Abstract
T cell activation through the antigen receptor (TCR) requires sustained signalling from signalosomes within lipid rafts microdomains in the plasma membrane. In a proteomic analysis of lipid rafts from human T cells, we identified stomatin-like protein 2 (SLP-2) as a candidate molecule involved in T cell activation through the antigen receptor. Here, we show that SLP-2 expression in human primary lymphocytes is up-regulated following in vivo and ex vivo activation. In activated T cells, SLP-2 interacts with components of TCR signalosomes and with polymerized actin. More importantly, up-regulation of SLP-2 expression in human T cell lines and primary peripheral blood T cells increases effector responses while down-regulation of SLP- 2 expression correlates with loss of sustained TCR signalling and decreased T cell activation. Our data suggest that SLP-2 is an important player in T cell activation by ensuring sustained TCR signalling, which is required for full effector T cell differentiation, and point to SLP-2 as a potential target for immunomodulation.
Keywords: Human, T Cells, Cell Activation
Introduction
CD4+ T cell activation involves the formation of a highly organized interface between the T cell and the antigen-presenting cell (APC) known as the immunological synapse (IS) (1-4). This process requires differential polarization of surface and intracellular molecules to the IS proximal pole (5) or to the antipodal pole of the T cell (6, 7), in a cytoskeleton-dependent manner (8-10). It also affects cellular organelles as recently shown by two elegant reports of the translocation of mitochondria to the IS during T cell activation (11), and to the uropod during T cell locomotion (12). In addition, T cell polarization is highly dynamic as illustrated by the early detection of signalling TCR microclusters or signalosomes in the outer ring of the synapse (13) and their subsequent movement to the centre of the synapse where they are internalized and destined to degradation (2, 3, 14), thus ensuring signal down-regulation (15).
To identify molecules that act as a functional bridge between TCR signalosomes and the cytoskeleton and cellular organelles, we performed a proteomic analysis of lipid raft microdomains from human T cells undergoing activation through their TCR. With this approach, we isolated stomatin-like protein 2 (SLP-2) (also known as STOML2 or EPB72). This finding has been corroborated by other groups using lipid rafts and/or mitochondrial fractions from different cell lineages of several species (16, 17).
SLP-2 is a member of the highly conserved family of stomatin proteins whose homologs span from archae to humans and include stomatin, SLP-1, and SLP-3 (18-22). SLP-2 shares with the other stomatins a central SPFH (stomatin/prohibitin/flotillins/HflK-HflC) domain that may mediate interactions with cell membranes (23-25). However, SLP-2 is unique among stomatins in that it does not have a putative transmembrane domain, but has six myristoylation/palmitoylation sites and an N-terminal mitochondria targeting sequence (25, 26). These features likely determine its detection in mitochondria and plasma membrane.
The function of stomatins, including SLP-2, is currently unknown. It has been suggested that they may be involved in the organization of the peripheral cytoskeleton, and in the assembly of multi-chain receptors such as ion channels (25, 27-30) and mechanosensation receptors (31-37). In this context, one would expect that SLP-2 be involved in the regulation of signalling from these receptors. Here, we report that SLP-2 plays an important role in human T cell activation by contributing to sustain TCR signalling. This effect correlates with the interaction of SLP-2 with TCR signalosome components, and with polymerized actin. More important, modulation of SLP-2 levels translate into changes in effector T cell responses, suggesting that SLP-2 may be a useful immunotherapeutic target.
Material and methods
Cells
Human peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood of normal donors using Ficoll-Hypaque gradients (Amersham Pharmacia Biotech, Uppsala, Sweden). Cells were washed in supplemented RPMI 1640 and resuspended at 1 x 106 cells/ml. PBMC blasts were generated by culturing PBMC with Phorbol Myristate Acetate (PMA; 1 ng/ml) and Ionomycin (100 ng/ml) for 72 hours at 37°C, 5 % CO2. T cells blasts were rested 48 hours before use. Primary T cells were isolated from PBMC using a Pan T cell Isolation Kit (Miltenyi Biotech, Auburn, CA). Jurkat T cells (E6.1) were obtained from American Type Culture Collection (Manassas, VA) and cultured in supplemented RPMI 1640 medium. The B lymphoblastoid cell line LG2, used as APC in some of these experiments, was kindly provided by Dr. Eric Long (NIAID, NIH, Rockville, MD) and cultured in standard supplemented RPMI 1640 media.
Plasmids, siRNA and T cell transfectants
Human SLP-2 cDNA was subcloned into the pEGFPN1 expression vector (Clontech Inc. Palo Alto, CA) to create an in-frame translational fusion of SLP-2 and gfp at the 3′ end. Subsequently, the SLP-2-gfp was placed into the doxycyclineinducible pBig2i vector (38). Stable transfectants were generated by electroporating linearized plasmid into Jurkat E6.1 T cells and screened for stable expression. Doxycycline (Sigma, St. Louis, MO) was added in culture overnight at 1μg/ml to induce SLP-2-gfp expression. Expression of SLP-2-gfp was monitored by direct flow cytometry (Becton Dickinson, CA). RNA interference targeting SLP-2 was performed using siRNAs obtained from Ambion (Austin, TX) (cat # 20643 and 20467) and nucleofected into Jurkat T cells, PBMC or PBMC blasts using either the Nucleofector kit for cell lines or for human primary T cells (Amaxa, Gaithersberg, MD). As controls for SLP-2 siRNA, we used scrambled siRNAs provided by the commercial supplier (Ambion). These control siRNAs have no significant similarity to any known gene sequences from mouse, rat, or human and no toxicity to cells, have been shown to lack any significant effect in cell proliferation and apoptosis assays, and do not modulate the mRNA levels of “housekeeping” genes (18S rRNA, GAPDH, and cyclophilin) up to 48 hr after transfection. siRNAs that target different exons of SLP-2 were also mixed in order to establish if better knock-down of SLP-2 could be achieved. Through this process, it was established that combining the two siRNAs (cat # 20643 and 20467) produced the highest level of knockdown. GFP cDNA in the targeting vector was used as a control for nucleofection efficiency. After siRNA or control nucleofection, T cells were rested for 24 hours until optimum down-regulation of SLP-2 was observed before proceeding with any functional assays.
