Abstract
Topoisomerase II (Top2) is a ubiquitous nuclear enzyme that relieves torsional stress in chromosomal DNA during various cellular processes. Agents that target Top2, involving etoposide, doxorubicin, and mitoxantrone, are among the most effective anticancer drugs used in the clinic. Mammalian cells possess two genetically distinct Top2 isoforms, both of which are the target of these agents. Top2α is essential for cell proliferation and is highly expressed in vigorously growing cells, whereas Top2β is nonessential for growth and has recently been implicated in treatment-associated secondary malignancies, highlighting the validity of a Top2α-specific drug for future cancer treatment; however, no such agent has been hitherto reported. Here we show that NK314, a novel synthetic benzo[c]phenanthridine alkaloid, targets Top2α and not Top2β in vivo. Unlike other Top2 inhibitors, NK314 induces Top2-DNA complexes and double-strand breaks (DSBs) in an α isoform-specific manner. Heterozygous disruption of the human TOP2α gene confers increased NK314 resistance, whereas TOP2β homozygous knock-out cells display increased NK314 sensitivity, indicating that the α isoform is the cellular target. We further show that the absence of Top2β does not alleviate NK314 hypersensitivity of cells deficient in non-homologous end-joining, a critical pathway for repairing Top2-mediated DSBs. Our results indicate that NK314 acts as a Top2α-specific poison in mammalian cells, with excellent potential as an efficacious and safe chemotherapeutic agent. We also suggest that a series of human knock-out cell lines are useful in assessing DNA damage and repair induced by potential topoisomerase-targeting agents.
DNA topoisomerase II (Top2)2 is a ubiquitous nuclear enzyme that alters the topological structure of DNA and chromosomes through a transient DNA double-strand break (DSB) and subsequent religation of the DSB (1, 2). The enzyme has been implicated in many aspects of DNA metabolism, including DNA replication, repair, transcription, and chromosome condensation/segregation (1, 3). Top2 has been of considerable interest to human medicine, because it is an important target for cancer chemotherapy (4). Top2-targeting agents, involving etoposide, doxorubicin, and mitoxantrone, are among the most effective and widely used anticancer drugs in cancer chemotherapy (5, 6). These agents are referred to as “Top2 poisons,” because they convert the essential enzyme into a highly cytotoxic DNA-damaging agent through the formation of “cleavage complex” (also called “cleavable complex”), in which a Top2-linked DNA strand-passing intermediate is stabilized, allowing the generation of a DSB (7, 8).
Mammalian cells possess two genetically distinct Top2 isoforms (9, 10). Despite their similar structural features (∼70% identity at the amino acid level) and biological properties, the two isoforms are differentially regulated and play different roles in living cells. Top2α is most abundantly expressed in rapidly growing tissues and its expression is cell cycle-regulated, peaking in G2/M, whereas Top2β is expressed in virtually all tissues and it is expressed throughout the cell cycle (11–13). Top2α has been shown to be essential for cell proliferation and embryonic development (14, 15). In mitosis, only Top2α associates with chromosomes, playing a unique role in chromosome segregation that cannot be substituted by Top2β (14–18). By contrast, despite its apparent roles in transcription (for example, Ref. 19), Top2β is dispensable for cell survival (20), although it has been implicated in neuronal differentiation (20–24).
The fact that Top2α, relative to Top2β, is highly expressed in tumor cells (21, 23, 25, 26) implies the validity of α isoform-specific Top2 inhibitor in cancer treatment. Indeed, two studies suggested that Top2α, rather than Top2β, was the determinant of cytotoxic effects of etoposide (27, 28). Even more intriguingly, Azarova et al. (27) suggested that Top2β may be responsible for the development of secondary malignancy associated with etoposide treatment. Hence, it is quite reasonable to expect that α isoform-specific Top2 poisons will be efficacious and safe chemotherapeutic agents with reduced risk of treatment-related secondary malignancies. To our knowledge, however, no such agent has been reported thus far.
NK314 is a novel synthetic benzo[c]phenanthridine alkaloid that exhibits strong antitumor activity (29). We previously reported that the drug stabilizes Top2 cleavage complexes and induces rapid DSBs to cause G2 arrest in tumor cells (29, 30). In this article, we find that NK314 acts as an α isoform-specific Top2 poison in living mammalian cells. We demonstrate that NK314 induces Top2-DNA complexes and chromosomal DSBs in a Top2α-dependent manner. Furthermore, with the use of a series of human gene knock-out cell lines, we genetically investigate DNA damage and repair after NK314 treatment. This is the first report on the discovery and characterization of an α isoform-specific Top2 poison.
EXPERIMENTAL PROCEDURES
Topoisomerase Inhibitors—NK314 and etoposide were synthesized at Nippon Kayaku (Tokyo, Japan). Doxorubicin hydrochloride and mitoxantrone hydrochloride were purchased from Kyowa Hakko Kogyo Co., Ltd. (Tokyo, Japan) and Wyeth K.K. (Tokyo, Japan), respectively. Camptothecin, amsacrine, and XK469 were purchased from Sigma. ICRF-193 was purchased from Funakoshi (Tokyo, Japan). Etoposide, mitoxantrone, amsacrine, ICRF-193, and XK469 were dissolved in dimethyl sulfoxide (DMSO). NK314 and doxorubicin were dissolved in distilled water. All the drugs were stored frozen in aliquots at –20 °C.
Cells and Culture Conditions—All cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. The human pre-B cell line Nalm-6 and its derivatives were cultured in ES medium (Nissui Seiyaku Co., Tokyo, Japan) supplemented with 10% calf serum (Hyclone, Logan, UT) and 50 μm 2-mercaptoethanol. The human cervical carcinoma cell line HeLaS3 was maintained in Eagle's minimal essential medium (Asahi Glass Co., Ltd., Chiba, Japan) containing 10% fetal bovine serum and 50 μg/ml kanamycin sulfate. The kidney cancer cell line ACHN (ATCC CRL 1611) was maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum and 1% non-essential amino acids. The non-small cell lung cancer cell line H460 (ATCC HTB 177) was maintained in RPMI 1640 medium (Asahi Glass Co., Ltd.) supplemented with 10% fetal bovine serum, 1 mm sodium pyruvate, and 10 mm Hepes. The colorectal adenocarcinoma cell line DLD-1 was obtained from JCRB Cell Bank (Tokyo, Japan) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. Mouse embryonic fibroblasts (MEFs) (Top2β+/+ and Top2β–/– cells) were cultured in 25-cm2 tissue culture flasks in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 1× penicillin/streptomycin.
