Abstract
Background
Oxidative stress may cause gastrointestinal cancers. The evidence on whether antioxidant supplements are effective in preventing gastrointestinal cancers is contradictory.
Objectives
To assess the beneficial and harmful effects of antioxidant supplements in preventing gastrointestinal cancers.
Search methods
We identified trials through the trials registers of the four Cochrane Review Groups on gastrointestinal diseases, The Cochrane Central Register of Controlled Trials in The Cochrane Library (Issue 2, 2007), MEDLINE, EMBASE, LILACS, SCI‐EXPANDED, and The Chinese Biomedical Database from inception to October 2007. We scanned reference lists and contacted pharmaceutical companies.
Selection criteria
Randomised trials comparing antioxidant supplements to placebo/no intervention examining occurrence of gastrointestinal cancers.
Data collection and analysis
Two authors (GB and DN) independently selected trials for inclusion and extracted data. Outcome measures were gastrointestinal cancers, overall mortality, and adverse effects. Outcomes were reported as relative risks (RR) with 95% confidence interval (CI) based on random‐effects and fixed‐effect model meta‐analysis. Meta‐regression assessed the effect of covariates across the trials.
Main results
We identified 20 randomised trials (211,818 participants), assessing beta‐carotene (12 trials), vitamin A (4 trials), vitamin C (8 trials), vitamin E (10 trials), and selenium (9 trials). Trials quality was generally high. Heterogeneity was low to moderate. Antioxidant supplements were without significant effects on gastrointestinal cancers (RR 0.94, 95% CI 0.83 to 1.06). However, there was significant heterogeneity (I2 = 54.0%, P = 0.003). The heterogeneity may have been explained by bias risk (low‐bias risk trials RR 1.04, 95% CI 0.96 to 1.13 compared to high‐bias risk trials RR 0.59, 95% CI 0.43 to 0.80; test of interaction P < 0.0005), and type of antioxidant supplement (beta‐carotene potentially increasing and selenium potentially decreasing cancer risk). The antioxidant supplements had no significant effects on mortality in a random‐effects model meta‐analysis (RR 1.02, 95% CI 0.97 to 1.07, I2 = 53.5%), but significantly increased mortality in a fixed‐effect model meta‐analysis (RR 1.04, 95% CI 1.02 to 1.07). Beta‐carotene in combination with vitamin A (RR 1.16, 95% CI 1.09 to 1.23) and vitamin E (RR 1.06, 95% CI 1.02 to 1.11) significantly increased mortality. Increased yellowing of the skin and belching were non‐serious adverse effects of beta‐carotene. In five trials (four with high risk of bias), selenium seemed to show significant beneficial effect on gastrointestinal cancer occurrence (RR 0.59, 95% CI 0.46 to 0.75, I2 = 0%).
Authors' conclusions
We could not find convincing evidence that antioxidant supplements prevent gastrointestinal cancers. On the contrary, antioxidant supplements seem to increase overall mortality. The potential cancer preventive effect of selenium should be tested in adequately conducted randomised trials.
Keywords: Humans, Dietary Supplements, Dietary Supplements/adverse effects, Antioxidants, Antioxidants/administration & dosage, Antioxidants/adverse effects, Gastrointestinal Neoplasms, Gastrointestinal Neoplasms/mortality, Gastrointestinal Neoplasms/prevention & control, Liver Neoplasms, Liver Neoplasms/mortality, Liver Neoplasms/prevention & control, Pancreatic Neoplasms, Pancreatic Neoplasms/mortality, Pancreatic Neoplasms/prevention & control, Randomized Controlled Trials as Topic
Plain language summary
Antioxidant supplements cannot be recommended for gastrointestinal cancer prevention
Our body cannot synthesize all compounds that are essential for health. Therefore such compounds must be taken through diet. Oxidative stress may cause cell damage that is implicated in chronic diseases like cancer. Gastrointestinal cancers are among the most common cancers worldwide. The poor prognosis of patients diagnosed with gastrointestinal cancers made primary prevention a potentially attractive approach. The evidence on whether antioxidant supplements are effective in decreasing gastrointestinal cancers is contradictory.
In this review prevention with antioxidant supplements of oesophageal, gastric, small intestinal, colorectal, pancreatic, liver, and biliary tract cancers is assessed. The review includes 20 randomised clinical trials. In total, 211,818 participants were randomised to antioxidant supplements (beta‐carotene, vitamin A, vitamin C, vitamin E, and selenium) versus placebo. Trial quality was exceptionally good.
Based on properly designed and conducted randomised clinical trials, convincing evidence that beta‐carotene, vitamin A, vitamin C, and vitamin E or their combinations may prevent gastrointestinal cancers is not found. A total of 2057 of 95084 participants (2.2%) randomised to antioxidant supplements and 1548 of 78935 participants (2.0%) randomised to placebo developed gastrointestinal cancers. These antioxidant supplements even seem to increase mortality. A total of 17114 of 122,501 participants (14.0%) randomised to antioxidant supplements and 8799 of 78693 participants (11.2%) randomised to placebo died. Selenium alone may have preventive effects on gastrointestinal cancers. This finding, however, is based on trials with flaws in their design and needs confirmation in properly conducted randomised clinical trials.
Background
Our body cannot synthesize all compounds that are essential for health. Therefore they must be taken through diet. Oxidative stress may cause cell damage that is implicated in chronic diseases like cancer (Sies 1985; Ames 1995). Antioxidants are compounds that can protect against oxidative stress (Diplock 1994; Poppel 1997; Papas 1999; Tamimi 2002; Willcox 2004). Laboratory and epidemiologic studies suggest a role of antioxidants in cancer prevention (Schrauzer 1977; Peto 1981). The possibility to improve health and prevent diseases by ameliorating excessive oxidative stress has attracted the attention of researchers in the last decades (Willcox 2004). Even though a healthy diet provides a sufficient amount of antioxidants, there are a number of people who regularly take antioxidant supplements (Balluz 2000; Millen 2004; Radimer 2004; Lichtenstein 2005; Nichter 2006).
Gastrointestinal cancers Gastrointestinal cancers are among the most common cancers and the leading cause of cancer death worldwide (Ferlay 2004). The poor prognosis of patients diagnosed with gastrointestinal cancers made chemoprevention an attractive approach. It is, therefore, understandable that antioxidant prevention of gastrointestinal cancers has drawn much attention (Garcea 2003; Sharma 2004; Grau 2006).
Oesophageal cancer is characterized by low likelihood of cure (Enzinger 2003). Prevention is complicated by the fact that the two major histologic types, ie, squamous‐cell carcinoma and adenocarcinoma, differ substantially (Fitzgerald 2006; Holmes 2007). The role of oxidative stress in the aetiology of two histological types of oesophageal cancer is unclear (Tzonou 1996; Terry 2000; Mayne 2001). Antioxidants were discussed as protective agents in studies of oesophageal squamous‐cell carcinoma as well as Barrett's oesophagus, a precancerous condition for oesophageal adenocarcinoma (Cheng 1996; Terry 2000; Sihvo 2002; Mehta 2005; Stoner 2007).
Gastric cancer is the fourth most common cancer and second leading cause of cancer death in the world (Ferlay 2004). Helicobacter pylori is the important aetiological agent of gastric cancer (Sugiyama 2004). Eradication of Helicobacter pylori and chemoprevention with antioxidants emerged as alternative strategies in reducing the incidence and mortality of gastric cancer (Leung 2006; SIT 2006; Plummer 2007).
Small intestinal cancers are rare (Neugut 1998), and prevention with antioxidant supplements has, according to our knowledge, not been extensively tested.
Colorectal cancer is the third most common cancer worldwide (Ferlay 2004). It develops in multiple steps (Potter 1999). Most colorectal cancers arise from adenomas, as a result of a series of molecular changes that transform normal colonic epithelial cells into colorectal cancer (Janne 2000; Lynch 2002). The transition from normal mucosa to carcinoma offer opportunities for prevention (Gwyn 2002). Observational studies postulate that diet may be associated with colorectal cancer. A diet rich in antioxidants is claimed to be able to lower the risk of colorectal cancer (Boyle 1985; Bostick 1993; Kune 2006). Antioxidants were the first agents evaluated in prevention of colorectal cancer (Grau 2006). However, antioxidant supplements have no significant effect on primary or secondary prevention of colorectal adenoma (Bjelakovic 2006).
Pancreatic cancer has a poor prognosis. Possible aetiologic factors for pancreatic cancer include chronic pancreatitis, smoking, diabetes, and other medical conditions (Lowenfels 2006). Chronic inflammation, resulting in chronic phagocytic activity, one of the major endogenous sources of free radicals, is associated with cancer of several organs (Collins 1987; Shimoda 1994; Holzinger 1999). Experimental and observational studies have shown that antioxidants might be effective in the prevention of pancreatic cancer (Doucas 2006).
Hepatocellular carcinoma incidence has increased over the last decades. Cirrhosis and aflatoxins are the main risk factors for its development (Yates 2007). Viral or chemical damage to the liver results in oxidative damage that may inhibit apoptosis and promote hepatocarcinogenesis (Patel 1998; Tabor 1999; MacDonald 2001; Sasaki 2006). The liver is well endowed with antioxidant mechanisms to combat oxidative stress, including micronutrients, such as vitamin E and vitamin C, and some enzymes that metabolise reactive metabolites and reactive oxygen species (Kaplowitz 2000). Whether additional antioxidant supplements could be beneficial is not clear.
The role of antioxidant supplements in preventing biliary tract cancers is not sufficiently investigated. There are only a few experimental studies dealing with this question (Takeda 2002).
Antioxidant supplements Vitamin A is essential for growth. Since cancer involves disturbances in normal tissue growth and differentiation, it was one of the first vitamins to be evaluated with respect to carcinogenesis. Later studies indicated that protective effects were only observed for dietary vitamin A from plant sources (beta‐carotene) (Peto 1981; Ziegler 1989). Vitamin C has antioxidative properties with possible cancer preventive potential (Hanck 1988). Vitamin E acts as a free radical scavenger to prevent lipid peroxidation of polyunsaturated fatty acids and block nitrosamine formation (Oshima 1982; Poppel 1997). Vitamin E supplementation can increase production of humoral antibodies and may have antitumour proliferation capacities, possibly by modulating gene expression (Knekt 1994). Selenium, a trace element, is also important for antioxidant defences of the body as an integral component of metalloprotein enzymes. It is a component of selenoproteins, which have important enzymatic functions (Hughes 2000; Rayman 2000). There is an inverse relationship between selenium intake and cancer mortality (Schrauzer 1977). In the USA, cancer mortality rates are significantly higher in low selenium regions (Clark 1991).
The evidence on whether antioxidant supplements are effective in decreasing gastrointestinal cancers is contradictory (Nomura 1987; Dawsey 1994; Yu 1997). We conducted a systematic Cochrane review on the issue published in 2004 and were unable to demonstrate convincing beneficial effects of antioxidant supplements (beta‐carotene, vitamin A, vitamin C, vitamin E, and selenium) on gastrointestinal cancers (Bjelakovic 2004a; Bjelakovic 2004b). Our results even suggested that these supplements, with the possible exception of selenium, may increase mortality (Bjelakovic 2004a; Bjelakovic 2004b). This present review is an update of the former review.
Objectives
To assess the beneficial and harmful effects of antioxidant supplements in preventing gastrointestinal cancers (oesophageal, gastric, small intestine, colorectal, pancreatic, liver, and biliary tract cancers).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised trials, irrespective of blinding, publication status, publication year, or language.
Types of participants
Adult participants (age 18 years or over) who were:
participants from the general population irrespective of age, sex, or ethnic origin; or
participants at high risk of developing gastrointestinal cancers (with premalignant conditions, or living in areas with high incidence of gastrointestinal cancers); or
participants coming from other patient groups, primarily with non‐gastrointestinal diseases.
Types of interventions
We considered for inclusion trials that compared antioxidant supplements (ie, beta‐carotene, vitamin A, vitamin C, vitamin E, and selenium) at any dose, duration, and route of administration versus placebo or no intervention.
The antioxidants could have been administered:
singly; or
in any combination among themselves; or
in combination with other vitamins; or
in combination with trace elements without antioxidant function.
Concomitant interventions were allowed if used equally in both intervention arms of the trial.
Studies concerning antioxidant supplements in prevention of other organ system disease (cardiovascular, respiratory, urinary tract, etc.) were considered if data on the occurrence of gastrointestinal cancers during the trial could be obtained.
Types of outcome measures
Our primary outcome measures were: (1) Number of patients developing gastrointestinal cancers. We determined whether supplementation with antioxidants, administered separately or in combination, influenced the incidences of any of the gastrointestinal cancers (oesophageal, gastric, small intestinal, colorectal, pancreatic, liver, and biliary tract cancers) and all gastrointestinal cancers combined. (2) Overall mortality.
Our secondary outcome measures were: (3) Any adverse effects as reported in the trials. Incidence and types of adverse effects connected with the active intervention. (4) Quality‐of‐life measures. (5) Health economics.
Search methods for identification of studies
The trials search co‐ordinators of The Cochrane Colorectal Cancer Group, The Cochrane Hepato‐Biliary Group, The Cochrane Inflammatory Bowel Disease Group, and The Cochrane Upper Gastrointestinal and Pancreatic Diseases Group provided us with searches of their respective trials registers on antioxidant supplements and prevention of oesophageal, gastric, small intestinal, colorectal, pancreatic, liver, and biliary tract cancers. We also conducted electronic searches in the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 2, 2007), MEDLINE (1966 to October 2007), EMBASE (Excerpta Medica Database) (1985 to October 2007), LILACS (1982 to October 2007), the Science Citation Index Expanded (SCI‐EXPANDED) (1945 to October 2007) (Royle 2003). All search strategies are given in Table 1. In addition, we obtained a search result in the Chinese Biomedical Database (1978 to October 2007).
1. Table.
| Database | Search performed | Search strategy |
| The Cochrane Central Register of Controlled Trials on the Cochrane Library | October 2007 | Digestive system neoplasms / explode all trees (MeSH), antioxidants / explode all trees (MeSH), (#1 and #2). |
| The Controlled Trials Registers of the four Cochrane gastrointestinal groups | October 2007 | 'oesophageal cancer' or 'gastric cancer' or 'stomach cancer' or 'bowel cancer' or 'colorectal cancer' or 'colon cancer' or 'rectal cancer' or 'pancreatic cancer' or 'hepatocellular carcinoma' or 'liver cancer' or 'biliary tract cancer' AND 'antioxidant*' or 'vitamin* and supplement* and random*'. |
| MEDLINE | October 2007 | #1 explode "Digestive‐System‐Neoplasms"/ all subheadings #2 retinol or beta carotene or ascorbic acid or alpha tocopherol or selenium or vitamin* or antioxidant* #3 TG = ANIMAL #4 random* #5 ((#1 and #2) not #3) and random* |
| EMBASE | October 2007 | #1 explode "digestive‐system‐tumor"/ all subheadings #2 retinol or beta carotene or ascorbic acid or alpha tocopherol or selenium or vitamin* or antioxidant* #3 random* #4 #1 and #2 and #3 |
| LILACS | October 2007 | #1 antioxidantes and cancer |
| The Web of Science (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | Accessed 01 October 2007 | #1 ‐ > [TS=(retinol or beta‐carotene or ascorbic acid or alpha tocopherol or selenium or vitamin* or antioxidant*) DocType=All document types; Language=All languages; Database(s)=SCI‐EXPANDED, SSCI, A&HCI; Timespan=1945‐2003] #2 ‐ ((o)esophageal or gastric or small intestin* or colorectal or pancreatic or liver or biliary tract) and cancer* #3 ‐ > TS=(random*) DocType=All document types; Language=All languages; Database(s)=SCI‐EXPANDED; Timespan = 1945‐2003 #4 ‐ 62 #1 and #2 and #3 |
| The Chinese Biomedical Database | October 2007 | (retinol or beta‐carotene or ascorbic acid or alpha tocopherol or selenium or vitamin* or antioxidant*) and #1 ((o)esophageal or gastric or small intestin* or colorectal or pancreatic or liver or biliary tract) and cancer* |
We scanned reference lists from review articles retrieved from the searches above in order to identify additional trials.
We contacted DSM, Roche, Bristol‐Meyers Squibb, BASF AIS, Hoechst, Bayer, Aventis, Takeda, and Lederle Laboratories, manufacturers of antioxidant supplements, to ask for unpublished randomised trials. Of these, Roche suggested some published trials, which we knew of. No other information was received.
Data collection and analysis
Inclusion criteria application We retrieved the identified material for assessment. GB and DN independently applied the inclusion criteria to all potential studies. We performed this without blinding. No discrepancy occurred in the trial selection.
Data extraction Participant characteristics, diagnosis, and interventions We recorded the following data from the individual randomised trials: first author; country of origin; country income category (low, middle, high) (World Bank 2006); number of participants; characteristics of participants: age range (mean or median) and sex ratio; participation rate; dropout rate; trial design (parallel or factorial); type of antioxidant; dose; duration of supplementation; duration of follow‐up (ie, duration of intervention plus post‐intervention follow‐up); co‐interventions; and the occurrence of gastrointestinal cancers (oesophageal, gastric, small intestinal, colorectal, pancreatic, liver, and biliary tract cancers).
Trial characteristics We recorded the date, location, sponsor of the trial (known or unknown and type of sponsor) as well as publication status.
Assessment of methodological quality We assessed the methodological quality defined as the confidence that the design and report restrict bias in the intervention comparison based on the randomisation, blinding, and follow‐up (Schulz 1995; Moher 1998; Kjaergard 2001). The following definitions were used: Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding (or masking)
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo or active drugs.
Unclear, if the trial was described as double blind, but the method of blinding was not described.
Not performed, if the trial was not double blind.
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Trials with adequate generation of the allocation sequence, adequate allocation concealment, adequate blinding, and adequate follow‐up were considered low‐bias risk trials (high methodological quality) (Kjaergard 2001; Gluud 2006a). Trials with one or more unclear or inadequate quality components were classified as high‐bias risk trials (low methodological quality) (Kjaergard 2001; Gluud 2006a).
We also reported on whether the investigators had performed a sample‐size calculation and used intention‐to‐treat analysis (Gluud 2001).
We used the classification of quality for sensitivity analyses and not as exclusion criteria.
Statistical analyses We performed the meta‐analyses according to the recommendations of The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). For the statistical analyses, we used RevMan Analyses (RevMan 2003), STATA 8.2 (STATA Corp, College Station, Tex), Sigma Stat 3.0 (SPSS Inc, Chicago, Ill), and Stats‐Direct (StatsDirect Ltd, Altrincham, England).
We analysed the data with both random‐effects (DerSimonian 1986) and fixed‐effect (DeMets 1987) models meta‐analyses. We present the results of the random‐effects model analysis if the two models concur regarding statistical significance (P < 0.05). If not, we present both analyses. Results are presented as the relative risk (RR) with 95% confidence intervals (CI). We assessed heterogeneity with I2, which describes the percentage of total variation across studies due to heterogeneity rather than chance (Higgins 2002). I2 can be calculated as I2 = 100% × (Q‐df)/Q (Q = Cochran's heterogeneity statistics, df = degrees of freedom). I2 ranged between 0% (ie, no observed heterogeneity) and 100% (maximal heterogeneity) (Higgins 2002). We used the STATA metareg command (Sharp 1998) for the random‐effects meta‐regression analyses to assess potential covariates predicting intertrial heterogeneity, ie, the covariates that are statistically associated with estimated intervention effects. The included covariates were type and dose of supplement, duration of supplementation, bias risk (low or high), and primary or secondary prevention. Trials with participants coming from the general population, areas with high incidence of gastrointestinal cancers, and other patient groups with non‐gastrointestinal diseases were considered primary prevention trials. Trials in participants with premalignant conditions of the gastrointestinal tract were considered secondary prevention trials. We performed univariate and multivariate analyses including all covariates.
All our analyses followed the intention‐to‐treat principle. We accounted all of the participants for each trial and performed the analyses irrespective of how the original trialists had analysed the data. Participants lost to follow‐up were considered survivors. For trials with a factorial design, we based our results on 'at‐margins' analysis, comparing all groups that received antioxidant supplements with groups that did not receive antioxidant supplements (McAlister 2003). To determine the effect of a single antioxidant we performed 'inside the table' analysis (McAlister 2003) in which we compared the single antioxidant arm with the placebo/no intervention arm. In the trials with parallel group design with more than two arms and additional therapy, we compared only the arms supplemented with antioxidants with the placebo arm (Higgins 2006).
Comparison of intervention effects was conducted with test of interaction (Altman 2003).
We performed adjusted rank correlation (Begg 1994) and regression asymmetry test (Egger 1997) for detection of bias. A P < 0.10 was considered significant.
Results
Description of studies
Search results We identified a total of 1096 references through the four gastrointestinal disease Cochrane Groups (n = 90), the Cochrane Central Register of Controlled Trials in The Cochrane Library (n = 221), MEDLINE (n = 224), EMBASE (n = 269), LILACS (n = 75), Science Citation Index Expanded (SCI‐expanded) (n = 96), the Chinese Biomedical Database (n = 36), and reading references (n = 85). We excluded 411 duplicates and 374 clearly irrelevant references through reading abstracts. Accordingly, 311 references were retrived for further assessment. Of these, we excluded 58 references because they did not fulfill our inclusion criteria. Reasons for exclusion are listed in the table 'Characteristics of excluded studies'. In total, 253 references describing 24 randomised trials fulfilled our inclusion criteria. Among these references, 11 references described 4 ongoing trials. These trials are listed under 'Characteristics of ongoing studies'. The remaining 242 references, describing 20 trials, fulfilled our inclusion criteria and provided data for the analyses. Details of the trials are shown in the table 'Characteristics of included studies'.
Trial design Nine trials used factorial designs (one trial 'half replicate of two‐by‐two‐by‐two‐by‐two', 4 trials 'two‐by‐two‐by‐two', and 4 trials 'two‐by‐two') and 11 trials used the two‐ or more‐armed parallel group trial designs. One 'two‐by‐two‐by‐two' factorial trial proceeded as a 'two‐by‐two' factorial trial. Two of the 'two‐by‐two' factorial trials proceeded as two‐armed trials (Pocock 1991) (Table 2).
2. Table.
| Trial | Design of the trials | Number of arms | Antioxidant vitamin supplements plus any additional non‐antioxidant interventions in the experimental arm/arms | Control group | Analysis reported |
| Munoz 1985 | Parallel. | 2 | Vitamin A, vitamin B2, and zinc. | Placebo. | |
| Yu 1991 | Parallel. | 2 | Selenium. | Placebo. | |
| NIT1 1993 | Half replicate of a 2x2x2x2 factorial trial. | 8 | A) Vitamin A, vitamin B2, vitamin B3, and zinc. B) Vitamin A, vitamin C, zinc, and molybdenum. C) Vitamin A, beta‐carotene, vitamin E, selenium, and zinc. D) Vitamin C, vitamin B2, vitamin B3, and molybdenum. E) Beta‐carotene, vitamin E, selenium, vitamin B2, and vitamin B3. F) Beta‐carotene, vitamin C, vitamin E, selenium, and molybdenum. G) Vitamin A, beta‐carotene, vitamin C, vitamin E, selenium, zinc, vitamin B2, vitamin B3, and molybdenum. | Placebo. | Four‐way. |
| NIT2 1993 | Parallel. | 2 | 13 vitamins (vitamin A, beta‐carotene, vitamin E, vitamin C, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin D, folic acid, niacinamide, biotin, pantothenic acid) and 13 minerals (calcium, phosphorus, iodine, iron, magnesium, copper, manganese, potassium, chloride, chromium, molybdenum, selenium, and zinc). | Placebo. | |
| NPCT 1996 | Parallel. | 2 | Selenium. | Placebo. | |
| PHS 1996 | 2x2 factorial trial changed into two‐armed trial. | Initially 4, then changed into 2. | A) Aspirin. B) Beta‐carotene. C) Beta‐carotene and aspirin. | Placebo. | Two‐way. |
| Yu 1997 | Parallel. | 2 | Selenium. | Placebo. | |
| Correa 2000 | 2x2x2 factorial trial. | 8 | A random half of the patients were treated with anti‐Helicobacter pylori treatment medication (which consisted of amoxicillin, metronidazole, and bismuth subsalicylate) before the start of the 2x2 factorial design of beta‐carotene and/or vitamin C versus placebo. A) Beta‐carotene. B) Vitamin C. C) Beta‐carotene plus anti‐Helicobacter pylori treatment. D) Vitamin C plus anti‐Helicobacter pylori treatment. E) Beta‐carotene and vitamin C. F) Beta‐carotene and vitamin C plus anti‐Helicobacter pylori treatment. G) anti‐Helicobacter pylori treatment. | Placebo. | Eight‐way. |
| Li 2000 | Parallel. | 2 | Selenium. | Placebo. | |
| HPS 2002 | 2x2 factorial trial. | 4 | A) Vitamin E, vitamin C, and beta‐carotene. B) Simvastatin. C) Vitamin E, vitamin C, beta‐carotene, and simvastatin. | Placebo. | Two‐way. |
| ATBC 2003 | 2x2 factorial trial. | 4 | A) Vitamin E. B) Beta‐carotene. C) Beta carotene and vitamin E. | Placebo. | Four‐way |
| Zhu 2003 | Parallel. | 3 (A fourth group assessing folate and vitamin B12 was disregarded.) | A) Beta‐carotene (natural). B) Beta‐carotene (synthetic). | Placebo. | |
| CARET 2004 | 2x2 factorial trial changed into two‐armed trial. | Initially 4, then changed into 2. | Vitamin A and beta‐carotene. | Placebo. | |
| Li 2004 | Parallel. | 2 | Selenium, synthetic allitridum (garlic extract). | Placebo. | |
| SUVIMAX 2004 | Parallel. | 2 | Beta‐carotene, vitamin C, vitamin E, selenium, and zinc [as gluconate]) . | Placebo. | |
| HOPE TOO 2005 | 2x2 | 4 | A) Vitamin E. B) Ramipril (angiotensin‐converting enzyme inhibitor). C) Vitamin E plus ramipril. | Placebo. | Two‐way. |
| WHS 2005 | 2x2x2 factorial trial. | Initially 8, changed into 4. | A) Beta‐carotene (abandoned after 22.8 months). B) Vitamin E. C) Beta‐carotene and vitamin E. D) Beta‐carotene and aspirin. E) Vitamin E and aspirin. F) Beta‐carotene, vitamin E, and aspirin. G) Aspirin. | Placebo. | Two‐way. |
| SIT 2006 | 2x2x2; 2x2 | 8 plus 4 | A) Amoxicillin and omeprazole, garlic, vitamin capsule* and selenium. B) Amoxicillin and omeprazole, garlic, vitamin placebo C) Amoxicillin and omeprazole, vitamin capsule* and selenium. D) Amoxicillin and omeprazole and vitamin placebo E) Garlic, vitamin capsule*, and selenium. F) Garlic and vitamin placebo. G) Vitamin capsule* and selenium. H) Garlic and selenium placebo. I) Garlic. J) Vitamin placebo. K) Selenium. *The vitamin capsule contained vitamin C (250 mg), vitamin E (100 IU), and selenium from yeast (37.5 µg) . | Placebo. | Two‐way |
| Plummer 2007 | Parallel | 2 | Beta‐carotene, vitamin C, and vitamin E. | Placebo. | |
| WACS 2007 | 2x2x2 | 8 Another arm testing a combination of folic acid (2.5 mg), vitamin B6 (50 mg), and vitamin B12 (1 mg) was disregarded. | A) Beta‐carotene, vitamin C, and vitamin E. B) Beta‐carotene placebo, vitamin C, and vitamin E. C) Beta‐carotene, vitamin C, and vitamin E placebo. D) Beta‐carotene placebo, vitamin C, and vitamin E placebo. E) Beta‐carotene, vitamin C placebo, and vitamin E. F) Beta‐carotene placebo, vitamin C placebo, and vitamin E. G) Beta‐carotene, vitamin C placebo, and vitamin E placebo. | Placebo. | Eight‐way |
Participants A total of 211,818 participants were randomised in the 20 trials. The number of participants in each trial ranged from 216 to 39876. We were not able to extract relevant data on the sex of the participants from two trials. The percentage of men was 58% of the trials reporting sex. The age varied from 18 to 84 years with a mean age of 56.5 years.
There were five trials with 122,411 participants from the general population (PHS 1996; ATBC 2003: CARET 2004; SUVIMAX 2004; WHS 2005), four trials (Munoz 1985; Yu 1991; NIT1 1993; Li 2004) with 37701 healthy participants living in areas at higher risk of developing gastrointestinal cancers, and four trials (NPCT 1996: HPS 2002; HOPE TOO 2005: WACS 2007) with 39560 participants with non‐gastrointestinal diseases; all were considered as primary prevention trials. There were seven trials (NIT2 1993; Yu 1997; Correa 2000; Li 2000; Zhu 2003; SIT 2006: Plummer 2007) with 12102 participants with premalignant conditions of the gastrointestinal tract; these were considered as secondary prevention trials.
Experimental interventions The route of antioxidant administration was oral for all the trials. Antioxidants were administered either alone, or in combination with other antioxidants, or with or without other vitamins, minerals or other interventions (Table 2). The types, doses, dose regimens, and duration of supplementation with antioxidants were as follows: beta‐carotene 6 mg to 30 mg (12 trials), vitamin A 1500 µg to 15000 µg (4 trials), vitamin C 120 to 2000 mg (8 trials), vitamin E 30 to 600 mg (10 trials), daily or on alternate days for 1.1 to 12 years; selenium 50 to 228 µg (9 trials), daily for two to four years (Figure 1). In one trial antioxidant supplements (vitamin A, riboflavin, and zinc) were given once weekly (Munoz 1985). One trial administered beta‐carotene 30 mg daily for the first year and 30 mg two times a week for the second (Zhu 2003). One trial administered selenium 100 µg on alternate days for one month of each year during two years (Li 2004).
1.
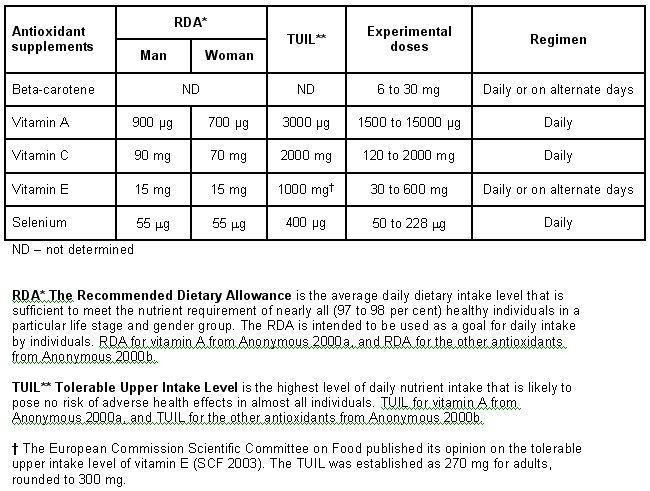
Recommended dietary allowance, tolerable upper intake level, experimental doses, and regimen used in antioxidant supplements
Beta‐carotene, vitamin C, vitamin E, or selenium were administered as a single antioxidant supplement (Table 2). Beta‐carotene, vitamin A, vitamin C, vitamin E, and selenium formed different combinations of antioxidant supplements only or were administered together with non‐antioxidant supplements (Table 3 and Table 2). The administered combinations consisting only of antioxidant supplements were: beta‐carotene and vitamin A; beta‐carotene and vitamin C; beta‐carotene and vitamin E; beta‐carotene, vitamin C, and vitamin E; vitamin C, vitamin E, and selenium; beta‐carotene, vitamin C, and vitamin E, and selenium (see 'Characteristics of included studies' and Table 2).
