Abstract
The present investigation extends previous work on the relationship between daily stressors and memory failures in a naturalistic setting by examining whether this relationship varies across levels of neuroticism. A daily diary study of 333 older adults (mean age = 73.27 years, SD = 7.17) in the Veterans Affairs Normative Aging Study (see A. Spiro & R. Bossé, 2001, for additional information) was used to examine whether there were neuroticism differences in cognitive reactivity to daily stressors. Multilevel models indicated that on days when people high in neuroticism experienced stressors, particularly interpersonal stressors, they were more likely to report memory failures compared to those who were lower in neuroticism. The findings may have important implications for age-related cognitive decline.
Keywords: neuroticism, daily stressors, interpersonal stressors, memory failures, intraindividual variability
Neuroticism, and the constant elevated levels of negative affect that accompany the trait over years or decades, can lead to a negative emotion “hair trigger” in older adulthood (Kendler, Thornton, & Gardner, 2001; Wilson, Evans, et al., 2003). This process suggests that as people high in neuroticism grow older, they become more susceptible to elevated negative affect. Therefore, they may become hypersensitive to stress. Indeed, Mroczek, Spiro, Griffin, and Neupert (2006) reported that older adults high in neuroticism were the most reactive to stressors (compared with younger and middle-aged adults). More recently, Mroczek and Spiro (2007) found that both a high level of and an increasing rate of change in neuroticism in older men were associated with elevated mortality risk, which points to the potential of high neuroticism to influence physical health outcomes. Mroczek and Spiro (2003) also found that older men with high levels of neuroticism complained of more memory problems than did those with low levels of neuroticism. Given these findings and the previously documented associations between laboratory-based stressors and cognition (e.g., Lupien & Lepage, 2001; McEwen, 2000; Sapolsky, 1999) as well as naturalistic stressors and memory failures (Neupert, Almeida, Mroczek, & Spiro, 2006a), it is important to examine whether older adults high in neuroticism exhibit patterns of hypersensitivity to daily stressors with respect to their daily memory. Although this process of hypersensitivity is often observed for negative affect, it has yet to be established with daily memory failures. Because the sample in the present study consisted of older adults, we were able to examine this process in people for whom age-related declines in memory (whether real or perceived) might be particularly salient.
The tendency of people high in neuroticism to react to stressful situations with high negative affect is called the stress reactivity effect (Bolger & Schilling, 1991; Suls, 2001), and it is perhaps the central concept in understanding individual differences in mood regulation (Mroczek et al., 2006). When people high in neuroticism experience stressful events, they tend to experience them as more aversive and react with much higher levels of negative affect than do those low in this trait (Bolger & Schilling, 1991; Bolger & Zuckerman, 1995; David & Suls, 1999; Gunthert, Cohen, & Armeli, 1999). Suls (2001) called this process hyperreactivity, or a large change in negative affect in response to a stressor. What is not known is whether hyperreactivity can also be observed with respect to cognition; in the present investigation, we focus on the specific cognitive domain of memory failures. We have defined cognitive reactivity as the within-person increase in memory failures associated with daily stressors (Neupert, Stawski, & Almeida, 2008), and we examine whether this increase is more pronounced for those high in neuroticism. This definition is distinct from the idea of intrusive thoughts and avoidant cognitions in response to stressful life events (Klein & Boals, 2001), but it is similar to the definition of cognitive reactivity that is posed in the clinical literature where it is defined as “a change in one or more cognitive indices in response to an emotion evocation challenge” (Fresco, Segal, Buis, & Kennedy, 2007, p. 448). We were especially interested in cognitive reactivity within a sample of older adults, given the age-related differences in many domains of cognitive performance (e.g., Salthouse, 1996; Salthouse, Babcock, & Shaw, 1991).
There are two theoretical underpinnings of hyperreactivity (Mroczek & Almeida, 2004) that are important for the present study. First, people high in neuroticism report more stressful events in their lives, which implies that there is greater exposure to stress or to the creation of stressful situations (Bolger & Zuckerman, 1995; Ormel & Wohlfarth, 1991). Optimal self-regulation requires an ability to identify and avoid situations that may elevate negative affect, and people high in neuroticism may lack this particular regulatory ability (Mroczek et al., 2006). However, certain life span theories of emotion regulation are consistent with the idea of lessened emotional reactivity to stress with age (Carstensen, 1995; Carstensen, Isaacowitz, & Charles, 1999; Labouvie-Vief & DeVoe, 1991; Lang, Staudinger, & Carstensen, 1998). For example, socioemotional selectivity theory (Carstensen et al., 1999) posits that there is better regulation of emotion among older adults, and better emotion regulation is a key aspect of optimal aging (Baltes & Baltes, 1990; Baltes, Lindenberger, & Staudinger, 1998; Heckhausen & Schulz, 1995; Magai, 2001). Reduced emotional reactivity to daily stressors in older adults compared to younger adults has been previously documented (Neupert, Almeida, & Charles, 2007), but the role of neuroticism in cognitive reactivity among older adults remains unexplored.
The second relevant theoretical underpinning of hyperreactivity is that people high in neuroticism are more likely to appraise stressors as threats instead of challenges, which increases the probability of experiencing negative affect as a response to a stressful event (Lazarus & Folkman, 1984; Suls, 2001). If things are seen as a threat, there is a risk of activating threat-response systems, such as Gray’s (1991) fight-or-flight response, when they are not necessary. Such overactivation of threat response systems not only results in elevated negative affect but also prolongs their physiological consequences, such as elevated levels of stress hormones (e.g., cortisol) and immune system parameters (e.g., interleukin-6). Chronic levels of these hormones indicate dysregulation and can ultimately cause both cardiovascular and neurological damage (Kendler et al., 2001; Wilson, Bienas, Mendes de Leon, Evans, & Bennett, 2003). This physical dysregulation is a consequence of psychological dysregulation. Because previous research has found that stress and its associated hormones are linked with poorer cognitive performance (e.g., Lupien, Maheu, Tu, Fiocco, & Schramek, 2007; Wolf, Schommer, Hellhammer, McEwen, & Kirschbaum, 2001), it is important to understand whether personality characteristics associated with overactivation of the stress response (i.e., neuroticism) are linked to the observed daily relationship between stressors and memory failures in older adults (e.g., Neupert et al., 2006a).
Neuroticism is associated with more variability in behavior and experience (Eid & Diener, 1999; H. J. Eysenck & Eysenck, 1985; Moskowitz & Zuroff, 2004), and indeed the low end of the neuroticism dimension is typically termed stability (Robinson & Tamir, 2005). Previous research has found that neuroticism is associated with more variability in laboratory-based cognitive performance (Robinson & Tamir, 2005). Neuroticism is also associated with increased perceived stress (e.g., Hooker, Monahan, Shifren, & Hutchinson, 1992) and heightened reactions to stressors (e.g., Mroczek & Almeida, 2004). Furthermore, neuroticism has been linked to cognitive performance, such that lower levels of neuroticism are related to better episodic memory performance (e.g., Meier, Perrig-Chiello, & Perrig, 2002). Perrig-Chiello, Perrig, and Staehelin (2000) found that neuroticism was a significant predictor of memory decline for men over 75 years old, and Wilson, Evans, et al. (2003) found that people high in neuroticism had twice the risk of developing Alzheimer’s disease as did those low in neuroticism. The present study extends previous work by examining naturally occurring stressors and memory failures for 8 consecutive days to test for interindividual differences in intraindividual variability and covariation; that is, we examined neuroticism differences in intraindividual variability in daily stressors and memory failures as well as the within-person covariation between stressors and memory failures over time within a sample of older adults.