Pulsed-field Gel Electrophoresis (PFGE) Analysis—Cells were treated with Top2 inhibitor for 1 h, and chromosome-sized DNA was prepared from the cells using the CHEF Genomic DNA Plug Kit (Bio-Rad). Briefly, cells (1 × 106) were embedded to agarose plugs, and the plugs were treated with lysis buffer (10 mm Tris-HCl (pH 8.0), 500 mm EDTA, 1% Sarkosyl) in the presence of 1 mg/ml proteinase K at 50 °C for 2 days, and washed three times with 50 mm EDTA. Plugs were loaded onto a 1% agarose gel prepared in 0.5× TBE buffer and electrophoresed using the CHEF-Mapper system (Bio-Rad). The running conditions were as follows: 14 °C, 60–120 s switch time, angle of 120°, and a voltage gradient of 6 V/cm. The gel was stained with ethidium bromide (1 μg/ml) and photographed under ultraviolet light.
Western Blot—Western blot analysis was carried out as described previously (31). Mouse anti-human Top2 monoclonal antibody (7B9) was kindly provided by Dr. Akihiko Kikuchi (32). Mouse monoclonal antibody against human Top2α or -β, and mouse anti-human Ku70 monoclonal antibody were purchased from BD Transduction Laboratories (Bedford, MA). Mouse anti-actin monoclonal antibody was purchased from Sigma. Levels of expression were quantified using a Fuji Image analyzer LAS-1000UVmini and a MultiGauge software (Fuji Film Co., Tokyo, Japan).
ICE Bioassay—Top2-DNA complex was detected by ICE bioassay essentially as described (33). Briefly, exponentially growing cells were treated with Top2 drug for 30 min. After removing the drug-containing medium, the cells were dissolved by immediate addition of 3 ml of 1% Sarkosyl in TE (pH 7.5). After gentle douncing, the lysates were layered onto a 2-ml cushion of CsCl (1.5 g/ml) and centrifuged at 90,000 × g for 16–20 h at 25 °C. DNA pellets were collected and dissolved in TE buffer, followed by shearing using an ultrasonic generator to reduce viscosity. DNA concentrations were determined from absorbance at 260 nm, and equal amounts of DNA were blotted to nitrocellulose or polyvinylidene difluoride membranes using a slot blot apparatus. Top2 protein (Top2α or Top2β) covalently bound to DNA was immunodetected with anti-human Top2α monoclonal antibody (BD Transduction Laboratories) or anti-human Top2β monoclonal antibody (TopoGEN, Inc., Columbus, OH), respectively, using the ECL Western Blotting Detection System (GE Healthcare).
TARDIS Assay—Drug-stabilized Top2 complexes were measured using the TARDIS assay essentially as described by Errington et al. (28). Briefly, cells (105 cells/well) were seeded into 6-well tissue culture dishes and grown for 48 h. NK314 or etoposide was added to exponentially growing cells at appropriate concentrations, and the cells were collected by trypsinization 90 min after drug addition, washed with ice-cold PBS, mixed with low melting point agarose in PBS, and spread onto agarose-coated microscope slides. The slides were placed in lysis buffer containing 1% SDS, 80 mm potassium phosphate (pH 6.8), 10 mm EDTA, and protease inhibitors for 30 min, followed by incubation for 30 min in 1 m NaCl plus protease inhibitors. The slides were then washed in PBS and incubated with anti-Top2β (18513β) (34) or anti-Top2α and -β (4882; raised against the N-terminal 140 kDa of bovine Top2) antibody for 1 h in PBS-T (PBS containing 0.1% Tween 20) with 1% (w/v) bovine serum albumin. The slides were washed three times in PBS-T and incubated for 1 h with rabbit fluorescein isothiocyanate-conjugated secondary antibody. The slides were washed three times in PBS-T followed by an overnight wash in PBS containing protease inhibitors at 4 °C. The slides were stained with Hoechst 33258 (10 mm in PBS). Separate gray scale images were captured for Hoechst 33258 and fluorescein isothiocyanate fluorescence using an epifluorescence microscope attached to a cooled slow scan CCD camera. For each of three randomly chosen fields of view, images of Hoechst 33258 (blue) and fluorescein isothiocyanate (green) fluorescence were captured to give a total of ∼100 cells/dose for each antibody. 16-Bit images were then analyzed to quantify the levels of blue and green fluorescence. All images were corrected for stray light and camera background and were subjected to blue and green shade correction to compensate for variations in intensity of illumination and non-uniformities in light transmission (34). Graphing and statistical analysis was carried out using Graph-Pad Prism software (Cherwell Scientific, Oxford, UK).
Top2 Assays—Human Top2α and Top2β were purified as described previously (35) and used for in vitro Top2 assays. Decatenation assay was performed by using a Topo II Assay Kit (TopoGEN, Inc.). Briefly, 0.2 μg of kinetoplast DNA was incubated with Top2α or Top2β at 37 °C for 15 min in 20 μl of 50 mm Tris-HCl (pH 8.0), 120 mm KCl, 10 mm MgCl2, 0.5 mm dithiothreitol, 0.5 mm ATP, and 30 μg/ml bovine serum albumin. One unit of activity is defined as the amount of Top2 enzyme that decatenates 0.2 μg of kinetoplast DNA under standard conditions. To examine the inhibitory effect of NK314 and etoposide on Top2 catalytic activity, 0.2 μg of kinetoplast DNA was incubated with 2 units of Top2α or Top2β in 20 μl of reaction buffer containing 5% DMSO at 37 °C for 15 min in the presence or absence of NK314 or etoposide. The reaction was stopped by adding 5 μl of loading dye (5% Sarkosyl, 0.0025% bromphenol blue, and 25% glycerol) and electrophoresed in a 1% agarose gel containing 0.5 μg/ml of ethidium bromide in TBE buffer.
DNA cleavage assay was performed by using a Topo II Drug Screening Kit (TopoGEN, Inc.). Briefly, 0.2 μg of pRYG plasmid was incubated with 5 units of Top2α or Top2β in 20 μl of assay buffer containing 5% DMSO at 37 °C for 30 min in the presence or absence of NK314 or etoposide. DNA cleavage product was trapped by the addition of 2 μl of 10% SDS, and 2.5 μl of 10 mg/ml proteinase K was added to the sample, which was incubated for 30 min at 37 °C to digest Top2. The samples were mixed with 2.5 μl of loading buffer and cleaned up by adding an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1). After brief vortex mixing, the sample was spun in a microcentrifuge for 5 s. An aliquot (10 μl) of the upper aqueous phase was electrophoresed in a 1% agarose gel containing 0.5 μg/ml of ethidium bromide in TBE buffer.
