Abstract
Here we describe the epizootiology and pathology of spontaneous, fatal acute intestinal pseudoobstruction that occurred in a mouse colony of 1000 breeding pairs, mainly of the C57Bl/6 strain and free from known pathogenic agents. Most of the mice affected were dams in the second week of lactation. At necropsy, segments of the small intestines were distended with fluid contents. Widespread apoptosis of the villus epithelium of the small intestine and superficial epithelial cells of the large intestine, associated with strong expression of active caspase 3, was a distinctive feature. Necrotic enterocytes, mucosal erosions, and acute mucosal inflammation were prominent in some mice, and morphologic signs of toxemia were generally present. No light microscopic neuronal changes were apparent in the gut, and no etiologic agents were identified. These results indicate that sudden activation of apoptosis in the trophically stimulated gut epithelium during peak lactation was instrumental for the fatal outcome of the condition, but the primary cause of the motility dysfunction of the bowel was not established.
Abbreviation: IPO, intestinal pseudoobstruction
Intestinal pseudoobstruction (IPO) includes various motility disorders of the gastrointestinal tract and is characterized by signs of intestinal obstruction in the absence of mechanical blockage of the bowel.17 These conditions can be a cause of morbidity and mortality in both humans and some animal species. Overt structural damage to the enteric nervous system can occur during various human IPOs and equine grass sickness.5,17 Although IPO in humans can result from genetic and metabolic disorders or even an infectious cause, its etiology is frequently unknown.8,16,17 In particular, acute pseudoobstruction of the colon (Ogilvie's syndrome) occurs in patients with a wide array of predisposing factors and conditions.15 In such cases, IPO is considered to result from an alteration in the autonomic regulation of intestinal motor function, but the mechanisms through which intestinal motility is deranged are unknown.15
An acute idiopathic pseudoobstructive condition of the intestines with a high mortality rate in lactating mice has been reported as paresis of peristalsis.19,28 In those studies, pathologic evaluations were essentially limited to gross observations. We here describe the epizootiologic and pathologic features of acute fatal IPO among mice in an institutional breeding colony that was apparently analogous to those described in the previously cited reports. The cause of the IPO in our mice was not established. However, widespread apoptosis in the gut mucosa, perhaps due to sudden abrogation of trophic stimulation of the gut during lactation, was a prominent feature and likely was instrumental to the fatal outcome.
Materials and Methods
The colony of origin housed about 1000 breeding pairs of mice, more than 80% of which were of the C57BL/6 strain. Mice were housed under barrier conditions in polycarbonatecages with wood-chip bedding. The ambient conditions of the room were about 20 °C, relative humidity of 40% to 60%, and a 12:12-h light:dark cycle. Commercial pelleted food (SDS RM 3, Essex, UK) and sterilized water were available ad libitum. The colony was monitored microbiologically according to the recommendations of the Federation of European Laboratory Animal Science Associations and was known to be free from the following agents: Mouse hepatitis virus, Mouse rotavirus (EDIM), Mouse minute virus, Mouse parvovirus, Pneumonia virus of mice, Sendai virus, Theiler's encephalomyelitis virus, Ectromelia virus, Lymphocytic choriomeningitis virus, Mouse adenoviruses, Mouse cytomegalovirus, Reovirus type 3, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Pasteurellaceae, Mycoplasma pulmonis, streptococci β-haemolytic (except for group D), intestinal parasites and ectoparasites.25
Gross and histologic studies.
All 41 of the mice submitted for necropsy were found dead, except for 1 that was euthanized while moribund. Many additional carcasses were excessively autolyzed and therefore not submitted for postmortem examination. In addition, 5 healthy female mice, 3 lactating and 2 nonlactating, were killed and used as controls for intestinal morphology. At necropsy, the gastrointestinal tract was either excised in toto or cut into several samples for histology. In the healthy mice and most of the sick mice, the heart, kidneys, liver, lung, pancreas, spleen, and selected lymph nodes also were examined histologically.
