Abstract
Respiratory deficient mutants of Saccharomyces cerevisiae have been instrumental in identifying an increasing number of nuclear gene products that promote pre- and post-translational steps of the pathway responsible for biogenesis of the mitochondrial ATP synthase. In this article we have attempted to marshal current information about the functions of such accessory factors and the roles they play in expression and assembly of the mitochondrially encoded subunits of the ATP synthase. We also discuss evidence that the ATP synthase may be build up from three separate modules corresponding to the F1 ATPase, the stator and F0.
I. Structure of the Saccharomyces cerevisiae ATP synthase
Depending on the organism and tissue origin, mitochondria display large variations in their metabolic activities. Central to the function of all mitochondria, however, is conservation of chemical energy in the form of ATP. This process known as oxidative phosphorylation is catalyzed by the respiratory chain and the proton-translocating ATP synthase (F1-F0 complex) that utilizes the energy of the proton gradient, produced during electron transport, for ATP synthesis [1]. The ATP synthase consists of the hydrophilic F1 ATPase attached to a hydrophobic unit, referred to as F0, located in the inner membrane. F1 and F0 are physically connected by means of a central stalk and a peripheral stalk or stator [2, 3]. The structure and best approximation of the subunit arrangement of the yeast ATP synthase are summarized below and illustrated in Fig. 1.
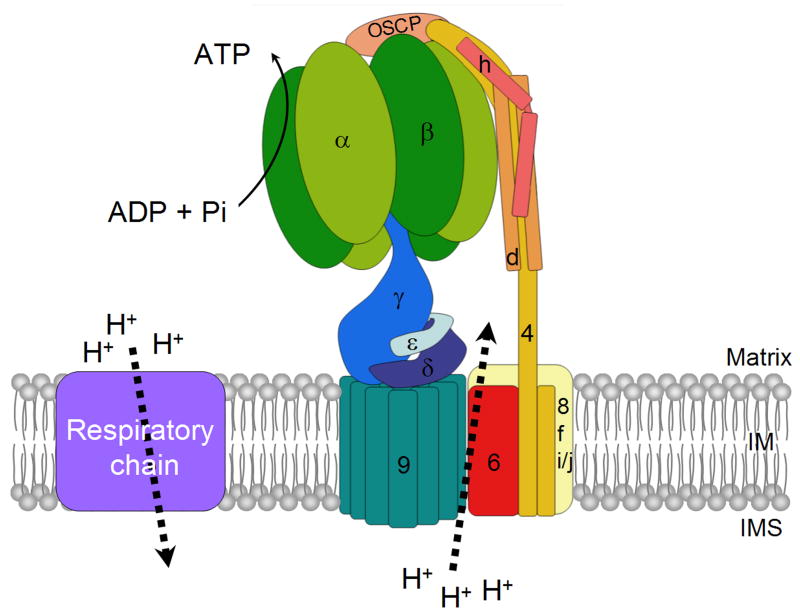
Figure 1.
The subunit organization of the mitochondrial ATP synthase.
F1 is composed of subunits α, β and the three central stalk subunits γ, δ and ε. The F0 sector contains the subunit 9 oligomer, subunit 6, subunits 8, f and i/j. The peripheral stalk consists of subunits 4, d, h and OSCP. The rotor is made up of the central stalk and the subunit 9 ring.
F1 is composed of five different subunits with a α3β3γδε stoichiometry. The bulk of this globular protein is made up of alternating α and β subunits that contain the three catalytic sites of the enzyme. The core of the spherical α3β3 hexamer is occupied by an elongated α-helical coiled-coil domain of the γ subunit. Another part of the γ subunit protrudes from the basal part of the F1 sphere where it interacts with the small δ and ε subunits to form the central stalk seen in negatively stained electron micrographs of the mitochondrial inner membrane. The stalk has a broad base that makes contact with subunit 9 of F0 [4-6].
F0 has not intrinsic ATPase activity. Its primary function is to use the electrochemical energy of the proton gradient generated during electron transport into a rotational movement of polymeric subunit 9. During this process protons are recycled from the intermembrane space to the matrix. Protons translocation occurs at an interface between subunit 9 (subunit c) and subunit 6 (subunit a) [2, 7]. Subunit 9 is a low-molecular weight proteolipid with a hairpin structure consisting of two transmembrane α-helices separated by a small loop of polar residues that extends into the matrix [8, 9]. The mammalian and yeast ATP synthases have ten copies of subunit 9 arranged in a ring [5]. In other ATP synthases the ring can have as many as 15 copies of subunit 9 [10, 11]. The subunit 9 ring is engaged in two functionally important interactions. One interaction, between the polar loop region of subunit 9 and the γδε subunits of the stalk, acts to provide a physical link between F1 and F0. The second interaction is between the subunit 9 ring and subunit 6, a transmembrane protein present in one copy. The stepwise rotation of the ring during each catalytic cycle causes the stationary subunit 6 to disengage from its pre-existing interaction and to form a new interface with the next subunit 9 of the ring [12, 13].
The stator is made up of OSCP, subunits 4, d, and h [3, 14, 15]. Subunit 4 (subunit b) has two transmembrane domains that make contact with subunit 6 of F0 [16]. The stator defined by these four proteins probably makes contact with some other ATP synthase components that are located in the membrane such as subunits 8, f, and i [17]. The exact demarcation between F0 and the stator is difficult to ascertain in the absence of high resolution structural information on the arrangement of these small hydrophobic proteins. This may turn out to be a semantic issue when a more complete structure is available. OSCP is tethered to the apical region of the α3β3 hexamer and is connected to the stator through subunits 4 and h. The external part of the stator that extends along the periphery of the α3β3 hexamer to the surface of the membrane includes subunits 4, d, and h [3, 15]. An important glimpse into the architecture of the stator has emerged from the X-ray crystal structure of a long domain composed of the soluble regions of the bovine subunits b, d, and F6 (4, d, and h in the yeast nomenclature, respectively) [15]. This extended structure is composed of apposed and overlapping α-helices contributed by each of these three subunits. This assembly is thought to provide the rigidity needed to prevent movement of α3β3 hexamer with respect to the γ subunit as the latter progresses through the three catalytic sites of F1 during catalysis of ATP synthesis [15].
The role of the stator is to join F1 to subunit 6. The combination of the stator, subunit 6 and the α3β3 hexamer constitutes the stationary part of the enzyme while the γδε stalk and the subunit 9 ring function as the motor fuelled by the translocation of protons between subunits 6 and 9. The directionality of proton flow and rotation of the ring is determined by whether the ATP synthase is engaged in ATP synthesis or hydrolysis. The rotary movement of the stalk causes the γ subunit to successively interact with and to induce conformational changes at the three catalytic sites located in the core of the α3β3 hexamer. The conformational changes alter the affinity for substrate and product and are an intrinsic feature of the mechanism by which ADP and inorganic phosphate are esterified or hydrolyzed [1, 18].
In addition to catalyzing ATP synthesis and maintaining a membrane potential through ATP-dependent translocation of protons from the matrix, the ATP synthase also contributes to the gross morphology of the mitochondrial inner membrane. The ATP synthase has been shown to exist as a dimer [19], a feature that has been proposed to be important in determining the curvature of the inner membrane [20]. Mutational analysis and cross-linking experiments have shown that dimerization of the ATP synthase depends on subunits e, g [19, 21]. Another small protein, subunit k, is found only in the dimeric form of the ATP synthase but is not required for dimerization [19].
Finally, three other proteins (If1p, Stf1p and Stf2p) have been implicated in regulation of the ATP synthase by modulating its hydrolytic activity [22-24].
II. Genetic origin of yeast mitochondrial ATP synthase
With a few exceptions such as green algae, in which the entire ATP synthase is of nuclear origin [25], the ATP synthase of most Eukaryotes is derived from both mitochondrial and nuclear genes. Generally, the subunits making up the stator and some of the F0 subunits are products of nuclear genes. This is also true of the entire complement of F1 subunits [26]. The α subunit of the plant F1, however, is encoded in mitochondrial DNA [27, 28]. The nuclear gene products are synthesized on cytoplasmic ribosomes as precursors with targeting sequences that direct their import and subsequent sorting into the appropriate mitochondrial compartment [29].
Subunits 6, 8 and 9 of plant and yeast F0 are encoded by the mitochondrial ATP6, ATP8 (AAP1) and ATP9 genes, respectively [30-33]. The ATP9 gene in animals was transferred to the nucleus and only the genes for subunits 6 and 8 have been retained in the mitochondrial genome [34]. An interesting situation exists in filamentous fungi such as Neurospora crassa and Aspergillus nidulans that have both, a mitochondrial and nuclear copy of ATP9 [35-37]. The nuclear gene is constitutively expressed during vegetative growth whereas the mitochondrial copy is expressed only in germinating spores. These organisms probably represent an intermediate evolutionary stage at which mitochondrial ATP9 has been successfully transferred to the nucleus but for reasons that remains unclear, a functional mitochondrial copy of the gene has been retained [38].
