Abstract
It is now widely accepted that certain types of cognitive functions are intimately related to synchronized neuronal oscillations at both low (α/θ) (4–7/8–13 Hz) and high (β/γ) (18–35/30–70 Hz) frequencies. The thalamus is a key participant in many of these oscillations, yet the cellular mechanisms by which this participation occurs are poorly understood. Here we describe how, under appropriate conditions, thalamocortical (TC) neurons from different nuclei can exhibit a wide array of largely unrecognised intrinsic oscillatory activities at a range of cognitively-relevant frequencies. For example, both metabotropic glutamate receptor (mGluR) and muscarinic Ach receptor (mAchR) activation can cause rhythmic bursting at α/θ frequencies. Interestingly, key differences exist between mGluR- and mAchR-induced bursting, with the former involving extensive dendritic Ca2+ electrogenesis and being mimicked by a non-specific block of K+ channels with Ba2+, whereas the latter appears to be more reliant on proximal Na+ channels and a prominent spike afterdepolarization (ADP). This likely relates to the differential somatodendritic distribution of mGluRs and mAChRs and may have important functional consequences. We also show here that in similarity to some neocortical neurons, inhibiting large-conductance Ca2+-activated K+ channels in TC neurons can lead to fast rhythmic bursting (FRB) at ~40 Hz. This activity also appears to rely on a Na+ channel-dependent spike ADP and may occur in vivo during natural wakefulness. Taken together, these results show that TC neurons are considerably more flexible than generally thought and strongly endorse a role for the thalamus in promoting a range of cognitively-relevant brain rhythms.
Keywords: acetylcholine, metabotropic glutamate receptors, lateral geniculate nucleus, intralaminar nuclei, oscillations, EEG, cognition, perception, memory
Introduction
Since the discovery of the EEG by Hans Berger in the early part of the last century (Berger 1929) oscillatory brain activity and its potential relationship with a range of behavioural variables has been a dominant theme in neuroscience research. In the 50–60 years following inception of the EEG, the main focus of research on brain oscillations was, unsurprisingly, the classical alpha (α) (8–13 Hz) rhythm. This rhythm, the first EEG oscillation to be documented, is concentrated at occipital sites, reflecting its origins in the visual system, and is most pronounced during periods of relaxed wakefulness (Berger 1929; Adrian and Matthews, 1934; Adrian and Yamagiwa 1935; Hughes and Crunelli 2005). Because the α rhythm is particularly evident when the eyes are closed, it has been widely considered to represent a simple idling of the visual cortex. However, its expression is not exclusively restricted to the eyes-closed condition (Mulholland 1965) and an extremely large body of psychophysical literature spanning several decades has shown that α activity is inseparably linked to a host of perceptual and cognitive phenomena (Lindsley 1952; Lansing 1957; Anliker 1963, 1966; VanRullen and Koch 2003). For example, α rhythm frequency is robustly correlated with both reaction time (Surwillo 1961) and perceived simultaneity (Kristofferson 1967) and α activity is strongly linked with various aspects of long term memory (Klimesch 1996, 1999).
Despite a recent tangible re-emergence of interest in the significance and mechanisms of α rhythms (Schürmann et al. 2000; Makeig et al. 2002; VanRullen and Koch 2003; Hughes et al. 2004; Hughes and Crunelli 2005; Mazaheri and Jensen 2006; VanRullen et al. 2006; Palva and Palva 2007; Becker et al. 2008), research on brain oscillations in the last 10–20 years has mainly focused on fast oscillations in the β/γ (18–35/30–70 Hz) band (Gray et al. 1989; Gray and Singer 1989; Whittington et al. 1995; Başar-Eroglu et al. 1996; Roelfsema et al. 1997; Tallon-Baudry et al. 1996, 1997; Buhl et al. 1998; Fisahn et al. 1998; Csicsvari et al. 2003; Cunningham et al. 2003, 2004; Hajos et al. 2004; Mann et al. 2005; Traub et al. 2005; Bartos et al. 2007; Fries et al. 2007; Jensen et al. 2007). Initial interest in these oscillations was largely motivated by the finding that following an appropriate visual stimulus, local field potential (LFP) recordings in the cat primary visual cortex (i.e. V1) can exhibit robust oscillations at around 40 Hz (i.e. in the γ band) that are tightly phased-related to local neuronal firing (Gray and Singer 1989). During these oscillations neurons with overlapping receptive fields and similar response characteristics were found to be synchronized with zero time-lag which suggested that γ activity may provide a means to temporarily connect groups of neurons which are functionally related (Gray et al. 1989). Zero time-lag synchronization during γ oscillations was also found to extend across different cortical territories and was noted to be especially strong between areas that perform related functions (Roelfsema et al. 1997). Ultimately, these and other findings led to the transient coupling of distributed neuronal assemblies by γ oscillations being widely touted as a solution to the binding problem (see for example Engel and Singer 2001), i.e. how the brain creates a stable and coherent percept from a distinct but related array of sensory signals, and ensured that the study of fast brain oscillations has been maintained as an area of strong interest in neuroscience.
The role of the thalamus in cognitively relevant brain oscillations
Although the neocortex is clearly involved in shaping the ultimate EEG α rhythm signal (Hughes and Crunelli 2005), ever since the early days of EEG research, the thalamus has been suggested as an important site for its generation. However, the first real evidence for this was provided by experiments on dogs in the early 1970s (Lopes da Silva et al. 1973). These showed that naturally occurring α activity in the visual cortex is accompanied by coherent α oscillations in the primary visual thalamus (i.e. the lateral geniculate nucleus, LGN) (Lopes da Silva et al. 1973). Furthermore, because these LGN oscillations sometimes occurred independently of cortical α rhythms, it appeared that the thalamus was able to autonomously produce α activity. Similar results were later obtained in cats, both for an equivalent of the occipital α rhythm in the visual system (Chatila et al. 1992, 1993; Rougeul-Buser and Buser 1997) as well as for an analogue of the somatosensory μ rhythm (Bouyer, et al. 1982, 1983; Rougeul-Buser and Buser 1997). More recently, an abundance of human imaging data has emerged which also strongly supports a central role for the thalamus in the generation of EEG α activity (summarized in Hughes and Crunelli 2005; see also Feige et al. 2005; Goncalves et al. 2006).
