Abstract
The Klenow fragment of Escherichia coli DNA polymerase I houses catalytic centers for both polymerase and 3′–5′ exonuclease activities that are separated by about 35 Å. Upon the incorporation of a mismatched nucleotide, the primer terminus is transferred from the polymerase site to an exonuclease site designed for excision of the mismatched nucleotides. The structural comparison of the binary complexes of DNA polymerases in the polymerase and the exonuclease modes, together with a molecular modeling of the template strand overhang in Klenow fragment, indicated its binding in the region spanning residues 821–824. Since these residues are conserved in the “A” family DNA polymerases, we have designated this region as the RRRY motif. The alanine substitution of individual amino acid residues of this motif did not change the polymerase activity; however, the 3′–5′ exonuclease activity was reduced 2–29-fold, depending upon the site of mutation. The R821A and R822A/Y824A mutant enzymes showed maximum cleavage defect with single-stranded DNA, mainly due to a large decrease in the ssDNA binding affinity of these enzymes. Mismatch removal by these enzymes was only moderately affected. However, data from the exonuclease-polymerase balance assays with mismatched template-primer suggest that the mutant enzymes are defective in switching mismatched primer from the polymerase to the exonuclease site. Thus, the RRRY motif provides a binding track for substrate ssDNA and for nonsubstrate single-stranded template overhang, in a polarity-dependent manner. This binding then facilitates cleavage of the substrate at the exonuclease site.
The faithful synthesis of DNA by E. coli DNA polymerase I (pol I)2 is accomplished by a combination of two processes: (a) correctly incorporating matched nucleotides and (b) removing incorrectly incorporated mismatched nucleotides with its 3′–5′ exonuclease activity (1). The incorporation of a mismatched nucleotide on a growing primer strand stalls further DNA synthesis and decreases the affinity of the polymerase for the template-primer. This results in shuttling of the primer to the exonuclease active site (exo site). Thus, error correction by DNA polymerases that contain both the polymerase and the exonuclease activities involves at least the following four steps: (a) sensing of the misincorporated nucleotide at the polymerase site (pol site), (b) transfer of the mismatched primer from the pol to the exo site, (c) excision of the incorrect nucleotide, and (d) transfer of the corrected primer terminus to the pol site for further nucleotide incorporation. The mechanistic details of these steps are not fully understood. However, the availability of DNA polymerase structures, with DNA bound in either the polymerase or the exonuclease mode, provides some clues to the possible structural alterations of the polymerase protein during DNA synthesis.
The active site of DNA polymerases catalyzing the polymerase reaction has a configuration that allows non-sequence-specific contacts with both DNA and the incoming dNTP. It has been suggested that the hydrogen-bonding interactions of certain amino acid side chains in the minor groove of the template-primer govern the sequence-independent binding of DNA with the protein (2). These interactions most likely play a primary role in the ability of polymerase to distinguish between the matched and the mismatched DNA base pairs (3). In the case of matched base pairs, two hydrogen bond accepting groups (N3 in purines and O2 in pyrimidines) occupy a topologically similar position. This position, however, is clearly different in the case of mismatched DNA base pairs (2). The altered position of these two (N3 and O2) atoms most likely perturbs the interactions between protein side chains and DNA, leading to steric hindrance or a loss of hydrogen bond(s). This mechanism may be one of the several mispair-sensing mechanisms utilized by polymerases that result in the shuttling of the primer to the exo site, followed by excision of the mismatched nucleotide. Thus, efficient proofreading may require the presence of specific amino acid residues within the polymerase domain, which bind the primer strand and also govern its partitioning between the two sites. Using KF and T4 DNA polymerase, several attempts have been made to understand the mechanisms of mismatch sensing by the polymerases and switching of the primer moiety between the pol and 3′–5′ exo sites (4–8).
We have previously demonstrated that the amino acid residues of the J-helix (Gln677 in particular) participate in the partitioning of the primer moiety between the pol and exo sites (8). Thus, substitution of the C-terminal proline (Pro680) by a glycine significantly reduced polymerase activity and concurrently increased exonuclease activity (8). The effect of mutating Pro680 to Gly was eventually interpreted to be an indirect effect of altered conformation of the Gln677 side chain when it was found that mutation of Gln677 to Ala caused both increased 3′–5′ exonuclease activity and a failure of the primer to transfer from the exo to the pol site (9).
Although the mechanism of primer shuttling and the involvement of amino acid side chains of pol I in this process are beginning to unfold, the status of the template strand stabilization during this process is still unclear. The UV-mediated cross-linking of DNA with KF and identification of the cross-linking site within the protein have revealed the position of template overhang binding during the polymerase reaction (10, 11). However, the position of the template overhang when the primer is bound to the exo site is not known. In addition, it is well established that pol I cleaves ssDNA quite efficiently (1). However, the mode of binding of ssDNA during its exonucleolytic cleavage is also not known, although it is presumed to be the same as that of the primer strand of the mismatch-containing template primer duplex. It has also been established that KF melts about 4 base pairs prior to the transfer of a primer moiety from the pol to the exo site (12). In addition, it is known that the distance between the two active sites is around 30–35 Å (13). Therefore, mere melting of 4 terminal base pairs of the duplex DNA may not suffice for the successful localization of the primer terminus at the exo site. Thus, other conformational adjustments within the protein and/or DNA (such as the repositioning of the template strand) may be necessary to facilitate shuttling of the primer strand to the exo site.
In an attempt to identify the amino acid residues that bind (or stabilize) the template strand during mismatch removal, we used comparative molecular modeling and sequence alignment techniques. We identified a conserved amino acid sequence designated as the RRRY motif (residues 821–824 of pol I) as a potential candidate to interact with the template strand. The role of this motif was assessed by means of site-directed mutagenesis, followed by the biochemical characterization of the polymerase and the exonuclease activities of mutant enzymes. It was observed that the alanine substitutions for any of the four amino acid residues of RRRY motif did not alter the polymerase activity. However, the exonuclease activity of mutant enzymes was significantly reduced with ssDNA and was moderately affected with a terminally mismatched template-primer. The mutant enzymes R821A and R822A/Y824A were found to be the most affected with all DNA substrates. Based on these results, we propose the involvement of the RRRY motif in the binding of ssDNA and template overhang during the exonuclease reaction. Furthermore, the binding of ssDNA requires the participation of RRRY motif, most likely by recognizing the polarity element of the DNA substrate.
EXPERIMENTAL PROCEDURES
Molecular Modeling—Molecular modeling was carried out to determine a tentative position of the template strand when the primer is bound at the exo site. For this purpose, we first superposed the crystal structure of Thermus aquaticus DNA polymerase I (KlenTaq polymerase mode complex; Protein Data Bank file 3ktq) onto the KF (exonuclease mode complex; Protein Data Bank file 1kln). This superposition showed that although the primer moves a distance of ∼35 Å to the exo site, the 5′-end of the template shows a displacement of ∼20 Å (Fig. 1B). However, the position of the template overhang could not be inferred, because the length of the template strand in the structure of the KF editing complex (14) was too short. To overcome this problem, we used the RB69 polymerase (a B family polymerase) crystal structures, which are available for both the polymerase and exonuclease mode complexes (15, 16). The coordinates of double-stranded DNA in the editing mode complex of RB69 DNA polymerase crystal structure were extracted and superposed on the DNA in the KF editing complex (Fig. 1B). We identified the Arg821, Arg822, Leu823, and Tyr824 residues of KF as forming a potential site for template overhang binding (Fig. 1B). In addition, the sequence alignment showed that these residues are highly conserved among members of the pol I family (Fig. 1C).
