Abstract

A new class of one-component Pd precatalysts bearing biarylphosphine ligands is described. These precatalysts are air- and thermally-stable, are easily activated under normal reaction conditions at or below room temperature, and ensure the formation of the highly active mono-ligated Pd(0) complex necessary for oxidative addition. The use of these precatalysts as a convenient source of LPd(0) in C-N cross-coupling reactions is explored. The reactivity that is demonstrated in this study is unprecedented in palladium chemistry.
Although phosphine-ligated Pd(0) complexes constitute the active catalysts in many C–N bond-forming cross-coupling methodologies,1,2 such complexes are usually difficult to prepare and extremely air-sensitive. Pd2(dba)3, developed as a stable source of Pd(0), includes coordinating dba ligands that can significantly retard the formation of active catalyst and/or diminish its reactivity.3 The use of a Pd(II) salt such as Pd(OAc)2, which circumvents problems of precatalyst instability, requires in situ reduction in order to generate the active Pd(0) complex. In light of the complications in forming phosphine-ligated Pd(0) complexes, we sought to develop a precatalyst scaffold constituting the source of Pd and phosphine ligand, which could form the active, mono-ligated Pd complex under mild conditions and without the need for exogenous additives.4 Herein, we report the development of a new class of air- and moisture-stable, one-component, Pd precatalysts that is activated under standard reaction conditions and ensures the formation of the active, L1Pd(0) (L = biarylphosphine) complex. We also demonstrate these precatalysts to be convenient Pd sources for facile C–N bond-forming reactions. Finally, we show the efficient oxidative addition of PhCl to a LPd(0) complex at −40 °C.

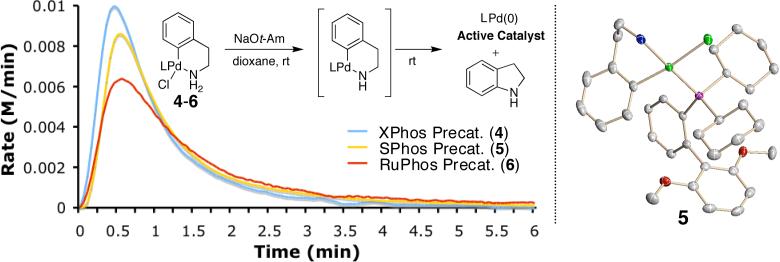
Our group has recently reported the isolation of a phosphineligated Pd(II) complex bearing a free amine.5 Building on this result, we proposed that an intramolecularly coordinated amine complex would provide an stable, mono-ligated Pd precatalyst. Precatalysts bearing ligands 1,6a 2,6b and 3,6c (4, 5 and 6, respectively) were prepared in excellent yields via the route illustrated in Figure 1. Yields of >85% were obtained for each step of this sequence without the need for a glovebox and using only recrystallization for purification. The X-ray crystal structure of 5 is shown in Figure 2. Calorimetric analysis (Figure 2) shows that activation of 4-6 is complete after ca. 3 minutes when the complexes are treated with NaOt-Am in dioxane at rt. In general, this activation process occurs readily with weak bases (e.g., K2CO3) at 80 °C, with alkoxide bases at room temperature, and with HMDS bases at −20 °C, as judged by 31P NMR.
Figure 1.
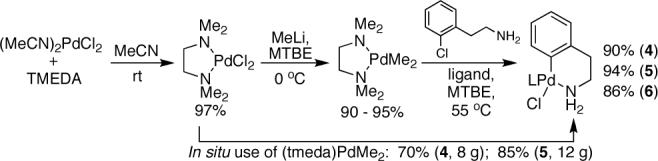
Synthesis of precatalysts 4-6.
Figure 2.
Calorimetric scans of the addition of precatalysts 4-6 to NaOt-Am in dioxane at room temperature; X-ray crystal structure of 5.
Figure 3 shows the progress of cross-coupling reactions of 4-chloroanisole and aniline using 0.1% Pd at 80 °C employing ligand 1 with different Pd sources and different methods of activation. The use of Pd(OAc)2/PhB(OH)2,6a [Pd(allyl)Cl]2, and Pd2dba3 as the Pd source effects a maximum conversion of ca. 25% before loss of catalytic activity. However, the use of 4 allows complete conversion to be achieved in 35 min. These results demonstrate the limitations of Pd(II) activation methods and the deleterious nature of the dba ligand in cross-coupling reactions. Using precatalyst 4, a highly active, L1Pd(0) complex is readily accessed in the absence of potentially inhibitory complexing ligands and without the need for exogenous additives.