Tissue specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. The ‘Swiss roll’ technique was applied for embedding of gastrointestinal tracts excised in toto23. Luxol fast blue–cresyl violet and Bielchowsky silver staining were used to visualize the morphology of neurons in the enteric wall, and Giemsa, Gram, and Warthin–Starry stains were used to search for microbes.
Immunohistochemistry.
Paraffin sections of the gut from 6 lactating mice dead from IPO and 2 euthanized healthy female control mice (1 lactating and 1 nonlactating) were cut and pretreated for antigen retrieval (Target Retrieval Solution, Dako A/S, Glostrup, Denmark). Endogenous peroxidase was quenched with 3% hydrogen peroxide, and nonspecific protein binding was inhibited with goat serum (Vector Laboratories, Burlingame, CA). The primary antibody was a rabbit monoclonal antibody against active caspase 3 (clone C92-605, BD Biosciences, Erembodegem, Belgium) used at a dilution of 1:40. The 2-step horseradish peroxidase-conjugated EnVision method (Dako) was used for demonstration of immunoreactivity, with amino-ethylcarbazole as chromogen and hematoxylin as counterstain.
Transmission electron microscopy.
Tissue specimens (3 × 1 × 1 mm) from the jejunum of 6 mice with IPO, 1 of which was euthanized when moribund, and of the control mice, were immersed in 2% buffered glutaraldehyde in 0.1 M PBS (pH 7.4) for at least 24 h, and postfixed in 1% osmium tetroxide for 2 h. In the euthanized mice, tissues were immersed in fixative within 1 to 2 min after death. Sections (1 μm thick) were stained with toluidine blue, and ultrathin sections were stained with uranyl acetate and lead citrate. The specimens were examined by transmission electron microscopy (EM 420, Philips, Eindhoven, Holland) at 60 kV.
Additional studies.
Intestinal samples from 7 affected mice were cultured both aerobically and anaerobically on 5% sheep-blood agar plates at 37 °C for 48 h. The bacterial isolates were identified by standard laboratory procedures at the National Veterinary Institute (Uppsala, Sweden). Blood from the euthanized mouse with IPO and from the 5 healthy control mice was collected by terminal cardiocentesis under anesthesia with CO2. Calcium levels were determined at the Department of Clinical Chemistry (Swedish University of Agricultural Sciences, Uppsala, Sweden) by using commercial kits (catalog no. 981367, Konelab Corporation, Vantaa, Finland).
Results
History of IPO in the study colony.
The mortality commenced in September 1998 and was monitored for a 3.5-y period, during which a total of 148 mice died. Initially, 7 lactating dams were found dead unexpectedly and without any preceding event. Mice continued to die during the winter months, but the mortality ebbed in the spring to continue sporadically, apparently without any seasonal trend. Premonitory signs occurred only rarely and included abdominal distension, arrested lactation, labored breathing, apathy, and hypothermia. There was no clustering of mortality at particular areas of the animal rooms. The pups from affected mothers remained healthy, provided they were nursed by foster dams. The vast majority of the dead mice were of the C57BL/6 strain (78.9%). Other affected mice were NMRI (4.1%), CD1 (1.4%) or hybrids of C57Bl/6 with 129 (8.2%), CBA (5.4%), and an unidentified strain (2.0%), respectively. Of the 139 mice that died for which gender was recorded, 136 were female. Breeding status (lactating or pregnant) was recorded for 124 female mice, of which 123 were lactating and 1 was pregnant. Among the 99 lactating mice for which data regarding the time of death relative to delivery were available, mortality was highest during the second week of lactation (57 of 99 mice), with a peak at postpartum day 10 (Figure 1). Data regarding age were available for 42 mice only (median age, 3 mo; range, 2 to 9 mo).
Figure 1.
