Abstract
Mucopolysaccharidosis I (MPS I) and MPS VII are due to deficient activity of the glycosaminoglycan-degrading lysosomal enzymes α-L-iduronidase and β-glucuronidase, respectively, and result in abnormal bones and joints. Here, the severity of skeletal disease in MPS I and MPS VII dogs and the effects of neonatal gene therapy were evaluated. For untreated MPS VII dogs, the lengths of the second cervical vertebrae (C2) and the femur were only 56% and 84% of normal, respectively, and bone dysplasia and articular erosions, and joint subluxation were severe. Previously, we reported that neonatal intravenous injection of a retroviral vector (RV) with the appropriate gene resulted in expression in liver and blood cells, and high serum enzyme activity. In this study, we demonstrate that C2 and femurs of RV-treated MPS VII dogs were longer at 82% and 101% of normal, respectively, and there were partial improvements of qualitative abnormalities. For untreated MPS I dogs, the lengths of C2 and femurs (91% and 96% of normal, respectively) were not significantly different from normal dogs. Qualitative changes in MPS I bones and joints were generally modest and were partially improved with RV treatment, although cervical spine disease was severe and was difficult to correct with gene therapy in both models. The greater severity of skeletal disease in MPS VII than in MPS I dogs may reflect accumulation of chondroitin sulfate in cartilage in MPS VII, or could relate to the specific mutations. Neonatal RV-mediated gene therapy ameliorates, but does not prevent, skeletal disease in MPS I and MPS VII dogs.
Keywords: Gene therapy, lysosomal storage disease, mucopolysaccharidosis, α-L-iduronidase, β-glucuronidase, glycosaminoglycan, dysostosis multiplex
Introduction
The mucopolysaccharidoses (MPS) are lysosomal storage diseases that result in accumulation of glycosaminoglycans (GAG). MPS I is due to deficient activity of α-L-iduronidase (IDUA; EC 3.2.1.76) and results in the accumulation of heparan and dermatan sulfate. MPS VII is due to deficient activity of β-D-glucuronidase (GUSB; EC 3.2.1.31) and results in the accumulation of chondroitin, heparan, and dermatan sulfate. Clinical manifestations of MPS can include bone and joint disease, visual and auditory deficiencies, cardiac disease, upper airway obstruction, hepatosplenomegaly, and mental retardation [1–2].
The skeletal disease of MPS is referred to as dysostosis multiplex, and manifests as shortened bones, thickened bones, bones that are abnormally shaped, and bones with articular erosions. In humans, MPS I results in facial dysmorphia, kyphoscoliosis, stiff joints, and hip dysplasia with subluxation that causes a waddling gait [3–8]. MPS I dogs display collapsed intervertebral spaces and vertebral disc herniation, vertebral ankylosis, osteopenia, focal articular erosions, degenerative joint disease, and joint effusions [9–10]. Although both humans and dogs with MPS I have stunted growth, bone length measurements have not been performed. MPS I mice have increased bone mineral density and bone diameter, but relatively normal bone lengths [11–12].
MPS VII also results in dysostosis multiplex. Humans have small vertebral bodies, kyphosis, widened ribs, shortened and thickened long bones, atlantoaxial instability leading to cervical cord compression, and hip dysplasia with subluxation [13–15]. MPS VII dogs have similar abnormalities to those found in humans, and also have curvature of long bones and patellar luxation [16–17]. MPS VII mice have thickening of long bones, and the femurs are ~80% of normal length [18–19].
The mechanisms that lead to bone and joint disease are not clear. Short bones are likely due to abnormalities in the growth plate, which is very disorganized in MPS VII mice and dogs [19] but is only mildly abnormal in MPS I mice [20]. Degenerative changes may be due to upregulation of proteases, as the synovium and articular cartilage of MPS VII dogs have increased levels of matrix metalloproteinases (MMPs) [21–22].
Current treatments for MPS I include hematopoietic stem cell transplantation (HSCT) [23] and enzyme-replacement therapy (ERT) [24–25]. Human MPS I patients who received HSCT at 1.5 years of age had improved joint mobility at 5 years of age, but kyphosis and hip subluxation progressed [8]. HSCT in a 12-year-old patient with MPS VII improved ambulation, although radiographic abnormalities were not ameliorated 1.5 years later [26]. In MPS I dogs that received HSCT at 5 months of age, radiographic abnormalities were reduced at 20 months but intervertebral space narrowing was still present [9–10]. HSCT to newborn MPS VII mice improved bone lengths to 87% of normal [27]. ERT to humans with MPS I improved growth [28], but details on the effect on radiographs have not been reported. ERT did not improve radiograph abnormalities in MPS I dogs, but this study was complicated by the presence of antibodies directed against the human IDUA [24]. ERT to MPS VII mice resulted in partial improvement of bone lengths to 88% of normal [18]. Thus, existing therapies reduce, but do not prevent, skeletal disease in MPS.
Gene therapy has been studied as an alternative treatment for MPS in animal models. We have previously injected gamma retroviral vectors (RV) intravenously (IV) into newborn MPS I and MPS VII mice and dogs [11, 17, 19, 29–30]. This resulted in transduction of ~20% and ~3% of hepatocytes in mice and dogs, respectively, which secreted enzyme into blood. In addition, ~1% of blood cells were transduced and expressed the RV in dogs [31]. Neonatal administration of RV normalized bone diameters in MPS I mice [11], and resulted in bones that were 86% to 93% of normal in MPS VII mice [19]. Although neonatal RV treatment in MPS VII dogs reduced skeletal disease at ~1 year of age [17, 19], later evaluation and quantification of the effect on bone lengths has not been reported. In addition, no data have been presented on the effect of gene therapy on skeletal disease in MPS I dogs. Here, we evaluated the radiographic manifestations of disease in bones and joints after neonatal gene therapy in MPS I and MPS VII dogs.
