Abstract
Dendritic cell migration from the airway to lymph nodes is a key event in the development of airway immunity during infection, allergy, and vaccination. To identify the best approaches to investigate DC migration to lung-draining lymph nodes, we directly compared three methods previously used to track DC migration: airway administration of fluorescent OVA, latex beads, or carboxyfluorescein succinimidyl ester (CFSE). We show that two of the methods employed in optimal conditions—administration of fluorescent OVA or latex particles—have broadly relevant utility in studies of pulmonary DC migration, both in the presence and absence of inflammatory mediators. However, CFSE was of limited value because it induced a robust airway inflammatory response upon instillation. Unexpectedly, antigen-loaded tracers with distinct physical properties differently affected the populations that acquired the tracers and the overall T cell response. Specifically, soluble OVA and OVA formulated as a particulate after conjugation to latex beads were acquired in different proportions in vivo by the two characterized subsets of pulmonary DCs: CD11bhiCD103− and CD11bloCD103+langerin+ DC populations. Consequently, and in line with recent studies that these two subsets of DCs respectively activate CD4+ and CD8+ lymphocyte populations, the physical nature of the antigen delivery vehicle strongly influenced the degree of CD4+ versus CD8+ OVA-specific T cell activation. This finding suggests that changes in the physical presentation of the same antigen delivered to the airway during natural immune responses or vaccinations may markedly affect the character of the T cell response that ensues.
Keywords: lymphatic, lung, mediastinal lymph node, dendritic cells
Introduction
Pulmonary dendritic cells (DCs) acquire antigen in the airway and lung and then migrate through lymphatic vessels to the mediastinal lymph node (MLN), where they present antigen and stimulate an immune response (Holt, 1993; Vermaelen and Pauwels, 2003). Because the airway frequently encounters foreign substances, the antigen-presenting role of DCs is critical for the maintenance of pulmonary health. Ideally, harmless antigens induce tolerance, whereas harmful antigens promote effector immune responses (de Heer et al., 2005). One component that influences tolerance versus effector responses is the state of DC maturation (Brimnes et al., 2003). In addition, other cellular mechanisms affect the immunological outcome in response to airway antigens (Hammad and Lambrecht, 2007).
Besides a small number of plasmacytoid DCs, there are two major DC subsets found in the alveolar space and lung parenchyma: CD11c+CD11bloCD103+DC (here referred to as CD103+ DC or CD11blo DC) and CD11c+CD11bhiCD103− DC (here called CD11bhi DC) (Kirby et al., 2006; Jakubzick et al., 2008). Recent studies are beginning to shed light on their functional roles. Suggesting that the two populations possess markedly distinct functional roles, one group found that CD103+ DCs nearly exclusively promote the proliferation of naïve CD8+ T cells (Belz et al., 2007; del Rio et al., 2007), whereas CD11bhi DC preferentially seem to induce proliferation of CD4+ T cells (del Rio et al., 2007). CD11bhi DCs secrete a substantial number of chemokines during homeostatic and inflammatory conditions, whereas CD103+ DCs mainly secrete chemokines associated with Th2 responses (Beaty et al., 2007). Furthermore, the distribution of these two cell subtypes differs across the pulmonary parenchyma. CD103+ DCs are located along the mucosal lining and vascular wall, while CD11bhi DCs are mainly within perivascular regions (Sung et al., 2006). CD103+ DCs express mRNA for langerin, produce IL-12 upon TLR stimulation, and express tight junction proteins that help them traverse the epithelium and acquire antigen (Sung et al., 2006).
There are three published labeling methods to track DC migration from the lungs to the draining lymph nodes: airway administration of carboxyfluorescein diacetate succinimidyl ester (CFSE), OVA-FITC, or latex particles (Legge and Braciale, 2003; Belz et al., 2004; de Heer et al., 2004; Jakubzick et al., 2006). However, a qualitative and quantitative comparison of these approaches has not been performed. The three methods label DCs in a completely different manner. CFSE labels cells non-selectively, as it spontaneously penetrates cell membranes and irreversibly couples to cellular proteins. OVA-FITC is a soluble protein taken up by pinocytosis, and fluorescent latex particles (0.5 µm diameter) are taken up by macropinocytosis or phagocytosis. Here we report on the advantageous and disadvantageous features of each labeling method. In addition, in the course of our study, we uncover a distinct propensity for the two pulmonary DC subsets to acquire soluble versus particulate antigen. Since the two subsets of DCs stimulate CD4+ and CD8+ T cells selectively (Belz et al., 2007; del Rio et al., 2007), we show that skewing antigen delivery to the two types of DCs by altering the physical presentation of the same antigen impacts the relative magnitude of CD4+versus CD8+ T cell proliferation in response to the antigen.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Research Laboratories and used for studies at 7–8 weeks of age. CX3CR1gfp/gfp mice (Jung et al., 2000) (originally a gift from Dan Littman) were maintained in our colony and were fully backcrossed at least 10 generations on the C57BL/6 background. Mice were housed in a specific pathogen-free environment at Mount Sinai School of Medicine and used in accordance with protocols approved by the Institutional Animal Care and Utilization Committee.