Oscillations in the β/γ band are present in a wide variety of brain areas but are most commonly associated with the neocortex (Gray et al. 1989; Gray and Singer 1989; Gray and McCormick 1996; Buhl et al. 1998; Cunningham et al. 2004; Traub et al. 2005), hippocampus (Whittington et al. 1995; Fisahn et al. 1998; Csicsvari et al. 2003; Hajos et al. 2004; Mann et al. 2005) and olfactory bulb (Eeckman and Freeman 1990). As such, the thalamus has not traditionally been considered as a key player in the generation of fast oscillations. Indeed, the initial inability to observe fast oscillations in the LGN suggested that they were neither reflected in the thalamus nor that the thalamus was involved in their generation (Gray and Singer 1989). However, a subsequent study in anesthetized cats showed that over half of neurons in the LGN show robust oscillatory activity at around 50 Hz (Ghose and Freeman 1992). Later work also revealed the presence of fast oscillations in the LGN, not only under anaesthesia but also during natural wakefulness (Steriade et al. 1996). Crucially, this latter study also demonstrated that such oscillations are present in a variety of different thalamic nuclei, occur in tight synchrony with rhythmic activity in related cortical areas, and are highly correlated with oscillatory phenomena in individual TC neurons (Steriade et al. 1996; see also Steriade et al. 1991). This suggested that the thalamus may play a more active role in the generation of fast oscillations than had been previously thought.
Activation of metabotropic glutamate receptors (mGluRs) or muscarinic Ach receptors (mAchRs) induces rhythmic bursting at α/θ frequencies in TC neurons
Given that the thalamus is involved in synchronized oscillations at both low and high cognitively-relevant frequencies, a key goal is to understand the intrinsic properties in thalamic neurons that are central to this involvement. Under normal conditions, TC neurons recorded intracellularly in vitro show two distinct modes of firing (Llinás and Jahnsen 1982). When these cells are relatively hyperpolarized (≤~−65 mV) a brief injection of positive current leads to a transient depolarization lasting around ~100–200 ms which is crowned by a high-frequency burst of action potentials (i.e. burst mode) (Fig. 1A, top left) (Llinás and Jahnsen 1982). This transient depolarization is typically referred to as a low-threshold Ca2+ potential (LTCP) or low-threshold spike (LTS) and is generated by a T-type Ca2+ current (Coulter et al. 1989; Crunelli et al. 1989; Hernandez-Cruz and Pape 1989; Suzuki and Rogawski 1989). In contrast, when TC neurons are relatively depolarized (≥~−60 mV), a brief injection of positive current leads to tonic firing or single spike activity (i.e. tonic or relay mode, see below) (Fig. 1A, top right) (Llinás and Jahnsen 1982). The discovery of these two modes of firing has laid the foundation for several basic ideas regarding how the thalamus operates with broad agreement existing that LTCPs are mainly associated with low frequency oscillations during sleep and anaesthesia (McCarley et al. 1983; Domich et al. 1986; Nunez et al. 1992; Steriade et al. 1993; Crunelli et al. 2006; but see Sherman 2001) whereas tonic firing occurs more commonly during wakefulness and is important for the faithful relay of sensory information to the neocortex.
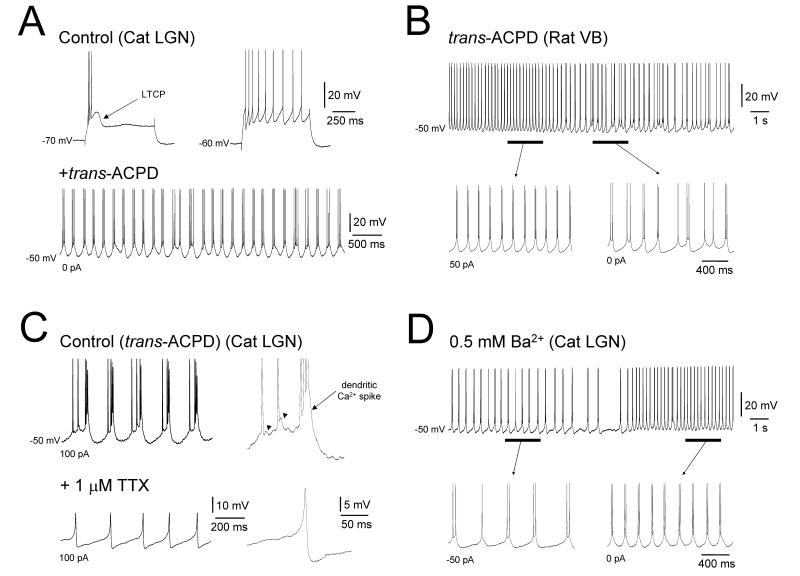
Figure 1. mGluR-activation induces HT bursting at α/θ frequencies in TC neurons.
A. Top: intracellular recordings form a cat LGN TC neuron in vitro showing basic burst (left) and tonic (right) modes of firing following the injection of a brief positive current step elicited from −70 mV and −60 mV, respectively. Bottom: application of the mGluR agonist, trans-ACPD (100 μM), brings about a third mode of firing termed HT bursting. B. Whole-cell patch clamp recording from a rat VB TC neuron in vitro exhibiting HT bursting in the presence of 25 μM trans-ACPD. The underlined sections are expanded below and show HT bursting at two different levels of steady injected current as indicated. C. Top: intracellular recording of mGluR-induced HT bursting in a cat LGN TC neuron in vitro. The trace to the right is an enlargement of a single HT burst which shows evidence of dendritic Ca2+ spike involvement as well as small spike ADPs (see arrowheads). Bottom: following a block of action potentials with 1 μM TTX, dendritic Ca2+ spikes become clearly evident. D. Intracellular recording of a cat LGN TC neuron in vitro in the presence of 0.5 mM Ba2+ showing activity that is essentially indistinguishable from mGluR-induced HT bursting. Again, the underlined sections are expanded below and show Ba2+-induced bursting at two distinct levels of steady injected current as indicated.