FIGURE 1.
Modeling the interactions of template overhang with Klenow fragment in exonuclease mode. A and B show the superimposition of the polymerase mode complex of KlenTaq (Protein Data Bank file 1clq) and the exonuclease mode complex of KF (Protein Data Bank file 1kln). The primer in the polymerase mode is in yellow, and the template is in red, whereas the primer in the exonuclease mode is in dark orange and the template is in cyan. The two white stars in A indicate the approximate locations of the pol and exo active sites in KF. Note that the length of the template strand in KF structure is not long enough to infer interaction of the template overhang with any amino acid residues in the editing mode. To overcome this limitation, we extracted the coordinates of template-primer from the editing complex of RB69 DNA polymerase (Protein Data Bank file 1clq) and superposed it onto the DNA of KF editing complex, using the double-stranded DNA as the point of reference for superposing (B). The template strand in RB69 exonuclease mode is colored in magenta, and the primer is green. The superposition of the DNA (from the exonuclease mode DNA-bound complex of RB69 polymerase) on the polymerase and the exonuclease complex of KlenTaq and KF, respectively, shows an ∼35-Å movement of the primer strand from the pol site to the exo site. A movement of ∼20 Å of the 5′-end of the template strand from its position in the polymerase mode to exonuclease mode is also noted (B). The short template strand in the exonuclease mode DNA-bound crystal structure of KF is now extended by superposition of longer template strand from RB69 exonuclease mode complex. A cluster of charged residues of the RRRY motif (labeled as RRLY, as in the KF sequence) appear in the close vicinity of the template overhang and are rendered in space-filled spheres in A and B. These residues are located within potentially interacting distance with the template overhang. The residue Phe771 of KF is also shown in space-filled spheres in A. Note the large spatial separation between residue Phe771 and the RRRY cluster. It may be pointed out here that the RB69 DNA polymerase is a member of the B family of polymerases, and unlike KF (a member of the A family polymerases), its 3′–5′ exonuclease domain is located on the opposite side of the polymerase active site. The primary amino acid sequence alignment of the region containing RRRY motif from various members of the A family polymerases is shown in C. The conservation of the RRRY motif is emphasized by outlining this region, which is flanked by a conserved proline on the C-terminal side. The significance of other comparatively less conserved residues (e.g. YV and TL) is not clear at this time.
In Vitro Site-directed Mutagenesis, Expression, and Purification of Wild Type (WT) and Mutant Enzymes—The mutant enzymes were generated from plasmid (pCJ141) generously provided by C. Joyce of Yale University (New Haven, CT). This plasmid encodes KF and contains a D424A mutation, which confers a deficiency in the 3′–5′ exonuclease activity. To obtain an exonuclease-efficient enzyme, Ala424 was reverted to Asp424. Desired mutations on the plasmids were introduced by a PCR-based protocol described in Stratagene's QuikChange site-directed mutagenesis kit, using pfu-turbo polymerase and PCR-grade dNTPs obtained from Roche Applied Science. The PCR product was treated with DpnI to digest the methylated non-mutated parental strand. Mutant plasmids were isolated from maintenance strain CJ406, and the desired mutations were confirmed by DNA sequencing. The plasmids confirmed for mutation at the desired site were transformed into the expression strain CJ376 also obtained from C. Joyce. Expression and purification of the WT and of mutant enzymes R821A, R822A, L823A, Y824A, and R822A/Y824A was carried out by methods described previously (9, 10, 17).
Specific Polymerase Activity under Steady-state Conditions—Primer extension assay was used to determine the specific polymerase activity of the WT and mutant enzymes. The DNA polymerase activity was assessed in a standard reaction mixture containing 50 mm Tris-HCl (pH 7.8), 1 mm dithiothreitol, 0.01% bovine serum albumin, 0.2 mm EDTA, 1.5 μm 32/14-mer template-32P-5′-end labeled primer, 50 μm each dATP and dCTP (two incoming nucleotides), and 7.5 nm WT or mutant enzymes. The template-primer used for polymerase activity was generated by annealing a 32-mer template (5′-TGC GCG TTA TAC CGC AGT CGG AG T GGC TAA CG-3′) with 14-mer primer (5′-CGT TAG CCA CTC CG-3′) in a ratio of 2:1. Reactions were initiated by the addition of 6 mm MgCl2 (final concentration) and incubated at 25 °C. Aliquots were taken out at various time points, and the reactions were quenched by the addition of Sanger's gel loading dye. The extension products were resolved on a 16% polyacrylamide, 8 m urea gel. Here and in subsequent assays, the gel was exposed to a PhosphorImager, and the radioactive bands were visualized by scanning the PhosphorImager plate (GE Healthcare). The band intensities were quantitated by ImageQuant (GE Healthcare). The amount of dNTPs incorporated was plotted as a function of time for each enzyme. The data were fitted to a straight line using GraphPad Prism (GraphPad Inc.). The slopes provided the rate of dNTP incorporation. In order to determine the Km(dNTP) for individual enzymes, rates (velocities) were obtained by the above procedure, with dNTP concentrations varying from 0.1 to 10 μm. The rates were then plotted as a function of the dNTP concentration using the Lineweaver-Burk plot, and the Km(dNTP) values were calculated.
Exonuclease Activity with ssDNA and dsDNA Containing One Mismatch under Steady-state Conditions—Steady-state conditions were first used to estimate 3′–5′ exonuclease activity rates for ssDNA and dsDNA containing 1 mismatch. For steady-state rate determination, a 10-fold molar excess of ssDNA or 1 mismatch-containing dsDNA over the WT or mutant enzymes was used. The exonuclease activity was assessed in a standard reaction mixture containing 50 mm Tris-HCl (pH 7.8), 1 mm dithiothreitol, 0.01% bovine serum albumin, 0.2 mm EDTA, 100 nm 32P-5′-end-labeled 16-mer ssDNA (5′-CGT TAG CCA CTC CAG G-3′) or 32P-5′-end-labeled 14-mer primer (5′-CGT TAG CCA CTC CG-3′) annealed to 33-mer template (5′-TGC GCG TTA TAC GGC ACC CTG GAG TGG CTA ACG-3′) providing one mismatch at the 3′-end of the primer, and 10 nm WT or mutant enzyme. The reactions were initiated by the addition of MgCl2 (6 mm final concentration) and quenched by the addition of Sanger's gel loading dye at desired time intervals. Products were resolved on a 16% polyacrylamide, 8 m urea gel. The visualization and quantitation of radioactive bands was carried out as described above. The rates of cleavage of ssDNA were calculated as described by Derbyshire et al. (18). To calculate the mismatch removal rate, the remaining fraction of 14-mer primer was plotted against time. The data points were fitted to a single exponential decay by means of nonlinear regression analysis, using GraphPad Prism (here and in subsequent experiments).
ssDNA and One-mismatch Cleavage Rates of WT and Mutant Enzymes under Pre-steady-state Conditions—The exonuclease degradation rate of ssDNA was determined with 1 nm 32P-5′end-labeled 16-mer (5′-CGT TAG CCA CTC CAG G-3′) and 125 nm WT or mutant enzymes. The cleavage rate of one mismatch was determined with 1 nm 33/14-mer template-primer containing terminal mismatch and 75 nm WT or mutant enzyme. The rates of ssDNA cleavage and mismatch removal of terminal nucleotide were calculated as described above. The reported rates for the cleavage activity of the WT and mutant enzymes are an average of the values from at least two independent experiments.