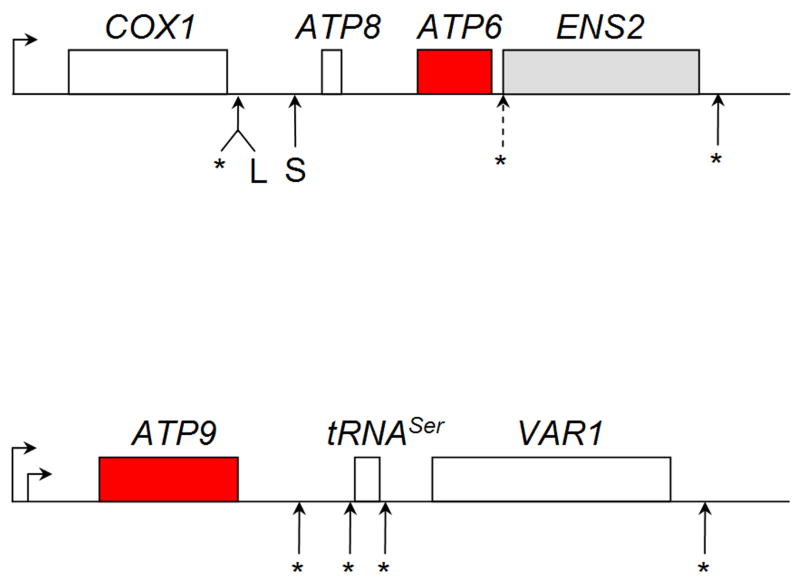
In S. cerevisiae ATP9 is co-transcribed with tRNAser and VAR1 from two closely spaced start sites, each corresponding to a conserved nonanucleotide sequence. The more prevalent transcript is initiated 0.63 kb and the lesser 0.55 kb upstream of the ATP9 initiation codon. This polycistronic transcript is processed in several steps by endonucleolytic cleavages to produce the tRNAser and the ATP9 and VAR1 mRNAs (Fig. 2). The major ATP9 messenger is a 0.9 kb RNA with 0.63 kb of 5′ untranslated sequence [39-41].
Figure 2.
Polycistronic transcripts of mitochondrial ATP synthase genes in S. cerevisiae. ATP6 and ATP8 are co-transcribed with COX1, encoding subunit 1 of cytochrome c oxidase and, in some strains, ENS2 that codes for a DNA endonuclease. The 5.2 and 4.6 kb mRNAs result from cuts at L and S, respectively. ATP9 is co-transcribed with tRNAser and VAR1, encoding a subunit protein of mitochondrial ribosomes. Transcription initiation sites are designated by horizontal arrows. Cleavage sites of the polycistronic transcripts that produce the mature messengers are shown by asterisks.
ATP6 and ATP8 are co-transcribed with COX1, encoding subunit 1 of cytochrome oxidase, from a site located 500 nucleotides upstream of the COX1 initiation codon (Fig. 2) [42, 43]. ENS2, a gene that codes for a DNA endonuclease, is located downstream of ATP6 in some strains of yeast, and when present, is co-transcribed along with COX1, ATP6 and ATP8 [44]. The primary polycistronic transcript is cleaved at two sites between COX1 and ATP8 resulting in the release of the COX1 mRNA and bicistronic mRNAs with ATP8 and ATP6. One cleavage is close to the 3′-end of the COX1 mRNA and yields the longer 5.2 kb ATP8/ATP6 mRNA. The shorter 4.6 kb mRNA is produced by cleavage at a site some 600 nucleotides downstream from the 3′-end of the COX1 mRNA [40, 45, 46]. Most strains of yeast have approximately equal amounts of the two mRNAs. ENS2, when present, is part of the ATP8/6 mRNA [47].
Mitochondria have 10 times more subunit 9 than subunits 6 or 8. In vivo labeling of mitochondrial gene products in yeast, indicates a substantially greater incorporation of the radioactive precursor into subunit 9 than subunits 6 and 8. This is probably a function of a higher concentration of the ATP9 relative to the ATP8/6 mRNAs. As discussed later in this article, translation of each gene depends on a separate translational activator. This could also account for the 10 times higher expression of subunit 9. At present, however, quantitative data on the relative concentrations of the mRNAs and their rates of translation are not available.
III. ATP synthase biogenesis is assisted by accessory proteins
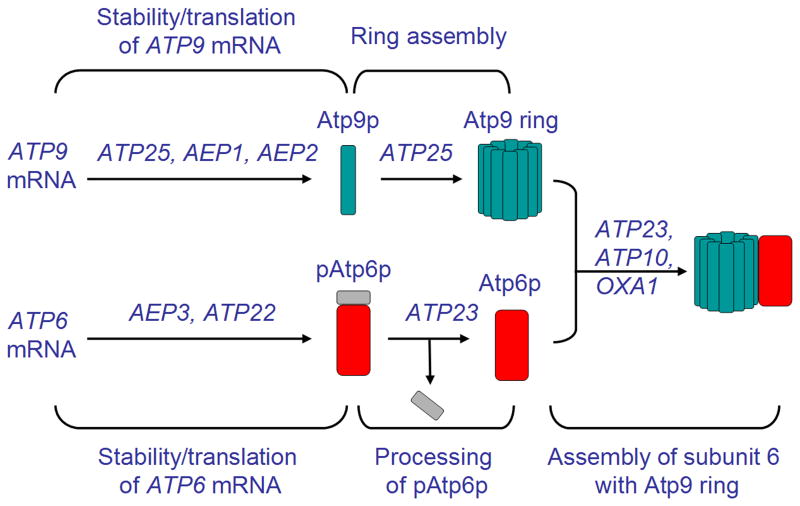
Biogenesis of a functional ATP synthase is an elaborate process, dependent on both subunit recognition/interaction events and the assistance of chaperones that promote certain assembly steps. An understanding of how this important mitochondrial enzyme is assembled is emerging, largely as a result of studies of ATP synthase deficient mutants of yeast. Efforts along these lines in several laboratories have revealed that lesions in the ATP synthase can stem not only from mutations in the structural genes but in a substantial number of nuclear genes that code for accessory factors with essential functions at pre- and post-translational stages of the assembly pathway (Fig. 3) [48-53].
Figure 3.
Assembly of subunit 6 with Atp9 ring is dependent on nuclear encoded accessory proteins (see text for description).
ATP synthase mutants can display one of two different biochemical phenotypes. The hallmark of mutants impaired in F1 assembly is the absence of ATPase activity in their mitochondria. The second phenotypic class is represented by mutants defective in F0 assembly, which leads to a loss of inhibition of the ATPase by oligomycin [54]. This antibiotic blocks the proton channel of F0 and inhibits F1-F0, but not F1 because the hydrolytic reaction is obligatorily coupled to proton tranlocation though F0. Mutants with defective F0 contain a fully assembled F1 oligomer, physically and enzymatically indistinguishable from the wild type enzyme.
III.1. Chaperones of F1 assembly
Three genes in yeast are known to be involved in assembly of the α3β3 hexamer of F1. ATP11 and ATP12 code for mitochondrial proteins that interact with the β and α subunits, respectively, and promote their assembly into the oligomeric F1 ATPase [48, 55, 56]. The absence of either protein causes the α and β subunits to aggregate into insoluble inclusion bodies in the mitochondrial matrix. Such aggregates are also detected in α or β subunit mutants but not in γ, δ or ε subunit mutants [57-59]. This suggests the existence of a “solubility checkpoint” during assembly of the F1 ATPase, the critical step being oligomerization of the α and β subunits, followed by additions of the other three subunits.
Atp11p and Atp12p are conserved in eukaryotes but do not appear to be present in bacteria. Recent X-ray crystallographic structures of these chaperones will be helpful in elucidating the details of how they function (S. Ackerman, private communication). Mutations in the third gene, FMC1, also elicit aggregation of the α and β subunits but only when cells are grown at 37° [49]. At 30°, fmc1 mutants assemble F1 and grow normally on non-fermentable carbon sources. A second interesting property of fmc1 mutants is their suppression by overexpression of ATP12. Based on these observations Fmc1p was proposed to be required for correct folding of Atp12p.
III.2. Factors required for expression of subunits 6, 8 and 9 of F0
Most of the known ATP synthase-specific accessory factors target the endogenously encoded subunits of F0. At present 9 such factors are known to affect the stability and/or translation of the mitochondrial ATP6 and ATP9 mRNAs.