Recently, we found that activating metabotropic gluatamate receptors (mGluRs), either pharmacologically or via electrically stimulating corticothalamic fibres, led to around 25% of TC neurons in the cat LGN recorded in vitro exhibiting a third mode of firing which we termed high-threshold (HT) bursting (Fig. 1A, bottom) (Hughes et al. 2002, 2004). HT bursting occurs rhythmically at ~3–15 Hz, thus encompassing both the α (8–13 Hz) and θ (4–7 Hz) bands, and unlike LTCP-mediated bursting is present when neurons are relatively depolarized (>−55 mV) (Hughes et al. 2002, 2004; Hughes and Crunelli 2005). Importantly, single unit recordings of LGN TC neurons from freely moving cats showed that activity with indistinguishable properties to HT bursting occurs coherently with α oscillations in the intact brain (Hughes et al. 2004; Hughes and Crunelli 2005, 2007). We have since found that following an equivalent activation of mGluRs, HT bursting with broadly similar properties to those noted in the cat LGN can also be observed in other areas of the cat thalamus, including the the ventrolateral (VL) nucleus, i.e. the motor thalamus, and the ventrobasal complex (VB), i.e. the somatosensory thalamus, where it may play a role in promoting synchronized μ oscillations (Bouyer et al. 1982; Hughes and Crunelli 2005). mGluR-induced HT bursting is also present in TC neurons from principal thalamic nuclei of both the rat and mouse (Fig. 1B).
Following application of the Na+ channel blocker tetrodotoxin (TTX) to block action potentials, mGluR-induced HT bursting is replaced by a residual oscillation comprising rhythmic dendritic Ca2+ spikes (Fig. 1C) (Jahnsen and Llinás 1984; Williams and Stuart 2000; Hughes et al. 2004), indicating that these events are the primary driving force behind burst activity (Hughes et al. 2004). Indeed, dendritic Ca2+ spikes can be regularly observed in HT bursting cells even before TTX treatment (Fig. 1C) whilst a blockade of Ca2+ channels with Ni2+ converts HT bursting into regular tonic firing (Hughes et al. 2004; Crunelli et al. 2006). Because the mGluR subtype that is responsible for inducing HT bursting (i.e. mGluR1a; (Hughes et al. 2004) is located at distal sites on TC neurons (Godwin et al. 1996; Erisir et al. 1997b) and thought to be negatively coupled to leak K+ channels (von Krosigk et al. 1999; Turner and Salt 2000; Hughes et al. 2002), it is reasonable to assume that mGluR-dependent HT bursting is reliant on a strong suppression of dendritic K+ conductance which in turn facilitates the generation of local Ca2+ spikes. In support of this, activity in TC neurons that is essentially indistinguishable from mGluR-induced HT bursting can also be instated by non-selectively reducing K+ conductance through the application 0.5 mM Ba2+ (Fig. 1D), whereas artificially reducing linear K+ conductance solely at the soma, using the dynamic clamp technique (see Hughes et al. 1999, 2002 and Blethyn et al. 2006), is unable to recreate HT bursting (data not shown).
Because several other types of receptors on TC neurons are also negatively coupled to leak K+ channels (McCormick 1992), we recently asked whether their activation might also lead to HT bursting in TC neurons. In particular, we were interested to test whether or not pharmacologically activating muscarinic Ach receptors (mAchRs), which play a central role in arousal regulation through their effects in the thalamocortical system (McCormick 1992), would have similar effects on TC neuron firing to activating mGluRs. Indeed, through an effect largely mediated by M1/M3 receptors, the ACh receptor agonist carbachol (Cch) applied in vitro brings about HT bursting at ~3–15 Hz in subsets of TC neurons from a variety of principal thalamic nuclei including the LGN, VB and VL (Fig. 2) as well as from the centrolateral (CL) nucleus, a member of the non-specific intralaminar nuclei (data not shown).
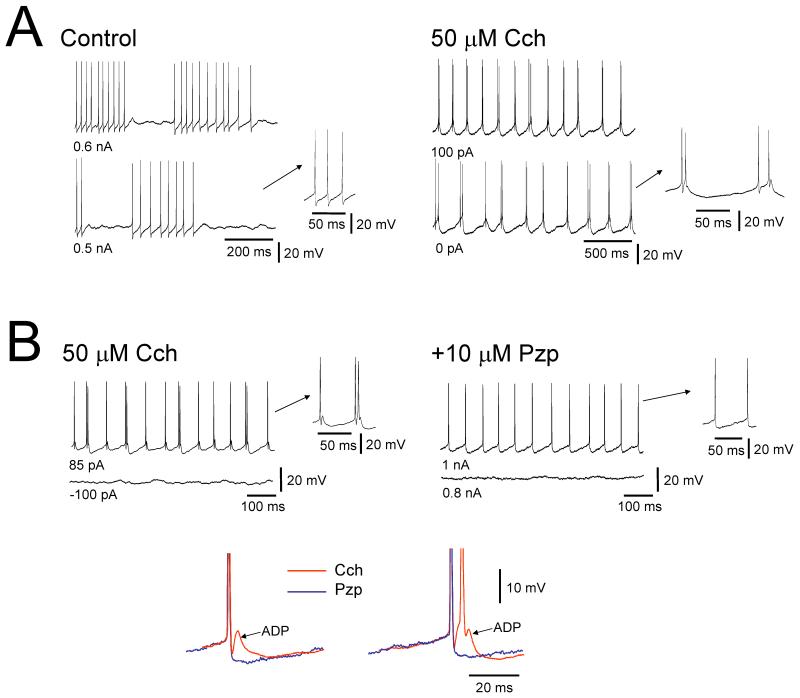
Figure 2. HT bursting can also be instated by mAchR activation but with distinct properties to those induced by mGLuR-activation.