Antibodies
An antiserum against human SLP-2, generated by immunization of rabbits with a peptide spanning amino acids 343 to 356 (ProSci Inc., Poway, CA), was used for immunoprecipitation of SLP-2. A pre-immunization serum was used as control for rabbit antisera immunoprecipitations (ProSci Inc.). Commercially available antibodies against SLP-2 were purchased from Protein Tech Group (Chicago, IL). Antisera against Lck, LAT, and phospho-LAT were obtained from Upstate Biotechnology (Lake Placid, NY). Anti-ZAP-70 and anti-phospho-ZAP-70 (Y292) antisera, and anti-Ras-GAP antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoblotting for ERK-1/2 was done using a rabbit polyclonal immunoaffinity purified antiserum (Stressgen Biotechnologies, Victoria, BC, Canada). Anti-PLC-γ1, anti-phospho-PLC-γ1, anti-active ERK-1 and ERK-2 E10, and anticaspase 3 antisera were obtained from Cell Signalling Technology (Beverly, MA). CD45 was immunoblotted using a mouse monoclonal antibody (clone 69) (BD Biosciences, Mississauga, ON, CA). Actin was immunoblotted using an affinity-purified goat polyclonal antiserum (Santa Cruz Biotechnology Inc.), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was immunoblotted with a mouse monoclonal antibody from Chemicon International (Temecula CA). To ensure that the Western Blot signals were within the linear range of detection, we performed signal titration over multiple exposures over a range of time periods from 30 seconds to 30 minutes. Signal saturation was detected by image acquisition software (Alpha Innotech). Densitometry and molecular weight calibration were done with the Phoretix 1D Database package.
Lipid raft isolation
Jurkat T cells or blast PBMC were stimulated with staphylococcal enterotoxin E superantigen (SEE) and LG-2 cells as antigen-presenting cells (APC) (at a 5:1 ratio; 60×106 T cells with 12×106 APCs). Whole cell lysates were prepared with 0.5% Triton-X-100, and lipid rafts isolated by sucrose gradient ultracentrifugation as described (39). Lipid rafts from the same cell equivalents per sample were pelleted by centrifugation of the 1mL raft fraction for 1 hour at 14,000 rpm and 4°C, and were resuspended in lysis buffer and sample buffer for biochemical analysis.
Cell lysate preparation
Jurkat T cells or PBMC were stimulated with superantigens at final concentration of 1 μg/ml, and APC at a 5:1 ratio, at 37°C, for 1, 5, 15, 30 and 60 min (40, 41). Cells were pelleted in PBS containing sodium o-vanadate (400 μM) and EDTA (400 μM), and lysed in lysis buffer (1% Triton X-100, 150mM NaCl, 10mM Tris (pH7.6), 5mM EDTA, 1mM sodium o-vanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 25 μM p-nitrophenyl-p'- guanidinobenzoate) at 4°C for 30min. Lysates were cleared of debris (14,000rpm, 4°C, 10min), and used for immunoprecipitation of target molecules using Ab-coated protein A or G agarose beads. Whole cell lysates from human tissues were obtained from ProSci Inc. (Poway, CA).
Cross-linked immunoprecipitation
Protein A agarose beads were coated with appropriate Abs at 4°C overnight. Beads were washed four times with room-temperature lysis buffer and four times with PBS. Immunoprecipitating antibodies were cross-linked to the beads in 1mg/ml of DSP (Pierce) in PBS, at room temperature rotating for 30mins. The cross-linked antibody-beads were neutralized with 1M Tris, pH8.0 at room-temperature for 5mins, washed once with lysis buffer and four times with PBS, and then used for immunoprecipitation. After immunoprecipitation, beads were pelleted and resuspended in sample buffer without β-Mercaptoethanol. Samples were boiled, pelleted, and supernatant collected, run in SDS-PAGE, and immunoblotted with the indicated Abs.
Tonsil B cells
Highly purified human tonsil B cells by RosetteSep B cell enrichment cocktail (StemCell Technologies,Vancouver, BC, Canada) were fractionated by a seven step Percoll gradient. With this procedure, four fractions were obtained (labeled f1 to f4). All fractions were phenotyped for naïve B cells (based on expression of IgM and IgD) and memory B cells (CD20bright and CD27+). The proportion of naïve resting B cells increases from fraction 1 to 4 (from 11% to 66% on average) while the proportion of memory/activated B cells decreases from fraction 1 to 4 (from 30% to 3% on average). In addition, each fraction was cultured in vitro to determine spontaneous Ig production. Only fractions 1 and 2 were able to spontaneously secrete Ig (data not shown). Whole cell lysates from B cells in each fraction were prepared and immunoblotted with appropriate specific antibody.
T cell functional assays
SLP-2-gfp, SLP-2 siRNA or control transfected T cells (0.2×106 cells/group) were plated in triplicate on 96 well plates with the B lymphoblast LG2 (0.1×106 cells/group) as APC and SEE in the presence or absence of 1000 ng/mL doxycycline, at 37°C for 24 hours. Supernatants were collected, and measurement of IL-2 by ELISA was performed following manufacturer specifications (BD Biosciences, Mississauga ON, CA). Results are presented as mean ± standard deviation and statistical significance was determined by two-tailed t tests for each given time point.