Figure 3.

Effect of Pd source on the cross-coupling of aniline and 4-chloroanisole using 0.1% Pd and ligand 1 at 80 °C.
Because of their low nucleophilicity, electron-deficient anilines are typically difficult substrates to employ in C–N cross-coupling reactions. Using 4, numerous highly electron-deficient anilines were successfully coupled with unactivated aryl chlorides in excellent yields (Table 1). 2-NO2, 2-CO2Et, 4-CF3, and 4-CN anilines had not been previously employed in C–N cross-coupling reactions involving aryl chlorides. Further, we have achieved the first C–N cross-coupling reactions of anilines with aryl chlorides bearing unprotected aldehydes. The success of these reactions reveals the advantages of using a highly reactive Pd source in C–N cross-coupling reactions.
Table 1.
Cross-Coupling Reactions of Electron-Deficient Anilines with ArCl Using a Weak Base.a
 |
 |
 |
 |
 |
 |
ArCl (1 mmol), amine (1.2 mmol), K2CO3 (1.4 mmol); average isolated yields of 2 runs.
b) 2 h reaction time,
c) using precatalyst 6.
The ability to achieve high yields from cross-coupling reactions using low catalyst loadings with short reaction times is of great synthetic importance. Table 2 shows the use of 4 in several C–N cross-coupling reactions of unactivated aryl chlorides with anilines using 0.1 mol% Pd. Few examples of C–N cross- coupling reactions using anilines have been previously demonstrated using catalyst loadings of less than 1 mol% Pd. The products displayed in Table 3 are each obtained in excellent yield in fewer than 10 min despite the use of such low catalyst loadings.
Table 2.
Rapid C–N Bond-Forming Reactions with 0.1% Catalyst.a
 |
 |
 |
ArCl (1 mmol), amine (1.2 mmol), base (1.2 mmol); average isolated yields of 2 runs.
Table 3.
C–N Bond-Forming Reactions Using ArCl at or below Room Temperaturea
 |
 |
 |
 |
 |
 |
ArCl (1 mmol), amine (1.2 mmol), base (1.2 mmol); average yield of 2 runs.
b) LHMDS (2.4 mmol)
c) NaOt-Am (1.02 mmol). d) in DME.
To show the ease with which 4-6 can be activated, we performed numerous C–N cross-coupling reactions at or below room temperature (Table 3). These precatalysts are particularly useful for substrate combinations that are incompatible with elevated reaction temperatures. Previously, 4 days were required to achieve a 61% yield in the formation of the coupling product of dibutylamine and 3-chlorophenethyl alcohol.7 Using 4, we can now obtain an isolated yield of 94% in only 4 hours. Similarly, the less reactive 4-chlorophenethyl alcohol can now be successfully employed an analogous reaction. In addition, we have successfully demonstrated the compatibility of esters of secondary alcohols in room temperature cross-coupling reactions using an alkoxide base. Our ability to perform amination reactions of an aryl chloride at −10 °C further illustrates the ease with which these precatalysts undergo activation.
To demonstrate the reactivity of a L1Pd(0) complex in the absence of competitive coordinating ligands, we prepared the N-Me derivatives of precatalysts 5 and 4 (7 and 9, respectively).8 As shown in Figure 4, chlorobenzene undergoes facile oxidative addition to SPhosPd(0) generated by deprotonation of 7 at −40 °C.9 This suggests that aryl chlorides should be usable in low temperature C–C cross-coupling reactions using precatalysts 4-6. Previously, only aryl iodides have been employed in cross-coupling reactions conducted at such low temperatures.10
Figure 4.

31P{1H} NMR spectra of: (a) 7 in toluene/PhCl at −40 °C and (b) 7 in toluene/PhCl w/ LHMDS (1 equiv.) after 110 min at −40 °C.
