that T cells may sometimes recognize these differences.
These findings may indicate that the TCR-like Fab antibody has a more exclusive fine specificity than certain TCRs have with regard to MHC/peptide complex recognition. Structural studies performed with TCR-like antibodies may have important implications in cancer immunotherapy because most peptides studied are of low affinity and therefore modifications are used to design peptides with increased affinity for class I MHC to enhance CTL stimulation.[65,66]
To demonstrate the ability of the D2 Fab TCR-like antibody to serve as a targeting moiety for antibody-based approaches we fused the D2 gene to a potent truncated form of Pseudomonas exotoxin (PE38) and tested the cytotoxic activity on TARP positive and negative cancer cells. We have shown that the cytotoxic effect of the D2 Fab-PE38 or D2 Fab-PE38KDEL fusion protein correlated with TARP and HLA-A2 expression. In the fusion with PE38KDEL the cytotoxic activity was improved as shown previously, because the C-terminal sequence contains the common consensus ER retention signal. These results further indicate the fine specificity of the TCR-like antibody and suggest the possibility that TCR-like antibody may be utilized as an immunotherapeutic modality that can mimic the fine specificity of T cells in instances when T cells fail to do so such as in cancer. In this study we have shown the in vivo activity of the TCR-like-Toxin fusion molecule in a very simplified model when tumor cells and immunotoxin were mixed and injected into immuno-deficient mice. This model was selected because of the limited properties of the current Fab-toxin fusion which possess a relative low affinity. The real test for such molecules after their optimization (for example: affinity maturation) would be their ability, when given systemically, to affect the growth of an established tumor.
TCR-like antibodies represent our ability to transform the unique fine specificity but low intrinsic affinity of TCRs into high affinity soluble antibody molecules endowed with a TCR-like specificity toward human tumor or viral epitopes. These molecules may prove to be crucial useful tools for studying MHC class I antigen presentation in health and disease as well as for therapeutic purposes in cancer, infectious diseases, and autoimmune disorders. This approach combines the best two features of our immune response; the unique specificity of T cell with the high affinity and specificity properties of antibodies. Like killer T-cells, TCR-like antibodies will recognize diseased cells which express specific disease related MHC-peptide complexes derived from intracellular proteins, however they possess a higher affinity, and are not inhibited by the body's auto-regulatory processes that govern the activity of T cells.
4. Materials and Methods
2.1 Production of Biotinylated scMHC/peptide Complexes
Single-chain MHC/peptide complexes were produced by in vitro refolding of inclusion bodies produced in E. coli, as described previously [35, 36]. Biotinylation was performed using the BirA enzyme (Avidity, Denver, CO) as previously described [67]
2.2 Selection of Phage-Antibodies on Biotinylated Complexes
A large human Fab library containing 3.7 × 1010 different Fab clones was used for the selection [38]. Phage (1013) was first preincubated for 1 hr at room temperature in PBS containing 2% nonfat dry milk with streptavidin–coated paramagnetic beads (200 μl; Dynal, Oslo) to deplete streptavidin binders. Streptavidin–coated paramagnetic beads (200 μl; Dynal, Oslo) were also incubated in PBS+2% milk for 1 hr at room temperature. The remaining phages were subsequently incubated for 1 hr with decreasing amounts of biotinylated scMHC-peptide complexes. Streptavidin magnetic beads were added, and the mixture was incubated for 15 min with continuous rotation. A magnetic force was applied to pull down phages bound to biotinylated complexes. After 10 washes of the streptavidin-bound complexes with PBS/0.1% Tween and 2 washes with PBS, bound phages were eluted by incubation for 7 min with 1 ml of Triethylamine (TEA) (100mM). The elusion mixture was neutralized by the addition of 100 μl of Tris-HCl (1M, pH 7.4) and used to infect E. coli TG1 cells (OD600=0.5) for 30 min at 37°C.
Selected phages were rescued using M13KO7 helper phage (5x1011 cfu).
The diversity of the selected antibodies was determined by DNA fingerprinting. The Fab DNA of different clones was PCR-amplified using the primers pUC-reverse (5′-AGCGGATAACAATTTCACACAGG-3′) and fd-tet-seq24 (5′-TTTGTCGTCTTTCCAGACGTTAGT-3′). The resulting PCR fragments were digested with BstNI (NEB) (2 hr, 37 °C) and analyzed by agarose gel electrophoresis.