Labeling and tracking pulmonary dendritic cells
Optimized delivery
We standardized and optimized an intranasal (IN) delivery method to compare all three labeling methods without the need to account for variances in delivery technique. Mice were completely anesthetized with avertin using 300 µl per mouse with tert-amyl alcohol content at 2.5% and 2,2,2 tribromoethanol (TCI America, T1420) at a concentration of 50 mg/kg. In this standardized delivery, each mouse was held in a vertical position perpendicular to the bench. Its tongue was pulled out and held with the thumb and the middle finger without obstructing the nasal cavity. While holding the tongue, 30 µl of labeling solution was delivered IN. The mouse position and tongue were held for an additional 30 sec post delivery to ensure solution entry into the lungs and prevent swallowing. Subsequently, the mouse was rested on the cage frame in a prone position. We ensured that the resting mouse had a clear breathing space between the cage bars. After 20 min of observation during recovery from anesthesia, the mouse was returned to its cage over a paper towel to prevent bedding inhalation and suffocation.
Final concentration used in the IN delivery
For CFSE labeling; 5 µM CFSE was prepared by using PBS to dilute a 50 µM CFSE stock in DMSO (Invitrogen, cat#C1157) (Legge and Braciale, 2003; Fainaru et al., 2005).
OVA-FITC was delivered at 5 mg/ml (Molecular Probes, cat#O23020) (Vermaelen and Pauwels, 2003).
Fluorescent latex particle labeling: Plain yellow-green fluorescent 0.5 µm latex particles (Polysciences, cat#17152) were diluted 1:25 in PBS (Jakubzick et al., 2006), for a final administration of approximately 3.64 × 10^8 particles per mouse. Where specified, tracer solutions delivered IN were supplemented with 1µg LPS (Sigma, cat#L-8274) or 1µg pertussis toxin (Sigma, cat#P7208) per mouse, respectively.
OVA-FITC
OVA-FITC from Molecular Probes (cat # O23020) was reconstituted at 5 mg/ml and dialyzed extensively against PBS. As an alternative source of OVA, Dr. Thomas Moran kindly provided us with OVA sterilely extracted from chicken eggs (Brimnes et al., 2003). This source of sterile OVA and Sigma OVA (Grade V, cat# A5503) was conjugated to FITC using the FluoReporter FITC protein labeling kit from Invitrogen (cat# F6434) per manufacturer’s instructions.
Immunohistochemistry
Lung was perfused intratracheally with 1 ml PBS:OCT (1:1) and then embedded in OCT. Sections of 7 µm thickness were collected onto printed slides (Carlson) and fixed with 4% paraformaldehyde. The slides were stained with rat-anti mouse MHC II antibody or rat IgG control Ab (BD Pharmingen) followed by CY3-conjugated anti-rat IgG (Jackson ImmunoResearch, cat# 712-165-150)
BAL collection and flow cytometry analysis
To obtain single-cell suspensions of bronchoalveolar lavage (BAL), immediately after sacrifice, the trachea of a mouse was exposed and the mouse was positioned upright by inserting one side of a blunt forceps behind the trachea. An 18-gauge needle attached to a 3 ml syringe was inserted through the largest upper-cartilage ring, and the forceps were gently clamped down on the inserted needle. The airways were flushed 4 times with 1 ml of 0.5 mM EDTA/HBSS. Collected cells were resuspended in FACS blocking solution and stained for 30 min with conjugated antibodies. The right mediastinal lymph node was excised, teased with needles and placed in collagenase D for 30 min at 37°C. Following digestion, 100 µl of 100 mM EDTA was added for 5 min. Cells were then filtered through a 70-µm nylon filter, washed, collected, and stained for FACS. For each mouse, BAL and LLN suspensions were run to completion in flow cytometric analysis to obtain the total number of DCs that acquired tracer in the BAL or migrated to the LLN. None of the samples were pooled. The following purified mAbs were used for staining: PE-conjugated mAbs to CD103 (#12-1031-82), CD8 (#553033), or B220 (#12-0452-82); PerCP-conjugated mAb to Gr-1 (recognizes Ly-6C and Ly-6G) (#552093), or CD11b (#550993); and APC-conjugated mAb to CD11c (#17-0114-82). Conjugated isotype-matched control mAbs were also obtained from eBiosciences or BD Pharmingen. In some samples, two minutes before live cell acquisition, 2 µl of 10 mg/ml stock solution of propidium iodide was added to identify the percent of live cells present in the BAL.
Proliferation of OVA-specific transgenic CD8+ and CD4+ T cells
Spleen and lymph node cells from OT-I and OT-II mice, in which the TCR of CD8+ and CD4+ cells are restricted to OVA (Hogquist et al., 1994; Barnden et al., 1998), were isolated and labeled with carboxyfluorescein diacetate succinimidyl diester (CFSE) (Molecular Probes). Half a million of these cells were transferred i.v. into recipient mice one day before IN delivery of latex particles not conjugated to protein, latex covalently coupled with egg-extracted OVA, or egg-extracted OVAFITC. Other mice received 200 µl i.p. 10 mg OVA mixed with Alum (1:1) to generate a positive control for robust T cell proliferation. Mice were sacrificed 3 days after IN deliveries and positive control i.p. injection. T cell proliferation (CFSE dye dilution) in the MLN was analyzed in single cell suspensions.
Statistics
Statistical analysis was conducted using InStat and Prism software. All bar graphs are expressed as the mean ± SEM. Statistical tests were performed using two-tailed Student’s t test and ANOVA. P < 0.05 was considered statistically significant.