A. Left traces: intracellular recordings from an LGN TC neuron in control conditions in vitro at different levels of steady injected current show conventional tonic firing. Right traces: following Cch application the output of the neuron at depolarized membrane potentials becomes characterised by rhythmic HT bursts. B. left traces: HT bursting in another LGN TC neuron induced by Cch application in vitro. Right traces: addition of the mAchR antagonist pirenzipine (Pzp) converts HT bursting to conventional tonic firing. The enlarged traces below reveal that mAchR-induced HT bursting is dependent on a prominent spike ADP which, unlike that present following mGluR activation (Fig.1C, top right), can drive additional action potentials. Note, however, the clear lack of involvement of dendritic Ca2+ spikes. Modified and reproduced from Lörincz et al. (2008) with permission.
mGluR- and mAchR-induced bursting exhibit subtle differences that may have important functional consequences
Interestingly, whilst the properties of HT bursting induced by Cch are broadly similar to those of HT bursting induced by mGluR activation, there is one notable distinction between the two types of activity. Specifically, whilst mGluR-induced HT bursting is overtly associated with dendritic Ca2+ spikes (Fig. 1C) (see above), this is rarely the case for mAChR-induced HT bursting (Fig. 2). Rather, HT bursting in this context seems to be generated by a prominent spike afterdepolarization (ADP) (Lörincz et al. 2008) (Fig. 2B). This ADP appears to be generated by Na+ channels (Hughes et al. 2004) and whilst also being present following mGluR1a activation (Fig. 1C, top right, arrowheads), it possesses a much greater amplitude and functional significance following mAchR activation (Fig. 2B, bottom).
A possible explanation for the distinction between mGluR- and mAchR-induced HT bursting relates to the differential distribution of these receptors on the somatodendritic axis of TC neurons (Fig. 3). mAchRs are located at relatively proximal sites (Erisir et al. 1997a,b) and their domain of influence may therefore not extend to the more distal regions where Ca2+ spike generation occurs (Jahnsen and Llinás 1984). On the other hand, they are ideally situated to modulate proximal Na+ channel-dependent events (Williams and Stuart 2000) explaining the appearance of a large spike ADP following Cch application. In contrast to mAchRs, and as mentioned above, mGluRs are a located more distally in a position that is presumably close to the dendritic Ca2+ spike generating machinery. However, because the domain of influence of mGluRs extends to the soma (von Krosigk et al. 1999; Turner and Salt 2000; Hughes et al. 2002), activation of these receptors can also affect proximal Na+ channel-dependent events, albeit to a lesser extent than mAchR activation. One interesting aspect of the difference between mGluR- and mAchR-induced HT bursting is that the former will obviously be associated with a large amount of dendritic Ca2+ influx. This hints at the possibility that whilst mAchR-induced HT bursting may perform a simple electrical pacemaker role (Lörincz et al. 2008), mGluR-induced bursting may also be associated with profound biochemical changes within the neuron, potentially leading to alterations in synaptic strength and gene expression.
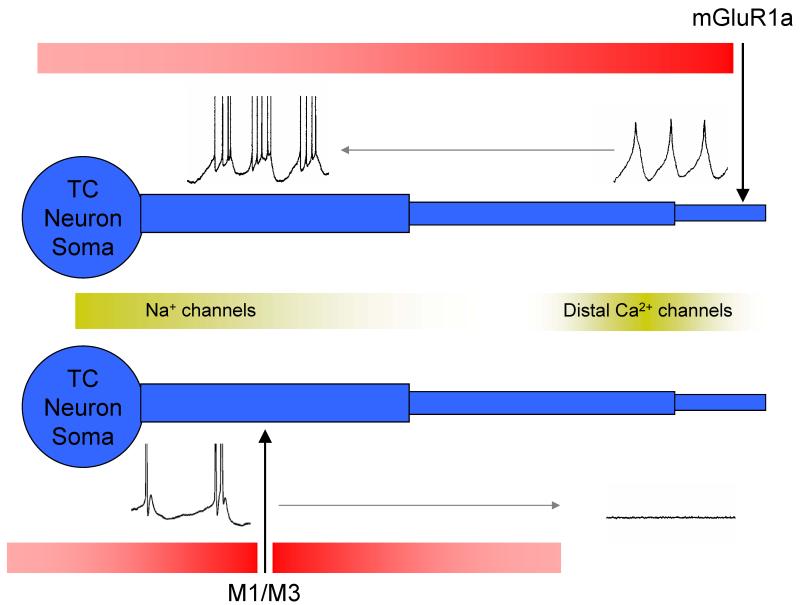
Figure 3. The differences between mGluR- and mAchR-induced HT bursting can be explained by a distinct somatodendritic receptor distribution.
Proposed scheme to explain the difference between mGluR- and mAchR-induced HT bursting. Top: mGluR1a receptors are located at distal sites, presumably close to the Ca2+ channels that underlie dendritic spike generation. Thus, suppression of K+ channels in this region facilitates the generation of Ca2+ spikes which then propagate to the soma where they interact with proximal Na+ channels to produce bursts of action potentials. Note that because the domain of influence of mGluR1a activation extends to the soma (red shaded bar), activating these receptors is able to depolarize the neuron sufficiently close to action potential threshold to facilitate bursting. Bottom: mAchRs are located at more proximal sites and are therefore unable to trigger distal Ca2+ spikes. However, they are ideally situated to enhance dendritic Na+ channel-dependent events, hence the appearance of a prominent spike ADP following activation of these receptors.