3. Table.
| Experimental antioxidant supplements | Oesophageal cancer | Gastric cancer | Colorectal cancer | Pancreatic cancer | Hepatocellular carcinoma |
| Beta‐carotene (PHS 1996; Correa 2000; ATBC 2003; Zhu 2003) | 0.75, 0.25 to 2.30 | 1.12, 0.79 to 1.59 | 1.09, 0.79 to 1.51 | 1.02, 0.54 to 1.90 | 1.92, 0.96 to 3.85 |
| Vitamin E (ATBC 2003; HOPE TOO 2005) | 1.46, 0.72 to 2.96 | 1.30, 0.90 to 1.88 | 1.10, 0.87 to 1.39 | 0.97, 0.67 to 1.39 | 1.33, 0.63 to 2.82 |
| Selenium (Yu 1991; NPCT 1996; Yu 1997; Li 2000; Li 2004) | 0.40, 0.08 to 2.07 | 0.76, 0.44 to 1.31 | 0.48, 0.22 to 1.05 | ND | 0.56, 0.42 to 0.76 |
| Beta‐carotene and vitamin A (CARET 2004) | 1.43, 0.90 to 2.29 | 0.89, 0.46 to 1.73 | 0.97, 0.76 to 1.25 | 1.33, 0.84 to 2.09 | 1.35, 0.51 to 3.54 |
| Beta‐carotene and vitamin C (Correa 2000) | ND | 2.90, 0.12 to 70.52 | ND | ND | ND |
| Beta‐carotene and vitamin E (ATBC 2003) | 1.23, 0.59 to 2.56 | 1.40, 0.98 to 2.01 | 1.20, 0.89 to 1.63 | 0.93, 0.65 to 1.35 | 1.25, 0.59 to 2.67 |
| Beta‐carotene, vitamin C, and vitamin E (HPS 2002; Plummer 2007) | 1.19, 0.71 to 2.01 | 1.25, 0.78 to 2.00 | 0.84, 0.65 to 1.07 | 1.00, 0.57 to 1.76 | 1.40, 0.44 to 4.41 |
| Vitamin A, riboflavin, and zinc (Munoz 1985) | 1.33, 0.30 to 5.91 | ND | ND | ND | ND |
| Vitamin C, vitamin E, and selenium (SIT 2006) | ND | 1.01, 0.60 to 1.68 | ND | ND | ND |
| Beta‐carotene, vitamin C, vitamin E, and selenium (SUVIMAX 2004) | 1.01, 0.14 to 7.16 | 1.01, 0.14 to 7.16 | 0.88, 0.49 to 1.58 | 0.67, 0.19 to 2.38 | 1.01, 0.06 to 16.12 |
| 26 vitamins/minerals (NIT2 1993) | 0.96, 0.76 to 1.22 | 1.19, 0.89 to 1.58 | ND | ND | ND |
| GI = gastrointestinal; ND = No data | |||||
| Relative risk, 95% confidence interval (random). |
Six trials added non‐antioxidant vitamins, ie, vitamin B12 and folic acid (Zhu 2003), vitamin B6, vitamin B12, and folic acid (WACS 2007), or non‐antioxidant vitamins and minerals (Munoz 1985; NIT1 1993; NIT2 1993; SUVIMAX 2004) to the experimental arms (see 'Characteristics of included studies' and Table 2).
Control interventions All trials used placebo capsules or tablets as control intervention.
Concomitant interventions The factorial designs of the trials permitted other interventions to be administered to some of the participants in the antioxidant experimental arms and in the control arms. Four trials primarily connected with the occurrence of cancers and cardiovascular diseases tested additional therapies: aspirin 100 mg to 325 mg, given daily or on alternate days (PHS 1996; WHS 2005); simvastatin (cholesterol lowering therapy) 40 mg (HPS 2002); or ramipril 10 mg (angiotensin‐converting enzyme inhibitor) (HOPE TOO 2005). Two trials assessed anti‐Helicobacter pylori interventions (Correa 2000; SIT 2006), two trials aged garlic extract 200 mg (Li 2004; SIT 2006), and one trial vitamin B6 50 mg, vitamin B12 1 mg, and folic acid 2.5 mg (WACS 2007) (Table 2).
Outcome measures All 20 trials examined gastrointestinal cancers. We were able to extract relevant data on the incidence of gastrointestinal cancers from 18 trials. We were not able to extract data on the incidence of gastrointestinal cancers for each arm separately from one trial (NIT1 1993), and the authors did not respond to our requests for further information. The data from WACS 2007 are not yet available.
Only 14 of the trials (70%) could provide data on overall mortality. Sponsorship Pharmaceutical companies were the provider or sponsor of antioxidant supplements in 17 trials. This information was not available in three trials (Yu 1991; Yu 1997; Li 2000). Roche was the sponsor or provider in 8 out of 17 trials (47%).
Risk of bias in included studies
For an overview of the methodological quality of the included trials see Figure 2.
2.
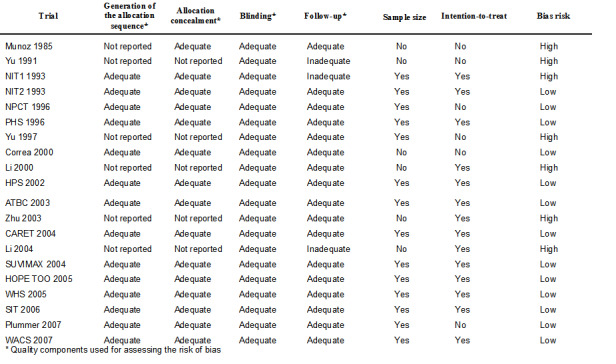
Table 05.
Bias risk of the trials
Gastrointestinal cancers Twelve trials out of the 18 (66.7%) providing data on gastrointestinal cancers reported adequate generation of the allocation sequence, 13 trials (72.3%) reported adequate allocation concealment, 18 trials (100%) used placebo and hence had presumed adequate blinding, and 16 trials (88.9%) reported adequate follow‐up. Twelve trials (66.7%) reported sample‐size calculations. Twelve trials (66.7%) based their analyses on the intention‐to‐treat principle.
There were 12 trials (66.7%) of low‐bias risk (high methodological quality) with adequate generation of the allocation sequence, allocation concealment, blinding, as well as follow‐up.
Overall mortality Among the 14 trials providing data on mortality, thirteen (92.9%) were of low‐bias risk (high methodological quality) with adequate generation of the allocation sequence, allocation concealment, blinding, as well as follow‐up. The 14th trial had inadequate follow‐up (NIT1 1993).
Effects of interventions
Gastrointestinal cancers Antioxidant supplements had no significant influence on gastrointestinal cancer occurrence (RR 0.94, 95% CI 0.83 to 1.06, I2 = 54.0%). Approximately 2.2% of the participants in the antioxidant group compared with 2.0% in the placebo group developed gastrointestinal cancers at the end of follow‐up.
Funnel plot asymmetry We analysed the antioxidant effect on gastrointestinal cancers for funnel plot asymmetry (Figure 3). From inspection of the figure, one may suspect bias. The asymmetry was statistically significant (P = 0.009) by Egger's test and (P = 0.096) by Begg's test.
3.
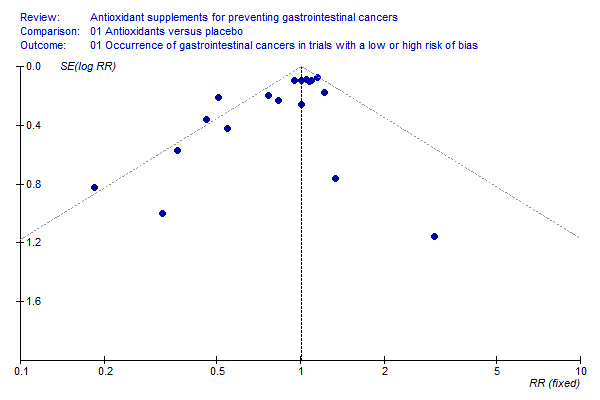
Funnel plot ‐ occurrence of gastrointestinal cancers
Meta‐regression analysis Univariate meta‐regression analyses revealed that the following covariates were significantly associated with estimated intervention effect on the occurrence of the gastrointestinal cancers: dose of beta‐carotene (RR 1.01, 95% CI 1.002 to 1.02; P = 0.012) and dose of selenium (RR 0.997, 95% CI 0.995 to 0.998, P < 0.0001). None of the other covariates (dose of vitamin A; dose of vitamin C; dose of vitamin E; bias risk of the trials; duration of supplementation; and primary or secondary prevention) were significantly associated with estimated intervention effect on gastrointestinal cancers.
In multivariate meta‐regression analysis including all covariates, dose of selenium was associated with a significantly lower estimated intervention effect on the gastrointestinal cancers (RR 0.996, 95% CI 0.994 to 0.999; P = 0.007). None of the other covariates was significantly associated with the estimated intervention effect on the gastrointestinal cancers.
Methodological quality and antioxidant effect on gastrointestinal cancer occurrence The trials with low risk of bias (n = 12) did not show a significant effect of antioxidant supplements on gastrointestinal cancers (RR 1.04, 95% CI 0.96 to 1.13, I2 = 19.6%). In the trials with high risk of bias (n = 6) antioxidant supplements significantly decreased gastrointestinal cancers (RR 0.59, 95% CI 0.43 to 0.80, I2 = 18.1%). The difference between the estimates obtained by trials with adequate and unclear or inadequate methodology was statistically significant by test of interaction (z = ‐3.53, P < 0.0005).
Generation of the allocation sequence In the trials with adequate generation of the allocation sequence, antioxidant supplements did not significantly influence gastrointestinal cancers (RR 1.05, 95% CI 0.98 to 1.13, I2 = 0%). In the trials with unclear or inadequate generation of the allocation sequence, antioxidant supplements showed a significant beneficial effect on gastrointestinal cancers (RR 0.59, 95% CI 0.43 to 0.80, I2 = 18.1%). The difference between the estimates obtained by trials with adequate and unclear or inadequate generation of the allocation sequence was statistically significant by test of interaction (z = ‐3.55, P < 0.0005).
Allocation concealment In the trials with adequate allocation concealment, antioxidant supplements did not significantly influence gastrointestinal cancers (RR 1.05, 95% CI 0.98 to 1.13, I2 = 0%). In the trials with unclear or inadequate allocation concealment, antioxidant supplements showed a significant beneficial effect on gastrointestinal cancers (RR 0.57, 95% CI 0.41 to 0.78, I2 = 19.6%). The difference between the estimates obtained by trials with adequate and unclear or inadequate allocation concealment was statistically significant by test of interaction (z = ‐3.64, P < 0.0003).
Blinding Blinding was assumed adequate in all the 18 trials due to the use of placebo.
Follow‐up In the trials with adequate follow‐up, antioxidant supplements did not significantly influence gastrointestinal cancers (RR 0.98, 95% CI 0.87 to 1.10, I2 = 49.8%). In the trials with unclear follow‐up, antioxidant supplements showed no significant effect on gastrointestinal cancers (RR 0.72, 95% CI 0.51 to 1.02, I2 = 0%). The difference between the estimates obtained by trials with adequate and unclear or inadequate follow‐up was not statistically significant by test of interaction (z = ‐1.65, P = 0.0989).
Type of antioxidant supplement Beta‐carotene (RR 1.04, 95% CI 0.80 to 1.35) or vitamin E (RR 1.11, 95% CI 0.93 to 1.34) given singly did not significantly influence gastrointestinal cancers. Different combinations of antioxidants, that is, beta‐carotene and vitamin A; beta‐carotene and vitamin C; beta‐carotene and vitamin E; vitamin A, riboflavin, and zinc; beta‐carotene, vitamin C, and vitamin E; vitamin C, vitamin E, and selenium; beta‐carotene, vitamin C, vitamin E, and selenium, or combinations of 26 vitamins/minerals did not significantly influence gastrointestinal cancers (RR 1.10, 95% CI 0.91 to 1.32; RR 2.90, 95% CI 0.12 to 70.52; RR 1.18, 95% CI 0.98 to 1.41; RR 1.33, 95% CI 0.30 to 5.91; RR 0.96, 95% CI 0.80 to 1.16; RR 1.01, 95% CI 0.60 to 1.68; RR 0.83, 95% CI 0.53 to 1.32; RR 1.05, 95% CI 0.88 to 1.25; respectively). Selenium given singly significantly decreased gastrointestinal cancers (RR 0.59, 95% CI 0.46 to 0.75, I2 = 0%). Selenium combined did not significantly influence gastrointestinal cancers (RR 1.02, 95% CI 0.87 to 1.19, I2 = 0%). Selenium given singly or combined significantly decreased gastrointestinal cancers (RR 0.86, 95% CI 0.75 to 0.98, I2 = 60.8%). Five out of the nine trials assessing selenium had high‐bias risk. The effect of selenium given singly or combined in 4 low‐bias risk trials was not significant (RR 0.89, 95% CI 0.68 to 1.18, I2 = 45.0%). For an overview of the effect of the different antioxidant supplements on different gastrointestinal cancers see (Table 3).
Occurrence of oesophageal cancer Antioxidant supplements did not significantly influence oesophageal cancer (RR 1.06, 95% CI 0.89 to 1.28, I2 = 0%). Approximately 0.36% of the participants in the antioxidant group compared to 0.38% in the placebo group developed oesophageal cancer at the end of follow‐up. Antioxidants administered singly, ie, beta‐carotene (RR 0.75, 95% CI 0.25 to 2.30); vitamin E (RR 1.46, 95% CI 0.72 to 2.96); selenium (RR 0.40, 95% CI 0.08 to 2.07), or in certain combinations as beta‐carotene and vitamin A (RR 1.43, 95% CI 0.90 to 2.29); beta‐carotene and vitamin E (RR 1.23, 95% CI 0.59 to 2.56); vitamin A, riboflavin, and zinc (RR 1.33, 95% CI 0.30 to 5.91); beta‐carotene, vitamin C, and vitamin E (RR 1.19, 95% CI 0.71 to 2.01); beta‐carotene, vitamin C, vitamin E and selenium (RR 1.01, 95% CI 0.14 to 7.16), or combination of 26 vitamins/minerals (RR 0.96, 95% CI 0.76 to 1.22) versus placebo for a period of 1.1 to 10.1 years, with a follow‐up up to 14.1 years, did not significantly influence oesophageal cancer.
Occurrence of gastric cancer Antioxidant supplements did not significantly influence gastric cancer (RR 1.14, 95% CI 0.97 to 1.33, I2 = 0%). Approximately 0.51% of the participants in the antioxidant group compared to 0.38% in the placebo group developed gastric cancer at the end of follow‐up. Antioxidants administered singly, ie, beta‐carotene (RR 1.12, 95% CI 0.79 to 1.59); vitamin E (RR 1.30, 95% CI 0.90 to 1.88); selenium (RR 0.76, 95% CI 0.44 to 1.31), or in certain combinations as beta‐carotene and vitamin A (RR 0.89, 95% CI 0.46 to 1.73); beta‐carotene and vitamin C (RR 2.90, 95% CI 0.12 to 70.52); beta‐carotene and vitamin E (RR 1.40, 95% CI 0.98 to 2.01); beta‐carotene, vitamin C, and vitamin E (RR 1.25, 95% CI 0.78 to 2.00); vitamin C, vitamin E, and selenium (RR 1.01, 95% CI 0.60 to 1.68); beta‐carotene, vitamin C, vitamin E, and selenium (RR 1.01, 95% CI 0.14 to 7.16), or combination of 26 vitamins/minerals (RR 1.19, 95% CI 0.89 to 1.58) versus placebo for a period of 2.1 to 12 years and follow‐up up to 14.1 years did not significantly influence gastric cancer.
Occurrence of small intestine cancer Only one trial had results about small intestine cancer (WHS 2005). Antioxidant supplements did not significantly influence small intestine cancer (RR 4.00, 95% CI 0.45 to 35.79).
Occurrence of colorectal cancer Antioxidant supplements did not significantly influence colorectal cancer (RR 0.97, 95% CI 0.86 to 1.09, I2 = 19.7%). Approximately 1.07% of the participants in the antioxidant group compared to 1.09% in the placebo group developed colorectal cancer at the end of follow‐up. Antioxidants administered singly, ie, beta‐carotene (RR 1,09 95%CI 0.79 to 1.51); vitamin E (RR 1.10, 95% CI 0.87 to 1.39); selenium (RR 0.48, 95% CI 0.22 to 1.05); or in combinations as beta‐carotene and vitamin A (RR 0.97, 95% CI 0.76 to 1.25); beta‐carotene and vitamin E (RR 1.20, 95% CI 0.89 to 1.63); beta‐carotene, vitamin C, and vitamin E (RR 0.84, 95% CI 0.65 to 1.07); beta‐carotene, vitamin C, vitamin E, and selenium (RR 0.88 95% CI 0.49 to 1.58) versus placebo for a period of 2.1 to 12 years and follow‐up up to 14.1 years did not significantly influence colorectal cancer.
Occurrence of pancreatic cancer Antioxidant supplements did not significantly influence pancreatic cancer (RR 1.16, 95% CI 0.90 to 1.50, I2 = 31.4%). Approximately 0.37% of the participants in the antioxidant group compared to 0.25% in the placebo group developed pancreatic cancer at the end of follow‐up. Antioxidants administered singly, ie, beta‐carotene (RR 1.02, 95% CI 0.54 to 1.90); vitamin E (RR 0.97, 95% CI 0.67 to 1.39); or in combinations as beta‐carotene and vitamin A (RR 1.33, 95% CI 0.84 to 2.09); beta‐carotene and vitamin E (RR 0.93, 95% CI 0.65 to 1.35); beta‐carotene, vitamin C, and vitamin E (RR 1.00, 95% CI 0.57 to 1.76); beta‐carotene, vitamin C, vitamin E, and selenium (RR 0.67, 95% CI 0.19 to 2.38) versus placebo for a period of 2.1 to 12 years and follow‐up up to 14.1 years did not significantly influence pancreatic cancer.
Occurrence of hepatocellular carcinoma Antioxidant supplements did not significantly influence hepatocellular carcinoma (RR 0.80, 95% CI 0.56 to 1.14, I2 = 38.5%). This effect was significantly beneficial in a fixed‐effect model (RR 0.76, 95% CI 0.60 to 0.96). Approximately 0.20% of the participants in the antioxidant group compared to 0.24% in the placebo group developed hepatocellular carcinoma at the end of follow‐up. Beta‐carotene administered singly (RR 1.92 95% CI 0.96 to 3.85), and vitamin E administered singly (RR 1.33, 95% CI 0.63 to 2.82) versus placebo for a period of 2 to 10.1 years and follow‐up up to 14.1 years did not significantly influence hepatocellular carcinoma. Antioxidants in combinations as beta‐carotene and vitamin A (RR 1.35, 95% CI 0.51 to 3.54), beta‐carotene and vitamin E (RR 1.25, 95% CI 0.59 to 2.67), beta‐carotene, vitamin C, and vitamin E (RR 1.40, 95% CI 0.44 to 4.41), or beta‐carotene, vitamin C, vitamin E, and selenium (RR 1.01, 95% CI 0.06 to 16.12) did not significantly influence hepatocellular carcinoma. Selenium administered singly versus placebo for two to four years significantly decreased hepatocellular carcinoma (RR 0.56, 95% CI 0.42 to 0.76, I2 = 0%). All four trials assessing selenium singly had high‐bias risk.
Occurrence of biliary tract cancer Antioxidant supplements did not significantly influence biliary tract cancers (RR 0.61, 95% CI 0.21 to 1.78, I2 = 0%. Approximately 0.019% of the participants in the antioxidant group compared to 0.034% in the placebo group developed biliary tract cancers at the end of follow‐up. Antioxidants administered in combination as beta‐carotene, vitamin C, vitamin E, and selenium had no significant influence on biliary tract cancers (RR 0.20, 95% CI 0.01 to 4.20).
Overall mortality Antioxidant supplements had no significant effect on mortality in a random‐effects model meta‐analysis (RR 1.02, 95% CI 0.97 to 1.07, I2 = 54.9%). Antioxidant supplements significantly increased mortality in the fixed‐effect model meta‐analysis (RR 1.04, 95% CI 1.02 to 1.07). A total of 17114 of 122,501 participants (14.0%) that were randomised to antioxidant supplements and 8799 of 78693 participants (11.2%) randomised to placebo died. To explore the reason for the difference between the two models, we excluded the trials administering selenium. After their exclusion, mortality was significantly higher in the antioxidant group with both the random‐effects (RR 1.06, 95% CI 1.01 to 1.10, I2 = 43.3%) and fixed‐effect model meta‐analyses (RR 1.06, 95% CI 1.03 to 1.09).
Funnel plot asymmetry We analysed the antioxidant effect on mortality for funnel plot asymmetry (Figure 4). The asymmetry was not statistically significant (P = 0.13) by Egger's test and (P = 0.15) by Begg's test.
4.
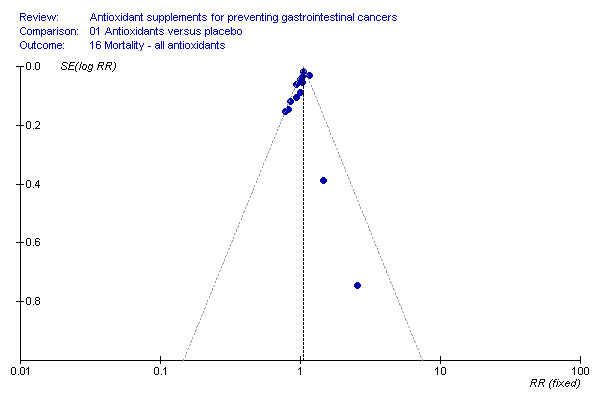
Funnel plot ‐ overall mortality
Meta‐regression analysis Univariate meta‐regression analyses revealed that the following covariates were significantly associated with estimated intervention effect on mortality: dose of beta‐carotene (RR 1.007, 95% CI 1.002 to 1.012; P = 0.003), dose of vitamin A (RR 1.000006, 95% CI 1.000001 to 1.000011, P = 0.009), and dose of selenium (RR 0.998, 95% CI 0.997 to 0.999, P = 0.002). None of the other covariates, ie, dose of vitamin C; dose of vitamin E; bias risk of the trials; duration of supplementation; and primary or secondary prevention, were significantly associated with estimated intervention effect on mortality.
In multivariate meta‐regression analysis including all covariates, dose of selenium was associated with the estimated intervention effect on mortality (RR 0.998, 95% CI 0.997 to 1.000, P = 0.043). None of the other covariates was significantly associated with the estimated intervention effect on mortality.
Methodological quality and antioxidant effect on overall mortality The effect of antioxidant supplements on mortality in trials with low risk of bias (high methodological quality) was not statistically significant in a random‐effects model meta‐analysis (RR 1.03, 95% CI 0.98 to 1.08, I2 = 53.5%). Antioxidant supplements significantly increased mortality in a fixed‐effect model meta‐analysis (RR 1.05, 95% CI 1.02 to 1.07). In the one trial with high risk of bias (NIT1 1993) antioxidant supplements did not significantly influence mortality (RR 0.94, 95% CI 0.84 to 1.06). The difference between the estimate of antioxidant effect in low‐bias and high‐bias risk trials was not significant by test of interaction (z = ‐1.42, P = 0.156).
Type of antioxidant supplement Antioxidants given singly, ie, beta‐carotene (RR 1.05, 95% CI 0.99 to 1.11), vitamin C (RR 0.97, 95% CI 0.77 to 1.23); vitamin E (RR 1.02, 95% CI 0.98 to 1.06); and selenium (RR 0.84, 95% CI 0.67 to 1.07) did not significantly influence mortality. Beta‐carotene used singly significantly increased mortality in a fixed‐effect model meta‐analysis (RR 1.06, 95% CI 1.02 to 1.10). Mortality in participants supplemented with beta‐carotene and vitamin A (RR 1.16, 95% CI 1.09 to 1.23), or beta‐carotene and vitamin E (RR 1.06, 95% CI 1.02 to 1.11) was significantly higher than in the placebo group. Antioxidants given in certain combinations, ie, beta‐carotene and vitamin C (RR 2.79, 95% CI 0.57 to 13.68); beta‐carotene, vitamin C, and vitamin E (RR 1.04, 95% CI 0.97 to 1.11); vitamin C, vitamin E, and selenium (RR 0.82, 95% CI 0.62 to 1.08); beta‐carotene, vitamin C, vitamin E, and selenium (RR 0.78, 95% CI 0.58 to 1.05), or combination of 26 vitamins/minerals (RR 0.94, 95% CI 0.85 to 1.05) versus placebo did not significantly influence mortality.
Non‐serious adverse effects Several adverse effects were recorded in the antioxidant group. Persistent yellowing of the skin and belching were significantly increased in participants supplemented with beta‐carotene (RR 29.14, 95% CI 21.60 to 39.32; RR 2.22, 95% CI 1.80 to 2.74; respectively). Transient yellowing of the skin (RR 1.85, 95% CI 0.74 to 4.67) and gastrointestinal upset (RR 1.03, 95% CI 1.00 to 1.06) were not significantly influenced. Haemorrhagic stroke was not significantly influenced by vitamin E (RR 1.01, 95% CI 0.82 to 1.23, I2 = 0%). Gastrointestinal upset in participants supplemented with selenium was not significantly different when compared to placebo (RR 1.51, 95% CI 0.78 to 2.95). Increased yellowing of the urine and the feeling of being hot and dry in participants taking a combination of 13 vitamins and 13 minerals was also not significantly different between the antioxidant and the placebo group.
Quality of life and cost‐effectiveness We did not find any data on quality of life in the randomised trials included in this review. We found cost‐effectiveness analyses in one trial (PHS 1996).
Discussion
Compared to our previous review (Bjelakovic 2004a), the number of included trials in the present review is expanded with six new trials (42.9%) adding 41293 participants (24.2%). Moreover, we have obtained updated results of longer follow‐up from three large‐scale randomised trials (ATBC 2003; CARET 2004; WHS 2005). Our results remain largely the same. Antioxidant supplements, ie, beta‐carotene, vitamin A, vitamin C, and vitamin E given singly or in combinations, do not seem to prevent gastrointestinal cancers. Beta‐carotene and vitamin A may increase the cancer risk. The studied antioxidants, other than selenium, also seem to increase overall mortality. Thus, the present results support our findings from 47 low‐bias risk trials showing increased mortality in participants undergoing primary or secondary prevention with similar antioxidant supplements (Bjelakovic 2007). Selenium might potentially reduce gastrointestinal cancers and mortality, but these observations run the risk of bias due to the low methodological quality of most of the assessed trials. Only one of the trials investigating selenium given as a single antioxidant had low‐bias risk (NPCT 1996). Although several hypotheses have been explored, the mechanisms involved in the possible cancer preventive role of selenium are largely unknown (Rayman 2005; Papp 2007). Recently, a randomised trial has shown that selenium may carry health risks, eg, increasing the risk of diabetes mellitus (Stranges 2007). Before therapeutic or preventive actions are considered, results of ongoing high‐quality randomised trials with selenium are needed (APPOSE 2001; SELECT 2003; HGPIN 2006).
Our review shows that beta‐carotene possesses significantly harmful effects on gastrointestinal cancers and seems to increase mortality when applied singly or in combination with vitamin A or vitamin E. A recent study suggests that beta‐carotene may act as a co‐carcinogen (Paolini 2003). In another review that we have performed, we have also demonstrated that beta‐carotene seems to increase mortality (Bjelakovic 2007). We were unable to identify trials assessing vitamin A alone in the prevention of gastrointestinal cancers. The combination of beta‐carotene and vitamin A was assessed in a high‐quality, large‐scale randomised trial having lung cancer prevention as the primary outcome (CARET 2004). Gastrointestinal cancer occurrence and overall mortality were significantly higher in the vitamin A supplemented group. Recent research in this field suggest that vitamin A might have pro‐oxidant abilities, which could lead to carcinogenesis (Murata 2000) and may even increase mortality (Bjelakovic 2007). This finding was corroborated in the present systematic review. The trials in which vitamin C was applied alone or in different combinations with beta‐carotene, vitamin A, vitamin E, and selenium found no significant effect on gastrointestinal cancers, or on overall mortality. Vitamin E did not significantly influence gastric, pancreatic, and colorectal cancer or overall mortality. According to recent meta‐analyses, vitamin E seems to increase overall mortality (Miller 2005; Bjelakovic 2007). Therefore, preventive use of vitamin E cannot be recommended.
The bias risk of the trials had a significant impact on our results. The low‐bias risk trials either showed significant harmful effects or no significant effects of antioxidant supplements on the primary outcome measures. On the contrary, trials with high‐bias risk found either no significant effects or significant beneficial effects. These observations are in accordance with several studies linking high‐bias risk with significant overestimation of beneficial effects (Schulz 1995; Moher 1998; Kjaergard 2001; Jüni 2001; Egger 2003; Gluud 2006a; Gluud 2006b) and underreporting of adverse effects (D'Amico 2003). Our meta‐regression analysis failed to identify bias risk as associated with risk of cancer, but we observed that trials with high risk of bias found a decreased risk of cancer.
We can only speculate what caused the significantly higher mortality among the participants supplemented with antioxidants. Based on our present results as well as the results of previous randomised trials and meta‐analysis it is likely that both cardiovascular diseases (ATBC 2003; Vivekananthan 2003) and cancers (ATBC 2003; CARET 2004) have led to increased mortality. We observed a non‐significant tendency towards increased occurrence of gastrointestinal cancers in the supplemented group of low‐bias risk trials (RR 1.04, 95% CI 0.96 to 1.13). Observational studies have shown possible detrimental effects of antioxidant supplements on cardiovascular mortality (Lee 2004), prostate cancer (Lawson 2007), and lung cancer (Slatore 2007). Cardiovascular diseases and cancers of the lung and gastrointestinal tract are the leading causes of death worldwide (Ferlay 2004; Lopez 2006; Mathers 2006). Reactive oxygen species in moderate concentrations are essential mediators of reactions by which unwanted cells are deleted from the body. Schulz et al found that the inhibition of reactive oxygen species formation in cells decreases the life span of nematodes (Schulz 2007). Excessive suppression of free radicals may have unwanted consequences to our health (Salganik 2001).
There are still many gaps in our knowledge of the mechanisms of bioavailability, biotransformation, and action of antioxidant supplements (Haenen 2002). Antioxidant supplements in pills are factory processed, biochemically unbalanced, and apparently unsafe compared to their naturally occurring counterparts (Herbert 1997; Seifried 2003). Antioxidant supplements also possess pro‐oxidant effects (Podmore 1998; Paolini 1999; Murata 2000; Lee 2003; Duarte 2005). A balanced diet typically contains safe levels of antioxidant vitamins and trace elements (Camire 1999). Some of the trials included in our review investigated the effects of antioxidant supplements administered at doses significantly higher than those found in a balanced diet. Some trials used dosages well above the recommended tolerable upper intake levels (Anonymous 2000a; Anonymous 2000b). The majority of the trials were conducted in middle‐ and high‐income countries among populations with already sufficient levels of antioxidant vitamins and trace elements. This might be a cause for the lack of protective effects and for the increase in mortality of antioxidant supplements.
For many years scientists have been concerned about possible harm caused by antioxidants (Herbert 1994; Schwartz 1996). However, large expectations and unconfined belief in the cancer preventive potential of the antioxidants have overwhelmed these concerns. The available data on adverse effects of antioxidant supplements are still limited (Mulholland 2007) and often underreported (Woo 2007). Less than 1% of all adverse effects associated with antioxidant supplements are notified (Woo 2007). Consumers presume antioxidant supplements to be safe and use them without physicians' supervision (Webb 2007). We find that it is high time that antioxidant supplements are moved from the free 'over the counter' market to the prescription regulatory market.