Small Interfering RNA (siRNA)-mediated Gene Knockdown—Top2 knockdown experiments were carried out in the same manner as described previously (36). The Top2α-targeting siRNA corresponds to nucleotides 76 to 96, and the Top2β siRNA to nucleotides 86 to 106 (relative to the first nucleotide of the start codon). These siRNAs were purchased from Invitrogen. As a nonspecific control, non-silencing control siRNA was used (sense, 5′-UUCUCCGAACGUGUCACGUdTdT-3′; antisense, 5′-ACGUGACACGUUCGGAGAAdTdT-3′; Qiagen, Japan). HeLa cells were transfected with siRNA using Lipofectamine (Invitrogen) according to the manufacturer's instructions. Top2α, Top2β, and non-silencing control siRNAs were added to achieve final concentrations of 1, 10, and 10 nm, respectively. Twenty-four hours after transfection, cells were collected by trypsinization and plated at 2.5 × 105 cells in 60-mm dishes and cultured for 48 h, followed by Western blot analysis or clonogenic assays. For clonogenic assays, cells were cultured for 1 h in the presence of NK314. After washing twice with PBS, the cells were trypsinized, collected in 15-ml Falcon tubes, and counted. Cells were plated at 102–105 cells/dish into 60-mm dishes. After a 2-week incubation, colonies were fixed with methanol, stained with methylene blue, and counted. The percent survival was determined by comparing the number of surviving colonies to untreated controls.
TOP2 Targeting Vectors—Targeting vectors were constructed by using a simplified construction method based on the MultiSite Gateway® Technology (Invitrogen) as described (37). Genomic DNA fragments were obtained by PCR amplification with ExTaq DNA polymerase (Takara Bio, Otsu, Japan) from Nalm-6 genomic DNA using the following primers: Top2A5F (5′-GGGGACAACTTTGTATAGAAAAGTTGAATGCTGCGGACAACAAACAAAGG-3′) and Top2A5R (5′-GGGGACTGCTTTTTTGTACAAACTTGAGCTGTCCAAATATGAGAGCTGGG-3′) for TOP2α 5′-arm, Top2A3F (5′-GGGGACAGCTTTCTTGTACAAAGTGGTGGAACTCAAGCCCTTCAATGGAG-3′) and Top2A3R (5′-GGGGACAACTTTGTATAATAAAGTTGTACAATACCACAGCCAATGGCCTG-3′) for TOP2α 3′-arm, Top2B5F (5′-GGGGACAACTTTGTATAGAAAAGTTGCTGGTTCGTGTAGAGGGGTCAAGG-3′) and Top2B5R (5′-GGGGACTGCTTTTTTGTACAAACTTGCTACCACATAATCCACGTGCCGTC-3′) for TOP2β 5′-arm, and Top2B3F (5′-GGGGACAGCTTTCTTGTACAAAGTGGCCCAAACTGGATGATGCTAATGATG-3′) and Top2B3R (5′-GGGGACAACTTTGTATAATAAAGTTGATGTAGCCTACGCTGTCTCCGGTC-3′) for TOP2β 3′-arm. The TOP2α and TOP2β targeting vectors were linearized with I-SceI or AhdI, respectively, prior to transfection.
Generation of Human Gene Knock-out Cell Lines—DNA transfection for gene targeting was performed in Nalm-6 cells as described previously (38–40). Briefly, to generate TOP2α+/– and TOP2β–/– cells, 4 × 106 wild-type cells were electroporated with 4 μg of linearized targeting vector, cultured for 22–24 h, and replated at a density of 0.5–1 × 106 per 90-mm dish into agarose medium containing 0.4 mg/ml hygromycin B (Wako Pure Chemical, Osaka, Japan). After a 2–3-week incubation, hygromycin-resistant colonies were isolated and genomic DNA was prepared from each clone. Primers used for PCR screening were TOP2Acc (5′-TTAATGCCACTCCTGTGCTGTTTG-3′) and universal primer B (5′-AGGTTCACTAGTACTGGCCATTG-3′) (37) for TOP2α targeting, and TOP2Bcc (5′-ATTGTGGCCCTCATGACTAGAAGGG-3′) and universal primer B for TOP2β targeting. To generate TOP2β–/– cells, TOP2β+/– cells were transfected with another targeting construct harboring a puromycin-resistance gene and selected with 0.5 μg/ml puromycin (Wako Pure Chemical, Osaka, Japan). Primers used for PCR screening were TOP2Bcc (see above) and universal primer A (5′-AATAATGGTTTCTTAGACGTGCG-3′) (37). LIG4–/–TOP2α+/– and LIG4–/–TOP2β–/– cells were similarly generated from LIG4–/– cells (37). Generation of RAD54–/– and LIG4–/–RAD54–/– cells will be described elsewhere.3
Drug Sensitivity Assays—Clonogenic assays using Nalm-6 and its derivative cell lines were performed as described previously (39). Briefly, exponentially growing cells were plated at 102–105 cells/dish into 60-mm dishes containing 5 ml of agarose medium with various concentrations of DNA-damaging agents. After a 2–3-week incubation at 37 °C, visible colonies were counted, and the percent survival was determined by comparing the number of surviving colonies to untreated controls.
For growth inhibition assays, 1 × 104 cells were seeded into 48-well plates and cultured for 96 h in growth medium containing various concentrations of topoisomerase inhibitor. Cell proliferation was measured by using the CellTiter-Glo® Luminescent Viability Assay kit (Promega) or methylene blue staining. Methylene blue staining was performed as previously described (41). Briefly, cells were stained with 0.05% methylene blue dissolved in 10 mm Tris buffer (pH 8.5) for 30 min, and washed thoroughly with distilled water. The stained dye was extracted with 3% HCl, and the absorbance at 660 nm was measured.
Survival Assays with MEFs—MEFs (9 × 104 cells) at 50–80% confluence were plated in each of a series of 60-mm dishes. Cells were cultured in these dishes for 48 h before adding NK314. Serial dilutions of NK314 were prepared, resulting in final concentrations of 4–1024 nm in the dishes. Two hours after drug addition, the drug-containing medium was removed and plates were washed with PBS. Cells were trypsinized, collected in 15-ml Falcon tubes, and counted. Cells were plated in duplicate at 2 × 102–5 × 104 cells per 90-mm dish. After 8 days, colonies were fixed with Carnoy's solution, stained with crystal violet, and counted.