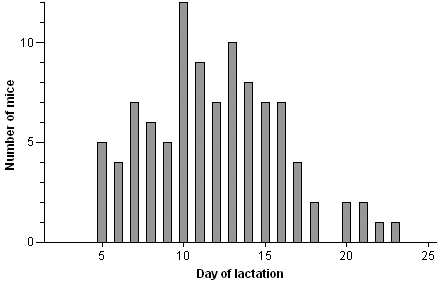
Day of death relative to day of lactation in lactating mice with signs of intestinal pseudoobstruction (n = 99).
Postmortem findings. Gross morphology.
The healthy control mice showed no lesions. In sick mice, the abdomen was markedly distended by enlarged intestinal loops (Figure 2), and the subcutaneous tissues were dry. The stomach frequently was dilated with watery fluid, and 1 mouse had a gastric rupture. The small intestine, predominantly the jejunum and ileum, exhibited dilated segments that ended abruptly or gradually. Dilated segments had dark green to brown, liquid, and frothy contents or contained pellets of dried ingesta. In general, the large intestines had sparse dry contents, but in some animals, the cecum was dilated with fluid contents (Figure 3). Other organs were unremarkable on gross examination.
Figure 2.
Skinned mouse with IPO. Markedly enlarged intestinal loops are visible through the unincised abdominal wall.
Figure 3.
Gastrointestinal tract of mouse with IPO. Segmentally dilated small intestine, most severely proximally. Note abrupt ending of dilation, fluid and frothy contents, pellets of dry ingesta, and empty colon. Moderate dilation of stomach and cecum also is apparent.
Light microscopy.
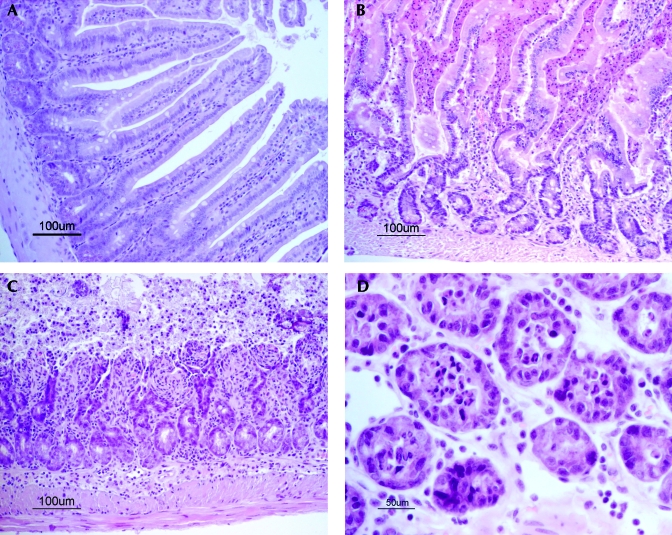
The gastrointestinal tract and other organs were normal in the healthy control mice. The small intestinal mucosa of the lactating healthy mice displayed tall villi lined by a moderately crowded columnar epithelium (Figure 4 A). Intestinal lesions in the IPO mice occurred segmentally. Areas of unaffected mucosa at sites showed epithelial features as in the control mice. The most severe lesions in the mice with IPO were in the dilated parts of the small intestines, but the nondilated segments as well as the large intestine also were affected. At many sites, the mucosa was lined by acidophilic enterocytes with pyknotic nuclei, consistent with apoptotic cells. In the small intestine, these cells appeared on distorted villi, and in the large intestines, in the surface epithelium. Detached acidophilic cells were abundant in the gut lumen (Figure 4 B).
Figure 4.
Histology of jejunum in (A) control mouse and (B through D) mice with IPO. (A) Regularly shaped villi lined by intact, moderately crowded columnar epithelium. (B) High numbers of detached acidophilic cells with pyknotic nuclei in the gut lumen between villi. (C) Eroded mucosa. Inflammatory cell infiltrate in the mucosa and submucosa. (D) Transversally cut mucosal crypts with luminal necrotic leukocytes and cellular debris. Hematoxylin and eosin stain; Bar, 100 μm (A through C), 50 μm (D).