Results
Relationship between administration of migration tracers and induction of inflammation in the airway and lung
We first analyzed which cells were labeled by CFSE, OVA-FITC, and green fluorescent latex particles by using an optimized intranasal delivery (described in methods). This method of delivery permits experimental comparison of all three labeling methods while enhancing consistency of delivery among mouse groups. However, whether the delivery was performed as described in the literature (intranasal or intratracheal) or by the optimized IN delivery, the same types of peripheral cells were labeled in the bronchoalveolar lavage (BAL) and lung (Vermaelen and Pauwels, 2003; Belz et al., 2004; Jakubzick et al., 2006) (data not shown).
In the BAL, gated live cells were plotted to reveal CD11c staining versus FL1, the FACS channel that identified all fluorescent tracers used. Treatment with PBS served as a control and allowed us to visualize CD11c+ autofluorescent macrophages (blue gate) and CD11c+ low autofluorescent DCs (green gate) (Jakubzick et al., 2006; Jakubzick et al., 2008). CFSE labeled all cells present in the BAL, as expected. In contrast, soluble OVA-FITC and latex particles were mainly found in phagocytic cells: CD11c−CD11bhi neutrophils and CD11chi macrophages and low autofluorescent dendritic cells (Fig. 1A) (Vermaelen and Pauwels, 2004). Most BAL DCs remained unlabeled when latex was administered as evident from the retention of FL1 low autofluorescent CD11c+ cells in the analysis (Fig. 1A and Table I). Other tracers labeled the vast majority of DCs. The proportion of labeled BAL DCs by each tracer is shown in Table I.
Figure 1. Labeling methods compared in BAL and Lung.
A) Flow cytometry of BAL gated on live cells 1 day post IN delivery of CFSE, OVA-FITC or latex particles without or with 1µg LPS, stained for CD11c versus the fluorescein fluorescent channel (left). Neutrophils are gated in red in plots depicting CD11c versus CD11b staining. Top panel shows IN delivery of PBS containing no tracer: DCs are gated in green and alveolar macrophages are gated in blue. In addition, the presence of non-latex+ DCs could be observed in the mice that received latex delivery (green gate). Representative data from seven experiments B) Lung histology of labeling methods top row without LPS: tracer is green; MHCII is red; and DAPI is blue. Naïve mouse lung (no tracer) bottom row: MHCII and control antibody are red and DAPI is blue. 200X magnification. C) Flow cytometry of BAL gated on live cells stained for CD11c versus CD11b one-day post IN delivery of commercially available OVA-FITC non-dialyzed and dialyzed. Non-FITC conjugated egg-extracted OVA was used as a control (left). D) Flow cytometry of BAL gated on live cells stained for CD11c versus CD11b one-day post IN delivery of FITC-conjugated, egg-extracted OVA and commercially available OVA. Bar graph measured the number of neutrophil infiltration into the BAL.
Table I. Percentage of tracer+ DCs.
The percentage of tracer+ DCs among the total DCs present in the BAL: n=8–10 mice per group. Standard error of the mean in parenthesis
| LABEL | DC no LPS % Tracer+ |
DC with LPS % Tracer+ |
|---|---|---|
| CFSE | 90.1 (5.1) | 99.5 (0.3) |
| OVA-FITC | 91.3 (0.6) | 98.5 (0.2) |
| Latex | 20.8 (3.9) | 25.9 (2.6) |
Even in the absence of added LPS, OVA-FITC and CFSE, but not latex particles, induced a robust inflammatory response, which was evident by an influx of neutrophils (Fig. 1A, neutrophil gates shown as red boxes). Similar to the latex particle delivery, only few neutrophils were recovered when mice were treated with sterile PBS (Fig. 1A, upper row). Within the lung, unlike OVA-FITC and latex particles that were evenly spread throughout the lung in phagocytic cells, CFSE appeared localized in patches (Fig. 1B).
We next explored two factors that could be contributing to the inflammatory response after the IN delivery of OVA-FITC in the BAL. One is that the commercially available OVA-FITC may contain excess FITC in the vial, which could be acting as an irritant. Alternatively, OVA-FITC might be contaminated with underlying inflammatory stimulants, such as LPS. To address these possibilities, commercial OVA-FITC was dialyzed extensively in PBS to remove excess FITC. Dialyzed OVA-FITC did not differ from non-dialyzed OVA-FITC in the level of inflammation observed in the BAL (Fig. 1C). Therefore, excess FITC was not inducing the neutrophil recruitment found in the airways. By contrast, IN delivery of a carefully prepared non-commercial source of egg extracted OVA, as previously described (Brimnes et al., 2003), eliminated its propensity to induce inflammation (Fig. 1C), suggesting that the commercially available popular source of ‘LPS-free OVA’ is contaminated with inflammatory stimulants that are not removed by dialysis. When FITC was conjugated to egg extracted OVA or another source of commercial OVA, robust inflammation was observed only with the commercial source (Fig. 1D). Thus, FITC-OVA can be used to study steady state migration of DCs from the airway, but the quality of the OVA is crucial, and we were unable to find a source of commercial OVA that failed to induce inflammation. In addition, latex particle administration was not associated with inflammation, suggesting that this method may also be favorable for analysis of DC migration in the steady state. By contrast, CFSE labeling is not suitable.
