Abstract
The need to obtain data from individual laboratory animals has forced many researchers to singly-house rodents and small animals. Isolation is an abnormal condition for many species, and adverse effects of single-housing on physiology and behavior threaten the validity of experimental results and generalization to humans, who are also social. This study assessed the practical use of a housing device - dubbed “Buddy Barrier” (BB) - that allows rats social stimulation in a paired-housing situation while at the same time permitting the collection of individual measures that traditionally require individual-housing. To assess stress responses to the BB, adult male rats were single or pair-housing for several days with and without a BB in the cage and fecal corticosterone metabolites (fCORT), food intake and body weight were monitored. Plasma corticosterone and adrenal catecholamine levels were also assessed at the end of the housing manipulation. Stress hormone measures did not differ in paired vs. singly-housed rats and paired rats quickly habituated to the BB taken in and out of the cage. Barring a trend for paired rats to eat more in the first 4hrs of the dark, there was no difference in 24hr intakes or body weight gain between singly and paired-housed rats. While the BB attenuated 24hr intakes in both groups, intakes normalized to non-BB conditions by the third BB reintroduction. A device such as the BB can enhance the welfare of animals by providing social enrichment without compromising the integrity of experimental manipulations in protocols that traditionally have required single-housing.
Keywords: corticosterone, feces, adrenal, stress hormones, HPA, ingestive behavior, feeding, body weight, RIA, HPLC, glucocorticoids, catecholamines, housing, rodent, enrichment, animal welfare, husbandry
Many experimental protocols that use rodents and other small animals require that the animals be isolated via single-housing for extended periods of time and, often, for the entire lab animals’ career. Unfortunately, isolation is not a normal condition in rodents that are, by nature, social creatures [1-4]. In fact, adverse effects of isolation are numerous and have been recognized for many years (see [4] for review). In the laboratory, such untoward effects have the potential to compromise the validity, reliability, and replicability of experimental results [4,5]. This is particularly important in behavioral rodent studies where it is expected, or actually assumed, that measures obtained from control groups reflect measures obtained from as “normal” a condition or conditions as possible. Additionally, if experimental results are meant to be extrapolated to humans who are also social animals, it makes more sense to allow for social conditions among animals during experimentation.
A non-exclusive list of consequences associated with isolation in laboratory animals include the induction of neurochemical and behavioral effects consistent with depression [6], anxiety [7], and psychosis [8], decreased exploration when isolated early in age [9,10], hyperactivity when isolated later in age [11,12], hypertension [5,13], increased or abnormal HPA axis responses [14-18], inadequate responses to aggressors and other stressors [19,20], compromised immune function [21], varied responses to pre-clinical drug tests [22,23] and to poisons [3], increased motivation for sucrose and drug rewards [24-27], suppression of running-induced neurogenesis [14], and decreased survival rate [10,28]. Congruent with the natural social nature of rodents, it is not surprising that rats prefer a location associated with the presence of another rat compared to a similar location paired without a rat [29].
The goal of this study was to assess the practical use of a rodent housing device that we have dubbed a “Buddy Barrier (BB)”, in protocols where individual measures are desired (i.e., where rodents are traditionally singly-housed). Two examples are protocols that call for the collection of fecal samples and of food intake measures from individual rats. Researchers have started to use fecal measures of corticosterone metabolites as a non-invasive method of assessing stress levels in animals [30-35] but rodents must be singly housed to do this. The same is true of ingestive studies where rodents must be isolated to obtain individual recordings. Therefore, in this study we chose to collect fecal samples and record food intake from rats housed in pairs via the intermittent use of a BB in the cage. In this way, the rats did not have to be completely isolated for the obtainment of individual samples. At the same time, by measuring corticosterone metabolites in feces (fCORT), we could determine to what extent, if any, the BB affected stress levels. We also subjected singly-housed rats to the BB to compare their response to that of paired rats. This allowed us to control for any effects due to the BB itself and/or the reduction in space produced by having a BB in the cage. We also compared fCORT levels, 4 and 24hr food intake, and body weight changes from these rats when the BB was in vs. out of the cage to evaluate habituation to this manipulation.
While there have been other studies examining the effect of single and group housing on stress and food intake, none have monitored these parameters when paired rats are intermittently exposed to a device that separates them but still allows them some social stimulation. In real life, researchers would want to keep rats paired for the duration of the study to avoid complete isolation but would also want to collect samples (feces, food, etc.) from individual rats which would normally requires single-housing.
At the end of the study when all of the rats were kept with a BB in the cage for several days, we obtained additional measures of stress including basal plasma CORT (pCORT), adrenal weights, and adrenal levels of catecholamines [35,36]. Ideally, it is our intent that BB would be used in experimental settings on an intermittent, not permanent basis. However, even if protocols required daily measures for an extended period of time, we believe that housing rats in pairs, with a BB in the cage so that some social interaction is possible, is preferable to complete isolation. Hence, in this study we allowed the BB to be in the cage for extended periods of time. In addition to improving the well-being of laboratory animals, the BB or similar device should benefit the researcher by substantially reducing the proportion of funding that must be allocated to housing expenses.
METHODS
Subjects
Male Sprague Dawley rats (n=20; Harlan, IN) weighing 220-250g at the onset of the study were housed in random pairs in clear 259mm × 476mm × 209mm polypropylene bedded cages with ad lib water and rat chow under a 12-h light/dark phase (lights off from 1100-2300). After two weeks of acclimation to the animal colony, the rats were weight-matched into one of two groups: an “Isolated” group or a “Paired” group. Those in the Isolated group were housed alone in a cage throughout the experiment. The Paired rats were now housed with a different cage mate from the first 2 wks in the colony in a cage of the same size as those housing the Isolated rats. Each Paired rat remained with its newly assigned cage mate for the entire duration of the study. The pairs were randomly assigned and one of each pair was identified from its cage mate by a black Sharpie® mark on its tail.
Buddy barriers (BBs) and BB placement procedures
The BBs are constructed from a perforated metal sheet running the length of the plastic cages and are anchored to narrow rods at the base of the plate (Figure 1A). The perforated holes are large enough to allow rats to see, hear, and smell each other as well as to have limited tactile contact (Figure 1B). The plate reaches through the cage lid to separate food placed on top on each side and a solid metal strip an inch from the bottom of the divider precluded transfer of feces or food from the bedding. The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved all materials for safety and sanitization in standard commercial cage washers. The reduced housing space resulting from placement of the temporary placement of the BB into the cage for each rat meets AAALAC guidelines. The study took place over a two-month period during which time the rats had a BB in the cage (BB condition) for several days followed by a period of consecutive days without a BB in the cage (NoBB condition). This was done for both groups, Paired and Isolated, as shown on the x-axis of Figures 2, 5 and 6 (only days when data were collected are shown and these are labeled “Day 1-39”-Saturdays and Sundays were not included in the “Day” count). Placing a BB in the Isolated groups’ cages served to control for any effect that decreased space, or the BB itself, might impose on the measures. The BBs were always inserted or removed during the hour prior to lights off (1000).