Histological analysis
Fragments of thymi and lymph nodes were fixed with 10% formalin, embedded in paraffin, and stained with Hematoxylin and Eosin. Immunohistochemical analysis for SLP-2 was performed with an antisera against SLP-2 at 1/1000 dilution following the streptavidin-biotin peroxidase method. CD5 and CD20 (monoclonal, mouse anti-human antibodies; Dako) stains were also performed to identify T and B lymphocytes respectively, and correlated with SLP-2 staining.
Results
Expression of SLP-2 in human lymphoid tissues
To explore the involvement of SLP-2 in lymphocyte activation, we first determined its expression in human lymphoid tissues using whole cell lysates from commercial sources. We detected SLP-2 mostly in lymph node and thymus lysates, and in lower amount, in tonsil lysates (figure 1A). Little expression was detected in lysates from unfractionated spleen and resting peripheral blood leukocytes. Such differences were not due to differences in protein loading as illustrated by immunoblotting of the same samples for GAPDH.
Figure 1. Expression of SLP-2 in human lymphoid tissues.
A) Whole cell lysates from the indicated organs were sequentially immunoblotted for SLP-2 and GAPDH. Equal loading of protein per lane was further confirmed by spectroscopy. Results are representative of four independent experiments. B) Lymph node samples from human non-specific B cell adenitis (top row) or T cell adenitis (middle row), and normal human thymus (bottom rows) were stained for SLP-2 using a C-terminus specific rabbit anti-human SLP-2 antisera or with appropriate controls (CD20bright for B cells, TdT for developing thymocytes), and biotinylated horse anti-rabbit secondary antibody and avidin (Vector Labs, Burlingame CA). High levels of SLP-2 expression were detected in the germinal centres (B cell area) of lymph nodes of non-specific B cell adenitis, in the paracortical (T cell) area of non-specific T cell adenitis, and in the cortex of the thymus. Much lower expression of SLP-2 was seen in cells outside these areas. The profile of intracellular localization of SLP-2 was predominantly associated to the periphery of the cell and some aggregates in the central area of the cytoplasm. C) Peripheral blood cells from a normal volunteer in whom expression of SLP-2 could be detected in resting peripheral blood mononuclear cells (PBMC) were used to fractionate these cells into T cells (T), B cells (B), and monocytes (M). Whole cell lysates from these subsets were prepared and sequentially immunoblotted for SLP-2 and GAPDH. Densitometric readings for SLP-2 normalized for GAPDH are shown.
In lymph nodes, SLP-2 was mostly detected in the germinal centres (B cell area) and in the paracortical (T cell) area (figure 1B), with a staining pattern of intracellular punctae and peripheral layer consistent with its targeting to mitochondria and plasma membrane association. In the thymus, SLP-2 was detected both in the cortex and in the medulla although the expression seemed to be higher in the cortex (figure 1B). In those individuals in which SLP-2 was detectable in peripheral blood mononuclear cells, it was expressed by monocytes, and to less extent, by T and B lymphocytes (figure 1C).
Up-regulation of SLP-2 expression in T and B lymphocytes upon activation
The high expression of SLP-2 in sites where lymphocyte signalling and activation take place (i.e., antigen activation in lymph nodes and tonsils, positive and negative selection in the thymus) prompted us to examine the effect of activation on SLP-2 expression. Although, in most volunteers tested, peripheral blood T cells express low levels or no SLP-2 under resting conditions, activation of these cells with bacterial superantigens led to a consistent and significant up-regulation of its expression after 36 hrs (figure 2A). Such an up-regulation was already detectable after 2 hours of stimulation through the TCR as observed when cross-linked antibodies against CD3 were used to activate T cells (data not shown). Similarly, in B cell preparations from human tonsils fractionated according to their activation status, SLP-2 was mostly detected in the fraction corresponding to activated/memory (CD20bright, CD27+) B cells (figure 2B). Seventy three percent of cells in this fraction (f1) were activated/memory B cells (as indicated by expression of CD27), and only 8% of cells in this fraction were naïve B cells. In contrast, 60% of the cells in fraction 4 were naïve B cells and only 11% were activated/memory B cells. Fractions 2 and 3 had progressively decreasing numbers of activated/memory B cells (52% and 32%, respectively) and increasing numbers of naïve B cells (17% and 45% respectively). Together, these data led us to conclude that SLP-2 expression is up-regulated by in vivo and ex vivo lymphocyte activation.
Figure 2. Up-regulation of SLP-2 expression in primary human T cells and B cells following activation.
A) Human peripheral blood T cells from two different volunteers were stimulated with syngeneic APC and SEE superantigen for 36 hours. Cell lysates were prepared and sequentially immunoblotted for SLP-2 and actin (as a loading control). Results are representative of three independent experiments. B) Human tonsil cells were prepared and fractionated through Percoll gradient. Whole cell lysates from the four fractions were immunoblotted for SLP-2 and actin. B cell markers for naïve and activated/memory B cells were used to determine the percentage of naïve and activated memory B cells in each fraction (shown in the table). Results are representative of two independent experiments.
SLP-2 interacts with components of TCR signalosomes and with polymerized actin during T cell activation
To study the contribution of SLP-2 to T cell activation, and since we had isolated SLP-2 from lipid rafts of activated T cells, we first analyzed the partitioning of SLP-2 within these microdomains following activation. To do this, we used Jurkat T cells that express SLP-2 under resting conditions. As shown in figure 3A, SLP-2 was detected in lipid rafts and its partitioning to these microdomains increased soon after activation, subsequently returning to basal levels. Such a profile of redistribution into lipid rafts was consistently documented in multiple experiments (figure 3B), and in primary human T cells at discrete time points (figure 3C). We failed to see a reciprocal change in the levels of SLP-2 in the soluble fractions in comparison to the increase in SLP-2 levels in the lipid raft fractions, either because the SLP-2 that redistributes into rafts came from the insoluble cytoskeletal pellet or because the amount SLP-2 that repartitions to the lipid raft fraction from the soluble fraction is small in comparison to the total amount of soluble SLP-2.