2.3 Expression and purification of soluble recombinant Fab antibodies
Soluble Fabs were purified from the periplasmic fraction of E.Coli BL21 cells using the hexahistidine tag fused to the CH1 domain of the Fabs (as described [37]). The homogeneity and purity of the purified Fabs were determined by analysis on non-reduced and reduced SDS-PAGE.
2.4 ELISA with purified Fab antibodies
The binding specificity of individual soluble Fab fragments was determined by ELISA using biotinylated scMHC-peptide complexes. ELISA plates (Falcon) were coated overnight with BSA-biotin (1μg/well). After having been washed, the plates were incubated (1 hr, RT) with streptavidin (1μg/well), washed extensively and further incubated (1 hr, RT) with 0.5 μg of MHC/peptide complexes. Plates were blocked for 30 min at RT with PBS/2% BSA and subsequently were incubated for 1 hr at RT with various concentrations of soluble purified Fab, and after washing, with 1:1000 HRP-conjugated/anti-human antibody. Detection was performed using TMB reagent (Sigma). The HLA-A2-restricted peptides used for specificity studies of the purified Fab antibodies are: the Cytomegalovirus(CMV)- derived peptide: NLVPMVATV, the Human T cell lymphotropic virus type 1 (HTLV-1)-derived peptide TAX: LLFGYPVYV, hTERT (540): ILAKFLHWL, gp100 derived peptides G9-209-2M: IMDQVPFSV and G9-280 :YLEPGPVTA, MART1 :AAGIGILTV and the Human Immunodeficiency Virus(HIV)-derived peptide Pol: ILKEPVHGV
2.4 Flow Cytometry
The EBV-transformed B-lymphoblast JY cells, the B cell line RMAS-HHD transfected with a single-chain β2M-HLA-A2 gene [68] or tumor cells were used to determine the reactivity of the recombinant Fab antibodies with cell surface-expressed HLA-A2/peptide complexes. About 106 JY cells were washed with serum-free RPMI and incubated overnight at 37°C in medium containing 100μM of the peptide. RMAS-HHD cells were washed with serum-free RPMI and incubated overnight at 26°C in medium containing 100μM peptide and were subsequently transfered to 37°C for two hours. The cells were incubated for 60-90 min at 4°C with recombinant Fab antibodies (50-150μg/ml) in 100μl. Cells were washed three times and incubated with FITC-labeled anti-human Fab or with PE- labeled anti-human Fab (Jackson). Adherent tumor cells were harvested by trypsinization and resuspended in cold RPMI. All subsequent washes and incubations were performed in ice-cold PBS as described above for JY and RMAS-HHD peptide-loaded cells.
Titrations of D2 Fab were on JY cells pulsed with 100μM peptide or at different concentrations of Fab. To determine minimal number of sites recognized by the recombinant Fab JY APCs were pulsed with various concentrations of the TARP29-37 peptide, and the binding of the PE-labeled D2 Fab was analyzed by flow cytometry. The level of fluorescence intensity on stained cells was compared with the fluorescence intensities of calibration beads with known numbers of PE molecules per bead (QuantiBRITE PE beads; BD Biosciences), providing a mean of quantifying PE-stained cells using a flow cytometry.
Anti-HLA antibodies BB7.2 and W6/32 are from the ATCC (American Type Culture Collection)[69], Analysis of the cells was performed by a FACStar flow cytometer (Becton Dickinson) and the results were analyzed with the WinMDI program (Trotter J., http://facs.scripps.edu/).
2.5 Expression and purification of Fab-PE38 fusion protein
The genes encoding the light and heavy chain of Fab D2 were cloned separately into a T7-promotor pET-based expression vector. The heavy chain gene was engineered to contain the PE38 recognition sequence at the COOH terminus (D2 heavy-PE38). These constructs were expressed separately in E. coli BL21 cells and upon induction with IPTG, intracellular inclusion bodies which contain large amounts of the recombinant protein accumulated. Inclusion bodies of both chains were purified, reduced, and subsequently refolded at a heavyPE38: light 2.5:1 ratio respectively in a redox-shuffling buffer system containing 0.1 M Tris, 0.5 M Arginine, 0.09 mM Oxidized Glutathione (pH 7.4). Correctly folded Fab was then isolated and purified by ion-exchange chromatography Sepharose and MonoQ (Pharmacia).
2.6 Cytotoxicity assays
Tumor cells were incubated with increasing concentrations of D2Fab-PE38 and the inhibition of protein synthesis was determined by measuring the uptake of 3H-Leucine into cellular proteins, as previously described [70]. IC50 was determined as the concentration of D2Fab -PE38 required to inhibit protein synthesis by 50%.
