Abstract
Pituitary adenylate cyclase-activating peptide (PACAP), a cAMP-activating agent, is highly expressed in the hypothalamus during the period when many neuroendocrine cells become differentiated from the neural stem cells (NSCs). Activation of the cAMP system in rat hypothalamic NSCs differentiated these cells into β-endorphin (BEP)-producing neurons in culture. When these in vitro differentiated neurons were transplanted into the paraventricular nucleus (PVN) of the hypothalamus of an adult rat, they integrated well with the surrounding cells and produced BEP and its precursor gene product, proopiomelanocortin (POMC). Animals with BEP cell transplants demonstrated remarkable protection against carcinogen induction of prostate cancer. Unlike carcinogen-treated animals with control cell transplants, rats with BEP cell transplants showed rare development of glandular hyperplasia, prostatic intraepithelial neoplasia (PIN), or well differentiated adenocarcinoma with invasion after N-methyl-N-nitrosourea (MNU) and testosterone treatments. Rats with the BEP neuron transplants showed increased natural killer (NK) cell cytolytic function in the spleens and peripheral blood mononuclear cells (PBMCs), elevated levels of antiinflammatory cytokine IFN-γ, and decreased levels of inflammatory cytokine tumor necrosis factor-α (TNF-α) in plasma. These results identified a critical role for cAMP in the differentiation of BEP neurons and revealed a previously undescribed role of these neurons in combating the growth and progression of neoplastic conditions like prostate cancer, possibly by increasing the innate immune function and reducing the inflammatory milieu.
Keywords: natural killer cells, rats, stem cells
The hypothalamus consists of several groups of hormone-secreting neurons that are critical for various neuroendocrine functions (1). Most of the neurons in the hypothalamus are derived from the proliferative neuroepithelium of the third ventricle (2). A study of cell development in the rat hypothalamus, using [3H]thymidine uptake assays, revealed that most of the neurons of the tuberomammillary and arcuate nuclei have late-forming starts, beginning after embryonic day 16 and continuing until birth (3). However, the inductive signal involved in the generation of specific neuronal cell types from these embryonic cells has not been identified. By using cells from the hypothalamus of rat embryos, it has been shown that cAMP-elevating agents protect against ethanol-induced death of β-endorphin (BEP) neurons (4). These findings have raised the question of whether this neurotrophic factor can be used to direct heterologous sets of neural stem cells (NSCs) into specific neuronal phenotype.
BEP neuronal cell bodies are primarily localized in the arcuate nuclei of the hypothalamus, and its terminals are distributed throughout the central nervous system. These neurons are involved in maintaining a variety of functions including stress regulation and immune functions (5, 6). Abnormalities in BEP neuronal function are correlated with various pathologies. For example, lower numbers of BEP neurons have been found in the postmortem brains of patients with schizophrenia and depression (7, 8), and a reduced BEP production due to proopiomelanocortin (POMC) gene mutation has been observed in many obese patients (9). It is noteworthy that a higher incidence of cancers and infection has been found under these pathological conditions (10–12). Furthermore, the endogenous function of BEP neurons is reported to be reduced in cancer patients (13). Therefore, we hypothesized that increased BEP neuronal activity might be beneficial to control the growth of tumors. In this study, we examined whether a cAMP analog, dbcAMP, and pituitary adenylate cyclase-activating peptide (PACAP) can be used to direct the differentiation of hypothalamus-derived NSCs into functional BEP neurons in vitro, and we determined the functionality of differentiated cells in vivo by transplanting them into the hypothalamus of male rats and evaluating their effects on carcinogen-induced prostate tumors and immune functions.
Results
cAMP-Elevating Agents Increased Differentiation of Hypothalamic NSCs to BEP Neurons in Cultures.
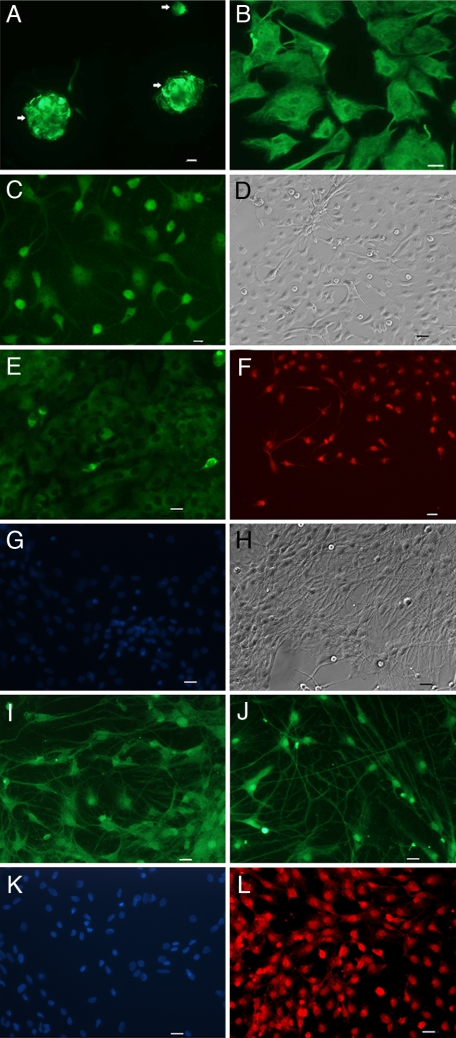
To examine the capacity of NSCs to generate BEP neurons, we purified neurons from embryonic hypothalamic tissues and grew neurospheres in cultures using stem cell-maintaining medium. It was determined whether or not PACAP and dbcAMP differentiate NSCs into neurons. An initial screening of the response of various doses (0.1–10 μM) of PACAP and dbcAMP alone revealed a moderate effect of these agents on neurosphere differentiation, because many cells remained as neurosphere-like structures. However, using a combined treatment of 10-μM concentrations of PACAP and dbcAMP, we found many neurospheres started forming single cells with various shapes (Fig. 1A) within a 3-day period. Many of these cells expressed significant amounts of vimentin (Fig. 1B) and α-internexin immunoreactivity (Fig. 1C), which are markers of early neuronal phenotypes (14). After 1 wk of PACAP and cAMP treatment, NSCs began to show filamentous structures (Fig. 1D) and to express neurofilament (NF)-M (Fig. 1E), a neuronal marker (15), indicating that the NSCs had begun to differentiate into neurons. Determination of the phenotype revealed that many of these cells (50–60%) were expressing BEP at this stage of differentiation (Fig. 1F). NSCs that were not treated with PACAP and cAMP showed no staining for BEP (IH).