Materials and Methods
Animals
NIH and USDA guidelines for the care and use of animals in research were followed in the animal colony of the School of Veterinary Medicine, University of Pennsylvania. MPS I dogs were treated with neonatal IV injection of 3 to 10×109 transducing units (TU)/kg of hAAT-cIDUA-WPRE, which is an RV expressing canine IDUA [11]. Radiographs from 3 males and 3 females whose clinical data were reported previously [30] were evaluated: I-99 (female; achieved 23 IDUA U/mL), I-101 (female; 27 U/mL), I-107 (female; 533 U/mL), I-140 (male; 888 U/mL), I-171 (male; 240 U/mL), and I-172 (male; 608 U/mL). A total of 4 males and 3 females were evaluated for the RV-treated MPS VII group. The RV-treated MPS VII dog designated M1287 (male; achieved 18,039 GUSB U/mL) received hepatocyte growth factor (HGF) to induce hepatocyte replication prior to neonatal administration of 12×109 TU/kg of hAAT-cGUSB-WPRE, which is an RV expressing canine GUSB, as described previously [17, 29]. All other RV-treated MPS VII dogs received neonatal IV injection of 3 to 88×109 TU/kg of hAAT-cGUSB-WPRE without preceding HGF and included M1328 (male; 429 U/mL), M1332 (female; 76 U/mL), M1337 (female; 294 U/mL), M1653 (female; 88 U/mL), M2065 (male; 2089 U/mL), and M2165 (male; 4027 U/mL). Clinical data on all animals except M1653, M2065, and M2165 were reported previously [17]. Untreated MPS I or MPS VII dogs, and heterozygous or homozygous normal dogs from the MPS I and the MPS VII colony were also evaluated. There were more females than males for the normal groups, as heterozygous females are maintained for breeding purposes, while heterozygous males are not maintained due to animal space constraints.
Statistics
All comparisons were done using SigmaStat 3.1 statistical analysis software (Systat Software, Inc., San Jose, CA).
Radiographs
Dogs were sedated with propofol to effect and positioned, the film was placed 40 inches from the emitter, and radiographs were obtained. Measurements were performed on X-ray film using a ruler or on digital radiographs using the Philips I-Site Enterprise 3.5 (March 2006) viewing software. Measurements of the lengths of vertebral bodies and the intervertebral spaces were performed on lateral views. Measurements of the radius, ulna, femur, and tibia were performed on lateral views of the limb positioned on the table. Lengths of metacarpals and metatarsals were measured on dorsopalmar and dorsoplantar views, respectively. Bone lengths and intervertebral space lengths were compared using one-way ANOVA with Tukey post-hoc analysis.
Radiograph scoring system
Radiographs were evaluated by a board-certified veterinary radiologist (VWK) who was blinded to the genotype and treatment of the animals. For most categories, radiographs were scored on a 0 to 2 scale, where 0 represented normal, 1 represented a moderate abnormality, and 2 represented a severe abnormality, and the Mann-Whitney U test was used for statistical comparisons. For luxation of the coxofemoral joint and patella, animals received a score of 0 if neither side was affected, 1 if one side was affected, and 2 if both sides were affected; this scoring system did not take into account the severity of luxation. Carpal bone subluxation was when the front paw deviated in a valgus (lateral) or varus (medial) direction or if the bones were not aligned normally. For cervical spine fusion, articular erosion of the carpi, and radial and ulnar curving, abnormalities were classified as 0 if absent and 2 if present, and statistical comparisons were performed with Fisher’s exact test.
Results
Neonatal gene therapy for MPS I and VII dogs
Six MPS I dogs that received neonatal gene therapy [30] achieved 23 to 888 U/mL of IDUA activity in serum. This was 2- to 68-fold the level in normal dogs, although high levels in serum do not necessarily result in normal enzyme activity in tissues. Six MPS VII dogs that received neonatal gene therapy [17, 29] achieved 76 to 4027 U/mL of serum GUSB activity, which was 0.3 to 15-fold the level in normal dogs. One MPS VII dog who received HGF in addition to neonatal gene therapy achieved 18,039 U/mL of GUSB activity (67-fold normal). RV-treated dogs with similar levels of serum enzyme activity had high enzyme activity as well as biochemical and histloogical evidence of improvement in lysosomal storage in many organs, as was reported previously [17,19, 30]. We have also measured urine GAG levels in the MPS VII dogs, and found that they were normalized in the RV-treated group (normal had 13.3 +/− 5.5 μg of GAG per mg of creatinine, N=5; untreated MPS VII dogs had 76 +/−52 μg of GAG per mg of creatinine, N=4; and RV-treated MPS VII dogs had 18 +/− 9.4 μg of GAG per mg of creatinine, N=4), and there were statistically significant differences between untreated and RV-treated dogs (p<0.05). Urine GAGs were not measured in the MPS I dogs. Thus, RV-treated dogs had evidence of reduced manifestations of disease in many sites.
RV-treated MPS I dogs were radiographed at 1 to 2.2 years of age, and RV-treated MPS VII dogs were radiographed at 1 to 7 years of age. Controls were heterozygous or homozygous normal dogs or untreated affected dogs from the same breeding colony. It should be noted that these are outbred dogs with substantial genetic variation.
Radiographic evaluation of vertebrae
As few untreated MPS VII dogs survive past 1 year of age, radiographs were obtained at 1 year for normal, untreated MPS I, and untreated MPS VII dogs to facilitate comparison of the severity of skeletal disease between the two models. For the RV-treated MPS I and MPS VII dogs, radiographs were obtained at 1 year to facilitate determination of whether or not the bones and joints were improved with RV treatment. In addition, some radiographs were obtained at a maximum age of 2.2 years and 7 years for the RV-treated MPS I and MPS VII dogs, respectively, which allowed the effect of aging upon skeletal disease to be evaluated. As detailed below, Fig. 1 shows representative examples of radiographs, while supplementary Figs. 1, 2, and 3 show many examples of radiographs obtained of the ventrodorsal cervical spine, the lateral cervical spine, and the lateral lumbar spine, respectively, at different ages for animals in each group. Fig. 2 shows average vertebral body and intervertebral space lengths ± one standard deviation (SD) for animals in different groups, and Fig. 3 shows subjective scores of a variety of parameters that were evaluated from radiographs.