KD(DNA) Determination for ssDNA and DNA Containing One Mismatch—DNA binding affinities for ssDNA and DNA with one mismatch were calculated from rates of exonucleolytic degradation obtained at different enzyme concentrations, ranging from 25 to 300 nm, as described before (9). The exonuclease rates were plotted as a function of enzyme concentration. The data were fitted to a hyperbola using GraphPad Prism to determine KD(DNA). The reported KD(DNA) values are derived from at least two independent experiments.
UV-mediated Cross-linking of Enzyme to ssDNA—The UV-mediated cross-linking was carried out as described by Singh and Modak (17). Briefly, the enzyme-ssDNA complexes were allowed to form for 5 min at 4 °C in a 30-μl reaction volume containing 50 mm Tris-HCl, pH 7.8, 1 mm dithiothreitol, 2 mm EDTA, 30 fmol of 32P-5′-end-labeled 14-mer, and 30 pmol of WT or mutant enzymes. The reaction mixtures were then exposed to UV light (254 nm) in a Spectrolinker (Spectronic Corp.) at 3700 microwatts/cm2 for 3 min. The reactions were terminated by the addition of protein solubilization medium containing SDS, followed by heating at 100 °C for 5 min. The cross-linked enzyme-DNA complexes were resolved on an 8% polyacrylamide gel containing SDS and were visualized by phosphorimaging (GE Healthcare).
Exonuclease-Polymerase Balance Assay—Determination of the alteration in the balance of the exonuclease and polymerase activities was carried out using 1 nm 33/14 template-primer containing a terminal mismatch and 50 μm each of two incoming dNTPs. This assay was performed essentially as described by Mendez et al. (19), with slight modifications. The WT and mutant enzyme concentrations were maintained at 75 nm, providing a single turnover reaction for both of the activities. The reactions in a standard buffer condition containing 50 mm Tris-HCl (pH 7.8), 1 mm dithiothreitol, 0.01% bovine serum albumin, 0.2 mm EDTA, were initiated by the addition of 6 mm MgCl2, together with the two dNTPs, and aliquots were removed at desired time intervals. The products of the exonuclease and polymerase activities were resolved on a 16% polyacrylamide gel containing 8 m urea and were quantitated as described above.
RESULTS
The present investigation was undertaken to determine if a specific region in pol I participates in the binding and/or stabilization of single-stranded template overhang during the process of mismatch excision. Several crystal structures of DNA polymerases have been reported where the template-primer is bound in the pol site (20, 21). In contrast, only two structures containing the DNA at the exo site have been reported. These structures are the template-primer-bound structure of RB69 DNA polymerase (16) and that of KF (14). In the exonuclease mode DNA-bound structure of RB69 DNA polymerase, the interactions of template overhang can be easily noted. However, for KF, a similar interacting region could not be discerned, due to its relatively short template strand, whose length is less than that of the primer strand (14) (Protein Data Bank file 1kln). To infer the interactions of the template strand with the enzyme, we used comparative molecular modeling and identified potential motif-spanning residues Arg821–Tyr824 of pol I (Fig. 1, A and B), which seemed likely to interact with the template strand during the primer excision reaction. The conservation of these residues across the members of the pol A family (Fig. 1C) further qualified this region as a motif, which we have named as the RRRY motif. Note that in E. coli pol I, this motif has the sequence RRLY. The alanine mutations of individual amino acids (R821A, R822A, L823A, and Y824A) together with a double mutant (R822A/Y824A) were then generated by site-directed mutagenesis, as detailed previously (9), and the expressed enzyme proteins were purified to homogeneity. The properties of the mutant enzymes were compared with that of the WT enzyme, in order to decipher the role of this motif in the exonuclease function of KF.
Polymerase Activity under Steady-state Conditions—The polymerase activity of WT KF and mutant enzymes (R821A, R822A, L823A, Y824A, and R822A/Y824A) was assessed under saturating template-primer and dNTP concentrations. The primer extension assay using two incoming nucleotides was used to estimate the polymerase activity. In this assay, 1.5 μm 33/14-mer template-primer (32P-label at the 5′-end of primer) was incubated with individual enzymes at 7.5 nm. The extension reaction was initiated by the simultaneous addition of 50 μm each of the next two incoming dNTPs and 6 mm MgCl2 (final concentration). A profile of primer extension is shown in Fig. 2A. The computed amount of dNTP incorporated by the WT and mutant enzymes shows little or no change in the polymerase activity of the mutant enzymes (Fig. 2A and Table 1). The two mutant enzymes, R822A and L823A, had approximately the same activity as that of the WT enzyme, whereas R821A, Y824A, and R822A/Y824A mutant enzymes showed slightly increased (19, 25, and 36%, respectively) polymerase activity (Table 1). The affinity for the substrate dNTP (Km(dNTP)) of these mutant enzymes was also found to be relatively unchanged (Table 1). Since no significant change in the polymerase activity of mutant enzymes was noted, it was inferred that the residues of RRRY motif are not required for the polymerase function. In addition, the alanine substitution in the mutant enzymes does not seem to perturb the overall structure of the enzyme, as judged by their intact polymerase activity.
FIGURE 2.
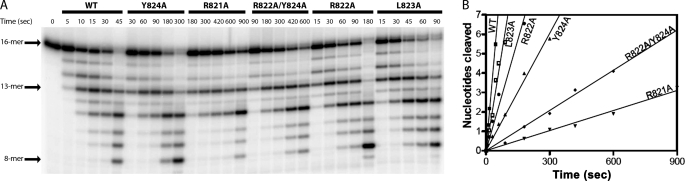
Time course of the polymerase (A) and the 3′–5′ exonuclease (B) activity of the WT and R821A mutant enzymes. DNA polymerase activity was assessed by primer extension assay using 1.5 μm 5′-32P-labeled 14-mer annealed to 32-mer template and 7.5 nm enzyme as described under “Experimental Procedures.” The amount of extension of 14-mer primer to 16-mer (after incorporation of two incoming nucleotides) was quantitated and plotted as a function of time. The gel image of only the WT and R821A enzyme is shown here. The polymerase activity of all of the other mutant enzymes was assessed in a similar manner. B depicts the time course of the 3′–5′ exonuclease activity, using 100 nm 16-mer ssDNA as a substrate and 10 nm enzyme. The gel image shows the pattern of degradation of the 5′-32P-labeled 16-mer DNA by WT and R821A mutant enzyme. Note that the reaction time for the mutant enzyme was much higher than that for the WT enzyme. The 3′–5′ exonuclease activity of other mutant enzymes was assessed similarly. The degradation rates were determined by quantification of the number of cleavage events required to produce a product of defined length, as described by Derbyshire et al. (18). A sum of the total number of cleavage events in the generation of various degradation products of 5′-32P-labeled 16-mer DNA was then plotted as a function of time and fitted to a straight line. The slope of the line provided the rates of polymerase and nuclease activities.