Fig. 1.
Immunocytochemical characterization of NSCs at different stages of differentiation. (A) Immunofluorescence staining of nestin, shown by arrows in NSCs at day 0. (B and C) At day 3, staining for vimentin (B) and α-internexin (C) in the early stage of differentiation. (D–F) At day 7, phase-contrast image (D) and staining for the neuronal marker NF-M (E) and BEP (F). (G) Control NSCs maintained in neuron culture medium without PACAP and cAMP showed no staining for BEP but showed DAPI staining. (H–L) At day 14, phase-contrast image (H), staining for neuronal markers MAP2 (I) and type III β-tubulin J, no staining for GFAP (K), and positive staining for BEP (L). Green staining is for NSCs or neural cell marker, red staining is for BEP, and blue staining is for nuclear DAPI. (Scale bars: 10 μm.)
The differentiated NSCs were further maintained in a defined-neuronal cell culture medium for a period of 1 wk to determine the permanency of the PACAP/cAMP effects. By the end of this treatment, all of these cells had a neuron-like appearance (Fig. 1I), and they expressed neuronal markers MAP2 (Fig. 1I; ref. 16) and type III β-tubulin (Fig. 1J), but not the astrocyte cell marker GFAP (Fig. 1K; ref. 17), suggesting that all NSCs were now differentiated into neurons. These cells also stained for BEP (Fig. 1L). A control experiment with excess antigen verified the specificity of BEP immunostaining in differentiated NSCs [supporting information (SI) Fig. S1A]. The staining of BEP for these cells merged well with the nuclear staining for DAPI (Fig. S1B), suggesting that the majority of NSCs is differentiated into BEP cells. However, these differentiated NSCs did not stain for neuropeptide Y, gonadotropin hormone-releasing hormone, or tyrosine hydroxylase (Fig. S1 C–E). These are some of the major peptides in the hypothalamus positively regulated by the cAMP and PACAP system (18–20). Therefore, activation of the cAMP system results in differentiation of NSCs to primarily BEP neurons.
cAMP Agent-Induced Differentiated Neurons Produced and Released BEP.
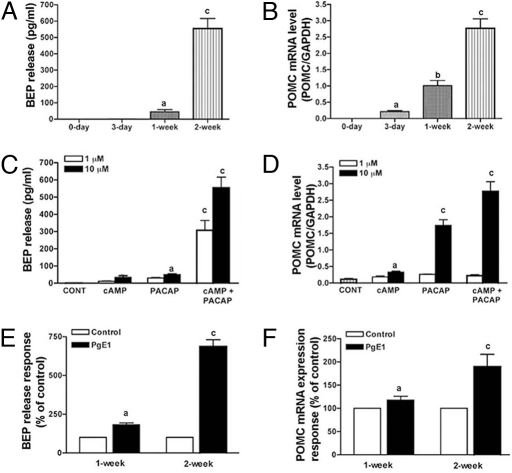
To clarify whether the differentiated NSCs replicate the functions of BEP neurons, we studied the dynamics of basal secretion of BEP during the period that the NSCs were differentiating in culture as well as after the completion of differentiation. In agreement with the immunohistochemical data, Fig. 2A shows that, immediately after the 1-wk treatment with PACAP and cAMP, NSCs secreted moderate amounts of BEP into the media. Furthermore, 1 wk after the weeklong treatment with PACAP/cAMP, NSCs secreted an amount of peptide in the media 10- to 12-fold greater than that which was produced immediately after the treatment. Like BEP release, the POMC mRNA levels in the cells were markedly increased at this time (Fig. 2B). Additionally, the amount of hormone released from these cells by PACAP and cAMP as a result of the amount of BEP neuron differentiation showed dose-dependency and synergistic effects when the two differentiating agents were combined (Fig. 2C). The expression patterns of POMC mRNA resembled the patterns of BEP released during PACAP and cAMP-induced differentiation (Fig. 2D). We also studied the ability of differentiated BEP neurons to respond to prostaglandin E1 (PGE1), which is known to elevate BEP release from hypothalamic cells (21). Fig. 2 E and F shows that BEP release and POMC expression of the differentiated neurons were increased by PGE1. These results suggest that the differentiated neurons produce and release BEP in culture.
Fig. 2.