RESULTS
NK314 Specifically Induces Top2α-DNA Complex—We previously showed that NK314 induced Top2 complexes and DSBs in several tumor cell lines (29, 30). In this article, we first performed PFGE analysis of genomic DNA from drug-treated Nalm-6 cells (Fig. 1A). We employed this human cell line for two reasons. First, Nalm-6 expresses roughly equal levels of Top2α and Top2β (Fig. 1B), which should be advantageous to analyze the relative contribution of Top2 isoforms to DNA damage and cytotoxicity. Second, the cell line enables rapid production of gene knock-out cell lines by gene targeting (37, 42) (see below). As expected, we found that NK314 rapidly induces chromosomal DSBs, and this DSB induction was significantly alleviated by pretreatment with ICRF-193 (Fig. 1A, lanes 3 and 4), a Top2 inhibitor that does not stabilize the cleavage complex (43, 44). Similar observations were made with the potent Top2 poison etoposide (Fig. 1A, lanes 5 and 6). These results indicate that, similar to etoposide, NK314 induces chromosomal DSBs in a Top2-dependent manner. To determine which isoform is responsible for NK314-induced cleavage complexes, we performed the ICE bioassay using isoform-specific antibodies. As shown in Fig. 1C, NK314 (3 μm) and etoposide (100 μm) induced similar levels of Top2α complexes in Nalm-6 cells. Notably, however, Top2β complexes were barely detectable in NK314-treated cells under the same condition. By contrast, similar levels of Top2α and Top2β complexes were detected in etoposide-treated cells. Essentially the same results were obtained with HeLa cells, as shown in Fig. 1D. NK314 at 3 μm induced Top2α, but not Top2β complexes, whereas other Top2 drugs (etoposide, amsacrine, and mitoxantrone) similarly induced Top2α and Top2β complexes. (Note that 25 times more DNA is blotted for Top2β detection (Fig. 1D), reflecting the fact that HeLa cells express very low levels of Top2β (see Fig. 1B).) In addition, those NK314-induced complexes were significantly reduced in cells pretreated with ICRF-193 (data not shown). These results indicate that, unlike other Top2 drugs such as etoposide, NK314 induces Top2-DNA complex in a manner highly specific to the α isoform. It should be noted, however, that NK314 at higher concentrations (10 μm in HeLa cells; Fig. 1D) can induce Top2β complexes, although to a much lesser extent than Top2α complexes. In fact, the TARDIS assay (28) on MEFs revealed Top2β complexes in NK314-treated cells, although less than in etoposide-treated cells (Fig. 1E).
FIGURE 1.
NK314 induces Top2 complexes and DSBs in a manner highly specific to theα isoform. A, PFGE analysis of Nalm-6 genomic DNA from untreated cells (None) or cells treated with NK314 (1 μm) or etoposide (100 μm) for 1 h. The cells were preincubated with (+) or without (–)10 μm ICRF-193 for 30 min. B, Western blot analysis for Top2 of Nalm-6 and HeLa cells using 7B9 antibody (31). Ku70 served as a loading control. C and D, ICE bioassay for detection of Top2 complex formation. Indicated amounts of DNA from drug-treated Nalm-6 (C) or HeLa (D) cells were blotted to polyvinylidene difluoride or nitrocellulose membrane. Top2 protein covalently bound to DNA was detected with Top2α- or Top2β-specific antibody. E, TARDIS analysis in MEF cells treated with 100 μm etoposide or 5 μm NK314 for 90 min. Total Top2 complexes (α + β) were detected with antibody 4882, whereas Top2β complexes (β) were detected with antibody 18513β. Mean fluorescence values were calculated for each treatment and normalized to the mean value for etoposide-treated MEFs. The data shown are from three to six independent experiments. F, effect of NK314 on Top2-mediated DNA cleavage. Supercoiled plasmid DNA was incubated with Top2α or Top2β or without enzyme (–Top2) in the absence (–solvent) or presence of 5% DMSO (None), NK314 (25–400 μm), or etoposide (100–400 μm). N, nicked circular DNA; L, linear DNA; S, supercoiled DNA; and R, relaxed DNA. G, inhibitory effect of NK314 on Top2 decatenation activity. Kinetoplast DNA was incubated with 2 units of purified Top2α or Top2β in the absence (5% DMSO; 0 μm) or presence of NK314 (3–100 μm).
We next sought to examine whether NK314 specifically inhibits Top2α in vitro. For this purpose, we performed a in vitro DNA cleavage assay using a plasmid with a Top2 cleavage consensus sequence (45). As shown in Fig. 1F, NK314 induced DNA cleavage in the presence of Top2α (lanes 6–8), as evidenced by the appearance of linear DNA (marked by L). Such Top2-mediated DNA cleavage, however, was hardly observed with the β isoform (lanes 13–15). By contrast, etoposide-induced DNA cleavage was similarly detected with both Top2α (lanes 9 and 10) and Top2β (lanes 16 and 17). We note that DNA binding activity of Top2β is not inhibited by NK314 in vitro, as evidenced by electrophoretic mobility shift assay (data not shown). We then performed a decatenation assay using kinetoplast DNA, a catenated DNA substrate. Etoposide inhibited the decatenation activity of both Top2α and Top2β (data not shown). Perhaps surprisingly, NK314 inhibited the decatenation activity of Top2β, to an extent similar to that of Top2α (Fig. 1G). Additionally, DNA relaxation assays showed that NK314 inhibited the relaxation activity of Top2β as well as Top2α (data not shown). The finding that NK314, in vitro, can inhibit the decatenation and relaxation activity of both Top2 isoforms may possibly explain the results of the above-mentioned DNA cleavage assay, in which similar amounts of supercoiled and relaxed DNA were observed with Top2α and Top2β by NK314 treatment, despite apparently different amounts of linear DNA product (Fig. 1F, lanes 6–8 versus lanes 13–15).
Top2α, Not Top2β, Is Responsible for NK314-induced DNA Damage and Cytotoxicity—Our finding that NK314 preferentially induces Top2α complexes suggests that the expression level of Top2α, rather than Top2β, determines the cytotoxic effect of NK314 in living mammalian cells. To test this, we performed gene knockdown experiments in HeLa cells using siRNA (36). siRNA-transfected cells were subjected to clonogenic survival assays 3 days after transfection, when optimal silencing of Top2 expression was achieved (Fig. 2A). As expected, cells transfected with Top2α siRNA exhibited increased resistance to NK314, relative to those transfected with control siRNA or Top2β siRNA, which had little or no influence on NK314 cytotoxicity (Fig. 2B).
FIGURE 2.
siRNA-mediated Top2α knockdown leads to increased NK314 resistance. A, Western blot analysis for Top2 in siRNA-transfected HeLa cells. Whole cell extract was prepared from mock-transfected cells (lane 1) or cells transfected with Top2α siRNA (lane 2), Top2β siRNA (lane 3), or control siRNA (lane 4). Actin served as a loading control. B, NK314 sensitivity of siRNA-transfected cells. Data are the mean ± S.D. of three independent assays. Where absent, error bars fall within symbols.