Figure 1.
Panel A) “Buddy Barrier” housing device used to avoid single housing of rats when individual measures such as food intake and fecal specimens are needed. Here it was also used in rats in the Isolated group to control for space restriction and novelty of the BB when the Paired group was exposed to it. Panel B) The BB allows for visual, audio, olfactory, and limited tactile contact between rats while food and feces are kept separated.
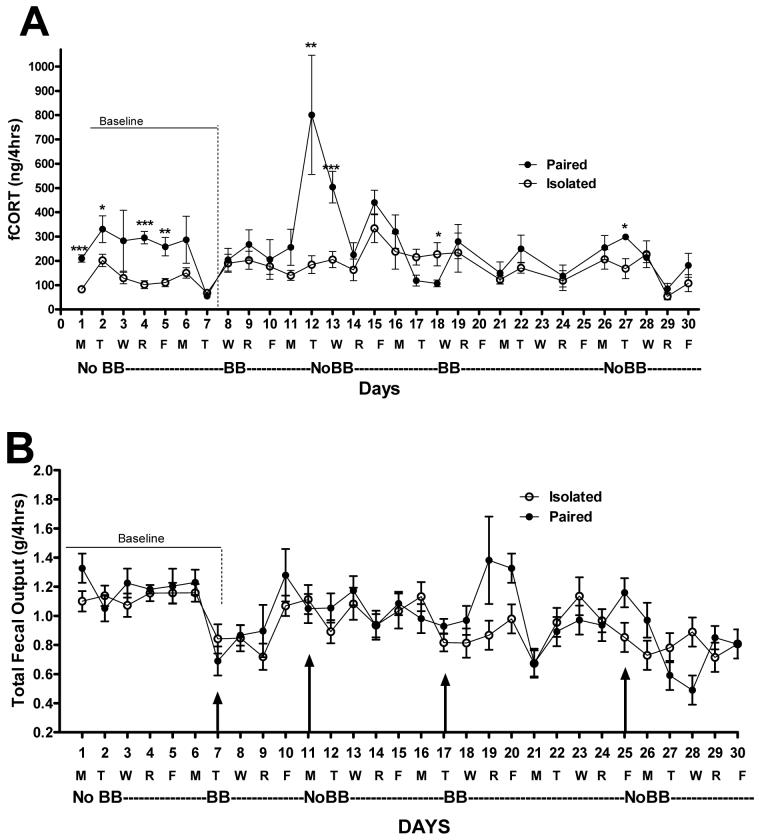
Figure 2.
Panel A) Daily levels of corticosterone extracted from a 0.20g sample of feces (fCORT) excreted by Paired and Isolated rats over a 4hr period on days when the buddy barrier was in the cage separating the Paired rats (BB) and when not in the cage allowing full social contact in Paired rats (NoBB). The 0.20g sample from Paired rats with no BB consisted of a mixture of both rats’ feces. A 6-9 hr time between circulating and fecal CORT was considered when the values where plotted. Therefore the BB conditions specified under each value reflects secretion of CORT under that BB condition (e.g., the BB was actually placed into the cage on the morning of Day 7 but the fCORT value plotted for Day 7 reflects circulating CORT levels occurring with No BB in the cage so the value was plotted under NoBB). Groups differed only on Day 17 and 26 (*p<0.05). Panel B) Total fecal output collected from Paired and Isolated rats over a 4hr period with the buddy barrier in the cage (BB) and not in the cage (NoBB). E.g., on Day 7, the BB was placed in the cage and feces collected after 4hr with the BB in the cage.
Fecal collection and CORT metabolite analyses
After a week following assignment of the rats into their groups, fecal collection began on Mondays-Fridays. Cage bedding was changed daily 1h prior to lights out so that feces could be collected from fresh bedding over the first 4hrs of the dark period (1100-1500h). Daily feces were collected for 30 days into individually labeled Whirlpak bags (Nasco, Fort Atkinson, WI) and stored in -20C. When feces were collected from Paired rats without the BB in the cage, the feces from both rats was stored in a single bag. Fecal samples were later dried, ground up, and mixed, then a sample of this mixture used for CORT metabolite analyses. Because there is a 6-9 hr lag between circulating CORT and excretion of fecal CORT metabolites [30] the collected fecal samples reflected circulating CORT levels secreted between 0200-0900h - the approximate time of the daily circulating CORT nadir. Feces were processed and CORT metabolites extracted as previously described [30] and stored at -80°C until assayed. CORT metabolite levels were quantified using a commercially available [125I]RIA (MP Biomedicals, Solon, OH, USA) for rat and mouse serum/plasma CORT that detects several rodent CORT metabolites in extract [30]. Prior to assay, fecal extracts were thawed and diluted (1:50) with steroid diluent provided in the RIA kit. All samples were run in duplicate, and samples from the three experimental conditions were counterbalanced across assays. Two control samples were analyzed in every assay (a ‘low’ pool at approximately 60% binding, and a ‘high’ pool at approximately 25% binding). Based on repeated analysis of these samples, intra- and inter-assay coefficients of variance were 10.1% (n=6) and 19.4% (n=7) for the low pool and 9.5% (n=7) and 14.2% (n=9) for the high pool. Total CORT metabolite excretions during the 4-hr collection interval are reported as group mean ± SEM. The values used in the analyses reflected amount of CORT extracted from 0.20 g of feces adjusted to total fecal weight for Isolated rats in both BB and NoBB conditions, and for Paired rats in the BB condition. For Paired rats when they were in the NoBB condition (i.e., not separated), the values represent CORT metabolites from a 0.20 g sample of a mixture of the Paired rats’ feces.