Mucosal erosions with adjacent acidophilic cells and pleomorphic or low columnar basophilic cells were prominent in some mice (Figure 4 C). In such areas, the lamina propria and deeper layers were congested and infiltrated by mixed inflammatory cells, chiefly neutrophils, and occasional hemorrhages were present. The gut lumen and crypts contained necrotic polymorphs (Figure 4 D), mucus, and numerous bacteria. In many of the mice, Paneth cells were prominent, and released granules were abundant.
No significant lesions were present in intestinal nerve plexuses, and the density of neuronal cell bodies appeared unchanged compared with that of control mice. Finely vacuolated neurons were present only rarely in both affected and control mice. In sections stained with Luxol fast blue and cresyl violet, neuronal Nissl substance was generally abundant.
The lymph follicles of the small intestine, mesenteric lymph nodes, and spleen exhibited excessive lymphocytolysis. Neutrophil stasis in small blood vessels at all levels of the intestinal wall, as well as in the liver, spleen, lung, heart and kidney, were prominent in mice with erosive intestinal lesions. No viral inclusion bodies, rods of Clostridium piliforme, or protozoan parasites or helminths were noted in any mouse.
Immunohistochemistry.
The intestinal mucosa of the control mice showed only occasional epithelial cells that stained positively for active caspase 3. In contrast, the jejunal and colonic epithelium of the sick mice displayed numerous strongly immunostained enterocytes, coinciding with the acidophilic enterocytes visible after staining with hematoxylin and eosin (Figure 5). Immunostaining was not seen in intestinal neuronal cell bodies.
Figure 5.
Immunostaining for active caspase 3, predominantly in the gut epithelium at the top of villi and in cells detached in the gut lumen. Stained by using a monoclonal antibody to active caspase 3 and conjugation with peroxidase, and visualized by using EnVision (Dako). Bar, 100 μm.
Transmission electron microscopy.
The jejunal mucosa of control mice exhibited normal ultrastructural features, as described previously.31 The following findings refer only to the ill mouse sampled immediately after euthanasia. Whereas light microscopy of the small intestine had shown erosive lesions and abundant epithelial acidophilic cells, electron microscopy revealed enterocytes, lining the mucosa or detached, with features of apoptosis.18,33 These features included electron-dense cytoplasm, visible microvilli, aggregation of organelles, and condensed and marginated nuclear chromatin (Figure 6 A). Apoptotic bodies were frequent (Figure 6 B). Other epithelial cells in the crypts and villi had numerous free ribosomes, many cytolysosomes, and swollen mitochondria with coiled cristae, highly electron-dense granules, and myeloid bodies, consistent with sublethal injury. Necrotic epithelial cells,10 characterized by electron-lucent cytoplasm, loss of cytoplasmic organelles, and fragmented nuclei, were also present.
Figure 6.
Transmission electron microscopy of upper jejunum from mouse with IPO. A. Apoptotic epithelial cell disintegrating in the gut lumen. Nucleus (N) with marginated, condensed chromatin. Densely packed organelles, including mitochondria and electron-dense vesicles, are visible. Superficial parts of intact surface epithelial cells are visible at left (*). Original magnification × 5600. (B) Parts of villus enterocytes. Apoptotic body (arrow) with condensed nuclear chromatin and densely packed organelles. The nucleus of an enterocyte is indicated (N). Original magnification × 1950.
Despite the autolytic changes in mice examined after delayed fixation, apoptotic cells and bodies were discernible in the epithelial lining and among detached cells. Neither virus particles nor intracellular bacteria were observed by electron microscopy of any mouse.
Additional studies.
Bacterial cultures of the small intestines yielded bacteria of the normal flora, and in 2 of the 7 mice, growth of Clostridium perfringens. Plasma calcium levels were within the normal range21 in the 1 mouse with IPO and the 5 control mice examined (median, 2.7 mmol/l; range, 2.6 to 3.5 mmol/l).