Certain potential limitations of this review warrant consideration. We are dealing with a group of trials, which by the nature of their topic, that is, preventive efforts over a number of years, may have inherent mistakes. We found a number of inconsistencies among the different reports of the individual trials. We tried in all cases to obtain clarification from the authors. However, this was not always possible. Diagnostic criteria and timing of screening differed among the trials or were not always well defined. We have compared the intervention effects of antioxidants of different types and their influence on different gastrointestinal cancers with different aetiology, biology, and epidemiology. Moreover, the examined populations varied. The effects of supplements were assessed in the general population, participants with premalignant conditions of gastrointestinal tract, and participants coming from other patient groups, primarily with non‐gastrointestinal diseases. The variable risk to develop cancer can influence the results. These populations mostly came from countries without overt deficiencies of specific supplements. Accordingly, we are unable to assess the influence of antioxidant supplements on gastrointestinal cancer occurrence in populations with specific needs. In general, the risk of individual cancer was below 1%. This may make it difficult to detect any effects ‐ beneficial or harmful.
The reporting of the occurrence of gastrointestinal cancers and overall mortality in the randomised trials was not always sufficient and consistent. Regarding gastrointestinal cancers, all included trials gave results. However, from one trial (NIT1 1993) we were unable to extract data for each arm separately. Data are awaited from another trial (WACS 2007). Of 20 trials included in our systematic review, only 14 (70%) reported overall mortality. We tried to obtain additional information from the authors but without success. Therefore, outcome reporting bias could influence the result of our meta‐analysis. Outcome reporting bias is defined as selective reporting of some results in trial publications and represent a threat to validity of meta‐analysis (Chan 2004a; Chan 2004b; Chan 2005; Williamson 2005; Furukawa 2007). We are well aware of the difficulties in collecting data on outcomes in clinical trials focusing on safety and efficacy evaluations. The worst result of outcome reporting bias and suppression of some significant or non‐significant findings could be the use of harmful interventions. Most of the included trials lacked detailed information on disease‐specific causes of mortality as well as separate reporting of cancers according to sex.
The choice of statistical model for performing meta‐analysis is important. The fixed‐effect model meta‐analysis assumes that the true intervention effect is the same in every randomised trial, ie, the effect is fixed across trials. The random‐effects model assumes that the effects being estimated based on the different randomised trials differ, but follow some general distribution. When there is no heterogeneity (I2 = 0%), then fixed‐ and random‐effects models meta‐analyses tend to give the same result. If heterogeneity increases, the estimated intervention effect and the corresponding 95% confidence interval will differ in the two models. The trials in the present review had clinical heterogeneity. This argues in favour of the random‐effects model. The standard random‐effects model used in RevMan Analysis is the DerSimonian and Laird method, which models the known differences between trials by incorporating a variance parameter tau to account for across‐trial variation (DerSimonian 1986). Adoption of the random‐effects model in meta‐analysis permits inferences to a broader population of studies than the fixed‐effect model does namely because it includes the parameter tau in the model. The use of the random‐effects model may come at a price. If there is between‐trial heterogeneity, then the weight of the large trials (usually providing more realistic estimates of intervention effects) is reduced. At the same time, the weight of small trials (usually providing more unrealistic estimates of intervention effects due to 'bias' (systematic errors) and 'chance' (random errors)) increases. Therefore, we also analysed our meta‐analyses with the fixed‐effect model.
We only examined certain antioxidant supplements that had been tested in randomised trials. Our results should not be translated to the potential effects of fruits and vegetables, which are rich not only in antioxidants but also in a number of other substances. In spite of intensive research, it is still not clear exactly which specific dietary constituents of fruits and vegetables might have anticarcinogenic properties. The results of randomised clinical trials on cancer prevention with high intake of fruits and vegetables are inconsistent and vary by cancer type and affected organ (Neuhouser 2003; Nouraie 2005). Recently published results of a randomised trial found non‐significant effect of high fruit and vegetable diet on colorectal adenoma recurrence (Lanza 2007). Results of epidemiologic studies differ significantly too, reporting beneficial or null effects (Larsson 2006; Freedman 2007; Koushik 2007; Kubo 2007; Takachi 2007).
Our results are in accordance with the results of other recently published meta‐analyses and systematic reviews (Caraballoso 2003; Vivekananthan 2003; Bjelakovic 2004a; Bjelakovic 2004b; Miller 2005; Bjelakovic 2006; Bjelakovic 2007), as well as recommendations of the United States Preventive Services Task Force and British Nutrition Foundation for the use of vitamin supplementation (McKevith 2003; Morris 2003; USPSTF 2003).
Authors' conclusions
Implications for practice.
There is no convincing evidence that the studied antioxidant supplements have beneficial effect on the occurrence of gastrointestinal cancers or on overall mortality. Beta‐carotene, vitamin A, vitamin C, and/or vitamin E seem to increase overall mortality. Therefore, we cannot recommend the use of these antioxidant supplements as a preventive measure.
Implications for research.
Selenium may potentially possess beneficial effects on gastrointestinal cancers. The potential anticarcinogenic effects of selenium have to be elucidated. Randomised clinical trials with selenium are ongoing and further trials may be needed.
The significant association between unclear or inadequate methodological quality and overestimation of intervention effects has again focused on the need for more objective assessment of preventive and therapeutic interventions.
National and international laws and regulations should require that anything sold to the public claiming health benefits is subjected to adequate assessment of benefits and harms before market release. We suggest that antioxidant supplements should be regulated as drugs.
Researches wishing to examine the influence of antioxidant supplements on gastrointestinal cancers or other diseases should adopt the CONSORT statement while performing and reporting randomised clinical trials (CONSORT ‐ Consolidated Standards of Reporting Trials: www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2008 | Amended | Converted to new review format. |
| 30 September 2007 | New search has been performed | Six new trials were added. |
| 30 September 2007 | New citation required but conclusions have not changed | Conclusions did not change. |
Notes
The Protocol for this Review was published with a title 'Antioxidats for preventing gastrointestinal cancers'.
Acknowledgements
We extend our gratitude to all participants in the randomised clinical trials. We thank Ronald L. Koretz, Contact Editor of The CHBG for very helpful comments on the review. We thank Mike Clarke, The Director of The UK Cochrane Centre; David Forman, The Co‐ordinating Editor of The Cochrane Upper Gastrointestinal and Pancreatic Diseases Group; John McDonald, The Co‐ordinating Editor of The Cochrane Inflammatory Bowel Disease Group, and Peer Wille‐Jørgensen, The Co‐ordinating Editor of The Cochrane Colorectal Cancer Group for their expert comments during preparation of the protocol. We thank Yan Gong and Bodil Als‐Nielsen for useful advice and comments, Yan Gong and Wendong Chen for translation of Chinese articles, and Maoling Wei, The Chinese Cochrane Centre, for performing searches on the Chinese Database and providing us with some articles. We are grateful to Jarmo Virtamo, Nea Malila, Julie Buring, Nancy Cook, Pelayo Correa, John A Baron, Howard Sesso, Serge Hercberg, Mark Thornquist, Matt Barnett, Gary Goodman, I‐Min Lee, Martyn Plummer and Mitchell H. Gail for the information on the trials they were involved in.
Data and analyses
Comparison 1. Antioxidants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Occurrence of gastrointestinal cancers in trials with a low or high risk of bias | 18 | 174019 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 1.1 Trials with low risk of bias | 12 | 163439 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.13] |
| 1.2 Trials with high risk of bias | 6 | 10580 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| 2 Occurrence of gastrointestinal cancers ‐ generation of the allocation sequence | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Adequate | 12 | 162948 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 2.2 Unclear/Inadequate | 6 | 10580 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.80] |
| 3 Occurrence of gastrointestinal cancers ‐ allocation concealment | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Adequate | 13 | 163558 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.13] |
| 3.2 Unclear/Inadequate | 5 | 9970 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 4 Occurrence of gastrointestinal cancers ‐ follow‐up | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Adequate | 16 | 166021 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.87, 1.10] |
| 4.2 Unclear/Inadequate | 2 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.02] |
| 5 Occurrence of all gastrointestinal cancers ‐ different antioxidants | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Beta‐carotene | 4 | 37046 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 5.2 Vitamin C | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Vitamin E | 1 | 14573 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.93, 1.34] |
| 5.4 Selenium | 5 | 11110 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.46, 0.75] |
| 5.5 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| 5.6 Beta‐carotene and vitamin C | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.12, 70.52] |
| 5.7 Beta carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.41] |
| 5.8 Vitamin A, riboflavin, and zinc | 1 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.30, 5.91] |
| 5.9 Beta‐carotene, vitamin C, and vitamin E | 2 | 22516 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.16] |
| 5.10 Vitamin C, vitamin E, and selenium | 1 | 3365 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.68] |
| 5.11 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.32] |
| 5.12 Combination of antioxidants (13 vitamins and 13 minerals) | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 6 Occurrence of different gastrointestinal cancers ‐ all antioxidants | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Occurrence of oesophageal cancer | 9 | 126288 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.89, 1.28] |
| 6.2 Occurrence of gastric cancer | 12 | 157300 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.97, 1.33] |
| 6.3 Occurrence of small intestine cancer | 1 | 39876 | Risk Ratio (M‐H, Random, 95% CI) | 4.00 [0.45, 35.79] |
| 6.4 Occurrence of colorectal cancer | 9 | 153972 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.09] |
| 6.5 Occurrence of pancreatic cancer | 6 | 142947 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.90, 1.50] |
| 6.6 Occurrence of hepatocellular carcinoma | 9 | 130674 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.14] |
| 6.7 Occurrence of biliary tract cancer | 2 | 52893 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.78] |
| 7 Occurrence of oesophageal cancer | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Beta‐carotene | 2 | 14741 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.25, 2.30] |
| 7.2 Vitamin E | 1 | 14573 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.72, 2.96] |
| 7.3 Selenium | 1 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.07] |
| 7.4 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.90, 2.29] |
| 7.5 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.59, 2.56] |
| 7.6 Vitamin A, riboflavin, and zinc | 1 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.30, 5.91] |
| 7.7 Beta‐carotene, vitamin C, and vitamin E | 1 | 20536 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.71, 2.01] |
| 7.8 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.16] |
| 7.9 Combination of antioxidants | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.22] |
| 8 Occurrence of gastric cancer | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Beta‐carotene | 4 | 37046 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.79, 1.59] |
| 8.2 Vitamin C | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Vitamin E | 1 | 14573 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.90, 1.88] |
| 8.4 Selenium | 1 | 5033 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.31] |
| 8.5 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.46, 1.73] |
| 8.6 Beta‐carotene and vitamin C | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.90 [0.12, 70.52] |
| 8.7 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.98, 2.01] |
| 8.8 Beta‐carotene, vitamin C, and vitamin E | 2 | 22516 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.78, 2.00] |
| 8.9 Vitamin C, vitamin E, and selenium | 1 | 3365 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.60, 1.68] |
| 8.10 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.16] |
| 8.11 Combination of antioxidants | 1 | 3318 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.89, 1.58] |
| 9 Occurrence of colorectal cancer | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Beta‐carotene | 3 | 36812 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.79, 1.51] |
| 9.2 Vitamin E | 2 | 24114 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.39] |
| 9.3 Selenium | 1 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.22, 1.05] |
| 9.4 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.25] |
| 9.5 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.89, 1.63] |
| 9.6 Beta‐carotene, vitamin C, and vitamin E | 1 | 20536 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.07] |
| 9.7 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.49, 1.58] |
| 10 Occurrence of pancreatic cancer | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Beta‐carotene | 2 | 36640 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.90] |
| 10.2 Vitamin E | 1 | 14573 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.67, 1.39] |
| 10.3 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.84, 2.09] |
| 10.4 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.35] |
| 10.5 Beta‐carotene, vitamin C, and vitamin E | 1 | 20536 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.57, 1.76] |
| 10.6 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.38] |
| 11 Occurrence of hepatocellular carcinoma | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Beta‐carotene | 1 | 14569 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.96, 3.85] |
| 11.2 Vitamin E | 1 | 14573 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.63, 2.82] |
| 11.3 Selenium | 4 | 9798 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.76] |
| 11.4 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.51, 3.54] |
| 11.5 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.59, 2.67] |
| 11.6 Beta‐carotene, vitamin C, and vitamin E | 1 | 20536 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.44, 4.41] |
| 11.7 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.06, 16.12] |
| 12 Occurrence of biliary tract cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.20] |
| 13 Mortality in trials with a low or high risk of bias | 14 | 201194 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.97, 1.07] |
| 13.1 Trials with low risk of bias | 13 | 171610 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.98, 1.08] |
| 13.2 Trials with high risk of bias | 1 | 29584 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
| 14 Mortality after excluding selenium trials | 9 | 150598 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.01, 1.10] |
| 14.1 Trials with low risk of bias | 9 | 150598 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.01, 1.10] |
| 14.2 Trials with high risk of bias | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Mortality ‐ different antioxidants | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Beta‐carotene | 4 | 39162 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.11] |
| 15.2 Vitamin C | 2 | 2523 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
| 15.3 Vitamin E | 3 | 26157 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.98, 1.06] |
| 15.4 Selenium | 1 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.07] |
| 15.5 Vitamin A and beta‐carotene | 1 | 18314 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.09, 1.23] |
| 15.6 Beta‐carotene and vitamin C | 1 | 492 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.57, 13.68] |
| 15.7 Beta‐carotene and vitamin E | 1 | 14565 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.02, 1.11] |
| 15.8 Beta‐carotene, vitamin C, and vitamin E | 3 | 30687 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.97, 1.11] |
| 15.9 Vitamin C, vitamin E, and selenium | 1 | 3365 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.62, 1.08] |
| 15.10 Beta‐carotene, vitamin C, vitamin E, and selenium | 1 | 13017 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.58, 1.05] |
| 15.11 Combination of antioxidants | 2 | 32902 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.05] |
| 16 Adverse effects ‐ beta‐carotene | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Transient yellowing of the skin | 4 | 91252 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.74, 4.67] |
| 16.2 Persistent yellowing of the skin | 1 | 29133 | Risk Ratio (M‐H, Random, 95% CI) | 29.14 [21.60, 39.32] |
| 16.3 Belching | 1 | 22071 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.80, 2.74] |
| 16.4 Gastrointestinal upset | 1 | 8171 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [1.00, 1.06] |
| 17 Adverse effects ‐ vitamin E | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Haemorrhagic stroke | 3 | 74985 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.82, 1.23] |
| 18 Adverse effects ‐ selenium | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Gastrointestinal upset | 1 | 1312 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.78, 2.95] |
1.1. Analysis.
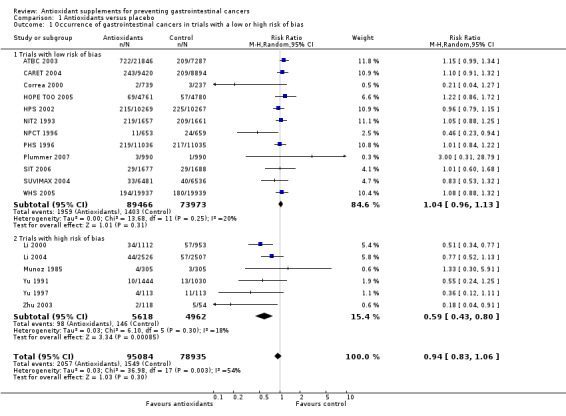
Comparison 1 Antioxidants versus placebo, Outcome 1 Occurrence of gastrointestinal cancers in trials with a low or high risk of bias.
1.2. Analysis.
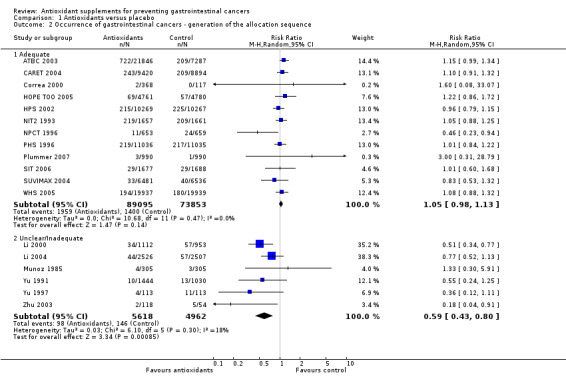
Comparison 1 Antioxidants versus placebo, Outcome 2 Occurrence of gastrointestinal cancers ‐ generation of the allocation sequence.
1.3. Analysis.
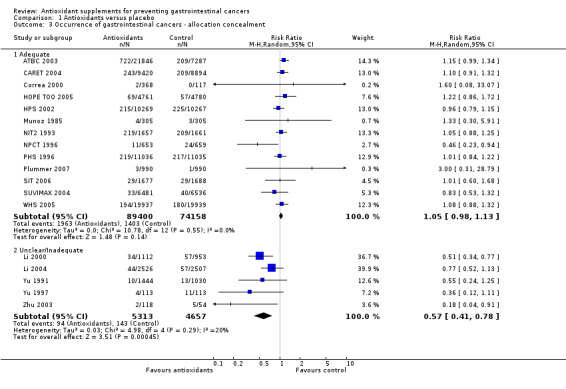
Comparison 1 Antioxidants versus placebo, Outcome 3 Occurrence of gastrointestinal cancers ‐ allocation concealment.
1.4. Analysis.
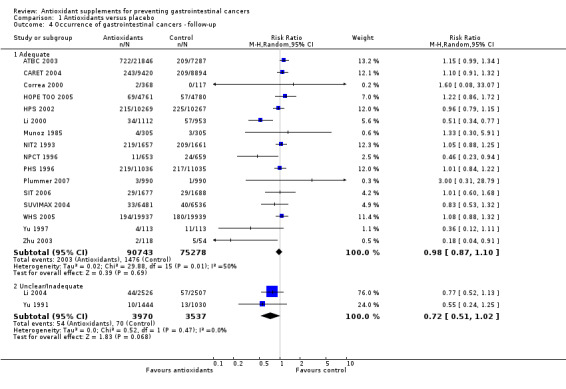
Comparison 1 Antioxidants versus placebo, Outcome 4 Occurrence of gastrointestinal cancers ‐ follow‐up.
1.5. Analysis.
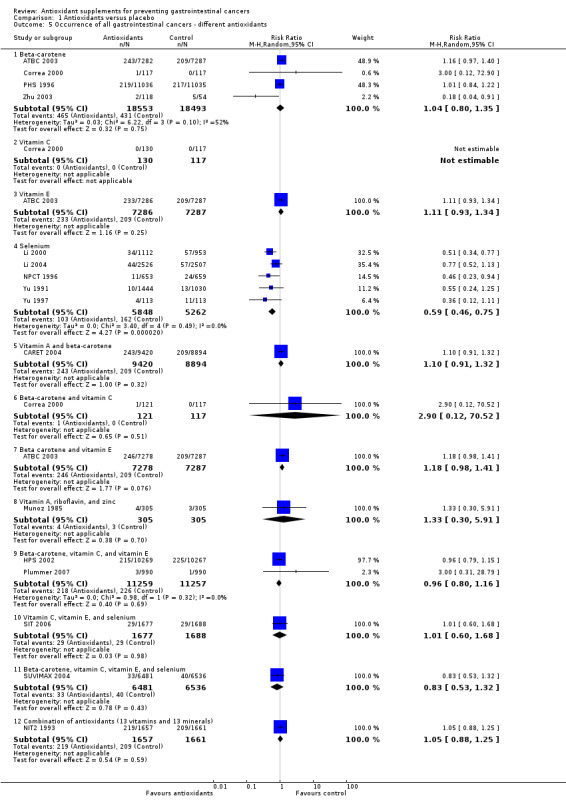
Comparison 1 Antioxidants versus placebo, Outcome 5 Occurrence of all gastrointestinal cancers ‐ different antioxidants.
1.6. Analysis.
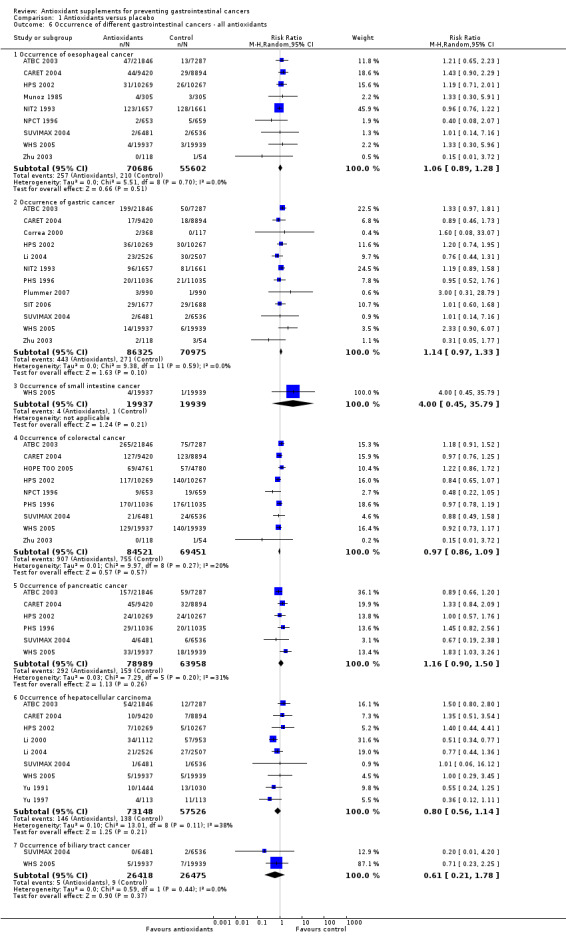
Comparison 1 Antioxidants versus placebo, Outcome 6 Occurrence of different gastrointestinal cancers ‐ all antioxidants.
1.7. Analysis.
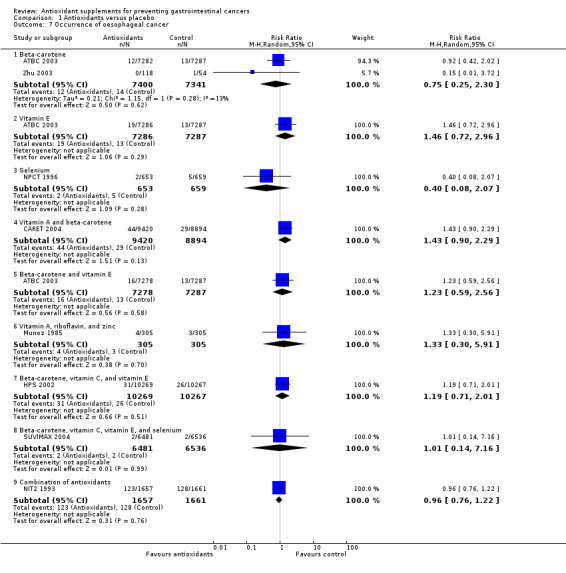
Comparison 1 Antioxidants versus placebo, Outcome 7 Occurrence of oesophageal cancer.
1.8. Analysis.
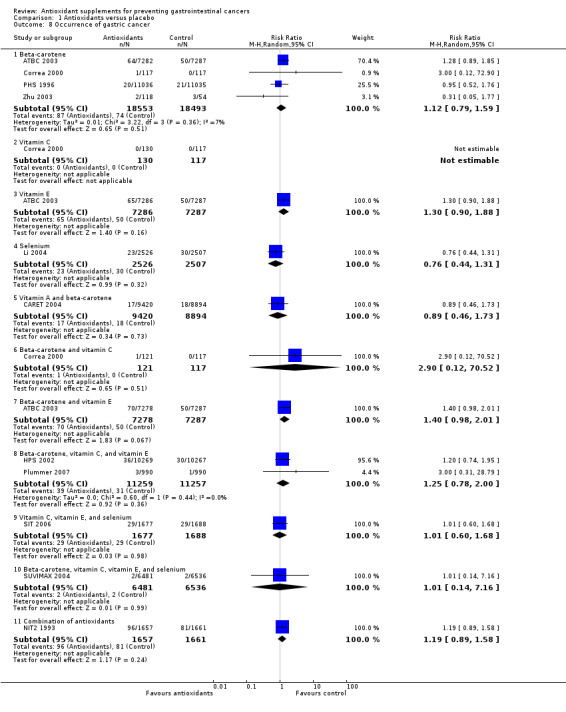
Comparison 1 Antioxidants versus placebo, Outcome 8 Occurrence of gastric cancer.
1.9. Analysis.
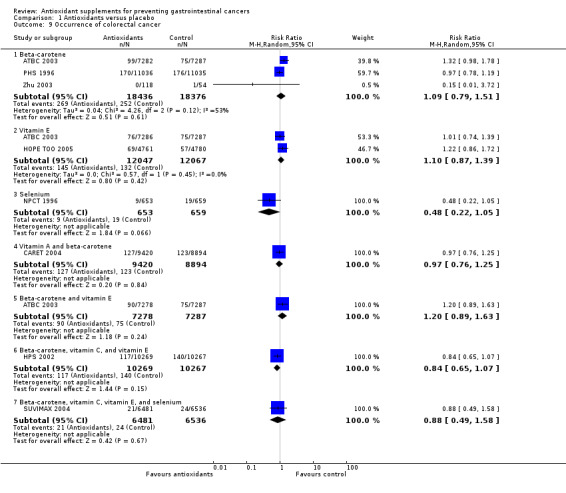
Comparison 1 Antioxidants versus placebo, Outcome 9 Occurrence of colorectal cancer.
1.10. Analysis.
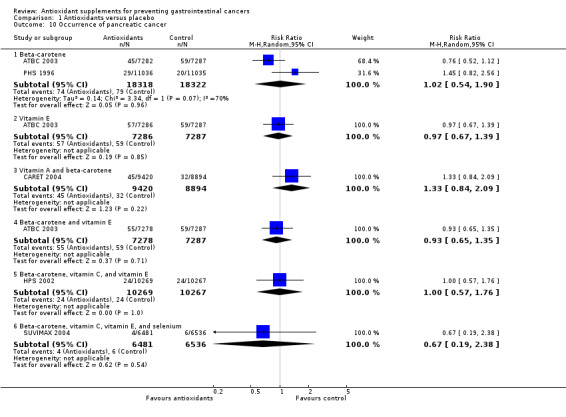
Comparison 1 Antioxidants versus placebo, Outcome 10 Occurrence of pancreatic cancer.
1.11. Analysis.
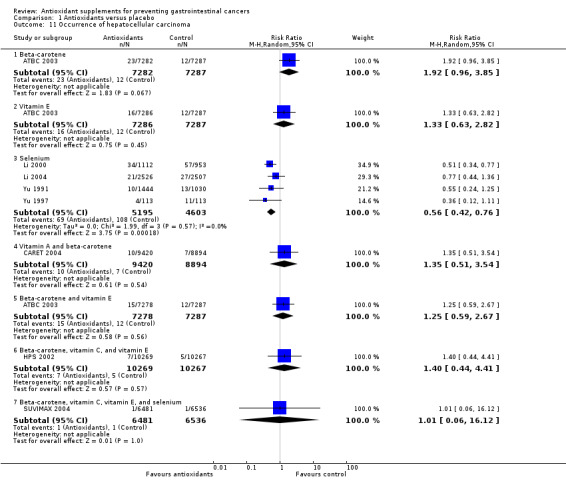
Comparison 1 Antioxidants versus placebo, Outcome 11 Occurrence of hepatocellular carcinoma.
1.12. Analysis.
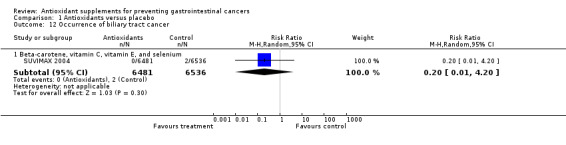
Comparison 1 Antioxidants versus placebo, Outcome 12 Occurrence of biliary tract cancer.
1.13. Analysis.
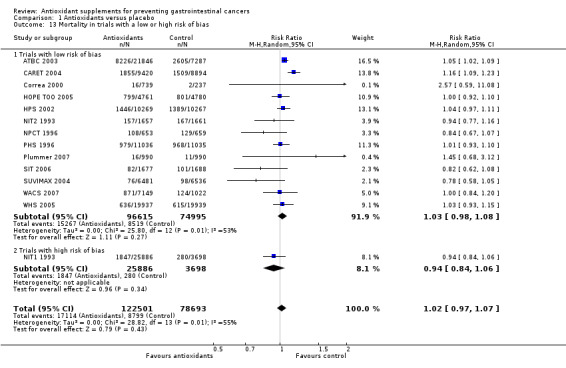
Comparison 1 Antioxidants versus placebo, Outcome 13 Mortality in trials with a low or high risk of bias.
1.14. Analysis.
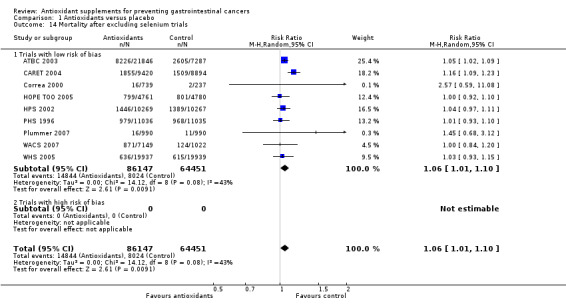
Comparison 1 Antioxidants versus placebo, Outcome 14 Mortality after excluding selenium trials.
1.15. Analysis.
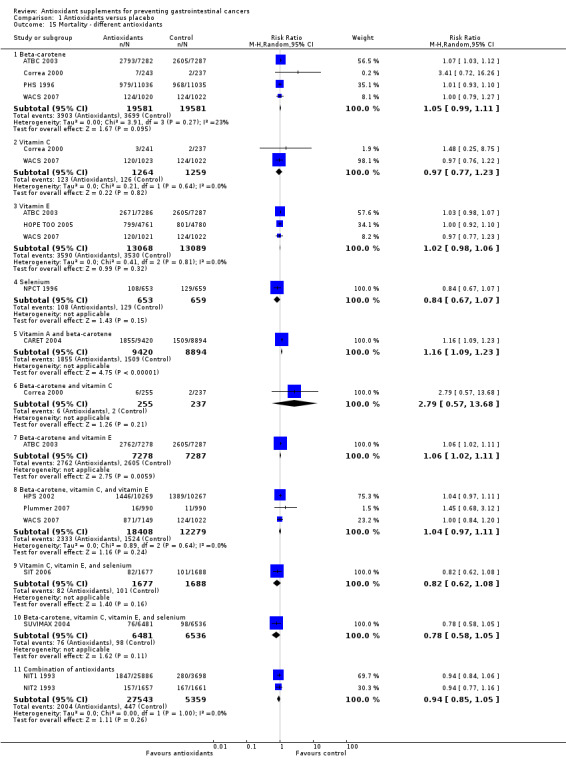
Comparison 1 Antioxidants versus placebo, Outcome 15 Mortality ‐ different antioxidants.
1.16. Analysis.
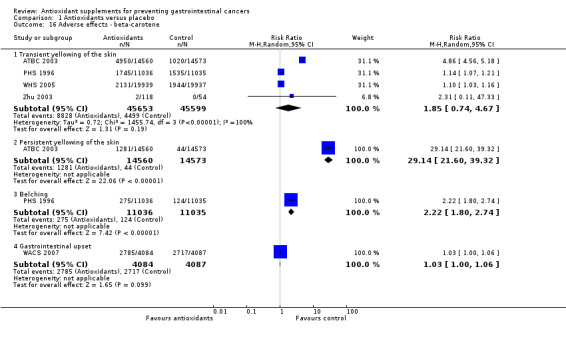
Comparison 1 Antioxidants versus placebo, Outcome 16 Adverse effects ‐ beta‐carotene.
1.17. Analysis.
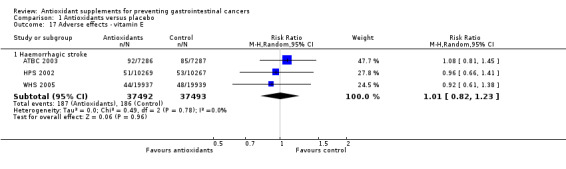
Comparison 1 Antioxidants versus placebo, Outcome 17 Adverse effects ‐ vitamin E.