In addition to the number of stressors one experiences, the type of stressor that one encounters may also be important. For example, interpersonal stressors (e.g., arguments) are associated with increased psychological distress, especially in women (Almeida & Kessler, 1998). More broadly, stressors that entail some sort of danger are associated with anxiety, whereas stressors that entail loss are associated with depression (Finlay-Jones, 1989). Neupert et al. (2006a) found that interpersonal stressors were associated with more memory failures as well as an increase in memory failures from one day to the next. Because the domain of the stressor is an important characteristic to examine (Lazarus, 1999), and because the interpersonal domain has been found to be particularly relevant to daily memory failures, we also examine whether the relationships between specific stressor domains and memory failures depend on neuroticism.
The relationship between stressors and memory failures could be due to a variety of mechanisms. Major life event stressors could be related to memory failures. For example, posttraumatic stress disorder has been associated with poorer cognitive functioning (e.g., Yehuda et al., 2005), and a positive association between life event stressors and daily memory failures has been reported in previous work (Neupert et al., 2006a). Physical health could also be important, given a link between poorer health status and lower cognitive functioning (e.g., Hultsch, MacDonald, Hunter, Levy-Bencheton, & Strauss, 2000). Depending on the cognitive task, gender differences could also play a role (e.g., Larrabee & Crook, 1993; West, Crook, & Barron, 1992). Because of the well-documented age differences in many cognitive measures (e.g., Salthouse, 1996), there could be age differences in memory failures. Therefore, age, life event stressors, self-rated physical health, and gender were used as covariates in the present study.
The main goal of the present study was to examine whether cognitive reactivity to daily stressors was associated with neuroticism in a sample of older adults. A microanalytic daily diary design was chosen because it allowed for the examination of within-person covariation of daily stressors and memory failures over time as well as the exploration of between-person differences (neuroticism) in within-person processes (the relationship between stressors and memory failures over time). Specifically, we hypothesized that people high in neuroticism would be more variable in their daily stressor exposure and memory failures; that is, they would exhibit more intraindividual variability than those low in neuroticism. We also hypothesized that people high in neuroticism would report more memory failures on days when stressors were experienced (i.e., be more reactive) compared to those lower in neuroticism. In addition to examining stressor exposure, we also examined whether these differences would be observed in stressor appraisal (i.e., subjective severity ratings). Further, we examined whether differential reactivity would be found for specific stressor domains (i.e., interpersonal, demands, network, health-related, and other).
Method
Participants
Participants for the analyses were drawn from the Veterans Affairs Normative Aging Study (NAS), a longitudinal study of normal aging processes in men that began in the 1960s (see Spiro & Bossé, 2001, for additional information). In 2001, 1,125 participants (882 men and 243 of their wives or female partners) completed a questionnaire that was used to assess personality, health behaviors, and life event stressors. Starting in August 2002, recruitment began for the 8-day daily diary study regarding stressors and memory failures (see Neupert, Almeida, Mroczek, & Spiro, 2006b, for more information). Between August 2002 and April 2003, we contacted 529 NAS respondents and their wives or female partners and invited them to participate. Of these, 374 agreed, and 333 (181 men, 152 women, 115 husband and wife or partner pairs) returned usable surveys. Most participants completed all 8 days of the study, yielding a compliance rate of 99% and resulting in 2,649 days available for analysis. Respondents who completed the diary did not differ significantly from those who refused or from NAS participants in the 2001 survey who were not included in the diary subsample in terms of age (M = 73.27 years, SD = 7.17, range: 44–89), self-rated health (M = 2.58, SD = 0.94, range: 1 [excellent] to 5 [poor]), number of life-event stressors (M = 3.36, SD = 3.59, range: 0–30), and neuroticism (M = 2.21, SD = 2.06, range: 0–9). Although diary participants ranged in age from 44–89 years, 97% of the sample was 60 years old or older. Further, those who were under the age of 60 were the wives or female partners of the male NAS participants.1 Because some of the participants were part of a reporting dyad, it was necessary to consider the possible dependency among observations. We conducted a multilevel model to test for the relationship between husbands’ and wives’ (or partners’) memory failures over time, and results indicated that the reports were not related (γ10 = .04, t = 0.92, p = .36). Thus, in all subsequent analyses we treat the participants as independent observations.
Procedure
Instructions indicating when to complete the diary (approximately half an hour before going to bed) and when to return the surveys (when all eight were completed) were sent to each participant. For 8 consecutive evenings, participants completed short semistructured questionnaires about their daily experiences (e.g., stressors and memory failures). At the conclusion of the 8-day period, participants returned the diaries. If they completed 5 or more of the 8 study days, they received $30; if they completed 4 or fewer days, they received $15.
Measures
The number of daily stressors was assessed through the Daily Inventory of Stressful Events (Almeida, Wethington, & Kessler, 2002). Participants answered questions regarding arguments, potential arguments, stressors that occurred at work and volunteer settings and at home, network stressors (stressors that occurred to a network of friends and family), health-related events, and other stressors (stressors that may not have fit into the other categories) each day. Several variables were computed from the frequency of stressors. For each person, for each day, we first computed the sum of all stressors reported (M = 0.85, SD = 1.10, range: 0–7); we also computed five dichotomous dummy variables that indicated whether the different types of stressors (i.e., interpersonal stressors, demands, network stressors, health stressors, and other) occurred. We chose to use the frequency of stressors as an index of stressor exposure, which is (a) consistent with previous work (e.g., Neupert et al., 2006a, 2006b), (b) maintains the maximum range of the exposure scale, and (c) allows for the examination of cumulative effects of multiple stressors. If participants reported that a stressor had occurred, they were also asked how stressful the event was for them (1 = not at all, 2 = a little, 3 = somewhat, 4 = very), which is a subjective appraisal of stressor severity (e.g., Lazarus, 1999). The sum of the subjective severity ratings was computed for each person for each day (M = 4.47, SD = 2.98, range: 1–28).
Participants’ everyday memory failures were assessed daily through the use of a shortened version of a questionnaire developed by Sunderland, Harris, and Baddeley (1983). The original consisted of 35 yes–no questions tapping five distinct aspects of everyday memory failures: Speech, Reading and Writing, Faces and Places, Actions, and Learning New Things. Sunderland et al. have shown that this self-report measure of everyday memory failures has good psychometric properties. To reduce participant burden, we selected 1 item from each of the 5 aspects, specifically choosing the ones that were most likely to be endorsed (see Neupert et al., 2006b, for a description of the pilot study and validation of the scale). The questions presented were
In the past 24 hours:
Did you go back to check whether you had done something that you meant to do?
Did you start to read something (a book or an article in a newspaper or a magazine) without realizing you had already read it before?
Did you find that a word was “on the tip of your tongue,” you knew what it was but could not quite find it?
Did you have difficulty picking up a new skill, for example, finding it hard to learn a new game or to work some new gadget after you had practiced once or twice?
Did you fail to recognize, by sight, close relatives or friends, or fail to recognize famous people seen on television or in photographs?
The diary also included an item that assessed everyday memory failures regarding medication adherence. Memory failure variables were constructed in a similar manner as the stressor variables; the frequency of all six memory failure items for each day was computed (M = 0.93, SD = 1.08, range: 0–6).