Blood collection and plasma CORT assay
After the last day of 24hr food intake measures (Day 39 on Fig.5B), the rats were kept in their cages with a BB for another 4 days (for a total of 9 consecutive days with the BB in the cage) after which they were sacrifice by guillotine decapitation between 0830-1030 (light phase; food withdrawn at 0800) in an order counterbalanced for group. Trunk blood was collected, centrifuged at 5,000 g for 15 min at 4° C and plasma stored in -20° C to be assayed for CORT by the UAB Clinical Nutrition Research Core Laboratory in 10ul aliquots using double-antibody RIA with reagents obtained from MP Biomedicals/ICN (Orangeburg, NY). Sensitivity for this assay was 11.0ng/ml, with a mean interassay coefficient of variation of 8.35%. Concentrations are reported as group mean ng/ml ± SEM.
Adrenal extraction and HPLC assay of adrenal catecholamines
After blood was collected, both adrenal glands were excised, weighed, snap-frozen in liquid nitrogen and stored in -80° until assayed using high-performance liquid chromatography (HPLC) with electrochemical detection (EC). After thawing, adrenal glands were homogenized in 0.2 N perchloric acid containing EDTA, sodium metabisulfite, and dihidroxybenzoacetic acid as the internal standard, then centrifuged at 12,000 g for 20 min at 4° C. Supernatants were then diluted in perchloric acid solution and injected into the HPLC system (4-channel CoulArray EC detector; ESA, Inc., MA). A C-18 reverse-phase column with a 4% methanol-based mobile phase at pH 4.3 was used with electrode potentials set at -100 mV, 150, 250, and 450 mV to separate norephinephrine, 3-methoxy-4-hydroxyphenylglycol (MHPG), epinephrine, and dopamine. Concentration values were corrected for dilution and adrenal weight, and reported as group mean ng/ml ± SEM.
Food intake and body weight measures
On the same day that feces began to be collected, we obtained 4hr measures of food intake on Monday-Friday for 30 days and 24 hr measures on the same Tuesday-Fridays for 39 days. Just prior to lights off, a pre-measured amount of Purina rat chow (Harlan Teklad Global Diets, Indianapolis, IN) was placed on the top of the cage lid. On days when the BBs were in the cage, food was also placed on the cage lid on either side of the BB which protruded through the metal cage lid to act as a food divider. Left over food and spillage (which was always minimal) was measured after a 4hr period and on the following day for a 24 hr recording. Ad lib water in two bottles per cage was available at all times (when BB was in and out of the cage). The data analyzed and graphed reflect group mean grams of intake derived from individual Isolated rats’ intakes when the BB was in and out of the cage. For the Paired rats, the data reflect individual rats’ intake when the BB was in the cage. However, when the BB was not in the cage, individual intakes could not be ascertained because the rats were not separated. Therefore, the summed amount of intake for each pair was divided in half and these values were used to compare intake to the Isolated group. Values were reported as mean kcals ± SEM. Body weights were recorded daily in the morning prior to presentation of pre-measured food for 30 days. We also obtained daily body weight changes by subtracting this number from the previous day’s body weight. Values were reported as mean grams ± SEM.
Statistics
Separate repeated-measures MANOVAs were conducted to determine differences between Isolated and Paired group concentrations of fCORT, of 4 and 24-hr food intake, of body weight, and body weight fluctuations. BB condition (BB or NoBB) was entered as the within subjects factor. Independent t-tests were used determined the days on which values differed significantly between groups. To determine differences in pCORT, adrenal weights, and adrenal catecholamines, ANOVAs were conducted using group (Isolated vs. Paired) as the between-groups factor. The alpha level was set at p<0.05 for all analyses.
RESULTS
Daily levels of fecal corticosterone metabolites (fCORT)
During baseline (Day 1-7) when the BB was still unknown to the rats, the Paired group had higher fCORT levels compared to the Isolated rats (Paired: 245.5 ± 14.1 vs. Isolated: 120.5 ± 9.9 ng/g; p<0.001). Once the BB was in the cage and during days with the BB in the cage, there was no longer a difference in fCORT levels between the Paired and Isolated rats (Paired: 196.7 ± 32.1 vs. Isolated: 175.7 ± 22.7ng/g; ns; data for Day 20, 23, and 25 are missing due to human error in collection procedures). Across days without the BB in the cage, a main effect of group emerged with Paired rats having higher fCORT levels than Isolated rats (p<0.01) to reveal a group × BB interaction (p<0.01). However, this was due completely to the fCORT surge noted on Day 12 in only the Paired rats (Fig. 2A, p<0.01). Interestingly, this is the first day with the BB lifted out of the cage and when Paired rats once again had total social contact. Without this day in the analysis, there were no differences in fCORT levels between Paired and Isolated rats during BB days (p = 0.58) but after Day 13, when there was a notable decline in the Paired rats’ fCORT levels, there was no longer even a trend for the groups to differ (Paired: 237.3 ± 30.8 vs. Isolated: 190.4 ± 21.8; p = 0.24, ns). Hence, once rats habituate to the BB placed into and removed from the cage, fCORT between groups do not differ.
Total fecal output
As for fCORT levels, there was no main effect of group (Isolated vs. Paired) on total feces excreted in the 4 hr period sampled but, as evident in Figure 2B, there was a main effect of BB when the baseline data were included. Fecal output was higher under no BB conditions than with a BB in the cage (p<0.01). However, as was also true of fCORT levels, when the baseline data (Days 1-6) were excluded there was no main effect of group (Isolated: 0.94 ± 0.05 vs. Paired: 0.97± 0.06 g/4hrs), and also no main effect of BB condition (BB: 0.96 ± 0.04 vs. No BB: 0.94 ± 0.05 g/4hrs; ns) on total fecal output. Groups approach a difference only on Day 20 but it was not statistically significant (p<0.06).
Plasma CORT levels
As shown in Figure 3, levels of CORT collected from plasma after 9 days of housing with a BB in the cage did not differ between groups (Isolated: 162.8 +/- 22.4 vs. Paired: 168.5 +/- 11.4 ng/ml, ns).
Figure 3.