Biochemical characterization of NSCs at different stages of differentiation. (A and B) BEP release (A) and POMC mRNA expression (B) in NSCs at different time periods of differentiation in the presence of PACAP (10 μM) and dbcAMP (10 μM). No BEP was detected in media samples collected from control cultures without vehicle (0-day) or the cultures treated with the differentiating agents for 3 days (3-day). (C and D) Dose-response and the synergistic effect of PACAP and dbcAMP on BEP neuron differentiation as determined by the ability of NSCs to release BEP (C) and express POMC mRNA (D) after treatment with the drug for 1 wk and then without the drugs for 1 wk. The control group was treated similarly with vehicle. (E and F) PgE1 (10 μM for 3 h)-induced BEP release (E) and POMC mRNA expression (F) in differentiated cells. Values are presented as a percentage of vehicle-treated control. n = 6–8. a, P < 0.05; b, P < 0.01; c, P < 0.001 vs. the rest.
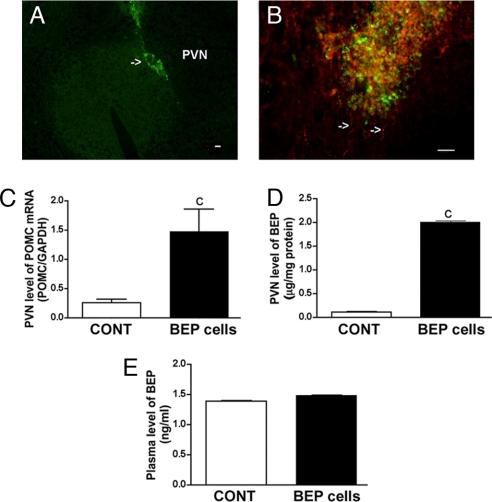
To determine whether the differentiated neurons maintained their neuronal phenotype in vivo, they were labeled with bromodeoxyuridine (BrdU) and transplanted into one lobe of the paraventricular nucleus (PVN) of the hypothalamus, a site containing very few BEP cell bodies (22). Two weeks after the transplantation, these cells remained at the site of transplantation in the PVN (Fig. 3A) and showed immunostaining for BEP (Fig. 3B). By determining levels of POMC mRNA in the PVN, we found that the expression of this gene was higher by a factor of 6 in the PVN where the BEP cells were transplanted than the lobe of the PVN into which nonviable BEP cells had been implanted (Fig. 3C). Determination of BEP protein levels in the hypothalamus and in plasma revealed that the transplants increased levels of these protein levels in the site of transplants (Fig. 3D) but not in the circulation (Fig. 3E).
Fig. 3.
Determination of the viability of NSC-derived BEP cells transplanted in the PVN. Young male rats were transplanted with in vitro differentiated BEP cells or nonviable BEP cell (CONT) unilaterally in the PVN. The viability of these transplanted cells was determined by immunocytochemical (A and B) and biochemical (C–E) procedures. (A) Representative photographs showing BrdU-stained cells at the site of transplantation. (B) High-power view showing immunofluorescence staining for BrdU (green), BEP (red), and merged (yellow) of transplanted cells. Many BEP-stained cells show long processes (arrows). (Scale bars: 10 μm.) (C–E) POMC mRNA levels (C) and BEP levels in the PVN (D) and BEP levels in plasma (E) of animals with BEP cell or CONT transplants. n = 6–8. c, P < 0.001 vs. CONT.
cAMP Agent-Induced Differentiated BEP Neurons Reduced N-Methyl-N-Nitrosourea (MNU)-Induced Prostate Tumors.
To determine the effect of BEP cell transplants on tumor growth and development, we implanted BEP cells in both lobes of the PVN of male rats and treated them with MNU and testosterone as described in ref. 23. For the control transplant, we used viable, non-BEP-producing fetal rat cortical cells rather than the nonviable BEP cells for a long-term transplant study. The viability of BEP cell transplant was tested by determining the plasma corticosterone response to LPS in the animal before euthanization. We hypothesized that, if BEP cell transplants were functional, they would have the ability to inhibit LPS-induced CRH release and therefore corticosterone release in the circulation. BEP neurons have been shown to inhibit CRH, which regulates the plasma level corticosterone (5). We found that LPS increased plasma corticosterone level in rats treated with control transplants and MNU and testosterone (saline-285 ± 15; LPS-478 ± 20; n = 11–12; P < 0.05) but not in rats treated with BEP cell transplants without MNU and testosterone (saline-305 ± 15; LPS-328 ± 14; n = 5–6) or with MNU and testosterone (saline-355 ± 35; LPS-398 ± 20; n = 8–9), suggesting that BEP neuron transplants were functional until the end of the treatment.