Neuroticism was measured by the EPI-Q (Floderus, 1974), which is a short version of the Eysenck Personality Inventory. The EPI-Q contains 18 dichotomous items (9 assessing Extraversion and 9 assessing Neuroticism) and has demonstrated suitable reliability and validity (Floderus-Myrhed, Pedersen, & Rasmuson, 1980; Janakiramaiah, 1983; Levenson, Aldwin, Bossé, & Spiro, 1988; Mroczek, Spiro, Aldwin, Ozer, & Bossé, 1993). The range of possible scores was from 0 to 9, with a higher score indicating a higher level of neuroticism (α = .71).
Covariates
We controlled for four covariates, which were assessed by a survey of NAS participants and their wives or partners in 2001. These included age, gender, life event stressors (measured by the Elders Life Stress Inventory, a 31-item self-administered scale; Aldwin, 1991), and a single-item global self-rating of health (ranging from 1 [excellent] to 5 [poor]).
Analyses
To analyze data that were obtained through a daily diary design, we used multilevel modeling. Multilevel modeling is frequently used by researchers to model intraindividual variability (e.g., Grzywacz, Almeida, Neupert, & Ettner, 2004; Neupert et al., 2006a, 2006b), that is, people’s variability around their own average. This technique was especially useful because we sought to examine intraindividual variability in stressors and memory failures and whether the covariation within individuals would depend on between-person differences in neuroticism. Models similar to the one below were used to address the research questions. The following model was used to examine neuroticism differences in the within-person covariation between daily stressor exposure and memory failures:
- Level 1: memory failuresit=β0it+β1it (daily stressors)+rit
- Level 2: β0i=γ00+γ01 (neuroticism)+μ0i
- β1i=γ10+γ11 (neuroticism)+u1i
In Level 1, memory failures for person i on day t is a function of the intercept, β0it, which is defined as the number of memory failures for person i on stressor-free days (i.e., daily stressors = 0). β1it is the expected change or shift in memory failures associated with the occurrence of stressors. The error term, rit, represents a unique effect associated with person i (i.e., individual fluctuation around the mean). In the Level 2 equations, γ00 is the mean number of memory failures for people with average levels of neuroticism (neuroticism was centered on the grand mean) on stressor-free days (i.e., daily stressors = 0), and γ10 is the average change in memory failure between days with and without stressors for people with average neuroticism levels. Neuroticism differences in the overall frequency of memory failures is represented by γ01, and the cross-level interaction examining neuroticism differences in the within-person slope (i.e., reactivity) of stressors and memory failures is represented by γ11. The degree to which people vary from the sample mean of memory failures is represented by u0i, and the degree to which people vary from the slope is represented by u1i.
Results
As previously reported by Neupert et al. (2006a), a preliminary analysis was conducted to ensure that there was sufficient variability between and within persons in the daily memory failures to warrant further analyses (e.g., Nezlek, 2001; Raudenbush & Bryk, 2002). A fully unconditional multilevel model (i.e., no predictors were included in the model) was conducted to obtain estimates of within-person (σ2 = .48, z = 33.95, p < .001) and between-person (τ00 = .70, z = 11.82, p < .001) variance. We then used the estimates to obtain the intraclass correlation coefficient, ρ = τ00 / (τ00 + σ2), which was .59, indicating that 59% of the variability in the sum of memory failures was between-person, and 41% was within-person. In other words, individuals varied around their own averages almost as much as they differed from others; thus, there was sufficient variability in the outcome variable.
To address the first hypothesis of neuroticism differences in intraindividual variability in daily stressors and memory failures, we computed correlations between individuals’ neuroticism scores and their within-person standard deviation scores for the daily variables. Results indicated that neuroticism was positively associated with intraindividual variability in overall stressor exposure, r(316) = .14, p < .05; the frequency of other stressors, r(316) = .13, p < .05; and the frequency of memory failures, r(316) = .17, p < .01; that is, people higher in neuroticism varied more than those lower in neuroticism in terms of these daily variables. Neuroticism was unrelated to variability in interpersonal stressors, r(316) = .08, p = .17; demands, r(316) = .07, p = .24; health stressors, r(316) = .09, p = .10; network stressors, r(316) = .08, p = .17; or subjective severity ratings of the stressors, r(234) = .04, p = .57. Because previous work (e.g., Eid & Diener, 1999) has shown that intraindividual variability can be related to mean level scores, we also conducted partial correlations on the three significant relationships from which we removed the mean level of stressor exposure, frequency of other stressors, and frequency of memory failures, respectively. Results from the partial correlations indicate that there were no neuroticism differences in intraindividual variability when mean levels were removed, stressor exposure, r(316) = −.03, p = .61; other stressors, r(316) = −.05, p = .34; memory failures, r(316) = .07, p = .20.
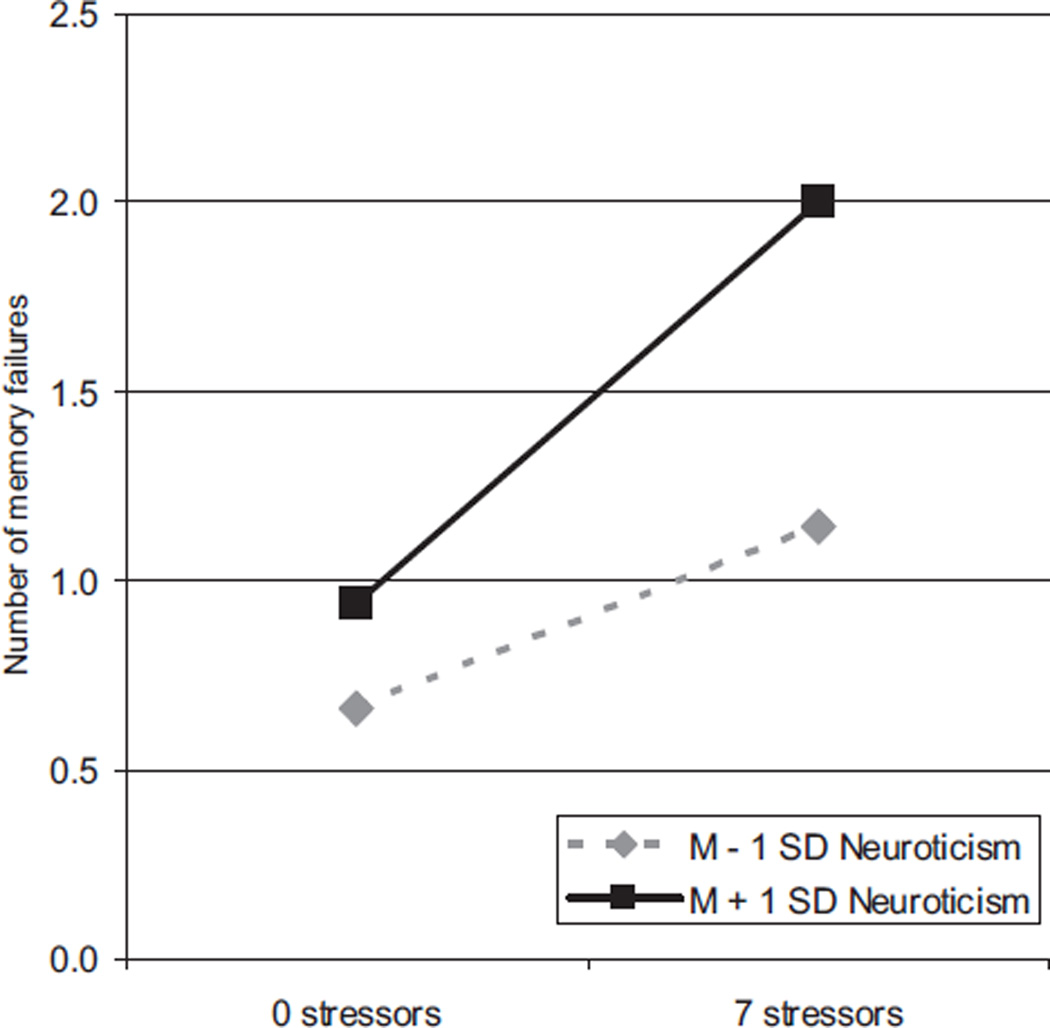