Discussion
The clinical signs and major gross findings of segmental dilation of the gut in the mice of this study were analogous to previous descriptions of paresis of peristalsis in mice.19,28 The disorder is consistent with acute intestinal pseudoobstruction (IPO), the term we prefer for this condition. The predominance of IPO cases among C57BL/6 mice mirrored the abundance of this breed at the colony, and the mortality evolved without any obvious seasonal pattern.
Neither of the above reports19,28 addressed the involvement of intestinal microorganisms. However, overgrowth of Clostridium perfringens type A was recently reported in lactating mice with bloating and sudden death from a necrohemorrhagic enteropathy at the top of lactation.20The colony in our study had been checked regularly for microorganisms, including rodent viruses such as rotaviruses and coronaviruses,25 and the observed pathology did not correspond to any disease caused by known rodent pathogens. Furthermore, no protozoan parasites, viral inclusions, or virus particles were found, and the pups from mice with IPO remained healthy, provided they were nursed by a foster dam. The possibility that the disorder primarily was caused by a pathogenic microorganism is therefore remote.
Our data showed a distinct predilection for dams in their second week of lactation, which coincides with the time of maximal milk yield in mice.12 A similar pattern was evident in previous reports.19,20,28 The association with lactation, as suggested previously,19 might indicate calcium deficiency as a possible inciting cause, but the plasma calcium level in the single mouse we measured was normal. Furthermore, to our knowledge, IPO is not a recognized feature of hypocalcemia in animals, and although hypocalcemia may occur with Ogilvie's syndrome in humans, it is only 1 of several electrolyte disturbances in patients with this disorder.15
The present report includes the first description of murine lactation-associated IPO at the histologic and ultrastructural levels. Routine histology and histochemistry did not reveal neuronal cell injury or myopathy, therefore suggesting that the motility disturbance might be purely functional.
Major histologic lesions of the intestinal mucosa included massive exfoliation of acidophilic and pyknotic epithelial cells, consistent with lethal cell damage. Mucosal erosions combined with acute inflammation were frequent, and ultrastructural features of necrosis of epithelial cells were evident. Epithelial acidophilia, pyknosis, and exfoliation likely were early changes that preceded erosions and inflammation. The acidophilic cells indicated apoptotic cell death, which was confirmed by ultrastructural findings and strong expression of activated caspase 3 in exfoliated and lining superficial enterocytes. Among the key enzymes triggering apoptosis, activated caspase 3 has been recognized as a marker of apoptotic cells in tissue sections.9 Apoptosis has been identified at 2 levels of the mammalian intestinal epithelium: in the lower crypt area, probably regulating cell numbers entering the crypt–villus axis, and regulating cell shedding at the villus tip.32 Consistent with previous studies,22,32 apoptotic and caspase-3–expressing cells were only occasionally present in the control mice in the present study.
Lymphocytolysis in lymphoid tissues, together with intravascular neutrophil stasis in extraintestinal organs, were frequent findings in the present mice. These changes are consistent with acute systemic inflammation in animal models of bacterial toxemia and septicemia.14,27 Bacterial proliferation in the gut lumen of the IPO mice was revealed through histology and was supported by the recovery of Clostridium perfringens from 2 of 7 mice. We consider it likely that a fatal toxemic state arose from intestinal bacterial overgrowth secondary to cessation of intestinal peristalsis in combination with breakdown of the mucosal barrier.
The cause of the pronounced apoptosis is unclear, and several factors may be implicated. The epizootiology of IPO among our mice indicates that lactation was critical for its development or at least for its fatal outcome. The intestinal epithelium of lactating mice is considered to respond by hyperplasia as an adaptation to increased metabolic demands during lactation.11,13,24 Suggested factors behind the trophic effects include both increased food intake, which is a prominent feature of murine lactation,11,13 and lactational hormones, such as prolactin.11 Experimental hyperprolactinemia, however, failed to produce mucosal hyperplasia in rats.24 Other peptides, including insulin-like growth factor 1 and enteroendocrine glucagon-like peptide 2, have powerful trophic effects on the intestinal mucosa of mice and other mammals.3,4,7,30 Glucagon-like peptide 2 is a potent stimulator of crypt epithelial cell growth and inhibits apoptosis of villus cells. Nutrient ingestion evokes the secretion of glucagon-like peptide 2, a response that is mediated through neural pathways.2,4 We infer that a sudden abrogation of trophic stimulation could lead to massive apoptosis in the stimulated gut mucosa. Hypothetically, an acute neurogenic disturbance of the gut, or perhaps even interruption of food intake, could trigger such mechanisms.