TABLE 1.
Relative specific activities of various mutant enzymes expressed as percentage of WT KF
| Enzyme | DNA polymerase activitya | Km(dNTP) | Exonuclease activity with ssDNAb | Exonuclease activity with one-mismatch DNAc |
|---|---|---|---|---|
| % | μm | % | % | |
| WT | 100 | 1.4 | 100 | 100 |
| R821A | 119 | 1.5 | 5 | 72 |
| R822A | 105 | 3.7 | 31 | 92 |
| L823A | 97 | 2.6 | 31 | 85 |
| Y824A | 125 | 2.0 | 25 | 43 |
| R822A/Y824A | 136 | 3.4 | 4 | 16 |
| F771A | 70 | NDd | 70 | 124 |
100% activity of the WT enzyme equals 315 pmol of dNTP incorporated by one pmol of enzyme in 1 min at 25 °C
100% exonuclease activity of WT enzyme equals 30 nucleotides cleaved by 1 pmol of enzyme in 1 min at 25 °C
100% activity of WT enzyme equals 8 nucleotides cleaved by 1 pmol of enzyme in 1 min at 25 °C. Details of all activity measurements and Km(dNTP) calculations are given under “Experimental Procedures”
ND, not determined
Exonuclease Activity under Steady-state Conditions—The exonuclease activity of the WT and mutant enzymes was determined under steady-state conditions, using 10 nm enzyme and 100 nm ssDNA. The activity of all mutant enzymes was affected, albeit to a varying degree (Fig. 2B and Table 1). R822A and L823A mutant enzymes showed ∼31% exonuclease activity, and the Y824A showed ∼25% exonuclease activity with ssDNA, in comparison with WT KF. The most significant loss of activity was noted with R821A and double mutant (R822A/Y824A) enzymes. These mutants had only about 4–5% of WT KF activity on ssDNA (Table 1). Since we had projected a role of the RRRY motif in the binding of the template overhang, after shuttling of primer terminus to the exonuclease site, the loss of exonuclease activity of the mutant enzymes with ssDNA suggests a role for these residues in the binding (or positioning) of ssDNA. Previously, Phe771 of pol I has been identified as the major site of interaction of template overhang in the enzyme-DNA-dNTP (polymerase) ternary complex (10, 11). Therefore, we determined the exonuclease activity of F771A mutant enzyme on ssDNA to assess if this mutant enzyme exhibits a similar deficiency in its exonuclease activity. The F771A mutant enzyme showed ∼70% exonuclease activity, in comparison with WT enzyme (Table 1), ruling out the possibility that the template overhang binding site in the polymerase mode is used in the exonuclease mode.
The assessment of the exonuclease activity on dsDNA containing one mismatch showed only a moderate deficiency in mismatch cleavage rate by mutant enzymes, compared with that seen for ssDNA. Little or no effect on mismatch removal rate was noted by mutant enzymes R822A and L823A. The double mutant (R822A/Y824A) enzyme displayed maximum decrease in the exonuclease activity, followed by the Y824A and R821A enzymes (Table 1).
ssDNA Cleavage Activity of WT and Mutant Enzymes under Pre-steady-state Conditions—The time course of the exonucleolytic cleavage of the WT and mutant enzymes (125 nm) with 16-mer ssDNA (1 nm) is shown in Fig. 3A. The pattern of ssDNA cleavage by R821A and R822A/Y824A mutant enzymes (Fig. 3A) suggests that these mutant enzymes are quite compromised in the degradation of ssDNA. To estimate the exonuclease rates, the total number of nucleotides cleaved by the individual enzyme was plotted against time (Fig. 3B). The mutant enzymes, L823A and R822A, were moderately (∼2–3-fold) deficient in cleaving ssDNA, whereas Y824A, R822A/Y824A, and R821A mutant enzymes showed significant reduction in cleavage rates (Table 2). The R821A enzyme was maximally deficient, with a reduction of about 29-fold in cleavage activity, followed by about 17-fold reduction in the activity of double mutant enzyme (Table 2).
FIGURE 3.
Determination of 3′–5′ exonuclease activity of WT and mutant enzymes using ssDNA under single turnover conditions. The exonucleolytic degradation of 5′-32P-labeled 16-mer (1 nm) by the WT and mutant enzymes is compared under saturating concentrations (125 nm) of the various enzymes (A). Note that the reaction times for some mutant enzymes differed from those used for WT enzymes. Thus, a longer time was needed to observe exonuclease activity of R821A and R822A/Y824A mutant enzymes. The degradation rates were determined as described in the legend to Fig. 2, and a plot obtained is shown in B. The rates calculated from these data are listed in Table 2.
TABLE 2.
The rates for the exonuclease cleavage and DNA binding affinities of the WT and mutant enzymes
|
Enzyme
|
Exonuclease
ratesa
|
KD(DNA)
|
||||
|---|---|---|---|---|---|---|
| With ssDNA | With one-mismatch dsDNA | With ssDNA | With one-mismatch dsDNA | |||
| s–1 | nm | |||||
| WT | 0.116 ± 0.009 | 0.122 ± 0.007 | 68 ± 18 | 29 ± 5 | ||
| R821A | 0.004 ± 0.001 | 0.056 ± 0.003 | >1000 | 57 ± 4 | ||
| R822A | 0.042 ± 0.010 | 0.099 ± 0.020 | 111 | NDb | ||
| L823A | 0.057 ± 0.019 | 0.118 ± 0.024 | 140 | ND | ||
| Y824A | 0.020 ± 0.003 | 0.067 ± 0.0125 | 184 ± 48 | 48 ± 6 | ||
| R822A/Y824A | 0.007 ± 0.001 | 0.029 ± 0.005 | >1000 | 38 ± 5 | ||
Rates with the indicated DNA substrates were determined under single turnover conditions, as described under “Experimental Procedures,” and are derived from the data shown in Figs. 3, 4, and 6. The values reported here are the averages derived from at least two independent experiments
ND, not determined
ssDNA Binding Affinity of WT and Mutant Enzymes—To understand if the decrease in the exonuclease activity of mutant enzymes was due to decreased affinity for ssDNA, we determined the ssDNA binding affinity (KD(ssDNA)) of the WT and mutant enzymes. For this purpose, the cleavage rates of ssDNA (1 nm) were determined using increasing concentrations (25–300 nm) of WT and mutant enzymes. A representative degradation pattern of ssDNA by WT and R821A mutant enzyme is shown in Fig. 4, A and B, respectively. The rates of degradation for each enzyme concentration were calculated from these figures as described by Derbyshire et al. (18). The rates of degradation, with increasing enzyme concentration, of WT and R821A enzyme are shown as insets in Fig. 4, C and D. The rates obtained for all of the enzymes were then plotted as a function of enzyme concentration, and the data were fitted to a hyperbola, to determine the KD(ssDNA) (Fig. 4, C–F). The DNA binding affinities, expressed as KD(ssDNA), are presented in Table 2. The KD(ssDNA) of the WT enzyme was ∼68 nm, which is very close to a previously reported value obtained by the same method (9). The mutant enzymes R822A, L823A and Y824A showed an ∼2–3-fold increase in KD(ssDNA), which accounts for the ∼2–3-fold decrease in exonuclease activity of these polymerases, except for Y824A, which showed a decrease of ∼6-fold in exonuclease activity. The most significant decrease in DNA binding affinity was noted for R821A and R822A/Y824A mutant enzymes. In fact, the rates of cleavage on ssDNA with R821A and R822A/Y824A remained linear, even when enzyme concentration as high as 1 μm was used (data not shown). Thus, the KD(ssDNA) for these mutant enzymes was greater than 1 μm, in agreement with the maximum decrease in ssDNA cleavage activity of these enzymes.