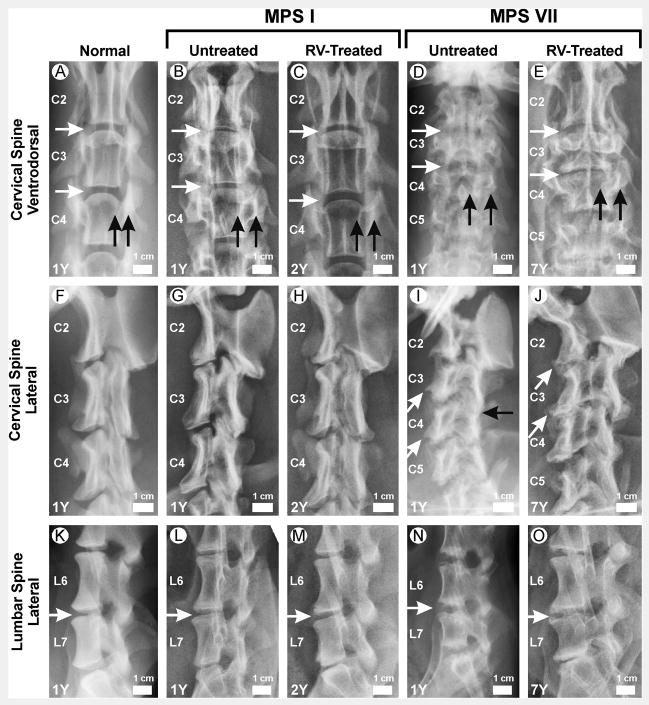
Figure 1. Radiographs of vertebrae.
Radiographs from male dogs were obtained at the ages in years (Y) shown in the lower left corner. The genotype and treatment status are indicated above the panels. RV-treated dogs received neonatal IV injection of an RV expressing the appropriate gene. Examples of RV-treated MPS I dogs are from I-171 in panels C and H and I-172 in panel M, while all examples of MPS VII dogs are from M1328. The scale bar shown in each image is 1 cm, and the cranial aspect is at the top and the caudal aspect at the bottom. A–E. Ventrodorsal view of the cervical spine. The second cervical vertebra (C2), C3, and C4 are indicated. The horizontal white arrows indicate intervertebral spaces. Black vertical arrows indicate the medial and lateral borders of the pedicle, and the space between the arrows was used to assess width. F–J. Lateral view of the cervical spine. Slanted white arrows indicate caudoventral vertebral body beaking. The black horizontal arrow indicates fusion of the articular facet joint. K–O. Lateral lumbar spine. The sixth lumbar vertebra (L6) and L7 are indicated. Horizontal white arrows indicate intervertebral spaces.
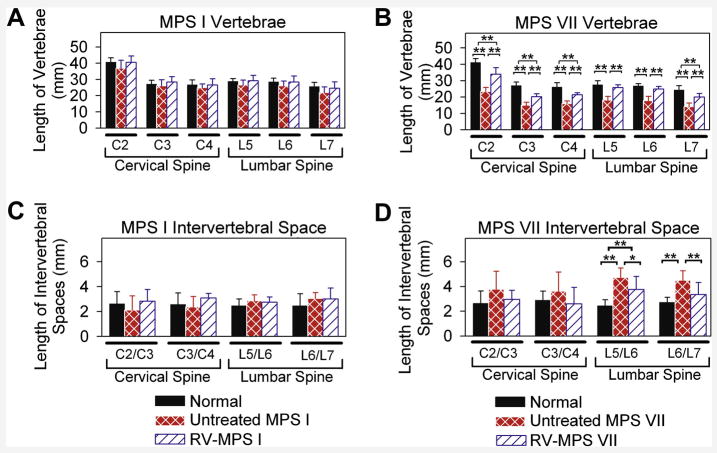
Figure 2. Axial skeleton measurements.
Measurements of vertebral body and intervertebral space lengths were obtained for the cervical and lumbar spine in post-pubertal dogs at 0.7 to 3 years after birth, and values for males and females were combined. Some RV-treated MPS VII dogs were evaluated at several ages, and values (which did not vary substantially) were averaged to give a single value for that animal. Values in several animals were then averaged and the mean +/− the standard deviation was calculated. The means for several animals in each group +/− one SD are shown; * indicates p of 0.01 to 0.05 and ** indicates p<0.01 for comparison of the groups connected by a bracket using ANOVA with Tukey post-hoc analysis. A. Vertebral body lengths for MPS I dogs. Vertebral body lengths of C2 (second cervical vertebra), C3, C4, L5 (fifth lumbar vertebra), L6, and L7 were measured for 8 heterozygous normal (1 male, 7 female), 7 untreated MPS I (5 male, 2 female), and 6 RV-treated MPS I (3 male, 3 female) post-pubertal dogs at 0.6 to 1 year of age. B. Vertebral body lengths for MPS VII dogs. Lengths were measured for 12 heterozygous normal (3 male, 9 female), 15 untreated MPS VII (11 male, 4 female), and 7 RV-treated MPS VII (4 male, 3 female) dogs. C and D. Intervertebral space lengths for MPS I and VII dogs. Intervertebral space lengths were measured for the same animals whose values are shown in panels A and B.
