The first colloquium on ‘Ion Channels and Cancer' took place between 25 and 28 November 2007, at the Schloss Ringberg in Tegernsee, Germany, and was organized by W. Stühmer and M. Djamgoz.
Introduction
The colloquium on ‘Ion Channels and Cancer' at the Schloss Ringberg in Tegernsee, Germany, was the first formal meeting to bring together nearly 60 international scientists, oncologists and representatives of the pharmaceutical industry who share a common interest: understanding the role of ion channels in the development and progression of cancer. This field has been growing steadily during the past decade or so, building on the initial observations that ion channels are involved in both mitogenesis (DeCoursey et al, 1984) and malignancy (Pardo et al, 1999). A rapidly increasing number of ion-channel types are now known to be expressed in various cancers, and some ion channels are selectively expressed in aggressive cancers and are intimately involved in metastasis (reviewed by Diss et al, 2004; Fiske et al, 2006; Kaczmarek, 2006; Roger et al, 2006; Schönherr, 2005; Villalonga et al, 2007).
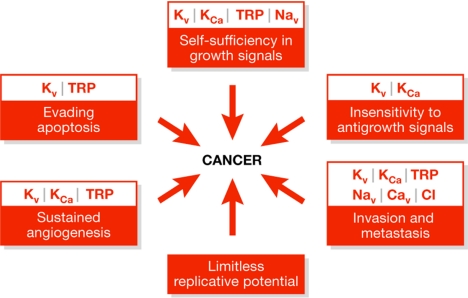
This two-day colloquium covered the mechanisms behind, and the functional consequences of, the flux of the main ions involved in cellular homeostasis: K+, Na+, Ca2+, H+ and Cl−. There was also a strong emphasis on linking the function of the ion channels to cellular behaviours that are important during cancer development and progression (Fig 1). The first day focused on ion-channel involvement in cell proliferation, transformation and apoptosis, whereas the second day dealt with cellular behaviours crucial to metastatic cell spread, such as motility and invasion. In addition to the discussions about the ion channels directly implicated in cancer, other talks covered related topics—for example, autoimmune disease, cell activation and vascular permeability—which highlighted mechanisms and pathways potentially relevant to oncology.
Figure 1.
A schematic diagram illustrating how the ion channels discussed at the Ringberg Colloquium might be linked with various aspects of the ‘six hallmarks of cancer', as originally outlined by Hanahan & Weinberg (2000). TRP, transient receptor potential.
One of the important take-home messages touched on by both the speakers and during the discussion periods was the need for the field to make the transition from basic research to the clinic. As ion channels are viable pharmacological targets for many diseases, there was overwhelming confidence that they will have tremendous potential in diagnosis, prognosis and, importantly, therapy. Indeed, this optimism is supported by some recent publications (Fiske et al, 2006; Gray & Macdonald, 2006; Hoang et al, 2007; Roger et al, 2006; Schönherr, 2005; Villalonga et al, 2007).
Potassium channels
Various types of K+ channel are expressed in non-tumorous cells where they are involved in fundamental cell behaviours such as proliferation and apoptosis (see, for example, Lang et al, 2005). K+ channels are also expressed in tumours (Pardo et al, 2005; Villalonga et al, 2007) and therefore there is much interest in understanding their contribution to cancer progression. The group led by H. Ouadid-Ahidouch (Amiens, France) has been studying the roles of Kv10.1, a voltage-gated potassium channel, and KCa3.1, a calcium-activated potassium channel, in breast cancer. Blocking these channels leads to the accumulation of cells in the G1 phase of the cell cycle. Ouadid-Ahidouch proposed that Kv10.1 allows entry into G1, and then KCa3.1 drives progression through G1 and into S phase. In agreement with this model, growth factors such as insulin-like growth factor 1 (IGF1) increased the expression of both Kv10.1 and KCa3.1, and induced cell proliferation. The current hypothesis is that KCa3.1 activation during G1 results in a strong hyperpolarization of the cell and increases the driving force for Ca2+ entry, possibly through the vanilloid receptor-related transient receptor potential channel 6 (TRPV6). For studies on colon cancer, K. Kunzelmann (Regensburg, Germany) used the human T84 intestinal epithelial cell model. Among the various K+ channels expressed, only voltage-gated K+channels—Kv1.5, Kv3.4 and Kv10.1—were involved in proliferation, possibly through the regulation of intracellular pH or Ca2+. After treatment with carcinogens, mouse colon cells showed increased expression of Kv1.3, Kv1.5, Kv3.1 and Kv10.1. Indeed, Kv10.1 has been detected in human colonic tumours and is associated with a poor prognosis (Ousingsawat et al, 2007). In addition, recent experiments using a mouse with a defect in the adenomatosis polyposis coli (APC) tumour suppressor gene—loss of which is an early event during cancer progression—showed increased KCa1.1 and Kv10.1 expression, which might have clinical implications.