FIGURE 4.
Single-stranded DNA binding affinity for the WT and mutant enzymes. Exonuclease cleavage pattern for the WT (A) and R821A (B) was obtained with ssDNA substrate (1 nm). The WT enzyme concentrations ranged from 25 to 150 nm, whereas the R821A mutant enzyme concentrations varied between 50 and 300 nm. The rates of degradation of ssDNA by varying enzyme concentrations were determined as described in the legend to Fig. 3 and are shown in insets of C (WT) and D (R821A). These rates of ssDNA degradation were then plotted as a function of enzyme concentration (C and D for WT and R821A, respectively) and were fit to a hyperbola to determine KD(ssDNA). Note that the time needed to develop a ssDNA degradation pattern for R821A mutant enzyme ranged from 3 to 15 min. E, the hyperbolic fit of the rates determined by varying the concentration of other mutant enzymes with 1 nm DNA. These plots provided the KD(ssDNA) for R822A, L823A, and Y824A mutant enzymes. The KD(ssDNA) of the double mutant (R822A/Y824A) was determined in similar fashion, and the plot is shown in F. Values of KD(ssDNA) for all enzymes are summarized in Table 2.
UV-mediated Cross-linking of the WT and Mutant Enzymes with ssDNA—Various mutant enzymes of the RRRY motif exhibit variable loss of affinity for ssDNA. As an additional means of assessing the binding affinities, we determined the extent of UV-mediated cross-linking of individual enzymes to ssDNA (Fig. 5A). To determine the cross-linking efficiency of WT and mutant enzymes, the intensities of the radioactive bands were quantified. The cross-linking of the individual mutant enzyme was then expressed as a percentage of WT cross-linking to ssDNA (Fig. 5B). It is clear from the results that the cross-linking of R821A and R822A/Y824A mutant enzymes to ssDNA was maximally affected (∼60% reduction), whereas R822A, L823A, and Y824A mutant enzymes showed a moderate loss (∼20–30%) in the cross-linking with ssDNA. The cross-linking results also indicate the presence of additional sites of ssDNA binding in the enzyme. It should be noted here that the extent of UV cross-linking depends on both the binding affinity and the cross-linkable groups, the latter of which are common to the WT and mutant enzymes, except at the site(s) of mutation. Overall, the UV cross-linking results, coupled with the KD(ssDNA) values in Table 2, strongly suggest a role for Arg821, Arg822, and Tyr824 of the RRRY domain in the binding of ssDNA, such that its exonucleolytic cleavage at the exo site is facilitated.
FIGURE 5.
Cross-linking of ssDNA to the WT and mutant enzymes. The upper row of bands in A represents the autoradiograph of 32P-labeled 16-mer cross-linked to WT and different mutant enzymes, whereas the lower group of bands in A depicts the Coomassie Blue staining of the proteins used in the cross-linking assay. The cross-linked complexes were resolved by electrophoresis on an 8% SDS-polyacrylamide gel. The gel was exposed to a PhosporImager, and the amount of cross-linking was quantitated using ImageQuant (GE Healthcare). The amount of ssDNA-enzyme cross-linking was expressed as a percentage of WT cross-linking and shown as a bar chart in B.
Exonuclease Activity of WT and Mutant Enzymes on Template-Primer Containing One Mismatch under Single Turnover Conditions—To examine the role of individual residues of the RRRY motif in the process of stabilization of the template overhang in the exonuclease mode, we determined the exonuclease activity of the WT and mutant enzymes using template-primer containing a single mismatch at the 3′-end of the 32P-5′-end-labeled primer.
The time course of exonucleolytic degradation of the template-primer containing a single mismatch at the 3′-end by WT and mutant enzymes is shown in Fig. 6A. The rates of mismatch cleavage for various enzymes were estimated from these data by plotting the fraction of uncleaved 14-mer primer as a function of time and fitting the data points to single exponential decay (Fig. 6B). The rates of nucleotide cleavage from a template-primer containing one mismatch by the WT and mutant enzymes are presented in Table 2. Both the R822A and L823A mutant enzymes showed no significant decrease in mismatch cleavage rate, whereas R821A and Y824A mutant enzymes showed an ∼2-fold decrease in the exonuclease activity with this substrate. The double mutant enzyme R822A/Y824A was the most deficient, with an ∼4-fold slower cleavage rate than the WT KF. Overall, the effect of mutation on the mismatch removal was not as prominent as that noted earlier for ssDNA degradation.
FIGURE 6.
Exonuclease cleavage rates of WT and mutant enzymes with template-primer containing terminal mismatch (33/14). The reaction conditions were identical to those used for ssDNA. The exonuclease activity was assayed using a 75 nm concentration of the indicated enzyme and 1 nm 33/14 mismatch template-primer. The reactions were quenched after different time intervals (note that the time of incubation for R821A and R882A/Y824A was increased to 60 and 90 s) by the addition of Sanger's stop dye, containing 95% formamide. The products were resolved on 16% polyacrylamide, 8 m urea gel (A). To determine the rates of degradation by the WT and mutant enzymes on one-mismatch template-primer, the amount of primer remaining was plotted as a function of time and fit to a single-exponential decay, using nonlinear regression (B). C shows the representative hyperbolic fit of the rates at various concentrations of WT and R822A/Y824A double mutant enzymes with one-mismatch DNA substrate. The rates at various enzyme concentrations were determined in a manner similar to that shown in B.
DNA Binding Affinity of the WT and Mutant Enzymes with DNA Containing a Terminal Mismatch—The DNA binding affinity of WT and mutant enzymes was determined essentially as described before (9). The rates for mismatch cleavage activity were obtained by varying the enzyme concentration, with template-primer at 1 nm concentration. These rates were plotted against the enzyme concentration and were fitted to a hyperbola (Fig. 6C) for WT and R822A/Y824A mutant enzymes. The KD(DNA) values for WT and mutant enzymes with single mismatch template-primer are shown in Table 2. The KD(DNA) of the WT KF determined here is in close agreement with that reported before (9). The comparison of KD(DNA) of the WT and mutant enzymes shows that there is a relatively small change in the binding affinity of mismatch-containing template-primer to enzymes containing mutation of amino acid residues belonging to the RRRY motif.