ATP6 factors - Mutations in NCA2 and NCA3 when present together express a cold-sensitive growth defect on respiratory substrates that has been ascribed to a deficiency of ATP synthase [60-62]. Both Nca2p and Nca3p have been localized to mitochondria. The nca2, nca3 double mutant overexpresses the primary transcript containing both COX1 and ATP8/6 but has less of the longer 5.2 kb mRNA [61, 62]. The reason for the deficit of the 5.2 kb transcript is not clear but could be due to inefficient processing of the primary transcript. Since the mutant exhibits reduced levels of subunits 6 and 8, the mutations could also affect translation of the ATP8/6 mRNA. This would imply that translation of the longer transcript is favored. The relative translation rates of the two mRNAs, however, are not known.
The stability of the 4.6 and 5.2 kb mRNAs is also dependent on AEP3, which codes for a peripherally associated inner membrane protein [47]. RNA turnover is much more severe in aep3 than in nca2, nca3 double mutants. The steady-state concentration of the long ATP8/6 transcript in the aep3 mutant is reduced to about one third of wild type while the short transcript is undetectable. The presence of normal levels of COX1 mRNA in aep3 mutants excludes a transcriptional defect. The mutants display some accumulation of the primary COX1/ATP8/ATP6 precursor suggesting that there may be a secondary effect of the mutation on processing as well. However, because of the presence of normal amounts of COX1 mRNA, a processing defect by itself cannot account for the phenotype of the mutant [47].
NAM1 (MTF2) is the fourth factor reported to contribute to the stability of the ATP8/6 mRNA [63, 64]. This gene was identified as a high-copy suppressor of mitochondrial splicing deficient mutants [63]. Subsequent studies indicated that nam1 mutants had lower concentrations of COB, COX1 and ATP8/6 mRNAs [65]. A defect in transcription of these regions of the genome was excluded based on the presence of normal amounts of the stable lariat form of group II introns emanating from COB and COX1. Furthermore, the effect of the nam1 mutation was confined primarily to the ATP8/6 mRNAs when present in the background of an intronless mitochondrial genome [65]. This suggests that Nam1p is likely to be an ATP8/6 stability factor, but because of the effect of the mutation on cytochrome b mRNA in the intron-containing background, this factor may also have a secondary function in RNA processing. Nam1p has been localized to mitochondria where it is loosely associated with matrix side of the inner membrane [66]. It is interesting that mutations in the N-terminal region of the mitochondrial RNA polymerase (Rpo4p), which specifically reduce the concentrations of the COX1 and COB mRNAs, are suppressed by overexpression of NAM1 [67]. Significantly, two-hybrid assays indicate that Nam1p interacts with the N-terminal domain of the polymerase. These observations suggest that stability factors such as Nam1p may interact with the cognate RNAs during transcription and that their role may be to feed the nascent RNA to the translational machinery [67]. Stability may, therefore, be the property of an mRNA that is protected from degradation by virtue of being engaged by the translational machinery.
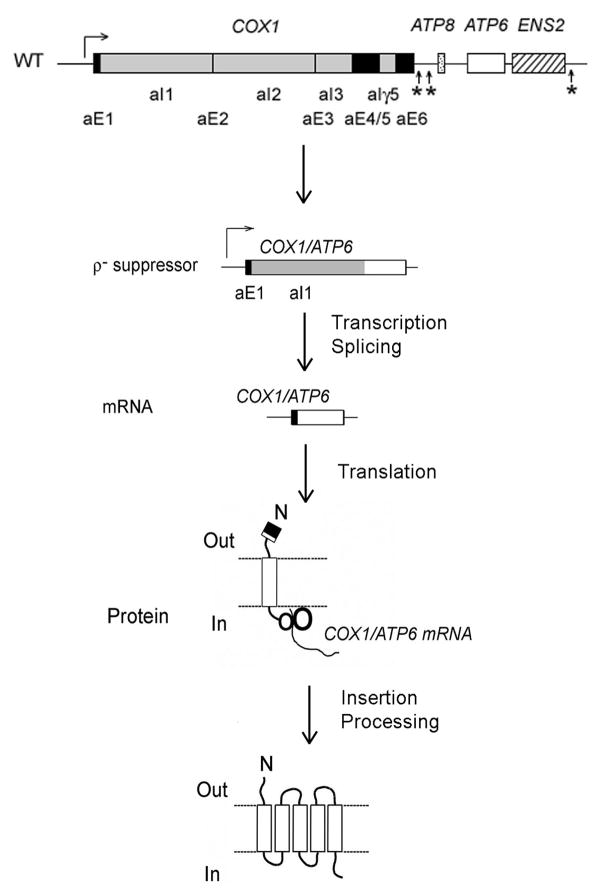
Expression of a number of yeast mitochondrial mRNAs requires transcript-specific translational activators some of which have been shown to interact with the 5′ UTRs of the mRNAs [68]. This is also true of the ATP8/6 mRNAs. Translation of subunit 6 but not subunit 8 is strictly dependent on the product of ATP22 [52, 69]. This is supported by the absence of subunit 6, but not subunits 8 and 9, in atp22 mutants and suppression of an atp22 null mutant by a ρ- genome in which the ATP6 coding sequence starting with the fourth codon was fused to a sequence consisting of the 5′-UTR, the first exon and the entire first intron of COX1 (Fig. 4) [69]. The ability of this recombinant genome to suppress the atp22 null mutant depends on its co-existence with normal mitochondrial DNA, which supplies all the other genes necessary for respiration. The fusion gene expresses a hybrid RNA, which after splicing removes the COX1 intron. This novel mRNA is translated with the assistance of the COX1 translational activators, Pet309p [70] and Mss51p [71]. The translation product is then cleaved at the normal processing site of subunit 6 (still intact in the hybrid protein) to produce mature subunit 6 and a polypeptide consisting of the sequence encoded by the first exon of COX1 plus the 6 residues of the subunit 6 N-terminal presequence (Fig. 4). The ability of the fusion gene to suppress the atp22 mutant confirms that only translation of ATP6 in the bicistronic mRNA is activated by Atp22p [69].
Figure 4.
Mechanism of suppression of an atp22 null mutant by a ρ- genome in which the ATP6 coding sequence was fused to the 5′-UTR, the first exon and the entire first intron of the COX1 gene (see text for description).
ATP9 factors - Several factors have been inferred to influence the stability and translatability of the ATP9 mRNA. ATP25 codes for a protein that is proteolytically cleaved approximately midway in the sequence [72]. Both halves of the proteins are detected in mitochondria and there is compelling evidence that each half is involved in a different aspect of subunit 9 biogenesis. Analyses of the atp25 mutants, including a temperature-sensitive mutant, have provided unambiguous evidence that the C-terminal half of the Atp25p (cAtp25p) functions as an RNA stability factor [72]. When grown at the non-permissive temperature the atp25 ts mutant lacks the 0.9 kb ATP9 mRNA completely, although the tRNAser and of the 1.5 kb ATP9 precursor from which the tRNA has been excised are present in normal amounts. The mutant synthesizes both subunits 8 and 6 but not subunit 9. Although this phenotype indicates that cAtp25p is an ATP9 mRNA-specific stability factor, it does not exclude the possibility that it may also be involved in translation of subunit 9.
AEP2/ATP13 also codes for a mitochondrial protein that has been implicated in expression of subunit 9 [41, 73-75]. Mutations in this gene cause severe deficits of the ATP9 mRNA and of subunit 9. The aep2 mutants have somewhat higher concentrations of the ATP9 precursor with the unprocessed tRNAser [73, 74]. These observations suggest that Aep2p may be involved in ATP9 mRNA processing/stability [41, 74]. Other evidence, however, is more consistent with a role of the Aep2p in translation of the ATP9 mRNA. Some aep2 mutants have been reported to contain 20-30% of the mRNA even though there is not detectable synthesis of subunit 9 [73]. Additionally, revertants of an aep2 ts mutant were ascertained to have a T->C point mutation in mitochondrial DNA 16 nucleotides upstream of the ATP9 initiation codon [51]. The proximity of the mutation to the start of the gene suggested that Aep2p activates translation by interacting with the mRNA and that the observed increase in turnover of the messenger is a secondary effect due to a block in recognition of the mRNA by the translational apparatus [51]. Obviously, a definitive statement about the function of this protein will require more work.
The third mitochondrial protein implicated in subunit 9 expression is encoded by AEP1/NCA1 [76, 77]. There are two conflicting studies dealing with the function of Aep1p. The earlier study suggested that Aep1p is a subunit 9 specific translation factor as a ts aep1 allele was found to contain normal levels of the ATP9 mRNA but to be lacking in the translation product [76]. Another study, however, proposed Aep1p to be an ATP9 mRNA stability factor based on the results obtained with a different ts mutant, in which there was a severe reduction of the ATP9 mRNA at the restrictive temperature [77]. The explanation for this discrepancy may lie in the different experimental conditions used in the two studies. The selective effect of the aep1 ts allele on translation but not the abundance of the ATP9 mRNA was observed when the mutant was exposed to the restrictive temperature for 3 hours [76]. In contrast loss of the ATP9 mRNA was sustained when the ts mutant was incubated under restrictive conditions for longer times [77]. This would suggest that the translational defect of aep2 mutants has a secondary effect on mRNA stability.