Other apoptosis-inducing factors also should be considered. The excessive distension of the gut might have resulted in ischemia of parts of the intestinal mucosa, and local ischemia can induce apoptosis of enterocytes.26,29 Because the lesions were compatible with toxemia, apoptosis of the gut epithelium could have resulted from bacterial toxins, as shown in experimental gram-negative endotoxemia in mice and cats.1,6
The present study extends our understanding of this fatal murine pseudoobstructive enteropathy, but the cause of the condition remains unknown. The results obtained warrant more detailed studies on the enteric nervous system, especially on the possible involvement of gut neuropeptides in the pathogenesis of the motility dysfunction.
Acknowledgments
We thank Tapio Nikkilä (Department of Biomedical Sciences and Veterinary Public Health) for technical assistance with electron microscopy.
References
- 1.Alscher KT, Phang PT, McDonald TE, Walley KR. 2001. Enteral feeding decreases gut apoptosis, permeability, and lung inflammation during murine endotoxemia. Am J Physiol Gastrointest Liver Physiol 281:G569–G576 [DOI] [PubMed] [Google Scholar]
- 2.Bjerknes M, Cheng H. 2001. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proc Natl Acad Sci USA 98:12497–12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botsios DS, Vasiliadis KD. 2003. Factors enhancing intestinal adaptation after bowel compensation. Dig Dis 21:228–236 [DOI] [PubMed] [Google Scholar]
- 4.Brubaker PL, Anini Y. 2003. Direct and indirect mechanisms regulating secretion of glucagon-like peptide 1 and glucagon-like peptide 2. Can J Physiol Pharmacol 81:1005–1012 [DOI] [PubMed] [Google Scholar]
- 5.Cottrell DF, McGorum BC, Pearson GT. 1999. The neurology and enterology of equine grass sickness: a review of basic mechanisms. Neurogastroenterol Motil 11:79–92 [DOI] [PubMed] [Google Scholar]
- 6.Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA. 2000. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med 161:1705–1712 [DOI] [PubMed] [Google Scholar]
- 7.Dahly EM, Guo Z, Ney DM. 2003. IGF 1 augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol 285:R800–R808 [DOI] [PubMed] [Google Scholar]
- 8.Debinski HS, Kamm MA, Talbot IC, Khan G, Kangro HO, Jeffries DJ. 1997. DNA viruses in the pathogenesis of sporadic chronic idiopathic intestinal pseudo-obstruction. Gut 41:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan WR, Garner DS, Williams SD, Funkes-Shippy CL, Spath IS, Blomme EAG. 2003. Comparison of immunohistochemistry for activated caspase 3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC3 subcutaneous xenographs. J Pathol 199:221–228 [DOI] [PubMed] [Google Scholar]
- 10.Ghadially FN. 1988. Ultrastructural pathology of the cell and matrix, 3rd edn, vol. 1. London: Butterworths; p 440 [Google Scholar]
- 11.Hammond KA. 1997. Adaptation of the maternal intestine during lactation. J Mammary Gland Biol Neoplasia 2:243–252 [DOI] [PubMed] [Google Scholar]
- 12.Hanrahan JP, Eisen EJ. 1970. A lactation curve for mice. Lab Anim Care 20:101–104 [PubMed] [Google Scholar]
- 13.Harding JD, Cairnie AB. 1975. Changes in intestinal cell kinetics in the small intestine of lactating mice. Cell Tissue Kinet 8:135–144 [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. 1997. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 7:247–253 [DOI] [PubMed] [Google Scholar]