K+ channels are also important in melanoma. R. Schönherr (Jena, Germany) discussed the importance of KCa3.1 and Kv10.1 in controlling the proliferation of several human melanoma cell lines. In addition, a microarray comparison between melanocytes and melanoma of 80 different K+ channel genes revealed a consistent upregulation of KCa3.1. Further studies on fresh melanoma tissue revealed the expression of KCa3.1 in 80% of the cases and Kv10.1 in 50% of the cases. Under hypoxia, which is commonly found in tumours, KCa3.1 was upregulated and this might lead to tumour progression. A. Arcangeli (Florence, Italy) provided insight into the significance of the interaction between β1 integrin and Kv11.1—also known as HERG—which is upregulated in several cancers, including glioma, leukaemia, and stomach and colorectal cancers. This macromolecular complex not only regulates downstream signalling pathways—for example, tyrosine kinases and GTPases—but also leads to signalling through conformational changes rather than ion flux. In acute myeloid leukaemia (AML), in which Kv11.1 expression is known to correlate with a poor prognosis, the Kv11.1–β1 integrin complex associates with the vascular endothelial growth factor (VEGF) receptor 1 to regulate VEGF secretion, cell proliferation and cell migration, resulting in more efficient invasion of the peripheral circulation and extra-medullary sites in immunodeficient mice (Pillozzi et al, 2007). Such Kv11.1–β1 integrin complexes also occur in paediatric acute lymphoblastic leukaemia, in which a block of Kv11.1 signalling leads to an increase in apoptosis, indicating that Kv11.1 could be a viable therapeutic target.
Transient receptor potential channels
It is becoming increasingly clear that TRP channels are involved in cancer progression (Bödding, 2007; Prevarskaya et al, 2007). In relation to prostate cancer, N. Prevarskaya (Villeneuve d'Ascq, France) described the role of classic TRP channels (TRPC), vanilloid TRP channels (TRPV) and melastatin TRP channels (TRPM) in the development of androgen independence. Among other things, androgen independence leads to apoptosis resistance, making the tumour difficult to treat clinically. Prevarskaya's group showed that Ca2+ entry on activation of different TRP channels could either stimulate proliferation through TRPC6 and TRPV6, or induce apoptosis through TRPC1/TRPC4 and TRPM8. Interestingly, the expression and subcellular distribution of TRPM8 is regulated by androgens, and is dependent on metastatic potential. Therefore, TRPM8 could lead to Ca2+ entry through the plasma membrane or Ca2+ release from intracellular stores, and this could contribute to the development of androgen independence (Bidaux et al, 2007). D. Bates (Bristol, UK) described the role of TRP channels in vascular permeability and angiogenesis. Both TRPC3 and TRPC6 channels are expressed in rodent microvasculature in which activation by VEGF or diacyl-glycerol leads to increased intracellular Ca2+ and vascular permeability. A dominant negative TRPC6 construct expressed in human microvascular endothelial cells abolished both the increased intracellular Ca2+ response to VEGF and cell migration. Such a role implies TRP involvement in the cancer process as angiogenesis is an important aspect of tumour growth and metastasis formation.