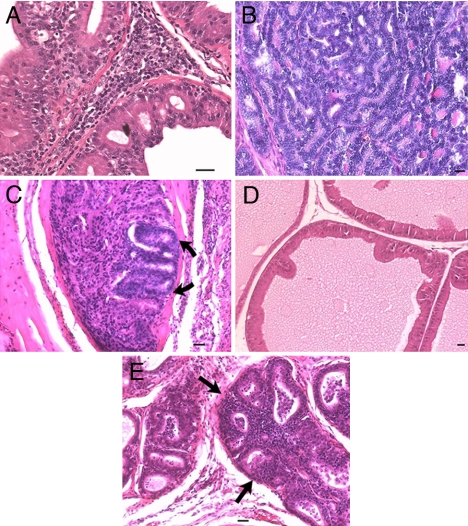
We found that total weight of prostates in rats treated with BEP neuron transplants and carcinogen did not significantly differ from those in rats treated with BEP neuron transplants and vehicle but differed from those in rats treated with control transplants plus carcinogen (total prostate weight: BEP neurons plus vehicle, 213 ± 13 mg/100 g of body weight; BEP neurons plus carcinogen, 396 ± 50 mg/100 g of body weight; CONT plus carcinogen, 908 ± 109 mg/100 g of body weight; P < 0.001, CONT plus carcinogen vs. the rest; n = 11–14). The prostates of rats receiving control cell transplants plus carcinogen displayed glandular hyperplasia (Fig. 4A), prostatic intraepithelial neoplasia (PIN) (Fig. 4B), and occasionally well differentiated adenocarcinoma with invasion (Fig. 4C). These lesions were primarily localized in the dorsolateral and anterior prostate. Similar to the non-carcinogen-treated controls, rats receiving BEP neuronal transplants and carcinogen showed either normal prostatic morphology (Fig. 4D) or mild epithelial atypia with glandular crowding (Fig. 4E). The incidence of adenocarcinoma was markedly lower (only 1 of 17 rats examined; P < 0.03) in the carcinogen plus BEP neuron transplant group than those rats receiving carcinogen and control cell transplants (22 of 24 rats examined; Table 1).
Fig. 4.
Evaluation of the effect of BEP cell transplants on the MNU and testosterone-induced prostate cancers. Adult male rats were transplanted with in vitro differentiated BEP cells or cortical cells (CONT) bilaterally in the PVN of male rats. These rats were then treated with MNU and testosterone treatments and used for determination of histopathology of prostates. (A–C) Prostates of rats transplanted with CONT showed lesions ranging from epithelial hyperplasia with mild atypia (A) to high-grade PIN (B) and occasionally invasive adenocarcinoma (shown by arrows; C). (D and E) Prostates of rats transplanted with BEP cells demonstrated very mild changes, ranging from minimal (D) to moderate hyperplasia (shown by arrows; E). (Scale bars: 10 μm.)
Table 1.
Effect of BEP cell transplants on prostatic neoplasia
| Treatment | % normal (n) | % atypia (n) | % hyperplasia (n) | % neoplasia (n) |
|---|---|---|---|---|
| BEP cells + vehicle | 67 (14) | 33 (7) | 0 | 0 |
| CONT + carcinogen | 3 (1) | 0 | 48 (11)* | 48 (11)* |
| BEP cells + carcinogen | 65 (11) | 29 (5) | 6 (1) | 0 |
BEP cells or cortical cells (controls; CONT) were transplanted into the PVN of male rats. The rats were then treated with MNU and testosterone or with vehicle.
*, P < 0.03; % neoplasia and % hyperplasia in CONT vs. BEP cells as determined by Fisher's exact test.
BEP Neuronal Transplants Increased Natural Killer (NK) Cell Cytolytic Function and Altered Production of IFN-γ and TNF-α.
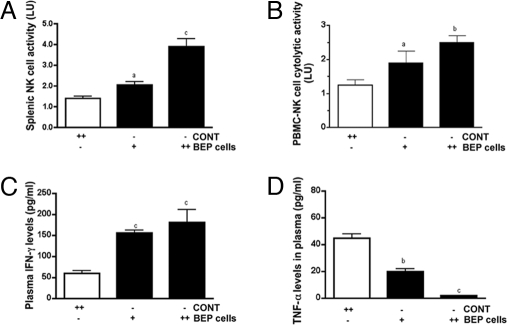
Because NK cells with potent cytotoxic activity are known to effectively kill prostate cancer cells (24), we investigated whether BEP cell transplants altered the NK cell cytolytic function. The effect of BEP cells on inflammatory and antiinflammatory cytokine levels in circulation was also studied, because this peptide is known to have potent antiinflammatory effects in the body (25). Additionally, epidemiologic studies, together with laboratory and clinical studies, suggest that infection and inflammation contribute to the early development of prostate cancer (26, 27). These issues were investigated by determining whether BEP cells can elevate NK cell function and alter circulatory levels of inflammatory and antiinflammatory cytokines in rats. To characterize the influence of BEP cell transplants influence on NK cells, the effects of these transplants either in one PVN or in both PVN of rats were determined. Data showed that BEP neuron-induced activation of splenic NK cell cytolytic function was greater when these cells were transplanted in both PVNs as compared with only one PVN (Fig. 5A). BEP transplants produced similar dose-response effects on NK cell cytolytic activity in peripheral blood mononuclear cells (PBMCs) (Fig. 5B) and on IFN-γ levels in plasma of rats (Fig. 5C). In contrast, BEP transplants dose-dependently reduced levels of TNF-α levels in the plasma of rats (Fig. 5D). These results suggest that BEP cell transplants are effective in activating NK cell cytolytic functions and reducing the body's inflammatory milieu.
Fig. 5.
Evaluation of the effects of BEP cell transplants on immune functions. Rats were transplanted with BEP cells or cortical cells (CONT) into one PVN (+) or two PVN (++) for 4 wk. Shown are dose-response effects of BEP cell transplants on splenic NK cell cytolytic activity (A), PBMC-derived NK cell cytolytic activity (B), plasma IFN-γ (C), and plasma TNF-α (D). n = 5–7 rats. a, P < 0.05; b, P < 0.01; c, P < 0.001 vs. the CONT.