Exonuclease-Polymerase Balance Assay—To determine whether the mutant enzymes had an altered rate of nucleotide turnover and/or a defect in switching between the exonuclease and the polymerase active centers, the balance (exo-pol coupled) assays were carried out using one mismatch-containing template primer. Two incoming dNTPs were also provided in the reaction mixture. In this case, the incorporation of dNTP, as determined by the extension of primer, occurs only when the terminal mismatched nucleotide is cleaved prior to switching over to the polymerization reaction. Since the polymerase reaction is several orders of magnitude faster than the exonuclease reaction, any alteration in the rate of dNTP incorporation (product of primer extension) between the WT and mutant enzymes indicates a defect either in the initial switching of TP to the exo site or its subsequent switching back to the pol site.
A time course of the cleavage of mismatched termini, followed by the extension of the matched primer by WT, R821A, R822A/Y824A, and L823A mutant enzymes is shown in Fig. 7. The presence of only two nucleotides in the reaction mixture permits the product extension to 17-mer, representing the addition of four nucleotides (one A and three Gs; Fig. 7A). It is worth noting that there is a complete absence of 13-mer (the product of exonucleolytic cleavage of the mismatch) at any time. This is due to its rapid extension by polymerase activity as soon as it is made available. The rate of incorporation of dNTPs by the WT and L823A enzymes is calculated to be 0.3 and 0.2 s-1, respectively, whereas it is 0.1 and 0.08 s-1 for the R821A and R822A/Y824A mutant enzymes, respectively (Fig. 7B). The reduced rate of dNTP incorporation seen with the mutant enzymes in this assay reflects the correspondingly reduced amount of a matched template-primer made available for extension at the pol site of these enzymes. These results indicate that the slower rate of polymerization (turnover of dNTPs) observed with the mutant enzymes is due to a delayed availability of the matched primer, which was the result of a slower rate of primer transfer and/or alignment from the pol to the exo site for cleavage.
FIGURE 7.
Exonuclease-polymerase balance assay of WT and mutant enzymes with template-primer containing terminal mismatch (33/14). Primer extension assays were performed under reaction conditions identical to those used for determining exonucleolytic activity using template-primer containing terminal mismatch, except that reactions were initiated by the addition of 6 mm MgCl2 and 50 μm each of two incoming dNTPs, and aliquots were removed at the indicated times. The products of the exonuclease and polymerase activities were resolved on a 16% polyacrylamide gel containing 8 m urea. The sequence of the 33/14 DNA substrate is shown at the top in A. A shows the gel image of only the WT and R822A/Y824A enzymes. The lanes marked 1–4 in A show the degradation pattern of WT (lanes 1 and 2) and R822A/Y824A mutant enzyme (lanes 3 and 4) with no dNTPs added. B, a plot of the amount of primer extended, as a function of time, fitted to a one-phase exponential association for the WT, L823A, R821A, and R822A/Y824A enzymes. This plot provided the rates for primer extension.
DISCUSSION
The kinetic partitioning of DNA by DNA polymerases to accomplish the removal of misincorporated nucleotide has been extensively studied (22, 23). Although the kinetic analyses of WT enzymes give insights into the overall functioning of an enzyme, these data do not provide much information about the orchestrated participation of enzyme components, such as the domains and the specific amino acid residues in the catalytic process. There exists an abundance of structural and biochemical data pertaining to the synthesis of DNA by DNA polymerases. These data have significantly aided in our understanding of mechanisms of template-primer and substrate nucleotide binding as well as the events involved in the synthetic process, namely the phosphodiester bond formation. In contrast, the structural data related to the binding of DNA in the exonuclease mode are scarce and in most instances have some aberrant property. For example, nearly a dozen or so DNA-bound crystal structures that contain mismatched primer templates have been reported (14, 16, 24). However, none of these structures exhibit primer moiety bound at the exo site. It is rather intriguing that despite the template-primer containing as many as six terminal mismatches, the primer moiety in the structure is still bound in the polymerase domain (24). One possible reason for this anomaly may be the fact that the DNA polymerase selected for such studies does not have a catalytically competent 3′-5′ exo site. For A family DNA polymerases, only one crystal structure (that of KF) with the primer strand bound to the 3′–5′ exo site has been reported (14). However, in this structure, the length of the primer is longer than that of the template strand. The other structure with DNA bound at the 3′–5′ exo site is that of RB69 DNA polymerase, which belongs to the B family of DNA polymerases. There are some basic differences in the architectural arrangement of the polymerase and the exonuclease domains in the enzymes of the A and the B families. In the A family, the exonuclease domain is located behind the palm subdomain, whereas in the B family enzymes (e.g. RB69 and T4 DNA polymerases), it is located in front of the polymerase domain. Despite nearly opposite location of the exonuclease domain with respect to the pol site in the two families, the distance between the pol and exo sites is approximately the same (30–40 Å). Therefore, it is possible that the mechanism of shuttling of the primer strand between the two sites may be similar in members of both families.
Both kinetic measurements and structural data indicate that the polymerases undergo conformational adjustments upon DNA and dNTP binding. In addition, the comparison of the DNA-bound structures of RB69 DNA polymerase in the polymerase and the exonuclease modes shows a 40° transition of the helical axis of DNA (15). A similar change can be noted upon the superposition of the polymerase mode DNA-bound crystal structure of KlenTaq (the KF equivalent of T. aquaticus DNA polymerase I) (21) and the exonuclease mode DNA-bound structure of KF (Fig. 1A). These observations suggest the requirement for a structural repositioning of not only the primer moiety but also of the template during a shift from the polymerase to the exonuclease mode of DNA binding. These structures also indicate that the binding of ssDNA template overhang in the exonuclease mode DNA-enzyme complex is different from the one established for the polymerase reaction by biochemical and structural data (10, 11, 21, 25). In order to tentatively identify the interactions between the KF and template overhang in the exonuclease mode, we resorted to comparative molecular modeling and sequence alignment. For this purpose, we started with two structures, representing the polymerase and the exonuclease modes of DNA-bound RB69 DNA polymerase (Protein Data Bank files 1ig9 and 1clq, respectively), for our studies. These structures have visible electron density for a sufficiently long 5′ terminus of the template overhang and therefore can be considered suitable for modeling the template overhang interactions with KF. Since the stem region of DNA duplex in the RB69 DNA polymerase structures (15, 16, 26) superimposes well on the equivalent region of KF editing complex (14), a model of KF editing complex with sufficient template overhang length was generated and used to discern the interactions between the template overhang and the protein. We identified a conserved sequence (RRRY), topologically located between the polymerase and the 3′–5′ exonuclease domain of KF (Fig. 1B) as a potential site of interaction, with template overhang in the exonuclease mode binding of DNA with pol I. The functional requirement of this motif for the exonuclease (or polymerase) activity was then investigated.