Characterization of newly emigrated DCs and other DCs in the mediastinal lymph node
Next we tracked the migration of labeled DCs to the mediastinal lymph node (MLN). Plotting migratory FL1+ (CFSE+, OVA-FITC+, and Latex particle+) cells versus an unstained channel facilitated the visualization of tracer+ migratory DCs, as this approach condensed the events corresponding with migratory DCs to a readily identifiable gate (Fig. 2A). From the perspective of detection, the latex particle method of tracing DC migration demonstrated a slight advantage over CFSE+ and OVA-FITC+ cells, since the latex+ cells were more brightly fluorescent than cells labeled with the other tracers, though all labeled populations could be sufficiently discerned (Fig. 2A). As expected, in all methods we observed accumulation of CD11chi DCs in the lung-draining lymph nodes bearing the respective tracers (Fig. 2B).
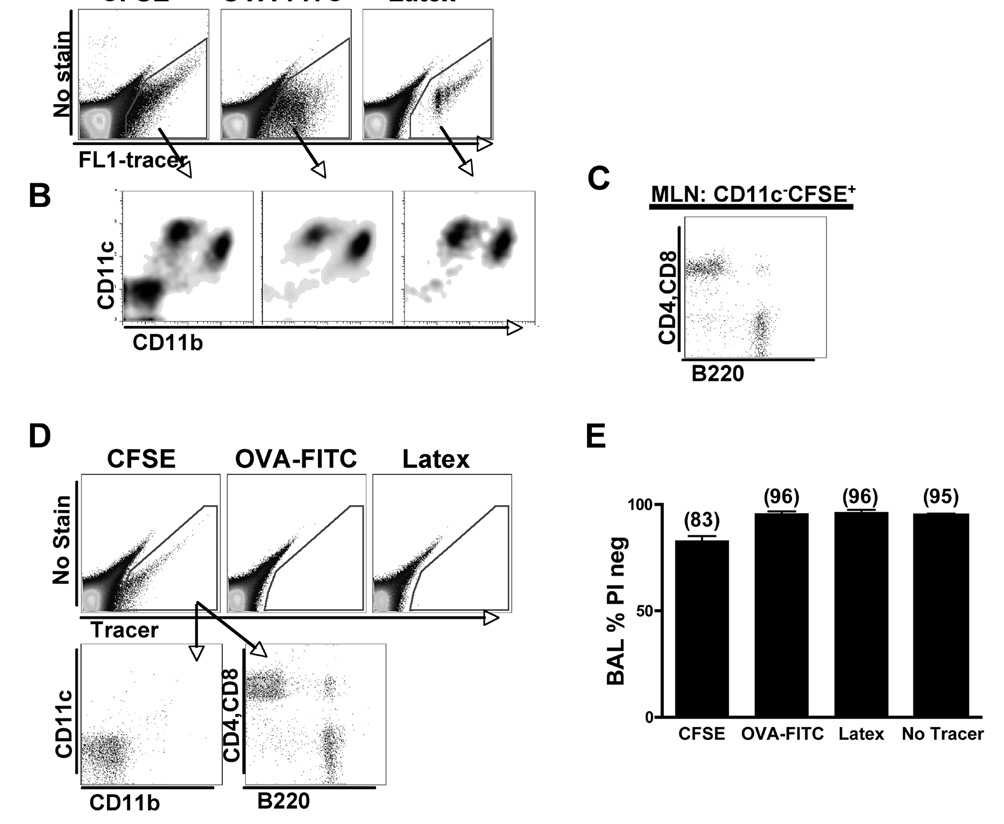
Figure 2. Tracer+ pulmonary DCs in the MLN.
A) Gated live cells from MLN were plotted as an empty channel versus FITC and then all tracer+ cells were gated. B) Gated MLN tracer+ cells were plotted as CD11c versus CD11b. These mice were treated with 1µg LPS. Data are representative of four experiments. C) CD11c−CD11b−CFSE+ cells from the MLN were stained for T cell markers, CD4 and CD8, and for a B cell marker, B220. D) Gated live cells from inguinal LN (distal LN) were plotted as an empty channel versus tracer one-day post IN delivery of CFSE, commercial OVA-FITC and latex particles. Lower plots show the CFSE+ cells from the inguinal LN plotted as CD11c versus CD11b and CD4+CD8 versus B220. Data are representative of three independent experiments. E) Analysis of the percent of live cells present in the BAL one-day post IN delivery of labeling methods. The percentage of live cells was analyzed by gating on negatively stained propidium iodide cells: n=5–7 mice per group.
Only the CFSE labeled mice tracked additional cells besides pulmonary DCs, since we found CFSE+CD11c−CD11b− labeled cells in the MLNs (Fig. 2B). Propidium iodide staining demonstrated that the CFSE+CD11c− CD11b− cells were not dead (data not shown). Staining for B220 to identify B cells and CD4 and CD8 to identify T cells indicated that these cells were lymphocytes (Fig. 2C). CFSE also leaked into the blood (data not shown), suggesting that this label may induce partial injury to the lung. CFSE+ circulating B and T cells spread to distal lymph nodes, such as inguinal lymph nodes (Fig. 2D). Systemic dissemination of labeled cells was not observed in OVA-FITC or latex particle-labeling methods (Fig. 2D). To follow-up on the possibility that CFSE labeling induced injury, we examined the percentage of dead cells among labeled cells in the BAL 1 day post-delivery. The CFSE labeling method induced the greatest amount of cell death. Only in the BAL of CFSE labeled mice was the fraction of live cells lower than baseline (no tracer) where 83% of cells were live compared to 96% in OVA-FITC and latex particle labeled mice (Fig. 2E). These findings suggest that CFSE induces cellular death, inflammation, and partial tissue damage even when administered in the absence of LPS. In addition, CFSE labeled lymphocytes are observed and spread systemically.