Figure 3. Increased translocation of SLP-2 into lipid rafts with TCR-dependent, T cell activation.
A) E6.1 Jurkat T cells were activated with APC and SEE superantigen for the indicated times. Membrane lipid rafts and the detergent-soluble fraction were isolated by sucrose gradient centrifugation and immunoblotted for SLP-2, ERK-1/-2 (as control for the quality of the detergent-soluble fraction), and GM-1 (as a control for the quality of lipid rafts). B) The SLP-2 signal (mean ± s.d.) for lipid raft fractions from T cells activated for the indicated times in three different experiments was quantified by densitometry. C) Lipid rafts from blast PBMC activated with APC and SEE superantigen for 5 and 15 minutes were used to prepare membrane lipid rafts and detergent-soluble fraction by sucrose gradient centrifugation, and immunoblotted for SLP-2, ERK-1/-2 (as control for the quality of the detergent-soluble fraction), and GM-1 (as a control for the quality of lipid rafts).
Next, we tested if SLP-2 interacted with the components of TCR signalosomes during activation for up to 60 minutes, a time window that covers the formation of TCR signalling microclusters and mature IS (figure 4A). In complementary co-immunoprecipitation studies, we observed that SLP-2 steadily associated with the CD3-ε chain of the TCR complex under resting conditions and during the 60 minutes of stimulation. Detailed quantitation studies of this association indicated that about 0.09% of the cellular SLP-2 associated with the TCR complex (data not shown). In these studies, we also found that SLP-2 interacted with Lck, ZAP-70, LAT and PLC-γ1 during the 30 minute period following stimulation in vitro. The SLP-2-associated pool of these molecules became phosphorylated/activated in a sequential manner, a profile compatible with their temporal involvement in early TCR signalling. sSuch profile of interactions was only observed in specific SLP-2 immunoprecipitations with cross-linked antibodies but not with non-specific control antibodies. Also, these interactions were preserved under stringent lysis conditions, and were selective because no association of SLP-2 with other surface receptors (CD45, CTLA-4) or with control intracellular molecules (Ras-GAP, caspase 3) was detected (figure 4A). In addition, such interactions were observed using a different detergent (octylglucoside - 2%), and in primary human T cells (data not shown). Since polarization of signalling molecules and organelles to the IS is cytoskeleton dependent, we examined the association of SLP-2 with actin. We found that SLP-2 interacted with actin under resting conditions and upon TCR stimulation (figure 4B). Such an association was not observed using a control pre-immune serum, ruling out a non-specific interaction, and mostly involved T cell actin given the T:APC ratio (5:1) used in these experiments. Furthermore, the association between SLP-2 and actin during T cell activation seems to involve mostly the polymerized form of actin because cytochalasin D, an inhibitor of actin polymerization, completely prevented this interaction (figure 4B). We observed a lower level of association between SLP-2 and actin at time 0 in T cells treated with cytochalasin D, likely reflecting a basal level of interaction between these molecules under steady state conditions. It is important to note that T cell activation in this experimental system is dependent on actin polymerization, because inhibition of actin polymerization with cytochalasin D led to inhibition of IL-2 production in a dose-dependent manner (figure 4C).
Figure 4. Association of SLP-2 with components of the TCR signalosome and actin cytoskeleton during T cell activation.
A) Jurkat T cells were stimulated with APC and SEE superantigen for the indicated times. Next, whole cell lysates were prepared and used for immunoprecipitation (ip) of SLP2 with cross-linked anti-SLP-2 antibodies. Pre-immune serum immunoprecipitation (lane ‘C’) was used as a negative control. The resulting ip were sequentially immunoblotted for CD3-ε, lck, phospho-ZAP-70, ZAP-70, phospho-LAT, LAT, phospho-PLC-γ1, PLC-γ1, CD45, Ras-GAP, caspase 3, and SLP-2. B) E6.1 Jurkat T cells pretreated with cytochalasin D (+CD) (10 μM) for 30 minutes were stimulated with APC and SEE for the indicated times. Whole cell lysates were prepared and used for immunoprecipitation of SLP-2 and sequentially immunoblotted for SLP-2 and actin. C) Inhibition of actin polymerization with cytochalasin down-regulated the IL-2 response of Jurkat T cells to APC and SEE stimulation. Supernatants from 24 hour cultures of T cells pre-treated with the indicated concentrations of cytochalasin D and stimulated with APC and different concentrations of SEE superantigen were used to measure IL-2 production by ELISA (mean ± s.d.). Results in this figure are representative of at least 4 separate experiments.
SLP-2 contributes to sustain T cell signalling
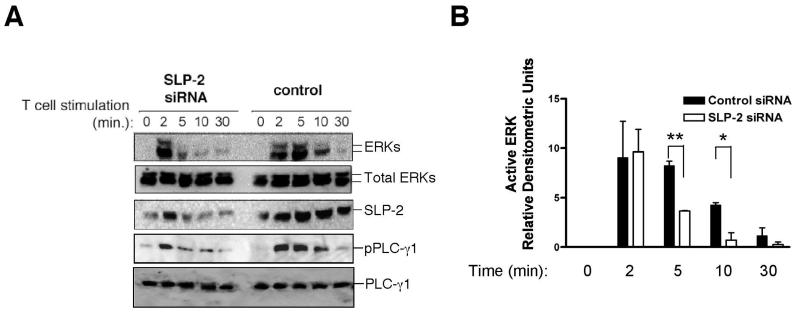
Next, we looked at the functional correlates of SLP-2 expression and its interactions with the components of the TCR signalosome. We reasoned that, if SLP-2 played a role in signalling from the TCR signalosomes, then knocking down SLP-2 would decrease TCR signalling. To test this hypothesis, we knocked down SLP-2 expression by RNA interference using two different sets of siRNAs. With this technique, we achieved between 65-85% reduction of SLP-2 levels. Under these conditions, we found that SLP-2 down-regulation correlated with a significantly shorter timeline of TCR signalling (Figure 5A). That is, at equal levels of early TCR signalling (as measured by similar levels of initial ERK-1/-2 activation), down-regulation of SLP-2 caused a remarkably shorter duration of ERK activation in response to TCR stimulation, already apparent at 5 minutes and still significant at 10 minutes of stimulation. The effect of SLP-2 down-regulation on TCR-dependent signaling was also observed for the kinetic profile of activation of PLC-γ1 (Figure 5A), and was statistically significant across multiple experiments looking at the profile of ERK activation in response to TCR signalling using Jurkat T cells and primary T cells (figure 5B).