Discussion
The main finding was that, under appropriate conditions, NSCs can be generated from rat embryonic hypothalamic tissues and propagated by cAMP-elevating agents to produce BEP neurons in cultures. Like in vivo, these cells go on to produce and secrete BEP, and they respond positively to the neuromodulator challenge. When transplanted in the hypothalamus, BEP cells survive and produce the peptide hormone. These BEP cell transplants inhibit prostate tumor development, possibly by increasing the NK cell activity, reducing the body's inflammatory milieu, and by yet unknown immune surveillance mechanisms. These results identify a critical role for cAMP in the differentiation of BEP neurons and reveal a previously uncharacterized role of these neurons in controlling prostate tumor growth.
PACAP belongs to the peptide family that includes secretin, glucagons, growth hormone-releasing factor, and vasoactive-intestinal peptide (28). Recent data suggest that PACAP affects developmental processes. PACAP gene expression and PACAP immunoreactivity are widely distributed in neurons within the neonatal rat brain (29). Activation of PACAP receptors regulates the proliferation of developing neuroblasts. PACAP and its receptors are expressed in the embryonic neural tube, where they seem to regulate neurogenesis (30). The activation of PACAP signaling in vitro has been shown to enhance NSC proliferation/survival through a PKA-independent mechanism. In contrast, PACAP has been shown to promote NSC self-renewal and neurogenesis through a mechanism dependent on PKA activation (31). Our results demonstrate that PACAP and dbcAMP, which is also an activator of PKA, interact to control the differentiation and functional maturation of BEP neurons.
We have previously shown that dbcAMP acts as a neurotropic factor for immature BEP neurons (4). Also, studies of transduction pathways identified the cAMP system as an important second messenger system in the regulation of hormone secretion and POMC gene regulation (32). Because PACAP is a potent inducer of cellular levels of cAMP in developing neurons (30), the interactive actions we observed between this peptide and dbcAMP support a role of the cAMP signaling system in the regulation of early differentiation of BEP neurons.
Chronic high levels of stress are known to enhance carcinogenesis in rat models (33). Furthermore, behavioral interventions aimed at reducing stress and increasing optimism in cancer patients have been documented to enhance immunity and to reduce tumor growth (34, 35). These studies have identified the importance of stress regulation in the management of cancer growth. The data presented here show that the NSC-derived BEP neurons, when transplanted in the PVN, remain at the site of transplantation and are well integrated with the other cells at this site. They seem to be functional as significant amounts of POMC mRNA and BEP peptide were detected in the tissue containing the transplanted cells but not in the tissue containing control cells. The transplanted ex vivo produced BEP neurons also showed remarkable anti-tumor activity. These data suggest that stress-relieving BEP neurons have the ability to suppress the growth of prostate cancers.
In this study, we found that ex vivo-produced BEP neuronal transplants significantly increased NK cell cytolytic activity in the spleen and in the PBMC. In addition, the transplants increased IFN-γ levels in plasma while reducing TNF-α levels. These data are in agreement with the finding that administration of BEP peptide in the PVN increases NK cell function (6). Because the transplants increased BEP levels in the hypothalamus but not in plasma, it is possible that the peptide may have inhibited sympathetic input to the spleen to increase NK cell cytolytic function (6). The NK cell is a critical component of the innate immune system and plays a central role in host defense against tumor cells. The importance of the NK cell in controlling tumor growth and metastasis of cancer cells has been clearly demonstrated in severe combined immunodeficiency mice (36). Therefore, the possibility arises that the higher level of NK cell cytolytic activity may have caused unfavorable conditions for prostate cancer cell growth. In addition, in the BEP cell-treated animals, the lower inflammatory milieu that was achieved by the higher level of antiinflammatory IFN-γ and lower level of inflammatory TNF-α may have also been involved in inhibiting prostate cancer growth. Proliferative inflammatory atrophy, a prostate cancer precursor lesion, ties the inflammatory response to prostatic carcinogenesis. Somatic epigenetic alterations, present in all prostate cancers, also seem to arise in the setting of inflammation (25). We hypothesize that BEP neuronal transplants inhibit prostate tumor development possibly by increasing the NK cell cytolytic activity and/or ameliorating the inflammation. These data provide strong evidence that hypothalamic BEP neurons play a critical role in controlling tumor growth. Because neuronal differentiation from NSC persists in the adult (37), the BEP-inducing therapies by cAMP-activating agents may hold promise as an adjuvant treatment for cancer.
Methods
Preparation of Neurospheres from the Fetal Rat Hypothalamus.
Mediobasal hypothalamic tissues from fetal rats (embryonic day 17) of the Sprague–Dawley (SD) strain (Charles River Laboratories, Wilmington, MA) were dissociated by mechanical dispersion as described in ref. 4. Neurons were separated from glial cells by filtering mixed hypothalamic cells through a 48-μm nylon mesh. Hypothalamic cells were then sedimented at 400 × g for 10 min; pellets were resuspended in Hepes-buffered DMEM (HDMEM, 4.5 g/liter glucose); and cells were cultured into 25-cm2 polyornithine-coated tissue culture flasks (2.5 million cells per flask) in HDMEM containing 10% FBS and antibiotics (1% penicillin/streptomycin). On day 2, the culture medium was replaced with HDMEM containing 10% FBS, 33.6 μg/ml uridine, and 13.6 μg/ml 5-flurodeoxyuridine to prevent the overgrowth of astroglial cells. On day 3, the culture medium was replaced with HDMEM containing serum supplement (SS; 30 nM selenium, 20 nM progesterone, 1 μM iron-free human transferrin, 5 μM insulin, and 100 μM putrescin) and antibiotics. These chemicals were obtained from Sigma (St. Louis, MO), except FBS, which was purchased from HyClone (Logan, UT). Cells were maintained for the next 2 days in this medium. By this time, the cultures were ≈85–90% neurons, as determined by MAP2 positivity.