To address the second hypothesis regarding neuroticism differences in the relationship between daily stressors and memory failures, we tested a multilevel model controlling for between-person differences in age, gender, life-event stressors, and self-reported health, with all variables entered simultaneously (see Table 1). Age, life event stressors, self-reported health, and neuroticism were centered around their grand means, such that the grand mean of the sum of the memory failures (γ00 = 0.89 in the concurrent day model) indicates the number of memory failures for a man (i.e., gender = 0) of average age and neuroticism with average health and average life event stressors when no daily stressors were experienced (i.e., sum of stressors was 0). In terms of between-person differences, older adults (γ01), men (γ05), people higher in neuroticism (γ03), and those who experienced more life event stressors (γ02) reported more memory failures, but self-rated health (γ04) was not significantly related to daily memory failures. In terms of within-person associations, on days when people experienced more daily stressors, they reported more memory failures compared to stressor-free days (γ10).2 Specifically, each daily stressor was associated with 0.11 more memory failures in the concurrent day model, so people who experienced one stressor for 9 days could expect to experience one additional memory failure. Importantly, this within-person association was qualified by a significant cross-level interaction with neuroticism (γ11). As can be seen from Figure 1, people higher in neuroticism exhibited greater reactivity to daily stressors than did those lower in neuroticism. That is, people with more neuroticism experienced the sharpest increase in the number of memory failures when daily stressors occurred. The percent of within-person variance accounted for by the sum of daily stressors was calculated by obtaining the change in within-person variance estimates (σ2) from the fully unconditional (unconstrained) model to the current constrained model and then dividing the result by the unconstrained estimate, . The percentage of between-person variance accounted for by the Level 2 predictors was calculated in a similar fashion with the between-person variance estimates (τ00), (τ00uc − τ00c)/τ00uc. This model accounted for 7% of the within-person and 27% of the between-person variance in memory failures.
Table 1.
Unstandardized Estimates (and Standard Errors) of Daily Stressors and Memory Failures
| Variable | Concurrent day | Lagged model |
|---|---|---|
| Fixed effectsa | ||
| Number of memory failures, β0 | ||
| Intercept, γ00 | .89 (.06)*** | .65 (.05)*** |
| Age γ01 | .03 (.01)*** | .02 (.00)*** |
| Life-event stressors, γ02 | .05 (.01)*** | .04 (.01)*** |
| Neuroticism, γ03 | .07 (.02)** | .05 (.02)* |
| Self-rated health, γ04 | −.00 (.05) | −.01 (.04) |
| Gender, γ05 | −.19 (.09)* | −.13 (.07) |
| Within-person daily stressor slope, β1 | ||
| Intercept, γ10 | .11 (.02)*** | .08 (.02)*** |
| Neuroticism, γ11 | .02 (.01)* | .02 (.01)* |
| Previous day memory failures, β2 | ||
| Intercept, γ20 | .14 (.03)*** | |
| Random effectsa | ||
| Level of memory failures, τ00 | .51*** | .24*** |
| Reactivity slope, τ11 | .03** | .03** |
| Slope of previous day, τ22 | .04*** | |
| Within-person fluctuation, σ2 | .45*** | .40*** |
n = 333 participants; 2,649 days.
p < .05.
p < .01.
p < .001.
Figure 1.
Predicted values based on extreme observed scores (0 stressors, 7 stressors) for the cross-level interaction of neuroticism differences in the within-person covariation of the sum of daily stressors and memory failures over time. The figure is adjusted for age, gender, self-rated health, and number of life event stressors. People higher in neuroticism (M = 1 SD) were more reactive to daily stressors than were those lower in neuroticism (M – 1 SD).
The previous model addressed neuroticism differences in the relationship between stressor exposure and memory failures, but we were also interested in possible neuroticism differences in the relationship between stressor appraisal (operationalized as subjective severity ratings) and memory failures. We estimated a model identical to the one above, but we replaced the sum of stressors with the sum of severity ratings. Results indicated that although days with more subjectively severe stressors were associated with more memory failures (γ10 = .05, t = 4.26, p < .001), this relationship did not vary by neuroticism (γ11 = .00, t = 0.11, p = .91).
Because it has been previously reported that daily stressors are associated with a change in memory failures from one day to the next (Neupert et al., 2006a), we also estimated a model to examine whether people higher in neuroticism would experience a greater increase in the number of memory failures from one day to the next. We conducted a lagged analysis where memory failures on day t predicted memory failures on day t = 1. This model is equivalent to a change score model in terms of the Level 1 variables (Raudenbush & Bryk, 2002). The lagged model in Table 1 is identical to the concurrent day model with the addition of previous day memory failures as a predictor. As indicated by the γ10 estimate, daily stressors were associated with an increase in memory failures from one day to the next, and the γ11 estimate indicates that people higher in neuroticism experienced a greater increase in memory failures from one day to the next compared with those who were lower in neuroticism. This model explained 17% of the within-person and 66% of the between-person variance in memory failures.
We estimated a similar model to test whether the subjective severity of stressors would be associated with an increase in memory failures from one day to the next. Although subjective severity was associated with an increase in memory failures (γ10 = .04, t = 2.74, p = .006), this increase did not differ by neuroticism (γ11 = .00, t = 1.05, p = .30).