III.3. Protein-dependent post-translational steps of ATPase assembly
Processing of the subunit 6 precursor
ATP23 codes for a mitochondrial metalloprotease that processes the precursor form of subunit 6 [53, 78]. The Atp23p protease is associated with the inner membrane such that its C-terminal domain is localized in the intermembrane space [53]. Null mutants in this gene are deficient in oligomcyin-sensitive ATPase and have a subunit 6 with a retarded electrophoretic migration indicative of impaired maturation of this F0 constituent. Processing of the subunit 6 precursor is also arrested in atp23 mutants with a Q->E mutation in a conserved HEXXH motif known from studies of other metalloproteases to abolish their proteolytic activity [79, 80]. Surprisingly the mutant harboring the Q->E mutation is able to assemble a functional ATP synthase complex and grows as well as wild type on non-fermentable substrates. These results indicate that removal of the subunit 6 presequence is not essential for biogenesis of the ATP synthase and that Atp23p, in addition to its proteolytic activity, provides another function essential for F0 assembly.
While the phenotype of the Q->E atp23 mutant indicates that maturation of the subunit 6 precursor does not interfere with its assembly or activity, it does not answer the question of whether the presequence of subunit 6 is necessary for assembly of F0. This was resolved by analyzing the phenotype of mutants in which the codons for amino acids 2-9 or 2-10 of the subunit 6 precursor were deleted from the ATP6 gene [81]. Such leaderless atp6 mutants grow as well as wild type on respiratory substrates and have mitochondria that synthesize ATP with the efficiency similar to wild type. Despite the apparent lack of an effect of the mutations on growth and mitochondrial metabolism, assembly of the ATP synthase is some two times less efficient in the mutants [81]. The reduced amount of ATP synthase correlates with a less efficient interaction of subunit 6 with the subunit 9 ring of the F0 sector and accumulation of a subunit 6/8 complex, destined for eventual proteolytic elimination. These results suggest that the presequence either targets subunit 6 to the subunit 9 ring or signals insertion of the subunit 6 precursor into a microcompartment of the membrane for more efficient interaction with the subunit 9 ring [81].
Formation of the subunit 9 ring
The subunit 9 ring has been assumed to form spontaneously [82]. Recent studies, however, indicate that assembly of this structural component is a protein-assisted process [72]. As indicated above, following import into mitochondria, Atp25p is cleaved after residue 292 producing two polypeptides of 32 kDa and 35 kDa, both of which are required for F0 assembly [72]. Null mutations in ATP25 block translation of subunit 9 by causing an increased rate of ATP9 mRNA turnover. Mutants expressing only the 35 kDa C-terminal polypeptide are able to translate subunit 9 but the newly translated protein does not assemble into the ring [72]. These results suggest that cAtp25p confers stability to the ATP9 mRNA while the function of the 32 kDa N-terminal peptide is related to oligomerization of Atp9p into a proper size ring structure [72].
In a recent study from Yoshida's lab, the uncI gene of Propionigenium modestum, the function of which has long remained mysterious, was shown to be indispensable for assembly of the ring in a heterologous bacterial system [83, 84]. The product of bacterial uncI is a small hydrophobic protein (130 residues in E. coli) for which several homologues exist in S. cerevisiae. One of these reading frames, YHL007c-a, has been localized to mitochondria. The other gene, VMA21, has been implicated in assembly of the vacuolar H+-ATPase [85]. YHL007c-a, because of its localization, seems the more likely candidate to be the functional homologue of uncI. This, however, needs to be verified experimentally.
Assembly of subunit 6 with the ring
Three proteins have been linked to the interaction of subunit 6 with the subunit 9 ring. Mutations in ATP10 arrest F0 assembly at a post-translational stage without affecting oligomerization of the F1 subunits into an active ATPase [50, 86]. The product of this gene is an integral component of the inner membrane. Mutations in ATP6 leading to an Ala -> Val subsitution at the 11th residue from the C-terminus of subunit 6 partially correct the assembly defect of atp10 null mutants [50]. This evidence pointing to a genetic interaction of Atp10p and subunit 6 was confirmed by cross-linking experiments showing that Atp10p forms a physical complex with newly translated but unassembled subunit 6 [86].
Yeast allowed to incubate for a period of time in the presence of chloramphenicol accumulate F1 and the other nuclear gene products of the ATP synthase. Mitochondria isolated from such chloramphenicol treated cells are able to assemble a complex that consists minimally of F1, subunit 6 and the subunit 9 ring but probably contains subunits of the stator also [86]. A 48 kDa subunit 9 ring and a 54 kDa complex of subunit 6 and the ring are detected by SDS-PAGE as radiolabeled products following in organello translation of the mitochondrial gene products in the presence of 35S-methionine [86]. Because of their hydrophobicity the subunit 9 ring and the ring/subunit 6 complex fail to be completely depolymerized by SDS. When this assay was used to test for the presence of the 48 kDa and 54 kDa complexes in an atp10 mutant, only the smaller complex consisting of subunit 9 was detected. This evidence indicates that Atp10p is required for the interaction of the ring with subunit 6 but not for ring formation.
Assembly of the ring/subunit 6 complex has also been shown to depend on Atp23p [78] and Oxa1p [87]. Deletion of the ATP23 coding region prevents assembly of F0 even though mutations that abolish the proteolytic activity of Atp23p do not. This was inferred to indicate that in addition to processing the subunit 6 precursor, Atp23p contributes in some other way towards assembly of the ATP synthase [53, 78]. The non-proteolytic function of Atp23p has been probed immunochemically by Western analysis of intermediates formed in atp23 and atp10 null mutants. Mitochondria from both mutants were found to accumulate two novel complexes that were absent in the wild type strain. The larger and less abundant complex was detected with a subunit 9 antibody and an antibody against the α subunit of F1 while the smaller complex reacted only with the subunit 9 antibody [78]. Neither complex contained subunit 6. This evidence suggests that ATP synthase assembly in the atp10 and atp23 mutants is arrested at the same or closely related stage. A partial functional overlap of Atp10p and Atp23p is also indicated by a less efficient processing of the subunit 6 precursor in the atp10 mutant and a partial rescue of the atp10 mutant by ATP23 [53].
Oxa1p is a protein translocase involved in insertion of certain mitochondrial and nuclear gene products into the inner membrane [88, 89]. For example, translocation of the hydrophilic N-terminal domain of subunit 2 of cytochrome oxidase across the inner membane and insertion of the first transmembrane domain both require Oxa1p [90, 91]. Stuart and coworkers have shown that oxa1 mutants contain oligomeric subunit 9 and form the ring/F1 intermediate but are unable to proceed to the downstream step at which this intermediate interacts with subunit 6 [87]. In the same study Oxa1p was found to associate with the newly formed subunit 9 ring prior to its integration into a fully assembled ATP synthase. A tenable interpretation of these observations is that the post translational/insertional interaction of the ring/F1 intermediate with Oxa1p makes the ring competent to further assemble with subunit 6 in an Atp10p and Atp23p dependent manner. This idea is supported the ability of Oxa1p to form a stable complex with the ring [87] and by a report showing that some oxa1 mutants defective in cytochrome oxidase biogenesis are able to express significan amounts of the ATP synthase [92].
IV. Experimental obstacles in the analysis of ATP synthase assembly
The order in which the subunits of F0 and the peripheral stalk interact with one another, remains largely a question of guesswork. The answers to this and related questions have been impeded by the instability of the mitochondrial genome in assembly-arrested mutants [93]. As a result a large percentage of ATP synthase deficient cells convert to secondary ρ- and ρ0 mutants that fail to express all three of the mitochondrially encoded F0 subunits and are, therefore, unsuitable for biochemical studies. This experimental difficulty is minimized in mutants with a partial loss of function, sufficient to express a clear biochemical phenotype, but not to produce an overwhelming number of ρ- and ρ0 cells. Temperature conditional ATP synthase mutants are an alternative as they too tend to sustain less damage in their mitochondrial DNA than do null or stringent point mutants. Another complication is the rapid turnover of some F0 and stator subunits, especially of subunit 6, in ATP synthase deficient mutants [16, 94-100]. This too, makes it very difficult to analyze intermediates that would otherwise be expected to accumulate in mutants arrested at a particular step of the assembly pathway. Although the turnover problem has been more difficult to overcome, some partial answers can be obtained by examining the early steps of the pathway following in organello translation of the endogenous gene products [78, 86, 87].