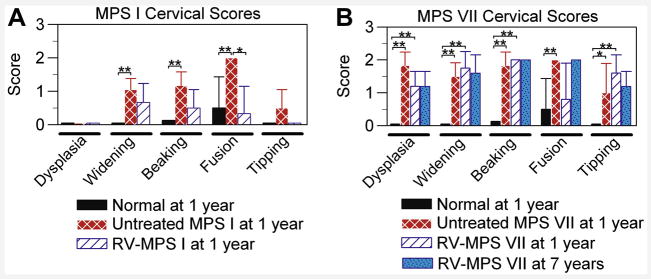
Figure 3. Axial skeleton radiographic scores.
Radiographs were scored for abnormalities at 1 year of age for most groups, where 0 indicates normal and 2 indicates severely abnormal. Both males and females were evaluated, means +/− SD are shown, and * indicates p<0.05 and ** indicates p<0.01 for comparison of the groups connected by a bracket using the Mann-Whitney U test. A. Radiographic scores for MPS I dogs. The scores are shown for 8 normal dogs at 1 to 2 years (2 male, 6 female), 6 untreated MPS I dogs at 1 year (4 male, 2 female), and 6 neonatal RV-treated MPS I dogs at 1 year (3 male, 3 female). Vertebral body dysplasia, pedicle widening, caudoventral vertebral body beaking, articular facet joint fusion, and vertebral body tipping were evaluated. B. Radiographic scores for MPS VII dogs. The scores are shown for 8 normal dogs at 1 to 2 years (2 male, 6 female), 6 untreated MPS VII dogs at 1 year (5 male, 1 female), and 5 RV-treated dogs at 1 year (2 male, 3 female), and statistical comparisons were performed for the 3 groups with the Mann-Whitney U test. Two of the seven RV-treated MPS VII dogs that were alive at 1 year were not evaluated as radiographs were not obtained at the appropriate age. RV-treated MPS VII dogs were also scored for 5 dogs at 4 to 7 years (2 males and 2 females at 7 years and 1 female at 4 years), and comparison of values with those in RV-treated MPS VII dogs at 1 year using the Mann-Whitney U test failed to find significant differences.
Lengths of vertebrae in untreated MPS I and MPS VII dogs
The lengths of the cervical vertebrae from untreated MPS I dogs (Figs. 1B and 2A) were only slightly shorter than in normal dogs from the same colony (Figs. 1A and 2A), as the second cervical vertebrae (C2), C3, and C4 were 37±5 mm (91% of normal for the same colony), 26±4 mm (96% normal), and 25±2 mm (93% of normal), respectively. The failure to observe significant differences between values in untreated MPS I and normal dogs may reflect the small number of animals in each group, and that values from dogs of both genders were pooled due to the small total numbers of animals. For normal animals, male vertebral body heights were 107±2% of those found in females.
In contrast to the results with MPS I, lengths of C2, C3, and C4 were markedly reduced in untreated MPS VII vertebrae (Figs. 1D and 2B) at 23±3 mm (57% of normal), 15±2 mm (56% normal), and 16±2 mm (62% normal), respectively (p<0.01 for comparison of MPS VII vs. normal for all vertebrae). Similarly, the fifth lumbar (L5), L6, and L7 vertebral body lengths were modestly reduced at 92%, 92%, and 85% of normal in untreated MPS I dogs (Figs. 1L and 2A; not significant vs. normal), but were markedly shortened at 66%, 67%, and 58% of normal in untreated MPS VII dogs (Figs. 1N and 2B; p<0.01 vs. normal).
Qualitative evaluation of the cervical spine in untreated MPS I and MPS VII dogs
A variety of parameters were subjectively scored from 0 (normal) to 2 (severely abnormal) on radiographs, as shown in Fig. 3. The structure of the cervical vertebrae from MPS I dogs appeared normal (Figs. 1B and 1G) and received a dysplastic score of 0±0 (Fig. 3A). In contrast, the cervical vertebrae from MPS VII dogs were markedly abnormal (Figs. 1D and 1I) with a dysplastic score of 1.8±0.4 (Fig. 3B; p<0.01 vs. normal). The cervical spine was assessed for widening, which refers to the thickening of bone around the pedicle and can be appreciated on the ventrodorsal projection for the MPS I dog shown in Fig. 1B. Widening occurred in both MPS I (score of 1.1±0.3; p<0.001 vs. normal) and MPS VII (score of 1.5±0.4; p<0.001 vs. normal) dogs. Beaking refers to the caudoventral osteophytosis that results in a bird-like beak, which can be appreciated in the MPS VII dog shown in Fig. 1I, and may be due to herniation of the nucleus pulposus [5, 32]. Beaking was present in both MPS I (1.2±0.4; p<0.001 vs. normal) and MPS VII (1.8±0.4; p<0.001 vs. normal) dogs. Fusion refers to articular facet joint space fusion, which can be appreciated in the MPS VII dog shown in Fig. 1I. Fusion was severe in both MPS I (2±0; p=0.01 vs. normal) and MPS VII (2±0; p=0.01 vs. normal) dogs. Tipping refers to a rotation of the vertebrae so that the normal end-to-end conformation is lost, and the cranial surface of one vertebra is elevated relative to the caudal aspect of the preceding vertebra. Although tipping was very modest in MPS I dogs at 0.5±0.5 (not significant vs. normal), it was moderate in MPS VII dogs (1±0.9; p=0.04 vs. normal). We conclude that both canine models of MPS had substantial abnormalities in the cervical spine, although disease was more severe in MPS VII than in MPS I.