Figure 5. Down-regulation of SLP-2 prevents sustained T cell signalling.
A) Jurkat T cells were nucleofected with two different sets of siRNA for SLP-2 or control siRNA and used for stimulation with APC and SEE for the indicated times. Cell lysates were prepared and immunoblotted for dually phosphorylated, active ERK-1/-2 (pERKs), total ERK-1/-2 (tERKs), phospho-PLC-γ1, total PLC-γ1, and SLP-2. B) Signals for activated ERK-1/-2 and total ERK were quantified for each group at the indicated time points for three independent experiments and plotted as normalized densitometric units (mean ± s.d.) for active ERK-1/-2. *: p<0.05, **: p<0.01.
Modulation of IL-2 responses by SLP-2
Since SLP-2 down-regulation prevented sustained signalling, and since sustained TCR signalling is required for full T cell activation, we predicted that changes in the levels of SLP-2 expression would translate in changes in an effector T-cell response such as IL-2 production. We tested this hypothesis in human T cell lines and primary T cells. To determine the effect of SLP-2 overexpression on IL-2 production, we transfected Jurkat T cells with a doxycycline-inducible SLP- 2-gfp cDNA. In these cells, SLP-2 expression is increased due to basal leakiness of the vector promoter. Such an expression can be further up-regulated with the addition of doxycycline to the culture, as demonstrated by FACS (Figure 6A). We found that de novo over-expression of SLP-2 (as corroborated by FACS) significantly increased IL-2 production of these T cells in response to SEE and APC, compared to the parental E6.1 T cell line expressing low levels of SLP-2 (figure 6A).
Figure 6. Changes in the level of SLP-2 expression in Jurkat T cells regulate IL-2 responses.
A) Jurkat T cells were transfected with a doxycycline-inducible SLP-2-gfp cDNA. Leakiness of the transfected cDNA was apparent as shown by FACS (WTSLP2gfp (-D)). Further over-expression of SLP-2 was induced by addition of doxycycline (1 μg/ml) to the culture for 18 hours (WTSLP2gfp (+D)). Non-transfected parental E6.1 Jurkat T cells (which express lower levels of SLP-2) were used as controls (Parental). Subsequently, these T cells were stimulated with APC and SEE superantigen for 24 hours. Cultures supernatants at that time were collected and used to measure IL-2 production by ELISA (mean ± s.d.). Over-expression of SLP-2-gfp was confirmed by FACS. B) Down-regulation of SLP-2 levels in Jurkat T cells was achieved by RNA interference as detailed in the Material and Methods section. The response of these T cells to APC and SEE was measured by IL-2 production. Down-regulation of SLP-2 expression after siRNA was confirmed by Western blotting.
To test whether knocking-down the expression of SLP-2 decreased IL-2 responses, we nucleofected Jurkat T cells with three different SLP-2 siRNA constructs. Down-regulation of SLP-2 upon RNA interference was documented by Western blotting as shown in figure 6B. On average, with a reduction of SLP-2 levels between 70% and 80%, we observed that the IL-2 response to APC and SEE significantly decreased compared to the parental SLP-2 expressing Jurkat T cell line (figure 6B).
Finally, we corroborated the effect of SLP-2 expression on IL-2 responses in primary human T cells from normal volunteers. Human naïve T cells express low levels of SLP-2 but the levels are up-regulated significantly upon activation. Thus, to induce high expression of SLP-2 in primary human T lymphocytes, we pre-activated these cells with a mitogenic combination of PMA and ionomycin for 3 days, followed by a resting period. At this point, these effector T cells and their naïve T cell counterparts from the same donor were nucleofected with SLP-2 siRNA or control siRNA, re-stimulated with SEE and APC for 24 hours, and their IL-2 response examined. As shown in figure 7A, knocking-down the expression of SLP-2 by more than 60% correlated with a significant decrease in the IL-2 response of the effector T cells (around 10-100 times) as illustrated by the ‘shift to the right’ of the dose-response curve to SEE (p<0.001). Little effect was observed for SLP-2 siRNA in resting T cells in which SLP-2 expression is almost absent (figure 7A), ruling out an off-target (non-SLP-2-dependent) effect of the SLP-2 siRNAs. SLP-2 siRNA had no effect on the IL-2 response of human T cells to mitogenic stimulation with PMA and ionomycin, which bypasses early TCR signalling events (figure 7B). Together, the findings from Jurkat T cells and from primary T cells demonstrate that T cell activation can be modulated by changing the levels of SLP-2. Furthermore, we confirmed in primary human T cells that the up-regulation of SLP-2 expression correlated with a more sustained profile of TCR-dependent ERK activation (figure 7C), with stronger phospho-ERK signal still detectable by 30 minutes post-activation, complementing the results shown in figure 5 of less sustained ERK activation in Jurkat T cells when SLP-2 was down-regulated by siRNA.