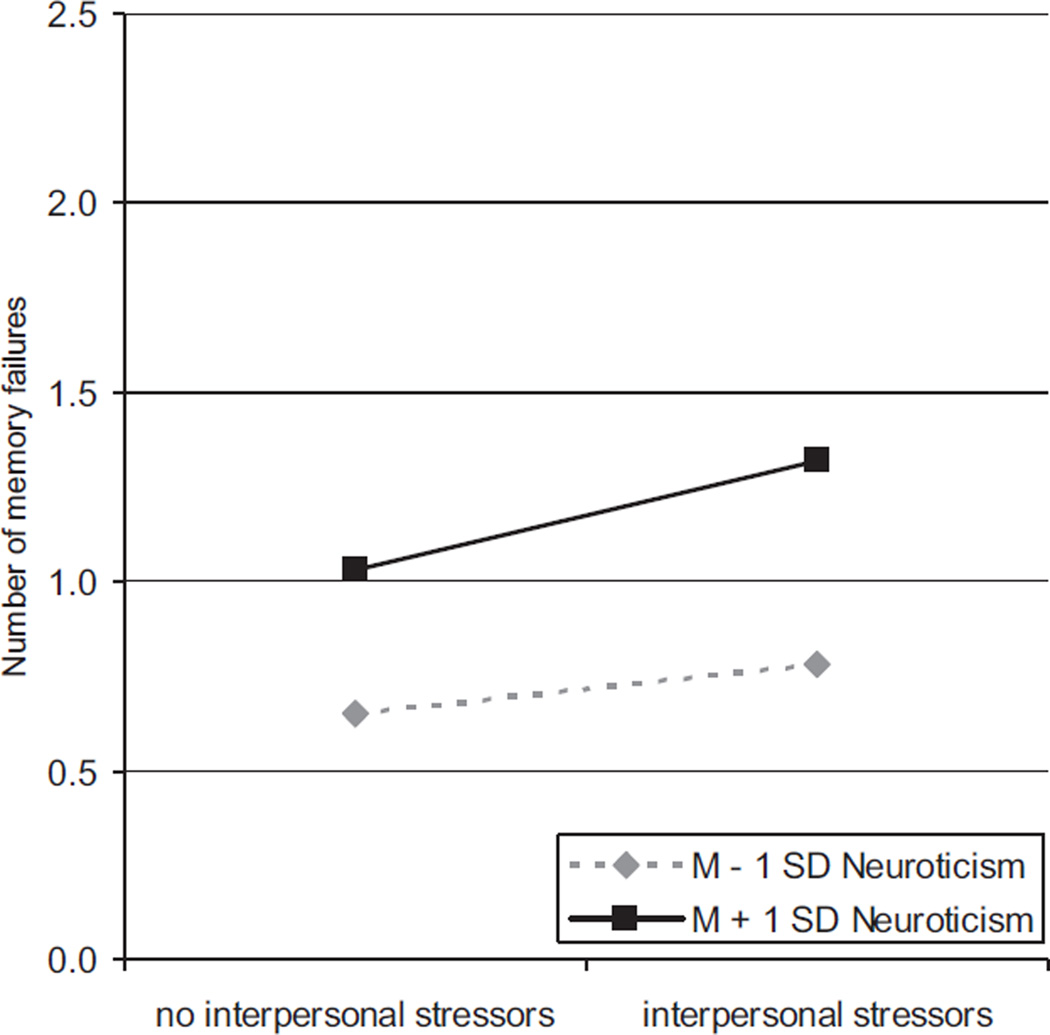
We also examined the role of neuroticism in relation to specific stressor domains and memory failures by modeling memory failures as a function of exposure to daily interpersonal stressors, demands (in the work or volunteer domain or the home domain), network stressors, health-related events, and other stressors and then by testing for cross-level interactions with neuroticism in each model. Findings from these models are shown in Table 2. Interpersonal stressors were positively associated with memory failure frequency (γ10), and Figure 2 shows that people higher in neuroticism were more reactive to interpersonal stressors than were those lower in neuroticism (γ11). Demands, network stressors, health-related stressors, and other stressors were not uniquely related to memory failures. We found no cross-level interactions with neuroticism for demands, network stressors, or health-related stressors, but the relationship between other stressors and memory failures did differ by neuroticism. It is important to note, however, that the random effect of the slope between other stressors and memory failures did not vary significantly across people. Therefore, this interaction should be interpreted with caution. The pattern of differences in reactivity to other stressors was similar to that of interpersonal stressors. The interpersonal stressor model accounted for 6% of the within-person and 26% of the between-person variance in memory failures, and the other stressor model accounted for 4% of the within-person and 12% of the between-person variance in memory failures.
Table 2.
Unstandardized Estimates (and Standard Errors) of Specific Daily Stressor Domain Exposure and the Frequency of Memory Failures
| Variable | Interpersonal | Demand | Network | Health | Other |
|---|---|---|---|---|---|
| Fixed effectsa | |||||
| Number of memory failures,β0 | |||||
| Interceptb,γ00 | .93 (.06) | .99 (.06) | .98 (.06) | .98 (.06) | .98 (.06) |
| Ageb,γ01 | .03 (.01) | .03 (.01) | .03 (.01) | .03 (.01) | .03 (.01) |
| Life event stressorsb,γ02 | .05 (.01) | .05 (.01) | .05 (.01) | .05 (.01) | .05 (.01) |
| Neuroticismb,γ03 | .09 (.02) | .11 (.02) | .10 (.02) | .10 (.02) | .10 (.02) |
| Self-rated health,γ04 | −.00 (.05) | −.02 (.05) | −.01 (.05) | −.01 (.05) | −.01 (.05) |
| Gender,γ05 | −.19 (.09)* | −.21 (.10)* | −.19 (.10)* | −.18 (.10) | −.19 (.10) |
| Within-person daily stressor domain slope,β1 | |||||
| Intercept,γ10 | .21 (.04)*** | .07 (.12) | .08 (.05) | .04 (.06) | .08 (.06) |
| Neuroticism,γ11 | .04 (.02)* | −.00 (.05) | .03 (.02) | .04 (.03) | .05 (.03)* |
| Random effectsa | |||||
| Level of memory failures, τ00b | .52 | .53 | .56 | .53 | .53 |
| Reactivity slope, τ11 | .08* | .01 | .05 | .13* | .03 |
| Within-person fluctuation,σ2b | .45 | .47 | .46 | .46 | .46 |
n = 333 participants; 2,649 days.
For all domains, p < .001.
p < .05.
p < .001.
Figure 2.
Predicted values for the cross-level interaction of neuroticism differences in the within-person covariation of interpersonal stressors and memory failures over time. The figure is adjusted for age, gender, self-rated health, and number of life event stressors. People higher in neuroticism (M + 1 SD) were more reactive to interpersonal stressors than those lower in neuroticism (M – 1 SD).
Discussion
The current study examined neuroticism differences in intraindividual variability and covariation of daily stressors and memory failures in a sample of older adults. We examined the fluctuation of daily stressors and memory failures over the course of 8 consecutive days, and we were able to examine whether neuroticism was related to that intraindividual variability. In addition, we examined the within-person covariation between daily stressors and memory failures and sought to determine if the hyperreactive effect for those high in neuroticism with respect to affect (i.e., a large change in negative affect in response to a stressor) could be extended to memory failures.
In line with our expectations, people higher in neuroticism were more variable in their daily stressor exposure and reports of memory failures when we considered intraindividual standard deviations. This finding supports the general notion that neuroticism is characterized by instability and is associated with more variability in behavior and experience (Eid & Diener, 1999; H. J. Eysenck & Eysenck, 1985; Moskowitz & Zuroff, 2004). Our finding regarding daily stressors fits with the idea that optimal self-regulation requires an ability to identify and avoid situations that may elevate negative affect. Even though socioemotional selectivity theory (Carstensen et al., 1999) proposes that older adults in general may demonstrate better emotion-regulation with age, this finding suggests that older adults with high neuroticism are not able to effectively regulate their daily stressor experiences. It is important to note, however, that these relationships were no longer significant when the mean levels of the stressor and memory failure variables were removed. This supports the finding of Salthouse, Nesselroade, and Berish (2006) who documented that the correlations between age and intraindividual variability in cognitive performance were no longer significant after the mean levels of cognitive performance were removed. Although Salthouse et al. (2006) used laboratory-based cognitive performance tasks and examined age differences rather than daily memory failures and neuroticism differences, the general trend for individual differences in intraindividual variability to be attenuated after controlling for mean levels in the variability scores is consistent. In our study, the attenuation suggests that people high in neuroticism are more likely to report consistently higher levels of daily stressor exposure and memory failures. Future work that is able to tease apart potential trait-like (e.g., individual differences in intraindividual variability) and contextual differences (e.g., intraindividual variability associated with study measures such as laboratory-based cognitive tasks vs. daily memory failures) will shed light on this issue.