1.18. Analysis.
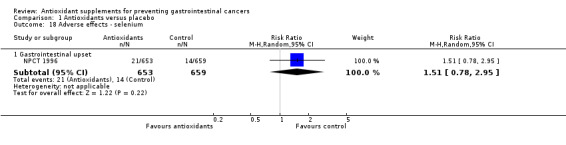
Comparison 1 Antioxidants versus placebo, Outcome 18 Adverse effects ‐ selenium.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ATBC 2003.
| Methods | Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study (ATBC). Randomised, double‐blind, placebo‐controlled trial with two‐by‐two factorial design. Generation of the allocation sequence: adequate, centrally by computer. Randomisation performed in blocks of eight within each of the study areas. Allocation concealment: adequate, coded capsule packs maintained centrally. Blinding: adequate, identical placebo capsules. Follow‐up: adequate, no losses to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: Finland. Number of participants randomised: 29,133 males. Inclusion criteria: male smokers (five or more cigarettes daily), aged 50 to 69 years, averaged 57.2 years of age at study entry, who lived in south‐western Finland. Exclusion criteria: men with a prior cancer or with other serious illness, or who used vitamin E, vitamin A, or beta‐carotene supplements in excess of predefined doses (> 20 mg, > 20000 IU, or > 6 mg, respectively), or anticoagulants. |
|
| Interventions | Participants were randomly assigned in four groups to receive: group 1: alpha‐tocopherol 50 mg (n = 7286); group 2: beta‐carotene 20 mg (n = 7282); group 3: alpha‐tocopherol and beta‐carotene, (n = 7278); group 4: placebo (n = 7287); daily for five to eight years (median 6.1 years). All participants took a single capsule daily. The four trial intervention groups were well balanced for all baseline characteristics evaluated. The two‐by‐two factorial design allowed assessment of the two intervention agents independently, with one‐half of participants receiving alpha‐tocopherol (n = 14,564) and the other half not (n = 14,569); similarly, half of the participants received beta‐carotene (n = 14,560) and half did not (n = 14,573). The study was conducted between 1985 and 1993. The active intervention continued through April 30, 1993 and postintervention follow‐up until April 30, 2001. Mean follow‐up time was 14.1 years. |
|
| Outcomes | The primary outcome measure was: incidence of lung cancer. Secondary outcome measures were: occurrence of other major cancers, overall and cause specific mortality, and occurrence of other diseases. |
|
| Notes | Compliance with treatment was assessed by counts of the remaining capsules at each visit, by measurement of serum alpha‐tocopherol and beta‐carotene levels after three years of supplementation, and by measurements in random serum samples throughout the study. Compliance with treatment was excellent with four out of five active participants taking more than 95% of the scheduled capsules. Dropout rate and compliance were similar between all four groups. All capsules were supplied by Hoffmann‐La Roche, Basel, Switzerland. Additional information received through personal communication with the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
CARET 2004.
| Methods | The Beta‐Carotene and Retinol Efficacy Trial (CARET). Randomised, double‐blind, placebo‐controlled trial with two‐by‐two factorial design in a pilot phase and then one‐by‐one. Generation of the allocation sequence: adequate, permuted block design with random block size chosen uniformly among 8, 10, 12, 14, and 16. Allocation concealment: adequate, locked central database with the link between study identifier and intervention assignment; all data analyses were performed centrally; the analyses that involved intervention assignment were performed only by the Co‐ordinating Center's statisticians using coded intervention assignment unknown to the statisticians; analyses involving the coded intervention assignments were seen only by CARET's Data and Safety Monitoring Board, Co‐ordinating Center statisticians, and a single CARET investigator who saw no participants. Blinding: adequate, identical placebo capsules provided by a sponsor. Follow‐up: adequate. The losses to follow‐up were less than 2% at the end of treatment. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United States of America. Number of participants randomised: 18314; 12025 males and 6289 females. Inclusion criteria: smokers, former smokers, and workers exposed to asbestos at high risk of developing lung cancer. A total of 4060 male workers, mean age 57 years, exposed to asbestos and 14254 heavy smokers (44% of whom were women), mean age 58 years, were randomised. The participants agreed to limit their supplemental intake of vitamin A to less than 5500 IU per day and to take no supplemental beta‐carotene. |
|
| Interventions | CARET builds on the experience of two pilot studies performed in Seattle (1985‐1988). The first pilot study initiated a phase III trial of the safety and efficacy of the study vitamins in 816 asbestos‐exposed participants randomised to a daily combination of 15 mg 13‐carotene and 25,000 IU retinol or a placebo medication. Participants were eligible up to age 74 and were not required to have a history of cigarette smoking; otherwise, the eligibility criteria were the same as for the asbestos‐exposed population in CARET. The second pilot study was a phase II trial of the comparative safety of the study vitamins in heavy smokers. The eligibility criteria were identical to those for heavy smokers in CARET. Overall 539 men and 490 women were randomised to one of four intervention groups: group 1: a daily combination of 30 mg 13‐carotene and 25,000 IU retinol; group 2: 30 mg 13‐carotene only; group 3: 25,000 IU retinol only; group 4: placebo medication. All 1845 participants in the two pilot studies continue to be followed for outcomes in CARET, together with approximately 16,000 additional participants. Participants of CARET trial were randomly assigned to receive: group 1: combination of 30 mg beta‐carotene and 25,000 IU vitamin A (n = 9420); group 2: placebo, (n = 8894). Both formulations were given as capsules. Beta‐carotene beadlets were combined with retinyl palmitate in a single capsule and dispensed in bottles, which were weighed and their content checked. The design projected active intervention until late 1997. The CARET active intervention was stopped 21 months earlier because of clear evidence of no benefit and substantial evidence of possible harm. The average duration of follow‐up was 10.0 years. |
|
| Outcomes | The primary outcome measure was: the occurrence of lung cancer. Other outcomes reported are: mortality rates and occurrence of other cancers. |
|
| Notes | Compliance was assessed by weighing the returned bottles to estimate the number of capsules remaining (in 85% of the assessments), or by relying on the participants own estimates (in 15% of the assessments). Compliance with treatment was excellent. Among the active participants, the mean rate of capsule consumption was 93% through five years of follow‐up, with no significant differences between the treatment groups. Participants who stopped receiving trial vitamins for any reason other than death were defined as inactive participants and were still followed for outcomes and counted in the analysis. As of December 15, 1995 ascertainment of vital status for more than 98% was complete. Active agents and placebos were purchased from Hoffmann‐La Roche and formulated by Tischon Corporation. Data were extracted from the primary publication, but additional information was received through personal communication with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Correa 2000.
| Methods | Randomised, controlled, partially double‐blind, chemoprevention trial with two‐by‐two‐by‐two factorial design. Generation of the allocation sequence: adequate, computer‐generated lists. Participants were randomly assigned in a single step, using a permuted block design, to one of eight different treatment regimens. Allocation concealment: adequate, coded capsules. Blinding: adequate, using identical placebo capsules. Follow‐up: adequate, the average rate of loss was 4.3% per year over the six‐year trial. Two hundred twenty‐one participants withdrew from the study before their 72‐month evaluation: 102 quitted treatment, 59 were lost to follow‐up, 34 dropped out of the study because of pregnancy and other medical conditions, 18 died of causes unrelated to gastric cancer, and eight developed cancer other than gastric cancer. In one participant, the 72‐month biopsy specimen was inadequate for histologic evaluation and determination of outcome. A total of 684 participants came to the 36‐month biopsy; of those, 92% (631) came for the 72‐month biopsy, there was a dropout rate of 2.6% per year for the last three years of the trial. Overall 24 participants from the placebo group, 25 from anti‐Helicobacter pylori (anti‐HP), 34 from the beta‐carotene (BC), 23 from the ascorbic acid (AA), 20 from the anti‐HP + BC, 23 from anti‐HP + AA, 17 from BC + AA, and 37 from anti‐HP + BC + AA were lost to follow‐up. Intention‐to‐treat analysis: no. Sample size calculations: no. |
|
| Participants | Country: Colombia. Number of participants randomised: 976; 46% males, aged 29 to 69 years, mean age 51.1 years. Inclusion criteria: preliminary histologic diagnosis of multifocal atrophic gastritis with or without intestinal metaplasia and dysplasia, good health. Exclusion criteria: normal histology, non‐atrophic gastritis, gastric cancer. Before randomisation, participants were classified into one of three strata: atrophy (without metaplasia), intestinal metaplasia, or dysplasia according to baseline histologic diagnosis. |
|
| Interventions | Participants were randomly assigned to a dietary supplement of beta‐carotene (30 mg once per day) and/or ascorbic acid (1 g twice a day) or their corresponding placebos, for a six‐year period. The prevalence of Helicobacter pylori infection among all gastric biopsy specimens was 97%. Anti‐Helicobacter pylori treatment consisting of amoxicillin (500 mg three times per day), metronidazole (375 mg three times per day), and bismuth subsalicylate (262 mg three times per day) was given for 14 days to half of the study participants assigned randomly. This treatment was not blinded or placebo controlled because an appropriate placebo was not available for bismuth subsalicylate. Participants were divided in eight treatment groups to receive: group 1: placebo (n = 117); group 2: anti‐Helicobacter pylori treatment, which consisted of amoxicillin, metronidazole, and bismuth subsalicylate (n = 120); group 3: beta‐carotene (n = 117); group 4: ascorbic acid (n = 130); group 5: Helicobacter pylori treatment consisting of amoxicillin, metronidazole, and bismuth subsalicylate, and additionally beta‐carotene (n = 126); group 6: Helicobacter pylori treatment consisting of amoxicillin, metronidazole, and bismuth subsalicylate, and additionally ascorbic acid (n = 111); group 7: beta‐carotene and ascorbic acid (n = 121); group 8: Helicobacter pylori treatment, consisting of amoxicillin, metronidazole, and bismuth subsalicylate, and additionally beta‐carotene and ascorbic acid (n = 134). Gastric biopsy specimens taken at baseline were compared with those taken at 72 months. |
|
| Outcomes | The primary outcome measures were: progression, no change or regression of gastric precancerous lesions (preliminary histologic diagnosis of multifocal atrophic gastritis with or without intestinal metaplasia and dysplasia). For our purposes we extracted data about the occurrence of gastric cancer. We have also extracted data on overall mortality for all antioxidants as well as for beta‐carotene and vitamin C. |
|
| Notes | Compliance with treatment was constantly encouraged and monitored by a social worker who interviewed the participants and recorded pill counts every three months. In addition, blood levels of beta‐carotene and ascorbic acid were measured every three months in a 20% random sample of the participants. Compliance with treatment among participants who completed the study was high for all intervention modalities (mean compliance for ascorbic acid, 91.8%; for beta‐carotene, 92.3%; and for anti‐Helicobacter pylori treatment, 99.1%). Active medication and placebos were provided like identical coded tablets by Hoffmann‐La Roche Inc. (Nutley, NJ). Additional information received through personal communication with the authors. Data were extracted from the article: Correa, et al. Re: Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti‐Helicobacter pylori therapy. Journal of the National Cancer Institute 2001; 93: 559. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
HOPE TOO 2005.
| Methods | The Heart Outcomes Prevention Evaluation Study (HOPE). Randomised, double‐blind, placebo‐controlled trial with two‐by‐two factorial design. Generation of the allocation sequence: adequate, by computer. Allocation concealment: adequate, randomisation is done by a telephone call to a central office. After receipt of appropriate baseline data over the telephone, the patient is randomised. Blinding: adequate, identical placebo capsules. Follow‐up: adequate. At the end of the initial HOPE trial, vital status was ascertained for 9535 (99.9%) of 9541 randomised patients. At the end of the HOPE‐TOO trial, vital status was ascertained for 4724 (99.8%) of 4732 patients who participated in the extension trial. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: International, North America, South America, Europe (19 countries) Number of participants randomised: 9541; 6996 males and 2545 females, 55 years old or older, mean age 66 years. Inclusion criteria: 55 years or older, had a history of CV disease (coronary artery disease, stroke or peripheral arterial disease) or diabetes in the presence of at least one additional CV risk factor (total cholesterol > 5.2 mmol/l, HDL cholesterol =0.9 mmol/l, hypertension, defined as use of medications to treat high blood pressure, or blood pressure at time of recruitment > 160 mmHg systolic or > 90 mmHg diastolic, known microalbuminuria, or current smoking). Exclusion criteria: Dipstick‐positive proteinuria, diabetic nephropathy, serum creatinine > 200 mmol/l, history of congestive heart failure, or known left ventricular ejection fraction (< 40%), hyperkalemia, uncontrolled hypertension, myocardial infarction, unstable angina or stroke within 1 month before trial enrollment, and use of or intolerance to vitamin E or angionetsin‐converting‐enzyme (ACE) inhibitors. |
|
| Interventions | Patients were randomly assigned to receive: group 1: 400 IU of vitamin E (RRR‐a‐tocopheryl acetate) daily from natural sources (n = 4761); group 2: matching placebo (n = 4780); group 3: an angiotensin‐converting–enzyme inhibitor (ramipril 10 mg) (n = 4645); group 4: matching placebo (n = 4652), once a day for a four to six years, mean 4.5 years. The Heart Outcomes Prevention Evaluation [HOPE] trial was conducted between December 21, 1993, and April 15, 1999. The Heart Outcomes Prevention was extended (HOPE‐The Ongoing Outcomes [HOPE‐TOO]) between April 16, 1999, and May 26, 2003. Of the initial 267 HOPE centres that had enrolled 9541 patients, 174 centres participated in the HOPE‐TOO trial. Of 7030 patients enrolled at these centres, 916 were deceased at the beginning of the extension of the trial, 1382 refused participation, 3994 continued to take the study intervention, and 738 agreed to passive follow‐up. The mean follow‐up period was 7 years. |
|
| Outcomes | The primary outcome measures were: cancer occurrence, cancer deaths, major cardiovascular events (myocardial infarction, stroke, and cardiovascular death). The secondary outcome measures were: unstable angina, congestive heart failure, revascularization or amputation, death from any cause, complications of diabetes, and cancer. |
|
| Notes | Compliance with treatment was checked by measuring the plasma vitamin E levels in randomly selected patients. The rate of compliance with the assigned regimen was high throughout the study. The percentages of patients who were taking vitamin E in the vitamin E and placebo groups, respectively, were 94.2% and 1.0% at 1 year, 93.3% and 1.7% at 2 years, 91.3% and 2.0% at 3 years, 90.2% and 2.7% at 4 years, and 89.2% and 3.4% at the final visit. There was no significant interaction between the study treatments (ramipril and vitamin E) for the primary, secondary, and other study outcomes. At the end of the initial HOPE trial, vital status was ascertained for 9535 (99.9%) of 9541 randomised patients. Funded by the Medical Research Council of Canada, Natural Source Vitamin E Association, Negma, Hoechst‐Marion Roussel, AstraZeneca, King Pharmaceuticals, and the Heart and Stroke Foundation of Ontario. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
HPS 2002.
| Methods | Heart Protection Study. Randomised, double‐blind, placebo‐controlled trial with two‐by‐two factorial design. Generation of the allocation sequence: adequate, computer generated random numbers. Allocation concealment: adequate, central telephone randomisation system. Blinding: adequate, identical placebo capsules. Follow‐up: adequate. 99.7% of the participants were with complete follow‐up for average of 5 years in vitamins allocated group and 99.6% in placebo group. Overall, 25 participants allocated to vitamins group and 35 participants to placebo group were lost to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United Kingdom. Number of participants randomised: 20536; 15454 males and 5082 females at an age 40 to 80 years. Inclusion criteria: adults with coronary disease, other occlusive arterial disease, or diabetes, and non‐fasting blood total cholesterol concentrations of at least 3.5 mmol/L. Exclusion criteria: other life‐threatening conditions, such as chronic liver disease, severe renal disease, severe heart failure, severe chronic airways disease, or diagnosed cancer (other than non‐melanoma skin cancer). In addition, anyone already taking high‐dose vitamin E supplements or in whom such supplements were considered indicated, was not to be randomised. |
|
| Interventions | Participants were randomly assigned to receive: group 1: 600 mg vitamin E, 250 mg vitamin C, and 20 mg beta‐carotene daily (n = 10,269); or group 2: matching placebo capsules (n = 10,267), daily during the scheduled 5‐year treatment period. |
|
| Outcomes | The primary outcome measures were: major coronary events (for overall analyses) and fatal or non‐fatal vascular events (for subcategory analyses), with subsidiary assessments of cancer and of other major morbidity. | |
| Notes | Compliance with treatment was assessed at each follow‐up by reviewing the calendar packed tablets remaining and for those who had stopped, the reasons for doing so were sought. An average of 83% of the participants in each treatment group remained compliant during the scheduled five‐year treatment period. To assess the effects of the treatment allocation on blood concentrations of the vitamins being studied, assays were performed in non‐fasting samples collected from about 5% of the participants at the initial screening visit and at an average of about three years of follow‐up (the approximate mid‐point of the study). Vitamins were provided by Roche. Data were extracted from the primary publication, but additional information was received through personal communication with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Li 2000.
| Methods | Randomised, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not described. Allocation concealment: unclear, not described. Blinding: adequate, matching placebo. Follow‐up: adequate, no losses to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: no. |
|
| Participants | Country: China. Number of participants randomised: 2065 males, aged 20 to 65 years. Inclusion criteria: males living in Qidong, Jiangsu province (a high risk area for liver cancer), HBsAg positive, AFP negative with normal liver function. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: group 1: sodium selenite 0.5 mg (228 µg of selenium) (n = 1112); group 2: placebo (n = 953); per day for three years. |
|
| Outcomes | The primary outcome measure was the occurrence of liver cancer. | |
| Notes | Compliance with treatment: good, between 81.5% in the first year and 79.8% in the third year. Data were extracted from the primary publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li 2004.
| Methods | Randomised double‐blind, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not reported. Allocation concealment: unclear, not reported. The whole process was conducted by the double‐blind method. Blinding: adequate, identical looking placebo capsules and placebo tablets. Follow‐up: inadequate. Intention‐to‐treat analysis: no. Sample size calculations: yes. |
|
| Participants | Country: China. Number of participants randomised: 5033, aged 34 to 74 years, 3250 men and 1783 women. Inclusion criteria: 34 to 74 years old, stomach disorder, or tumours in other family members, or smoking and/or alcohol consumption. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: group 1: selenium (sodium selenite) 100 µg and synthetic allitridum 200 mg (n = 2526); group 2: placebo (n = 2507); Each participant took orally two soft intestinal dissolving capsules that contained synthetic allitridum every day and one table of sodium selenite which contained selenium every other day for one month of each year during two years and at the same time each participant in the control group was given two placebo capsules which contained corn oil and one starch table of identical appearance to that in intervention group. Participants were treated from 1989 to 1991, and followed during next five years (1192 to 1997), overall 7 years. Allitridum is an extract of garlic. |
|
| Outcomes | The primary outcome measure was the occurrence of gastric cancer. | |
| Notes | Compliance with treatment was checked by interviews face to face and by measuring thiamine concentration in the urine. Compliance with treatment was excellent. Mean compliance estimated for the taking of the medication was 93%. Synthetic allitridum was made from in Dongfeng Pharmaceutical Factory, Lianyuguang, China, and the soft intestinal dissolving capsule of allitridum turned out in the Weihai Branch Factory of Shangdong Xinhua Pharmaceutical Factory, Zibo, China. The tables of sodium seletine came from the Office of Shangdong Endemic Diseases, and were manufactured by Hezhe Pharmaceutical Factory, Hezhe, China. Authors reported that the major side effects of the drugs were garlic‐odour and heartburn, but did not provide numbers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Munoz 1985.
| Methods | Randomised, double‐blind, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not described. Allocation concealment: adequate, the capsules were packed individually on two and three blister charts, respectively. On the reverse side of the blisters was self‐adhesive label with the individual's study number. The participants, barefoot doctors, and field supervisors were unaware of the treatment assignment. The code for the treatment assignment was not opened until the histological evaluation was completed. Blinding: adequate, identical‐looking gelatin capsules. Follow‐up: adequate, 567 persons (93%) attended the final endoscopic examination. Intention‐to‐treat analysis: no. Sample size calculations: no. |
|
| Participants | Country: China, Huixian, Henan Province. Number of participants randomised: 610, 50% males, aged 35 to 64 years. Inclusion criteria: inhabitants of Huixian, Henan province of China with high risk of oesophageal cancer. Exclusion criteria: none stated. In September, 1983 a random population sample was drawn from two production brigades (Meng Zhuang and Dong Xia Feng) in Huixian, Henan province. |
|
| Interventions | Participants were randomly assigned to receive: group 1: 15 mg (50,000 IU) retinol, 200 mg riboflavine, and 50 mg zinc (as zinc gluconate), (n = 305); group 2: matching placebo (n = 305). once a week for 13.5 months. The final examination was in October/November 1984, 13.5 months after the beginning of the trial and included endoscopy. Two biopsy specimens were taken at random from the middle and lower third of the oesophagus, and additional ones from any macroscopic lesion. Of 567 persons (93%) who attended the final endoscopic examination, histological slides were evaluated from 552 persons (90.5%). In the other 15 the available material was inadequate. |
|
| Outcomes | The primary outcome measures were the prevalence of precancerous lesions of the oesophagus, as well as occurrence of oesophageal cancer. | |
| Notes | In the early stages of the trial compliance was assessed by vitamin measurements in blood two months after the start of the trial from a sample of 100 participants stratified by age, sex, production brigade, and treatment. Follow‐up forms and remaining capsules were inspected every two months by fields supervisors. Compliance was excellent. The final examination on 567 (93%) participants included oesophagoscopy and at least two biopsies. All vitamin and placebo preparations were provided by Hoffmann‐La Roche, Basel, Switzerland. Data were extracted from the primary publication. During the trial one patient died, but authors did not report from which arm and we were unable to obtain this information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
NIT1 1993.
| Methods | Nutrition Intervention Trial (NIT); The General Population Trial, in Linxian, China. Randomised, placebo‐controlled trial with one‐half replicate of a two‐by‐two‐by‐two‐by‐two factorial design. Generation of the allocation sequence: adequate, random numbers generated by independent data management centre in USA. The randomisation sequence was known only at data management centre until the intervention ended. Allocation concealment: adequate, sequentially numbered coded bottles. Pill containers were labelled in the USA at the pill distribution centre. Blinding: adequate, identical placebo pills. Follow‐up: inadequate, losses to follow‐up not reported. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: China, Henan Province of north central China, Linxian County. Number of participants randomised: 29584; 45% males, aged 40 to 69 years. Inclusion criteria: residents willing to take part in a multi‐year, daily pill‐taking regimen. Exclusion criteria: debilitating disease or prior oesophageal or stomach cancer. |
|
| Interventions | Participants were randomly assigned to receive one of eight vitamin/mineral supplement combinations in the form of individual oral tablets. The eight intervention groups (each with 3677 to 3709 participants) were derived from a one‐half replicate of a two‐by‐two‐by‐two‐by‐two factorial design which allowed to asses four factors (ie, nutrient combinations) in a single experiment. The four factors designated by the letters A, B, C, D were: A ‐ retinol (as palmitate) 5000 IU, zinc (as zinc oxide) 22.5 mg; B ‐ riboflavin (vitamin B2) 3.2 mg and niacin (vitamin B3) 40 mg; C ‐ ascorbic acid 120 mg and molybdenum (as molybdenum yeast complex) 30 µg; D ‐ beta carotene 15 mg, selenium (as selenium yeast) 50 µg, and alpha‐tocopherol 30 mg. Doses of each nutrient varied from one to two times US Recommended Daily Allowances (RDAs). The eight intervention groups were defined by the following combinations of supplements; AB, AC, AD, BC, BD, CD, ABCD, or placebo and packed in coded bottles containing a one‐month supply. Bottles were distributed monthly beginning in March 1986 and continuing through May 1991, average 5.25 years. | |
| Outcomes | The primary outcome measures were: cancer occurence, cancer mortality, and overall mortality. | |
| Notes | Compliance with study treatment was assessed by monthly pill counts and biochemical measures. Compliance was excellent throughout the study. The overall pill disappearance rate was 93% for all participants, with no difference by treatment group (range 92% to 93%) and little change during the trial. All vitamin/mineral supplements and placebos were provided by Hoffmann‐La Roche, Basel, Switzerland and Lederle Laboratories, Inc. Data were extracted from the primary publication. Additional information was received through personal communication with the authors. However, we did not receive information on gastrointestinal cancer occurrence per group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
NIT2 1993.
| Methods | The Dysplasia Trial. Randomised, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: adequate, random numbers generated by independent data management centre in USA. Allocation concealment: adequate, sequentially numbered coded bottles. Pill containers were labelled in the USA at the pill distribution centre. Blinding: adequate, identical placebo pills. Follow‐up: adequate, the morbidity and mortality follow‐up rates were 99%. Intention‐to‐treat analysis: yes. Sample size calculation: yes. |
|
| Participants | Country: China, Henan Province of north central China, Linxian County. Number of participants randomised: 3318; 1461 males and 1857 females at age 40 to 69 years, mean age 54 years. Inclusion criteria: place of living in one of the three northern Linxian communes (Yaocun, Rencun, or Donggang), provided consent, diagnosis of oesophageal dysplasia on a balloon cytology examination. Exclusion criteria: taking vitamins of any type regularly, or antitumour B (a traditional Chinese drug consisting of a mixture of six medical herbs), history of malignancy or other debilitating disease. |
|
| Interventions | Participants were randomly assigned to receive: group 1: 13 vitamins and 13 minerals (vitamin A (acetate) 10000 IU; vitamin E (dl‐alpha tocopherol acetate) 60 IU, vitamin C (ascorbic acid) 180 mg, vitamin B1 5 mg, vitamin B2 5.2 mg, vitamin B6 6 mg, vitamin B12 18 µg, vitamin D 800 IU; beta‐carotene 15 mg, folic acid 800 µg, niacinamide 40 mg, biotin 90 µg, pantothenic acid 20 mg, calcium 324 mg, phosphorus 250 mg, iodine 300 µg, iron 54 mg, magnesium 200 mg, copper 6 mg, manganese 15 mg, potassium 15.4 mg, chloride 14 mg, chromium 30 µg, molybdenum 30 µg, selenium (sodium selenate) 50 µg, and zinc 45 mg (n = 1657); group 2: placebo (n = 1661); for a period of 6 years. The doses were typically two to three times the US Recommended Daily Allowances (RDAs), but ranged from 0.26 to seven times the RDA depending on the vitamin or mineral. Each participant was given three pills daily, including one capsule beta‐carotene or placebos and two tablets of vitamin/mineral supplement, or placebos. |
|
| Outcomes | The primary outcome measures were: cancer occurrence, cancer mortality, and overall mortality. | |
| Notes | Compliance with treatment was assessed by counting unused pills for all trial participants and by assessing nutrient levels in blood collected from samples of individuals randomly selected without replacement every three months throughout the trial. Compliance with treatment was excellent. The overall pill disappearance rate was 94% in both groups with slight decline (from 96% in year 1 to 92% in year 6 in both groups) over the duration of the trial. Data were extracted from the primary publication. Active medications and placebos were provided: beta‐carotene as Solatane by Hoffmann‐La Roche, Inc., Nutley, N.Y., and vitamin/mineral supplement as Centrum Lederle Laboratories, Inc., Pearl River, N.Y. Additional information was received through personal communication with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
NPCT 1996.
| Methods | Nutritional Prevention of Cancer Trial (NPCT). Randomised, double‐blind, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: adequate, computer generated random numbers. Allocation concealment: adequate, treatment group assignment was made centrally. The co‐ordinating centre held all treatment information in blinded form. Medications were distributed using sealed pill bottles. Blinding: adequate, identical placebo tablets. Follow‐up: adequate. At the end of the blinded period of treatment no participants were lost to vital follow‐up, and only 7 participants (3 in the selenium group and 4 in the placebo group) declined to provide additional information about the illness. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United States of America. Number of participants randomised: 1312; 75% males, aged 18 to 80 years, mean age 63 years. Inclusion criteria: history of two or more basal cell skin cancers (BCC) or one squamous cell skin cancer (SCC), with one of this occurring within the year prior the randomisation, life expectancy of at least five years and no internal malignancies treated within the previous five years. Exclusion criteria: history of significant liver or kidney disorders. Recruitment began on September 15, 1983 and continued each year through 1991. |
|
| Interventions | Patients were randomly assigned to receive: group 1: 200 µg of selenium supplied in a 0.5 g high‐selenium bakers yeast tablet (n = 653); group 2: placebo (n = 659); The end of a blinded period of treatment was on February 1, 1996. Mean length of treatment was 4.5 years and follow‐up 7.4 years. |
|
| Outcomes | The primary outcome measures were: incidences of basal cell and squamous cell carcinoma of the skin. In 1990 secondary outcome measures were identified, which included: total mortality and cancer mortality, as well as the incidence of the lung, colorectal, and prostate cancers. | |
| Notes | Compliance with treatment: excellent, 79.3% of the participants (80.3% in the placebo group and 78.4% in the selenium group) missed taking a pill less than twice a month. Trial medications were provided by Nutrition 21 (La Jolla, CA), through 1995 and by Cypress Systems (Fresno, CA) thereafter. Data about the gastrointestinal cancer occurrence were extracted from the article: Duffield‐Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev 2002;11(7):630‐9. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
PHS 1996.
| Methods | Physicians Health Study (PHS). Randomised, double‐blind, placebo‐controlled trial with two‐by‐two factorial design. Generation of the allocation sequence: adequate, by computer in blocks. Allocation concealment: adequate, the shipping department sent out calendar packs (which were identical whether active or placebo) to individual participants depending on this code. All of the calendar packs were in coded boxes, supplied by the drug manufacturer, so that the shippers did not know which drug they were shipping. Blinding: adequate, identical placebo capsules. Follow‐up: adequate. By December 31, 1995, the scheduled end of the trial, less than 1% of the participants were lost to follow up. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United States of America. Number of participants randomised: 22071 US male physicians at age 40 to 84 years, mean age 53 years. Inclusion criteria: US male physicians willing to take part in this trial. Exclusion criteria: chronic liver disease or evidence of abnormal liver function, severe renal disease or evidence of impaired renal function, inflammatory muscle disease or evidence of muscle problems (creatine kinase > 750 IU/L); concurrent treatment with cyclosporin, fibrates, or high‐dose niacin; child‐bearing potential; severe heart failure; some life‐threatening condition other than vascular disease or diabetes (eg, severe chronic airways disease or any cancer other than non‐melanoma skin cancer); or conditions that might limit long‐term compliance (eg, severely disabling stroke, dementia, or psychiatric disorder). |
|
| Interventions | Physicians were randomly assigned to one of the four groups to receive: group 1: active aspirin 325 mg on alternate days plus beta‐carotene placebo; group 2: active beta‐carotene 50 mg on alternate days plus aspirin placebo; group 3: both active agents; or group 4: both placebos. The randomised aspirin component of the trial was terminated early, on 25 January 1988. The beta‐carotene component continued uninterrupted until its scheduled end in December 1995. A total of 11036 physicians were assigned at random to receive beta‐carotene and 11035 to receive beta‐carotene placebo. Time from randomisation to the end of the trial averaged 12 years, and time of follow‐up 12.9 years. |
|
| Outcomes | The primary outcome measures were: overall and within subgroups, occurrence of malignant neoplasms (except non melanoma skin cancer), incidence of cardiovascular disease, and overall mortality. | |
| Notes | Compliance with treatment was checked by random serum assessments obtained at unannounced visits to trial participants. Compliance with treatment excellent, the average per cent of pills taken was 97% in both the active and placebo groups. There was 85% compliance with beta‐carotene treatment after five years and 78% after 12 years. The use of vitamin A supplements was reported by only 6% of the placebo group even by the end of trial. Active trial packs and matching placebos were provided by: aspirin (Bufferin) by Bristol Meyers; beta‐carotene (Lurotin), BASF corporation. Additional information received through personal communication with the authors. Data were extracted from the article: Cook et al. Effects of beta‐carotene supplementation on cancer incidence by baseline characteristics in the Physicians' Health Study (United States). Cancer Causes and Control 2000; 11: 617‐26, with extended follow‐up of 12.9 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Plummer 2007.