Figure 7. Modulation of human peripheral blood T cell responses by SLP-2.
A) Peripheral blood lymphocytes from a normal volunteer were isolated and used as resting cells, or after three days of ex vivo activation with PMA and ionomycin and 48hr of resting. Cells were nucleofected with SLP-2 siRNA or gfp control and used 24 hours later for stimulation with autologous APC and SEE. IL-2 production after 24 hours was assessed by ELISA (mean ± s.d.). Additional control of cells nucleofected without any DNA was used to rule out non-specific effect of nucleofection. Inlet figure shows Western blot for SLP-2 to confirm knockdown of SLP-2, and actin as a loading control in the two groups tested. Similar results were obtained in 4 separate experiments. ***: p<0.001. B) Down-regulation of SLP-2 does not affect the IL-2 response to mitogenic stimulation with PMA and ionomycin. Peripheral blood lymphocytes from the experiment shown in figure 7A were used after three days of ex vivo activation and 48hr of resting. Cells were nucleofected with SLP-2 siRNA and used 24 hours later for stimulation with PMA and ionomycin. IL-2 production after 24 hours was assessed by ELISA (mean ± s.d.). Cells nucleofected without any DNA or with non-sense siRNA were used as controls. The effect of SLP-2 siRNA on SLP-2 levels are shown in the right inlet panel of figure 7A. C) Resting and blast human peripheral blood T cells were stimulated with APC and SEE for the indicated times. Cell lysates were prepared and immunoblotted for dually phosphorylated, active ERK-1/-2 (pERKs), total ERK-1/-2 (tERKs), and SLP-2.
Discussion
The results presented in this paper show that SLP-2 expression in T and B lymphocytes is upregulated by activation in vivo and ex vivo. Upon expression in T cells, SLP-2 interacts with molecules involved in early TCR signalling and with polymerized actin, and contributes to sustain TCR-dependent signalling. Consistent with these findings, one can modulate effector T cell responses such as IL-2 production by changing the levels of SLP-2: over-expression of this protein increases IL-2 responses whereas down-regulation of its expression correlates with decreased IL-2 responses, both in T cell lines as well as in primary human T cells. To our knowledge, these results provide the first biological evidence of a function for SLP-2 in an eukaryotic cell type - that of sustaining TCR signalling and enhancing T cell activation. The findings of SLP-2 partitioning in lipid rafts, its interactions with TCR signalosome components and polymerized actin, and its contribution to sustain TCR-dependent signalling are consistent with the emerging role assigned to proteins containing an SPFH domain. This domain has been linked to protein interactions with cell membranes (23-25). In addition, proteins containing such a domain are enriched in lipid rafts, and have been previously implicated in the regulation of cell signalling (42, 43). For example, prohibitin is indispensable for the activation of the Ras-ERK signalling pathway (44), and a similar role has been proposed for the flotillins (45-47). Together this evidence positions SLP-2 in the proper subcellular compartment to participate in TCR-dependent signaling.
The structural basis of the molecular interactions identified for SLP-2 is currently under study. The primary sequence of SLP-2 predicts that there are at least two cellular pools of this protein: one in mitochondria (25) and the other associated with the plasma membrane (16, 48-50). Preliminary data from our laboratory confirm these two pools and suggest that both pools of SLP-2 coalesce in the peripheral area of the IS, a region where signalling TCR microclusters accumulate (14) (MGK, CDL and JM, preliminary observations). Such an observation is also consistent with the recent report of mitochondrial polarization to the IS during T cell activation (11). The primary sequence of SLP-2 does not stand out with an enzymatic domain or with a conventional protein-protein interaction domain that could explain its direct interactions with signaling molecules. It is therefore plausible to assume that the interactions of SLP-2 with TCR signalosomes and with polymerized actin involve indirect associations between SLP-2 and other molecules, with SLP-2 potentially acting as a scaffolding/assembling protein.
How can SLP-2 contribute to sustain TCR-dependent signalling? We propose two, nonmutually exclusive ways by which SLP-2 can do that. One way is that SLP-2 contributes to the assembly of the multimolecular signalosomes on the transmembrane adapter LAT. Such a facilitating role in signalosome assembly would “stabilize” signalosomes for sufficient time to deliver sustained signaling. This possible mechanism is supported by the observed sequential interactions between SLP-2 and signalling molecules and polymerized actin, and by the prevention of sustained TCR signalling seen with down-regulation of SLP-2 expression. This possibility is also consistent with the formation of TCR signalosomes within lipid rafts (51-53), where SLP-2 is enriched, and with the recent claim that SPFH family members bind cholesterol, and contribute to the formation of signalling-permissive protein-lipid complexes (30, 54). Another way to explain how SLP-2 contributes to sustain TCR-dependent signalling is by claiming that SLP2 modulates mitochondrial function, which is in itself linked to TCR signalling as indicated by the polarization of mitochondria to the IS. The specific role that SLP-2 plays in mitochondrial function is unknown but may be linked to the assembly of the electron transport supercomplexes in lipid rafts of the inner mitochondrial membrane in a way analogous to what we propose for the SLP-2 pool in the plasma membrane. According to this hypothesis, polymerization of actin in response to TCR signalling would bring TCR signalosomes and mitochondria to the IS. In this way, the energetic requirements of TCR signalling can be met because it warrants close proximity between the TCR signalosomes and the compartmentalized mitochondria. This possibility is in line with the changes in mitochondria positioning during cell responses through fission and fusion, processes that make use of the cytoskeleton and/or microtubules (12, 55). We cannot rule out that SLP-2 may scaffold mitochondria with the cytoskeleton through its interaction with mitochondrial external membrane proteins (56) such mitofusin-2 (although we have so far failed to document SLP-2-mitofusin-2 association in T cells). This proposed role for SLP-2 in T cell activation points to a novel regulatory mechanism of signal transduction based on cytoskeleton-dependent interaction between signalosomes and cellular organelles (9), a mechanism that may be applicable to other cell surface receptors in addition to the TCR (preliminary observations).