2.7 In vivo studies
Athymic nude mice, 4 weeks old, were obtained from Harlan Sprague Dawley and housed under sterile conditions. 16 mice were injected subcutaneously with 17β-estradiol 60-day release pellets (0.72mg/pellet, innovative Research of America, Sarasota, Florida), 24h before tumor injection. 8 mice were implanted with 8 × 106 (n=8) freshly harvested MCF-7 cells. MCF-7 tumor cells were mixed with either 1μg D2 Fab-PE38 diluted in PBS or 1μg TA2 Fab-PE38 diluted in PBS or only PBS, and were immediately injected s.c. Animals were kept for at least 2 weeks after the appearance of the last tumor in the control group (a total of 33 days).
5. Acknowledgments
This work was funded by The Israel Science Foundation (Y.R.)
REFERENCES
- 1.Saffran DC, Reiter RE, Jakobovits A, Witte ON. Target antigens for prostate cancer immunotherapy. Cancer Metastasis Rev. 1999;18:437–449. doi: 10.1023/a:1006333222424. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty NG, Stevens RL, Mehrotra S, Laska E, Taxel P, Sporn JR, Schauer P, et al. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol.Immunother. 2003;52:497–505. doi: 10.1007/s00262-003-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroers R, Shen L, Rollins L, Xiao Z, Sonderstrup G, Slawin K, Huang XF, et al. Identification of MHC class II-restricted T-cell epitopes in prostate-specific membrane antigen. Clin.Cancer Res. 2003;9:3260–3271. [PubMed] [Google Scholar]
- 4.Dannull J, Diener PA, Prikler L, Furstenberger G, Cerny T, Schmid U, Ackermann DK, et al. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res. 2000;60:5522–5528. [PubMed] [Google Scholar]
- 5.Kiessling A, Schmitz M, Stevanovic S, Weigle B, Holig K, Fussel M, Fussel S, et al. Prostate stem cell antigen: Identification of immunogenic peptides and assessment of reactive CD8+ T cells in prostate cancer patients. Int.J.Cancer. 2002;102:390–397. doi: 10.1002/ijc.10713. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Takaue Y, Takei M, Kato K, Kanai S, Harada Y, Tobisu K, et al. Induction of tumor specific cytotoxic T lymphocytes in prostate cancer using prostatic acid phosphatase derived HLA-A2402 binding peptide. J.Urol. 2001;166:1508–1513. [PubMed] [Google Scholar]
- 7.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, Itoh K, et al. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003;57:80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, et al. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39:54–59. doi: 10.1002/(sici)1097-0045(19990401)39:1<54::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Murphy GP, Tjoa BA, Simmons SJ, Jarisch J, Bowes VA, Ragde H, Rogers M, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate. 1999;38:73–78. doi: 10.1002/(sici)1097-0045(19990101)38:1<73::aid-pros9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br.J.Cancer. 2004;91:688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J.Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 12.Brinkmann U, Vasmatzis G, Lee B, Yerushalmi N, Essand M, Pastan I. PAGE-1, an X chromosome-linked GAGE-like gene that is expressed in normal and neoplastic prostate, testis, and uterus. Proc.Natl.Acad.Sci.U.S.A. 1998;95:10757–10762. doi: 10.1073/pnas.95.18.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson P, Bera TK, Essand M, Kumar V, Duray P, Vincent J, Lee B, Pastan I. GDEP, a new gene differentially expressed in normal prostate and prostate cancer. Prostate. 2001;48:231–241. doi: 10.1002/pros.1102. [DOI] [PubMed] [Google Scholar]
- 14.Bera TK, Iavarone C, Kumar V, Lee S, Lee B, Pastan I. MRP9, an unusual truncated member of the ABC transporter superfamily, is highly expressed in breast cancer. Proc.Natl.Acad.Sci.U.S.A. 2002;99:6997–7002. doi: 10.1073/pnas.102187299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essand M, Vasmatzis G, Brinkmann U, Duray P, Lee B, Pastan I. High expression of a specific T-cell receptor gamma transcript in epithelial cells of the prostate. Proc.Natl.Acad.Sci.U.S.A. 1999;96:9287–9292. doi: 10.1073/pnas.96.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfgang CD, Essand M, Vincent JJ, Lee B, Pastan I. TARP: a nuclear protein expressed in prostate and breast cancer cells derived from an alternate reading frame of the T cell receptor gamma chain locus. Proc.Natl.Acad.Sci.U.S.A. 2000;97:9437–9442. doi: 10.1073/pnas.160270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh S, Terabe M, Pendleton CD, Bhattacharyya A, Bera TK, Epel M, Reiter Y, et al. Human CTLs to wild-type and enhanced epitopes of a novel prostate and breast tumor-associated protein, TARP, lyse human breast cancer cells. Cancer Res. 2004;64:2610–2618. doi: 10.1158/0008-5472.can-03-2183. [DOI] [PubMed] [Google Scholar]