Sodium channels
Voltage-gated Na+ channels (VGSCs) are now known to be upregulated in many types of cancer, including breast, lung—both small-cell and non-small-cell—and prostate cancers, leukaemia (reviewed by Fiske et al, 2006; Roger et al, 2006) and, more recently, cervical cancer (Diaz et al, 2007) and mesothelioma (Fulgenzi et al, 2006). Although VGSCs potentiate cell behaviours necessary for the metastatic cascade, the mechanisms through which they act are not well understood. J.-Y. Le Guennec (Tours, France) presented evidence that VGSC (Nav1.5) activity increased the invasiveness of human breast cancer cells through increased cysteine cathepsin activity. Cysteine cathepsins are proteolytic enzymes with many roles in cancer metastasis (Mohamed & Sloane, 2006). Interestingly, analogous results were found for prostate and non-small-cell lung cancers, which also express VGSCs, indicating that the involvement of cysteine cathepsins might be a general mechanism in carcinomas. Also studying breast cancer, S. Fraser (London, UK) reported an increase in Nav1.5 amplitude after acute oestrogen application. Oestrogen is a steroid hormone known to be important during breast cancer progression. The mechanism by which it increases Nav1.5 amplitude seems to be through a recently characterized plasma-membrane G-protein-coupled receptor for oestrogen signalling, GPR30, mediated by protein kinase A activation. As the application of oestrogen reduced cell adhesiveness in a VGSC-dependent manner, these findings indicate that the action of oestrogen on VGSCs might be important during breast cancer metastasis. Non-conduction functioning of the VGSC and its possible importance in cancer was discussed by L. Isom (Ann Arbor, MI, USA). VGSCs, which are composed of a central pore-forming α-subunit and auxiliary β (β1–4)-subunits, are part of larger multi-protein complexes that can include components of the cytoskeleton, as well as transport proteins, kinases, phosphatases and extracellular matrix proteins. Therefore, individual components might have different functions and modulation of the complex could contribute to disease conditions, including cancer. For example, β1-null mice suffer many abnormalities, including epilepsy, ataxia, neuronal pathfinding errors and premature death. The exact involvement of the β-subunits might be many and complex, as β-subunits are also known to function independently of the α-subunit. For example, cleavage of the β2-subunit by the β-site of APP cleaving enzyme (BACE1) and γ-secretase results in the release of an intracellular domain of β2, and an increase in VGSC (Nav1.1) mRNA and protein levels in neuroblastoma cells (Kim et al, 2007).
Other ion channels and associated mechanisms
J. Pouysségur (Nice, France) highlighted some of the signalling mechanisms that contribute to tumour resistance and survival in nutrient-depleted and acidic microenvironments. For example, the carbonic anhydrases CAIX and CAXII control internal pH to keep it alkaline, thus promoting cell survival and migration. Hypoxia-inducible factor 1 (HIF1), the expression of which is associated with a poor prognosis, controls the expression of many genes, including BH-3-only proteins BNIP3 and BNIP3L, which influence tumour survival by inducing autophagy (see, for example, Dayan et al, 2006). The regulation of voltage-gated Ca2+ channels (VGCCs) by growth factors was discussed by E. Fitzgerald (Manchester, UK). Growth factors were found to upregulate VGCCs through Ras/extracellular-signal-regulated kinase (ERK) in dorsal root ganglion (Woodall et al, 2008). Both growth factors and the Ras/ERK pathway are extremely important for cancer progression and metastasis although the contribution of VGCCs to the cancer process requires further study. Similarly, L. Kaczmarek (New Haven, CT, USA) described the role of Na+-activated K+ channels, known as slick and slack, in electrically excitable cells. It has been known for some time that protein synthesis at the synapse is Na+-dependent, and the hypothesis being pursued is that changes in conformation of the slack channel produced by increased intracellular Na+ might influence the translation of neuronal mRNAs. Whether such pathways occur during cancer remains to be explored. A. Schwab (Münster, Germany) showed elegantly the involvement of KCa3.1 channels in cell migration. Their physiological function in cell migration is to induce localized changes in cell volume at the rear part of motile cells and thereby support the retraction of this cell pole. By using Madin–Darby canine kidney cells transfected with KCa3.1 with an extracellular haemagglutinin (HA)-tag, single channels could be detected in the plasma membrane by ‘quantum dot' labelling (Nechyporuk-Zloy et al, 2006, 2008). These experiments showed that the channels move in a diffusive manner and are not ‘trapped' in the membrane. Such a technique might allow the labelling of any ion channel in the plasma membrane at the single-molecule level.
D. Beech (Leeds, UK) presented work detailing the control of ion-channel expression in smooth muscle cells by repressor element 1-silencing transcription factor (REST). Importantly, REST is known to control the expression of several ion channels—for example, Nav1.2—and has been implicated in disease development, including cancer (reviewed by Cheong et al, 2006). Vascular smooth muscle cells switch between a quiescent (contractile) and activated (proliferating/migratory) phenotype; crucial to this is the upregulation of KCa3.1, which results from the downregulation of REST. Increased KCa3.1 leads to membrane hyperpolarization and Ca2+ entry through heteromultimeric TRPC1/TRPC5 channels, which are controlled by sphingosine-1-phosphate. However, other ion channels such as Kv1.3 are also important in activated cells and were also found to be regulated by REST. Therefore, REST might have a greater role in the control of ion-channel expression than previously thought. An additional example of the relevance of ion channels in oncology occurs during cancer stem-cell selection in neuroblastoma. E. Wanke (Florence, Italy) reported studies on clonal selection in these tumours and the correlation with changes in electrophysiological properties, so-called ‘electrophysiological clusters of differentiation' (ECD; Marzi et al, 2007). Under hypoxia or in the presence of the antiblastic etoposide, cell lineages developed with different ECD. These results might help to explain the documented regression of neuroblastoma from a malignant to a benign phenotype, either spontaneously or with antiblastic treatment. There are further clinical implications because—as long as the cancer stem-cell model holds true—the only possibility of completely eradicating a tumour is by depleting the stem-cell population. Therefore, distinguishing these cells from healthy cells is of pivotal importance.