Inhibiting large conductance Ca2+-activated K+ channels brings about intrinsic bursting at ~40 Hz in TC neurons
Prominent spike ADPs similar to those observed during mAChR-induced HT bursting are also a common feature of rhythmic bursting in several other types of neurons. For example, such events play a central role in repetitive bursting at 40–80 Hz in pyramidal cells of the electrosensory lateral line lobe of the weakly electric fish, an activity which, interestingly, is blocked by selectively applying TTX to the proximal apical dendritic region (Lemon and Turner 2000). Similarly, spike ADPs are an important determinant of the so-called chattering or fast rhythmic bursting (FRB) activity which occurs at 20–80 Hz, is present in a subset of layer II/III neocortical pyramidal neurons (Gray and McCormick 1996; Brumberg et al. 2000) and which, again, is highly sensitive to an inhibition of Na+ channels (Brumberg et al. 2000). With regard to FRB, it appears that persistent rather than transient Na+ channels are the key component in generating ADPs because potentiating these channels by applying either the Na+ channel toxin, ATX II (Brumberg et al. 2000), or the NO donor, S-nitroso-N-acetylpenicillamine (SNAP) (Traub et al. 2003), can transform regular spiking (RS) layer II/III neurons into FRB cells, with this transformation being reversed by the putative persistent Na+ channel blocker, phenytoin (Traub et al. 2003). More specifically, it appears that a delicate balance between afterhyperpolarization (AHP)-generating currents and persistent Na+ channels is what determines the mode of firing in these cells because both experimental and modelling studies show that a transformation from RS to FRB behaviour can also be readily achieved by blocking large conductance Ca2+-activated (BK) K+ channels with iberiotoxin (Ibtx) (Traub et al. 2003).
Given that TC neurons have the clear capacity to generate Na+ channel-dependent spike ADPs and that a high immunoreactivity specifically for BK channels is present in the dorsal thalamus of the rodent brain (Sausbier et al. 2006), we recently tested whether application of Ibtx to TC neurons of the LGN maintained in vitro could induce FRB. In doing so, we found that Ibtx, applied at 100 nM, was able to consistently and reversibly induce FRB at 20–60 Hz in all TC neurons tested (Fig 4A). Furthermore, as with layer II/III neocortical cells, this type of activity involved a clear spike ADP (Fig 4B) and was preferentially blocked by TTX, being abolished well before a full block of action potentials was achieved (Fig 5). Interestingly, we have found that manipulations which reduce the supply of intracellular Ca2+, either by decreasing [Ca2+]o to 0 mM or chelating intracellular Ca2+ with EGTA or BAPTA (Fig 4C), are also able to induce FRB-like behaviour in TC neurons, in a similar way to that predicted by simulation studies to occur for cortical pyramidal neurons (Traub et al. 2003). This finding is consistent with experiments showing that disrupting Ca2+-induced Ca2+-release in TC neurons can also bring about a similar behaviour to FRB (Budde et al. 2000) and hints at a complex management of firing in these cells which may be potentially influenced by various regulatory systems which couple to the cyclic ADP ribose pathway (Budde et al. 2000), via increases in intracellular cyclic GMP (Graeff et al. 1998), or to protein kinase A (PKA) (Traub et al. 2003), via stimulation of cyclic AMP.
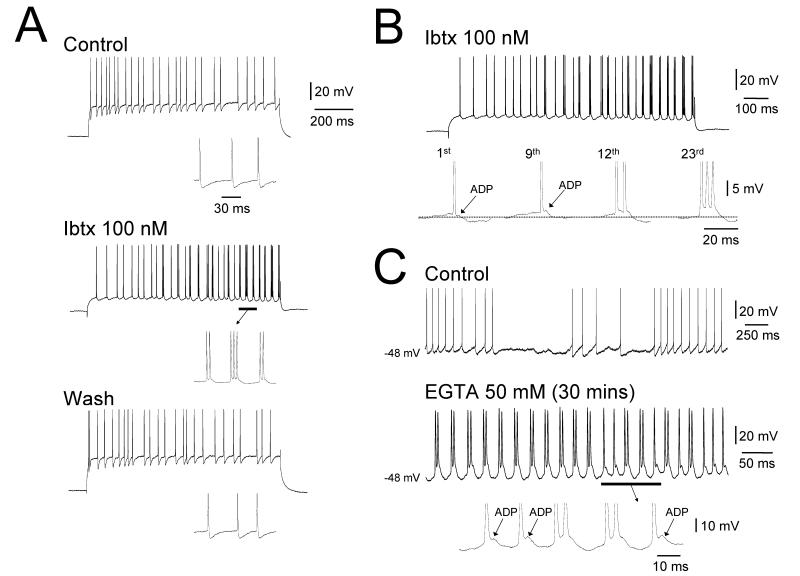
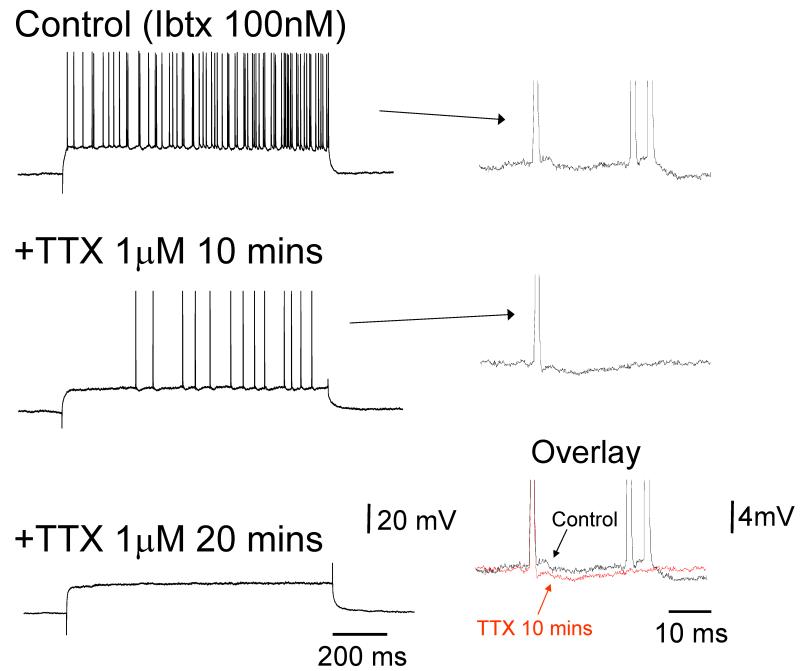
Figure 4. Inihition of BK channels leads to rhythmic bursting at ~40 Hz in TC neurons.