V. Modular assembly of ATP synthase
As indicated earlier in this article, the ATP synthase consists of three structurally and functionally distinct parts, F0, F1 and the stator. There is evidence to suggest that the ATP synthase may be assembled from these structural modules. It is clear that assembly of the F1 ATPase occurs independent of F0 and the stator [17, 101, 102].
Similarly, oligomerization of subunit 9 takes place even when there is no net assembly of the ATP synthase complex or of a functional F0 unit. The subunit 9 ring is readily detected in yeast atp10 mutants and some other mutants that are blocked in completing assembly of F0 [86]. The subunit 9 ring has also been shown to be present in human ρo mutants, which express this protein from a nuclear gene [103]. Like yeast, human ρo cells, however, are unable to assemble a functional F0 because they lack subunit 6 and 8 [34].
Oligomerization of subunit 9 into the ring is probably the initiating event in F0 assembly. The order in which others F0 subunits assemble is much less obvious. It is not entirely evident if assembly of subunit 6 with the ring is an early or late event. It has been assumed that the interaction of subunit 6 with the ring must be one of the last steps during F0 assembly because of the deleterious effect of an unproductive proton channel on mitochondrial membrane potential [104, 105]. However, a ring/subunit 6 complex would not necessarily have to function as a proton conduit if the channel is blocked until assembly of the ATP synthase is completed.
A late interaction of subunit 6 with the ring is supported by its proteolysis in almost all mutants arrested in assembly of F0 or the stator [16, 95-100]. This has been interpreted to indicate that integration of subunit 6 into the ATP synthase, concomitant with its protection against proteolytic degradation, occurs after other subunits of F0 and the stator have been assembled. This evidence is also indirect and may simply indicate that unassembled subunit 6 is more prone to proteolysis than other proteins when it is not part of the mature ATP synthase. Furthermore, subunit 6 is not unique in this respect as the steady-state levels of other F0 subunits are also markedly decreased in many F0 and stator mutants [17].
There is evidence that the mitochondrially encoded subunits of yeast F0 can interact with one another to form different complexes even when assembly of ATP syntase is arrested. An antibody against subunit 6 coprecipitates subunit 9 and a small fraction of subunit 8 from mitochondria of wild type cells labeled in organello with 35S-methionine when there is no assembly of the ATP synthase [81]. The subunit 6/subunit 8 complexes consist of a heterogeneous mixture of large aggregates. Less of the subunit 6/subunit 9 complex but considerably more of the aggregated subunit 6/subunit 8 complex is precipitated by the subunit 6 antibody from mitochondria of a mutant with a N-terminal truncation of subunit 6 that decreases the amount of ATP synthase to one half of the normal level [81]. These results were interpreted to indicate that subunit 6 is able to form a complex with subunit 9 even when assembly of F0 is not completed. Because this step is less efficient in the leaderless mutant, more of the subunit 6 is available to interact with subunit 8.
In other studies subunit 8 mutants were shown to contain subunit 9 but to be depleted of subunit 6. Mutants lacking subunit 6, on the other hand, contained both subunits 9 and 8. These results suggested that subunit 8 interacts with subunit 9 before subunit 6 [106]. It should be borne in mind, however, that subunit 6 is especially prone to proteolysis in ATP synthase mutants. The rapid turnover is seen not only under steady state conditions but also occurs in some mutants when radiolabeled subunit 6 is translated during a short pulse in cells poisoned with cycloheximide or in isolated mitochondria (Zeng, unpublished). At present the jury is still out on the question of the order in which subunits 6 and 8 as well as some of the nuclearly encoded products of F0 such as subunits f and i(j), interact with the subunit 9 ring. Nor is it excluded that some F0 subunits may pre-assemble with one another before they associate with the ring. The already mentioned subunit 6/8 complex that accumulates in the leaderless subunit 6 mutant could be a bona fide intermediate that undergoes a secondary aggregation and eventual proteolysis as a result of its less efficient assembly with the subunit 9 ring.
The stator of the yeast ATP synthase consists of 4 subunits three of which (d, h, and OSCP) are hydrophilic proteins. Subunit 4, however, has both a membrane anchoring domain and a hydrophilic domain that makes contact with its partners to form a long structure extending from the surface of the inner membrane to the apical region of F1 [17]. The homologous stator subunits purified from the bovine ATP synthase have been shown to interact with each other in a stoichiometric fashion to form a partial stalk [14]. Because only the hydrophilic domain of subunit 4 was used in these studies it was not possible to ascertain if the preformed F1-stator complex could further assemble with the F0 subunits. The reconstitution of a structure characteristic of the stator from the constituent polypeptides suggests that part or the entire stator may self-assemble in vivo. Assembly of the stator is most likely initiated with transport and insertion of subunit 4 into the inner membrane. The hydrophilic region of subunit 4, external to the membrane, may be all that is necessary for the interaction of the other stator components. This could take place in a progressive manner or some preassembly of the hydrophilic stator subunits might occur in the soluble matrix phase prior to their interaction with subunit 4. This scenario is supported by experiments in which the stability of the stator subunits was examined following depletion of one subunit [94]. The assumption in this study was that unassembled subunits would be more rapidly degraded than subunits that were part of an assembled structure. The order of assembly deduced from this approach was subunit 4, followed by OSCP and terminating with subunit d. This experimental approach has the usual caveat that the turnover rate of a particular subunit is a function not only of whether or not it is assembled but is also influenced by how resistant or susceptible it is to proteolysis.
Mutations in the stator subunits have no effect on assembly of F1 but do affect its ability to interact with the subunit 9 ring. The F1 oligomer of stator mutants is recovered in the soluble protein fraction, even when mitochondria are disrupted by a brief sonic treatment to release matrix proteins. This indicates either that the ring/F1 complex is extremely labile or that the stator is crucial for the initial interaction of F1 with the ring. The importance of the stator for stability of the ring/F1 complex is also seen in the requirement of OSCP not only for oligomycin-sensitivity but for binding of F1 to F0 in OSCP-depleted inner membranes vesicles [107]. Even though these observations imply that the F1 module interacts with the ring after the stator has been incorporated into F0, they do not exclude the possibility that some of the stator subunits may be associated with F1 when it attaches to F0. For example, the hydrophilic subunits of the stator may preassemble with F1 before this complex interacts with the membrane embedded part of the stator (subunit 4) and F0 (subunit 9 ring). It may be significant that the stator reconstituted from the hydrophilic subunits of the bovine ATP synthase has been reported to form a 1:1 complex with F1 [14].
VI. Concluding comments
From everything said here, it is apparent that our understanding of how the ATP synthase is assembled is still fragmentary. The biogenesis of the ATP synthase is not easily studied biochemically because of the extensive turnover of key subunits of F0 and the stator in assembly-arrested mutants. This circumstance hampers reconstruction of the sequence of subunit interactions from an analysis of the intermediates that might otherwise be detected in different assembly-arrested mutants. Despite this drawback substantial progress has been made and some key events identified from studies of ATP synthase mutants. In addition to revealing a multiplicity of factors needed for expression of the mitochondrially encoded subunits, such strains have also been useful in identifying chaperones that play important but still not totally understood roles in oligomerization of the α3β3 hexamer of F1 and the subunit 9 ring of F0. Future studies will need to address questions related to the 1) interdependence or lack thereof of F0 and stator assembly, 2) the stage at which F1 interacts with the ring and the dependence of this interaction on the presence of the stator, 3) the sequence in which subunits of F0 interact with each other, and 4) the extent to which hydrophilic subunits of the stator preassemble in the matrix before being incorporated into the stalk through their interaction with the membrane anchored subunit 4? While genetic approaches combined with biochemical analysis of mutant phenotypes will facilitate arriving at answer to some of these questions, biochemical strategies, alternative to those used in the past, will also need to be devised.