People higher in neuroticism were more cognitively reactive to daily stressors; that is, on days when they experienced stressors, they reported more memory failures compared to those lower in neuroticism. This finding is consistent with the idea that optimal self-regulation requires an ability to identify and avoid situations that may elevate negative affect, and people high in neuroticism may lack this particular regulatory ability (Mroczek et al., 2006). It is possible that daily stressors are related to memory failures in older adults through cognitive interference. Wegner (1988) argued that stress impairs mental control, specifically, the ability to concentrate. Consistent with these perspectives, Sarason, Pierce, and Sarason (1996) as well as M. W. Eysenck and Calvo (1992) have advanced cognitive interference as a mechanism responsible for effects of stress on cognition. Cognitive interference, according to Sarason et al. and Eysenck and Calvo, can be task-oriented worries (e.g., worrying about performance quality) and off-task thoughts (e.g., thinking about a negative event that may have just happened). These cognitions, in turn, limit the capacity to process and store information. Similarly, M. W. Eysenck, Derakshan, Santos, and Calvo (2007) developed attentional control theory, which states that anxiety impairs efficient functioning of the goal-directed attentional system. Although the current study did not include measures of actual cognitive deficits, the self-reported memory failures were primarily related to attention (e.g., Pollina, Greene, Tunick, & Puckett, 1993; Sunderland et al., 1983). Because of age-related differences in the capacity to process and store information (Sapolsky, 1999), any situation that vies for the attention of older adults could put them at increased risk of memory failures, especially if high levels of neuroticism predispose them to intrusive or negative thoughts. On days when people reported high ratings of subjective stressor severity, they also reported more memory failures compared to days with lower severity ratings. This finding highlights the importance of stressor appraisal in addition to exposure for older adults’ daily memory experiences. The effect of stressor appraisal was consistent across levels of neuroticism, as we did not find any interactions with neuroticism for within-person cognitive reactivity. The lack of difference by neuroticism is in contrast with the idea that people high in neuroticism are more likely to appraise stressors as threats instead of challenges, which increases the probability of negative responses to a stressful event (Lazarus & Folkman, 1984; Suls, 2001). However, this is the first time that cognitive reactivity to daily stressors has been examined as a function of neuroticism within a sample of older adults, and perhaps neuroticism differences in stressor appraisal are more central to affective responses across a wider range of ages. Because we were able to examine both stressor exposure and appraisal in this study, we document the neuroticism differences in cognitive reactivity for stressor exposure in older adults and consider the role of more subjective experiences such as stressor appraisal.
We also examined whether specific stressor domain was an important consideration in this process. Indeed, people high in neuroticism were especially reactive to interpersonal stressors. Interpersonal conflicts or tensions can be particularly detrimental to individuals’ psychological (Rook, 1984; Sherman, 2003), physical (Kiecolt-Glaser, 1999), and cognitive (Neupert et al., 2006a) functioning. It is possible that interpersonal tensions are especially distracting for older adults because of the motivation to maintain important social ties that possibly increases with age (Lang, 2001). Blanchard-Fields and Beatty (2005) conducted experimental studies, and suggested that older adults may be more likely than younger age groups to forgo self-enhancing attributions for relationship dilemmas when there is a possibility of repairing the relationship. In addition, Sorkin and Rook (2006) found that it may be easier for older adults to respond to an interpersonal stressor in ways that reduce or shorten their emotional distress than it is for them to respond in ways that increase a sense of coping efficacy. Because effort and attention are directed toward the interpersonal conflict, less attention may be available for tasks requiring memory. This process could be especially relevant to older adults high in neuroticism because although older adults in general are better at regulating their emotions (e.g., Carstensen et al., 1999) and therefore may not experience depletion in cognitive resources when faced with an interpersonal stressor, it appears that those high in neuroticism are more vulnerable.
Physiological mechanisms could also underlie the relationship between interpersonal stressors and memory failures. Kiecolt-Glaser, Malarkey, Cacioppo, and Glaser (1994) examined the physiological impact of close personal relationships that are chronically abrasive and stressful and found that they may provoke persistent physiological alterations. They speculated that heightened sympathetic nervous system activity is one key mechanism fueling endocrine and immune alterations. Similar to the effects that interpersonal stressors have on physical health (e.g., immune functioning), these stressors may also affect memory through a physiological process. If this is the case, neuroticism and interpersonal stressors may be acting as a double-whammy; that is, the potential physiological alterations associated with the dysregulation of neuroticism and the ramifications of interpersonal stressors are creating a synergistic effect on daily memory failures. This is especially important within the context of the observed age differences in the number of memory failures in this study, with older-old reporting more memory failures than were young-old. It is possible that older-old adults with high neuroticism who experience frequent interpersonal stressors may be at an especially high risk of frequent memory failures. We examined the possibility that the neuroticism differences in cognitive reactivity would be further qualified by age, but none of the interactions were significant. Because we were restricted to a primarily older adult sample, future work that incorporates younger, middle-aged, and older adults would be better equipped to address this question and to examine other possible mechanisms that may relate to neuroticism differences in cognitive reactivity.
In addition to interpersonal stressors, people high in neuroticism were also more cognitively reactive to other stressors (i.e., stressors which did not fit in the other categories). Future work will be important for researchers to determine if there is a specific attribute of these miscellaneous stressors that is important for the daily memory failures of older adults high in neuroticism. Because most of the participants lived in the Boston area and completed the study during the winter months, many of the other stressors were weather-related. Additionally, a subsample of participants (n = 32) were completing the diaries when the Columbia Shuttle exploded on February 1, 2003. As reported elsewhere (Neupert et al., 2006b), this event was recorded by some of the participants as a stressor which fell into the “other” category. Although speculative, these events appear to generally capture truly external circumstances which are beyond one’s control. Because control beliefs are important for reactivity to daily stressors (e.g., Neupert et al., 2007), future work that incorporates general as well as event-specific control beliefs will be able to shed light on the role that high neuroticism plays in cognitive reactivity to these types of events.
Limitations
The findings of this study should be considered in light of its limitations. Although we were able to address questions of daily stressor exposure and subjective severity, we did not have information regarding the objective severity of the events. We relied on subjective reports of severity (appraisals) to provide some additional context regarding the stressors, but we acknowledge that we were not able to fully capture the surrounding context of the events. Although previous research has documented that subjective severity is more closely tied to psychological well-being than objective severity is (e.g., Almeida, Neupert, Banks, & Serido, 2005), comparative work has not yet been done with daily memory. Additionally, information on neuroticism and the covariates (i.e., life events, health) was collected 1 to 2 years before participants completed the diary project. We wanted to include these potentially important variables and therefore used the closest assessment available, but we acknowledge that it is possible that they may have changed before the diary study began.
Because the diaries were sent and returned all at once and administered via paper and pencil, we cannot be certain that participants correctly followed the instructions in regard to when to complete the diaries (i.e., at the end of each day, about 30 min before going to bed). Although Stone, Shiffman, Schwartz, Broderick, and Hufford (2003) noted high rates of noncompliance with paper diaries when the study protocol called for four assessments each day for 21 consecutive days, we assert that our participants were more likely to be compliant because of the decreased burden associated with our protocol (i.e., one assessment per day for 8 days), and previous research has reported high levels of compliance with paper diaries when only one assessment per day is required (e.g., Sherliker & Steptoe, 2000). In addition, given the NAS participants’ history of compliance with this longitudinal study over the past 40 years (of the survivors, over 90% are continuing participants, and most of the men who are continuing participants report to the Boston Veterans Affairs Medical Center every 3 years for a biomedical exam and complete a number of questionnaires that are either mailed a month in advance or administered the day of the exam), it is unlikely that they waited until the 8th day to complete all of their diaries.