Clinical potential
E. Stevens (Cambridge, UK) gave an encouraging talk that highlighted the ongoing potential for developing ion-channel modulators as cancer drugs. Particularly relevant are the advances in selective strategies and screening technologies such as automated electrophysiology. The potential of ion-channel inhibitors was ably shown by H. Sontheimer (Birmingham, AL, USA); looking at the role of ion channels in glioma invasion, Sontheimer and colleagues found that a chloride channel (ClC-3) involved in the invasive process is inhibited indirectly, but specifically, by a scorpion toxin, chlorotoxin (Cltx). Importantly, a recently developed, radiolabelled Cltx compound (I131-Cltx) has successfully completed phase I clinical trials for patients with late-stage gliomas (Mamelak et al, 2006) and a multi-centre phase II trial is now underway. Similarly, G. Chandy (Irvine, CA, USA) described the selective targeting of a type of voltage-gated K+ channel (VGPC), Kv1.3, which is expressed in CCR7−CD45RA− effector/memory T cells (TEM) that are involved in inflammatory autoimmune diseases such as multiple sclerosis and rheumatoid arthritis (Beeton et al, 2006). By using a modified form of the sea anemone toxin ShK (ShK-186) that shows high selectivity for Kv1.3 compared with other VGPCs, tests in animal models indicated a long-term loss of TEM cells and an improvement in autoimmune disease symptoms. Importantly, ShK-186 spares the CCR7+CD45RA− T-cell population that protects against infection and cancers. Kv10.1 was the focus of the presentation by L. Pardo (Göttingen, Germany). Immunohistochemical studies on various types of human tumour revealed Kv10.1 expression in more than 75% of cases but, interestingly, low (or lack of) expression in healthy tissue. Antibodies against Kv10.1, used for in vivo imaging in mice, revealed specific labelling of tumours. In addition, the inhibition of Kv10.1 expression and/or function reduced tumour progression, whereas a specific antibody blocking Kv10.1 activity reduced tumour growth, indicating this could have therapeutic applications.
Concluding remarks
The talks and posters presented at the meeting outlined the remarkable array of processes through which ion channels contribute to the survival of cancer cells, their growth, and progression to the metastatic phase. The topics included the involvement of ion channels in cell proliferation and the development of resistance to apoptosis; their ability to combat the inhospitable surroundings that a cancer cell must endure, including hypoxia and oxidative stress; the various mechanisms involving ion channels that contribute to the metastatic process, including adhesion, motility, invasion, secretion, metabolism and gene expression. Finally, some of the signalling pathways important during carcinogenesis and that have been linked with ion channels—such as growth factors, steroid hormones and protein kinases—were explored. Therefore, although there is much left to understand regarding the involvement of ion channels in the metastatic process, it is encouraging that many of the approaches already adopted have clinical potential in terms of both improved diagnosis/prognosis and therapy.

Scott P. Fraser

Luis A. Pardo
Acknowledgments
We are grateful to W. Stühmer and M. Djamgoz for organizing this excellent and insightful meeting, aided by the outstanding effort of K. Börst. We thank all the speakers for allowing their work to be cited, praise the commentators for their perceptive comments and discussions, and apologize to colleagues whose work might not have been covered fully owing to space limitations. We are grateful for the support of the Max-Planck-Gesellschaft, which was instrumental in facilitating the meeting.