Acknowledgments
Some of the studies described in this review were supported by NIH Grant HL22174
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyer PD. The binding change mechanism for ATP synthase--some probabilities and possibilities. Biochim Biophys Acta. 1993;1140:215–250. doi: 10.1016/0005-2728(93)90063-l. [DOI] [PubMed] [Google Scholar]
- 2.Boyer PD. The ATP synthase--a splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 3.Walker JE, Dickson VK. The peripheral stalk of the mitochondrial ATP synthase. Biochim Biophys Acta. 2006;1757:286–296. doi: 10.1016/j.bbabio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Walker JE, Fearnley IM, Gay NJ, Gibson BW, Northrop FD, Powell SJ, Runswick MJ, Saraste M, Tybulewicz VL. Primary structure and subunit stoichiometry of F1-ATPase from bovine mitochondria. J Mol Biol. 1985;184:677–701. doi: 10.1016/0022-2836(85)90313-4. [DOI] [PubMed] [Google Scholar]
- 5.Stock D, Leslie AG, Walker JE. Molecular architecture of the rotary motor in ATP synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons C, Montgomery MG, Leslie AG, Walker JE. The structure of the central stalk in bovine F(1)-ATPase at 2.4 Å resolution. Nat Struct Biol. 2000;7:1055–1061. doi: 10.1038/80981. [DOI] [PubMed] [Google Scholar]
- 7.Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE. The rotary mechanism of ATP synthase. Curr Opin Struct Biol. 2000;10:672–679. doi: 10.1016/s0959-440x(00)00147-0. [DOI] [PubMed] [Google Scholar]
- 8.Dmitriev OY, Jones PC, Fillingame RH. Structure of the subunit c oligomer in the F1Fo ATP synthase: model derived from solution structure of the monomer and cross-linking in the native enzyme. Proc Natl Acad Sci U S A. 1999;96:7785–7790. doi: 10.1073/pnas.96.14.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillingame RH, Dmitriev OY. Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ. Biochim Biophys Acta. 2002;1565:232–245. doi: 10.1016/s0005-2736(02)00572-2. [DOI] [PubMed] [Google Scholar]
- 10.Pogoryelov D, Yu J, Meier T, Vonck J, Dimroth P, Muller DJ. The c15 ring of the Spirulina platensis F-ATP synthase: F1/F0 symmetry mismatch is not obligatory. EMBO Rep. 2005;6:1040–1044. doi: 10.1038/sj.embor.7400517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogoryelov D, Reichen C, Klyszejko AL, Brunisholz R, Muller DJ, Dimroth P, Meier T. The oligomeric state of c rings from cyanobacterial F-ATP synthases varies from 13 to 15. J Bacteriol. 2007;189:5895–5902. doi: 10.1128/JB.00581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillingame RH, Angevine CM, Dmitriev OY. Coupling proton movements to c-ring rotation in F1Fo ATP synthase: aqueous access channels and helix rotations at the a-c interface. Biochim Biophys Acta. 2002;1555:29–36. doi: 10.1016/s0005-2728(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 13.Fillingame RH, Angevine CM, Dmitriev OY. Mechanics of coupling proton movements to c-ring rotation in ATP synthase. FEBS Lett. 2003;555:29–34. doi: 10.1016/s0014-5793(03)01101-3. [DOI] [PubMed] [Google Scholar]
- 14.Collinson IR, Skehel JM, Fearnley IM, Runswick MJ, Walker JE. The F1F0-ATPase complex from bovine heart mitochondria: the molar ratio of the subunits in the stalk region linking the F1 and F0 domains. Biochemistry. 1996;35:12640–12646. doi: 10.1021/bi960969t. [DOI] [PubMed] [Google Scholar]
- 15.Dickson VK, Silvester JA, Fearnley IM, Leslie AG, Walker JE. On the structure of the stator of the mitochondrial ATP synthase. EMBO J. 2006;25:2911–2918. doi: 10.1038/sj.emboj.7601177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spannagel C, Vaillier J, Arselin G, Graves PV, Velours J. The subunit f of mitochondrial yeast ATP synthase--characterization of the protein and disruption of the structural gene ATP17. Eur J Biochem. 1997;247:1111–1117. doi: 10.1111/j.1432-1033.1997.01111.x. [DOI] [PubMed] [Google Scholar]
- 17.Velours J, Arselin G. The Saccharomyces cerevisiae ATP synthase. J Bioenerg Biomembr. 2000;32:383–390. doi: 10.1023/a:1005580020547. [DOI] [PubMed] [Google Scholar]
- 18.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 19.Arnold I, Pfeiffer K, Neupert W, Stuart RA, Schagger H. Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J. 1998;17:7170–7178. doi: 10.1093/emboj/17.24.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen RD, Schroeder CC, Fok AK. An investigation of mitochondrial inner membranes by rapid-freeze deep-etch techniques. J Cell Biol. 1989;108:2233–2240. doi: 10.1083/jcb.108.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paumard P, Vaillier J, Coulary B, Schaeffer J, Soubannier V, Mueller DM, Brethes D, di Rago JP, Velours J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto T, Yoshida Y, Tagawa K. Regulatory proteins of F1F0-ATPase: role of ATPase inhibitor. J Bioenerg Biomembr. 1990;22:27–38. doi: 10.1007/BF00762843. [DOI] [PubMed] [Google Scholar]
- 23.Walker JE. The regulation of catalysis in ATP synthase. Curr Opin Struct Biol. 1994;4:912–918. doi: 10.1016/0959-440x(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 24.Hong S, Pedersen PL. ATP synthase of yeast: structural insight into the different inhibitory potencies of two regulatory peptides and identification of a new potential regulator. Arch Biochem Biophys. 2002;405:38–43. doi: 10.1016/s0003-9861(02)00303-x. [DOI] [PubMed] [Google Scholar]
- 25.Funes S, Davidson E, Claros MG, van Lis R, Perez-Martinez X, Vazquez-Acevedo M, King MP, Gonzalez-Halphen D. The typically mitochondrial DNA-encoded ATP6 subunit of the F1F0-ATPase is encoded by a nuclear gene in Chlamydomonas reinhardtii. J Biol Chem. 2002;277:6051–6058. doi: 10.1074/jbc.M109993200. [DOI] [PubMed] [Google Scholar]
- 26.Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- 27.Braun CJ, Levings CS. Nucleotide Sequence of the F1-ATPase alpha Subunit Gene from Maize Mitochondria. Plant Physiol. 1985;79:571–577. doi: 10.1104/pp.79.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutry M, Briquet M, Goffeau A. The alpha subunit of a plant mitochondrial F1-ATPase is translated in mitochondria. J Biol Chem. 1983;258:8524–8526. [PubMed] [Google Scholar]
- 29.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 30.Macreadie IG, Novitski CE, Maxwell RJ, John U, Ooi BG, McMullen GL, Lukins HB, Linnane AW, Nagley P. Biogenesis of mitochondria: the mitochondrial gene (aap1) coding for mitochondrial ATPase subunit 8 in Saccharomyces cerevisiae. Nucleic Acids Res. 1983;11:4435–4451. doi: 10.1093/nar/11.13.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macino G, Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980;20:507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- 32.Hensgens LA, Grivell LA, Borst P, Bos JL. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979;76:1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dewey RE, Schuster AM, Levings CS, Timothy DH. Nucleotide sequence of F0-ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci U S A. 1985;82:1015–1019. doi: 10.1073/pnas.82.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 35.Viebrock A, Perz A, Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1:565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Boogaart P, Samallo J, Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982;298:187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]
- 37.Brown TA, Ray JA, Waring RB, Scazzocchio C, Davies RW. A mitochondrial reading frame which may code for a second form of ATPase subunit 9 in Aspergillus nidulans. Curt Genet. 1984;8:489–492. doi: 10.1007/BF00410434. [DOI] [PubMed] [Google Scholar]
- 38.Bittner-Eddy P, Monroy AF, Brambl R. Expression of mitochondrial genes in the germinating conidia of Neurospora crassa. J Mol Biol. 1994;235:881–897. doi: 10.1006/jmbi.1994.1046. [DOI] [PubMed] [Google Scholar]
- 39.Zassenhaus HP, Martin NC, Butow RA. Origins of transcripts of the yeast mitochondrial var 1 gene. J Biol Chem. 1984;259:6019–6027. [PubMed] [Google Scholar]
- 40.Christianson T, Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983;258:14025–14033. [PubMed] [Google Scholar]
- 41.Finnegan PM, Payne MJ, Keramidaris E, Lukins HB. Characterization of a yeast nuclear gene, AEP2, required for accumulation of mitochondrial mRNA encoding subunit 9 of the ATP synthase. Curr Genet. 1991;20:53–61. doi: 10.1007/BF00312765. [DOI] [PubMed] [Google Scholar]