To specifically analyze migrating pulmonary DCs within all CD11chi DCs in the MLN, all CD11chi MLN DCs were gated and then plotted using an unstained channel versus FL-1 (Fig. 3A). In the lung draining lymph node, migrating CD11bloCD103+ DCs express more CD11c than the migrating CD11bhi DCs (Fig. 2B). Therefore, it would be inappropriate to gate on migrating DCs by using CD11chi as the only criterion because the total number of CD11chiCD11bhi DCs would be missed. Therefore, to exclude analysis of CD11cintCD11b− plasmacytoid DCs and focus on pulmonary DCs that migrate from lung, we found it best to gate on the migrating DCs by plotting CD11c versus CD11b as shown in figure 3A, top row.
Figure 3. Time course analysis of migrating tracer+ cell in the MLN and the percentage and number of tracer+ cells found in the MLN.
A) All live cells are plotted as CD11c versus CD11b and then all CD11chi cells in the MLN were gated, two main subsets are observed: CD11chiCD11blo and CD11chiCD11bhi DCs. Within these subsets, tracer+ migratory DCs were extracted. Next, all CD11chi cells were plotted as an empty channel versus tracer and then all tracer+ cells were gated on (migrating DCs). Small gray arrow in the latex method top panel represents the CD11cintCD11b− cells, where the majority of these cells are B220+ plasmacytoid DCs. These mice were treated with 1µg LPS. Data are representative of five independent experiments. B) Gated latex particle+ DCs were stained for CD103 or CD8 versus CD11b. C) CD11c+ BAL DCs were gated by low autofluorescence (macrophages are the highly autofluorescent) using empty FITC fluorescent channel, then the DCs were stained for CD103 or CD8 versus CD11b in naïve and mice treated with 1µg LPS. D) Time course indicating the total number of migrated pulmonary DCs, CD11chiFITC+ cells in the MLN post IN delivery of tracer with LPS E) The percentage and number of tracer+ cells (CFSE+, EE=FITC conjugated egg extracted OVA, commercial OVA-FITC+ and latex particle+) in CD11chi DCs in all labeling methods one-day post IN delivery with or without LPS: n=8–10 mice per group. Results are expressed as mean ±SEM (error bars); difference between EE and commercial OVA-FITC treated mice with or without LPS was significant as was latex particle non-LPS and LPS treated mice (*p<0.05, **p<0.001, ***p<0.0001).
The migrating pulmonary DCs in the MLN displayed the same surface phenotype observed in the BAL and parenchymal lung: CD11chiCD11bloCD103+ and CD11chiCD11bhi populations (Fig. 3B and data not shown) (Vermaelen and Pauwels, 2004; Sung et al., 2006). All the tracer-bearing CD11chiCD11blo DCs in the MLN were CD103+ and CD8α− regardless of the labeling method employed (Fig. 3B, displayed data from latex particle method). An important difference between analysis of MLN and lung is the way in which DCs are identified by flow cytometry (Vermaelen and Pauwels, 2004). In contrast to the MLN where all CD11chi cells are DCs (Fig. 3A), in the lung, both DCs and macrophages are CD11chi. Therefore, lung DCs were distinguished from macrophages by another criteria—autofluorescence (Fig. 3C) (Vermaelen and Pauwels, 2004).
Time course analysis of tracers present in the mediastinal lymph node
A time course demonstrated that peak presence of tracer+ DCs for CFSE and OVA-FITC labeling was at day 1 post IN delivery (Fig. 3D). Presence of latex+ DCs was similar at day 1 and day 2. Thus, all data were analyzed one day after IN deliveries.
In general, for the tracers that induced inflammation in the airway on their own, DC migration was correspondingly more robust and the effects of LPS on the magnitude of DC migration were less marked (Fig. 3E). LPS addition, for example, did not alter the percentage of CFSE+ DCs in the MLN (Fig. 3E, upper). However, for all tracers, including CFSE, the total number of tracer+ DCs in the MLN was increased when LPS was added (Fig. 3E, lower). That LPS prompted a significant increase in DC migration from the airway was most clearly observed when the effects of LPS were compared to the use of the non-inflammatory, egg-extract (EE) preparation of OVA (Brimnes et al., 2003) or to latex particles as tracers. For both of these tracers, which appear to measure steady state migration, addition of LPS augmented the number of tracer-bearing DCs in the MLN by 10-fold. Latex particle as a tracer was restricted to fewer DCs in all conditions than the other methods. This was not due to latex beads impairing DC migration but rather to the fact that fewer DCs were labeled with latex beads to begin with, since the proportional difference in labeled DCs found in the LN was similar to the proportional difference in original labeling (Table I).
Effects of pertussis toxin on the accumulation of tracer-bearing DCs in the MLNs
To determine whether CFSE, soluble OVA-FITC or latex particles were freely migrating down the lymphatic vessels and accessing MLN DCs in a migration-independent manner, pertussis toxin was used to inhibit DC migration from the lungs to the MLN. To ensure robust DC migration, all tracers were studied in the presence of LPS, and half of the mice were treated with pertussis toxin. The percentage of neutrophil recruitment in the BAL after LPS stimulation was surprisingly not significantly altered in the presence of pertussis toxin (Fig. 4A). Hence, pertussis toxin delivered IN did not substantially inhibit local inflammation. However, pertussis toxin significantly inhibited the accumulation of tracer+ (CFSE+, OVA-FITC+, or Latex particle+) cells in the MLN, by an average of 92%, 97%, 100% for the 3 tracers, respectively (Fig. 4B). The accumulation of CFSE+CD11c−CD11b− lymphocytes in the MLN was less affected by pertussis toxin than the accumulation of CFSE+ DCs. Thus, all 3 tracers accumulate in the MLN DCs as a result of specific transport by DCs from the airway or lung.