Individual mutations of the amino acid residues of the RRRY motif to alanine resulted in little or no change in the polymerase activity of KF (Fig. 2A and Table 1), suggesting that these residues do not play a critical role in the DNA synthesis reaction. Furthermore, these results also suggest that the mutations in this region are unlikely to affect the overall structure of the Klenow fragment. The 3′–5′ exonuclease activity of the WT and mutant enzymes was first assessed using the simplest substrate, ssDNA. The results demonstrate that the mutant enzymes exhibited variably decreased 3′–5′ exonuclease activity (Table 1). The maximum decrease in exonuclease activity (∼29-fold) was observed with R821A mutant enzyme, followed by the double mutant (R822A/Y824A; ∼17-fold in comparison with the WT KF). The effect of the mutation at other individual positions (Arg822, Leu823, and Tyr824) on the exonuclease activity was moderate. The decrease in ssDNA cleavage activity corresponded to the decreased ssDNA binding affinity (KD(ssDNA)) of the mutant enzymes. This observation suggested that the involvement of the RRRY motif residues in the binding of ssDNA. Since single-stranded template overhang stabilization in the polymerase mode has been shown to occur along the residues of the O1-helix (mainly Phe771) (10, 11), we also examined the status of the exonuclease activity of F771A mutant enzyme. Mutation of Phe771 did not significantly alter exonuclease activity (Table 1), suggesting that the template overhang stabilizing region, unlike the RRRY motif, has no influence on exonuclease activity.
The assessment of the 3′–5′ exonuclease activity, with template-primer containing a terminal mismatch, showed a rather interesting pattern for various mutant enzymes (Tables 1 and 2). Only the double mutant (R822A/Y824A) enzyme displayed a significant reduction (∼4–5-fold) in the cleavage of mismatch. The R821A and Y824A mutant enzymes showed only a 2-fold reduction, whereas the rest of the mutant enzymes, namely R822A and L823A, were only slightly affected. In general, the overall extent of activity reduction was not as prominent as that observed with ssDNA. The KD(DNA) determination with R821A and the double mutant enzymes, for one-mismatch-containing template-primer, did not reveal significant change in DNA binding affinity (Table 2), suggesting that the decrease in mismatch removal by these enzymes may be a result of improper template-primer positioning.
The most likely scenario for the observed differences in the exonuclease activities of mutant enzymes with ssDNA and one-mismatch-containing DNA is that the binding mode of these two substrates is different. The approach of the primer terminus of the mismatched DNA and that of the 3′-OH terminus of ssDNA at the 3′–5′ exo site must occur in a similar manner. However, the binding of the distal regions of the two substrates (i.e. primer strand of the mismatched DNA and the ssDNA) probably occurs at different regions or motifs of polymerase protein. A tentative model depicting this scenario is presented in Fig. 8, where the primer moiety of the mismatched DNA and the ssDNA approach the catalytic site from opposite sides. In fact, the distal region of ssDNA substrate and the template overhang strand (a nonsubstrate strand) of the mismatched DNA appear to bind at the same RRRY motif of the enzyme. Furthermore, the binding of ssDNA at this motif seems to be dictated by the 5′–3′ polarity recognition. In addition, the stem region of the one-mismatch-containing dsDNA provides additional stability for binding of mismatched duplex in the palm region of the enzyme. The binding of the stem region of dsDNA in the binary complexes in both polymerase and exonuclease mode has been seen to occur in the same general region of the enzyme in two crystal structures (14, 15, 21). Furthermore, in the exonuclease mode DNA-bound crystal structure, the 40° rotation of helical axis of DNA does not change the overall ionic environment around the stem region of the duplex. Regardless of the differences in the binding mode of two substrates, it is clear from the present results that the binding track for the primer moiety of the mismatched DNA does not involve the RRRY motif.
FIGURE 8.
Proposed mode of binding of mismatched template-primer and ssDNA. The binding of template (solid lines) and primer (broken lines) moieties of matched template-primer is shown in A. B, the proposed binding mode of the primer (at the 3′ exo site) and template overhang in mismatched template-primer. Suggested binding of ssDNA is depicted in C. The strand polarity and the position of polymerase and exonuclease active centers are also indicated. Note that the position of template overhang is altered in the polymerase and exonuclease modes of DNA binding. It is stabilized at Phe771 of the O1-helix in the polymerase mode, whereas it binds to the RRRY motif (labeled and depicted as a filled ellipse) during the exonuclease reaction. Note that the binding of ssDNA is probably governed by the strand polarity (5′-3′), and the 5′ portion of the ssDNA and 5′ template overhang in mismatched DNA share a common binding region, the RRRY motif.
The results of the ssDNA cross-linking to various mutant enzymes (Fig. 5) also showed some reduction in the cross-linking of ssDNA to R821A and R822A/Y824A, in comparison with WT enzyme. A similar reduction was not noted with other mutant enzymes, including F771A (data not shown). These results provide further support for the involvement of the RRRY motif in the binding of ssDNA substrate.
To assess whether the reduction in the cleavage rate of a mismatched substrate is biologically relevant, we carried out an exonuclease-polymerase balance assay, where the extension of a mismatched template primer by the desired enzyme is monitored. The catalytic events involved in this assay can be divided into the following steps: (a) initial binding of template-primer and recognition of the mismatch at the pol site, (b) switching of the mismatched primer terminus to the exo site, (c) cleavage of the mismatched terminal nucleotide at the exo site, (d) switching of the matched terminus back to the pol site, and (e) extension of the primer terminus. In this assay, the polymerase reaction is entirely dependent on the available quantity of the matched template primer produced by the exonuclease reaction. The results of the balance assay with WT or mutant enzymes clearly show that the WT or L823A enzymes exhibit similar patterns, whereas the R821A and R822A/Y824A enzymes show a loss of 3–4-fold in the primer extension, as judged by the nucleotide polymerization rate of 0.3 and 0.2 s-1 by WT and L823A, versus 0.1 and 0.08 s-1 for the latter two enzymes, respectively. Since the polymerase activity of WT and all of the mutant enzymes are nearly equal (Fig. 2A and Table 1), the reduction of activity in the mutant enzymes is concluded to be a direct result of the reduced quantity of available matched primer termini at a given time. The possibility of a delay in switching the primer termini from the exo to pol site, after mismatch cleavage, is also ruled out by the fact that there is not even a trace quantity of matched primer (13-mer band in the gel in Fig. 7A) among the products, in both WT and mutant enzymes. This suggests that the matched primer termini are instantly switched to and extended at the pol site. The delayed availability of matched primer with the two mutant enzymes thus could result from either the inefficient switching of mismatched primer to the exo site or a defect (possibly an alteration in geometry at the active site) in the cleavage of the mismatched primer moiety. A catalytic defect at the exo site seems highly unlikely, since the location of the RRRY motif is far removed from the active center of the exonuclease site as well as from the primer strand itself. Therefore, it is logical to conclude that the observed effects in the mutant enzymes are mediated through the unstable binding of the nonsubstrate template strand, which in turn leads to a delayed positioning of the primer strand at the exo site. Early partitioning studies carried out with one-mismatch-containing fluorescent template primers, had shown that the primer moieties were distributed between the pol and exo sites in a ratio of about 45:55 (27). Thus, the mismatch-cleaving activity may also be regulated by kinetic partitioning, upon initial binding at the pol site, as well as by the availability of a functional RRRY motif, which stabilizes the nonsubstrate template moiety, thereby facilitating the efficient transfer of the primer moiety to the exo site.
As regards to the role that the RRRY motif plays in the ssDNA cleavage activity, the same general mechanism may be applicable (i.e. this motif facilitates the initial binding and subsequent orientation of ssDNA at the 3′–5′ exo site). The biological utility of ssDNA binding at this motif is less certain. Nevertheless, it may be pointed out that the participation of the 3′–5′ exonuclease activity during the process of strand displacement synthesis and, particularly, in the cleavage of the displaced strand (ssDNA), as noted during Okazaki fragment maturation, has been implicated recently (28). However, this aspect requires further exploration.