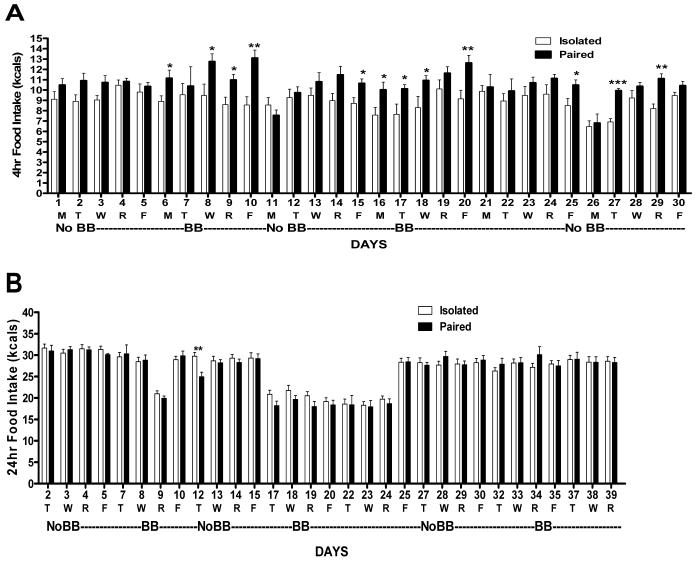
Panel A) Daily Food intake during the first 4hrs in the dark of Isolated and Paired rats with and without a BB in the cage. There was no main effect of group across BB conditions but individual t-tests revealed increased intake in Paired vs. Isolated rats on some days (*p<0.05; **p<0.01, ***p<0.001). Panel B) Serial 24-hour chow intake of Isolated vs. Paired rats on days with the buddy barrier in the cage (BB) and without (NoBB). Days omitted (1, 6, 11, 16, 26, and 31) were Mondays, when no 24 hr data was obtained (Sunday-Monday). NoBB data for Paired rats were derived from the total intake of both rats in the cage divided by 2.
Adrenal weight and catecholamine levels
After 9 consecutive days with a BB in the cage, mean adrenal weights did not differ between groups (Isolated: 6.32 ± 0.9 vs. Paired: 6.16 ± 0.6 mg/100g; ns; not shown). As depicted in Figure 4, no differences were detected between Isolated and Paired rats in amount of adrenal content of norepinephrine (NE; Fig. 4A), the NE metabolite, 3-methoxy-4-hydroxyphenylglycol (MHPG; Fig. 4B), nor of epinephrine (Epi; Fig. 4C) or dopamine (DA; Figure 4D).
Figure 4.
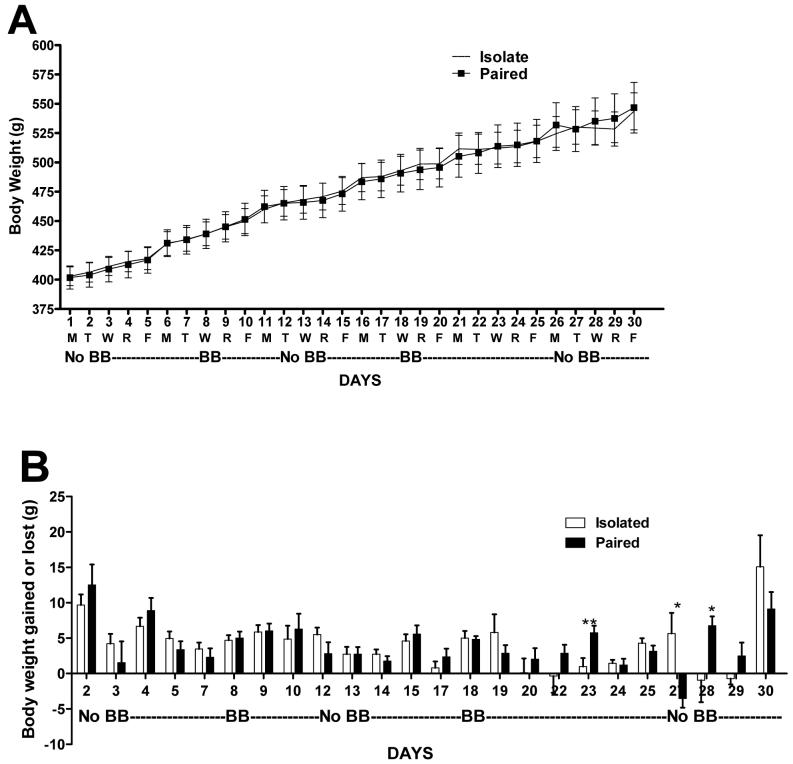
Panel A) Daily mean body weights of Paired and Isolated rats on days with the buddy barrier in the cage (BB) and without (NoBB). Panel B) Daily weight gain or lost in the groups across BB conditions. Omitted days were Mondays as weights were not recorded on Sunday to subtract from Monday’s weights; *p<0.05, **p<0.01 difference in weight gained between groups.
4hr food intake
There is often an interest in recording food intakes over shorter intervals than 24 hrs. Therefore, we also obtained data representing the first 4hrs of food intake in the dark (1100 - 1500). Including the baseline data (Days 1-6), there was no main effect of group or of BB condition. Without the baseline (Days 1-6) data included in the analyses, there was still no main effect of group (Isolated: 8.77 ± 0.5 vs. Paired: 10.33 ± 0.7 kcals; ns) on 4hr food intake. Although not significant, inspection of Figure 5A suggests a trend for Paired rats to eat more than isolate rats (denoted * if significant with individual t-tests on Fig. 5A). The significant increases occurred as frequently on BB days than on NoBB days, explaining the lack of a group × BB interaction. When baseline data (Day 1-6) was not excluded from the analysis, a main effect of BB condition was found with 4hr food intake increased when the BB was in the cage vs. when it was not across groups (BB: 9.97 ± 0.5 vs. NoBB: 9.13 ± 0.3 kcals; p<0.01).
24-hour food intake
As evident in Figure 5B, the trend to increase intake in the first 4 hours observed in the Paired rats on some days (Fig.5A) disappeared by 24 hrs so that there was no group difference in intake or interaction with BB conditions. This was also true if baseline data (Day 2-7) was excluded from the analysis (Isolated: 25.3 ± 0.7 vs. Paired: 25.4 ± 0.9 kcals; ns). Only on Day 12, when fCORT levels where elevated in the Paired rats, did food intake differ between groups; it was lower in Paired vs. Isolated rats (p<0.01; Fig. 5B). While there was no group difference, there was a main effect of BB condition on 24hr intake such that intake was decreased in both groups on days when the BB was in the cage vs. when it was not (BB: 23.2 ± 0.8 vs. NoBB: 27.5 ± 0.6 kcals; p<0.001). This was true whether baseline intake was included in the analysis or not. However, as evident by inspection of Fig. 5B, by the 3rd re-introduction of the BB (Day 34), 24hr intakes were the same as during NoBB conditions (BB: 28.4 ± 0.6 vs. NoBB: 28.05 ± 0.5 kcals; ns). In fact by Day 27, 24hr food intakes under both NoBB conditions (Day 27-33) and BB conditions (Day 34-39) are much more uniform compared to fluctuations under both conditions noted prior to this day (Fig. 5B).