References
- Beeton C et al. (2006) Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA 103: 17414–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaux G et al. (2007) Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J Clin Invest 117: 1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödding M (2007) TRP proteins and cancer. Cell Signal 19: 617–624 [DOI] [PubMed] [Google Scholar]
- Cheong A, Wood IC, Beech DJ (2006) Less REST, more vascular disease? Regulation of cell cycle and migration of vascular smooth muscle cells. Cell Cycle 5: 129–131 [DOI] [PubMed] [Google Scholar]
- Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, Mazure NM (2006) The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-α. Cancer Res 66: 3688–3698 [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD (1984) Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature 307: 465–468 [DOI] [PubMed] [Google Scholar]
- Diaz D, Delgadillo DM, Hernández-Gallegos E, Ramírez-Domínguez ME, Hinojosa LM, Ortiz CS, Berumen J, Camacho J, Gomora JC (2007) Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol 210: 469–478 [DOI] [PubMed] [Google Scholar]
- Diss JKJ, Fraser SP, Djamgoz MBA (2004) Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects. Eur Biophys J 33: 180–193 [DOI] [PubMed] [Google Scholar]
- Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA (2006) Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev 25: 493–500 [DOI] [PubMed] [Google Scholar]
- Fulgenzi G, Graciotti L, Faronato M, Soldovieri MV, Miceli F, Amoroso S, Annunziato L, Procopio A, Taglialatela M (2006) Human neoplastic mesothelial cells express voltage-gated sodium channels involved in cell motility. Int J Biochem Cell Biol 38: 1146–1159 [DOI] [PubMed] [Google Scholar]
- Gray LS, Macdonald TL (2006) The pharmacology and regulation of T type calcium channels: new opportunities for unique therapeutics for cancer. Cell Calcium 40: 115–120 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70 [DOI] [PubMed] [Google Scholar]
- Hoang BX, Shaw DG, Pham P, Levine SA (2007) Neuro-bioenergetic concepts in cancer prevention and treatment. Med Hypoth 68: 832–843 [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK (2006) Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771 [DOI] [PubMed] [Google Scholar]
- Kim DY et al. (2007) BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol 9: 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM (2005) Ion channels in cell proliferation and apoptotic cell death. J Membr Biol 205: 147–157 [DOI] [PubMed] [Google Scholar]
- Mamelak AN et al. (2006) Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent high-grade glioma. J Clin Oncol 24: 3644–3650 [DOI] [PubMed] [Google Scholar]
- Marzi F, D'Amico M, Biagiotti T, Giunti S, Carbone MV, Fredducci D, Wanke E, Olivotto M (2007) Purging of the neuroblastoma stem cell compartment and tumor regression on exposure to hypoxia or cytotoxic treatment. Cancer Res 67: 2402–2407 [DOI] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF (2006) Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer 10: 764–775 [DOI] [PubMed] [Google Scholar]
- Nechyporuk-Zloy V, Stock C, Schillers H, Oberleithner H, Schwab A (2006) Single plasma membrane K+ channel detection by using dual-color quantum dot labeling. Am J Physiol Cell Physiol 291: C266–C269 [DOI] [PubMed] [Google Scholar]
- Nechyporuk-Zloy V, Dieterich P, Oberleithner H, Stock C, Schwab A (2008) Dynamics of single potassium channel proteins in the plama membrane of migrating cells. Am J Physiol Cell Physiol 294: C1096–C1102 [DOI] [PubMed] [Google Scholar]
- Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L, Kunzelmann K, Schreiber R (2007) Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 13: 824–831 [DOI] [PubMed]
- Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W (1999) Oncogenic potential of EAG K+ channels. EMBO J 18: 5540–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W (2005) Role of voltage-gated potassium channels in cancer. J Membr Biol 205: 115–124 [DOI] [PubMed] [Google Scholar]
- Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V, Boddi V, Pegoraro L, Becchetti A, Arcangeli A (2007) VEGFR-1 (FLT-1), β1 integrin and hERG K+ channel form a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 110: 1238–1250 [DOI] [PubMed] [Google Scholar]
- Prevarskaya N, Skryma R, Bidaux G, Flourakis M, Shuba Y (2007) Ion channels in death and differentiation of prostate cancer cells. Cell Death Diff 14: 1295–1304 [DOI] [PubMed] [Google Scholar]
- Roger S, Potier M, Vandier C, Besson P, Le Guennec JY (2006) Voltage-gated sodium channels: new targets in cancer therapy? Curr Pharmaceut Design 12: 3681–3695 [DOI] [PubMed] [Google Scholar]
- Schönherr R (2005) Clinical relevance of ion channels for diagnosis and therapy of cancer. J Membr Biol 205: 175–184 [DOI] [PubMed] [Google Scholar]
- Villalonga N, Ferreres JC, Argiles JM, Condom E, Felipe A (2007) Potassium channels are a new target field in anticancer drug design. Recent Patents Anticancer Drug Discov 2: 212–223 [DOI] [PubMed] [Google Scholar]
- Woodall AJ, Richards MA, Turner DJ, Fitzgerald EM (2008) Growth factors differentially regulate Cav channels via ERK-dependent signalling. Cell Calcium (in press) [DOI] [PubMed] [Google Scholar]