Figure 4. Pertussis toxin significantly inhibited pulmonary DC migration to the MLN.
A) Representative dot plots in the BAL were stained for CD11c versus CD11b showing neutrophil infiltration after one-day post IN delivery of tracer with 1µg LPS. In addition, some mice were treated simultaneously with PBS (top plots) or 1µg of pertussis toxin (bottom plots). Bar graph summarizes the percentage of neutrophils present in BAL from PBS or pertussis toxin treated mice one-day post IN delivery of CFSE, commercial OVA-FITC and latex particles: n=5 mice per group. B) Dot plots display gated CFSE+, OVA-FITC+ and latex particle+ cells in the MLN stained for CD11c versus CD11b in PBS and 1µg pertussis toxin treated mice. The bar graph compiles the total number of CD11chitracer+ (CFSE+, OVA-FITC+ and latex particle+) cells in the MLN from LPS labeled mice treated with PBS or pertussis toxin: n=5 mice per group. Results are expressed as mean ±SEM (error bars); differences between PBS and pertussis toxin treated mice were significant for all tracers (**p<0.001, *** p<0.0001).
CD103+ DCs preferentially acquire and transport particulate material whereas CD11bhi DCs preferentially acquire and transport soluble antigen
During the course of these experiments, we observed an unexpected difference in the distribution of two tracers, OVA-FITC and latex particles, in the two pulmonary DC subsets after initial uptake. After administration of OVA-FITC, the major DC subset bearing this tracer in the MLN was the CD11bhi DC population. The frequency of OVA-FITC+ CD11bhi DCs was about 3 times greater than the frequency of OVA-FITC+ CD103+ DCs (Fig. 5A) and was also greater than the overall frequency of CD11bhi DCs in the lymph node (Fig. 5B). By contrast, CD103+ DCs preferentially transported latex particles. These differences were reflected by a similar difference in uptake of OVA or latex beads in the BAL and lung interstitium (data not shown). The fraction of latex+ CD103+ DCs was more than two times higher than the fraction of latex+CD11bhi DCs in the MLN (Fig. 5A). Coating latex beads with OVA did not change this pattern, indicating that the preference for different tracers was not dictated by OVA receptors, such as the mannose receptor (Kindberg et al., 1990; Burgdorf et al., 2006). It seems more likely, then, that the two subsets of DCs preferentially acquire and transport different classes of antigen—particulate versus soluble—to lymph nodes. CFSE showed no preferential transport by one DC subset or the other, consistent with its rather nonspecific means of labeling; instead the fraction of CFSE+ DC subsets in the MLN mirrored the overall distribution of the total DC subsets (Fig. 5B).
Figure 5. CD11bhi DCs preferentially acquires soluble protein, whereas CD11bloCD103+ DCs preferentially take up particles.
A) The percentage of CD11blo and CD11bhi DCs in CD11chitracer+ cells from IN delivery of OVA-FITC or latex particles: n=8–10 mice per group. Result is expressed as mean ±SEM (error bars); difference between OVA-FITC and latex particle treated mice is statistically significant for the percentage of FITC+CD11blo or FITC+CD11bhi DCs (*** p<0.0001). B) Left bar graph shows the percentage of CD11blo and CD11bhi DCs in CD11chitracer+ cells from IN delivery of CFSE and bar graph on the right shows the percentage of all (tracer+ and tracer−) CD11blo and CD11bhi DCs in the MLN from the three types of tracer delivered mice: n=8–10 mice per group.
Latex particle carrying antigen enhance CD8+ T cell proliferation
Two groups have shown in vitro that sorted CD103+ DCs enhanced antigen-specific CD8+ T cell proliferation, whereas CD11bhi DCs did not (Belz et al., 2007; del Rio et al., 2007). Since we demonstrated that the two pulmonary DC subsets preferentially carry different classes of antigen (Fig. 5), we hypothesized that latex particle-OVA versus soluble-OVA delivered IN would demonstrate altered outcomes in the immune response induced in vivo for the proliferation of CD8 or CD4 OVA-specific T cell. Thus, since latex particles are mainly carried by CD103+ DCs, and CD103+ DCs have been shown to cross-present in vitro, we expect more cross-presentation and expansion of CD8+ OT-I T cells in vivo in response to particulate OVA delivery over soluble form. To test this hypothesis, transgenic T cells with TCR specificity for OVA antigen, CD8+OT-I and CD4+ OT-II T cells were labeled with CFSE to detect proliferation and transferred intravenously at a 1:1 ratio in recipient mice. The following day, IN deliveries were given to three cohorts of mice: latex particles alone as a negative control, latex particles conjugated to egg-extracted OVA, or soluble egg-extracted OVA-FITC. Intraperitoneal injection of commercial OVA was given as a positive control. Three days post IN delivery, mice were sacrificed and analyzed for the induction and extent of T cell proliferation. In the MLN, Vα2+CFSE+ T cells were gated and then plotted to quantify the proliferation of either OVA-specific CD8+ T cells or CD4+T cells (Fig. 6). There was no proliferative response to plain latex particles and a strong proliferative reponse in mice that were injected intraperitoneal with commercial OVA. However, mice that received latex-conjugated OVA demonstrated enhanced 3:1 ratio of OVA-specific CD8+ T cell proliferation over OVA-specific CD4+ T cells. On the other hand, soluble OVA delivery induced a 1:1 proliferative response of OVA-specific CD8+ and CD4+ T cells (Fig. 6). Hence, these data shows that there are potential consequences in the kind of immune response produced if the carrier that contains the antigen of interest was altered or preferentially taken up by distinct pulmonary DC subsets.