| Methods | Randomized, double‐blind, placebo‐controlled, primary‐prevention trial with parallel group design. Generation of the allocation sequence: adequate, the random allocation sequence to determine treatment group was generated by Hoffmann‐La Roche, using random permuted blocks of size eight. Allocation concealment: adequate, all histologic diagnoses and assays on biologic samples were conducted blind to the treatment allocation. During the trial, the central study database at IARC did not contain data on treatment allocation. The data were added to the database after the trial had been completed. Blinding: adequate, the placebo was prepared in the form of capsules identical to those containing the vitamins. Follow‐up: adequate, overall 302 participants from active and 278 participants from placebo group dropped‐out during the trial. The number of participants who dropped out was slightly higher in the vitamin group than in the placebo group, but the difference was not statistically significant (P = 0.14, for difference of two proportions). Intention‐to‐treat analysis: no. Sample size calculations: yes. |
|
| Participants | Country: Venezuela Number of participants randomised: 1980, aged 35 to 69 years, 52.7% females. Inclusion criteria: population at high risk for stomach cancer in general good health, and permanent residents of Tachira State. Exclusion criteria: serious illness, including any type of cancer, those whose mental status made long‐term adherence to the treatment regimen unlikely and pregnant women. |
|
| Interventions | Participants were randomly assigned to receive: group 1: vitamin C (750 mg), vitamin E (600 mg), and beta‐carotene (18 mg) (n = 990); group 2: placebo (n = 990); daily for 3 years. The treatment was taken in the form of three capsules per day, one with each of the three main meals. Each capsule contained 250 mg vitamin C, 200 mg vitamin E, and 6 mg of beta‐carotene, for a daily dose of 750 mg of vitamin C (12.5 times the recommended daily allowance), 600 mg vitamin E (20 times the recommended daily allowance), and 18 mg beta‐carotene (considered the maximum dose if carotenoderma is to be avoided). |
|
| Outcomes | The primary outcome of the trial was the progression and regression of precancerous lesions of the stomach, as determined by histologic findings. | |
| Notes | Compliance for the intervention group was confirmed by the pill counts and measuring the biochemical markers of supplementation. Excellent compliance was indicated by pill counts when participants returned for their vitamin pills: 91% of all containers were returned with less than 10% of pills. There were clear increases in beta‐carotene and vitamin E levels in the treated group beyond the levels observed at baseline. In the placebo group, by contrast, no changes were observed. Participants who did not return for their supply of capsules were contacted first by telephone, then visited at home by social workers who enquired about the reasons for nonattendance, encouraged continuing participation, and provided the next month's supply of capsules. Both vitamin capsules and placebo were supplied by Hoffman‐La Roche. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SIT 2006.
| Methods | Shandong Intervention Trial Randomised, double‐blind, placebo controlled, primary prevention trial with stratified, factorial design 2x2x2 versus 2x2. Generation of the allocation sequence: adequate, randomized treatment assignments were generated at Westat in the United States. Allocation concealment: adequate, pill bottles bearing codes corresponding to assignments were then distributed to the study participants. Blinding: adequate, using identical placebo capsules. Follow‐up: adequate, overall 15 participants from placebo and 19 participants from active intervention group were lost to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: China (Linqu County, Shandong Province). Number of participants randomised: 3411, 1753 men and 1658 women aged 35 to 64 years. Inclusion criteria: participants aged 35 to 64 years willing to participate in 42‐month study, baseline gastroscopy with biopsies, known Helicobacter pylori status. Exclusion criteria: illness, bleeding disorders, cancers (except nonmelanoma skin cancer), heart failure, emphysema, renal or liver diseases, other life‐threatening illnesses, allergy to penicillin or related antibiotics. |
|
| Interventions | Participants were first divided on the basis of whether they showed serologic evidence of Helicobacter pylori infection at baseline (2285) or not (1126). Participants with serologic evidence of Helicobacter pylori at baseline were eligible to receive amoxicillin (1 g twice a day) and omeprazole (20 mg twice a day) in three capsules (two 500 mg amoxicillin and one 20 mg omeprazole) to be taken twice daily (before breakfast and dinner) for 2 weeks. Look‐alike placebo capsules containing lactose and starch for amoxicillin and sucrose and starch for omeprazole were given to serologically positive controls and to all seronegative participants. Approximately 3 months after initial treatment for Helicobacter pylori, supplementation with 100 IU alpha‐tocopherol, 250 mg vitamin C, and 37.5 µg selenium twice a day began its 39‐month course. Participants received this mixture in one capsule, to be taken twice daily before or after breakfast and dinner. From December 1995 to May 1996, this mixture also contained beta‐carotene (7.5 mg twice a day). Look‐alike placebo capsules contained cellulose, lactose, and magnesium stearate. In the garlic group, participants took two capsules twice a day before or after breakfast and dinner. Each capsule contains 200 mg Kyolic aged garlic extract and 1 mg steam‐distilled garlic oil. To prepare the extract, the manufacturer sliced garlic cloves and soaks them in aqueous ethanol (about 20%) for over 18 months at room temperature. The extract is then filtered, concentrated, and dried. The look‐alike placebo capsules contained cellulose, granulated sugar, caramel, and magnesium stearate. Bottles holding placebo capsules contained minute quantities of garlic oil so they would smell like garlic. HP‐seropositive at baseline (2258) entered 2x2x2 factorial of antibiotics, vitamins, and garlic. HP‐seronegative at baseline (1126) entered 2x2 factorial trial of vitamins, and garlic. Participants were randomised in 12 groups: group 1: amoxicillin and omeprazole, garlic, vitamin and selenium (n = 286); group 2: amoxicillin and omeprazole, garlic, vitamin and selenium placebo (n = 285); group 3: amoxicillin and omeprazole, garlic placebo, vitamin and selenium (n = 286); group 4: amoxicillin and omeprazole, garlic placebo, vitamin and selenium placebo (n = 285); group 5: amoxicillin and omeprazole placebo, garlic, vitamin and selenium (n = 285); group 6: amoxicillin and omeprazole placebo, garlic, vitamin and selenium placebo (n = 286); group 7: amoxicillin and omeprazole placebo, garlic placebo, vitamin and selenium (n = 286); group 8: amoxicillin and omeprazole placebo, garlic placebo, vitamin and selenium placebo (n = 286); group 9: amoxicillin and omeprazole placebo, garlic; vitamin and selenium (n = 282); group 10: amoxicillin and omeprazole placebo, garlic, vitamin and selenium placebo (n = 281); group 11: amoxicillin and omeprazole placebo, garlic placebo, vitamin and selenium (n = 281); group 12: amoxicillin and omeprazole placebo, garlic placebo, vitamin and selenium placebo (n = 282); daily for 7.3 years. |
|
| Outcomes | The primary outcome measures were: prevalence of dysplasia or gastric cancer, prevalence of severe chronic atrophic gastritis, intestinal metaplasia, dysplasia or gastric cancer, and average severity score. Secondary outcome measures were: rates of transition from baseline to final histopathologic states and the effects of treatments on these rates of transition; evidence of the effectiveness of amoxicillin and omeprazole in eradicating Helicobacter pylori, based on 13C‐urea breath tests 3 months following treatment, on annual serology, and on a final pathologic examination of biopsies to look for Helicobacter pylori; and blood pressure at the time of the final examination. | |
| Notes | Compliance with treatment was checked by measuring the plasma vitamin levels in randomly selected participants every 3 months and counting of the pills. Compliance with treatment was good. The average monthly proportion of participants taking all pills was 92.3%. Serum samples obtained from randomly selected participants demonstrate higher levels of vitamins C and E in participants assigned to vitamins 2 and higher levels of S‐allylcysteine in those assigned to garlic preparation. (Wakunaga of America, Co., Ltd, Mission Viejo, CA) provided the garlic preparation, Astra (East Asia Region) provided amoxicillin and omeprazole; and Sino‐American Shanghai‐Squibb Pharmaceuticals, Ltd. provided vitamin and mineral supplement. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SUVIMAX 2004.
| Methods | The SUpplementation en VItamines et Mine´raux AntioXydants (SU.VI.MAX) Study. Randomised, double‐blind, placebo‐controlled, primary‐prevention trial with parallel group design. Generation of the allocation sequence: adequate, random treatment allocation was performed by block‐sequence generation stratified by sex and age group by computer. Allocation concealment: adequate, capsule boxes were labelled with the participant's number, using partitioned organisation to ensure total security of the blind study. Blinding: adequate, identical placebo capsules. Follow‐up: adequate. Losses to follow‐up; 5.4 % in the intervention group and 6.2% in the placebo group. Overall, 739 participants in the active and 828 participants in the placebo group were lost to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: France. Number of participants randomised: 13017 French adults, 5141 men and 7876 women, aged from 35 to 60 years, mean age 48.95 years. Inclusion criteria: lack of disease likely to hinder active participation or threatened 5‐year survival; acceptance of possibility to be given placebo and acceptance of the constraints of participation; lack of previous regular supplementation with any of the vitamins and minerals in the supplement provided and absence of extreme beliefs or behaviour regarding diet. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: group 1: beta‐carotene 6 mg; vitamin C 120 mg; vitamin E 30 mg; selenium 100 µg; zinc 20 mg (n = 6481); group 2: placebo (n = 6536). All participants took a single daily capsule. Median follow‐up time was 7.5 years. | |
| Outcomes | The primary outcome measures were: major fatal and nonfatal ischaemic cardiovascular events and cancer of any kind, except for the basal cell carcinoma of the skin. The secondary outcome measure was: all cause mortality. | |
| Notes | Compliance for the intervention group was confirmed by measuring the biochemical markers of supplementation after 2 years and after 7 years for beta‐carotene, vitamin C and selenium. At the end of follow‐up, 74% of participants reported having taken at least two thirds of the capsules. There were no differences between the groups mean percentage of capsules taken, ie, 79% in each group). Sponsors of the trial: Fruit d'Or Recherche, Candia, Lipton, Kellogg's, Centre d'Information sur Canderel, Orangina, Este e Lauder, Cereal, Grands Moulins de Paris, CERIN, L'Ore al, Peugeot, Jet Service, RP Scherer, Sodexho, France Telecom, Santogen, Becton Dickinson, Fould Springer, Boehringer Diagnostic, Seppic Givaudan Lavirotte, Le Grand Canal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
WACS 2007.
| Methods | The Women's Antioxidant Cardiovascular Study Randomised, double‐blind, placebo‐controlled secondary prevention trial with two‐by‐two‐by‐two factorial design. Generation of the allocation sequence: adequate, centrally by computer. Allocation concealment: adequate, the randomisation allocation is coded. Shipping department sends out calendar packs (which are identical whether active or placebo) to individual participants depending on this code. All of the calendar packs are in coded boxes, supplied by the drug manufacturer, so that the shippers do not know which drug they are shipping. Blinding: adequate, identical placebo capsules and tablets. Follow‐up: adequate. Losses to follow‐up almost equal in the intervention arms (62 to 78 participants). Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United States of America. Number of participants randomised: 8171, women at high risk with either a history of vascular disease or at least 3 cardiovascular risk factors. Inclusion criteria: women 40 years or older, postmenopausal, or had no intention of becoming pregnant, had a self‐reported history of cardiovascular disease (CVD), or had at least 3 cardiac risk factors. The cardiac risk factors determining eligibility were self‐reported diagnosis of hypertension, high cholesterol level, or diabetes mellitus; parental history of premature myocardial infarction (MI) (before age 60 years); obesity (body mass index [BMI] >30 [calculated as weight in kilograms divided by height in meters squared]), current cigarette smoking; and inconsistent report of prior CVD. Exclusion criteria: self‐reported history of cancer (excluding nonmelanoma skin cancer) within the past 10 years, any serious non‐CVD illness, or were currently using warfarin sodium or other anticoagulants. |
|
| Interventions | Women were randomly assigned according to a 2 x 2 x 2 factorial design to take ascorbic acid (500 mg/d), vitamin E (600 IU every other day), and beta carotene (50 mg every other day) yielding eight treatment groups. A fourth arm was subsequently added to test a combination of folic acid 2.5 mg, vitamin B6 (50 mg, and vitamin B12 1 mg. Surviving members of the original cohort of 8171 women were invited to participate in this arm (referred to hereafter as the folic acid arm). Of 8026 survivors, a total of 5442 were additionally randomised to a folic acid/vitamin B6/vitamin B12 combination or its placebo on April 16, 1998, thus creating a total of 24 distinct treatment groups (16 groups in the folic acid arm, plus 8 groups not in that arm). group 1: beta‐carotene, vitamin C, and vitamin E (n = 1020); group 2: beta‐carotene placebo, vitamin C, and vitamin E (n = 1021); group 3: beta‐carotene, vitamin C, vitamin E placebo (n = 1023); group 4: beta‐carotene placebo, vitamin C, vitamin E placebo (n = 1023); group 5: beta‐carotene, vitamin C placebo, vitamin E (n = 1021); group 6: beta‐carotene placebo, vitamin C placebo, vitamin E (n = 1021); group 7: beta‐carotene, vitamin C placebo, vitamin E placebo (n = 1020); group 8: beta‐carotene placebo, vitamin C placebo, vitamin E placebo (n = 1022); for a mean duration of 9.4 years. |
|
| Outcomes | The primary outcome was a combined outcome measure of cardiovascular disease morbidity and mortality, including incident myocardial infarction, stroke, coronary revascularization procedures (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty), and cardiovascular mortality. The individual components of myocardial infarction, stroke, coronary revascularization, and cardiovascular disease death were prespecified secondary outcome measures. Information on transient ischaemic attack and total mortality was also collected and reviewed. | |
| Notes | Compliance was assessed through self‐report and defined as taking at least two‐thirds of study pills. Reported compliance was, on average, 76% at 4 years and 68% at 8 years of follow‐up for each antioxidant, with no significant difference between active and placebo groups at these times except for ascorbic acid at 8 years (70% versus 67% in the active vs placebo group; P = .01). Mean compliance over follow‐up was approximately 73% for all active and placebo agents. In 1999, blood samples were obtained from 30 local participants to evaluate biomarkers for compliance. Blood levels were elevated in each active vs placebo group. Vitamin C (ascorbic acid), was provided by BASF Corporation (Mount Olive, New Jersey), vitamin E (600 IU of natural vitamin E (d‐alpha tocopherol acetate) by Cognis Corporation (La Grange, Illinois), and beta carotene (50 mg of Lurotin, provided by BASF Corporation). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
WHS 2005.
| Methods | Women's Health Study (WHS). Randomised, double‐blind, placebo‐controlled trial with two‐by‐two‐by‐two factorial design in the beginning and than two‐by‐two. Generation of the allocation sequence: adequate, centrally by computer in batches of blocks of size 16. Allocation concealment: adequate, the randomisation allocation is coded. Shipping department sends out calendar packs (which are identical whether active or placebo) to individual participants depending on this code. All of the calendar packs are in coded boxes, supplied by the drug manufacturer, so that the shippers do not know which drug they are shipping. Blinding: adequate, identical placebo capsules. Follow‐up: adequate. Losses to follow‐up; 0.01% in beta‐carotene group and 0.005% in placebo group. Overall, 132 participants in the active group and 102 participants in the placebo group had unknown vital status. Intention‐to‐treat analysis: yes. Sample size calculations: yes. |
|
| Participants | Country: United States of America. Number of participants randomised: 39876 females aged 45 years or older, mean age 54.6 years. Inclusion criteria: female health professionals willing to take part in the trial. Age 45 years or older; no previous history of coronary heart disease, cerebrovascular disease, cancer (except nonmelanoma skin cancer), or other major chronic illnesses; no history of adverse effects from aspirin; no use of aspirin or nonsteroidal anti‐inflammatory drugs (NSAIDs) more than once a week, or willingness to forgo their use; no use of anticoagulants or corticosteroids; and no use of individual supplements of vitamin A, E, or beta carotene for more than once a week. Exclusion criteria: history of cancer (except non‐melanoma skin cancer), coronary heart disease, or cerebrovascular disease. |
|
| Interventions | Participants were randomly assigned to one of the eight treatment groups. The active agents were 100 mg of aspirin, given on alternate days; 600 IU of vitamin E, given on alternate days; and 50 mg of beta‐carotene, given on alternate days. group 1: aspirin 100 mg, beta‐carotene 50 mg, vitamin E 600 IU; group 2: aspirin 100 mg, beta‐carotene 50 mg, vitamin E placebo; group 3: aspirin 100 mg, beta‐carotene 50 mg placebo, vitamin E 600 IU; group 4: aspirin 100 mg, beta‐carotene placebo, vitamin E placebo; group 5: aspirin placebo, beta‐carotene 50 mg, vitamin E 600 IU; group 6: aspirin placebo, beta‐carotene 50 mg, vitamin E placebo; group 7: aspirin placebo, beta‐carotene placebo, vitamin E 600 IU; group 8: aspirin placebo, beta‐carotene placebo, vitamin E placebo; A total of 19939 women were assigned at random to receive beta‐carotene and 19937 to receive placebo in the beginning of April 1993. A total of 19937 women were assigned at random to receive vitamin E and 19939 to receive placebo. The beta‐carotene component of the trial was terminated early, on January 18, 1996. The aspirin and vitamin E components of the trial continued uninterrupted. The time from randomisation to the end of beta‐carotene component of the trial averaged 2.1 years. Authors published results of the beta‐carotene component of the trial on February 6, 1998, after a median total follow‐up of 4.1 years (2.1 years treatment plus 2.0 years follow‐up). From that time trials proceeded as two‐arm (vitamin E and placebo). Follow‐up and validation of reported end points were completed in February 2005. The average duration of follow‐up from randomisation to the end of the trial was 10.1 years (range, 8.2 to 10.9 years). |
|
| Outcomes | The primary outcome measures were: incidence of invasive cancer (except non‐melanoma skin cancer), myocardial infarction, and stroke. The secondary outcome measures were: non‐fatal myocardial infarction, non‐fatal stroke, death from cardiovascular causes, and death from any cause. | |
| Notes | Compliance with treatment was checked by random serum assessments. Compliance with treatment was excellent. At the time of termination of the beta‐carotene component, 87% of the active group have taken at least two thirds of the study capsules, while 9.9% of the women in the placebo group have taken beta‐carotene or vitamin A supplements outside the trial. The active agents were provided as follows: aspirin by Bayer AG, Leverkusen, Germany; vitamin E by Natural Source Vitamin E Association, Washington DC; and beta‐carotene by Lurotin, BASF Corporation, Wiandotte, MI. Data were extracted from the primary publication, but additional information was received through personal communication with the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yu 1991.
| Methods | Randomised clinical placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not reported. Allocation concealment: unclear, not reported. Blinding: adequate, identical placebo tablets. Follow‐up: inadequate. Intention‐to‐treat analysis: no. Sample size calculations: no. |
|
| Participants | Country: China. Number of participants randomised: 2474, aged 18 to 75 years. Inclusion criteria: members of families with high incidences of liver cancer. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: group 1: 200 µg of selenium in the form of selenized yeast tablet (n = 1444); group 2: identical placebo of yeast tablet (n = 1030); daily for two years. |
|
| Outcomes | The primary outcome measure was the occurrence of liver cancer. | |
| Notes | Compliance with treatment is not reported. Data were extracted from the primary publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yu 1997.
| Methods | Randomised clinical placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not reported. Allocation concealment: unclear, not reported. Blinding: adequate, identical placebo tablets. Follow‐up: adequate, no losses to follow‐up. Intention‐to‐treat analysis: yes. Sample size calculations: no. |
|
| Participants | Country: China. Number of participants randomised: 226, aged 21 to 63 years. Inclusion criteria: HBsAg carriers with normal liver function. Exclusion criteria: none stated. |
|
| Interventions | Participants were randomly assigned to receive: group 1: 200 µg of selenium in the form of selenized yeast tablet (n = 113); group 2: identical placebo of yeast tablet (n = 113); daily for four years. Patients were followed‐up eight years from 1987 to 1994. |
|
| Outcomes | The primary outcome measure was the occurrence of liver cancer. | |
| Notes | Compliance with treatment is not reported. Data were extracted from the primary publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhu 2003.
| Methods | Randomised double‐blind, placebo‐controlled trial with parallel group design. Generation of the allocation sequence: unclear, not reported. Allocation concealment: unclear, not reported. Blinding: adequate. Patients were allocated to four arms (see Interventions). The three arms (group 2 to 4), used in this review, seem to have been adequately placebo controlled. Follow‐up: adequate, 3.7 % of patients were lost to follow up, and the losses were similar between the treatment groups. Intention‐to‐treat analysis: yes. Sample size calculations: no. |
|
| Participants | Country: China. Number of participants randomised: 216; 137 males, 79 females, aged 28 to 77 years, mean age 55.6 years. Inclusion criteria: patients with atrophic gastritis. Exclusion criteria: history of malignant tumour, gastrointestinal operation, chronic heart, lung, liver, and renal disease, taking vitamin pills in the last three months. |
|
| Interventions | Patients were assigned to four treatment groups to receive: group 1: folate 20 mg per day plus vitamin B12, 1 mg intramuscularly, per month for one year, then folate 20 mg twice a week plus vitamin B12 1 mg per three months for the next year (n = 44); group 2: natural beta‐carotene, 30 mg per day in the first year, then 30 mg twice a week for the next year (n = 61); group 3: synthetic beta‐carotene administered as natural beta‐carotene (n = 57); group 4: placebo (n = 54). All patients were followed‐up from 1994 to 2001, in total seven years. |
|
| Outcomes | The primary outcome measures were the occurrence of gastrointestinal tumors and mortality. | |
| Notes | Compliance with treatment was checked by counting the remaining pills at each visit, as well as by measurement of relevant vitamin concentrations in serum randomly at 15 days, 3, 6, 12, and 24 months. Compliance, as assessed by quarterly pill counting and random blood sampling, was excellent throughout the trial. More than 90% of all patients took pills according to the protocol. After the supplementation, the serum levels of folic acid or relative carotenoids increased several times in the three active treated groups, but not in placebo. Folate and vitamin B12 were provided by Shanghai Huanghe Pharmacy and Shanghai First Pharmacy, China; natural beta‐carotene by Betatene Co. Australia and Henkel Co.; synthetic beta‐carotene by Shanghai Sixth Pharmacy and Henkel Co. Data were extracted from the primary publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ATBC: Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study CARET: The Beta‐Carotene and Retinol Efficacy Trial HOPE: Heart Outcomes Prevention Evaluation Study HOPE TOO: Heart Outcomes Prevention Evaluation Study The Ongoing Outcomes HPS: Heart Protection Study. NIT: Nutrition Intervetion Trial NPCT: Nutritional Prevention of Cancer Trial PHS: Physicians Health Study SIT: Shandong Intervention Trial SUVIMAX: The SUpplementation en VItamines et Mine´raux AntioXydants WACS: Women Antioxidant Cardiovascular Study WHS: Women's Health Study BCC: basal cell skin cancers SCC: squamous cell skin cancer RDA: recommended daily allowance HBsAg: hepatitis B surface antigen AFP: alpha fetoprotein US: United States
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bespalov 2004 | Randomised clinical trial of the drug karinat was carried out in patients with chronic multifocal atrophic gastritis. Karinat contains beta‐carotene 2.5 mg, alpha‐tocopherol 5 mg, ascorbic acid 30 mg and garlic powder 150 mg per tablet. Out of 66 patients, 34 received karinat and 32 placebo. This is phase II clinical trial. There were no participants with gastrointestinal cancers at the end of the trial. |
| Bostick 1993 | A prospective cohort study. |
| Bukin 1996 | Randomised clinical trial which studied the effects of beta‐carotene, vitamin E, and pharmaceutical complex of natural antioxidants on abnormally high ornithine decarboxylase activity in antral gastric mucosa of patients with atrophic gastritis accompanied by intestinal metaplasia. |
| Bussey 1982 | We have been unable to obtain data on gastrointestinal cancers from this trial. |
| Chuang 2002 | Randomised clinical trial to test test whether vitamin C and E supplements to triple therapy can improve the Helicobacter pylori eradication rate and gastric inflammation. Trial is of short follow‐up period without data on incidence of gastrointestinal cancers. |
| De Stefani 1999 | A case‐control study in Uruguay with patients already afflicted with cancer of the oral cavity, pharynx, larynx, and oesophagus. Patients were interviewed about their dietary habits. Based on food frequency questionnaire, intake of certain nutrients was calculated. |
| De Stefani 1999a | A case‐control study in Uruguay with patients already afflicted with cancer of the oral cavity, pharynx, larynx, and oesophagus. Patients were interviewed about their dietary habits and based on food frequency questionnaire intake of certain food groups was calculated. |
| De Stefani 2000 | A case‐control study in Uruguay. Intake of antioxidants and other dietary habits were obtained indirectly by face‐to‐face interviews with patients already having oesophageal cancer and control group of patients by the food frequency questionnaire. |
| DeCosse 1975 | Ascorbic acid, 3 g daily, was given to five patients who had active rectal adenomatous polyp formation long after ileorectal anastomosis for familial polyposis. This is not randomised clinical trial. |
| DeCosse 1989 | We have been unable to obtain data on gastrointestinal cancers from this trial. |
| ECP‐IM 1994 | We have been unable to obtain data on gastrointestinal cancers from this trial. |
| EUROSCAN 2000 | Randomised clinical trial in patients with head and neck cancer or with lung cancer, most of whom had a history of smoking. Patients were supplemented with vitamin A and N‐acetylcysteine. |
| Frank 1995 | An observational study of the demographic and psychosocial characteristics as well as the health behaviours, health status, and counselling practices of women physicians. |
| Greenberg 1990 | Randomised clinical trial of beta‐carotene in prevention of basal‐cell and squamous‐cell cancers of the skin. The trial did not provide data about the incidence of gastrointestinal cancers in supplemented and placebo group. |
| Ishikawa 1995 | Randomised trial for prevention of colorectal cancer where patients with multiple colorectal tumours were enrolled in two regimens. One was dietary guidance alone, and the other was dietary guidance plus eating wheat bran biscuits. |
| Jacobs 2001 | A large prospective cohort study that examined the association between colorectal cancer mortality and use of individual vitamin C and E supplements in the American's Cancer Society's Cancer Prevention Study II cohort. Intake of vitamin C and vitamin E was calculated based on self‐administrated questionnaire. |
| Jansen 1999 | Ecological analysis of data from The Seven Countries Study to investigate whether intake of fiber and plant foods contributes to cross cultural diferences in 25‐year colorectal‐cancer mortality in man. |
| Ji 1995 | A population‐based case control study, which examined the effects of diet on pancreatic cancer among the residents of Shanghai, newly diagnosed with this type of cancer. Information of usual adult dietary intake was obtained by interviewers, using a food frequency questionnaire. Intake of certain group foods was compared with incidence of pancreatic cancer. |
| Kirk 2006 | Randomised, double‐blind, placebo‐controlled crossover trial, evaluating the efficacy of a combined antioxidant preparation in the management of chronic pancreatitis. Patients with confirmed chronic pancreatitis (N = 36) were randomised to receive treatment with either antioxidants, which contains the antioxidants selenium, beta‐carotene, L‐methionine, and vitamins C and E, or placebo for 10 weeks. Each group of patients then switched to receive the alternative treatment for a further 10 weeks. Markers of antioxidant status were measured by blood sampling, whereas quality of life and pain were assessed using the SF‐36 questionnaire. Nineteen patients completed the full 20 weeks of treatment. This trial did not fulfil our inclusion criteria. |
| Krishnaswamy 1993 | A case‐control study. Serum selenium levels were measured in patients with oral or oesophageal cancer and compared with matched controls. |
| La Vecchia 2002 | A case‐control study in Italy concerning intake of lycopene in patients with histologically confirmed cancer of several organs (between them oesophageal and colorectal). Intake of antioxidants was calculated retrospectively based on the interview with patients using a food frequency questionnaire. |
| Lacroix 1987 | An observational study conducted to determine whether it is possible to increase plasma levels of retinol in cancer patients. Plasma levels of retinol were measured in 46 patients treated with chemotherapy for various malignancies and in 43 control individuals, before and after supplementation. |
| Lanza 1996 | An article describing the rationale, design, recruitment, and baseline participant characteristics of the Polyp Prevention Trial (Schatzkin 1996), a multicenter randomised controlled trial examining the effect of a low‐fat, high‐fiber, high‐vegetable and ‐fruit dietary pattern on the recurrence of colorectal adenomatous polyps. |
| Lanza 2001 | A multicenter randomised clinical trial, as a part of Polyp Prevention Trial (Schatzkin 1996), which examined results of dietary changes (implementation of four year high‐fiber, high‐fruit and ‐vegetable, low‐fat dietary intervention) on the recurrence of adenomatous polyps in large the bowel. |
| Levi 2000 | The association between dietary intake of various micronutrients and colorectal cancer risk was analysed using data from a case‐control study conducted between 1992 and 1997 in the Swiss Canton of Vaud. Dietary habits were investigated using a validated food frequency questionnaire. |
| Limburg 2005 | Randomised clinical trial of selenomethionine 200 microg daily and/or celecoxib 200 mg twice daily (2 x 2 factorial design) among residents of Linxian, People's Republic of China. Subjects had histologically confirmed mild or moderate esophageal squamous dysplasia at baseline. Esophagogastroduodenoscopy was performed before and after a 10‐month intervention. Per‐subject change (regression, stable, or progression) in the worst dysplasia grade was defined as the primary end point. Results were compared by agent group (selenomethionine versus placebo; celecoxib versus placebo). Two hundred sixty‐seven subjects fulfilled all eligibility criteria, and 238 (89%) completed the trial. Authors did not report incidence of oesophageal cancer. |
| Macrae 1999 | A review, discussing the results of several randomised clinical trials concerning the wheat bran fiber and development of adenomatous polyps. |
| Marotta 2003 | The aim of this study was to test the effect of antioxidants on enzymatic abnormalities and free radicals‐modified DNA adducts associated with pre‐malignant changes in HP‐negative chronic atrophic gastritis patients. 60 patients with and intestinal metaplasia underwent a GI endoscopy with biopsy samples for histology and for: alpha‐tocopherol, malonyldialdehyde, xanthine oxidase, ornithine decarboxylase and 8‐hydroxydeoxyguanosine. Patients were randomly allocated into three groups supplemented for 6 months with: vitamin E, 300 mg/day; Multivitamin, 2 tablets/day and a certified fermented papaya preparation 6 g (Immune‐Age FPP, Osato Research Institute, Gifu, Japan). Ten dyspeptic patients without histological abnormalities served as control. Histological and biochemical parameters were blindly repeated at 3 and 6 months. There are no results about the incidence of gastric cancer. This is phase II clinical trial. |
| Marotta 2004 | Randomised trial to test the effect of antioxidant supplementation on enzymatic abnormalities and free radical‐modified DNA adducts associated with premalignant changes in the gastric mucosa of elderly patients with HP‐negative atrophic gastritis (CAG). Sixty patients with atrophic gastritis and intestinal metaplasia underwent a nutritional interview and a gastroscopy with multiple biopsy samples in the antrum that were processed for histology and for assaying: alpha‐tocopherol, MDA, xanthine oxidase (XO), ornithine decarboxylase (ODC), and 8‐OHdG. Patients were randomly allocated into three matched groups and supplemented for 6 months with (1) vitamin E, 300 mg/day; (2) multivitamin, two tablets t.i.d.; and (3) Immun‐Age 6 g/day nocte (ORI, Gifu, Japan), a certified fermented papaya preparation with basic science‐validated antioxidant/immunomodulant properties. Ten dyspeptic patients served as controls. Histology and biochemistry were blindly repeated at 3 and 6 months. |
| Mayne 2001 | A case‐control study, which examined nutrient intake as a risk factor for oesophageal and gastric cancers. |
| Moriwaki 2002 | A review concerning current perspectives in prevention of liver cancer. |
| Muto 1996 | Randomised clinical trial in which 89 patients were studied after surgical resection of primary hepatoma or the percutaneous injection of ethanol. They were randomly assigned to receive either polyprenoic acid (600 mg daily) or placebo for 12 months. The primary outcome measure of the study was appearance of a histologically confirmed recurrence or new hepatoma. The reason for excluding this trial was existence of liver cancer at the time of randomisation. |
| Newsome 2000 | An observational study investigated serum retinol levels in patients with liver disease and hepatocellular carcinoma, assessing its importance as a risk factor for the development of hepatocellular carcinoma. |
| Nomura 1987a | A case‐control study comparing plasma selenium levels and risk of cancer by specific sites. |
| Pan 1993 | A case‐control study carried out on patients with newly diagnosed hepatocellular carcinoma and controls without hepatocellular carcinoma. Plasma levels of vitamin A, vitamin E, or beta‐carotene were compared among the two groups as well as education level, consumption of alcohol, and smoking status. |
| Podmore 1998a | Study involving healthy volunteers whose diets were supplemented with 500 milligrams per day of vitamin C for six weeks. The levels of oxidative damage to peripheral blood lymphocytes in terms of modified DNA bases were assessed. It is not a randomised trial. |
| Qu 2007 | Substudy of the Nutrition Prevention Trial already included in the analyses. |
| Rocchi 1997 | An observational study, which compared plasma liposoluble vitamins with tocopherol content in healthy and neoplastic liver tissue in humans. |
| Russo 1997 | A cross‐sectional observational study among patients with colorectal adenomas, comparing the incidence of adenomas with plasma selenium levels. |
| Sasazuki 2003 | Randomised clinical trial to examine the effect of vitamin C supplementation on serum pepsinogen level, Helicobacter pylori infection, and cytotoxin‐associated gene A status. Subjects aged 40 to 69 years living in one village in Akita prefecture, a high‐risk area for gastric cancer in Japan, were recruited through annual health check‐up programs. Among 635 participants diagnosed as having chronic gastritis on the basis of serum PG levels, after excluding ineligible cases, 439 subjects were assigned to one of four groups using a 2 x 2 factorial design (0 or 15 mg/day beta‐carotene and 50 or 500 mg/day vitamin C). However, based on the results from two beta‐carotene trials in the United States, beta‐carotene was discontinued (vitamin C supplementation was continued). Finally, 120 subjects in the low‐dose group (vitamin C 50 mg), and 124 subjects in the high‐dose group (vitamin C 500 mg) completed the 5‐year supplementation. Due to modification of protocol primary end point was not incidence of gastric cancer as it was first planned. |
| Schatzkin 1996 | A study describing the dietary intervention programme and participant baseline dietary characteristics of the Polyp Prevention Trial (PPT), a multicenter randomised trial examining the effect of a low‐fat, high‐fiber high‐vegetable and ‐fruit dietary pattern on the recurrence of colorectal adenomatous polyps, precursors of most colorectal malignancies. |
| Simone 2002 | An observational study with aim to determine whether short‐term supplementation of beta‐carotene or vitamin E would result in their respective accumulation in normal colonic mucosa and in adenomatous polyps and to determine whether the intake of beta‐carotene would interfere with the concentration of vitamin E in these target tissues. |
| Siriwardena 2007 | Randomised clinical trial of intravenous antioxidant (n‐acetylcysteine, selenium, vitamin C) therapy in patients with predicted severe acute pancreatitis. This trial did not fulfill our inclusion criteria. |
| Takshashi 2003 | The Hiraka Dietary Intervention Study is a community‐based randomized cross‐over trial designed to develop an effective dietary modification tool and system in an area with high mortality of stomach cancer and stroke. The participants were 550 healthy volunteers and were randomized into two groups with tailored dietary education to decrease sodium intake and to increase vitamin C and carotene intakes either in the first year (intervention group) or in the second year (control group). Dietary changes were assessed using a validated self‐administered diet history questionnaire, fasting blood samples, and 48‐hour urine samples, which were obtained before and after the one year period. |
| Terry 2000 | A nation‐wide, population‐based, case‐control study in Sweden. Intake of antioxidants in newly diagnosed patients with oesophageal cancer was calculated indirectly by interviews with patients about their dietary habits, by food frequency questionnaires, during the 20 years period prior to interview. |
| Weisburger 1991 | A review of the causes of the main human cancers, analysing the mechanisms of the protective effects of fruits and vegetables. |
| Whelan 1999 | The purpose of this case‐control study was to further investigate whether regular vitamin or calcium supplement intake influenced the incidence of recurrent adenomatous polyps in patients with previous neoplasia who were undergoing follow‐up colonoscopy. |
| Yang 2000 | A review discussing the problem of vitamin nutrition and gastroesophageal cancer. |
| Yu 1995 | An observational cohort study of 8436 men in Taiwan recruited between 1984 and 1986. Serum retinol levels were compared between patients with hepatocellular carcinoma and matched controls. |
| Yu 1999 | An observational study, which examined the association between plasma selenium levels and risk of hepatocellular carcinoma among chronic carriers of hepatitis B and/or C virus in a cohort of 7342 men in Taiwan. |
| Zheng 1995 | An observational prospective cohort study evaluating the association of retinol and antioxidant vitamins intake and the risk of cancers of upper digestive tract in Iowa Women's Health Study. |
PPT: Polyp Prevention Trial t.i.d: ter in die (three times a day).