Conclusions and Future Directions
These findings may have important implications for age-related cognitive decline. As previous research has demonstrated (Mroczek & Spiro, 2003), neuroticism tends to decline with age. As neuroticism declines, the effect on cognitive reactivity may also decline. An important question for future research, and one we plan to investigate longitudinally with a second burst of daily diary measurement, is whether long-term changes in neuroticism are associated with long-term changes in the short-term covariation between daily stressors and memory failures. Additionally, future studies that incorporate physiological measures of stress will be able to more clearly assess the role of physiological dysregulation as a mechanism in the relationship between neuroticism and cognitive reactivity.
Acknowledgment
The views expressed in this article are those of the authors, and they do not necessarily represent the views of the Department of Veterans Affairs. This study was supported by National Institute on Aging Grant R01-AG18436 awarded to Daniel K. Mroczek and by the Research Service of the Department of Veterans Affairs. The Veterans Affairs Normative Aging Study is supported by the Cooperative Studies Program/ERIC, U.S. Department of Veterans Affairs and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston.
Footnotes
Portions of this article were presented at the annual meeting of the Gerontological Society of America, November, 2006, in Dallas, Texas.
Analyses were conducted with only those participants who were aged 60 or older, and the pattern of results remained the same. All analyses reported here include the full sample of participants.
Analyses were also conducted with the stressor variables group-mean centered, such that the between-person differences in the level of daily stressors was removed from the estimate of the within-person relationship between stressors and memory failures. The pattern of results was identical to that reported here. Results of these analyses are available from Shevaun D. Neupert upon request.
Contributor Information
Shevaun D. Neupert, Department of Psychology, North Carolina State University
Daniel K. Mroczek, Department of Child Development and Family Studies, and Center on Aging and the Life Course, Purdue University
Avron Spiro, III, Veterans Affairs Boston Healthcare System, Boston, Massachusetts, and Department of Epidemiology, Boston University School of Public Health.
References
- Aldwin CM. The Elders Life Stress Inventory (ELSI): Research and clinical applications. In: Keller PA, Heyman SR, editors. Innovations in clinical practice: A sourcebook. Sarasota, FL: Professional Resource Press; 1991. pp. 355–364. [Google Scholar]
- Almeida DM, Kessler RC. Everyday stressors and gender differences in daily distress. Journal of Personality and Social Psychology. 1998;75:670–680. doi: 10.1037//0022-3514.75.3.670. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Neupert SD, Banks SR, Serido J. Do daily stress processes account for socioeconomic health disparities? Journal of Gerontology: Social Sciences. 2005;60:34–39. doi: 10.1093/geronb/60.special_issue_2.s34. [DOI] [PubMed] [Google Scholar]
- Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: An investigator-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM. Psychological perspectives on successful aging: The model of selective optimization with compensation. In: Baltes PB, Baltes MB, editors. Successful aging: Perspectives from the behavioral sciences. New York: Cambridge University Press; 1990. pp. 1–34. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Life-span theory in developmental psychology. In: Damon W, Lerner RM, editors. Handbook of child psychology: Volume 1: Theoretical models of human development. 5th ed. Hoboken, NJ: Wiley; 1998. pp. 1029–1143. [Google Scholar]
- Blanchard-Fields F, Beatty C. Age differences in blame attributions: The role of relationship outcome ambiguity and personal identification. Journal of Gerontology: Psychological Sciences. 2005;60:P19–P26. doi: 10.1093/geronb/60.1.p19. [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and problems of everyday life: The role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59:356–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science. 1995;4:151–155. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- David JP, Suls J. Coping efforts in daily life: Role of big five traits and problem appraisals. Journal of Personality. 1999;67:265–294. doi: 10.1111/1467-6494.00056. [DOI] [PubMed] [Google Scholar]
- Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. Journal of Personality and Social Psychology. 1999;76:662–676. [Google Scholar]
- Eysenck HJ, Eysenck MW. Personality and individual differences: A natural science approach. New York: Plenum; 1985. [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Finlay-Jones R. Anxiety. In: Brown GW, Harris TO, editors. Life events and illness. New York: Guilford Press; 1989. pp. 95–112. [Google Scholar]
- Floderus B. Psychosocial factors in relation to coronary heart disease and associated risk factors. Nordisk Hygienisk Tidskrift. 1974;(Suppl. 6):1–148. [Google Scholar]
- Floderus-Myrhed B, Pedersen N, Rasmuson I. Assessment of heritability for personality, based on a short-form of the Eysenck Personality Inventory: A study of 12,898 twin pairs. Behavior Genetics. 1980;10:153–162. doi: 10.1007/BF01066265. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Segal ZV, Buis T, Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. Journal of Consulting and Clinical Psychology. 2007;75:447–455. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- Gray JA. Neural systems, emotion, and personality. In: Madden J, editor. Neurobiology of learning, emotion, and affect. New York: Raven Press; 1991. pp. 273–306. [Google Scholar]
- Grzywacz JG, Almeida DM, Neupert SD, Ettner S. Socioeconomic status and health: A micro-level analysis of exposure and vulnerability to daily stressors. Journal of Health and Social Behavior. 2004;45:1–16. doi: 10.1177/002214650404500101. [DOI] [PubMed] [Google Scholar]
- Gunthert KC, Cohen LH, Armeli S. The role of neuroticism in daily stress and coping. Journal of Personality and Social Psychology. 1999;57:731–739. doi: 10.1037//0022-3514.77.5.1087. [DOI] [PubMed] [Google Scholar]
- Heckhausen J, Schulz R. A life-span theory of control. Psychological Review. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- Hooker K, Monahan D, Shifren K, Hutchinson C. Mental and physical health of spouse caregivers: The role of personality. Psychology and Aging. 1992;7:367–375. doi: 10.1037//0882-7974.7.3.367. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14:588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Janakiramaiah N. EPI-Q: A short questionnaire version of EPI. Indian Journal of Clinical Psychology. 1983;10:275–278. [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. American Journal of Psychiatry. 2001;158:582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. Stress, personal relationships, and immune function: Health implications. Brain, Behavior, and Immunity. 1999;13:61–72. doi: 10.1006/brbi.1999.0552. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Malarkey WB, Cacioppo JT, Glaser R. Stressful personal relationships: Immune and endocrine function. In: Glaser R, Kiecolt-Glaser JK, editors. Handbook of human stress and immunity. San Diego, CA: Academic Press; 1994. pp. 321–339. [Google Scholar]
- Klein K, Boals A. Expressive writing can increase working memory capacity. Journal of Experimental Psychology: General. 2001;130:520–533. doi: 10.1037//0096-3445.130.3.520. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, DeVoe M. Emotional regulation in adulthood and later life: A developmental view. In: Schaie KW, Lawton MP, editors. Annual review of gerontology and geriatrics: Behavioral science and aging. Vol. 11. New York: Springer; 1991. pp. 172–194. [Google Scholar]
- Lang FR. Regulation of social relationships in later adulthood. Journal of Gerontology: Psychological Sciences. 2001;56:P321–P326. doi: 10.1093/geronb/56.6.p321. [DOI] [PubMed] [Google Scholar]