Effect of gene therapy on the cervical spine in MPS I and MPS VII dogs
A major goal of this project was to determine if neonatal gene therapy with an RV expressing the appropriate gene could prevent bone disease in MPS I and MPS VII dogs. The caudal to cranial lengths of vertebrae and intervertebral spaces were determined for post-pubertal animals whose ages ranged from 0.7 to 3 years. Some individual RV-treated dogs were evaluated at several ages, and the values (which were similar) were averaged. There were no significant differences in the lengths of cervical or lumbar vertebral bodies in RV-treated MPS I vs. untreated MPS I dogs (Fig. 2A), which may reflect modest shortening in MPS I dogs. For RV-treated MPS VII dogs, the heights of C2 (82% of normal), C3 (75%), C4 (82%), L5 (93%), L6 (93%), and L7 (83%) were all significantly longer than in untreated MPS VII dogs (p<0.01 for all vertebrae vs. untreated MPS VII), but remained statistically shorter than in normal dogs for C2, C3, C4, and L7 (p<0.01), as shown in Fig. 2B. RV-treated MPS I dogs had a significant reduction in fusion at 1 year of age when compared with untreated MPS I dogs (Fig. 3A), although the modest improvements in widening, beaking, and tipping were not significant. RV-treated MPS VII dogs did not have a significant improvement in any of the parameters that were scored in Fig. 3B at 1 year of age. In general, the scores at 7 years were similar to those obtained at 1 year for MPS VII dogs. However, fusion appeared to worsen with age, although differences in the values obtained at 1 and 7 years were not significant.
Evaluation of intervertebral spaces
The intervertebral space lengths can be used to determine the caudal to cranial length of the non-calcified intervertebral discs. The intervertebral space lengths between C2 and C3 (C2/C3) and C3/C4 were not significantly different in MPS I or MPS VII dogs from the values in normal dogs (Fig. 2C and 2D). Although lumbar intervertebral space lengths were not significantly different between untreated MPS I and normal dogs, they were greater in untreated MPS VII dogs at 193% and 164% of normal at L5/L6 and L6/L7, respectively (p<0.01 vs. normal). Neonatal gene therapy to MPS VII dogs resulted in intervertebral space lengths that were reduced to 156% of normal for L5/L6 (p<0.05 vs. untreated MPS VII) and 129% of normal for L6/L7 (p<0.01 vs. untreated MPS VII), respectively, although the length at the L5/L6 intervertebral space remained greater than normal (p<0.01).
Three of six untreated MPS I dogs that were evaluated for 1 year or longer developed clinical signs of cord compression, which included weakness, hyperreflexia, and reduced proprioception of the limbs, as described previously [30]. Herniated discs can result in cord compression, and can be associated with intervertebral space narrowing. Indeed, some untreated MPS I mice had intervertebral space narrowing, as can be seen at C2/C3 in Fig. 1B. All cervical intervertebral spaces were evaluated for 6 untreated animals, and a total of 9 of 36 (25%) were narrowed for the untreated MPS I dogs. Although the number of narrowed cervical intervertebral spaces of 4 out of 36 evaluated (11%) from 6 RV-treated MPS I dogs was not significantly different from the frequency in MPS I dogs with Fisher’s exact test, no RV-treated MPS I dogs developed signs of cord compression. Thus, it is possible that cord compression was due to other causes such as thickening of the dura mater or ligaments of the spine, or ligament laxity resulting in subluxation of the bones, as has been reported in human patients with MPS [5–7, 14–15]. Magnetic resonance imaging will be performed in the future to attempt to identify the etiology of cord compression in untreated MPS I dogs. Quantification of the number of narrowed intervertebral space lengths in the cervical spine of MPS VII dogs was complicated by the fact that the marked dysplasia of the cervical vertebral bodies in the caudal regions made it difficult to define the boundaries of bone and disc. For this reason, intervertebral space narrowing was only evaluated at C2/C3 and at C3/C4. For untreated MPS VII dogs, 5 of 12 (42%) intervertebral disc spaces that were evaluated at 0.5 to 2 years of age were narrowed. This frequency was similar at 1 year in RV-treated MPS VII dogs (5 of 12 spaces were narrowed, or 42%) and at 7 years in RV-treated dogs (4 of 8 spaces were narrowed, or 50%; see C3/C4 in Figs. 1E and 1J). Clinical evaluation for cord compression in untreated MPS VII dogs was difficult, as these dogs did not walk beyond 6 months of age. No RV-treated MPS VII dogs developed clinical signs of cord compression for up to 7 years of evaluation.
Radiographic evaluation of the carpus
The appendicular skeleton was also evaluated radiographically. As detailed below, Fig. 4 shows representative radiographs, Fig. 5 shows severity scores for various abnormalities, and Fig. 6 quantifies long bone lengths. Additional examples of radiographs from dogs of each group are provided in the supplementary data section for the cubital joint (elbows; supplementary Fig. 4), carpal joint (supplementary Fig. 5), coxofemoral joint (hip; supplementary Fig. 6), lateral stifle joint (knee; supplementary Fig. 7), ventrodorsal stifle joint (supplementary Fig. 8), and radius/ulna (supplementary Fig. 9).
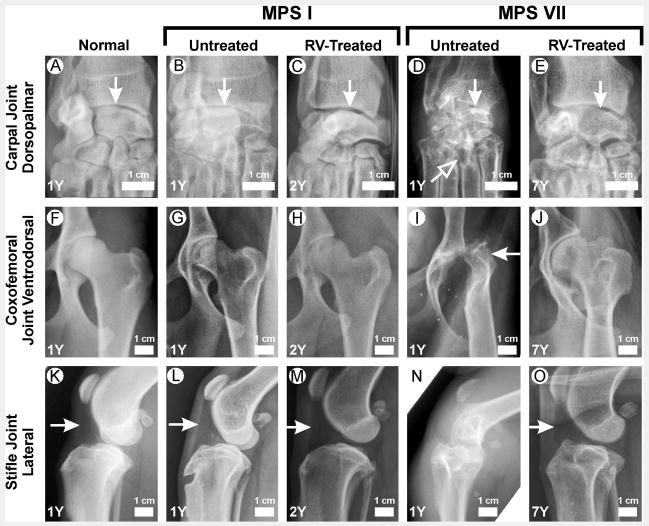
Figure 4. Evaluation of joints.