Enriched hypothalamic neurons were maintained in HDMEM containing 10% FBS, trypsinized using trypsin/EDTA (Sigma) solution weekly and cultured for 3 wk to develop neurosphere. These spheres were then separated and dissociated into single cells by trypsinization and cultured in suspension or in poly-l-ornithine-coated 24-well plates (20,000 cells per well) in stem cell medium [DMEM F-12: 0.1 μg/ml lymphokine inhibitory factor (LIF), 10 mM l-glutamine, 20 ng/ml rat bFGF, and 0.5% Mem amino acid solution (MAA); all chemicals were from Sigma, except bFGF, which was obtained from R & D Systems, Minneapolis, MN] for a period of 2 wk, during which time they grew and developed secondary spheres. These neurospheres can be maintained in cultures for several months by regularly changing the medium and by splitting the cells. The secondary spheres were resuspended and cultured on poly-l-ornithine-coated 24-well plates (20,000 cells per well; for biochemical studies) or in poly-l-ornithine-coated 8-well permanox slides (1,000 cells per slide; Nalg Nunc International, Naperville, IL; for histochemical studies). Differentiation experiments were performed by treating these cells for 1 wk with PACAP (1 or 10 μM; SynPep) and/or dbcAMP (1 or 10 μM; Sigma) and then suspending them in a defined cell culture medium without the drugs for 1 wk. At days 3, 7, and 14, the immunocytochemical, biochemical and/or qRT-PCR analyses were performed.
Immunohistochemical Characterization of NSCs.
Cell cultures were fixed in 4% paraformaldehyde for 30 min and then in 70% ethanol for an additional 30 min. Cells were incubated with primary antibodies overnight at 4°C. Primary antibodies used were monoclonal antibodies for nestin (BD Biosciences, San Jose, CA; 1 μg/ml), vimentin (clone V9, mouse ascites fluid, 0.22 μg/ml; Sigma; 1:40), α-internexin (Santa Cruz Biotechnology, Santa Cruz, CA; 1 μg/ml), MAP2 (2A plus 2B, clone AP-20, mouse ascites fluid, 0.72 μg/ml; Sigma), β-tubulin (clone SDL.3D10, 0.30 μg/ml, Sigma), GFAP (clone G-A-5, 45 μg/ml, Sigma), NF-M (145 kDa, 5 μg/ml, Chemicon International, Temecula, CA), RIP (anti-oligodendrocyte, clone NS-1, 1:1,000; Chemicon), TH (1:500; BD), polyclonal primary rabbit antibody for BEP (1:1,000; Peninsula Laboratories, San Carlos, CA), GnRH (1:500; Chemicon), and NPY (1:500; Peninsula Laboratories). The secondary antibody used to react with mouse primary antibodies was Alexa Fluor 488 donkey anti-mouse IgG (4 μg/ml) and with the rabbit primary antibody was Alexa Fluor 594 donkey anti-rabbit IgG (H+L) (4 μg/ml; both from Molecular Probes, Eugene, OR). Both of these secondary antibodies failed to stain cells in the absence of a primary antibody. Some cell cultures were mounted by using DAPI-containing Mounting Medium (Vector Laboratories, Burlingame, CA).
Expression levels of POMC mRNA in stem cells and differentiated cells were assayed by a quantitative RT-PCR (qRT-PCR) on an ABI PRISM 7700 Sequence Detector (Perkin–Elmer Applied Biosystems, Foster City, CA), as described in ref. 38. The immunoreactive BEP levels in culture media were measured by a RIA (4).
Animal Preparation and Surgery.
Pregnant female Sprague–Dawley rats were obtained from Charles River Laboratories (Wilmington, MA) and housed in a controlled environment and provided rodent chow meal and water ad libitum. Male pups of these dams were used in these series of studies.
In vitro differentiated BEP cells were dissociated by using 0.05% trypsin/EDTA, washed, and resuspended in HDMEM and SS medium for transplantation. Cortical cells were prepared (39) and maintained in cultures for 4 days, trypsinized, and resuspended in HDMEM and SS medium for transplantation. Cells were placed on ice throughout the grafting session. Cell viability, assessed by the Trypan Blue exclusion assay, was routinely >90%. The composition of the differentiated cultures, with respect to the absence of undifferentiated NSCs and the presence of mature BEP-producing cells, was verified before grafting by staining for nestin, MAP-2, GFAP, RIP (for oligodendrocytes), and BEP. In some experiments, BEP cells were frozen and thawed for three cycles and used as a nonviable BEP cells control.
Male rats between 35 and 60 days of age were anesthetized with sodium pentobarbital (50–70 mg/kg, i.p.; Henry Schein, Indianapolis, IN) and injected with 1.0 μl of cell suspension (20,000 cells per lobe) into either one or both PVN lobes (the coordinates were set 0.5 mm from the midline, 1.8 mm behind the bregma, 0.5 mm lateral of the bregma, and 7.5 mm below the cortex) using a 5-μl Hamilton syringe. Each injection was over a 5-min duration. After the injection, the cannula was left in place for 20 min to prevent cells from being sucked out during removal of the cannula. The cannula was then slowly removed over a 10-min period. The dura was closed with 9–0 suture, muscle was reapposed, and the skin was closed with wound clips. Animal surgery and care were performed in accordance with institutional guidelines and complied with National Institutes of Health policy.