A. Response of a TC neuron in the rat LGN in vitro to a positive current pulse in control conditions (top), following 100 nM Ibtx application (middle) and after Ibtx washout (bottom). Ibtx reversibly induces rhythmic burst firing at 30–40 Hz. In each panel, the underlined sections are enlarged below as indicated. B. A closer inspection of the response of the neuron shown in A following Ibtx application reveals that burst activity arises from the progressive build up of a spike ADP. C. Top trace: activity of a TC neuron from the cat LGN in control conditions in vitro after depolarization with steady current reveals conventional tonic firing. Bottom trace: after recording for 30 mins with 50 mM EGTA in the electrode, at the same level of injected current this neuron exhibits rhythmic burst firing at 40–50 Hz. Again, this bursting is associated with a prominent spike ADP as shown by the enlarged section below.
Figure 5. The spike ADP underlying rhythmic bursting at ~40 Hz in TC neurons is dependent on Na+ channels.
Top trace: response of a rat LGN TC neuron in vitro to a positive current step during application of 100 nM Ibtx showing rhythmic bursting at 20–60 Hz. Middle trace: after 10 minutes of TTX treatment the neuron reverts to a pattern of single spike activity. The enlarged sections to the right show that this is due to a preferential suppression of the spike ADP by TTX. Bottom trace: after 20 minutes TTX abolished all action potential output.
The finding that an inhibition of BK channels leads to rhythmic bursting at ~40 Hz in TC neurons in vitro is noteworthy because previous in vivo studies utilising single unit extracellular recordings have shown that, during both natural wakefulness and REM sleep, a subset of TC neurons in the cat CL nucleus exhibit rhythmic bursting at ~40 Hz with similar properties to those described here (Steriade and Glenn 1982; Steriade et al. 1993). Indeed, intracellular recordings of these cells obtained during barbiturate anaesthesia revealed that this type of bursting is intrinsic and involves a clear spike ADP which bears a striking resemblance to that seen in our in vitro recordings (Steriade et al. 1993). The additional lack of prominent spike AHP and narrow action potential width in these CL TC neurons led the investigators to speculate that their unusual behaviour arose from a combination of reduced Ca2+-activated K+ current and increased Na+ conductance, a suggestion which, again, is fully in line with our in vitro data. Mirroring these findings from the CL thalamus, we have recently noted that some TC neurons in the cat LGN also exhibit brief periods of rhythmic bursting at 40–60 Hz during natural wakefulness (Fig. 6). Thus, we suggest that rhythmic bursting in the γ band may occur in cells from a variety of both specific and non-specific thalamic nuclei where it could play a role in driving behaviourally-relevant, synchronized γ oscillations as is the case for FRB in the neocortex (Cunningham et al. 2004).
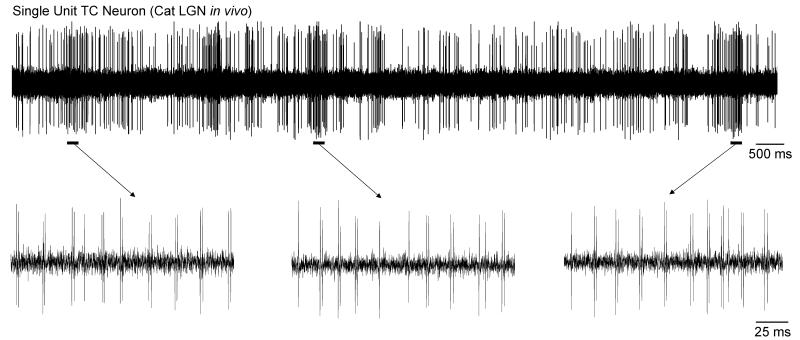
Figure 6. TC neurons recorded from the LGN of naturally waking cats can exhibit brief periods of rhythmic bursting at ~50 Hz.
Single unit recording of a TC neuron from the cat LGN during natural wakefulness. The underlined sections are enlarged below and reveal episodes of rhythmic bursting at 40–60 Hz.
Summary
Synchronized oscillations at both low (α/θ) (4–7/8–13 Hz) and high (β/γ) (18–35/30–70 Hz) frequencies have close links with a variety of cognitive and perceptual phenomena. Whilst such oscillations are known to involve the thalamus, very little is known about the way in which thalamic neurons engage in and promote oscillatory activity. We have shown here how, under certain conditions, TC neurons in a variety of nuclei and species can display several types of intrinsic oscillatory activity which have previously gone largely unrecognised (Sherman 2001; Llinás and Steriade 2006). In particular, TC neurons are able to generate two distinct types of rhythmic HT bursting at α/θ frequencies following either mGluR or mAchR activation, respectively, and can show a ~40 Hz activity, similar to the FRB behaviour of some layer II/III neocortical pyramidal cells (Brumberg et al. 2000; Traub et al. 2003; Cunningham et al. 2004), following a suppression of BK channels. Whilst intrinsic oscillatory activity is also a common and important feature of other brain areas, we propose that these novel modes of operation of TC neurons may be a key component in shaping synchronized brain oscillations at both low and high cognitively-relevant frequencies. Finally, we suggest that a disruption of the mechanisms that underlie these unusual forms of intrinsic oscillatory activity may be an important aspect of several pathological scenarios that affect cognitive performance (Llinás et al. 1999), or are associated with excessive rhythmicity in thalamocortical networks (Crunelli and Leresche 2002).