- 18.Wulfing C, Pluckthun A. Correctly folded T-cell receptor fragments in the periplasm of Escherichia coli. Influence of folding catalysts. J.Mol.Biol. 1994;242:655–669. doi: 10.1006/jmbi.1994.1615. [DOI] [PubMed] [Google Scholar]
- 19.Corr M, Slanetz AE, Boyd LF, Jelonek MT, Khilko S, al Ramadi BK, Kim YS, et al. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- 20.Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc.Natl.Acad.Sci.U.S.A. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen CJ, Denkberg G, Lev A, Epel M, Reiter Y. Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR-peptide-MHC interactions. J.Mol.Recognit. 2003;16:324–332. doi: 10.1002/jmr.640. [DOI] [PubMed] [Google Scholar]
- 22.Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y. Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies. J.Immunol. 2003;170:4349–4361. doi: 10.4049/jimmunol.170.8.4349. [DOI] [PubMed] [Google Scholar]
- 23.Cohen CJ, Hoffmann N, Farago M, Hoogenboom HR, Eisenbach L, Reiter Y. Direct detection and quantitation of a distinct T-cell epitope derived from tumor-specific epithelial cell-associated mucin using human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Cancer Res. 2002;62:5835–5844. [PubMed] [Google Scholar]
- 24.Denkberg G, Cohen CJ, Lev A, Chames P, Hoogenboom HR, Reiter Y. Direct visualization of distinct T cell epitopes derived from a melanoma tumor-associated antigen by using human recombinant antibodies with MHC- restricted T cell receptor-like specificity. Proc.Natl.Acad.Sci.U.S.A. 2002;99:9421–9426. doi: 10.1073/pnas.132285699. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Held G, Matsuo M, Epel M, Gnjatic S, Ritter G, Lee SY, Tai TY, et al. Dissecting cytotoxic T cell responses towards the NY-ESO-1 protein by peptide/MHC-specific antibody fragments. Eur.J.Immunol. 2004;34:2919–2929. doi: 10.1002/eji.200425297. [DOI] [PubMed] [Google Scholar]
- 26.Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P, Hoogenboom HR, Reiter Y. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–3194. [PubMed] [Google Scholar]
- 27.Niv R, Cohen CJ, Denkberg G, Segal D, Reiter Y. Antibody engineering for targeted therapy of cancer: recombinant Fv-immunotoxins. Curr.Pharm.Biotechnol. 2001;2:19–46. doi: 10.2174/1389201013378824. [DOI] [PubMed] [Google Scholar]
- 28.Reiter Y, Brinkmann U, Webber KO, Jung SH, Lee B, Pastan I. Engineering interchain disulfide bonds into conserved framework regions of Fv fragments: improved biochemical characteristics of recombinant immunotoxins containing disulfide-stabilized Fv. Protein Eng. 1994;7:697–704. doi: 10.1093/protein/7.5.697. [DOI] [PubMed] [Google Scholar]
- 29.Benhar I, Pastan I. Cloning, expression and characterization of the Fv fragments of the anti-carbohydrate mAbs B1 and B5 as single-chain immunotoxins. Protein Eng. 1994;7:1509–1515. doi: 10.1093/protein/7.12.1509. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann U, Pastan I. Immunotoxins against cancer. Biochim.Biophys.Acta. 1994;1198:27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin.Ther. 1999;21:309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 32.Lode HN, Reisfeld RA. Targeted cytokines for cancer immunotherapy. Immunol.Res. 2000;21:279–288. doi: 10.1385/IR:21:2-3:279. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka A, Ishida M, Murakami S, Ohno S. Sensitization of chemotherapy by anti-HER. Breast Cancer. 2004;11:105–115. doi: 10.1007/BF02968288. [DOI] [PubMed] [Google Scholar]
- 34.Pastan I, Kreitman RJ. Immunotoxins in cancer therapy. Curr.Opin.Investig.Drugs. 2002;3:1089–1091. [PubMed] [Google Scholar]
- 35.Denkberg G, Cohen CJ, Segal D, Kirkin AF, Reiter Y. Recombinant human single-chain MHC-peptide complexes made from E. coli By in vitro refolding: functional single-chain MHC-peptide complexes and tetramers with tumor associated antigens. Eur.J.Immunol. 2000;30:3522–3532. doi: 10.1002/1521-4141(2000012)30:12<3522::AID-IMMU3522>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 36.Denkberg G, Cohen CJ, Reiter Y. Critical role for CD8 in binding of MHC tetramers to TCR: CD8 antibodies block specific binding of human tumor-specific MHC-peptide tetramers to TCR. J.Immunol. 2001;167:270–276. doi: 10.4049/jimmunol.167.1.270. [DOI] [PubMed] [Google Scholar]