NSC-BEP Cell Transplants Viability.
A group of male rats with differentiated BEP cell transplant in one of the PVN lobe was used for detection of BEP immunoreactivity in the transplanted cells. These animals were anesthetized with sodium pentobarbital and perfused with 0.1 M PBS followed by 4% paraformaldehyde in PBS, postfixed, frozen in cryoprotectant, serially sectioned (40 μm), and double-stained for BrdU and BEP using immunohistochemistry methods. A separate group of rats with the differentiated BEP cell transplant or nonviable BEP cells transplant in one of the PVN lobe were used for measurement of POMC mRNA using the qRT-PCR analysis and BEP levels in the PVN and plasma using RIA.
NSC-BEP Cell Transplants and the Growth of Prostate Tumors Induced by MNU and Testosterone in Rats.
The effects of the BEP cell transplants on MNU and testosterone-induced prostate tumor growth were determined as described in ref. 23. Adult male rats were transplanted with BEP cells or cortical cells into both PVN at 90 days of age and then were injected i.p. with cyproterone acetate (50 mg/0.3 ml DMSO/kg; Sigma) for 21 consecutive days followed by daily i.p. injections of 100 mg/kg testosterone propionate (Steraloids, Newport, RI) in propylene glycol for 3 days. One day after the last testosterone injection, all rats received a single i.p. dose (50 mg/kg of body weight) of MNU (Sigma) dissolved in saline at 10 mg/ml. One week after MNU administration, rats received daily i.p. injection of testosterone (2 mg/kg of body weight) for 60 days. After this treatment period, rats were injected i.p. with 0.3 ml of saline or LPS (100 μg/ml saline/kg); 3 h later, they were killed, and trunk blood was collected for the corticosterone ELISA (Diagnostic System Laboratories, Webster, TX). Prostates were removed from the adhering connective tissue, washed several times with physiological saline, weighed, fixed with 10% neutral buffered formalin, and stained with hematoxylin/eosin for determination of tissue histopathology.
Innate Immune System Response to BEP Cell Transplants.
The immune system response to BEP cell transplants was determined by measuring the NK cell cytolytic activity in the spleen and PBMC and cytokine (IFN-γ and TNF-α) levels in the plasma after 4 wk of transplantation with BEP cells or cortical cells into one or both PVNs in male rats at 60–70 days of age. At the end of the experiment, these rats were decapitated, and the spleens and peripheral blood were obtained and used for isolation of splenocytes and PBMC to measure NK cell cytolytic activity as described in ref. 6. Plasma levels of INF-γ and TNF-α were measured by ELISA (Amersham Biosciences, Piscataway, NJ).
Statistics.
Means ± standard errors of the data are calculated and presented in the text. The significance of the differences between two experimental groups was analyzed by the t test. Multiple groups of data were analyzed by using one-way analysis of variance. The differences between groups were determined by using the Student–Newmann–Keuls test. The proportions of tumors of each type developed between treatment groups were compared by using the Fisher's exact test. A value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
We thank Kathleen Roberts for technical assistance and Kathy Manger for editorial assistance. This work was supported by National Institutes of Health grants AA00220, AA08757, AA15718, and ES-05022.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800289105/DCSupplemental.
References
- 1.Settle M. The hypothalamus. Neonatal Netw. 2000;19:9–14. doi: 10.1891/0730-0832.19.6.9. [DOI] [PubMed] [Google Scholar]
- 2.van Eerdenburg FJ, Rakic P. Early neurogenesis in the anterior hypothalamus of the Rhesus monkey. Brain Res Dev Brain Res. 1994;79:290–296. doi: 10.1016/0165-3806(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Bayer SA. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- 4.De A, Boyadjieva N, Pastorcic M, Reddy BV, Sarkar DK. cAMP and ethanol interact to control apoptosis and differentiation in hypothalamic β-endorphin neurons. J Biol Chem. 1994;269:26697–26705. [PubMed] [Google Scholar]
- 5.Plotsky PM, Thrivikraman KV, Meaney MJ. Central and feedback regulation of hypothalamic corticotropin releasing factor secretion. Ciba Found Symp. 1993;172:59–75. doi: 10.1002/9780470514368.ch4. [DOI] [PubMed] [Google Scholar]
- 6.Boyadjieva N, Advis JP, Sarkar DK. Role of BEP, corticotropin-releasing hormone and autonomic nervous system in mediation of the effect of chronic ethanol on natural killer cell cytolytic activity. Alcohol Clin Exp Res. 2006;30:1761–1767. doi: 10.1111/j.1530-0277.2006.00209.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein HG, Krell D, Emrich HM, Baumann B, Danos P, Diekmann S, Bogerts B. Fewer β-endorphin expressing arcuate nucleus neurons and reduced β-endorphinergic innervation of paraventricular neurons in schizophrenics and patients with depression. Cell Mol Biol. 2002;48(Suppl):OL259–OL265. [PubMed] [Google Scholar]
- 8.Zangen A, Nakash R, Roth-Deri I, Overstreet DH, Yadid G. Impaired release of β-endorphin in response to serotonin in a rat model of depression. Neuroscience. 2002;110:389–393. doi: 10.1016/s0306-4522(01)00612-1. [DOI] [PubMed] [Google Scholar]