Body weight
As shown in Figure 6A, mean body weights of Isolated and Paired rats did not differ throughout the study (Isolated: 561.2 ± 15.5 vs. Paired: 558.1 ± 16.9 g, ns, at time of sacrifice). There was also no effect of group or BB condition on daily body weight fluctuations (Figure 6B). Individual t-tests revealed significant weight change differences between groups only on Days 23, 27, and 28 but there was no group or BB condition pattern to these changes.
DISCUSSION
This study explored the practical use of a simple perforated housing unit that temporarily separates rats within a cage in an effort to avoid single-housing animals in research. This divider, dubbed “Buddy Barrier”, was used in a protocol that allowed us to measure stress hormone metabolites in feces and to record amount of 4 and 24hr food intakes from individual rats without isolating them. These are measures that traditionally require the life-long isolation of laboratory animals because data must be recorded from individual rats and is precluded by paired or group-housing. Because fCORT can be used to gage stress in animals, the procedure allowed us to determine how much, if any, stress was evoked by the intermittent use of the BBs over an extended period of time. The intermittent placement of a BB in and out of the same cages also allowed us to evaluate habituation to the BBs as defined by changes in CORT levels, food intake, and body weight. The overall motive in conducting this study was driven by the tenet that “stressed animals do not make good research subjects” ([37], pg.18). It was our desire to improve the welfare of laboratory animals that are, by nature, social animals.
Results revealed that fCORT levels during the circadian nadir did not differ between Isolated and Paired rats across BB conditions. Similarly, others studying singly and group-housed male mice found no differences in fCORT levels between singly and group house male mice [34]. In our study the fCORT levels revolved around a slightly higher amount in the Paired rats once the BBs started being used, however, this elevation was the result of one day of elevated fCORT levels the day the BB was first removed from the pair-housed males. After the first exposure to the BB, having the device in or out of the cage did not differentially affect fCORT levels. One might argue that fCORT measures are not as sensitive as plasma CORT measures. However, this may only be the case in measuring short-lived changes in glucocorticoid production (e.g. 1-2 hr changes) which are not readily identified in fecal samples that provide an integrated index of circulating steroid levels over many hours. When measuring longer-term changes in glucocorticoid production (e.g. several hours) there is ample evidence that fCORT measures can identify stress-related elevations in glucocorticoid production (e.g. long-term capture, chronic catheterization, daily husbandry disturbances such as cage lids opening, the noise of keys jingling and light cycle switching [31,38,39]. It should be noted that the early difference in fCORT levels of the Paired and Isolated rats during the baseline period (with no BB) may have been a result of decreased physical/social activity in the newly isolated males. Long-term voluntary exercise can elevate circulating glucocorticoid levels in rodents [40]. Loss of a social partner for the single-housed rats may have led to less physical activity and thus lowered glucocorticoid production for a short-period. Alternatively, elevated fCORT metabolite levels in the pair-housed rats during this time may have reflected increased activity between two novel social partners during the baseline period. On further examination (Fig. 2A), it is evident that the Isolated rats’ fCORT levels become more similar to those of the group-housed males during the experimental period, perhaps a result of increased activity in response to the novel BB.
No differences in total fecal output were noted between the BB and NoBB conditions, for either the pair- or single-housed males. These results suggest that metabolic rate is not highly affected by insertion of the BB. In terms of the practical use of the BB in experiments, it may be necessary for rat pairs to grow accustomed to each other for at least a one-week period before introducing the BB or similar device. Housing males with a novel cage mate and immediately introducing the BB may introduce an elevated level of stress that may slow habituation to the BB manipulation.
Other neurochemical indices of stress including pCORT, and adrenal catecholamines did not differ between isolated and paired rats housed with a BB in their cage for 9 consecutive days. Unfortunately the limited number of subjects precluded us from measuring these parameters in a group of rats without a BB in the cage prior to sacrifice. Nonetheless, we are primarily concerned with the “real life” application of the BB or similar device in research settings. Since samples such as feces or food intake would be collected with the BB in the cage, we can conclude that an extended period of time with a BB in the cage neither increases or decreases stress compared to single-housed animals. We cannot rule out that these stress indices would have differed from isolated rats with no BB in the cage but given the lack of fCORT differences between these groups regardless of the presence or absence of a BB, we doubt that any differences would be observed. Unlike our data showing a lack of stress differences between isolated and paired rats, one study using isolated vs. group-housed (6-8/cage) pubescent-aged male rats, found that 4 weeks of isolation augmented pCORT secretion and disrupted correlations between CORT and adrenocorticotropic hormone [17]. The inconsistency may be due to group vs. paired conditions. Indeed, our findings are more consistent with a study by Dallman’s group showing no difference in pCORT or pACTH levels obtained by decapitation in isolated vs. paired adult male rats. In cannulated (vs. decapitated rats), isolated rats had slightly elevated levels of these hormones in the morning but were similar to evening and noise-stimulated increases to the decapitated rats [41]. Despite a lack of stress reduction or enhancement between isolated and paired rats, consistency of housing conditions is essential [2]. All treatments or manipulations ought to be performed in either isolated or, more ideally for the animals’ welfare, in paired housing conditions.
While our data revealed no effect of the BB or of single vs. paired housing on basal stress hormone levels during the circadian nadir, it is important to consider that this does not rule out the possibility that isolation may predispose rodents to hyper-respond to an aversive stressor (e.g., footshock, restraint, or cold) or conversely, that social contact protects them from consequences of isolation. This was not tested here but several studies have found that isolation does indeed appear to predispose rats to increased physiological and behavioral stress-responses while group housing attenuates them [7,14-16,18,20,21].
A caveat concerns the generalization of our stress hormone results to female rats since we only studied male rats. Westenbroek et al. found that isolation alone, without the added effect of footshock stress, produced higher adrenal weights in female rats than in isolated males [20]. In mice, females housed individually for just one week engaged in behaviors consistent with increased anxiety compared with group-housed females [42]. If females are more susceptible to isolation-induced stress, use of the BB or similar device may prove even more beneficial as the rats would be able to be kept in pairs.