Characteristics of ongoing studies [ordered by study ID]
APPOSE 2001.
| Trial name or title | The Australian Prostate Cancer Prevention Trial Using Selenium (APPOSE) trial. |
| Methods | |
| Participants | Country: Australia. Inclusion criteria: men at risk of prostate cancer. The cohort size will be 2000 participants in each arm. |
| Interventions | Parallel group design. Participants were randomly assigned to receive either 200 microg selenium or matching placebo daily. |
| Outcomes | The primary outcome measure is incidence of prostate cancer. |
| Starting date | 2001 |
| Contact information | Anthony J. Costello, MD, Level 1, 77 Victoria Parade, Fitzroy, Melbourne 3065, Australia; email: cosurol@mpx.com.au |
| Notes |
HGPIN 2006.
| Trial name or title | High‐grade prostatic intraepithelial neoplasia (HGPIN) trial. |
| Methods | |
| Participants | Country: United States of America Inclusion criteria: men high‐grade prostatic intraepithelial neoplasia (HGPIN) must be identified by biopsy; the tissue must then be confirmed by centralized pathology review to show HGPIN and no cancer. Exclusion criteria: taking finasteride or any other drug known to affect PSA, or selenium supplements in excess of 50 µg/d. |
| Interventions | The patient is randomly assigned to 200 µg/d of selenium as L‐selenomethionine, or to placebo, with treatment scheduled for 3 years. |
| Outcomes | The primary outcome measure is incidence of prostate cancer. |
| Starting date | 1999 |
| Contact information | James R. Marshall, Roswell Park Cancer Institute, Buffalo, NY 14263. Phone: 716‐845‐8444; Fax: 716‐845‐8487. E‐mail: james.marshall@roswellpark.org |
| Notes |
PHS II 2000.
| Trial name or title | Physicians' Health Study II (PHS II). Randomised double‐blind, placebo‐controlled trial with two‐by‐two‐by‐two‐by‐two factorial design. |
| Methods | |
| Participants | Country: United States of America. Number of participants randomised: 15,000 US male physicians, aged 55 years and older. Inclusion criteria: willing and eligible physicians to take part in this trial, including all willing and eligible participants from Physician Health Study I. |
| Interventions | Physicians' Health Study II utilized a two‐by‐two‐by‐two‐by‐two factorial design to test alternate day beta‐carotene, alternate day vitamin E, daily vitamin C, and a daily multivitamin. During the first half of 2003 the beta‐carotene component of the ongoing Physicians' Healthy Study II has been terminated. It continues to examine a multivitamin, vitamin C, and vitamin E. |
| Outcomes | The primary outcome measures are the incidence of total and prostate cancer, cardiovascular and eye diseases. |
| Starting date | 1999 |
| Contact information | Charles H. Hennekens MD 1415 W. Camino Real, Boca Raton, FL 33486, United States of America |
| Notes |
SELECT 2003.
| Trial name or title | The selenium and vitamin E cancer prevention trial, SELECT). Randomised double‐blind, placebo‐controlled trial with two‐by‐two factorial design. |
| Methods | |
| Participants | Country: United States of America. Number of participants randomised: 32,400 males, aged 50 years or older. Inclusion criteria: age > 55 years for Caucasians and > 50 years for African‐Americans, digital rectal examination not suspicious for prostate cancer, total serum prostate specific antigen < 4.0 ng/ml, no prior history of prostate cancer or high‐grade prostatic intraepithelial neoplasia, no anticoagulation therapy, except low‐dose aspirin, normal blood pressure (systolic blood pressure < 150 mm Hg and diastolic blood pressure < 90 mm Hg), willing to restrict supplementation of selenium and vitamin E during participation. |
| Interventions | Participants were randomly assigned to receive either 200 µg of 1‐selenomethionine, 400 mg of racaemic alpha‐tocopherol, and an optional multivitamin containing no selenium or vitamin E. The racaemic mix of alpha‐tocopherol will include both the d‐ and l‐isomers. Participants will be divided in four group according to two‐by‐two factorial design: group 1: placebo (n = 8100); group 2: vitamin E (n = 8100); group 3: selenium (n = 8100); group 4: vitamin E and selenium (n = 8100). |
| Outcomes | The primary outcome measure is the clinical incidence of prostate cancer as determined by a clinical diagnostic work‐up, including yearly digital rectal examination
and serum prostate specific antigen level. Secondary outcome measures will include prostate cancer‐free survival, all cause mortality, and the incidence and mortality of other cancers and diseases potentially impacted by the chronic use of selenium and vitamin E. Other trial objectives will include periodic quality of life assessments, assessment of serum micronutrient levels and prostate cancer risk, and studies of the evaluation of biological and genetic markers with the risk of prostate cancer. |
| Starting date | 2001 |
| Contact information | Eric A. Klein, Section of Urologic Oncology, Department of Urology, Cleveland Clinic Foundation, Cleveland, OH, USA Tel.: +1‐216‐444‐5591; Fax: +1‐216‐445‐ 3532, e‐mail address: kleine@ccf.org. |
| Notes |
APOSE: The Australian Prostate Cancer Prevention Trial HGPIN: High‐Grade Prostatic Intraepithelial Neoplasia PHS II: Physicians' Health Study II SELECT: The selenium and vitamin E cancer prevention trial PSA: prostate‐specific antigen
Contributions of authors
Goran Bjelakovic conceived the idea for and drafted the protocol and the review. Dimitrinka Nikolova developed the search strategy and revised the protocol and the review. Rosa Simonetti revised the protocol and the review. Christian Gluud provided input during the protocol stage as Contact Editor and joined the team of authors during the preparation of the review providing strategy for data analyses, data interpretation, and revisions of the review. All co‐reviewers contributed to the data extraction, data verification, and update of the review.
Sources of support
Internal sources
The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Denmark.
External sources
Knowledge and Research Centre for Alternative Medicine (ViFAB), Denmark.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ATBC 2003 {published data only}
- Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, et al. Effects of alpha‐tocopherol and beta‐carotene supplements on cancer incidence in the Alpha‐Tocopherol Beta‐Carotene Cancer Prevention Study. American Journal of Clinical Nutrition 1995;62(6 Suppl):1427s‐30s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, et al. Alpha‐tocopherol and beta‐carotene supplements and lung cancer incidence in the alpha‐tocopherol, beta‐carotene cancer prevention study: effects of base‐line characteristics and study compliance. Journal of the National Cancer Institute 1996;88(21):1560‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Albanes D, Malila N, Taylor PR, Huttunen JK, Virtamo J, Edwards BK, et al. Effects of supplemental alpha‐tocopherol and beta‐carotene on colorectal cancer: results from a controlled trial (Finland). Cancer Causes & Control 2000;11(3):197‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Blumberg J. The Alpha‐Tocopherol, Beta‐Carotene Cancer Prevention Study in Finland. Nutrition Reviews 1994;52(7):242‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Freedman DM, Tangrea JA, Virtamo J, Albanes D. The effect of beta‐carotene supplementation on serum vitamin D metabolite concentrations. Cancer Epidemiology, Biomarkers & Prevention 1999;8(12):1115‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Hartman TJ, Dorgan JF, Woodson K, Virtamo J, Tangrea JA, Heinonen OP, et al. Effects of long‐term alpha‐tocopherol supplementation on serum hormones in older men. Prostate 2001;46(1):33‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leppala JM, Virtamo J, Fogelholm R, Huttunen JK, Albanes D, Taylor PR, et al. Controlled trial of alpha‐tocopherol and beta‐carotene supplements on stroke incidence and mortality in male smokers. Arteriosclerosis Thrombosis and Vascular Biology 2000;20(1):230‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liede KE, Haukka JK, Saxen LM, Heinonen OP. Increased tendency towards gingival bleeding caused by joint effect of alpha‐tocopherol supplementation and acetylsalicylic acid. Annals of Medicine 1998;30(6):542‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Malila N, Taylor PR, Virtanen MJ, Korhonen P, Huttunen JK, Albanes D, et al. Effects of alpha‐tocopherol and beta‐carotene supplementation on gastric cancer incidence in male smokers (ATBC Study, Finland). Cancer Causes & Control 2002;13(7):617‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Malila N, Virtamo J, Virtanen M, Albanes D, Tangrea J, Huttunen J. The effect of alpha‐tocopherol and beta‐carotene supplementation on colorectal adenomas in middle‐aged male smokers. Cancer Epidemiology, Biomarkers & Prevention 1999;8(6):489‐93. [MEDLINE: ] [PubMed] [Google Scholar]
- Malila N, Virtamo J, Virtanen M, Pietinen P, Albanes D, Teppo L. Dietary and serum alpha‐tocopherol, beta‐carotene and retinol, and risk for colorectal cancer in male smokers. European Journal of Clinical Nutrition 2002;56(7):615‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Michaud DS, Pietinen P, Taylor PR, Virtanen M, Virtamo J, Albanes D. Intakes of fruits and vegetables, carotenoids and vitamins A, E, C in relation to the risk of bladder cancer in the ATBC cohort study. British Journal of Cancer 2002;87(9):960‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautalahti MT, Virtamo JR, Taylor PR, Heinonen OP, Albanes D, Haukka JK, et al. The effects of supplementation with alpha‐tocopherol and beta‐carotene on the incidence and mortality of carcinoma of the pancreas in a randomized, controlled trial. Cancer 1999;86(1):37‐42. [MEDLINE: ] [PubMed] [Google Scholar]
- Stolzenberg‐Solomon RZ, Blaser MJ, Limburg PJ, Perez‐Perez G, Taylor PR, Virtamo J, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. Journal of the National Cancer Institute 2001;93(12):937‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teikari JM, Laatikainen L, Rapola JM, Virtamo J, Haukka J, Liesto K, et al. Retinal vascular changes following supplementation with alpha‐tocopherol or beta‐carotene. Acta Ophthalmologica Scandinavica 1998;76(1):68‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teikari JM, Laatikainen L, Virtamo J, Haukka J, Rautalahti M, Liesto K, et al. Six‐year supplementation with alpha‐tocopherol and beta‐carotene and age‐related maculopathy. Acta Ophthalmologica Scandinavica 1998;76(2):224‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teikari JM, Rautalahti M, Haukka J, Jarvinen P, Hartman AM, Virtamo J, et al. Incidence of cataract operations in Finnish male smokers unaffected by alpha tocopherol or beta carotene supplements. Journal of Epidemiology and Community Health 1998;52(7):468‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ATBC Cancer Prevention Study Group. The Alpha‐Tocopherol, Beta‐Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. Annals of Epidemiology 1994;4(1):1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- The ATBC Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. New England Journal of Medicine 1994;330(15):1029‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tornwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Alpha‐tocopherol (vitamin E) and beta‐carotene supplementation does not affect the risk for large abdominal aortic aneurysm in a controlled trial. Atherosclerosis 2001;157(1):167‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tornwall ME, Virtamo J, Haukka JK, Aro A, Albanes D, Huttunen JK. The effect of alpha‐tocopherol and beta‐carotene supplementation on symptoms and progression of intermittent claudication in a controlled trial. Atherosclerosis 1999;147(1):193‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tornwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Albanes D, Huttunen JK. Postintervention effect of alpha tocopherol and beta carotene on different strokes: a 6‐year follow‐up of the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Stroke 2004;35(8):1908‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Varis K, Sipponen P, Laxen F, Samloff IM, Huttunen JK, Taylor PR, et al. Implications of serum pepsinogen I in early endoscopic diagnosis of gastric cancer and dysplasia. Helsinki Gastritis Study Group. Scandinavian Journal of Gastroenterology 2000;35(9):950‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Varis K, Taylor PR, Sipponen P, Samloff IM, Heinonen OP, Albanes D, et al. Gastric cancer and premalignant lesions in atrophic gastritis: a controlled trial on the effect of supplementation with alpha‐tocopherol and beta‐carotene. The Helsinki Gastritis Study Group. Scandinavian Journal of Gastroenterology 1998;33(3):294‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, et al. Effects of supplemental alpha‐tocopherol and beta‐carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland). Cancer Causes & Control 2000;11(10):933‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, et al. Incidence of cancer and mortality following alpha‐tocopherol and beta‐carotene supplementation: a postintervention follow‐up. JAMA 2003;290(4):476‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Woodson K, Tangrea JA, Barrett MJ, Virtamo J, Taylor PR, Albanes D. Serum alpha‐tocopherol and subsequent risk of lung cancer among male smokers. Journal of the National Cancer Institute 1999;91(20):1738‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CARET 2004 {published data only}
- Alfano CM, Klesges RC, Murray DM, Bowen DJ, McTiernan A, Vander Weg MW, et al. Physical activity in relation to all‐site and lung cancer incidence and mortality in current and former smokers. Cancer Epidemiology, Biomarkers & Prevention 2004;13(12):2233‐41. [MEDLINE: ] [PubMed] [Google Scholar]
- Aliyu OA, Cullen MR, Barnett MJ, Balmes JR, Cartmel B, Redlich CA, et al. Evidence for excess colorectal cancer incidence among asbestos‐exposed men in the beta‐carotene and retinol efficacy trial. American Journal of Epidemiology 2005;162(9):868‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barnhart S, Keogh J, Cullen MR, Brodkin C, Liu D, Goodman G, et al. The CARET asbestos‐exposed cohort: baseline characteristics and comparison to other asbestos‐exposed cohorts. American Journal of Industrial Medicine 1997;32(6):573‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Thornquist M, Anderson K, Barnett M, Powell C, Goodman G, et al. Stopping the active intervention: CARET. Controlled Clinical Trials 2003;24(1):39‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brodkin CA, McCullough J, Stover B, Balmes J, Hammar S, Omenn GS, et al. Lobe of origin and histologic type of lung cancer associated with asbestos exposure in the Carotene and Retinol Efficacy Trial (CARET). American Journal of Industrial Medicine 1997;32(6):582‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cartmel B, Dziura J, Cullen MR, Vegso S, Omenn GS, Goodman GE, et al. Changes in cholesterol and triglyceride concentrations in the Vanguard population of the Carotene and Retinol Efficacy Trial (CARET). European Journal of Clinical Nutrition 2005;59(10):1173‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Challem JJ. Re: Risk factors for lung cancer and for intervention effects in CARET, the Beta‐Carotene and Retinol Efficacy Trial. Journal of the National Cancer Institute 1997;89(4):325‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chuwers P, Barnhart S, Blanc P, Brodkin CA, Cullen M, Kelly T, et al. The protective effect of beta‐carotene and retinol on ventilatory function in an asbestos‐exposed cohort. American Journal of Respiratory and Critical Care Medicine 1997;155(3):1066‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cullen MR, Barnett MJ, Balmes JR, Cartmel B, Redlich CA, Brodkin CA, et al. Predictors of lung cancer among asbestos‐exposed men in the beta‐carotene and retinol efficacy trial. American Journal of Epidemiology 2005;161(3):160‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goodman GE, Omenn GS. Carotene and retinol efficacy trial: lung cancer chemoprevention trial in heavy cigarette smokers and asbestos‐exposed workers. CARET Coinvestigators and Staff. Advances in Experimental Medicine and Biology 1992;320:137‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goodman GE, Omenn GS, Thornquist MD, Lund B, Metch B, Gylys‐Colwell I. The Carotene and Retinol Efficacy Trial (CARET) to prevent lung cancer in high‐risk populations: pilot study with cigarette smokers. Cancer Epidemiology, Biomarkers & Prevention 1993;2(4):389‐96. [MEDLINE: ] [PubMed] [Google Scholar]
- Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from Beta‐Carotene and Retinol Efficacy Trial. Cancer Epidemiology, Biomarkers & Prevention 2003;12(6):518‐26. [MEDLINE: ] [PubMed] [Google Scholar]
- Goodman GE, Thornquist M, Kestin M, Metch B, Anderson G, Omenn GS. The association between participant characteristics and serum concentrations of beta‐carotene, retinol, retinyl palmitate, and alpha‐tocopherol among participants in the Carotene and Retinol Efficacy Trial (CARET) for prevention of lung cancer. Cancer Epidemiology, Biomarkers & Prevention 1996;5(10):815‐21. [MEDLINE: ] [PubMed] [Google Scholar]
- Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL Jr, Omenn GS, et al. The Beta‐Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6‐year follow‐up after stopping beta‐carotene and retinol supplements. Journal of the National Cancer Institute 2004;96(23):1743‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goodman GE, Valanis B, Meyskens FL Jr, Williams JH Jr, Metch BJ, Thornquist MD, et al. Strategies for recruitment to a population‐based lung cancer prevention trial: the CARET experience with heavy smokers. Beta‐Carotene and Retinol Efficacy Trial. Cancer Epidemiology, Biomarkers & Prevention 1998;7(5):405‐12. [MEDLINE: ] [PubMed] [Google Scholar]
- King IB, Kristal AR, Schaffer S, Thornquist M, Goodman GE. Serum trans‐fatty acids are associated with risk of prostate cancer in Beta‐Carotene and Retinol Efficacy Trial. Cancer Epidemiology, Biomarkers & Prevention 2005;14(4):988‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Re: Risk factors for lung cancer and for intervention effects in CARET, the Beta‐Carotene and Retinol Efficacy Trial. Journal of the National Cancer Institute 1997;89(22):1722‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the Beta‐Carotene and Retinol Efficacy Trial (CARET). Cancer Epidemiology, Biomarkers & Prevention 2003;12(4):350‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the Beta‐Carotene and Retinol Efficacy Trial (CARET). Cancer Epidemiology, Biomarkers & Prevention 2003;12(4):350‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Omenn GS. CARET, the Beta‐Carotene and Retinol Efficacy Trial to prevent lung cancer in high‐risk populations. Public Health Reviews 1991;19(1‐4):205‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Omenn GS, Goodman G, Grizzle J, Thornquist M, Rosenstock L, Barnhart S, et al. CARET, the Beta‐Carotene and Retinol Efficacy Trial to prevent lung cancer in asbestos‐exposed workers and in smokers. Anticancer Drugs 1991;2(1):79‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman G, Grizzle J, Thornquist M, Rosenstock L, Barnhart S, et al. Recruitment for the Beta‐Carotene and Retinol Efficacy Trial (CARET) to prevent lung cancer in smokers and asbestos‐exposed workers. Western Journal of Medicine 1992;156(5):540‐4. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, Goodman G, Thornquist M, Barnhart S, Balmes J, Cherniack M, et al. Chemoprevention of lung cancer: the Beta‐Carotene and Retinol Efficacy Trial (CARET) in high‐risk smokers and asbestos‐exposed workers. IARC Scientific Publications 1996;136:67‐85. [MEDLINE: ] [PubMed] [Google Scholar]
- Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, et al. The Beta‐Carotene and Retinol Efficacy Trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos‐exposed workers. Cancer Research 1994;54(7 Suppl):2038s‐43s. [MEDLINE: ] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist M, Brunzell JD. Long‐term vitamin A does not produce clinically significant hypertriglyceridemia: results from CARET, the Beta‐Carotene and Retinol Efficacy Trial. Cancer Epidemiology, Biomarkers & Prevention 1994;3(8):711‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A. Risk factors for lung cancer and for intervention effects in CARET, the Beta‐Carotene and Retinol Efficacy Trial. Journal of the National Cancer Institute 1996;88(21):1550‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England Journal of Medicine 1996;334(18):1150‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Rosenstock L, Barnhart S, Gylys‐Colwell I, et al. The Carotene and Retinol Efficacy Trial (CARET) to prevent lung cancer in high‐risk populations: pilot study with asbestos‐exposed workers. Cancer Epidemiology, Biomarkers & Prevention 1993;2(4):381‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Redlich CA, Chung JS, Cullen MR, Blaner WS, Van‐Bennekum AM, Berglund L. Effect of long‐term beta‐carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the Beta‐Carotene and Retinol Efficacy Trial (CARET). Atherosclerosis 1999;143(2):427‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smigel K. Beta carotene fails to prevent cancer in two major studies; CARET intervention stopped. Journal of the National Cancer Institute 1996;88(3‐4):145. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sondik EJ. CARET study: women included. Science 1991;254(5030):360. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thornquist MD, Edelstein C, Goodman GE, Omenn GS. Streamlining IRB review in multisite trials through single‐study IRB cooperative agreements: experience of the Beta‐Carotene and Retinol Efficacy Trial (CARET). Controlled Clinical Trials 2002;23(1):80‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thornquist MD, Omenn GS, Goodman GE, Grizzle JE, Rosenstock L, et al. Statistical design and monitoring of the Beta‐Carotene and Retinol Efficacy Trial (CARET). Controlled Clinical Trials 1993;14(4):308‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thornquist MD, Patrick DL, Omenn GS. Participation and adherence among older men and women recruited to the Beta‐Carotene and Retinol Efficacy Trial (CARET). Gerontologist 1991;31(5):593‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thornquist MD, Urban N, Tseng A, Edelstein C, Lund B, Omenn GS. Research cost analyses to aid in decision making in the conduct of a large prevention trial, CARET. Beta‐Carotene and Retinol Efficacy Trial. Controlled Clinical Trials 1993;14(4):325‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valanis B, Blank J, Glass A. Mailing strategies and costs of recruiting heavy smokers in CARET, a large chemoprevention trial. Controlled Clinical Trials 1998;19(1):25‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Correa 2000 {published data only}
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti‐Helicobacter pylori therapy. Journal of the National Cancer Institute 2000;92(23):1881‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Correa P, Fontham ETH, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Re: chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplements and anti‐Helicobacter pylori therapy. Journal of the National Cancer Institute 2001;93(7):559‐60. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brown LM, You WC. Re: chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti‐Helicobacter pylori therapy. Journal of the National Cancer Institute 2001;93(7):559‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54(11):1536‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz B, Garay J, Correa P, Fontham ET, Bravo JC, Bravo LE, et al. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. American Journal of Gastroenterology 2001;96(12):3281‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HOPE TOO 2005 {published data only}
- Bosch J, Lonn E, Pogue J, Arnold JM, Dagenais GR, Yusuf S, HOPE/HOPE‐TOO Study Investigators. Long‐term effects of ramipril on cardiovascular events and on diabetes: results of the HOPE study extension. Circulation 2005;112(9):1339‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gerstein HC. Reduction of cardiovascular events and microvascular complications in diabetes with ACE inhibitor treatment: HOPE and MICRO‐HOPE. Diabetes/Metabolism Research and Reviews 2002;18(suppl 3):s82‐s5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in patients with diabetis mellitus: results of the HOPE study and MIRCO‐HOPE substudy. Lancet 2000;355(9200):253‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Heart Outcomes Prevention Evaluation Study Investigators. The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of an angiotensin‐converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. Canadian Journal of Cardiology 1996;12(2):127‐37. [PubMed] [Google Scholar]
- Lamy A, Yusuf S, Pogue J, Gafni A, Heart Outcomes Prevention Evaluation Investigators. Cost implications of the use of ramipril in high‐risk patients based on the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003;107(7):960‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long‐term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293(11):1338‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Mathew J, Pogue J, Johnstone D, Danisa K, Bosch J, et al. Relationship of electrocardiographic left ventricular hypertrophy to mortality and cardiovascular morbidity in high‐risk patients. European Journal of Cardiovascular Prevention and Rehabilitation 2003;10(6):420‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Roccaforte R, Yi Q, Dagenais G, Sleight P, Bosch J, et al. Effect of long‐term therapy with ramipril in high‐risk women. Journal of the American College of Cardiology 2002;40(4):693‐702. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, et al. Effects of vitamin E on cardiovascular and microvascular outcomes in high‐risk patients with diabetes: results of the HOPE study and MICRO‐HOPE substudy. Diabetes Care 2002;25(11):1919‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Annals of Internal Medicine 2001;134(8):629‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Franke J, Lonn EM, Hoogwerf BJ, et al. Progression of renal insufficiency in type 2 diabetes with and without microalbuminuria: results of the Heart Outcomes and Prevention Evaluation (HOPE) randomized study. American Journal of Kidney Diseases 2003;42(5):936‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Gerstein HC, Yi QL, Lonn EM, Hoogwerf BJ, Rashkow A, et al. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. Journal of the American Society of Nephrology 2003;14(3):641‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Lonn EM, Yi Q, Gerstein HC, Hoogwerf BJ, Pogue J, et al. Effects of vitamin E on cardiovascular outcomes in people with mild‐to‐moderate renal insufficiency: results of the HOPE study. Kidney International 2004;65(4):1375‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mann JF, Yi QL, Sleight P, Dagenais GR, Gerstein HC, Lonn EM, et al. Serum potassium, cardiovascular risk, and effects of an ACE inhibitor: results of the HOPE study. Clinical Nephrology 2005;63(8):181‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scandinavian Journal of Clinical and Laboratory Investigation 2005;Suppl 240:143‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Qilong Y, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. European Heart Journal 2004;25(1):17‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sharpe N. The HOPE TIPS: The HOPE Study Translated into Practices. Cardiovascular Drugs and Therapy 2005;19(3):197‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Teo KK, Mitchell LB, Pogue J, Bosch J, Dagenais G, Yusuf S, et al. Effect of ramipril in reducing sudden deaths and nonfatal cardiac arrests in high‐risk individuals without heart failure or left ventricular dysfunction. Circulation 2004;110(11):1413‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):154‐60. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Gerstein H, Hoogwerf B, Pogue J, Bosch J, Wolffenbuttel BH, et al. Ramipril and the development of diabetes. JAMA 2001;286(15):1882‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effect of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HPS 2002 {published data only}
- Armitage J, Collins R. Need for large scale randomised evidence about lowering LDL cholesterol in people with diabetes mellitus: MRC/BHF Heart Protection Study and other major trials. Heart 2000;84(4):357‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates CJ. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1781‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brown MJ. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1782. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleight P, Peto R. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1783‐4. [MEDLINE: ] [Google Scholar]
- Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. International Journal of Clinical Practice 2002;56(1):53‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Durrington PN. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1781. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Farmer JA. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomized, placebo‐controlled trial. Current Atherosclerosis Reports 2003;5(2):93‐4. [MEDLINE: ] [Google Scholar]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. European Heart Journal 1999;20(10):725‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kris‐Etherton PM. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Current Atherosclerosis Reports 2002;4(6):411‐2. [MEDLINE: ] [Google Scholar]
- Kulbertus H, Scheen AJ. Clinical study of the month. The MRC/BHF Heart Protection Study [L'etude clinique du mois. La MRC/BHF Heart Protection Study]. Revue Medicale de Liege 2002;57(9):613‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Kumana CR, Cheung BM, Lauder IJ. MRC/BHF Heart Protection Study. ACP Journal Club 2003;138(2):A15. [MEDLINE: ] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002; Vol. 360, issue 9326:23‐33. [MEDLINE: ] [DOI] [PubMed]
- MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. European Heart Jornal 1999;20(10):725‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 20,536 high‐risk individuals: a randomized placebo controlled trial. Lancet 2002;360(9326):7‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Robins SJ, Despres JP. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1780. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Buckley BM. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1780‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Violi F, Micheletta F, Iuliano L. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1782‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wald N, Law M. MRC/BHF Heart Protection Study. Lancet 2002;360(9347):1781. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li W, Zhu Y, Yan X, Zhang Q, Li X, Ni Z, et al. The prevention of primary liver cancer by selenium in high risk populations. (Zhonghua Yu Fang Yi Xue Za Zhi) Chinese Journal of Preventive Medicine 2000;34(6):336‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Li WG. Preliminary observations on effect of selenium yeast on high risk populations with primary liver cancer. (Zhonghua Yu Fang Yi Xue Za Zhi) Chinese Journal of Preventive Medicine 1992;26(5):268‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li H, Li HQ, Wang Y, Xu HX, Fan WT, Wang ML, et al. An intervention study to prevent gastric cancer by micro‐selenium and large dose of allitridum. Chinese Medical Journal 2004;117(8):1155‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Munoz 1985 {published data only}
- Munoz N, Wahrendorf J, Bang LJ, Crespi M, Thurnham DI, Day NE, et al. No effect of riboflavine, retinol, and zinc on prevalence of precancerous lesions of oesophagus. Randomised double‐blind intervention study in high‐risk population of China. Lancet 1985;2(8447):111‐4. [MEDLINE: ; 00039020] [DOI] [PubMed] [Google Scholar]
NIT1 1993 {published data only}