- 42.de Zamaroczy M, Bernardi G. The primary structure of the mitochondrial genome of Saccharomyces cerevisiae. Gene. 1986;47:155–177. doi: 10.1016/0378-1119(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 43.Foury F, Roganti T, Lecrenier N, Purnelle B. The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 1998;440:325–331. doi: 10.1016/s0014-5793(98)01467-7. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K, Morishima N, Shibata T. A maturase-like subunit of the sequence-specific endonuclease endo.SceI from yeast mitochondria. J Biol Chem. 1991;266:1977–1984. [PubMed] [Google Scholar]
- 45.Beilharz MW, Cobon GS, Nagley P. Physiological alteration of the pattern of transcription of the oli2 region of yeast mitochondrial DNA. FEBS Lett. 1982;147:235–238. doi: 10.1016/0014-5793(82)81049-1. [DOI] [PubMed] [Google Scholar]
- 46.Simon M, Faye G. Organization and processing of the mitochondrial oxi3/oli2 multigenic transcript in yeast. Mol Gen Genet. 1984;196:266–274. doi: 10.1007/BF00328059. [DOI] [PubMed] [Google Scholar]
- 47.Ellis TP, Helfenbein KG, Tzagoloff A, Dieckmann CL. Aep3p stabilizes the mitochondrial bicistronic mRNA encoding subunits 6 and 8 of the H+-translocating ATP synthase of Saccharomyces cerevisiae. J Biol Chem. 2004;279:15728–15733. doi: 10.1074/jbc.M314162200. [DOI] [PubMed] [Google Scholar]
- 48.Ackerman SH, Tzagoloff A. Identification of two nuclear genes (ATP11, ATP12) required for assembly of the yeast F1-ATPase. Proc Natl Acad Sci U S A. 1990;87:4986–4990. doi: 10.1073/pnas.87.13.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lefebvre-Legendre L, Vaillier J, Benabdelhak H, Velours J, Slonimski PP, di Rago JP. Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F1-ATPase in heat stress conditions. J Biol Chem. 2001;276:6789–6796. doi: 10.1074/jbc.M009557200. [DOI] [PubMed] [Google Scholar]
- 50.Paul MF, Barrientos A, Tzagoloff A. A single amino acid change in subunit 6 of the yeast mitochondrial ATPase suppresses a null mutation in ATP10. J Biol Chem. 2000;275:29238–29243. doi: 10.1074/jbc.M004546200. [DOI] [PubMed] [Google Scholar]
- 51.Ellis TP, Lukins HB, Nagley P, Corner BE. Suppression of a nuclear aep2 mutation in Saccharomyces cerevisiae by a base substitution in the 5′-untranslated region of the mitochondrial oli1 gene encoding subunit 9 of ATP synthase. Genetics. 1999;151:1353–1363. doi: 10.1093/genetics/151.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helfenbein KG, Ellis TP, Dieckmann CL, Tzagoloff A. ATP22, a nuclear gene required for expression of the F0 sector of mitochondrial ATPase in Saccharomyces cerevisiae. J Biol Chem. 2003;278:19751–19756. doi: 10.1074/jbc.M301679200. [DOI] [PubMed] [Google Scholar]
- 53.Zeng X, Neupert W, Tzagoloff A. The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol Biol Cell. 2007;18:617–626. doi: 10.1091/mbc.E06-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzagoloff A, Dieckmann CL. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990;54:211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang ZG, Ackerman SH. Identification of functional domains in Atp11p. Protein required for assembly of the mitochondrial F1-ATPase in yeast. J Biol Chem. 1996;271:4887–4894. doi: 10.1074/jbc.271.9.4887. [DOI] [PubMed] [Google Scholar]
- 56.Wang ZG, Ackerman SH. Mutational studies with Atp12p, a protein required for assembly of the mitochondrial F1-ATPase in yeast. Identification of domains important for Atp12p function and oligomerization. J Biol Chem. 1998;273:2993–3002. doi: 10.1074/jbc.273.5.2993. [DOI] [PubMed] [Google Scholar]
- 57.Paul MF, Ackerman S, Yue J, Arselin G, Velours J, Tzagoloff A, Ackermann S. Cloning of the yeast ATP3 gene coding for the gamma-subunit of F1 and characterization of atp3 mutants. J Biol Chem. 1994;269:26158–26164. [PubMed] [Google Scholar]
- 58.Giraud MF, Velours J. ATP synthase of yeast mitochondria. Isolation of the F1 delta subunit, sequence and disruption of the structural gene. Eur J Biochem. 1994;222:851–859. doi: 10.1111/j.1432-1033.1994.tb18932.x. [DOI] [PubMed] [Google Scholar]
- 59.Guelin E, Chevallier J, Rigoulet M, Guerin B, Velours J. ATP synthase of yeast mitochondria. Isolation and disruption of the ATP epsilon gene. J Biol Chem. 1993;268:161–167. [PubMed] [Google Scholar]
- 60.Pelissier PP, Camougrand NM, Manon ST, Velours GM, Guerin MG. Regulation by nuclear genes of the mitochondrial synthesis of subunits 6 and 8 of the ATP synthase of Saccharomyces cerevisiae. J Biol Chem. 1992;267:2467–2473. [PubMed] [Google Scholar]
- 61.Pelissier P, Camougrand N, Velours G, Guerin M. NCA3, a nuclear gene involved in the mitochondrial expression of subunits 6 and 8 of the Fo-F1 ATP synthase of S. cerevisiae. Curr Genet. 1995;27:409–416. doi: 10.1007/BF00311209. [DOI] [PubMed] [Google Scholar]
- 62.Camougrand N, Pelissier P, Velours G, Guerin M. NCA2, a second nuclear gene required for the control of mitochondrial synthesis of subunits 6 and 8 of ATP synthase in Saccharomyces cerevisiae. J Mol Biol. 1995;247:588–596. doi: 10.1006/jmbi.1995.0165. [DOI] [PubMed] [Google Scholar]
- 63.Asher EB, Groudinsky O, Dujardin G, Altamura N, Kermorgant M, Slonimski PP. Novel class of nuclear genes involved in both mRNA splicing and protein synthesis in Saccharomyces cerevisiae mitochondria. Mol Gen Genet. 1989;215:517–528. doi: 10.1007/BF00427051. [DOI] [PubMed] [Google Scholar]
- 64.Lisowsky T, Michaelis G. Mutations in the genes for mitochondrial RNA polymerase and a second mitochondrial transcription factor of Saccharomyces cerevisiae. Mol Gen Genet. 1989;219:125–128. doi: 10.1007/BF00261167. [DOI] [PubMed] [Google Scholar]
- 65.Groudinsky O, Bousquet I, Wallis MG, Slonimski PP, Dujardin G. The NAM1/MTF2 nuclear gene product is selectively required for the stability and/or processing of mitochondrial transcripts of the atp6 and of the mosaic, cox1 and cyt. b genes in Saccharomyces cerevisiae. Mol Gen Genet. 1993;240:419–427. doi: 10.1007/BF00280396. [DOI] [PubMed] [Google Scholar]
- 66.Wallis MG, Groudinsky O, Slonimski PP, Dujardin G. The NAM1 protein (NAM1p), which is selectively required for cox1, cyt. b and atp6 transcript processing/stabilisation, is located in the yeast mitochondrial matrix. Eur J Biochem. 1994;222:27–32. doi: 10.1111/j.1432-1033.1994.tb18837.x. [DOI] [PubMed] [Google Scholar]
- 67.Rodeheffer MS, Boone BE, Bryan AC, Shadel GS. Nam1p, a protein involved in RNA processing and translation, is coupled to transcription through an interaction with yeast mitochondrial RNA polymerase. J Biol Chem. 2001;276:8616–8622. doi: 10.1074/jbc.M009901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costanzo MC, Fox TD. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- 69.Zeng X, Hourset A, Tzagoloff A. The Saccharomyces cerevisiae ATP22 gene codes for the mitochondrial ATPase subunit 6-specific translation factor. Genetics. 2007;175:55–63. doi: 10.1534/genetics.106.065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manthey GM, Przybyla-Zawislak BD, McEwen JE. The Saccharomyces cerevisiae Pet309 protein is embedded in the mitochondrial inner membrane. Eur J Biochem. 1998;255:156–161. doi: 10.1046/j.1432-1327.1998.2550156.x. [DOI] [PubMed] [Google Scholar]
- 71.Decoster E, Simon M, Hatat D, Faye G. The MSS51 gene product is required for the translation of the COX1 mRNA in yeast mitochondria. Mol Gen Genet. 1990;224:111–118. doi: 10.1007/BF00259457. [DOI] [PubMed] [Google Scholar]
- 72.Zeng X, Barros MH, Shulman T, Tzagoloff A. ATP25, a New Nuclear Gene of Saccharomyces cerevisiae Required for Expression and Assembly of the Atp9p Subunit of Mitochondrial ATPase. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-08-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackerman SH, Gatti DL, Gellefors P, Douglas MG, Tzagoloff A. ATP13, a nuclear gene of Saccharomyces cerevisiae essential for the expression of subunit 9 of the mitochondrial ATPase. FEBS Lett. 1991;278:234–238. doi: 10.1016/0014-5793(91)80124-l. [DOI] [PubMed] [Google Scholar]