Our results with food intake revealed that during the first 4hrs of feeding in the dark, when rats tend to consume their larger meal, there was a non-significant trend for paired rats to consume more than isolated rats regardless of whether the BB was in or out of the cage. By 24hrs, however, this trend disappeared so there was no difference in intake between groups. Regarding any effect of the BB itself, 4hr intakes where greater in both Isolated and Paired groups but this had to be followed by a decrease in intake at later hours since 24hr intakes when the BB was in the cage was lower than on days without the BB. By the 3rd time that the BB was re-introduced, however, 24hr intakes became quite stable across days and did not differ from intakes when the BB was not in the cage (Fig. 5B). A conservative conclusion for the practical use of a BB or similar device in food intake studies using pair-housed rats is that intakes should be expected to be somewhat lower the first few times that the BB is in the cage. Hence, it is recommended that the rats be habituated to having the BB in and out of the cage for at least 3 times to habituate the animals and arrive at food intake levels comparable to those seen without the BB present. Food intake should be measured on days when the BB is in the cage to establish a baseline. There should be no need to measure intake when the BBs are out of the cage since experimental procedures would not be conducted under this condition. Of course, experimental and control groups should also always be tested under the same conditions when data is to be collected (i.e., paired, with a BB).
If body weight, and not food intake, is the main dependent variable of interest, there should be no concern that using the BB or similar device will affect these parameters. One caveat that should be considered however is that pairing rats may not be the same as group-housing rats (3+ per cage). Scalera et al found that housing more than two rats together resulted in decreased food intake and body weight gain compared to isolating or pairing rats [43]. We observed no difference in the mean body weight of rats assigned to the Isolated group despite the fact that they were paired in the animal colony and ostensible group housed from weaning at Harlan. It may be that having been housed with one other rat vs. in a group of more than one rat, prior to isolation, buffered these rats from losing weight.
Others have conducted food intake studies comparing isolated and socialized rodents but direct comparisons between our data and theirs is hampered by the fact that most of these studies did not include partial socialization as that afforded by the BB. Also, the animals were always either in one type of housing condition or another while in our study, the paired rats where together on some days and semi-separated on other days. Several studies have found that isolated animals eat more and gain more weight than socialized animals [10,11,24,44]. However, this is not a consistent finding. A study using female rats found no difference in food intake or body weight in rats that were isolated for part of the study vs. rats that were continuously allowed social interaction [45]. Others also failed to observe any effect of post-weaning isolation on body weight of female and male rats [18]. In a recent study using pubescent male rats, food intake did not change in isolated vs. group-housed rats but grouped rats gained weight more rapidly than isolated rats [17]. These latter studies are more in line with our observed trend for Paired rats to eat more than Isolated rats, at least in the first few hours of the dark phase. This is a time when rats take their largest meal [46] and the increase may have been socially facilitated as is true of humans who eat more in larger groups than in smaller groups or alone [47,48]. If a rat stops eating but sees his paired partner continue to eat, the other rat may continue eating.
However, as stated above, this trend for the Paired rats to eat more in the first 4 dark hours may be isolated to that period since by 24hrs there is no difference in total food intake between single and pair-housed rats. Alternately, our Isolated rats could be eating more than the Paired rats during the light phase. An early study found that rats isolated from the time of weaning ate more during normally low-intake times of the day (light phase) than socialized rats [24]. They also made more lever presses for food suggesting heightened motivation for food [24], a suggestion since supported by later studies [25]. An enhanced baseline motivation for food in isolated rats poses an important caveat when working with rodent models of binge-eating, obesity, and addiction. To this, the effect of pharmacologic agents on food intake in rats has also been shown to be influenced by single vs. group housing conditions [22].
The more notable effect we observed on food intake was that imposed not by isolation vs. paired-housing per se, but by the BB itself which decreased food intake compared to when the BB was absent. Because this pattern also occurred in the Isolated group it had to be an artifact of the BB itself. The metallic and circle-perforated pattern of the BB may have actually added complexity to the environment, especially since the holes were large enough for sensory interaction but not full contact. This may have kept the rats distracted from eating as much. Indeed, Collier et al. found that daily food intake decreased as housing became more complex in male rats and, also like our findings, isolation or social conditions had no effect [46]. Rats in enriched conditions also were observed to eat less and gain less weight than those kept in impoverished conditions [49]. A study using male mice also found food intake to decline if there were nestlets in the cage for enrichment but body weights remained the same [50]. No differences in intake are observed when cages of isolated mice are enriched [51,52]. A study by Lopak & Eikelboom however would argue against the possibility that novelty of the BB caused the suppression in intake. They manipulated novelty (a new rat cage partner) vs. isolation or pairing and found the latter, not novelty, to affect food intake [53]. We are unsure however, whether a novel animal equates to a novel object such as PVC piping, mouse igloos, or nestlets that were used in the aforementioned studies [50-52].
In any case, as stated above, it appears that the influence of the BB eventually dissipates as we found no differences in 24hr food intakes by the 3rd time the BB was placed into the cage. In sum, we believe that using the BBs to allow animals social interaction stimulation is worth the time they need to habituate to BB conditions. Even when initial changes in intake were observed, they were not enough to affect rate of body weight gain and body weights compared to rats kept in isolation. Still, as for any new device that gets introduced to the animals’ environment, the rats should be habituated to the BB or similar device prior to testing and once testing begins, control groups must be run under the same housing conditions.
Besides the collection of feces and food intake, there are other protocols and procedures where the BB or similar devices should be beneficial to both animals and researchers. The dividers could be used to decrease aggressive behavior between male rodents by gradually habituating fighting males to co-habitation via intermittent use of the BB. The BB can be used to allow stud males some social contact when they must be isolated. Colleagues at UAB have used the BB successfully in rats in a retina degeneration protocol. Fewer light bulbs were needed per cage, they afforded the rats some social contact, and they substantially reduced housing costs (Y. Wen, personal communication). A similar device was used successfully in transgenic mice (R. Kesterson, personal communication). In such studies, where large number of animals is typically required, use of BBs or similar devices can mean substantial grant-dollar savings. This is important given the flat growth rate of federal funding for biomedical research [54,55]. The device can also keep post-surgical animals separated when pairing or group housing may damage external implants (e.g., cannulae or probe guides) without having to completely isolate the rats. The BB or similar device would also allow researchers to use male rats in lieu of female rats if their primary reason for using female rats is to avoid disruption of the experiment due to aggressive behavior between males. Finally, for fecal steroid metabolite analyses of long-term changes in steroid production, the BB allows for individual fecal sample collections without completely isolating animals and in fact maintaining a great deal of social interaction between animals.