- 37.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, de Bruine AP, et al. large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J.Biol.Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 38.Pastan I. Targeted therapy of cancer with recombinant immunotoxins. Biochim.Biophys.Acta. 1997;1333:C1–C6. doi: 10.1016/s0304-419x(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 39.Kreitman RJ, Pastan I. Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem.J. 1995;307(Pt 1):29–37. doi: 10.1042/bj3070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat.Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 41.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 42.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J.Exp.Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol.Today. 2000;21:455–464. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 44.Esche C, Shurin MR, Lotze MT. The use of dendritic cells for cancer vaccination. Curr.Opin.Mol.Ther. 1999;1:72–81. [PubMed] [Google Scholar]
- 45.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat.Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 46.Aharoni R, Teitelbaum D, Arnon R, Puri J. Immunomodulation of experimental allergic encephalomyelitis by antibodies to the antigen-Ia complex. Nature. 1991;351:147–150. doi: 10.1038/351147a0. [DOI] [PubMed] [Google Scholar]
- 47.Stryhn A, Andersen PS, Pedersen LO, Svejgaard A, Holm A, Thorpe CJ, Fugger L, et al. Shared fine specificity between T-cell receptors and an antibody recognizing a peptide/major histocompatibility class I complex. Proc.Natl.Acad.Sci.U.S.A. 1996;93:10338–10342. doi: 10.1073/pnas.93.19.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krogsgaard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J, Ditzel H, et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85-99 complex. J.Exp.Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc.Natl.Acad.Sci.U.S.A. 1996;93:1820–1824. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 51.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 52.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu.Rev.Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 53.Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 54.Barbas CF, III, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc.Natl.Acad.Sci.U.S.A. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolfgang CD, Essand M, Lee B, Pastan I. T-cell receptor gamma chain alternate reading frame protein (TARP) expression in prostate cancer cells leads to an increased growth rate and induction of caveolins and amphiregulin. Cancer Res. 2001;61:8122–8126. [PubMed] [Google Scholar]
- 56.Williams A, Peh CA, Elliott T. The cell biology of MHC class I antigen presentation. Tissue Antigens. 2002;59:3–17. doi: 10.1034/j.1399-0039.2002.590103.x. [DOI] [PubMed] [Google Scholar]
- 57.Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr.Opin.Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 58.Rammensee HG, Falk K, Rotzschke O. Peptides naturally presented by MHC class I molecules. Annu.Rev.Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 59.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu.Rev.Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 60.Matsumura M, Fremont DH, Peterson PA, Wilson IA. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992;257:927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- 61.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu.Rev.Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 62.Parker KC, Bednarek MA, Hull LK, Utz U, Cunningham B, Zweerink HJ, Biddison, et al. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J.Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 63.Denkberg G, Klechevsky E, Reiter Y. Modification of a tumor-derived peptide at an HLA-A2 anchor residue can alter the conformation of the MHC-peptide complex: probing with TCR-like recombinant antibodies. J.Immunol. 2002;169:4399–4407. doi: 10.4049/jimmunol.169.8.4399. [DOI] [PubMed] [Google Scholar]
- 64.Clay TM, Custer MC, McKee MD, Parkhurst M, Robbins PF, Kerstann K, Wunderlich J, et al. Changes in the fine specificity of gp100(209-217)-reactive T cells in patients following vaccination with a peptide modified at an HLA-A2.1 anchor residue. J.Immunol. 1999;162:1749–1755. [PubMed] [Google Scholar]
- 65.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 66.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat.Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 68.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J.Exp.Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- 70.Brinkmann U, Pai LH, FitzGerald DJ, Willingham M, Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc.Natl.Acad.Sci.U.S.A. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]