- Blot WJ, Li JY. Some considerations in the design of a Nutritional Intervention Trial in Linxian, People's Republic of China. National Cancer Institute Monographs 1984;69:29‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition Intervention Trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease‐specific mortality in the general population. Journal of the National Cancer Institute 1993;85(18):1483‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin‐mineral intervention group. American Journal of Clinical Nutrition 1995;62(6 Suppl):1424s‐6s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li B, Taylor PR, Li JY, Dawsey SM, Wang W, Tangrea JA, et al. Linxian Nutrition Intervention Trials. Design, methods, participant characteristics, and compliance. Annals of Epidemiology 1993;3(6):577‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li JY. Vitamins and minerals in cancer: The Nutrition Intervention Trials in Linxian, China. Medecine Biologie Environnement 1998;26(2):187‐209. [Google Scholar]
- Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, et al. Prospective study of serum selenium levels and incident esophageal and gastric cancers. Journal of the National Cancer Institute 2000;92(21):1753‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Taylor PR, Li B, Dawsey SM, Li JY, Yang CS, Guo W, et al. Prevention of esophageal cancer: the Nutrition Intervention Trials in Linxian, China. Linxian Nutrition Intervention Trials Study Group. Cancer Research 1994;54(7 Suppl):2029s‐31s. [MEDLINE: ] [PubMed] [Google Scholar]
- Wang GQ, Dawsey SM, Li JY, Taylor PR, Li B, Blot WJ, et al. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the General Population Trial in Linxian, China. Cancer Epidemiology, Biomarkers & Prevention 1994;3(2):161‐6. [MEDLINE: ] [PubMed] [Google Scholar]
NIT2 1993 {published data only}
- Li JY, Li B, Blot WJ, Taylor PR. Preliminary report on the results of nutrition prevention trials of cancer and other common diseases among residents in Linxian, China. (Zhonghua Zhong Liu Za Zhi) Chinese Journal of Oncology 1993;15(3):165‐81. [MEDLINE: ] [PubMed] [Google Scholar]
- Li JY, Taylor PR, Li B, Dawsey S, Wang GQ, Ershow AG, et al. Nutrition Intervention Trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease‐specific mortality among adults with esophageal dysplasia. Journal of the National Cancer Institute 1993;85(18):1492‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mark SD, Liu SF, Li JY, Gail MH, Shen Q, Dawsey SM, et al. The effect of vitamin and mineral supplementation on esophageal cytology: results from the Linxian Dysplasia Trial. International Journal of Cancer 1994;57(2):162‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Taylor PR, Wang GQ, Dawsey SM, Guo W, Mark SD, Li JY, et al. Effect of nutrition intervention on intermediate endpoints in esophageal and gastric carcinogenesis. American Journal of Clinical Nutrition 1995;62(6 Suppl):1420s‐3s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NPCT 1996 {published data only}
- Clark LC, Combs GF Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Journal of American Medical Association 1996;276(24):1957‐63. [MEDLINE: ] [PubMed] [Google Scholar]
- Clark LC, Dalkin B, Krongrad A, Combs GF Jr, Turnbull BW, Slate EH, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double‐blind cancer prevention trial. British Journal of Urology 1998;81(5):730‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Combs GF Jr. Impact of selenium and cancer‐prevention findings on the nutrition‐health paradigm. Nutrition and Cancer 2001;40(1):6‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Combs GF Jr, Clark LC, Turnbull BW. Reduction of cancer mortality and incidence by selenium supplementation. Medizinische Klinik 1997;92(Suppl 3):42‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Combs GF, Clark LC, Turnbull BW. Reduction of cancer risk with an oral supplement of selenium. Biomedical and Environmental Sciences 1997;10(2‐3):227‐34. [MEDLINE: ] [PubMed] [Google Scholar]
- Duffield‐Lillico AJ, Reid ME, Turnbull BW, Combs Jr GF, Slate EH, Fischbach LA. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention 2002;11(7):630‐9. [PubMed] [Google Scholar]
- Redman C, Xu MJ, Peng YM, Scott JA, Payne C, Clark LC, et al. Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells. Carcinogenesis 1997;18(6):1195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reid ME, Duffield‐Lillico AJ, Garland L, Turnbull BW, Clark LC, Marshall JR. Selenium supplementation and lung cancer incidence: an update of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology, Biomarkers & Prevention 2002;11(11):1285‐91. [MEDLINE: ] [PubMed] [Google Scholar]
PHS 1996 {published data only}
- Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Archives of Internal Medicine 2002;162(12):1382‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Andreotti F, Burzotta F, De‐Stefano V, Maseri A, Iacoviello L. The G20210A prothrombin mutation and the Physicians' Health Study. Circulation 2000;101(21):E207‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Berger K, Kase CS, Buring JE. Interobserver agreement in the classification of stroke in the Physicians' Health Study. Stroke 1996;27(2):238‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buring JE, Hebert P, Romero J, Kittross A, Cook N, Manson J, et al. Migraine and subsequent risk of stroke in the Physicians' Health Study. Archives of Neurology 1995;52(2):129‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buring JE, Hennekens CH. Cost and efficiency in clinical trials: the U.S. Physicians' Health Study. Statistics in Medicine 1990;9(1‐2):29‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. American Journal of Clinical Nutrition 2001;74(4):549‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Ajani UA, Schaumberg DA, Manson JE, Buring JE, et al. Baseline self‐reported cataract and subsequent mortality in Physicians' Health Study I. Ophthalmic Epidemiology 2000;7(2):115‐25. [MEDLINE: ] [PubMed] [Google Scholar]
- Christen WG, Glynn RJ, Seddon JM, Manson JE, Buring JE, Hennekens CH. Confirmation of self‐reported cataract in the Physicians' Health Study. Ophthalmic Epidemiology 1994;1(2):85‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta‐carotene supplementation on cancer incidence by baseline characteristics in the Physicians' Health Study (United States). Cancer Causes & Control 2000;11(7):617‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frieling UM, Schaumberg DA, Kupper TS, Muntwyler J, Hennekens CH. A randomized, 12‐year primary‐prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the Physician's Health Study. Archives of Dermatology 2000;136(2):179‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, et al. Light‐to‐moderate alcohol consumption and mortality in the Physicians' Health Study enrollment cohort. Journal of the American College of Cardiology 2000;35(1):96‐105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Buring JE. Methodologic considerations in the design and conduct of randomized trials: the U.S. Physicians' Health Study. Controlled Clinical Trials 1989;10(4 Suppl):142S‐50S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long‐term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New England Journal of Medicine 1996;334(18):1145‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Eberlein K. A randomized trial of aspirin and beta‐carotene among U.S. physicians. Preventive Medicine 1985;14(2):165‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jonas S. The Physician's Health Study. A neurologist's concern. Archives of Neurology 1990;47(12):1352‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run‐in on the power of the Physicians' Health Study. Statistics in Medicine 1991;10(10):1585‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee IM, Manson JE, Ajani U, Paffenbarger RS Jr, Hennekens CH, Buring JE. Physical activity and risk of colon cancer: the Physicians' Health Study (United States). Cancer Causes & Control 1997;8(4):568‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liu S, Lee IM, Ajani U, Cole SR, Buring JE, Manson JE. Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: the Physicians' Health Study. International Journal of Epidemiology 2001;30(1):130‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lotufo PA, Chae CU, Ajani UA, Hennekens CH, Manson JE. Male pattern baldness and coronary heart disease: the Physicians' Health Study. Archives of Internal Medicine 2000;160(2):165‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lotufo PA, Lee IM, Ajani UA, Hennekens CH, Manson JE. Cigarette smoking and risk of prostate cancer in the Physicians' Health Study (United States). International Journal of Cancer 2000;87(1):141‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ma J, HennekensCH, Ridker PM, Stampfer MJ. A prospective study of fibrinogen and risk of myocardial infarction in the Physicians' Health Study. Journal of the American College of Cardiology 1999;33(5):1347‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians' health study: a prospective study. American Journal of Epidemiology 1995;142(2):166‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Perera FP, Mooney L A, Stampfer M, Phillips DH, Bell DA, Rundle A, et al. Associations between carcinogen‐DNA damage, glutathione S‐transferase genotypes, and risk of lung cancer in the prospective Physicians' Health Cohort Study. Carcinogenesis 2002;23(10):1641‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Physicians' Health Study Research Group. Peliminary report: findings from the aspirin component of the ongoing Physicians Health Study. New England Journal of Medicine 1988;318(4):262‐4. [DOI] [PubMed] [Google Scholar]
- Satterfield S, Greco PJ, Goldhaber SZ, Stampfer MJ, Swartz SL, Stein EA, et al. Biochemical markers of compliance in the Physicians' Health Study. American Journal of Preventive Medicine 1990;6(5):290‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Sesso HD, Gaziano JM, VanDenburgh M, Hennekens CH, Glynn RJ, Buring JE. Comparison of baseline characteristics and mortality experience of participants and nonparticipants in a randomized clinical trial: the Physicians' Health Study. Controlled Clinical Trials 2002;23(6):686‐702. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. New England Journal of Medicine 1989;321(3):129‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sturmer T, Glynn RJ, Lee IM, Christen WG, Hennekens CH. Lifetime cigarette smoking and colorectal cancer incidence in the Physicians' Health Study I. Journal of the National Cancer Institute 2000;92(14):1178‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tang D, Phillips DH, Stampfer M, Mooney LA, Hsu Y, Cho S, et al. Association between carcinogen‐DNA adducts in white blood cells and lung cancer risk in the Physicians Health Study. Cancer Research 2001;61(18):6708‐12. [MEDLINE: ] [PubMed] [Google Scholar]
- Vieth R. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. American Journal of Clinical Nutrition 2002;76(2):490‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plummer 2007 {published data only}
- Munoz N, Kato I, Peraza S, Lopez G, Carrillo E, Ramirez H, et al. Prevalence of precancerous lesions of the stomach in Venezuela. Cancer Epidemiology, Biomarkers & Prevention 1996;5(1):41‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Munoz N, Vivas J, Buiatti E, Kato I, Oliver W. Chemoprevention trial on precancerous lesions of the stomach in Venezuela: summary of study design and baseline data. IARC Scientific Publications 1996;139:125‐33. [MEDLINE: ] [PubMed] [Google Scholar]
- Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high‐risk population. Journal of the National Cancer Institute 2007;99(2):137‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sanjose S, Munoz N, Sobala G, Vivas J, Peraza S, Cano E, et al. Antioxidants, Helicobacter pylori and stomach cancer in Venezuela. European Journal of Cancer Prevention 1996;5(1):57‐62. [MEDLINE: ] [PubMed] [Google Scholar]
SIT 2006 {published data only}
- Gail MH, Pfeiffer RM, Brown LM, Zhang L, Ma JL, Pan KF, et al. Garlic, vitamin, and antibiotic treatment for Helicobacter pylori: a randomized factorial controlled trial. Helicobacter 2007;12(5):575‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gail MH, You WC. A factorial trial including garlic supplements assesses effect in reducing precancerous gastric lesions. Journal of Nutrition 2006;136(3 Suppl):813S‐5S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gail MH, You WC, Chang YS, Zhang L, Blot WJ, Brown LM, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Controlled Clinical Trials 1998;19(4):352‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double‐blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. Journal of the National Cancer Institute 2006;98(14):974‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- You WC, Chang YS, Heinrich J, Ma JL, Liu WD, Zhang L, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S‐allyl cysteine levels, and toxicity. European Journal of Cancer Prevention 2001;10(3):257‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhang L, Gail MH, Wang YQ, Brown LM, Pan KF, Ma JL, et al. A randomized factorial study of the effects of long‐term garlic and micronutrient supplementation and of 2‐wk antibiotic treatment for Helicobacter pylori infection on serum cholesterol and lipoproteins. American Journal of Clinical Nutrition 2006;84(4):912‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SUVIMAX 2004 {published data only}
- Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, Hercberg S. Dietary intakes and food sources of n‐6 and n‐3 PUFA in French adult men and women. Lipids 2004;39(6):527‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Barrere X, Valeix P, Preziosi P, Bensimon M, Pelletier B, Galan P, et al. Determinants of thyroid volume in healthy French adults participating in the SU.VI.MAX cohort. Clinical Endocrinology 2000;52(3):273‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bellisle F, Altenburg de Assis MA, Fieux B, Preziosi P, Galan P, Guy‐Grand B, et al. Use of 'light' foods and drinks in French adults: biological, anthropometric and nutritional correlates. Journal of Human Nutrition and Dietetics 2001;14(3):191‐206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertrais S, Galan P, Renault N, Zarebska M, Preziosi P, Hercberg S. Consumption of soup and nutritional intake in French adults: consequences for nutritional status. Journal of Human Nutrition and Dietetics 2001;14(2):121‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertrais S, Polo Luque ML, Preziosi P, Fieux B, Torra De Flot M, Galan P, et al. Contribution of ready‐to‐eat cereals to nutrition intakes in French adults and relations with corpulence. Annals of Nutrition & Metabolism 2000;44(5‐6):249‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bertrais S, Preziosi P, Mennen L, Galan P, Hercberg S, Oppert JM. Sociodemographic and geographic correlates of meeting current recommendations for physical activity in middle‐aged French adults: the Supplementation en Vitamines et Mineraux Antioxydants (SUVIMAX) Study. American Journal of Public Health 2004;94(9):1560‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boini S, Briancon S, Guillemin F, Galan P, Hercberg S. Impact of cancer occurrence on health‐related quality of life: a longitudinal pre‐post assessment. Health and Quality of Life Outcomes 2004;2(1):4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckert E, Czernichow S, Bertrais S, Paillard F, Tichet J, Galan P, et al. Blood lipid and lipoprotein levels: relationships with educational level and region of residence in the French SU.VI.MAX study. Preventive Medicine 2005;40(6):803‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cailhol J, Czernichow S, Mennen L, Boutron‐Ruault MC, Zarebska M, Franchisseur C, et al. Participation and medical follow‐up in screening of colorectal cancer in France within the SU.VI.MAX study [Dépistage du cancer colorectal par test Hémoccult : taux de participation et prise en charge médicale des sujets à test positif au sein de l'étude SU.VI.MAX]. Revue d'Epidémiologie et de Santé Publique 2002;50(3):321‐3. [MEDLINE: ] [PubMed] [Google Scholar]
- Chango A, Potier De Courcy G, Boisson F, Guilland JC, Barbe F. 5,10‐methylenetetrahydrofolate reductase common mutations, folate status and plasma homocysteine in healthy French adults of the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) cohort. The British Journal of Nutrition 2000;84(6):891‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Czernichow S, Bertrais S, Oppert JM, Galan P, Blacher J, Ducimetiere P, et al. Body composition and fat repartition in relation to structure and function of large arteries in middle‐aged adults (the SU.VI.MAX study). International Journal of Obesity and Related Metabolic Disorders 2005;29(7):826‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Czernichow S, Bertrais S, Preziosi P, Galan P, Hercberg S, Oppert JM, et al. Indicators of abdominal adiposity in middle‐aged participants of the SU.VI.MAX study: relationships with educational level, smoking status and physical inactivity. Diabetes & Metabolism 2004;30(2):153‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Czernichow S, Mennen L, Bertrais S, Preziosi P, Hercberg S, Oppert JM. Relationships between changes in weight and changes in cardiovascular risk factors in middle‐aged French subjects: effect of dieting. International Journal of Obesity and Related Metabolic Disorders 2002;26(8):1138‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron‐Ruault MC, Arnaud J, et al. Association of selenium with thyroid volume and echostructure in 35‐ to 60‐year‐old French adults. European Journal of Endocrinology 2003;148(3):309‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Duport N, Preziosi P, Boutron‐Ruault MC, Bertrais S, Galan P, Favier A, et al. Consequences of iron depletion on health in menstruating women. European Journal of Clinical Nutrition 2003;57(9):1169‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Arnaud MJ, Czernichow S, Delabroise AM, Preziosi P, Bertrais S, et al. Contribution of mineral waters to dietary calcium and magnesium intake in a French adult population. Journal of the American Dietetic Association 2002;102(11):1658‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Briancon S, Favier A, Bertrais S, Preziosi P, Faure H, et al. Antioxidant status and risk of cancer in the SU.VI.MAX study: is the effect of supplementation dependent on baseline levels?. British Journal of Nutrition 2005;94(1):125‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Favier A, Preziosi P, Bertrais S, Arnault N, Hercberg S. The bank of biological material in the SU.VI.MAX study [La biothèque dans l'étude SU.VI.MAX]. Revue d'Epidemiologie et de Sante Publique 2003;51(1 Pt 2):147‐50. [MEDLINE: ] [PubMed] [Google Scholar]
- Galan P, Preziosi P, Durlach V, Valeix P, Ribas L, Bouzid D, et al. Dietary magnesium intake in a French adult population. Magnesium Research 1997;10(4):321‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Galan P, Renault N, Aissa M, Adad HA, Rahim B, Potier de Courcy G, et al. Relationship between soup consumption, folate, beta‐carotene, and vitamin C status in a French adult population. International Journal for Vitamin and Nutrition Research 2003;73(5):315‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, Arnaud J, et al. Serum concentrations of beta‐carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. European Journal of Clinical Nutrition 2005;59(10):1181‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Galan P, Yoon HC, Preziosi P, Viteri F, Valeix P, Fieux B, et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Mineraux AntioXydants. European Journal of Clinical Nutrition 1998;52(6):383‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gauthier‐Chelle K, Mennen L, Arnault N, Rigalleau V, Hercberg S, Gin H. Comparison of the diet of self‐declared diabetics with non‐diabetic patients in the SU.VI.MAX study: did the diabetics modify their nutritional behavior?. Diabetes & Metabolism 2004;30(6):535‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guinot C, Latreille J, Malvy D, Preziosi P, Galan P, Hercberg S, et al. Use of multiple correspondence analysis and cluster analysis to study dietary behaviour: food consumption questionnaire in the SU.VI.MAX. cohort. European Journal of Epidemiology 2001;17(6):505‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guinot C, Malvy DJ, Latreille J, Ezzedine K, Galan P, Tenenhaus M, et al. Sun‐reactive skin type in 4912 French adults participating in the SU.VI.MAX. Photochemistry and Photobiology 2005;81(4):934‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hercberg S. Antioxidant micronutrients and chronic degenerative pathology: the role of complementary nutritional doses. Bulletin et Memoires de l'Academie Royale de Medecine de Belgique 1997;152(10‐11):379‐85. [MEDLINE: ] [PubMed] [Google Scholar]
- Hercberg S, Bertrais S, Czernichow S, Noisette N, Galan P, Jaouen A, et al. Alterations of the lipid profile after 7.5 years of low‐dose antioxidant supplementation in the SU.VI.MAX Study. Lipids 2005;40(4):335‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The SU.VI.MAX Study: a randomized, placebo‐controlled trial of the health effects of antioxidant vitamins and minerals. Archives of Internal Medicine 2004;164(21):2335‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, Malvy M, Briancon S, Ait HM, et al. The SU.VI.MAX trial on antioxidants. IARC Scientific Publications 2002;156:451‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, Roussel AM, Arnaud J, Richard MJ, et al. Background and rationale behind the SU.VI.MAX Study, a prevention trial using nutritional doses of a combination of antioxidant vitamins and minerals to reduce cardiovascular diseases and cancers. SUpplementation en VItamines et Mineraux AntioXydants Study. International Journal for Vitamin and Nutrition Research 1998;68(1):3‐20. [MEDLINE: ] [PubMed] [Google Scholar]
- Hercberg S, Hebel P, Preziosi P, Briancon S, Favier A, Galan P, et al. Motivations of volunteers for participation in an interventional study in the field of nutritional prevention: results of a pilot study of the SU.VI.MAX project. Revue d'Epidemiologie et de Sante Publique 1995;43(2):139‐46. [MEDLINE: ] [PubMed] [Google Scholar]
- Hercberg S, Preziosi P, Briancon S, Galan P, Triol I, Malvy D, et al. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: the SU.VI.MAX study‐design, methods, and participant characteristics. SUpplementation en VItamines et Mineraux AntioXydants. Controlled Clinical Trials 1998;19(4):336‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hercberg S, Preziosi P, Galan P, Faure H, Arnaud J, Duport N, et al. "The SU.VI.MAX Study": a primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers. SUpplementation on VItamines et Mineraux AntioXydants. Food and Chemical Toxicology 1999;37(9‐10):925‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lairon D, Bertrais S, Vincent S, Arnault N, Galan P, Boutron MC, et al. Dietary fibre intake and clinical indices in the French Supplementation en Vitamines et Mineraux AntioXydants (SU.VI.MAX) adult cohort. The Proceedings of the Nutrition Society 2003;62(1):11‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leclere J. Multinodular goiters. La Revue du Praticien 2005;55(2):167‐73. [MEDLINE: ] [PubMed] [Google Scholar]
- Lukasiewicz E, Mennen LI, Bertrais S, Arnault N, Preziosi P, Galan P, et al. Alcohol intake in relation to body mass index and waist‐to‐hip ratio: the importance of type of alcoholic beverage. Public Health Nutrition 2005;8(3):315‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Malkin JE, Morand P, Malvy D, Ly TD, Chanzy B, Labareyre C, et al. Seroprevalence of HSV‐1 and HSV‐2 infection in the general French population. Sexually Transmitted Infections 2002;78(3):201‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvy DJ, Favier A, Faure H, Preziosi P, Galan P, Arnaud J, et al. Effect of two years' supplementation with natural antioxidants on vitamin and trace element status biomarkers: preliminary data of the SU.VI.MAX study. Cancer Detection and Prevention 2001;25(5):479‐85. [MEDLINE: ] [PubMed] [Google Scholar]
- Malvy J, Guinot C, Preziosi P, Vaillant L, Tenenhaus M, Galan P, et al. Epidemiologic determinants of skin photoaging: baseline data of the SU.VI.MAX. cohort. Journal of the American Academy of Dermatology 2000;42(1 pt 1):47‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mennen LI, Bertrais S, Galan P, Arnault N, Potier de Couray G, Hercberg S. The use of computerised 24 h dietary recalls in the French SU.VI.MAX Study: number of recalls required. European Journal of Clinical Nutrition 2002;56(7):659‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mennen LI, Sapinho D, Bree A, Arnault N, Bertrais S, Galan P, et al. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. The Journal of Nutrition 2004;134(4):923‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, et al. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. International Journal of Cancer 2005;116(2):182‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mohammed‐Cherif S, Briancon S, Potier de Courcy G, Preziosi P, Fieux B, Zarebska M, et al. Factors determining the use of hormone replacement therapy in recent naturally postmenopausal women participating in the French SU.VI.MAX cohort. European Journal of Epidemiology 2000;16(5):477‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Preziosi P, Czernichow S, Gehanno P, Hercberg S. Workplace air‐conditioning and health services attendance among French middle‐aged women: a prospective cohort study. International Journal of Epidemiology 2004;33(5):1120‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rouillier P, Boutron‐Ruault MC, Bertrais S, Arnault N, Daudin JJ, Bacro JN, et al. Alcohol and atherosclerotic vascular disease risk factors in French men: relationships are linear, J‐shaped, and U‐shaped. Alcoholism, Clinical and Experimental Research 2005;29(1):84‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rouillier P, Boutron‐Ruault MC, Bertrais S, Arnault N, Daudin JJ, Bacro JN, et al. Drinking patterns in French adult men ‐ a cluster analysis of alcoholic beverages and relationship with lifestyle. European Journal of Nutrition 2004;43(2):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valeix P, Dos Santos C, Castetbon K, Bertrais S, Cousty C, Hercberg S. Thyroid hormone levels and thyroid dysfunction of French adults participating in the SU.VI.MAX study [Statut thyroïdien et fréquences des dysthyroïdies chez les adultes inclus dans l’étude SU.VI.MAX en 1994‐1995]. Annales d'Endocrinologie 2004;65(6):477‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Valeix P, Zarebska M, Bensimon M, Cousty C, Bertrais S, Galan P, et al. Ultrasonic assessment of thyroid nodules, and iodine status of French adults participating in the SU.VI.MAX study [Nodules thyroïdiens à l'échographie et statut en iode des adultes volontaires de l'étude SU.VI.MAX]. Annales d'Endocrinologie 2001;62(6):499‐506. [MEDLINE: ] [PubMed] [Google Scholar]
- Vazquez Martinez C, Galan P, Preziosi P, Ribas L, Serra LL, Hercberg S. The SUVIMAX (France) study: the role of antioxidants in the prevention of cancer and cardiovascular disorders. Revista Espanola de Salud Publica 1998;72(3):173‐83. [MEDLINE: ] [PubMed] [Google Scholar]
- Vercherin P, Gutknecht C, Guillemin F, Ecochard R, Mennen LI, Mercier M. Missing data mechanisms of the questionnaire SF‐36's items in the SU.VI.MAX study [Non‐réponses aux questionnaires de qualité de vie SF‐36 dans un échantillon de l'étude SU.VI.MAX]. Revue d'Epidémiologie et de Santé Publique 2003;51(5):513‐25. [MEDLINE: ] [PubMed] [Google Scholar]
- Vuillemin A, Boini S, Bertrais S, Tessier S, Oppert JM, Hercberg S, et al. Leisure time physical activity and health‐related quality of life. Preventive Medicine 2005;41(2):562‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vuillemin A, Oppert JM, Guillemin F, Essermeant L, Fontvieille AM, Galan P, et al. Self‐administered questionnaire compared with interview to assess past‐year physical activity. Medicine and Science in Sports and Exercise 2000;32(6):1119‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, et al. Effects of long‐term daily low‐dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arteriosclerosis, Thrombosis, and Vascular Biology 2004;24(8):1485‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WACS 2007 {published data only}
- Bassuk SS, Albert CM, Cook NR, Zaharris E, MacFadyen JG, Danielson E, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants. Journal of Women's Health 2004;13(1):99‐117. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Archives of Internal Medicine 2007;167(15):1610‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Gaziano JM, Spelsberg A, Ridker PM, Cook NR, Buring JE, et al. A secondary prevention trial of antioxidant vitamins and cardiovascular disease in women. Rationale, design, and methods. The WACS Research Group. Annals of Epidemiology 1995;5(4):261‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mason PJ, Manson JE, Sesso HD, Albert CM, Chown MJ, Cook NR, et al. Blood pressure and risk of secondary cardiovascular events in women: the Women's Antioxidant Cardiovascular Study (WACS). Circulation 2004;109(13):1623‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHS 2005 {published data only}
- Buring JE, Hennekens CH. The Women's Health Study: rationale and background. The Journal of Myocardial Ischemia 1992;4(3):30‐40. [Google Scholar]
- Buring JE, Hennekens CH. The Women's Health Study: summary of study design. The Journal of Myocardial Ischemia 1992;4(3):27‐9. [Google Scholar]
- Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low‐dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294(1):47‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karlson EW, Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Comparison of self‐reported diagnosis of connective tissue disease with medical records in female health professionals: the Women's Health Cohort Study. American Journal of Epidemiology 1999;150(6):652‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294(1):56‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee IM, Cook NR, Manson JE, Buring JE. Randomized beta‐carotene supplementation and incidence of cancer and cardiovascular disease in women: is the association modified by baseline plasma level. British Journal of Cancer 2002;86(5):698‐701. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Randomised beta‐carotene supplementation and incidence of cancer and cardiovascular disease in women: the Women's Health Study. Journal of the National Cancer Institute 1999;91:2102‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. American Journal of Clinical Nutrition 2000;72(4):922‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. Journal of Women's Health and Gender Based Medicine 2000;9(1):19‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low‐dose aspirin in the primary prevention of cardiovascular disease in women. New England Journal of Medicine 2005;352(13):1293‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yu 1991 {published data only}
- Yu SY, Zhu YJ, Li WG, Huang QS, Huang CZ, Zhang QN, et al. A preliminary report on the intervention trials of primary liver cancer in high‐risk populations with nutritional supplementation of selenium in China. Biological Trace Element Research 1991;29(3):289‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yu 1997 {published data only}
- Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biological Trace Element Research 1997;56:117‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhu 2003 {published data only}
- Zhu S, Mason J, Shi Y, Hu Y, Li R, Wahg M, et al. The effect of folic acid on the development of stomach and other gastrointestinal cancers. Chinese Medical Journal 2003;116(1):15‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Zhu S, Mason J, Shi Y, Hu Y, Li R, Wang M, et al. The interventional effect of folic acid on the development of gastric and other gastrointestinal cancers ‐Clinical trial and follow‐up for seven years. Chinese Journal of Gastroenterology 2002; Vol. 7, issue 2:73‐8.