We have shown that, following T cell activation, the levels of SLP-2 increase, and this translates into enhanced effector responses (e.g., IL-2 production). In contrast, decreasing SLP-2 expression decreases IL-2 responses. Such observations have, in our opinion, several important biological implications. One is that it may partially explain the long-standing finding that, at equal requirement for sustained signalling, primed T cells show much stronger signal transduction and functional responsiveness than naïve T cells. One may argue that this is, in part, due to primed T cells having higher levels of SLP-2 than naïve T cells (57, 58). Another implication is that the increased effector phenotype seen with neoplastic transformation may be due, in some cases, to higher SLP-2 expression (59-62). Finally, the ability to modulate effector T cell responses by regulating SLP-2 expression identifies SLP-2 as a potentially useful target for immunotherapy. This could take the form of down-regulation of SLP-2 expression to decrease T cell reactivity (e.g., in the course of autoimmune disease) or alternatively, enhancement of SLP-2 expression to increase T cell responsiveness (e.g., vaccine development).
Acknowledgments
We thank Drs. E. H. Ball, E. Cairns, M. Chalfie, T. L. Delovitch, H. M. McBride, B. Singh, and the members of the Madrenas laboratory for many insightful discussions.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Kidney Foundation of Canada (Allison Knudsen Research Award), and the Multi-Organ Transplant Program of the London Health Sciences Centre (to J.M.), NIH grant R01-AI43549 (to M.L.D.), NIH training grant T32-GM07308 (to S.V.), and FONACIT S12002000575 (to I.B.). M.G.K. is the recipient of a CIHR MD/PhD studentship, and J.M. holds a Canada Research Chair in Immunobiology.
References
- 1.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–314. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 3.Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK, Shaw AS. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 4.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 7.Arp J, Kirchhof MG, Baroja ML, Nazarian SH, Chau TA, Strathdee CA, Ball EH, Madrenas J. Regulation of T-cell activation by phosphodiesterase 4B2 requires its dynamic redistribution during immunological synapse formation. Mol CellBiol. 2003;23:8042–8057. doi: 10.1128/MCB.23.22.8042-8057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers W, Farris D, Mishra S. Merging complexes: properties of membrane raft assembly during lymphocyte signaling. Trends Immunol. 2005;26:97–103. doi: 10.1016/j.it.2004.11.016. Kirchhof et al.: SLP-2 and T cell activation 22. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Burkhardt JK. T-cell-receptor-dependent actin regulatory mechanisms. J Cell Sci. 2007;120:723–730. doi: 10.1242/jcs.000786. [DOI] [PubMed] [Google Scholar]
- 10.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 11.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gascoigne NR, Zal T. Molecular interactions at the T cell-antigenpresenting cell interface. Curr Opin Immunol. 2004;16:114–119. doi: 10.1016/j.coi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptorproximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 16.Sprenger RR, Speijer D, Back JW, De Koster CG, Pannekoek H, Horrevoets AJ. Comparative proteomics of human endothelial cell caveolae and rafts using two-dimensional gel electrophoresis and mass spectrometry. Electrophoresis. 2004;25:156–172. doi: 10.1002/elps.200305675. [DOI] [PubMed] [Google Scholar]
- 17.Dowling P, Meleady P, Dowd A, Henry M, Glynn S, Clynes M. Proteomic analysis of isolated membrane fractions from superinvasive cancer cells. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbapap.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Morrow JS. Identification and characterization of human SLP-2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J Biol Chem. 2000;275:8062–8071. doi: 10.1074/jbc.275.11.8062. [DOI] [PubMed] [Google Scholar]
- 19.Owczarek CM, Treutlein HR, Portbury KJ, Gulluyan LM, Kola I, Hertzog PJ. A novel member of the STOMATIN/EPB72/mec-2 family, stomatin-like 2 (STOML2), is ubiquitously expressed and localizes to HSA chromosome 9p13.1. Cytogenet Cell Genet. 2001;92:196–203. doi: 10.1159/000056902. [DOI] [PubMed] [Google Scholar]
- 20.Seidel G, Prohaska R. Molecular cloning of hSLP-1, a novel human brainspecific member of the band 7/MEC-2 family similar to Caenorhabditis elegans UNC-24. Gene. 1998;225:23–29. doi: 10.1016/s0378-1119(98)00532-0. [DOI] [PubMed] [Google Scholar]
- 21.Hiller NL, Akompong T, Morrow JS, Holder AA, Haldar K. Identification of a stomatin orthologue in vacuoles induced in human erythrocytes by malaria parasites. A role for microbial raft proteins in apicomplexan vacuole biogenesis. J Biol Chem. 2003;278:48413–48421. doi: 10.1074/jbc.M307266200. [DOI] [PubMed] [Google Scholar]
- 22.Green JB, Fricke B, Chetty MC, von During M, Preston GF, Stewart GW. Eukaryotic and prokaryotic stomatins: the proteolytic link. Blood Cells Mol Dis. 2004;32:411–422. doi: 10.1016/j.bcmd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24:425–427. doi: 10.1016/s0968-0004(99)01467-x. [DOI] [PubMed] [Google Scholar]
- 24.Morrow IC, Parton RG. Flotillins and the PHB Domain Protein Family: Rafts, Worms and Anaesthetics. Traffic. 2005;6:725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 25.Hajek P, Chomyn A, Attardi G. Identification of a novel mitochondrial complex containing mitofusin 2 and stomatin-like protein 2. J Biol Chem. 2007;282:5670–5681. doi: 10.1074/jbc.M608168200. [DOI] [PubMed] [Google Scholar]
- 26.Salzer U, Prohaska R. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood. 2001;97:1141–1143. doi: 10.1182/blood.v97.4.1141. [DOI] [PubMed] [Google Scholar]