The presence of an RRRY-like motif is not seen in RB69 polymerase at the structurally equivalent position. Thus, the region involved in stabilization of template strand overhang in the exonuclease mode of this polymerase cannot be inferred. However, the presence of a KKRY motif, which is superficially homologous to the RRRY motif, has been described in the polymerase mode of DNA-bound RB69 DNA polymerase (15). The KKRY motif of RB69 polymerase is located in the close vicinity of the polymerase active site and is conserved in B family polymerases. It may be pointed out that the RRRY motif of A family polymerases is located between the polymerase and the exonuclease domains and is therefore not structurally equivalent to the KKRY motif of B family polymerases (15, 26). Furthermore, the KKRY motif in RB69 polymerase has not been shown to be required for the exonuclease activity. A β-hairpin loop present in the polymerase domain of B family polymerases (15, 26) has been implicated in the shuttling of primer between the two sites. This assignment is based on the observation that the mutation of the residues of this loop in T4 and RB69 polymerases results in the deficient cleavage of mismatched termini (7, 26). However, ssDNA-cleaving activity of these mutant enzymes remained unaffected, implying the presence of as yet unidentified structural components functionally equivalent to the RRRY motif in B family polymerases.
In summary, we have shown the following. (a) A newly identified RRRY motif in the pol A family of polymerases is required for the binding and cleavage of ssDNA. (b) the RRRY motif is also required for the binding and stabilization of the template overhang of the mismatch-containing template-primer. This, in turn, facilitates the switching and orientation of the mismatched primer at the exo site for its cleavage. (c) The binding of both ssDNA and the template overhang of mismatched DNA seems to occur in the same polarity. Our observations on the ssDNA binding mode also raise a rather intriguing question relating to the biological utility of ssDNA cleavage activity of all polymerase-associated exonucleases.
Acknowledgments
We gratefully acknowledge the editorial assistance of Dr. Harold Calvin.
This work was supported, in whole or in part, by National Institutes of Health Grants GM 36307 (NIGMS) and AI-064477 (NIAID). A part of this work was presented at the annual meeting of FASEB (29). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: pol I, E. coli DNA polymerase I; exo site, exonuclease active site; pol site, polymerase active site; KF, Klenow fragment of E. coli DNA polymerase I; Klentaq, Klenow fragment equivalent of T. aquaticus DNA polymerase I; WT, wild type; ssDNA, single-stranded DNA; dsDNA, double-stranded DNA.
References
- 1.Kornberg, A., and Baker, T. (1992) DNA Replication, p. 130, W. H. Freeman and Co., New York
- 2.Brown, T., and Kennard, O. (1992) Curr. Biol. 2 299-299 [Google Scholar]
- 3.Seeman, N. C., Rosenberg, J. M., and Rich, A. (1976) Proc. Natl. Acad. Sci. U. S. A. 73 804-808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyce, C. M. (1989) J. Biol. Chem. 264 10858-10866 [PubMed] [Google Scholar]
- 5.Spacciapoli, P., and Nossal, N. G. (1994) J. Biol. Chem. 269 438-446 [PubMed] [Google Scholar]
- 6.Stocki, S. A., Nonay, R. L., and Reha-Krantz, L. J. (1995) J. Mol. Biol. 254 15-28 [DOI] [PubMed] [Google Scholar]
- 7.Baker, R. P., and Reha-Krantz, L. J. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 3507-3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuske, S., Singh, K., Kaushik, N., and Modak, M. J. (2000) J. Biol. Chem. 275 23759-23768 [DOI] [PubMed] [Google Scholar]
- 9.Singh, K., and Modak, M. J. (2005) Biochemistry 44 8101-8110 [DOI] [PubMed] [Google Scholar]
- 10.Srivastava, A., Singh, K., and Modak, M. J. (2003) Biochemistry 42 3645-3654 [DOI] [PubMed] [Google Scholar]
- 11.Turner, R. M., Jr., Grindley, N. D., and Joyce, C. M. (2003) Biochemistry 42 2373-2385 [DOI] [PubMed] [Google Scholar]
- 12.Catalano, C. E., Allen, D. J., and Benkovic, S. J. (1990) Biochemistry 29 3612-3621 [DOI] [PubMed] [Google Scholar]
- 13.Ollis, D. L., Brick, P., Hamlin, R., Xuong, N. G., and Steitz, T. A. (1985) Nature 313 762-766 [DOI] [PubMed] [Google Scholar]
- 14.Beese, L. S., Derbyshire, V., and Steitz, T. A. (1993) Science 260 352-355 [DOI] [PubMed] [Google Scholar]
- 15.Franklin, M. C., Wang, J., and Steitz, T. A. (2001) Cell 105 657-667 [DOI] [PubMed] [Google Scholar]
- 16.Shamoo, Y., and Steitz, T. A. (1999) Cell 99 155-166 [DOI] [PubMed] [Google Scholar]
- 17.Singh, K., and Modak, M. J. (2003) J. Biol. Chem. 278 11289-11302 [DOI] [PubMed] [Google Scholar]
- 18.Derbyshire, V., Pinsonneault, J. K., and Joyce, C. M. (1995) Methods Enzymol. 262 363-385 [DOI] [PubMed] [Google Scholar]
- 19.Méndez, J., Blanco, L., Lázaro, J., and Salas, M. (1994) J. Biol. Chem. 269 30030-30038 [PubMed] [Google Scholar]
- 20.Doublie, S., Tabor, S., Long, A. M., Richardson, C. C., and Ellenberger, T. (1998) Nature 391 251-258 [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., Korolev, S., and Waksman, G. (1998) EMBO J. 17 7514-7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, K. A. (1993) Annu. Rev. Biochem. 62 685-713 [DOI] [PubMed] [Google Scholar]
- 23.Kuchta, R. D., Benkovic, P., and Benkovic, S. J. (1988) Biochemistry 27 6716-6725 [DOI] [PubMed] [Google Scholar]
- 24.Johnson, S. J., and Beese, L. S. (2004) Cell 116 803-816 [DOI] [PubMed] [Google Scholar]
- 25.Kiefer, J. R., Mao, C., Braman, J. C., and Beese, L. S. (1998) Nature 391 304-307 [DOI] [PubMed] [Google Scholar]
- 26.Hogg, M., Aller, P., Konigsberg, W., Wallace, S. S., and Doublie, S. (2007) J. Biol. Chem. 282 1432-1444 [DOI] [PubMed] [Google Scholar]
- 27.Lam, W., Van der Schans, E., Joyce, C., and Millar, D. (1998) Biochemistry 37 1513-1522 [DOI] [PubMed] [Google Scholar]
- 28.Rossi, M., and Bambara, R. (2006) J. Biol. Chem. 281 26051-26061 [DOI] [PubMed] [Google Scholar]
- 29.Kukreti, P., Singh, K., and Modak, M. J. (2007) FASEB Annual Meeting, Washington, D. C., April 28, 2007, Abstract 661.1, Federation of American Societies for Experimental Biology, Bethesda, MD