Figure 6. OT-I cells proliferate more when CD103+ DCs present antigen compared with presentation by CD11bhi DCs.
Representative histograms show gated adoptively transferred Vα2+CFSE+ OT-I and OT-II cells in the MLN (top row) for IN delivery of non-conjugated latex particles as a negative control, i.p. injection of commercial OVA as a positive control, IN delivery of egg-extracted OVA-conjugated latex particles and egg-extracted soluble OVA-FITC. Below the first row, Vα2+CFSE+ gate was divided and viewed as a histogram for proliferation of CD8+ OT-I and CD4+ OT-II cells. Bar graph (on the left) demonstrates the ratio of the number of OT-I/OTII cells found in the MLN 4 days after adoptive transfer in mice treated with non-conjugated latex particles. Bar graph (right) the ratio of the number of OT-I/OT-II cells that proliferated in the MLN 4 days after adoptive transfer in mice treated with egg-extracted OVA-conjugated latex particles and egg-extracted OVA-FITC. Data are representative of three independent experiments. Result is expressed as mean ±SEM (error bars); difference between mice treated with EE OVA-conjugated latex particles and EE OVA-FITC is statistically significant (*** p<0.0001).
Discussion
In the literature, there are at least three different methods of labeling pulmonary DCs. These methods may impact experimental outcomes due to selective labeling, induction of cellular death, or inflammation. By making direct comparisons, we were able to provide information about the nuances of each technique that can further guide experimentation.
Besides a small fraction of plasmacytoid DCs, the lung contains two major DC subsets that do not alter their phenotype during migration to the MLN. The phenotype of these subsets can be distinguished by five markers: CD11c, CD103, CD11b, langerin, and CX3CR1. The CD103+ DCs express more CD11c than CD11bhi DCs. The expression of langerin, CD103 and CX3CR1 are specific to a given subset. CD103+ DCs express low levels of langerin (as seen in Langerin GFP mice, data not shown, (Sung et al., 2006)) but not CX3CR1 (Jakubzick et al., 2008). Inversely, CD11bhi DCs do not express langerin (Sung et al., 2006) but express high levels of CX3CR1 (Landsman et al., 2007; Jakubzick et al., 2006).
In the process of performing traditional intranasal and non-invasive intratracheal deliveries, an easier and more consistent technique was developed for use in this study that combines both forms of delivery (described in Methods). All labeling approaches traced migrating DCs, and the addition of LPS enhanced DC migration while pertussis toxin inhibited it. It has been claimed that airway administration of CFSE permits the study of steady state DC migration (Legge and Braciale, 2003), and this technique has been used in many important studies subsequently (Belz et al., 2004; Fainaru et al., 2005) without challenge to this claim. Our findings reveal otherwise and suggest that CFSE administration should be abandoned as a means to study DC migration, as it induced robust inflammation, caused cellular death and tissue damage, and also stained migrating lymphocytes.
Data obtained with two other tracers were more promising. A fluorochrome-labeled protein such as OVA can be used to study migration without itself inducing inflammation, but our findings highlight the need to establish that the protein is not contaminated with inflammatory stimuli. When we tested the same source of OVA commonly used to study DC migration from the airway, again presumed to be doing so in the steady state (Vermaelen and Pauwels, 2003), we found that this source induced inflammation as well. Since contaminants may vary from lot to lot in protein preparations, each lot likely should be tested independently, and the most reliable source of OVA may be that extracted directly from the egg, as previously indicated (Brimnes et al., 2003).
Latex beads were free of inflammatory stimulants as judged by failure to induce neutrophil influx into the lung. Thus, like the most optimal preparations of OVA, latex beads could be used to study DC migration without inducing inflammation. From a quantitative perspective, ‘clean OVA’ was superior to latex beads in that the limited uptake of latex beads in the airway reduced the magnitude of migrating DCs detected in the draining lymph node by several fold. However, in the absence of a ready source of uncontaminated protein tracers, latex beads serve as a particularly good option for studying DC migration. One of the distinguishing features of latex beads is their capacity to persist beyond the lifespan of the cell, in this case the migratory DC, that initially acquired it. Indeed, we observed that the numbers of latex bead+ DCs reached a plateau in the LLN, whereas the other, biodegradable tracers peaked and then decayed from the LLN. We have previously documented transfer of latex beads from migratory, adoptively transferred DCs in skin to other DCs in the draining lymph node {Angeli, 2006 #150}. Persistence of the tracer could be considered a disadvantage under some circumstances, but this feature may also be useful in charting the fate of dying DCs or aspects of antigen sharing and transfer between DCs and other DCs or other cells. We have designed methods to exploit this feature to an advantage in tracking, for example, fate of monocyte-derived cells in inflammatory diseases like atherosclerosis {Randolph, #151}.