- 27.Stewart GW, Hepworth-Jones BE, Keen JN, Dash BC, Argent AC, Casimir CM. Isolation of cDNA coding for an ubiquitous membrane protein deficient in high Na+, low K+ stomatocytic erythrocytes. Blood. 1992;79:1593–1601. [PubMed] [Google Scholar]
- 28.Stewart GW, Argent AC, Dash BC. Stomatin: a putative cation transport regulator in the red cell membrane. Biochim Biophys Acta. 1993;1225:15–25. doi: 10.1016/0925-4439(93)90116-i. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher PG, Forget BG. Structure, organization, and expression of the human band 7.2b gene, a candidate gene for hereditary hydrocytosis. J Biol Chem. 1995;270:26358–26363. doi: 10.1074/jbc.270.44.26358. [DOI] [PubMed] [Google Scholar]
- 30.Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstadt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Inaugural Article: Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 32.Rajaram S, Sedensky MM, Morgan PG. Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:8761–8766. doi: 10.1073/pnas.95.15.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, R OH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touchcell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- 34.Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Ma C, Delohery T, Nasipak B, Foat BC, Bounoutas A, Bussemaker HJ, Kim SK, Chalfie M. Identification of genes expressed in C. elegans touch receptor neurons. Nature. 2002;418:331–335. doi: 10.1038/nature00891. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr Biol. 2004;14:1888–1896. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2006 doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- 38.Baroja ML, Luxenberg D, Chau T, Ling V, Strathdee CA, Carreno BM, Madrenas J. The inhibitory function of CTLA-4 does not require its tyrosine phosphorylation. J Immunol. 2000;164:49–55. doi: 10.4049/jimmunol.164.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Darlington PJ, Baroja ML, Chau TA, Siu E, Ling V, Carreno BM, Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shan X, Balakir R, Criado G, Wood JS, Seminario MC, Madrenas J, Wange RL. Zap-70-independent Ca(2+) mobilization and Erk activation in Jurkat T cells in response to T-cell antigen receptor ligation. Mol Cell Biol. 2001;21:7137–7149. doi: 10.1128/MCB.21.21.7137-7149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, Tsoukas CD, McCormick JK, Madrenas J. Bacterial superantigens bypass Lckdependent T cell receptor signaling by activating a Galpha11-dependent, PLC-betamediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Rajendran L, Masilamani M, Solomon S, Tikkanen R, Stuermer CA, Plattner H, Illges H. Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc Natl Acad Sci U S A. 2003;100:8241–8246. doi: 10.1073/pnas.1331629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem. 2005;280:16125–16134. doi: 10.1074/jbc.M500940200. [DOI] [PubMed] [Google Scholar]
- 44.Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 45.Slaughter N, Laux I, Tu X, Whitelegge J, Zhu X, Effros R, Bickel P, Nel A. The flotillins are integral membrane proteins in lipid rafts that contain TCRassociated signaling components: implications for T-cell activation. Clin Immunol. 2003;108:138–151. doi: 10.1016/s1521-6616(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 46.Stuermer CA, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. Faseb J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 47.Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005 doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Da Cruz S, Xenarios I, Langridge J, Vilbois F, Parone PA, Martinou JC. Proteomic analysis of the mouse liver mitochondrial inner membrane. J Biol Chem. 2003;278:41566–41571. doi: 10.1074/jbc.M304940200. [DOI] [PubMed] [Google Scholar]
- 49.Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt S, Prokisch H, Schlunck T, Camp DG, 2nd, Ahting U, Waizenegger T, Scharfe C, Meitinger T, Imhof A, Neupert W, Oefner PJ, Rapaport D. Proteome analysis of mitochondrial outer membrane from Neurospora crassa. Proteomics. 2006;6:72–80. doi: 10.1002/pmic.200402084. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 52.Sommers CL, Samelson LE, Love PE. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays. 2004;26:61–67. doi: 10.1002/bies.10384. [DOI] [PubMed] [Google Scholar]
- 53.Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 54.Browman DT, Hoegg MB, Robbins SM. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 55.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 56.Hajek P, Chomyn A, Attardi G. Identification of a novel mitochondrial complex containing mitofusin 2 and stomatin-like protein 2. J Biol Chem. 2006 doi: 10.1074/jbc.M608168200. [DOI] [PubMed] [Google Scholar]
- 57.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/s0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 58.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 59.Cao W, Zhang B, Liu Y, Li H, Zhang S, Fu L, Niu Y, Ning L, Cao X, Liu Z, Sun B. High-level SLP-2 expression and HER-2/neu protein expression are associated with decreased breast cancer patient survival. Am J Clin Pathol. 2007;128:430–436. doi: 10.1309/C6X54HRB580EP2NQ. [DOI] [PubMed] [Google Scholar]
- 60.Cui Z, Zhang L, Hua Z, Cao W, Feng W, Liu Z. Stomatin-like protein 2 is overexpressed and related to cell growth in human endometrial adenocarcinoma. Oncol Rep. 2007;17:829–833. [PubMed] [Google Scholar]
- 61.Cao WF, Zhang LY, Liu MB, Tang PZ, Liu ZH, Sun BC. Prognostic significance of stomatin-like protein 2 overexpression in laryngeal squamous cell carcinoma: clinical, histologic, and immunohistochemistry analyses with tissue microarray. Hum Pathol. 2007;38:747–752. doi: 10.1016/j.humpath.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhang L, Ding F, Cao W, Liu Z, Liu W, Yu Z, Wu Y, Li W, Li Y. Stomatin-like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:1639–1646. doi: 10.1158/1078-0432.CCR-05-1858. [DOI] [PubMed] [Google Scholar]