- 74.Payne MJ, Schweizer E, Lukins HB. Properties of two nuclear pet mutants affecting expression of the mitochondrial oli1 gene of Saccharomyces cerevisiae. Curr Genet. 1991;19:343–351. doi: 10.1007/BF00309594. [DOI] [PubMed] [Google Scholar]
- 75.Finnegan PM, Ellis TP, Nagley P, Lukins HB. The mature AEP2 gene product of Saccharomyces cerevisiae, required for the expression of subunit 9 of ATP synthase, is a 58 kDa mitochondrial protein. FEBS Lett. 1995;368:505–508. doi: 10.1016/0014-5793(95)00727-q. [DOI] [PubMed] [Google Scholar]
- 76.Payne MJ, Finnegan PM, Smooker PM, Lukins HB. Characterization of a second nuclear gene, AEP1, required for expression of the mitochondrial OLI1 gene in Saccharomyces cerevisiae. Curr Genet. 1993;24:126–135. doi: 10.1007/BF00324676. [DOI] [PubMed] [Google Scholar]
- 77.Ziaja K, Michaelis G, Lisowsky T. Nuclear control of the messenger RNA expression for mitochondrial ATPase subunit 9 in a new yeast mutant. J Mol Biol. 1993;229:909–916. doi: 10.1006/jmbi.1993.1095. [DOI] [PubMed] [Google Scholar]
- 78.Osman C, Wilmes C, Tatsuta T, Langer T. Prohibitins interact genetically with Atp23, a novel processing peptidase and chaperone for the F1Fo-ATP synthase. Mol Biol Cell. 2007;18:627–635. doi: 10.1091/mbc.E06-09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jongeneel CV, Bouvier J, Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989;242:211–214. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- 80.Becker AB, Roth RA. An unusual active site identified in a family of zinc metalloendopeptidases. Proc Natl Acad Sci U S A. 1992;89:3835–3839. doi: 10.1073/pnas.89.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng X, Kucharczyk R, di Rago JP, Tzagoloff A. The leader peptide of yeast Atp6p is required for efficient interaction with the Atp9p ring of the mitochondrial ATPase. J Biol Chem. 2007;282:36167–36176. doi: 10.1074/jbc.M705436200. [DOI] [PubMed] [Google Scholar]
- 82.Arechaga I, Butler PJ, Walker JE. Self-assembly of ATP synthase subunit c rings. FEBS Lett. 2002;515:189–193. doi: 10.1016/s0014-5793(02)02447-x. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki T, Ozaki Y, Sone N, Feniouk BA, Yoshida M. The product of uncI gene in F1Fo-ATP synthase operon plays a chaperone-like role to assist c-ring assembly. Proc Natl Acad Sci U S A. 2007;104:20776–20781. doi: 10.1073/pnas.0708075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozaki Y, Suzuki T, Kuruma Y, Ueda T, Yoshida M. UncI protein can mediate ring-assembly of c-subunits of FoF1-ATP synthase in vitro. Biochem Biophys Res Commun. 2008;367:663–666. doi: 10.1016/j.bbrc.2007.12.170. [DOI] [PubMed] [Google Scholar]
- 85.Malkus P, Graham LA, Stevens TH, Schekman R. Role of Vma21p in assembly and transport of the yeast vacuolar ATPase. Mol Biol Cell. 2004;15:5075–5091. doi: 10.1091/mbc.E04-06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tzagoloff A, Barrientos A, Neupert W, Herrmann JM. Atp10p assists assembly of Atp6p into the F0 unit of the yeast mitochondrial ATPase. J Biol Chem. 2004;279:19775–19780. doi: 10.1074/jbc.M401506200. [DOI] [PubMed] [Google Scholar]
- 87.Jia L, Dienhart MK, Stuart RA. Oxa1 directly interacts with Atp9 and mediates its assembly into the mitochondrial F1Fo-ATP synthase complex. Mol Biol Cell. 2007;18:1897–1908. doi: 10.1091/mbc.E06-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonnefoy N, Chalvet F, Hamel P, Slonimski PP, Dujardin G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J Mol Biol. 1994;239:201–212. doi: 10.1006/jmbi.1994.1363. [DOI] [PubMed] [Google Scholar]
- 89.Altamura N, Capitanio N, Bonnefoy N, Papa S, Dujardin G. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase. FEBS Lett. 1996;382:111–115. doi: 10.1016/0014-5793(96)00165-2. [DOI] [PubMed] [Google Scholar]
- 90.He S, Fox TD. Membrane translocation of mitochondrially coded Cox2p: distinct requirements for export of N and C termini and dependence on the conserved protein Oxa1p. Mol Biol Cell. 1997;8:1449–1460. doi: 10.1091/mbc.8.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herrmann JM, Neupert W, Stuart RA. Insertion into the mitochondrial inner membrane of a polytopic protein, the nuclear-encoded Oxa1p. EMBO J. 1997;16:2217–2226. doi: 10.1093/emboj/16.9.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hell K, Neupert W, Stuart RA. Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 2001;20:1281–1288. doi: 10.1093/emboj/20.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Contamine V, Picard M. Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast. Microbiol Mol Biol Rev. 2000;64:281–315. doi: 10.1128/mmbr.64.2.281-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Straffon AF, Prescott M, Nagley P, Devenish RJ. The assembly of yeast mitochondrial ATP synthase: subunit depletion in vivo suggests ordered assembly of the stalk subunits b, OSCP and d. Biochim Biophys Acta. 1998;1371:157–162. doi: 10.1016/s0005-2736(98)00034-0. [DOI] [PubMed] [Google Scholar]
- 95.Vaillier J, Arselin G, Graves PV, Camougrand N, Velours J. Isolation of supernumerary yeast ATP synthase subunits e and i. Characterization of subunit i and disruption of its structural gene ATP18. J Biol Chem. 1999;274:543–548. doi: 10.1074/jbc.274.1.543. [DOI] [PubMed] [Google Scholar]
- 96.Velours J, Durrens P, Aigle M, Guerin B. ATP4, the structural gene for yeast F0F1 ATPase subunit 4. Eur J Biochem. 1988;170:637–642. doi: 10.1111/j.1432-1033.1988.tb13745.x. [DOI] [PubMed] [Google Scholar]
- 97.Norais N, Prome D, Velours J. ATP synthase of yeast mitochondria. Characterization of subunit d and sequence analysis of the structural gene ATP7. J Biol Chem. 1991;266:16541–16549. [PubMed] [Google Scholar]
- 98.Arselin G, Vaillier J, Graves PV, Velours J. ATP synthase of yeast mitochondria. Isolation of the subunit h and disruption of the ATP14 gene. J Biol Chem. 1996;271:20284–20290. doi: 10.1074/jbc.271.34.20284. [DOI] [PubMed] [Google Scholar]
- 99.Paul MF, Velours J, Arselin de Chateaubodeau G, Aigle M, Guerin B. The role of subunit 4, a nuclear-encoded protein of the F0 sector of yeast mitochondrial ATP synthase, in the assembly of the whole complex. Eur J Biochem. 1989;185:163–171. doi: 10.1111/j.1432-1033.1989.tb15098.x. [DOI] [PubMed] [Google Scholar]
- 100.Prescott M, Bush NC, Nagley P, Devenish RJ. Properties of yeast cells depleted of the OSCP subunit of mitochondrial ATP synthase by regulated expression of the ATP5 gene. Biochem Mol Biol Int. 1994;34:789–799. [PubMed] [Google Scholar]
- 101.Schatz G. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic “petite” mutant of Saccharomyces cerevisiae. J Biol Chem. 1968;243:2192–2199. [PubMed] [Google Scholar]
- 102.Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem. 1969;244:5027–5033. [PubMed] [Google Scholar]
- 103.Garcia JJ, Ogilvie I, Robinson BH, Capaldi RA. Structure, functioning, and assembly of the ATP synthase in cells from patients with the T8993G mitochondrial DNA mutation. Comparison with the enzyme in Rho0 cells completely lacking mtdna. J Biol Chem. 2000;275:11075–11081. doi: 10.1074/jbc.275.15.11075. [DOI] [PubMed] [Google Scholar]
- 104.Lai-Zhang J, Xiao Y, Mueller DM. Epistatic interactions of deletion mutants in the genes encoding the F1-ATPase in yeast Saccharomyces cerevisiae. EMBO J. 1999;18:58–64. doi: 10.1093/emboj/18.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duvezin-Caubet S, Rak M, Lefebvre-Legendre L, Tetaud E, Bonnefoy N, di Rago JP. A “petite obligate” mutant of Saccharomyces cerevisiae: functional mtDNA is lethal in cells lacking the delta subunit of mitochondrial F1-ATPase. J Biol Chem. 2006;281:16305–16313. doi: 10.1074/jbc.M513805200. [DOI] [PubMed] [Google Scholar]
- 106.Hadikusumo RG, Meltzer S, Choo WM, Jean-Francois MJ, Linnane AW, Marzuki S. The definition of mitochondrial H+ ATPase assembly defects in mit- mutants of Saccharomyces cerevisiae with a monoclonal antibody to the enzyme complex as an assembly probe. Biochim Biophys Acta. 1988;933:212–222. doi: 10.1016/0005-2728(88)90072-2. [DOI] [PubMed] [Google Scholar]
- 107.Tzagoloff A. Assembly of the mitochondrial membrane system. 3. Function and synthesis of the oligomycin sensitivity-conferring protein of yeast mitochondria. J Biol Chem. 1970;245:1545–1551. [PubMed] [Google Scholar]