Reinhardt points out in an eloquently written “call to arms” for more sensitive treatment of laboratory animals, that many researchers ignore the status of housing conditions despite the fact that stress evoked by such conditions can jeopardize the value of their data [56]. We posit that there are many of us who have great sensitivity for our animal subjects but are forced nonetheless to isolate them in order to obtain the data we need. Use of the BB or similar cage devices is only one, but a significant way of improving our subjects’ welfare. Any monetary savings to us in housing expenses is a secondary benefit. However, at a time when funding rates are flat, it behooves us to be able to divert housing expenses to other uses that at the end, will make the animals’ involvement in the research even more worthwhile.
ACKNOWLEDGEMENTS
We thank Dr. Sam Cartner, Jim Crawford, and Shellie Hyde of the UAB Animal Resources Program for their assistance with the development of the Buddy Barriers and assuring that they adhere to IACUC regulations. We thank Harry Stubblefield and Jerry Sewell for the construction and modifications of the BBs, respectively, and appreciate Dr. Bob Baker and Matt Giddings for their assistance with adrenal extractions. In addition, we thank Krishna Mehta, Cole Mateo, Janey Quinn, Collen Kovacsics, Jenna Smith, Alex Bruscke, Rachel Anolik and Kerry Michael for assistance in fecal sample preparation and extraction. The study was supported by the following grants: P30DK056336 (UAB CNRC); DK066007 and UAB-Support for Development & Application of Research Using Animal Models Grant (Boggiano); and internal Pennsylvania State University Funding (Cavigelli).
REFERENCES
- 1.Van Loo PL, Van de Weerd HA, Van Zutphen LF, Baumans V. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim. 2004;38:178–188. doi: 10.1258/002367704322968867. [DOI] [PubMed] [Google Scholar]
- 2.Gonder JC, Laber K. A renewed look at laboratory rodent housing and management. ILAR J. 2007;48:29–36. doi: 10.1093/ilar.48.1.29. [DOI] [PubMed] [Google Scholar]
- 3.Fox MW. In: Laboratory Animal Husbandry: Ethology, Welfare and Experimental Variables. Albany NY, editor. State University of New York Press; 1986. [Google Scholar]
- 4.Russell WMS. Shooting the clock: Timeless lessons of the past still guide today’s refinement initiatives. Science and Animal Care WARDS Newsletter. 1997;8:1–2. [Google Scholar]
- 5.Claasen V. Neglected Factors in Pharmacology and Neuroscience Research. Elsevier; Amsterdam, Netherlands: 1994. [Google Scholar]
- 6.Brenes Saenz JC, Villagra OR, Fornaguera Trias J. Factor analysis of forced swimming test, sucrose preference test and open field test on enriched, social and isolated reared rats. Behav Brain Res. 2006;169:57–65. doi: 10.1016/j.bbr.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Andrade CS, Guimarães FS. Anxiolytic-like effect of group housing on stress-induced behavior in rats. Depress Anxiety. 2003;18:149–152. doi: 10.1002/da.10124. [DOI] [PubMed] [Google Scholar]
- 8.Muchimapura S, Mason R, Marsden CA. Effect of isolation rearing on pre- and post-synaptic serotonergic function in the rat dorsal hippocampus. Synapse. 2003;47:209–217. doi: 10.1002/syn.10167. [DOI] [PubMed] [Google Scholar]
- 9.Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behavioral Processes. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Menich SR, Baron A. Social housing of rats: life-span effects on reaction time, exploration, weight, and longevity. Exp Aging Res. 1984;10:95–100. doi: 10.1080/03610738408258550. [DOI] [PubMed] [Google Scholar]
- 11.Sahakian BJ, Burdess C, Luckhurst H, Trayhurn P. Hyperactivity and obesity: the interaction of social isolation and cafeteria feeding. Physiol Behav. 1982;28:117–124. doi: 10.1016/0031-9384(82)90112-3. [DOI] [PubMed] [Google Scholar]
- 12.Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Gardiner SM, Bennett T. Factors affecting the development of isolation-induced hypertension in rats. Med Biol. 1978;56:277–281. [PubMed] [Google Scholar]
- 14.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrilovic L, Dronjak S. Activation of rat pituitary-adrenocortical and sympatho-adrenomedullary system in response to different stressors. Neuro Endocrinol Lett. 2005;26:515–520. [PubMed] [Google Scholar]
- 16.Nunes Mamede Rosa ML, Nobre MJ, Ribeiro Oliveira A, Brandao ML. Isolation-induced changes in ultrasonic vocalization, fear-potentiated startle and prepulse inhibition in rats. Neuropsychobio. 2005;51:248–255. doi: 10.1159/000085820. [DOI] [PubMed] [Google Scholar]
- 17.Perelló M, Chacon F, Cardinali DP, Esquifino AI, Spinedi E. Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sci. 2006;78:1857–1862. doi: 10.1016/j.lfs.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Von Frijtag JC, Schot M, van den Bos R, Spruijt BM. Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Dev Psychobiol. 2002;41:58–69. doi: 10.1002/dev.10057. [DOI] [PubMed] [Google Scholar]
- 20.Westenbroek C, Snijders TA, den Boer JA, Gerrits M, Fokkema DS, Ter Horst GJ. Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Horm Behav. 2005;47:620–628. doi: 10.1016/j.yhbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Bartolomucci A, Palanza P, Sacerdote P, Ceresini G, Chirieleison A, Panerai AE, Parmigiani S. Individual housing induces altered immuno-endocrine responses to psychological stress in male mice. Psychoneuroendocrin. 2003;28:540–558. doi: 10.1016/s0306-4530(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 22.Silva RC, Santos NR, Brandao ML. Influence of housing conditions on the effects of serotonergic drugs on feeding behavior in non-deprived rats. Neuropsychobio. 2003;47:98–101. doi: 10.1159/000070017. [DOI] [PubMed] [Google Scholar]
- 23.Karolewicz B, Paul IA. Group housing of mice increases immobility and antidepressant sensitivity in the forced swim and tail suspension tests. Eur J Pharmacol. 2001;415:197–201. doi: 10.1016/s0014-2999(01)00830-5. [DOI] [PubMed] [Google Scholar]
- 24.Morgan M, Einon D. Incentive motivation and behavioral inhibition in socially-isolated rats. Physiol Behav. 1975;15:405–409. doi: 10.1016/0031-9384(75)90205-x. 1. [DOI] [PubMed] [Google Scholar]