Radiographs are from male dogs at the ages in years (Y) shown in the lower left corner. The genotype and treatment status are indicated above the panels. Examples of radiographs of RV-treated MPS I dogs were from I-172 in panels C and H, and from I-171 in panel M. All examples of radiographs of the RV-treated MPS VII dog were from M1328. A–E. Carpal joint dorsopalmar. The metacarpals are at the bottom of the image, and the radius (right) and ulna (left) are on the top. The white vertical arrows indicate radiocarpal bones. The slanted white arrow indicates erosions of the 3rd metacarpal bone. F–J. Coxofemoral joint ventrodorsal. The pelvis is on the left side of the image. The horizontal white arrow indicates luxation of the femoral head. K–O. Stifle joint lateral. The femur is at the top of the image and the tibia is at the bottom. The white arrows indicate the left aspect of the triangular-shaped radiolucent fat pad present at the cranial aspect of the stifle joint, which can be visualized when no effusion is present. This triangle is absent in an untreated MPS VII dog (panel N) due to an effusion.
Figure 5. Appendicular skeleton radiographic scores. A. Radiographic scores for MPS I carpi.
Radiographic scores are shown for 8 normal dogs at 2 years of age (2 male, 6 female), 6 untreated MPS I dogs at 1 year (4 male, 2 female), and 6 RV-treated MPS I dogs at 1 year of age (3 male, 3 female). Carpal bone dysplasia, carpal bone lucency, articular bone erosions, carpal joint subluxation, and joint effusions were scored from 0 (normal or absent) to 2 (severely abnormal). B. Radiographic scores for MPS VII forelimb. Radiographic scores are shown for 8 normal dogs at 1 to 2 years of age (2 male, 6 female), 6 untreated MPS VII dogs at 1 year (5 male, 1 female), 5 RV-treated dogs at 1 year (2 males and 2 females at 7 years, and 1 female at 4 years), and 5 RV-treated dogs at >4 years (2 male, 3 female). C. Radiographic scores for MPS I hindlimb. Radiographic scores are shown for the same groups that are described in panel A. The coxofemoral joint was evaluated for dysplasia, subluxation, articular bone erosions, and degenerative joint disease (DJD). The stifle joints were evaluated for patellar luxation (pat. lux.) and effusions. D. Radiographic scores for MPS VII hindlimb. Radiographic scores are shown for the same animals described in panel B. E. Radiographic scores for MPS I femur and radius/ulna. Radiographic scores are shown for the same dogs described in panel A. Femoral head dysplasia, femoral erosions, femoral curvature, radius/ulna curving, and radius/ulna dysplasia were evaluated. F. Radiographic scores for MPS VII femur and radius/ulna. Radiographic scores are shown for the same dogs described in panel B.
Figure 6. Appendicular skeleton lengths.
Measurements of bone length were obtained for hindlimbs for post-pubertal dogs that were 0.7 to 3 years of age. Values did not differ in the RV-treated animals that were radiographed at 1 to 3 years of age. * indicates a p value of 0.01 to 0.05, and ** indicates a p value <0.01 for the statistical comparison of the groups that are connected with a bracket. A. Hindlimb bone lengths in MPS I dogs. Measurements of the femur, tibia, and metatarsals (MT) of the same dogs that are described in Fig. 5A are shown. B. Hindlimb bone lengths in MPS VII dogs. Measurements of the bones of the dogs described in Fig. 5B are shown.
Carpal bones from MPS I dogs were only mildly dysplastic and lucent, there were no articular erosions, and there were only modest effusions (Fig. 4B and 5A), but these parameters were all markedly abnormal in untreated MPS VII dogs (Fig. 4D and 5B). RV-treated MPS VII dogs had significant reductions in carpal bone dysplasia (p<0.05), articular erosions (p<0.01), and joint effusions (p<0.05) at 1 year of age. These improvements were generally maintained at 7 years, although articular erosions appeared to worsen with age.
Radiographic evaluation of the coxofemoral joint, stifle joint, and long bones
The coxofemoral joints, stifle joints, and long bones were also evaluated radiographically. For untreated MPS I dogs at 1 year, there was a significant but modest increase in dysplasia of the coxofemoral joint (Fig. 4G and 5C), the severity of stifle joint effusions (Fig. 5C), and dysplasia of the radius and ulna (Fig. 5E) compared with normal dogs (Fig. 4F, 4K, 5A, 5C, and 5E). The severity of stifle joint effusions was reduced in RV-treated MPS I dogs at one year (Fig. 5C) and improvements were maintained at 2 years. Untreated MPS I dogs that did not develop cord compression had a normal gait for up to 1.75 years of age, the oldest age of analysis. Five of six RV-treated MPS I dogs had a normal gait for the duration of evaluation, although I-99 developed luxated shoulders at 13 months of age.