- Lang FR, Staudinger UM, Carstensen LL. Perspectives on socioemotional selectivity in late life: How personality and social context do (and do not) make a difference. Journal of Gerontology: Psychological Sciences. 1998;53B:P21–P30. doi: 10.1093/geronb/53b.1.p21. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Crook TH. Do men show more rapid ageassociated decline in simulated everyday memory than do women? Psychology and Aging. 1993;8:68–71. doi: 10.1037//0882-7974.8.1.68. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Stress and emotion: A new synthesis. New York: Springer; 1999. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Levenson MR, Aldwin CM, Bossé R, Spiro A., III Emotionality and mental health: Longitudinal findings from the Normative Aging Study. Journal of Abnormal Psychology. 1988;97:94–96. doi: 10.1037//0021-843x.97.1.94. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: Can’t live with it, can’t live without it. Behavioural Brain Research. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Magai C. Emotions over the life span. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5th ed. San Diego, CA: Academic Press; 2001. pp. 399–426. [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Meier B, Perrig-Chiello P, Perrig W. Personality and memory in old age. Aging, Neuropsychology, and Cognition. 2002;9:135–144. [Google Scholar]
- Moskowitz DS, Zuroff DC. Flux, pulse, and spin: Dynamic additions to the personality lexicon. Journal of Personality and Social Psychology. 2004;86:880–893. doi: 10.1037/0022-3514.86.6.880. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A., III Modeling intraindividual change in personality traits: Findings from the Normative Aging Study. Journals of Gerontology: Psychological Sciences. 2003;58B:P153–P165. doi: 10.1093/geronb/58.3.p153. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A., III Personality change influences mortality in older men. Psychological Science. 2007;18:371–376. doi: 10.1111/j.1467-9280.2007.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A, III, Aldwin CM, Ozer DJ, Bossé R. Construct validation of optimism and pessimism in older men: Findings from the Normative Aging Study. Health Psychology. 1993;12:406–409. doi: 10.1037//0278-6133.12.5.406. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A, III, Griffin PW, Neupert SD. Social influences on adult personality, self-regulation, and health. In: Schaie KW, Carstensen LL, editors. Social structures, aging, and self-regulation in the elderly. New York: Springer; 2006. pp. 69–83. [Google Scholar]
- Neupert SD, Almeida DM, Charles ST. Age differencesin reactivity to daily stressors: The role of personal control. Journal of Gerontology: Psychological Sciences. 2007;62B:P216–P225. doi: 10.1093/geronb/62.4.p216. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, Mroczek DK, Spiro A., III Daily stressors and memory failures: Findings from the VA Normative Aging Study. Psychology and Aging. 2006a;21:424–429. doi: 10.1037/0882-7974.21.2.424. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, Mroczek DK, Spiro A., III The effects of the Columbia shuttle disaster on the daily lives of older adults: Findings from the VA Normative Aging Study. Aging & Mental Health. 2006b;10:272–281. doi: 10.1080/13607860500409682. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Stawski RS, Almeida DM. Considerations for sampling time in research on aging: Examples from research on stress and cognition. In: Hofer SM, Alwin DF, editors. Handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 492–505. [Google Scholar]
- Nezlek JB. Multilevel random coefficient analyses of event- and interval-contingent data in social and personality psychology research. Personality and Social Psychology Bulletin. 2001;27:771–785. [Google Scholar]
- Ormel J, Wohlfarth T. How neuroticism, long-term difficulties, and life situation change influence psychological distress. Journal of Personality and Social Psychology. 1991;60:744–755. doi: 10.1037//0022-3514.60.5.744. [DOI] [PubMed] [Google Scholar]
- Perrig-Chiello P, Perrig WG, Staehelin HB. Differential aspects of memory self-evaluation in old and very old people. Aging & Mental Health. 2000;4:130–135. [Google Scholar]
- Pollina LK, Greene AL, Tunick RH, Puckett JM. Dimensions of everyday memory in late adulthood. Current Psychology: Research & Reviews. 1993;12:46–56. doi: 10.1111/j.2044-8295.1992.tb02443.x. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Robinson MD, Tamir M. Neuroticism as mental noise: A relation between neuroticism and reaction time standard deviations. Journal of Personality and Social Psychology. 2005;89:107–114. doi: 10.1037/0022-3514.89.1.107. [DOI] [PubMed] [Google Scholar]
- Rook KS. The negative side of social interaction: Impact on psychological well-being. Journal of Personality and Social Psychology. 1984;46:1097–1108. doi: 10.1037//0022-3514.46.5.1097. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL, Shaw RJ. Effects of adult age on structural and operational capacities in working memory. Psychology and Aging. 1991;6:118–127. doi: 10.1037//0882-7974.6.1.118. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Nesselroade JR, Berish DE. Short-term variability in cognitive performance and the calibration of longitudinal change. Journal of Gerontology: Psychological Sciences. 2006;61B:P144–P151. doi: 10.1093/geronb/61.3.p144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: Relevance to aging. Experimental Gerontology. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Pierce GR, Sarason BR. Domains of cognitive interference. In: Sarason IG, Pierce GR, editors. Cognitive interference: Theories, methods, and findings. Hillsdale, NJ: Erlbaum; 1996. pp. 139–152. [Google Scholar]
- Sherliker L, Steptoe A. Coping with new treatments for cancer: A feasibility study of daily diary measures. Patient Education and Counseling. 2000;40:11–19. doi: 10.1016/s0738-3991(99)00047-6. [DOI] [PubMed] [Google Scholar]
- Sherman AM. Social relations and depressive symptoms in older adults with osteoarthritis. Social Science and Medicine. 2003;56:247–257. doi: 10.1016/s0277-9536(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Sorkin DH, Rook KS. Dealing with negative social exchanges in later life: Coping responses, goals, and effectiveness. Psychology and Aging. 2006;21:715–725. doi: 10.1037/0882-7974.21.4.715. [DOI] [PubMed] [Google Scholar]
- Spiro A, III, Bossé R. The Normative Aging Study. In: Maddox G, editor. Encyclopedia of aging. 3rd ed. New York: Springer; 2001. pp. 744–746. [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Controlled Clinical Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Suls J. Affect, stress, and personality. In: Forgas JP, editor. Handbook of affect and social cognition. Mahwah, NJ: Erlbaum; 2001. pp. 392–409. [Google Scholar]
- Sunderland A, Harris JE, Baddeley AD. Do laboratory tests predict everyday memory? A neuropsychological study. Journal of Verbal Learning and Verbal Behavior. 1983;22:341–357. [Google Scholar]
- Wegner DM. Stress and mental control. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. London: John Wiley & Sons; 1988. pp. 683–697. [Google Scholar]
- West RL, Crook TH, Barron KL. Everyday memory performance across the life span: Effect of age and noncognitive individual differences. Psychology and Aging. 1992;7:72–82. doi: 10.1037//0882-7974.7.1.72. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bienas JL, Mendes de Leon CF, Evans DA, Bennett DA. Negative affect and mortality in older persons. American Journal of Epidemiology. 2003;158:827–835. doi: 10.1093/aje/kwg224. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer’s disease. Neurology. 2003;61:1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Harvey PD, Stavitsky K, Kaufman S, Grossman RA, et al. Relationship between cortisol and agerelated memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30:678–687. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]