References to studies excluded from this review
Bespalov 2004 {published data only}
- Berspalov VG, Shcherbakov AM, Kalinovskii VP, Novik VI, Chepik OF, Aleksandrov VA, et al. Study of the antioxidant drug "Karinat" in patients with chronic atrophic gastritis [Izucenie antioksidatnogo preparata "Karinat" kak sredstva dla lecenih boljnih s hroniceskim atroficeskim gastritom]. Voprosii Onkologii 2004;50(1):81‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Bostick 1993 {published data only}
- Bostick RM, Potter JD, McKenzie DR, Sellers TA, Kushi LH, Steinmetz KA, et al. Reduced risk of colon cancer with high intake of vitamin E: the Iowa Women's Health Study. Cancer Research 1993;53(18):4230‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Bukin 1996 {published data only}
- Bukin YV, Poddubniy BK, Kuvshinov YP, Draudin‐Krylenko VA, Shabanov MA. The effects of certain vitamins and natural anti‐oxidants on ornithine decarboxylase activity and on atrophic and premalignant changes in the human gastric mucosa. Digestive Endoscopy 1996;8(3):184‐91. [MEDLINE: ] [Google Scholar]
Bussey 1982 {published data only}
- Bussey HJ, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, et al. A randomized trial of ascorbic acid in polyposis coli. Cancer 1982;50(7):1434‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chuang 2002 {published data only}
- Chuang CH, Sheu BS, Huang AH, Yang HB, Wu JJ. Vitamin C and E supplements to lansoprazole‐amoxicillin‐metronidazole triple therapy may reduce the eradication rate of metronidazole‐susceptible Helicobacter pylori infection. Helicobacter 2002;7(5):310‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Stefani 1999 {published data only}
- Stefani E, Ronco A, Mendilaharsu M, Deneo‐Pellegrini H. Diet and risk of cancer of the upper aerodigestive tract‐II. Nutrients. Oral Oncology 1999;35(1):22‐6. [DOI] [PubMed] [Google Scholar]
De Stefani 1999a {published data only}
- Stefani E, Deneo‐Pellegrini H, Mendilaharsu M, Ronco A. Diet and risk of cancer of the upper aerodigestive tract‐I. Foods. Oral Oncology 1999;35(1):17‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Stefani 2000 {published data only}
- Stefani E, Brennan P, Boffetta P, Ronco AL, Mendilaharsu M, Deneo‐Pellegrini H. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case‐control study in Uruguay. Nutrition and Cancer 2000;38(1):23‐9. [DOI] [PubMed] [Google Scholar]
DeCosse 1975 {published data only}
- Cosse JJ, Adams MB, Kuzma JF, Lo Gerfo P, Condon RE. Effect of ascorbic acid on rectal polyps of patients with familial polyposis. Wisconsin Medical Journal 1976;75(1):S8. [MEDLINE: ] [PubMed] [Google Scholar]
- DeCosse JJ, Adams MB, Kuzma JF, LoGerfo P, Condon RE. Effect of ascorbic acid on rectal polyps of patients with familial polyposis. Surgery 1975;78(5):608‐12. [MEDLINE: ] [PubMed] [Google Scholar]
DeCosse 1989 {published data only}
ECP‐IM 1994 {published data only}
- Reed PI. The ECP‐IM intervention study. European Journal of Cancer Prevention 1994;3(Suppl 2):99‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Reed PI, Johnston BJ. Primary prevention of gastric cancer ‐ The ECP‐IM intervention study. Acta Endoscopica 1995;25(1):45‐54. [Google Scholar]
EUROSCAN 2000 {published data only}
- Zandwijk N, Dalesio O, Pastorino U, de‐Vries N, Tinteren H. EUROSCAN, a randomized trial of vitamin A and N‐acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. Journal of the National Cancer Institute 2000;92(12):977‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frank 1995 {published data only}
- Frank E. The Women Physicians' Health Study: background, objectives, and methods. Journal of the American Medical Women's Association 1995;50(2):64‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Greenberg 1990 {published data only}
- Greenberg ER, Baron JA, Stukel TA, Stevens MM, Mandel JS, Spencer SK, et al. A clinical trial of beta carotene to prevent basal‐cell and squamous‐cell cancers of the skin. The Skin Cancer Prevention Study Group. New England Journal of Medicine 1990;323(12):789‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ishikawa 1995 {published data only}
- Ishikawa H, Akedo I, Suzuki T, Otani T, Sobue T. Interventional trial for colorectal cancer prevention in Osaka: an introduction to the protocol. Japanese Journal of Cancer Research 1995;86(8):707‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2001 {published data only}
- Jacobs EJ, Connell CJ, Patel AV, Chao A, Rodriguez C, Seymour J, et al. Vitamin C and vitamin E supplement use and colorectal cancer mortality in a large American Cancer Society cohort. Cancer Epidemiology Biomarkers and Prevention 2001;10(1):17‐23. [MEDLINE: ] [PubMed] [Google Scholar]
Jansen 1999 {published data only}
- Jansen MC, Bueno de Mesquita HB, Buzina R, Fidanza F, Menotti A, Blackburn H, et al. Dietary fiber and plant foods in relation to colorectal cancer mortality: the Seven Countries Study. International Journal of Cancer 1999;81(2):174‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ji 1995 {published data only}
- Ji BT, Chow WH, Gridley G, Mclaughlin JK, Dai Q, Wacholder S, et al. Dietary factors and the risk of pancreatic cancer: a case‐control study in Shanghai China. Cancer Epidemiology, Biomarkers & Prevention 1995;4(8):885‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Kirk 2006 {published data only}
- Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, et al. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. Journal of Gastrointestinal Surgery 2006;10(4):499‐503. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krishnaswamy 1993 {published data only}
- Krishnaswamy K, Prasad MPR, Krishna TP, Pasricha S. A case‐control study of selenium in cancer. Indian Journal of Medical Research 1993;98:124‐8. [PubMed] [Google Scholar]
La Vecchia 2002 {published data only}
- Vecchia C. Tomatoes, lycopene intake, and digestive tract and female hormone‐related neoplasms. Experimental Biology and Medicine 2002;227(10):860‐3. [DOI] [PubMed] [Google Scholar]
Lacroix 1987 {published data only}
- Lacroix A, Bhat PV, Karabatsos A, Couture P, Latreille J, Beaulieu R, et al. Plasma levels of retinol in cancer patients supplemented with retinol. Oncology 1987;44(2):108‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lanza 1996 {published data only}
- Lanza E, Schatzkin A, Ballard‐Barbash R, Corle D, Clifford C, Paskett E, et al. The polyp prevention trial II: dietary intervention program and participant baseline dietary characteristics. Cancer Epidemiology, Biomarkers & Prevention 1996;5(5):385‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Lanza 2001 {published data only}
- Lanza E, Schatzkin A, Daston C, Corle D, Freedman L, Ballard‐Barbash R, et al. Implementation of a 4‐Y, high‐fiber, high‐fruit‐and‐vegetable, low‐fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. American Journal of Clinical Nutrition 2001;74(3):387‐401. [DOI] [PubMed] [Google Scholar]
Levi 2000 {published data only}
- Levi F, Pasche C, Lucchini F, Vecchia C. Selected micronutrients and colorectal cancer: case‐control study from the Canton of Vaud, Switzerland. European Journal of Cancer 2000;36(16):2115‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Limburg 2005 {published data only}
- Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, et al. Randomized, placebo‐controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology 2005;129(3):863‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macrae 1999 {published data only}
- Macrae F. Wheat bran fiber and development of adenomatous polyps: evidence from randomized, controlled clinical trials. American Journal of Medicine 1999;106(1 A):38S‐42S. [DOI] [PubMed] [Google Scholar]
Marotta 2003 {published data only}
- Marotta F, Barretto R, Tajiri H, Bertuccelli J, Safran P, Bobadilla J, et al. Atrophic/metaplastic changes of gastric mucosa: a preliminary interventional trial comparing different antioxidant supplements. International Congress Series 2003;1255:291‐4. [Google Scholar]
Marotta 2004 {published data only}
- Marotta F, Barreto R, Tajiri H, Bertuccelli J, Safran P, Yoshida C, et al. The aging/precancerous gastric mucosa: a pilot nutraceutical trial. Annals of the New York Academy of Sciences 2004;1019:195‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mayne 2001 {published data only}
- Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan Tl, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiology, Biomarkers & Prevention 2001;10(10):1055‐62. [MEDLINE: ] [PubMed] [Google Scholar]
Moriwaki 2002 {published data only}
- Moriwaki H. Prevention of liver cancer: current strategies and future perspectives. International Journal of Clinical Oncology 2002;7(1):27‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muto 1996 {published data only}
- Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. New England Journal of Medicine 1996;334(24):1561‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Newsome 2000 {published data only}
- Newsome PN, Beldon I, Moussa Y, Delahooke TE, Poulopoulos G, Hayes PC, et al. Low serum retinol levels are associated with hepatocellular carcinoma in patients with chronic liver disease. Alimentary Pharmacology & Therapeutics 2000;14(10):1295‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nomura 1987a {published data only}
- Nomura A, Heilbrun LK, Morris JS, Stemmermann GN. Serum selenium and the risk of cancer, by specific sites: case‐control analysis of prospective data. Journal of the National Cancer Institute 1987;79(1):103‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Pan 1993 {published data only}
- Pan WH, Wang CY, Huang SM, Yeh SY, Lin WG, Lin DI, et al. Vitamin A, Vitamin E or beta‐carotene status and hepatitis B‐related hepatocellular carcinoma. Annals of Epidemiology 1993;3(3):217‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Podmore 1998a {published data only}
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, et al. Vitamin C exhibits pro‐oxidant properties. Nature 1998;392(6676):559. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Qu 2007 {published data only}
- Qu CX, Kamangar F, Fan JH, Yu B, Sun XD, Taylor PR, et al. Chemoprevention of primary liver cancer: a randomized, double‐blind trial in Linxian, China. Journal of the National Cancer Institute 2007;99(16):1240‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rocchi 1997 {published data only}
- Rocchi E, Seium Y, Camellini L, Casalgrandi G, Borghi A, D'Alimonte P, et al. Hepatic tocopherol content in primary hepatocellular carcinoma and liver metastases. Hepatology 1997;26(1):67‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Russo 1997 {published data only}
- Russo MW, Murray SC, Wurzelmann JI, Woosley JT, Sandler RS. Plasma selenium levels and the risk of colorectal adenomas. Nutrition and Cancer 1997;28(2):125‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasazuki 2003 {published data only}
- Kim MK, Sasaki S, Sasazuki S, Okubo S, Hayashi M, Tsugane S. Lack of long‐term effect of vitamin C supplementation on blood pressure. Hypertension 2002;40(6):797‐803. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kim MK, Sasazuki S, Sasaki S, Okubo S, Hayashi M, Tsugane S. Effect of five‐year supplementation of vitamin C on serum vitamin C concentration and consumption of vegetables and fruits in middle‐aged Japanese: a randomized controlled trial. Journal of the American College of Nutrition 2003;22(3):208‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sasaki S, Tsubono Y, Okubo S, Hayashi M, Kakizoe T, Tsugane S. Effects of three‐month oral supplementation of beta‐carotene and vitamin C on serum concentrations of carotenoids and vitamins in middle‐aged subjects: a pilot study for a randomized controlled trial to prevent gastric cancer in high‐risk Japanese population. Japanese Journal of Cancer Research 2000;91(5):464‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasazuki S, Sasaki S, Tsubono Y, Okubo S, Hayashi M, Kakizoe T, et al. The effect of 5‐year vitamin C supplementation on serum pepsinogen level and Helicobacter pylori infection. Cancer Science 2003;94(4):378‐82. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubono Y, Okubo S, Hayashi M, Kakizoe T, Tsugane S. A randomized controlled trial for chemoprevention of gastric cancer in high‐risk Japanese population; study design, feasibility and protocol modification. Japanese Journal of Cancer Research 1997;88(4):344‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugane S, Tsubono Y, Okubo S, Hayashi M, Kakizoe T. A pilot study for a randomized controlled trial to prevent gastric cancer in high‐risk Japanese population: study design and feasibility evaluation. Japanese Journal of Cancer Research 1996;87(7):676‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schatzkin 1996 {published data only}
- Schatzkin A, Lanza E, Freedman LS, Tangrea J, Cooper MR, Marshall JR, et al. The polyp prevention trial I: rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiology, Biomarkers & Prevention 1996;5(5):375‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Simone 2002 {published data only}
- Simone F, Pappalardo G, Maiani G, Guadalaxara A, Bugianesi R, Conte AM, et al. Accumulation and interactions of beta‐carotene and alpha‐tocopherol in patients with adenomatous polyps. European Journal of Clinical Nutrition 2002;56(6):546‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Siriwardena 2007 {published data only}
- Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n‐acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut 2007;56(10):1439‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takshashi 2003 {published data only}
- Takashashi Y, Sasaki S, Takahashi M, Okubo S, Hayashi M, Tsugane S. A population‐based dietary intervention trial in a high‐risk area for stomach cancer and stroke: changes in intakes and related biomarkers. Preventive Medicine 2003;37(5):432‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terry 2000 {published data only}
- Terry P, Lagergren J, Ye WM, Nyren O, Wolk A. Antioxidants and cancers of the esophagus and gastric cardia. International Journal of Cancer 2000;87(5):750‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Weisburger 1991 {published data only}
- Weisburger JH. Nutritional approach to cancer prevention with emphasis on vitamins, antioxidants, and carotenoids. American Journal of Clinical Nutrition 1991;53(1):S226‐S237. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Whelan 1999 {published data only}
- Whelan RL, Horvath KD, Gleason NR, Forde KA, Treat MD, Teitelbaum SL, et al. Vitamin and calcium supplement use is associated with decreased adenoma recurrence in patients with a previous history of neoplasia. Diseases of the Colon and Rectum 1999;42(2):212‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang CS. Vitamin nutrition and gastroesophageal cancer. Journal of Nutrition 2000;130(2):338S‐9S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yu 1995 {published data only}
- Yu MW, Hsieh HH, Pan WH, Yang CS, Chen CJ. Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Research 1995;55(6):1301‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Yu 1999 {published data only}
- Yu M W, Horng IS, Hsu KH, Chiang YC, Liaw Y F, Chen CJ. Plasma selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. American Journal of Epidemiology 1999;150(4):367‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zheng 1995 {published data only}
- Zheng W, Sellers TA, Doyle TJ, Kushi LH, Potter JD, Folsom AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. American Journal of Epidemiology 1995;142(9):955‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
APPOSE 2001 {published data only}
- Costello AJ. A randomized, controlled chemoprevention trial of selenium in familial prostate cancer: rationale, recruitment, and design issues. Urology 2001;57(4):182‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
HGPIN 2006 {published data only}
- Marshall JR, Sakr W, Wood D, Berry D, Tangen C, Parker F, et al. Design and progress of a trial of selenium to prevent prostate cancer among men with high‐grade prostatic intraepithelial neoplasia. Cancer Epidemiology, Biomarkers & Prevention 2006;15(8):1479‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
PHS II 2000 {published data only}
- Christen WG, Gaziano JM, Hennekens CH. Design of Physicians' Health Study II a randomized trial of beta‐carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Annals of Epidemiology 2000;10(2):125‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SELECT 2003 {published data only}
- Hoque A, Albanes D, Lippman SM, Spitz MR, Taylor PR, Klein EA, et al. Molecular epidemiologic studies within the Selenium and vitamin E Cancer Prevention Trial (SELECT). Cancer Causes & Control 2001;12(7):627‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA. Clinical models for testing chemopreventative agents in prostate cancer and overview of SELECT: the Selenium and vitamin E Cancer Prevention Trial. Recent Results in Cancer Research 2003;163:212‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA. Selenium and vitamin E Cancer Prevention Trial. Annals of the New York Academy of Sciences 2004;1031:234‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA, Lippman SM, Thompson IM, Goodman PJ, Albanes D, Taylor PR, et al. The Selenium and vitamin E Cancer Prevention Trial. World Journal of Urology 2003;21(1):21‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the Selenium and vitamin E Cancer Prevention Trial. Urologic Oncology 2003;21(1):59‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the Selenium and vitamin E Cancer Prevention Trial: rationale and design. Prostate Cancer and Prostatic Diseases 2000;3(3):145‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, et al. SELECT: the next prostate cancer prevention trial. Selenum and vitamin E Cancer Prevention Trial. The Journal of Urology 2001;166(4):1311‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lippman SM, Goodman PJ, Klein EA, Parnes HL, Thompson IM Jr, Kristal AR, et al. Designing the Selenium and vitamin E Cancer Prevention Trial (SELECT). Journal of the National Cancer Institute 2005;97(2):94‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ames 1995
- Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proceedings of the National Academy of Sciences of the United States of America Sci USA 1995;92(12):5258‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2000a
- Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington DC 2000:1‐800.
Anonymous 2000b
- Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of DRIs, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. Institute of Medicine. Dietary reference intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington, DC 2000:1‐529.
Balluz 2000
- Balluz LS, Kieszak SM, Philen RM, Mulinare J. Vitamin and mineral supplement use in the United States. Results from the third National Health and Nutrition Examination Survey. Archives of Family Medicine 2000;9:258‐62. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Bjelakovic 2006
- Bjelakovic G, Nagorni A, Nikolova D, Simonetti RG, Bjelakovic M, Gluud C. Meta‐analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma. Alimentary Pharmacology & Therapeutics 2006;24(2):281‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bjelakovic 2007
- Bjelakovic G, Nikolova D, Gluud LL, Simoneti RG, Gluud C. Mortality in randomized trials on antioxidant supplements for primary and secondary prevention. Systematic review and meta‐analysis. JAMA 2007;297(8):842‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boyle 1985
- Boyle P, Zaridze DG, Smans M. Descriptive epidemiology of colorectal cancer. International Journal of Cancer 1985;36(1):9‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Camire 1999
- Camire ME, Kantor MA. Dietary supplements: nutritional and legal considerations. Food Technology 1999;53(7):87‐95. [Google Scholar]
Caraballoso 2003
Chan 2004a
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2004b
- Chan AW, Krleza‐Jeric' K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. Canadian Medical Association Journal 2004;171(7):735‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2005
- Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ (Clinical Research Ed) 2005;330(7494):753. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 1996
- Cheng K, Day N. Nutrition and esophageal cancer. Cancer Causes and Control 1996;7(1):33‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clark 1991
- Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in U.S. counties. Archives of Environmental Health 1991;46(1):37‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collins 1987
- Collins RH, Feldman M, Fordtran JS. Colon cancer, dysplasia and surveillance in patients with ulcerative colitis. A critical review. New England Journal of Medicine 1987;316:1654‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
D'Amico 2003
- D'Amico G, Pietrosi G, Tarantino I, Pagliaro L. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta‐analysis. Gastroenterology 2003;124(5):1277‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dawsey 1994
- Dawsey SM, Wang GQ, Taylor PR, Li JY, Blot WJ, Li B, et al. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the Dysplasia Trial in Linxian, China. Cancer Epidemiology, Biomarkers & Prevention 1994;3(2):167‐72. [MEDLINE: ] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diplock 1994
- Diplock AT. Antioxidants and disease prevention. Molecular Aspects of Medicine 1994;15:293‐376. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Doucas 2006
- Doucas H, Garcea G, Neal CP, Manson MM, Berry DP. Chemoprevention of pancreatic cancer: a review of the molecular pathways involved, and evidence for the potential for chemoprevention. Pancreatology 2006;6(5):429‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duarte 2005
- Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radical Research 2005;39(7):671‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2003
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technolology Assessment 2003;7(1):1‐76. [MEDLINE: ] [PubMed] [Google Scholar]
Enzinger 2003
- Enzinger PC, Mayer RJ. Esophageal cancer. New England Journal of Medicine 2003;349(23):2241‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferlay 2004
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No 5, version 2.0. IARC Press, Lyon, France, 2004.
Fitzgerald 2006
- Fitzgerald RC. Molecular basis of Barrett's oesophagus and oesophageal adenocarcinoma. Gut 2006;55(12):1810‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freedman 2007
- Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. International Journal of Cancer 2007;121(12):2753‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297(5):468‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcea 2003
- Garcea G, Dennison AR, Steward WP, Berry DP. Chemoprevention of gastrointestinal malignancies. ANZ Journal of Surgery 2003;73:680‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluud 2001
- Gluud C. Alcoholic hepatitis: no glucocorticosteroids?. In: Leuschner U, James OFW, Dancygier H editor(s). Steatohepatitis (NASH and ASH) ‐ Falk Symposium 121. Lancaster: Kluwer Academic Publisher, 2001:322‐42. [Google Scholar]
Gluud 2006a
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluud 2006b
- Gluud C. The culture of designing hepato‐biliary randomised trials. Journal of Hepatology 2006;44(3):607‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grau 2006
- Grau MV, Rees JR, Baron JA. Chemoprevention in gastrointestinal cancers: current status. Basic & Clinical Pharmacology & Toxicology 2006;98(3):281‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gwyn 2002
- Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. American Journal of Gastroenterology 2002;97(1):13‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haenen 2002
- Haenen GR, Bast A. The use of vitamin supplements in self‐medication. Therapie 2002;57(2):119‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Hanck 1988
- Hanck AB. Vitamin C and cancer. Progress in Clinical and Biological Research 1988;259:307‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Herbert 1994
- Herbert V. The antioxidant supplement myth. American Journal of Clinical Nutrition 1994;60(2):157‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herbert 1997
- Herbert V. The value of antioxidant supplements vs their natural counterparts. Journal of the American Dietetic Association 1997;97(4):375‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. In: The Cochrane Library, Issue 4, 2006. Chichester, UK. John Wiley & sons, Ltd, 2006.
Holmes 2007
- Holmes RS, Vaughan TL, Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Seminars in Radiation Oncology 2007;17(1):2‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Holzinger 1999
- Holzinger F, Z'graggen K, Büchler MW. Mechanisms of biliary carcinogenesis: a pathogenetic multi‐stage cascade towards cholangiocarcinoma. Annals of Oncology 1999;10(Suppl 4):S122‐S6. [MEDLINE: ] [PubMed] [Google Scholar]
Hughes 2000
- Hughes D. Dietary antioxidants and human immune function. Nutrition Bulletin 2000;25:35‐41. [Google Scholar]
Janne 2000
- Janne PA, Mayer RJ. Chemoprevention of colorectal cancer. New England Journal of Medicine 2000;342:1960‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ (Clinical Research Ed) 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplowitz 2000
- Kaplowitz N. Cell death at the millennium. Implications for liver diseases. Clinics in Liver Disease 2000;4(1):1‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knekt 1994
- Knekt P. Vitamin E and cancer prevention. In: Balz Frei editor(s). Natural Antioxidants in Human Health and Disease. San Diego: Academic Press, Inc., 1994:199‐239. [Google Scholar]
Koushik 2007
- Koushik A, Hunter DJ, Spiegelman D, Beeson WL, Brandt PA, Buring JE, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. Journal of the National Cancer Institute 2007;99(19):1471‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kubo 2007
- Kubo A, Corley DA. Meta‐analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. American Journal of Gastroenterology 2007;102(10):2323‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kune 2006
- Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutrition and Cancer 2006;56(1):11‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lanza 2007
- Lanza E, Yu B, Murphy G, Albert PS, Caan B, Marshall JR, et al. The polyp prevention trial continued follow‐up study: no effect of a low‐fat, high‐fiber, high‐fruit, and ‐vegetable diet on adenoma recurrence eight years after randomization. Cancer Epidemiology, Biomarkers & Prevention 2007;16(9):1745‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larsson 2006
- Larsson SC, Håkansson N, Näslund I, Bergkvist L, Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiology, Biomarkers & Prevention 2006;15(10):1998‐2001. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lawson 2007
- Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health – AARP diet and health study. Journal of the National Cancer Institute 2007;99(10):754‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2003
- Lee BM, Park KK. Beneficial and adverse effects of chemopreventive agents. Mutation Research 2003;523‐4:265‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR Jr. Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes?. American Journal of Clinical Nutrition 2004;80(5):1194‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leung 2006
- Leung WK, Sung JJ, Leung WK, Sung JJ. Chemoprevention of gastric cancer. European Journal of Gastroenterology and Hepatology 2006;18(8):867‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lichtenstein 2005
- Lichtenstein AH, Russell RM. Essential nutrients: food or supplements? Where should the emphasis be. JAMA 2005;294(3):351‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367(9524):1747‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowenfels 2006
- Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Practice & Research Clinical Gastroenterology 2006;20(2):197‐209. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lynch 2002
- Lunch JP, Hoops TC. The genetic pathogenesis of colorectal cancer. Hematology/oncology Clinics of North America 2002;16(4):775‐810. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacDonald 2001
- MacDonald GA. Pathogenesis of hepatocellular carcinoma. Clinics in Liver Disease 2001;5(1):69‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine 2006;3(11):e442. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McAlister 2003
- McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials. A systematic review. JAMA 2004;289(19):2545‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKevith 2003
- McKevith B, Kelly C, Stanner S, Hughes J, Buttriss J. The Food Standards Agency's antioxidants in food programme‐a summary. Journal of Human Nutrition and Dietetics 2003;16(4):257‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mehta 2005
- Mehta S, Johnson IT, Rhodes M. Systematic review: the chemoprevention of oesophageal adenocarcinoma. Alimentary Pharmacology & Therapeutics 2005;22(9):759‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Millen 2004
- Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. Journal of the American Dietetic Association 2004;104(6):942‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142(1):37‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad A, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analysis. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morris 2003
- Morris CD, Carson S. Routine vitamin supplementation to prevent cardiovascular disease: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2003;139(1):56‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mulholland 2007
- Mulholland CA, Benford DJ. What is known about the safety of multivitamin‐multimineral supplements for the generally healthy population? Theoretical basis for harm. American Journal of Clinical Nutrition 2007;85(1):318S‐322S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murata 2000
- Murata M, Kawanishi S. Oxidative DNA damage by vitamin A and its derivative via superoxide generation. The Journal of Biological Chemistry 2000;275(3):2003‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neugut 1998
- Neugut AI, Jacobson JS, Suh S, Mukherjee R, Arber N. The epidemiology of cancer of the small bowel. Cancer Epidemiology, Biomarkers & Prevention 1998;7(3):243‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Neuhouser 2003
- Neuhouser ML, Patterson RE, Thornquist MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta‐carotene and retinol efficacy trial (CARET). Cancer Epidemiology, Biomarkers & Prevention 2003;12(4):350‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Nichter 2006
- Nichter M, Thompson JJ. For my wellness, not just my illness: North Americans' use of dietary supplements. Culture, Medicine and Psychiatry 2006;30(2):175‐222. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nomura 1987
- Nomura A, Heilbrun LK, Morris JS, Stemmermann GN. Serum selenium and the risk of cancer, by specific sites: case‐control analysis of prospective data. Journal of the National Cancer Institute 1987;79(1):103‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Nouraie 2005
- Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, Albanes D, et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiology, Biomarkers & Prevention 2005;14(9):2087‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oshima 1982
- Oshima H, Berezial J. Monitoring N‐nitrosamino acids excreted in the urine and feces of rats as an index of endogenous nitrozation. Carcinogenesis 1982;3(1):115‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paolini 1999
- Paolini M, Pozzetti L, Pedulli GF, Marchesi E, Cantelli‐Forti G. The nature of prooxidant activity of vitamin C. Life Sciences 1999;64(23):PL 273‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paolini 2003
- Paolini M, Abdel‐Rahman SZ, Sapone A, Pedulli GF, Perocco P, Cantelli‐Forti G, et al. Beta‐carotene: a cancer chemopreventive agent or a co‐carcinogen?. Mutation Research 2003;543(3):195‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Papas 1999
- Papas A. Antioxidant Status, Diet, Nutrition and Health. London, New York, Washington DC: Boca Raton FL: CRC: Press, 1999. [Google Scholar]
Papp 2007
- Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxidants & Redox Signaling 2007;9(7):775‐806. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 1998
- Patel T, Roberts LR, Jones BA, Gores GJ. Dysregulation of apoptosis as a mechanism of liver disease: An overview. Seminars in Liver Disease 1998;18(2):105‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Peto 1981
- Peto R, Doll R, Buckley JD, Sporn MB. A dietary carotene materially reduces human cancer rates?. Nature 1981;290(5803):201‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pocock 1991
- Pocock SJ. Clinical Trials. A Practical Approach. John Wiley and Sons, 1991. [Google Scholar]
Podmore 1998
- Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro‐oxidant properties. Nature 1998;392(6676):559. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poppel 1997
- Poppel G, Berg H. Vitamins and cancer. Cancer Letters 1997;114(1‐2):195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Potter 1999
- Potter JD. Colorectal cancer: molecules and populations. Journal of the National Cancer Institute 1999;91(11):916‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Radimer 2004
- Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999‐2000. American Journal of Epidemiology 2004;160(4):339‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rayman 2000
- Rayman M. The importance of selenium to human health. Lancet 2000;356(9225):233‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rayman 2005
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. The Proceedings of the Nutrition Society 2005;64(4):527‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19:591‐603. [DOI] [PubMed] [Google Scholar]
Salganik 2001
- Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. Journal of the American College of Nutrition 2001;20(5 Suppl):464S‐72S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasaki 2006
- Sasaki Y. Does oxidative stress participate in the development of hepatocellular carcinoma?. Journal of Gastroenterology 2006;41(12):1135‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SCF 2003
- European Commission Scientific Committee on Food. Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Vitamin E. 2003.
Schrauzer 1977
- Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies. III. Statistical association with dietary selenium intakes. Bioinorganic Chemistry 1977;7(1):35‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. [7823387] [DOI] [PubMed] [Google Scholar]
Schulz 2007
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress respiration and increasing oxidative stress. Cell Metabolism 2007;6(4):280‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schwartz 1996
- Schwartz JL. The dual roles of nutrients as antioxidants and prooxidants: their effects on tumor cell growth. Journal of Nutrition 1996;126(4 Suppl):1221S‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seifried 2003
- Seifried HE, McDonald SS, Anderson DE, Greenwald P, Milner JA. The antioxidant conundrum in cancer. Cancer Research 2003;63(15):4295‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Sharma 2004
- Sharma N, Trope B, Lipman TO. Vitamin supplementation: what the gastroenterologist needs to know. Journal of Clinical Gastroenterology 2004;38(10):844‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharp 1998
- Sharp SJ. Metaanalysis regression. Stata Technical Bulletin 1998;42:16‐22. [Google Scholar]
Shimoda 1994
- Shimoda R, Nagashima M, Sakamoto M, Yamaguchi N, Hirohashi S, Yokota J, et al. Increased formation of oxidative DNA damage, 8‐hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Research 1994;54(12):3171‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Sies 1985
- Sies H. Introductory remarks. In: Sies H editor(s). Oxidative Stress. Orlando: Academic Press, 1985:1‐7. [Google Scholar]
Sihvo 2002
- Sihvo EI, Salminen JT, Rantanen TK, Ramo OJ, Ahotupa M, Farkkila M, et al. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. International Journal of Cancer 2002;102(6):551‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Slatore 2007
- Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long‐term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. American Journal of Respiratory and Critical Care Medicine 2007;177(5):524‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoner 2007
- Stoner GD, Wang LS, Chen T. Chemoprevention of esophageal squamous cell carcinoma. Toxicology and Applied Pharmacology 2007;224(3):337‐49. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stranges 2007
- Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long‐term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Annals of Internal Medicine 2007;147(4):217‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sugiyama 2004
- Sugiyama T. Development of gastric cancer associated with Helicobacter pylori infection. Cancer Chemotherapy and Pharmacology 2004;54(Suppl 1):S12‐S20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tabor 1999
- Tabor E, Bisceglie AM. Hepatitis B. Hepatocellular carcinoma. Clinics in Liver Disease 1999;3(2):327‐48. [Google Scholar]
Takachi 2007
- Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S, et al. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center‐Based Prospective Study. American Journal of Epidemiology 2007;167(1):59‐70. [DOI] [PubMed] [Google Scholar]
Takeda 2002
- Takada M, Ku Y, Habara K, Ajiki T, Suzuki Y, Kuroda Y. Inhibitory effect of epigallocatechin‐3‐gallate on growth and invasion in human biliary tract carcinoma cells. World Journal of Surgery 2002;26(6):683‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tamimi 2002
- Tamimi RM, Lagiou P, Adami HO, Trichopoulos D. Prospect for chemoprevention of cancer. Journal of Internal Medicine 2002;251:286‐300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tzonou 1996
- Tzonou A, Lipworth L, Garidou A, Signorello LB, Lagiou P, Hsieh C, et al. Diet and risk of esophageal cancer by histologic type in a low‐risk population. International Journal of Cancer 1996;68(3):300‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
USPSTF 2003
- U.S. Preventive Services Task Force. Routine vitamin supplementation to prevent cancer and cardiovascular disease: recommendations and rationale. Annals of Internal Medicine 2003;139(1):51‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vivekananthan 2003
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta‐analysis of randomised trials. Lancet 2003;361(9374):2017‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Webb 2007
- Webb GP. Nutritional supplements and conventional medicine; what should the physician know?. The Proceedings of the Nutrition Society 2007;66(4):471‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Willcox 2004
- Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Critical Reviews in Food Science and Nutrition 2004;44(4):275‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williamson 2005
- Williamson PR, Gamble C, Altman DG, Hutton JL. Outcome selection bias in meta‐analysis. Statistical Methods in Medical Research 2005;14(5):515‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 2007
- Woo JJ. Adverse event monitoring and multivitamin‐multimineral dietary supplements. American Journal of Clinical Nutrition 2007;85(1):323S‐4S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
World Bank 2006
- World Bank List of Economies (July 2006). http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS (accessed 31 October 2007).
Yates 2007
- Yates MS, Kensler TW. Keap1 eye on the target: chemoprevention of liver cancer. Acta Pharmacologica Sinica 2007;28(9):1331‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ziegler 1989
- Ziegler RG. A review of epidemiologic evidence that carotenoids reduce the risk of cancer. Journal of Nutrition 1989;119(1):116‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bjelakovic 2004a
Bjelakovic 2004b
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta‐analysis. Lancet 2004;364(9441):1219‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