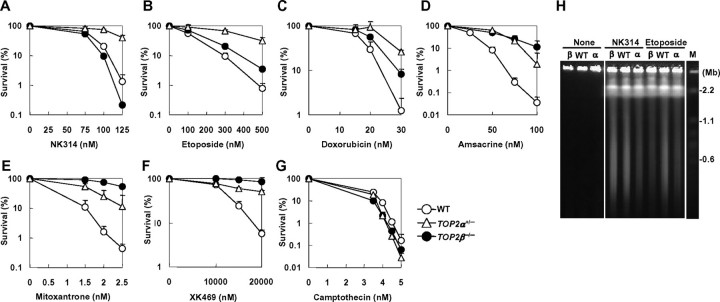
To further investigate the relative contribution of each Top2 isoform to NK314-induced cytotoxicity, we generated human TOP2α+/– and TOP2β–/– cells by gene targeting in the Nalm-6 cell line (Figs. 3A and 4A). Heterozygous disruption of TOP2α and homozygous disruption of TOP2β were confirmed by Southern and Western blot analysis (Figs. 3, B and C, and 4, B and C; note that Top2α levels in TOP2α+/– cells were decreased to ∼50% that of wild-type cells). TOP2β gene disruption did not affect Top2α levels, and vice versa; and cell proliferation was marginally affected by these gene-targeting events (Figs. 3, C and D, and 4, C and D). Using these mutant human cell lines, we performed clonogenic survival assays in the presence of NK314 or other Top2-targeting drugs. As shown in Fig. 5, TOP2α+/– cells exhibited increased resistance to NK314 as well as to other Top2 inhibitors, etoposide, doxorubicin, amsacrine, mitoxantrone, and XK469. In sharp contrast, TOP2β–/– cells did not display an increased resistance, but slightly increased sensitivity to NK314. Such increased sensitivity was not observed with other Top2 inhibitors; rather, increased resistance was observed in TOP2β–/– cells (Fig. 5, B–F), confirming that these inhibitors all target Top2β as well as Top2α in a cell. Of note, neither TOP2α nor TOP2β gene targeting significantly affected cellular sensitivity to camptothecin, a potent topoisomerase I (Top1) poison (7) (Fig. 5G), serving as controls to indicate that increased sensitivity/resistance to Top2 inhibitors observed in the mutant cell lines results from changes in Top2 expression status itself. To examine whether NK314 actually induces Top2α-dependent chromosomal DSBs, we carried out PFGE analysis using genomic DNA from drug-treated cells. As shown in Fig. 5H, NK314 induced chromosomal DSBs at lower levels in TOP2α+/– cells than in wild-type (TOP2α+/+) or TOP2β–/– cells. (Note that TOP2β–/– cells show wild-type levels of DSBs.) By contrast, etoposide induced lower levels of DSBs in both TOP2α+/– and TOP2β–/– cells than in wild-type cells. Finally, to test the possibility that Top1 could be involved in NK314 cytotoxicity, we performed heterozygous disruption of the human TOP1 gene. The TOP1+/– cells had ∼30% decreased Top1 levels and showed increased resistance to the Top1 poison camptothecin (data not shown). However, the TOP1+/– mutant exhibited wild-type levels of sensitivity to NK314 (and etoposide), clearly eliminating the possibility that NK314 targets Top1 in vivo (data not shown).
FIGURE 3.
Targeted disruption of the human TOP2α gene. A, scheme for TOP2α gene targeting. The targeting vector was designed to replace exons 5 to 7 with a hygromycin-resistance (Hygr) gene. Triangles represent loxP sequences. DT-A, a gene encoding diphtheria toxin A fragment. B, Southern blot analysis of EcoRI-digested genomic DNA from wild-type (+/+) and TOP2α heterozygous (+/–) cells using the probe shown in A. C, Western blot analysis for Top2 in mutant cell lines. Whole cell extract from 1 × 105 cells was loaded on a 7.5% SDS-polyacrylamide gel. Levels of expression were quantified using an image analyzer. Ku70 served as a loading control. D, growth curves of wild-type and mutant cell lines. Data are the mean ± S.D. of three independent experiments. Where absent, error bars fall within symbols.
FIGURE 4.
Targeted disruption of the human TOP2β gene. A, scheme for TOP2β gene targeting. The targeting vector was designed to replace exons 9 to 11 with a hygromycin-resistance (Hygr) or puromycin-resistance (Puror) gene. Symbols are as described in the legend to Fig. 3. B, Southern blot analysis of KpnI/NcoI-digested genomic DNA from wild-type (+/+) and TOP2β heterozygous (+/–) and homozygous (–/–) cells using the probe shown in A. C, Western blot analysis for Top2 in mutant cell lines. Whole cell extract from 1 × 105 cells was loaded on a 7.5% SDS-polyacrylamide gel. Levels of expression were quantified using an image analyzer. Ku70 and Actin served as loading controls. D, growth curves of wild-type and mutant cell lines. Data are the mean ± S.D. of three independent experiments. Where absent, error bars fall within symbols.
FIGURE 5.
NK314, unlike other Top2 inhibitors, specifically targets the α isoform. A–G, sensitivities of wild-type, TOP2α+/–, and TOP2β–/– cells to topoisomerase inhibitors: NK314 (A), etoposide (B), doxorubicin (C), amsacrine (D), mitoxantrone (E), XK469 (F), and camptothecin (G). Data are the mean ± S.D. of three independent experiments. Where absent, error bars fall within symbols. H, PFGE analysis of Nalm-6 genomic DNA from untreated cells (None) or cells treated with NK314 (1 μm) or etoposide (30 μm) for 1 h. WT, wild-type; α, TOP2α+/–; and β, TOP2β–/– cells.
Together, these results show that Top2α is responsible for NK314-induced cytotoxicity and chromosomal DSBs in living cells. We also performed survival assays for NK314 using MEFs, and confirmed no contribution of Top2β to NK314-induced cytotoxicity (Fig. 6).
FIGURE 6.
Loss of Top2β does not affect NK314 sensitivity in MEFs. Clonogenic survival assays were performed using NK314-treated Top2β+/+ (WT) and Top2β–/– MEFs. Data are the mean ± S.D. of three independent experiments. Where absent, error bars fall within symbols.
Human Cells Deficient in Non-homologous End-joining Are Hypersensitive to NK314, Although to a Lesser Extent Than Etoposide and Doxorubicin—Repair of drug-induced DNA damage, in general, is one of the key factors that determine the cellular sensitivity to, and the efficacy of, the drug. It is thus important to elucidate the repair mechanisms responsible for drug-induced DNA damage and, ideally, such work should be performed in the context of human somatic cells, not model organisms. In this respect, we previously reported that Top2-mediated DNA damage is predominantly repaired by the non-homologous end-joining (NHEJ) pathway in higher eukaryotes, contrasting with the situation in yeast, where homologous recombination repairs virtually all Top2-mediated DNA damage (37, 39, 46, 47). In mammalian cells, NHEJ and homologous recombination are the two major pathways of DSB repair, and these pathways are roughly equally important for the repair of radiation-induced DSBs (48–51).