ACKNOWLEDGEMENTS
This work was supported by the Wellcome Trust grants 71436, 78403 awarded to VC and 78311 awarded to SWH.
REFERENCES
- Adrian ED, Matthews BHC. The Berger Rhythm: potential changes from the occipital lobes in man. Brain. 1934;57:355–85. doi: 10.1093/brain/awp324. [DOI] [PubMed] [Google Scholar]
- Adrian ED, Yamagiwa K. The origin of the Berger rhythm. Brain. 1935;58:323–51. [Google Scholar]
- Anliker J. Variations in alpha voltage of the electroencephalogram and time perception. Science. 1963;140:1307–9. doi: 10.1126/science.140.3573.1307. [DOI] [PubMed] [Google Scholar]
- Anliker J. Simultaneous changes in visual separation threshold and voltage of cortical alpha rhythm. Science. 1966;153:316–18. doi: 10.1126/science.153.3733.316. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Başar-Eroglu C, Strüber D, Schürmann M, Stadler M, Başar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int. J. Psychophysiol. 1996;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Becker R, Ritter P, Villringer A. Influence of ongoing alpha rhythm on the visual evoked potential. Neuroimage. 2008;39:707–16. doi: 10.1016/j.neuroimage.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektroenkaphogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten. 1929;87:527–70. [Google Scholar]
- Blethyn KL, Hughes SW, Tóth TI, Cope DW, Crunelli V. Neuronal basis of the slow (<1 Hz) oscillation in neurons of the nucleus reticularis thalami in vitro. J. Neurosci. 2006;26:2474–86. doi: 10.1523/JNEUROSCI.3607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer JJ, Rougeul A, Buser P. Somatosensory rhythms in the awake cat: a single unit exploration of their thalamic concomitant in nucleus ventralis posterior and vicinity. Arch. Ital. Biol. 1982;120:95–110. [PubMed] [Google Scholar]
- Bouyer JJ, Tilquin C, Rougeul A. Thalamic rhythms in cat during quiet wakefulness and immobility. Electroencephalogr. Clin. Neurophysiol. 1983;55:180–7. doi: 10.1016/0013-4694(83)90186-4. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J. Neurosci. 2000;20:4829–43. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Sieg F, Braunewell KH, Gundelfinger ED, Pape HC. Ca2+-induced Ca2+ release supports the relay mode of activity in thalamocortical cells. Neuron. 2000;26:483–92. doi: 10.1016/s0896-6273(00)81180-0. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Tamás G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513:117–26. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila M, Milleret C, Buser P, Rougeul A. A 10 Hz alpha-like rhythm in the visual cortex of the waking cat. Electroencephalogr. Clin. Neurophysiol. 1992;83:217–22. doi: 10.1016/0013-4694(92)90147-a. [DOI] [PubMed] [Google Scholar]
- Chatila M, Milleret C, Rougeul A, Buser P. Alpha rhythm in the cat thalamus. C. R. Acad. Sci. III. 1993;316:51–8. [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J. Physiol. 1989;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Cope DW, Hughes SW. Thalamic T-type Ca2+ channels and NREM sleep. Cell Calcium. 2006;40:175–90. doi: 10.1016/j.ceca.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002;3:371–82. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J. Physiol. 1989;413:543–61. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–22. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J. Neurosci. 2003;23:9761–9. doi: 10.1523/JNEUROSCI.23-30-09761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7152–7. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J. Physiol. 1986;379:429–49. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckman FH, Freeman WJ. Correlations between unit firing and EEG in the rat olfactory system. Brain Res. 1990;528:238–44. doi: 10.1016/0006-8993(90)91663-2. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends. Cogn. Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: a comparison with corticogeniculate terminals. J. Comp. Neurol. 1997a;377:535–49. [PubMed] [Google Scholar]
- Erişir A, Van Horn SC, Sherman SM. Relative numbers of cortical and brainstem inputs to the lateral geniculate nucleus. Proc. Natl. Acad. Sci. U.S.A. 1997b;94:1517–20. doi: 10.1073/pnas.94.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J. Neurophysiol. 2005;93:2864–72. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–9. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–16. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ghose GM, Freeman RD. Oscillatory discharge in the visual system: does it have a functional role? J. Neurophysiol. 1992;68:1558–74. doi: 10.1152/jn.1992.68.5.1558. [DOI] [PubMed] [Google Scholar]
- Godwin DW, Van Horn SC, Eriir A, Sesma M, Romano C, Sherman SM. Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J. Neurosci. 1996;16:8181–92. doi: 10.1523/JNEUROSCI.16-24-08181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, Kuijer JP, Maurits NM, Hoogduin JM, Van Someren EJ, Heethaar RM, Lopes da Silva FH. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage. 2006;30:203–13. doi: 10.1016/j.neuroimage.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Graeff RM, Franco L, De Flora A, Lee HC. Cyclic GMP-dependent and -independent effects on the synthesis of the calcium messengers cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem. 1998;273:118–25. doi: 10.1074/jbc.273.1.118. [DOI] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–7. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–13. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1698–702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J. Neurosci. 2004;24:9127–37. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Cruz A, Pape H-C. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J. Neurophysiol. 1989;61:1270–83. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–58. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Tóth TI, Williams SR, Crunelli V. All thalamocortical neurones possess a T-type Ca2+ ‘window’ current that enables the expression of bistability-mediated activities. J. Physiol. 1999;517:805–15. doi: 10.1111/j.1469-7793.1999.0805s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005;11:357–72. doi: 10.1177/1073858405277450. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Crunelli V. Just a phase they’re going through: The complex interaction of intrinsic high-threshold bursting and gap junctions in the generation of thalamic alpha and theta rhythms. Int. J. Psychophysiol. 2007;64:3–7. doi: 10.1016/j.ijpsycho.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Lörincz M, Cope DW, Blethyn KL, Kékesi KA, Parri HR, Juhász G, Crunelli V. Synchronized oscillations at alpha and theta frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–68. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J. Physiol. 1984;349:227–47. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–24. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kristofferson AB. Successiveness discrimination as a two-state, quantal process. Science. 1967;158:1337–9. doi: 10.1126/science.158.3806.1337. [DOI] [PubMed] [Google Scholar]