Technically, airway DC migration assays should always include the step of acquiring and analyzing the cells from BAL in each individual mouse post IN delivery of any tracer for two reasons: analysis of neutrophil infiltration (for the dinduction of inflammation or not) and macrophage acquisition of tracers to ensure proper tracer delivery. Fortunately, alveolar macrophages make up most of the cells in the BAL of a naïve mouse and they are the first to acquire the tracers. In addition, they do not migrate, unlike DCs (MacLean et al., 1996; Jakubzick et al., 2006), making them a good source of cells to analyze proper installation. For example, if an improper delivery were performed such that the mouse swallowed the tracer instead of inhaling it, then BAL analysis would clearly display this. If, for example, only 30% of the macrophages were carrying the beads (normally 70–95% of alveolar macrophages acquire tracers, including latex beads), then an improper instillation of tracer should be assumed. A poor instillation would lead to a decreased number of migrating DCs found in the MLN compared to a proper delivery and might lead to incorrect conclusions if adequate delivery were assumed.
We unexpectedly found preferential antigen acquisition and migration in the DC subsets: CD103+ DCs preferentially acquired latex beads (±OVA) whereas CD11bhi DCs preferentially acquired soluble OVA. The analysis of in vivo T cell proliferation after the delivery of latex particles or soluble protein led to the observation that higher levels of antigen carried by CD103+ DCs enhanced CD8+ T cell proliferation compared to the same antigen mainly carried by CD11bhi DCs, pointing to the likelihood that these two DC subsets have distinct roles in promoting CD8+ T cell or CD4+ T cell proliferation in vivo, as observed previously in vitro and ex vivo (Belz et al., 2007; del Rio et al., 2007). A possible explanation for the differential uptake of particles versus soluble OVA may be found in the fact that CD103+ DCs preferentially line the lung mucosa, express tight junction proteins, adhesion molecules, and endocytose avidly (10). This location makes them prime candidates to acquire incoming particulates into the lungs. It remains unclear how much this apparent preference for particulates versus soluble proteins by different DCs will hold up when a greater variety of antigens and delivery systems are tested. Pathogens in particular may possess mechanisms that lead to alterations in this outcome. CD103+ DCs appear to be the initial harbingers of Mycobacterium tuberculosis to lymph nodes, though this later shifts to CD11bhi DCs as weeks go by (Wolf et al., 2007). Our findings may have more relevance in vaccine formulations, which like our tracers, are often comprised of purified proteins complexed with manufactured delivery vehicles. It will be of particular interest to determine if nanoparticle-based airway vaccines are mainly acquired by CD8+ T cell-inducing CD103+ DCs as we observe for latex particles. Since the quality of the T cell response dictates effectiveness of a vaccine in rendering protection and memory, it will be particular important to pay attention to how the physical nature of the delivery vehicle affects the course of the initial and ongoing T cell response.
Acknowledgements
This work was supported by NIH grant AI49653 to GJR, an institutional training grant to CJ, and a Primary Caregiver Supplement to CJ by NIAID. GJR is an established investigator of the American Heart Association.
Abbreviations
- BAL
bronchoaveolar lavage
- DC
dendritic cell
- MLN
mediastinal lymph node
- IN
Intranasal
- IT
Intratracheal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Beaty SR, Rose CE, Jr, Sung SS. Diverse and Potent Chemokine Production by Lung CD11bhigh Dendritic Cells in Homeostasis and in Allergic Lung Inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Kool M, Lambrecht BN. Dendritic cell subsets and immune regulation in the lung. Semin Immunol. 2005;17:295–303. doi: 10.1016/j.smim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- Fainaru O, Shseyov D, Hantisteanu S, Groner Y. Accelerated chemokine receptor 7-mediated dendritic cell migration in Runx3 knockout mice and the spontaneous development of asthma-like disease. Proc Natl Acad Sci U S A. 2005;102:10598–10603. doi: 10.1073/pnas.0504787102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. Lung dendritic cell migration. Adv Immunol. 2007;93:265–278. doi: 10.1016/S0065-2776(06)93007-7. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Holt PG. Regulation of antigen-presenting cell function(s) in lung and airway tissues. Eur Respir J. 1993;6:120–129. [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, Merad M, Randolph GJ. Blood Monocyte Subsets Differentially Give Rise to CD103+ and CD103− Pulmonary Dendritic Cell Populations. J Immunol. 2008;180:3019–3027. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindberg GM, Magnusson S, Berg T, Smedsrod B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990;270:197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Raynes JG, Kaye PM. CD11b regulates recruitment of alveolar macrophages but not pulmonary dendritic cells after pneumococcal challenge. J Infect Dis. 2006;193:205–213. doi: 10.1086/498874. [DOI] [PubMed] [Google Scholar]
- Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- MacLean JA, Xia W, Pinto CE, Zhao L, Liu HW, Kradin RL. Sequestration of inhaled particulate antigens by lung phagocytes. A mechanism for the effective inhibition of pulmonary cell-mediated immunity. Am J Pathol. 1996;148:657–666. [PMC free article] [PubMed] [Google Scholar]
- Sung SJ, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta 7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction proteins. J. Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- Vermaelen K, Pauwels R. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am J Respir Cell Mol Biol. 2003;29:405–409. doi: 10.1165/rcmb.2003-0008OC. [DOI] [PubMed] [Google Scholar]
- Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry. 2004;61A:170–177. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]