The ability to generate L1Pd(0) complexes in the absence of competing ligands is also very useful for mechanistic investigations. Precatalyst 9 was employed at room temperature to conduct a direct Hammett study of the oxidative addition of aryl chlorides to the XPhosPd(0) complex (see Supporting Info). The slope of ρ = +2.3 for Hammett's correlation is consistent with results previously obtained from (PPh3)2Pd(0) with aryl iodides, suggesting a concerted 3-centered transition state for oxidative addition.11 This is the first information of this type available for the reaction of aryl chlorides with monodentate ligands.12
In summary, we have developed a new class of Pd precatalysts bearing biarylphosphine ligands that are particularly useful in cases where a highly active Pd complex is required to promote a difficult cross-coupling reaction or where functional group instability requires the use of low temperatures. We have additionally demonstrated that an unactivated aryl chloride can undergo oxidative addition to SPhosPd(0) at temperatures as low as −40 °C. The use of these precatalysts should greatly expand the general scope of Pd-catalyzed cross-coupling reactions.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (NIH) for support (GM-058160). M. R. B. thanks the NIH for a postdoctoral fellowship (GM-F32-75685). We also thank Amgen, Merck, and Boehringer Ingelheim for unrestricted funds; and Dr. Timothy E. Barder for solving the X-ray structure of 5.
Footnotes
Supporting Information Available: Procedural, spectral, and crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.a Jiang L, Buchwald SL. In: Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. de Meijere A, Diederich F, editors. Wiley-VCH; Weinheim: 2004. [Google Scholar]; b Hartwig JF. Synlett. 2006:1283. [Google Scholar]
- 2.With NHC ligands: Marion N, Navarro O, Mei J, Stevens ED, Scott NM, Nolan SP. J. Am. Chem. Soc. 2006;128:4101. doi: 10.1021/ja057704z.
- 3.a Amatore C, Broeker G, Jutand A, Khalil F. J. Am. Chem. Soc. 1997;119:5176. [Google Scholar]; b Fairlamb IJS, Kapdl AR, Lee AF. Org. Lett. 2004;6:4435. doi: 10.1021/ol048413i. [DOI] [PubMed] [Google Scholar]
- 4.Pd precatalysts: Zim D, Buchwald SL. Org. Lett. 2003;5:2413. doi: 10.1021/ol034561h. (b) Ref. 2. Stambuli JP, Kuwano R, Hartwig JF. Angew. Chem. Int. Ed. 2002;41:4746. doi: 10.1002/anie.200290036.Andreu MG, Zapf A, Beller M. Chem. Commun. 2000:2475.Bedford RB, Cazin CSJ, Coles SJ, Gelbrich T, Horton PN, Hursthouse MB, Light ME. Organometallics. 2003;22:987.
- 5.Biscoe MR, Barder TE, Buchwald SL. Angew. Chem. Int. Ed. 2007;46:7232. doi: 10.1002/anie.200702122. [DOI] [PubMed] [Google Scholar]
- 6.a Huang X, Anderson KW, Zim D, Jiang L, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2003;125:6653. doi: 10.1021/ja035483w. [DOI] [PubMed] [Google Scholar]; b Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]; c Milne JE, Buchwald SL. J. Am. Chem. Soc. 2004;126:13028. doi: 10.1021/ja0474493. [DOI] [PubMed] [Google Scholar]
- 7.Harris MC, Huang X, Buchwald SL. Org. Lett. 2002;4:2885. doi: 10.1021/ol0262688. [DOI] [PubMed] [Google Scholar]
- 8.7 generates N-Me indoline as a byproduct, which will not undergo ensuing C–N bond-formation. Thus, formation of 8 can be monitored.
- 9.Complex 8 has been previously isolated by our group. See: Barder TE, Biscoe MR, Buchwald SL. Organometallics. 2007;26:2183.
- 10.Martin R, Buchwald SL. J. Am. Chem. Soc. 2007;129:3844. doi: 10.1021/ja070830d. [DOI] [PubMed] [Google Scholar]
- 11.a Amatore C, Pfluger F. Organometallics. 1990;9:2276. [Google Scholar]; b Jutand A, Mosleh A. Organometallics. 1995;14:1810. [Google Scholar]
- 12.The use of a bidentate ligand gave the higher correlation value of ρ = +5.2. See: Portnoy M, Milstein D. Organometallics. 1993;12:1665.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.