- 25.Hall FS, Humby T, Wilkinson LS, Robbins TW. The effects of isolation-rearing on sucrose consumption in rats. Physiol Behav. 1997;62:291–297. doi: 10.1016/s0031-9384(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 26.Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology. 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- 27.Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology. 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- 28.Shaw DC, Gallagher RH. Group or singly housed rats? In Standards in Laboratory Animal Management. Potters Bar; U.K.: 1984. [Google Scholar]
- 29.Crowder WF, Hutto CWJ. Operant place conditioning measures examined using two nondrug reinforcers. Pharmacol Biochem Behav. 1992;41:817–824. doi: 10.1016/0091-3057(92)90233-6. [DOI] [PubMed] [Google Scholar]
- 30.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrin. 2005;184:153–163. doi: 10.1677/joe.1.05935. [DOI] [PubMed] [Google Scholar]
- 31.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci. 2006;45:17–21. [PubMed] [Google Scholar]
- 32.Good T, Khan MZ, Lynch JW. Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus) Physio & Behav. 2003;80:405–411. doi: 10.1016/j.physbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- 34.Hunt C, Hambly C. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiol Behav. 2006;87:519–526. doi: 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Möstl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002;23:67–74. doi: 10.1016/s0739-7240(02)00146-7. [DOI] [PubMed] [Google Scholar]
- 36.Vollmer RR, Baruchin A, Kolibal-Pegher SS, Corey SP, Stricker EM, Kaplan BB. Selective activation of norepinephrine- and epinephrine-secreting chromaffin cells in rat adrenal medulla. Am J Physiol. 1992;263:R716–721. doi: 10.1152/ajpregu.1992.263.3.R716. [DOI] [PubMed] [Google Scholar]
- 37.American Medical Association . Use of Animals in Biomedical Research -The Challenge and Response - An American Medical Association White Paper. Group on Science and Technology, AMA; Chicago: 1992. [Google Scholar]
- 38.Harper JM, Austad SN. Effect of capture and season on fecal glucocorticoid levels in deer mice (Peromyscus maniculatus) and red-backed voles (Clethrionomys gapperi) Gen Comp Endocrinol. 2001;123:337–344. doi: 10.1006/gcen.2001.7682. [DOI] [PubMed] [Google Scholar]
- 39.Royo F, Björk N, Carlsson H-E, Mayo S, Hau J. Impact of chronic catheterization and automated blood sampling (Accusampler) on serum corticosterone and fecal immunoreactive corticosterone metabolites and immunoglobulin A in male rats. J Endocrinol. 2004;180:145–153. doi: 10.1677/joe.0.1800145. [DOI] [PubMed] [Google Scholar]
- 40.Borer KT, Bestervelt LL, Mannheim M, Brosamer MB, Thompson M, Swamy U, Piper WM. Stimulation by voluntary exercise of adrenal glucocorticoid secretion in mature female hamsters. Phys Behav. 1992;51:713–718. doi: 10.1016/0031-9384(92)90106-c. [DOI] [PubMed] [Google Scholar]
- 41.Fagin KD, Shinsako J, Dallman MF. Effects of housing and chronic cannulation on plasma ACTH and corticosterone in the rat. Am J Physiol. 1983;245:E515–E520. doi: 10.1152/ajpendo.1983.245.5.E515. [DOI] [PubMed] [Google Scholar]
- 42.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 43.Scalera G. Taste preferences, body weight gain, food and fluid intake in singly or group-housed rats. Physiol Behav. 1992;52:935–943. doi: 10.1016/0031-9384(92)90374-b. [DOI] [PubMed] [Google Scholar]
- 44.Georgsson L, Barrett J, Gietzen D. The effects of group-housing and relative weight on feeding behaviour in rats. Scand J Lab Anim Sci. 2001;28:201–209. [Google Scholar]
- 45.Perez C, Canal JR, Dominguez E, Campillo JE, Guillen M, Torres MD. Individual housing influences certain biochemical parameters in the rat. Lab Anim. 1997;31:357–361. doi: 10.1258/002367797780596158. [DOI] [PubMed] [Google Scholar]
- 46.Collier G, Johnson DF, Mitchell C. The relation between meal size and the time between meals: effects of cage complexity and food cost. Physiol Behav. 1999;67:339–346. doi: 10.1016/s0031-9384(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 47.de Castro JM, Brewer EM. The amount eaten in meals by humans is a power function of the number of people present. Physiol Behav. 1992;51:121–151. doi: 10.1016/0031-9384(92)90212-k. [DOI] [PubMed] [Google Scholar]
- 48.Lumeng JC, Hillman KH. Eating in larger groups increases food consumption. Arch Dis Child. 2007;92:384–387. doi: 10.1136/adc.2006.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiala B, Snow FM, Greenough WT. “Impoverished” rats weigh more than “enriched” rats because they eat more. Dev Psychobiol. 1977;10:537–541. doi: 10.1002/dev.420100607. [DOI] [PubMed] [Google Scholar]
- 50.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. Nesting material as environmental enrichment has no adverse effects on behavior and physiology of laboratory mice. Physiol Behav. 1997;62:1019–1028. doi: 10.1016/s0031-9384(97)00232-1. [DOI] [PubMed] [Google Scholar]
- 51.Robertson KL, Rowland NE. Effect of two types of environmental enrichment for singly housed mice on food intake and weight gain. Lab Anim Sci. 2005;34:29–32. doi: 10.1038/laban1005-29. [DOI] [PubMed] [Google Scholar]
- 52.Moons CP, Van Wiele P, Odberg FO. To enrich or not to enrich: providing shelter does not complicate handling of laboratory mice. Contemp Top Lab Anim Sci. 2004;43:18–21. [PubMed] [Google Scholar]
- 53.Lopak V, Eikelboom R. Pair housing induced feeding suppression: individual housing not novelty. Physiol Behav. 2000;71:329–333. doi: 10.1016/s0031-9384(00)00347-4. [DOI] [PubMed] [Google Scholar]
- 54.Loscalzo J. The NIH budget and the future of biomedical research. NEJM. 2006;354:1665–1667. doi: 10.1056/NEJMp068050. [DOI] [PubMed] [Google Scholar]
- 55.Dove A. Biomedical research faces flat budget for 2008. Nat Med. 2007;13:228–229. doi: 10.1038/nm0307-228b. [DOI] [PubMed] [Google Scholar]
- 56.Reinhardt V. Compassion for animals in the laboratory: impairment or refinement of research methodology? J Appl Anim Welf Sci. 2003;6:123–130. doi: 10.1207/S15327604JAWS0602_04. [DOI] [PubMed] [Google Scholar]