- Lansing RW. Relation of brain and tremor rhythms to visual reaction time. Electroencephalogr. Clin. Neurophysiol. 1957;9:497–504. doi: 10.1016/0013-4694(57)90037-8. [DOI] [PubMed] [Google Scholar]
- Lemon N, Turner RW. Conditional spike backpropagation generates burst discharge in a sensory neuron. J. Neurophysiol. 2000;84:1519–30. doi: 10.1152/jn.2000.84.3.1519. [DOI] [PubMed] [Google Scholar]
- Lindsley DB. Psychological phenomena and the electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 1952;4:443–456. doi: 10.1016/0013-4694(52)90075-8. [DOI] [PubMed] [Google Scholar]
- Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–8. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. U.S.A. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva FH, van Lierop TH, Schrijer CF, van Leeuwen WS. Organization of thalamic and cortical alpha rhythms: spectra and coherences. Electroencephalogr. Clin. Neurophysiol. 1973;35:627–39. doi: 10.1016/0013-4694(73)90216-2. [DOI] [PubMed] [Google Scholar]
- Lörincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8–13 Hz) rhythms in sensory thalamic nuclei in vitro. J. Neurosci. 2008;28:660–71. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–4. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–17. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O. Posterior alpha activity is not phase-reset by visual stimuli. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2948–52. doi: 10.1073/pnas.0505785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Benoit O, Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J. Neurophysiol. 1983;50:798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J. Clin. Neurophysiol. 1992;9:212–23. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- Mulholland TB. Occurrence of the electroencephalographic alpha rhythm with eyes open. Nature. 1965;206:746. doi: 10.1038/206746a0. [DOI] [PubMed] [Google Scholar]
- Nuñez A, Amzica F, Steriade M. Intrinsic and synaptically generated delta (1–4 Hz) rhythms in dorsal lateral geniculate neurons and their modulation by light-induced fast (30–70 Hz) events. Neuroscience. 1992;51:269–84. doi: 10.1016/0306-4522(92)90314-r. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–8. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Engel AK, König P, Singer W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature. 1997;385:157–61. doi: 10.1038/385157a0. [DOI] [PubMed] [Google Scholar]
- Rougeul-Buser A, Buser P. Rhythms in the alpha band in cats and their behavioural correlates. Int. J. Psychophysiol. 1997;26:191–203. doi: 10.1016/s0167-8760(97)00764-2. [DOI] [PubMed] [Google Scholar]
- Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem. Cell Biol. 2006;125:725–41. doi: 10.1007/s00418-005-0124-7. [DOI] [PubMed] [Google Scholar]
- Schürmann M, Demiralp T, Başar E, Başar Eroglu C. Electroencephalogram alpha (8–15 Hz) responses to visual stimuli in cat cortex, thalamus, and hippocampus: a distributed alpha network? Neurosci. Lett. 2000;292:175–178. doi: 10.1016/s0304-3940(00)01456-7. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–6. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Amzica F, Timofeev I. Synchronization of fast (30–40 Hz) spontaneous oscillations in intrathalamic and thalamocortical networks. J Neurosci. 1996;16(8):2788–808. doi: 10.1523/JNEUROSCI.16-08-02788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curró Dossi R, Nuñez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J. Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Curró Dossi R, Contreras D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (approximately 40 HZ) spike-bursts at approximately 1000 HZ during waking and rapid eye movement sleep. Neuroscience. 1993;56:1–9. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- Steriade M, Dossi RC, Paré D, Oakson G. Fast oscillations (20–40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4396–400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Glenn LL. Neocortical and caudate projections of intralaminar thalamic neurons and their synaptic excitation from midbrain reticular core. J. Neurophysiol. 1982;48:352–71. doi: 10.1152/jn.1982.48.2.352. [DOI] [PubMed] [Google Scholar]
- Surwillo WW. Frequency of the ‘Alpha’ Rhythm, Reaction Time and Age. Nature. 1961;191:823–824. [Google Scholar]
- Suzuki S, Rogawski MA. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7228–32. doi: 10.1073/pnas.86.18.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J. Neurosci. 1997;17:722–34. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996;16:4240–9. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Cunningham MO, Whittington MA. Persistent gamma oscillations in superficial layers of rat auditory neocortex: experiment and model. J. Physiol. 2005;562:3–8. doi: 10.1113/jphysiol.2004.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Buhl EH, Gloveli T, Whittington MA. Fast rhythmic bursting can be induced in layer 2/3 cortical neurons by enhancing persistent Na+ conductance or by blocking BK channels. J. Neurophysiol. 2003;89:909–21. doi: 10.1152/jn.00573.2002. [DOI] [PubMed] [Google Scholar]
- Turner JP, Salt TE. Synaptic activation of the group I metabotropic glutamate receptor mGlu1 on the thalamocortical neurons of the rat dorsal lateral geniculate nucleus in vitro. Neuroscience. 2000;100:493–505. doi: 10.1016/s0306-4522(00)00280-3. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Koch C. Is perception discrete or continuous? Trends Cogn. Sci. 2003;7:207–213. doi: 10.1016/s1364-6613(03)00095-0. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Reddy L, Koch C. The continuous wagon wheel illusion is associated with changes in electroencephalogram power at approximately 13 Hz. J. Neurosci. 2006;26:502–7. doi: 10.1523/JNEUROSCI.4654-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Monckton JE, Reiner PB, McCormick DA. Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus. Neuroscience. 1999;91:7–20. doi: 10.1016/s0306-4522(98)00557-0. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–5. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J. Neurosci. 2000;20:1307–17. doi: 10.1523/JNEUROSCI.20-04-01307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]