To investigate repair mechanisms of NK314-induced DSBs in human somatic cells, we employed a series of knock-out mutant cell lines, involving those lacking Rad54 and/or DNA ligase IV (Lig4), which are key components of homologous recombination and NHEJ, respectively (38, 52–54). As shown in Fig. 7A, RAD54–/– cells showed only slightly increased sensitivity to NK314, whereas LIG4–/– cells showed much higher sensitivity to NK314, and LIG4–/–RAD54–/– cells were slightly more hypersensitive than LIG4–/– cells. These results indicate that the NHEJ pathway is important for repairing NK314-induced DSBs, whereas the homologous recombination pathway only plays a minor role in the repair. Similar results were obtained with etoposide and doxorubicin (Fig. 7, B and C), consistent with the notion that Top2-mediated DSBs rely heavily on NHEJ repair in animal cells. It should be noted, however, the NHEJ dependence of NK314 appears to be less prominent than that of other Top2 inhibitors; for instance, IC90 comparison indicated that LIG4–/– cells were ∼20 times more sensitive than wild-type cells to etoposide, but only 2.7 times more sensitive to NK314 (Fig. 7, H and I). More specifically, LIG4–/– cells did not show increased resistance to NK314, unlike other inhibitors, at very low concentrations. This may imply that a repair pathway(s) other than NHEJ can efficiently repair NK314-induced DSBs. Alternatively, or additionally, NK314, compared with other inhibitors, might require substantial amounts in a cell to exert cytotoxicity (see “Discussion”).
FIGURE 7.

NHEJ-deficient human cells are hypersensitive to NK314, although less prominent than to other Top2 inhibitors. A–C, sensitivities of wild-type, RAD54–/–, LIG4–/–, and LIG4–/–RAD54–/– cells to NK314 (A), etoposide (B), and doxorubicin (C). D and E, sensitivities of wild-type, TOP2β–/–, LIG4–/–, and LIG4–/–TOP2β–/– cells to NK314 (D) and etoposide (E). F and G, sensitivities of wild-type, TOP2α+/–, LIG4–/–, and LIG4–/–TOP2α+/– cells to NK314 (F) and etoposide (G). In all assays, cells were allowed for colony formation in agarose medium containing the indicated concentrations of drugs. Data are the mean ± S.D. of three to six independent experiments. Where absent, error bars fall within symbols. H, summary of sensitivity assays shown in A and B. I, summary of sensitivity assays shown in D–G.
We next sought to examine whether the less prominent NHEJ dependence of NK314 could result from its nature as an α isoform-specific poison. If NHEJ repair was much more important for Top2β-mediated DNA damage than for Top2α-mediated DNA damage, then NHEJ-deficient cells would show greatly increased etoposide resistance in the absence of Top2β, perhaps with similar survival curves to those for NK314. To test this possibility, we performed TOP2α and TOP2β gene targeting in a LIG4–/– background (Figs. 3 and 4). The resulting LIG4–/–TOP2α+/– and LIG4–/–TOP2β–/– cells had normal growth properties (Figs. 3D and 4D). As shown in Fig. 7E, TOP2β disruption only slightly alleviated etoposide hypersensitivity of LIG4–/– cells, to an extent similar to that observed in wild-type (LIG4+/+) cells. This finding suggests that Top2β-mediated DNA damage does rely on NHEJ repair, implying that the above-mentioned less prominent NHEJ dependence of NK314 cannot be attributable to α isoform-specific poisoning by this drug. As shown, TOP2β disruption had no effect on NK314 sensitivity of LIG4–/– cells (Fig. 7D), whereas TOP2α heterozygous disruption conferred greatly increased resistance to NK314 and etoposide in the LIG4–/– background (Fig. 7, F and G). These observations further support the conclusion that NK314 only targets the α isoform of Top2 in vivo.
NK314 Cytotoxicity Is Less Dependent on Exposure Time Than Etoposide Cytotoxicity—As the above described clonogenic survival assays were all performed in the presence of relatively low drug concentrations (i.e. continuous drug exposure), we next performed these experiments after a 1-h treatment of cells with NK314 or etoposide. Again, LIG4–/– cells were found to be highly sensitive to NK314 and etoposide, whereas RAD54–/– cells showed no increased sensitivity to these agents (Fig. 8, A and B). These results do confirm that NHEJ is indeed important for repairing Top2-mediated DNA damage. Similarly importantly, it should be emphasized that ∼20 times higher concentration was required for etoposide to achieve a 90% inhibition in wild-type Nalm-6 cells (5068 nm for 1-h treatment versus 277 nm for continuous exposure), whereas at most a 5 times higher concentration was required for NK314 (457 versus 98 nm) (Fig. 8C). Interestingly, the difference was more prominent in the LIG4–/– mutant (1329 versus 14 nm for etoposide, and 201 versus 36 nm for NK314) (Fig. 8C), suggesting that the cytotoxic effect of NK314 is less dependent on exposure time than that of etoposide. To further confirm this, we treated various human cancer cell lines with NK314 or etoposide and compared the IC50 values for short (1 h) and long (72 h) exposures. As expected, the concentration of etoposide to achieve 50% growth inhibition was 10–50 times higher for a short exposure than for a long exposure, whereas that of NK314 was only ∼2–3 times higher (Fig. 8D). Together, these results suggest that, compared with etoposide, NK314 exerts its cytotoxic effect in an exposure time-independent manner.
FIGURE 8.
NK314 cytotoxicity is less dependent on exposure time than etoposide cytotoxicity. A and B, sensitivities of wild-type, RAD54–/–, LIG4–/–, and LIG4–/–RAD54–/– cells to NK314 (A) and etoposide (B). Sensitivity was measured by treating cells with Top2 inhibitor for 1 h, followed by colony formation in drug-free agarose medium. Data are the mean ± S.D. of three independent experiments. Where absent, error bars fall within symbols. C, summary of sensitivity assays shown in A and B. D, inhibitory effect of NK314 and etoposide on proliferation of various cancer cell lines. Cell proliferation was measured by using CellTiter-Glo (for Nalm-6) or methylene blue staining (for other cell lines). Cells were cultured for 72 h (for Nalm-6, 96 h) in the presence of Top2 inhibitor (long exposure, L), or for 1 h in the presence of Top2 inhibitor, followed by culture in drug-free medium for 71 h (for Nalm-6, 96 h) (short exposure, S). Exposure time dependence of the growth inhibitory effect of NK314 or etoposide was determined by dividing IC50 (S) by IC50 (L).