- 15.Jetmore AB, Timmcke AE, Gathright JB, Jr, Hicks TC, Ray JE, Baker JW. 1992. Ogilvie's syndrome: colonoscopic decompression and analysis of predisposing factors. Dis Colon Rectum 35:1135–1142 [DOI] [PubMed] [Google Scholar]
- 16.Kapur RP. 2001. Neuropathology of paediatric chronic intestinal pseudo-obstruction and related animal models. J Pathol 194:277–288 [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurthy S, Schuffler MD. 1987. Pathology of neuromuscular disorders of the small intestine and colon. Gastroenterology 93:610–639 [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Abbas AK, Fausto N, editors. 2005. Robbins and Cotran pathologic basis of disease, 7th edn. Philadelphia: Elsevier Saunders; p 26–32 [Google Scholar]
- 19.Kunstyr I. 1986. Paresis of peristalsis and ileus lead to death in lactating mice. Lab Anim 20:32–35 [DOI] [PubMed] [Google Scholar]
- 20.Krugner-Higby L, Girard I, Welter J, Gendron A, Rhodes JS, Garland T., Jr 2006. Clostridial enteropathy in lactating outbred Swiss-derived (ICR) mice. J Am Assoc Lab Anim Sci 45:80–87 [PubMed] [Google Scholar]
- 21.Loeb F, Quimby FW. 1989. The clinical chemistry of laboratory animals. Oxford: Pergamon Press; p 438 [Google Scholar]
- 22.Marshman E, Ottewell PD, Potten CS, Watson AJM. 2001. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol 195:285–292 [DOI] [PubMed] [Google Scholar]
- 23.Moolenbeek C, Ruitenberg EJ. 1981. The ”Swiss roll”: A simple technique for histological studies of the rodent intestine. Lab Anim 15:57–59 [DOI] [PubMed] [Google Scholar]
- 24.Muller E, Dowling RH. 1981. Prolactin and the small intestine. Effect of hyperprolactinaemia on mucosal structure in the rat. Gut 22:558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. 2002. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim 36:20–42 [DOI] [PubMed] [Google Scholar]
- 26.Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. 1998. Programmed cell death induced by ischemia–reperfusion in rat intestinal mucosa. Am J Physiol 274:G270–G276 [DOI] [PubMed] [Google Scholar]
- 27.O'Malley J, Matesic LE, Zink MC, Strandberg JD, Mooney ML, DeMaio A, Reeves RH. 1998. Comparison of acute endotoxin-induced lesions in A/J and C57BL/6J mice. J Hered 89:525–530 [DOI] [PubMed] [Google Scholar]
- 28.Rollman C, Olshan K, Hammer J. 1998. Abdominal distension in lactating mice: paresis (paralysis) of peristalsis in lactating mice. Lab Anim (NY) 27:19–20 [Google Scholar]
- 29.Saegesser F, Sandblom P. 1975. Ischemic lesions of the distended colon. A complication of obstructive colorectal cancer. Am J Surg 129:309–315 [DOI] [PubMed] [Google Scholar]
- 30.Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. 2005. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide 2 in mice. Gastroenterology 128:1340–1353 [DOI] [PubMed] [Google Scholar]
- 31.Toner PG, Carr KE, Wyburn GM. 1971. The digestive system—an ultrastructural atlas and review London: Butterworth; p 55–204 [Google Scholar]
- 32.Watson AJM, Pritchard DM. 2000. Lessons from genetically engineered animal models. VII. Apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am J Physiol Gastrointest Liver Physiol 278:G1–G5 [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Sheng Z-Y, Hu S, Gao J-C, Yu S, Liu Y. 2002. The influence of apoptosis of mucosal epithelial cells on intestinal barrier integrity after scald in rats. Burns 28:731–737 [DOI] [PubMed] [Google Scholar]