- 9.Pankov IA, et al. Screening of mutations in genes of pro-opiomelanocortin in patients with constitutional exogenous obesity. Vopr Med Khim. 2002;48:121–130. [PubMed] [Google Scholar]
- 10.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 12.Grinshpoon A, et al. Cancer in schizophrenia: Is the risk higher or lower? Schizophr Res. 2005;73:333–341. doi: 10.1016/j.schres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Lissoni P, et al. Evidence for altered opioid activity in patients with cancer. Br J Cancer. 1983;56:834–837. doi: 10.1038/bjc.1987.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan MP, Chin SS, Fliegner KH, Liem RK. Alpha-internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10:2735–2748. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans J, et al. Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J Neurophysiol. 2002;87:1076–1085. doi: 10.1152/jn.00088.2001. [DOI] [PubMed] [Google Scholar]
- 17.Raju T, Bignami A, Dahl D. In vivo and in vitro differentiation of neurons and astrocytes in the rat embryo: Immunofluorescence study with neurofilament and glial filament antisera. Dev Biol. 1981;85:344–357. doi: 10.1016/0012-1606(81)90266-9. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res Mol Brain Res. 1996;41:157–162. doi: 10.1016/0169-328x(96)00086-1. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno Y, et al. Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: Lack of evidence for involvement of hypothalamic neuropeptide gene expression. J Neuroendocrinol. 1998;10:611–616. doi: 10.1046/j.1365-2826.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 20.Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Boyadjieva N, Sarkar DK. Effects of ethanol on basal and prostaglandin E1-induced increases in β-endorphin release and intracellular cAMP levels in hypothalamic cells. Alcohol Clin Exp Res. 1997;21:1005–1009. [PubMed] [Google Scholar]
- 22.Hisano S, Kawano H, Nishiyama T, Diakoku S. Immunoreactive ACTH/beta-endorphin neurons in the tubero-infundibular hypothalamus of rats. Cell Tissue Res. 1982;224:303–314. doi: 10.1007/BF00216875. [DOI] [PubMed] [Google Scholar]
- 23.Arunkumar A, Vijayababu MR, Venkataraman P, Senthilkumar K, Arunakaran J. Chemoprevention of rat prostate carcinogenesis by diallyl disulfide, an organosulfur compound of garlic. Biol Pharm Bull. 2006;29:375–379. doi: 10.1248/bpb.29.375. [DOI] [PubMed] [Google Scholar]
- 24.Stagg J, Smyth MJ. NK cell-based cancer immunotherapy. Drug News Perspect. 2007;20:155–163. doi: 10.1358/dnp.2007.20.3.1092096. [DOI] [PubMed] [Google Scholar]
- 25.Stanojević S, Mitić K, Vujić V, Kovacević-Jovanović V, Dimitrijević M. Beta-endorphin differentially affects inflammation in two inbred rat strains. Eur J Pharmacol. 2006;549:157–165. doi: 10.1016/j.ejphar.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Nelson WG. Prostate cancer prevention. Curr Opin Urol. 2007;17:157–167. doi: 10.1097/MOU.0b013e3280eb110f. [DOI] [PubMed] [Google Scholar]
- 27.Sutcliffe S, Platz EA. Inflammation in the etiology of prostate cancer: An epidemiologic perspective. Urol Oncol. 2007;25:242–249. doi: 10.1016/j.urolonc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HS, Hannibal J, Fahrenkrug J. Expression of pituitary adenylate cyclase activating polypeptide (PACAP) in the postnatal and adult rat cerebellar cortex. NeuroReport. 1998;9:2639–2642. doi: 10.1097/00001756-199808030-00039. [DOI] [PubMed] [Google Scholar]
- 30.DiCicco-Bloom E, et al. An expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Dev Biol. 2000;219:197–213. doi: 10.1006/dbio.2000.9604. [DOI] [PubMed] [Google Scholar]
- 31.Ohta S, Gregg C, Weiss S. Pituitary adenylate cyclase-activating polypeptide regulates forebrain neural stem cells and neurogenesis in vitro and in vivo. J Neurosci Res. 2006;84:1177–1186. doi: 10.1002/jnr.21026. [DOI] [PubMed] [Google Scholar]
- 32.Lundblad JR, Roberts JL. Regulation of proopiomelanocortin gene expression in pituitary. Endocr Rev. 1988;9:135–158. doi: 10.1210/edrv-9-1-135. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel D, Sephton SE. Psychoneuroimmune and endocrine pathways in cancer: Effects of stress and support. Semin Clin Neuropsychiatry. 2001;6:252–265. doi: 10.1053/scnp.2001.26995. [DOI] [PubMed] [Google Scholar]
- 34.Ah DV, Kang DH, Carpenter JS. Stress, optimism, and social support: Impact on immune responses in breast cancer. Res Nurs Health. 2007;30:72–83. doi: 10.1002/nur.20164. [DOI] [PubMed] [Google Scholar]
- 35.Carlson LE, Speca M, Patel KD, Faris P. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Dewan MZ, et al. Natural killer cells in breast cancer cell growth and metastasis in SCID mice. Biomed Pharmacother. 2005;59(Suppl 2):S375–S379. doi: 10.1016/s0753-3322(05)80082-4. [DOI] [PubMed] [Google Scholar]
- 37.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of proopiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. J Neurochem. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- 39.Noh JS, Gwag BJ. Attenuation of oxidative neuronal necrosis by a dopamine D1 agonist in mouse cortical cell cultures. Exp Neurol. 1997;146:604–608. doi: 10.1006/exnr.1997.6569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.