DISCUSSION
Etoposide, doxorubicin, and mitoxantrone all target both isoforms of Top2, and are among the most effective anticancer drugs in clinical use; however, these drugs often cause serious side effects, such as secondary malignancies. Recently, Azarova et al. (27) presented evidence that in the absence of Top2β less melanomas developed, suggesting that the β isoform is involved in the development of these malignancies. This, together with the fact that Top2α, the isoform essential for cell growth, is typically highly expressed in rapidly growing cancer cells, strongly supports the idea that Top2α-specific drugs may be a valuable novel approach for cancer treatment. In this article, we have reported for the first time an α isoform-specific Top2 inhibitor, NK314. We have shown that NK314 induces Top2 complexes and chromosomal DSBs in a Top2α-dependent manner. Furthermore, with the use of human gene knock-out mutants, we have genetically investigated isoform specificity of, and the repair mechanisms for DNA damage induced by, NK314. Our results unequivocally indicate that NK314 specifically induces Top2α-mediated DSBs, which are preferentially repaired by the NHEJ pathway. Although further work is required to establish that NK314 is a promising drug candidate for cancer treatment, our finding that NK314 is a specific Top2α poison suggests that NK314 may serve as an anticancer agent that does not cause secondary malignancies. It will thus be particularly interesting to examine whether NK314 treatment does or does not lead to deleterious chromosomal translocations or chromosomal rearrangements.
In the present study, we have conducted genetic analyses using gene knock-out human cell lines to investigate isoform specificity of NK314. Our data suggest that the α isoform is responsible for NK314 cytotoxicity. Additionally, we have examined the relative contribution of Top2 isoforms to cytotoxicity of other Top2 drugs. For instance, our results confirm that the α isoform is the major determinant of etoposide and doxorubicin cytotoxicity; in contrast, the β isoform does significantly contribute to mitoxantrone and XK469 cytotoxicity (see Fig. 5, B–F). (Note that XK469 was reported to be a β isoformspecific inhibitor in previous work (55), but in our cell lines this is not the case.) It should be emphasized that because these human cell lines have been created by targeted gene disruption, the isogenicity between the cell lines is otherwise completely retained. Furthermore, the Nalm-6 cell line has normal p53 status (56) and expresses nearly equal levels of Top2α and Top2β (Fig. 1B). These human cell mutants described here will be invaluable for studying the role of each topoisomerase in the cytotoxicity of potential anticancer agents.
Acknowledgments
We thank Dr. Akihiko Kikuchi for providing the anti-human Top2 monoclonal antibody 7B9. We also thank Haruna Kamekawa for help in performing some of the Top2 assays.
This work was supported in part by Yokohama City University Strategic Research Project Grants W18006 and K19009 (to N. A.), and by Grant-in-Aids from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan Project numbers 18590063, 18058019, and 18018034 (to N. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Top2, topoisomerase II; DMSO, dimethyl sulfoxide; DSB, double-strand break; ICE, in vivo complex of enzyme; MEF, mouse embryonic fibroblast; NHEJ, non-homologous end-joining; PFGE, pulsed-field gel electrophoresis; siRNA, small interfering RNA; TARDIS, trapped in agarose DNA immunostaining; PBS, phosphate-buffered saline.
A. Kurosawa, H. Koyama, S. Iiizumi, S. So, S. Nakamura, K. Iwabuchi, M. Lieber, and N. Adachi, unpublished results.
References
- 1.Wang, J. C. (1996) Annu. Rev. Biochem. 65635 –692 [DOI] [PubMed] [Google Scholar]
- 2.Dong, K. C., and Berger, J. M. (2007) Nature 4501201 –1205 [DOI] [PubMed] [Google Scholar]
- 3.Wang, J. C. (2002) Nat. Rev. Mol. Cell Biol. 3430 –440 [DOI] [PubMed] [Google Scholar]
- 4.Li, T. K., and Liu, L. F. (2001) Annu. Rev. Pharmacol. Toxicol. 4153 –77 [DOI] [PubMed] [Google Scholar]
- 5.Osheroff, N. (1989) Biochemistry 286157 –6160 [DOI] [PubMed] [Google Scholar]
- 6.Cummings, J., and Smyth, J. F. (1993) Ann. Oncol. 4533 –543 [DOI] [PubMed] [Google Scholar]
- 7.Liu, L. F. (1989) Annu. Rev. Biochem. 58351 –375 [DOI] [PubMed] [Google Scholar]
- 8.Nitiss, J. L., and Wang, J. C. (1996) Mol. Pharmacol. 501095 –1102 [PubMed] [Google Scholar]
- 9.Austin, C. A., and Marsh, K. L. (1998) Bioessays 20215 –226 [DOI] [PubMed] [Google Scholar]
- 10.Champoux, J. J. (2001) Annu. Rev. Biochem. 70369 –413 [DOI] [PubMed] [Google Scholar]
- 11.Woessner, R. D., Mattern, M. R., Mirabelli, C. K., Johnson, R. K., and Drake, F. H. (1991) Cell Growth & Differ. 2209 –214 [PubMed] [Google Scholar]
- 12.Padget, K., Pearson, A. D., and Austin, C. A. (2000) Leukemia (Basingstoke) 141997 –2005 [DOI] [PubMed] [Google Scholar]
- 13.Adachi, N., Nomoto, M., Kohno, K., and Koyama, H. (2000) Gene (Amst.) 24549 –57 [DOI] [PubMed] [Google Scholar]
- 14.Akimitsu, N., Adachi, N., Hirai, H., Hossain, M. S., Hamamoto, H., Kobayashi, M., Aratani, Y., Koyama, H., and Sekimizu, K. (2003) Genes Cells 8393 –402 [DOI] [PubMed] [Google Scholar]
- 15.Carpenter, A. J., and Porter, A. C. (2004) Mol. Biol. Cell 155700 –5711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen, M. O., Larsen, M. K., Barthelmes, H. U., Hock, R., Andersen, C. L., Kjeldsen, E., Knudsen, B. R., Westergaard, O., Boege, F., and Mielke, C. (2002) J. Cell Biol. 15731 –44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linka, R. M., Porter, A. C., Volkov, A., Mielke, C., Boege, F., and Christensen, M. O. (2007) Nucleic Acids Res. 353810 –3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaly, N., Chen, X., Dentry, J., and Brown, D. L. (1996) Chromosome Res. 4457 –466 [DOI] [PubMed] [Google Scholar]