For untreated MPS VII dogs, there was severe dysplasia and luxation of the coxofemoral joint at 1 year (Fig. 4I and 5D). Indeed, 6 of 7 (86%) untreated MPS VII dogs had markedly luxated femoral heads at 1 year, and all were unable to stand or walk by 6 months of age. RV-treated MPS VII dogs had a significant reduction in dysplasia of the coxofemoral joint (Fig. 4J and 5D; p=0.02 vs. untreated MPS VII). Although the reduction in the score for luxation in RV-treated MPS VII dogs was not significant at 1 year, only 1 of 7 (14%) RV-treated MPS VII dogs had severe subluxation at 1 year (p=0.029, data not shown). In addition, all four RV-treated MPS VII dogs that were evaluated at 7.5 years could run, as shown in the accompanying video; M1287 and M1328 did have an abnormal gait that could be due to the hip dysplasia that is present to some degree in normal dogs from the colony. Untreated MPS VII dogs also had severe articular erosions and degenerative joint disease of the coxofemoral joint (Fig. 4I), patellar luxation, stifle effusions (Fig. 4N), dysplasia and articular erosions of the distal femur (Fig. 4N), curving of the femur, and dysplasia of the radius and ulna. Scores for RV-treated MPS VII dogs at 1 year that were significantly better than for untreated MPS VII dogs at 1 year included articular erosion of the coxofemoral joint, patellar luxation, stifle effusions, articular erosion of the distal femur, and curving of the femur (Fig. 5D and 5F). In general, scores at 7 years of age in RV-treated MPS VII dogs were similar to those found at 1 year in RV-treated MPS VII dogs. Although scores of articular erosions of the coxofemoral joint and femur tended to increase in RV-treated dogs at 7 years, these differences were not significantly different from those observed in RV-treated dogs at 1 year using the Mann-Whitney U test.
Lengths of long bones
Lengths of long bones of the hindlimb (Fig. 6A and 6B) and the forelimb (supplementary Fig. 10) were determined. Lengths of the radius, ulna, metacarpals, femur, tibia and metatarsals in untreated MPS I dogs were all >92% of normal, and no value was significantly different from those in normal dogs from the same colony. In contrast, untreated MPS VII dogs had significant reductions in lengths of the ulna (88% of normal; p<0.01 vs. normal), the second metacarpal (MC2)(86%; p<0.01), MC3 (85%; p<0.01), MC4 (85%; p<0.01), MC5 (81%; p<0.01), femur (84%; p<0.01), tibia (90%; p<0.01), the second metatarsal (MT2) (84%; p<0.05), MT3 (83%; p<0.01), MT4 (85%; p<0.05), and MT5 (83%; p<0.01). RV-treated MPS VII dogs had lengths that were near-normal for all of these bones (not significant vs. normal), and were significantly longer than in untreated MPS VII dogs for the ulna, all metacarpals that were evaluated, the femur, the tibia, and the second to fourth metatarsals.
Discussion
Skeletal disease is more severe in MPS VII than in MPS I dogs
The first goal of this study was to compare the severity of skeletal disease in MPS I and MPS VII dogs by evaluating skeletal radiographs. MPS VII dogs exhibited more severe shortening of vertebral bodies and long bones, and were more abnormal in subjective evaluation of dysplasia, articular erosions, subluxation, and effusions. There are two possible explanations for why bones are less severely affected in MPS I than in MPS VII dogs: 1) the mutation in MPS I dogs could be relatively attenuated and, therefore, analogous to that in a Hurler/Scheie or a Scheie patient; or 2) the accumulation of chondroitin sulfate in cartilage and other sites in MPS VII but not MPS I may be particularly important for skeletal disease.
It is possible that skeletal disease is less severe in the MPS I dog model because the mutation allows some residual enzyme activity to remain. MPS I dogs have a 5′ splice site mutation in intron 1 [33], and liver extracts from untreated MPS I dogs had some activity against the artificial substrate 4-methylumbelliferyl α-L-idopyranosiduronic acid in vitro, as the fluorescence of samples increased with time during a 3-hour incubation (Ping Wang, MEH, KPP, unpublished data), although the activity was low. The enzyme activity was calculated to be 1.1 U/mg (5% of the value in normal dogs [30]), although it is possible that another enzyme could exhibit some activity against the artificial substrate but not degrade the endogenous substrate. Liver from MPS I mice with an insertion into exon 6 of the 14-exon gene had 0.01 U/mg (0.2% of normal), and liver from MPS I cats with a 3 nt deletion in the coding sequence had 0.6 U/mg (2.4% normal) [34]. Since MPS I dogs and cats had >60-fold as much enzyme as did knockout MPS I mice, this raises the possibility that there may be some residual enzyme activity in the large animal models. Although these MPS I dogs do not have detectable IDUA protein on immunoblot [33], it is possible that protein and correctly-spliced RNA are present at low levels. Our attempts to amplify IDUA RNA using several different forwards exon 1 and reverse exon 2 primers were not successful in normal dogs (M. Tittiger, KPP, unpublished data), making it difficult to assess this possibility. The dog model of MPS VII has a missense R166H mutation and GUSB activity is <1% of normal [35]. Thus, skeletal disease may be less severe in MPS I dogs than in MPS VII dogs because MPS I dogs maintain a low level of enzyme activity.
Alternatively, MPS VII may result in more severe skeletal disease than MPS I because chondroitin sulfate accumulates in the former but not the latter model. Chondroitin sulfate contains β-glucuronic acid and, therefore, is metabolized by GUSB, while it does not contain iduronic acid and, therefore, is not metabolized by IDUA. Chondroitin sulfate is the major GAG in articular cartilage and in the growth plate where bone elongation occurs [36–37]. In contrast, levels of dermatan sulfate and heparan sulfate, which are metabolized by both GUSB and IDUA, are very low in the cartilage and growth plates [38–40]. Chondroitin sulfate accumulates in the urine of MPS VII dogs [16] and in the growth plate of MPS VII mice but not MPS I mice (Jason Metcalf, KPP, unpublished data). Indeed, MPS VII mice that have <1% of normal GUSB activity [41–42] have bone lengths that are extremely short at ~80% of normal [18–19], while MPS I mice with a knock-out mutation that results in <0.2% of normal IDUA activity had long bone lengths that are almost normal at >95% of normal [11, 12, 43]. Evaluation of skeletal disease in other murine MPS models supports the hypothesis that accumulation of chondroitin sulfate and/or keratan sulfate are important for bone disease. For example, skeletal disease was very severe in MPS VI mice with knock-out of N-acetylgalactosamine 4-sulfatase [44], which is important for degradation of chondroitin 4-sulfate. Conversely, skeletal disease was mild or absent in MPS II mice [45] with deficiency of iduronate sulfatase, which is important for degradation of the sulfated iduronic acid-containing dermatan and heparan sulfate. Skeletal disease was also mild in MPS IIIA [46] and MPS IIIB [47] mice with deficiency of heparan N-sulfatase and α-N-acetyl-glucosaminidase, respectively, both of which are important for degradation of heparan sulfate. Interestingly although skeletal disease is severe in human MPS IVA patients that cannot degrade chondroitin 6-sulfate or keratan sulfate due to deficiency of N-acetylgalactosamine 6-sulfatase, MPS IVA mice have bones that are nearly normal in length [48]; the lack of skeletal disease in MPS IVA mice was attributed to chondroitin 6-sulfate being <2% of the total chondroitin sulfate in murine cartilage [49]. Thus, data from other mouse models are consistent with the hypothesis that accumulation of chondroitin sulfate is associated with long bone shortening. It is unclear whether chondroitin sulfate plays a specific role in activating signal transduction pathways, or if other GAGs have the potential to affect bones, but simply do not accumulate in the cartilage and growth plates.