- 19.Ju, B. G., Lunyak, V. V., Perissi, V., Garcia-Bassets, I., Rose, D. W., Glass, C. K., and Rosenfeld, M. G. (2006) Science 3121798 –1802 [DOI] [PubMed] [Google Scholar]
- 20.Yang, X., Li, W., Prescott, E. D., Burden, S. J., and Wang, J. C. (2000) Science 287131 –134 [DOI] [PubMed] [Google Scholar]
- 21.Tsutsui, K., Hosoya, O., Sano, K., and Tokunaga, A. (2001) J. Comp. Neurol. 431228 –239 [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui, K., Sano, K., Kikuchi, A., and Tokunaga, A. (2001) J. Biol. Chem. 2765769 –5778 [DOI] [PubMed] [Google Scholar]
- 23.Lyu, Y. L., and Wang, J. C. (2003) Proc. Natl. Acad. Sci. U. S. A. 1007123 –7128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandvliet, D. W., Hanby, A. M., Austin, C. A., Marsh, K. L., Clark, I. B., Wright, N. A., and Poulsom, R. (1996) Biochim. Biophys. Acta 1307239 –247 [DOI] [PubMed] [Google Scholar]
- 25.Capranico, G., Tinelli, S., Austin, C. A., Fisher, M. L., and Zunino, F. (1992) Biochim. Biophys. Acta 113243 –48 [DOI] [PubMed] [Google Scholar]
- 26.Watanabe, M., Tsutsui, K., and Inoue, Y. (1994) Neurosci. Res. 1951 –57 [DOI] [PubMed] [Google Scholar]
- 27.Azarova, A. M., Lyu, Y. L., Lin, C. P., Tsai, Y. C., Lau, J. Y., Wang, J. C., and Liu, L. F. (2007) Proc. Natl. Acad. Sci. U. S. A. 10411014 –11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Errington, F., Willmore, E., Tilby, M. J., Li, L., Li, G., Li, W., Baguley, B. C., and Austin, C. A. (1999) Mol. Pharmacol. 561309 –1316 [DOI] [PubMed] [Google Scholar]
- 29.Onda, T., Toyoda, E., Miyazaki, O., Seno, C., Kagaya, S., Okamoto, K., and Nishikawa, K. (2007) Cancer Lett. 25999 –110 [DOI] [PubMed] [Google Scholar]
- 30.Guo, L., Liu, X., Nishikawa, K., and Plunkett, W. (2007) Mol. Cancer Ther. 61501 –1508 [DOI] [PubMed] [Google Scholar]
- 31.Uegaki, K., Adachi, N., So, S., Iiizumi, S., and Koyama, H. (2006) DNA Repair (Amst.) 5303 –311 [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi, A., Miyaike, M., Kuroda, K., Nozaki, N., Tanaka, M., Hibino, M., Fujii, Y., Kato, S., and Kikuchi, A. (2002) J. Biochem. (Tokyo) 132409 –416 [DOI] [PubMed] [Google Scholar]
- 33.Trask, D. K., and Muller, M. T. (1988) Proc. Natl. Acad. Sci. U. S. A. 851417 –1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willmore, E., Frank, A. J., Padget, K., Tilby, M. J., and Austin, C. A. (1998) Mol. Pharmacol. 5478 –85 [DOI] [PubMed] [Google Scholar]
- 35.Austin, C. A., Marsh, K. L., Wasserman, R. A., Willmore, E., Sayer, P. J., Wang, J. C., and Fisher, L. M. (1995) J. Biol. Chem. 27015739 –15746 [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi, A., and Kikuchi, A. (2004) J. Cell Sci. 1171047 –1054 [DOI] [PubMed] [Google Scholar]
- 37.Iiizumi, S., Nomura, Y., So, S., Uegaki, K., Aoki, K., Shibahara, K., Adachi, N., and Koyama, H. (2006) BioTechniques 41311 –316 [DOI] [PubMed] [Google Scholar]
- 38.Adachi, N., Ishino, T., Ishii, Y., Takeda, S., and Koyama, H. (2001) Proc. Natl. Acad. Sci. U. S. A. 9812109 –12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.So, S., Adachi, N., Lieber, M. R., and Koyama, H. (2004) J. Biol. Chem. 27955433 –55442 [DOI] [PubMed] [Google Scholar]
- 40.So, S., Nomura, Y., Adachi, N., Kobayashi, Y., Hori, T., Kurihara, Y., and Koyama, H. (2006) Genes Cells 11363 –371 [DOI] [PubMed] [Google Scholar]
- 41.Kobori, O., Vuillot, M. T., and Martin, F. (1982) Int. J. Cancer 3065 –67 [DOI] [PubMed] [Google Scholar]
- 42.Adachi, N., So, S., Iiizumi, S., Nomura, Y., Murai, K., Yamakawa, C., Miyagawa, K., and Koyama, H. (2006) DNA Cell Biol. 2519 –24 [DOI] [PubMed] [Google Scholar]
- 43.Roca, J., Ishida, R., Berger, J. M., Andoh, T., and Wang, J. C. (1994) Proc. Natl. Acad. Sci. U. S. A. 911781 –1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andoh, T. (1998) Biochimie (Paris) 80235 –246 [DOI] [PubMed] [Google Scholar]
- 45.Spitzner, J. R., Chung, I. K., and Muller, M. T. (1990) Nucleic Acids Res. 181 –11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi, N., Iiizumi, S., So, S., and Koyama, H. (2004) Biochem. Biophys. Res. Commun. 318856 –861 [DOI] [PubMed] [Google Scholar]
- 47.Adachi, N., Suzuki, H., Iiizumi, S., and Koyama, H. (2003) J. Biol. Chem. 27835897 –35902 [DOI] [PubMed] [Google Scholar]
- 48.Haber, J. E. (2000) Trends Genet. 16259 –264 [DOI] [PubMed] [Google Scholar]
- 49.Lieber, M. R., Ma, Y., Pannicke, U., and Schwarz, K. (2003) Nat. Rev. Mol. Cell Biol. 4712 –720 [DOI] [PubMed] [Google Scholar]
- 50.Lieber, M. R. (1999) Genes Cells 477 –85 [DOI] [PubMed] [Google Scholar]
- 51.Lieber, M. R. (2008) J. Biol. Chem. 2831 –5 [DOI] [PubMed] [Google Scholar]
- 52.Heyer, W. D., Li, X., Rolfsmeier, M., and Zhang, X. P. (2006) Nucleic Acids Res. 344115 –4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grawunder, U., Zimmer, D., Fugmann, S., Schwarz, K., and Lieber, M. R. (1998) Mol. Cell 2477 –484 [DOI] [PubMed] [Google Scholar]
- 54.Grawunder, U., Zimmer, D., Kulesza, P., and Lieber, M. R. (1998) J. Biol. Chem. 27324708 –24714 [DOI] [PubMed] [Google Scholar]
- 55.Gao, H., Huang, K. C., Yamasaki, E. F., Chan, K. K., Chohan, L., and Snapka, R. M. (1999) Proc. Natl. Acad. Sci. U. S. A. 9612168 –12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.So, S., Adachi, N., and Koyama, H. (2007) DNA Cell Biol. 26517 –525 [DOI] [PubMed] [Google Scholar]