If indeed the reduced severity of skeletal disease in MPS I dogs as compared with MPS VII dogs is due to the specific GAGs that accumulate in each model, it is unclear as to why skeletal disease appears to be more severe in human MPS I-Hurler patients than in MPS I dogs and mice. This could reflect differences in the amounts of dermatan and/or heparan sulfate that accumulate in cartilage and growth plates in humans as compared with dogs and mice, or could reflect the fact that humans require more time to develop and GAGs could accumulate to greater levels during this period. Although bone lengths in MPS I dogs were not significantly shorter than in normal littermates, it should be kept in mind that the number of animals evaluated was small, and many bones were consistently shorter in MPS I dogs at 85% to 95% of normal. It is possible that analysis of more animals will identify significant differences between the groups.
Neonatal gene therapy reduces many manifestations of skeletal disease in dogs
A second goal of this study was to evaluate the long-term effects of neonatal gene therapy on the skeletal disease in MPS I and MPS VII dogs. Evaluation of the effect of gene therapy on skeletal disease in MPS I dogs was complicated by the mildness of skeletal disease in untreated MPS I dogs, the relatively short period (at most 2 years) of evaluation, and the small numbers of animals that were evaluated. In general, gene therapy probably reduced but did not eliminate many aspects of skeletal disease in MPS I dogs, but evaluation of more animals will be required to achieve significance for most parameters. In the MPS VII model, gene therapy clearly increased bone lengths and improved many parameters of skeletal disease over those observed in untreated MPS VII dogs, but bones remained shorter than in normal dogs and some parameters did not improve. The cervical spine was difficult to correct with gene therapy in both models, and remains a site where substantial morbidity may occur. In general, bones were similar at 7 years in RV-treated MPS VII dogs to those found at 1 year in RV-treated MPS VII dogs, but articular erosions tended to worsen.
Although this study does not evaluate the pathogenesis of bone and joint disease, previous studies suggested that cartilage and synovium upregulated expression of MMPs in MPS. Indeed, our laboratory has recently demonstrated that MMP and cathepsin activities are markedly increased in ligaments, articular cartilage, and synovium of MPS VII dogs as compared with normal dogs, and that values in MPS VII dogs are greater than in MPS I dogs (X. Ma, MEH, and KPP, unpublished data). These data are consistent with articular erosions and subluxation of joints being more severe in MPS VII than in MPS I dogs. The role that these destructive proteases plays in bone and joint disease in MPS I and MPS VII dogs is currently being further evaluated. The improvement in skeletal disease with RV therapy could reflect diffusion of enzyme from blood, bone marrow, or blood cells into these connective tissues. Indeed, we previously demonstrated that RV-treated MPS VII dogs had GUSB activity that was 60% of normal in synovial fluid [17].
Implications
This study demonstrates that neonatal gene therapy with an RV to MPS VII dogs can increase bone lengths, reduce many qualitative abnormalities of bone and joint disease, and enable animals to ambulate at 7 years. Nevertheless, skeletal disease was not completely corrected, and disease in the cervical spine remained severe. Achieving higher serum activity may not be effective, as the skeletal disease in M1287 with 18,039 U/ml of serum GUSB activity (see supplemental figures) was similar to that in RV-treated animals with much lower GUSB activity. It is likely that the enzyme does not diffuse well into the avascular cartilage, and that it will be difficult to fully correct skeletal disease with gene therapy unless it can be performed earlier (i.e. in utero) and/or result in transduction of chondrocytes. An important implication of this study is that skeletal disease in MPS I is less severe than in MPS VII in dogs, although it remains possible that this reflects a milder mutation in the MPS I dog than in the MPS VII dog. If indeed skeletal disease is intrinsically less severe in MPS I than in MPS VII, this may allow gene therapy to more effective in MPS I, as skeletal disease is relatively difficult to treat.
Supplementary Material
Acknowledgments
This work was supported by the Ryan Foundation, the National MPS Society, and the National Institutes of Health (DK66448 awarded to KPP, DK54481 awarded to MEH, and RR02512 awarded to MEH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neufeld EF, Muenzer J. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill; 2001. pp. 3421–3452. [Google Scholar]
- 2.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 3.Masterson EL, Murphy PG, O’Meara A, Moore DP, Dowling FE, Fogarty EE. Hip dysplasia in Hurler’s syndrome: orthopedic management after bone marrow transplantation. J Ped Ortho. 1996;16:731–733. doi: 10.1097/00004694-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson RE, Howell RR, McKusick VA, Suskind R, Hanson JW, Elliott DE, Neufeld EF. The iduronidase-deficient mucopolysaccharidoses: clinical and roentgenographic features. Pediatrics. 1976;57:111–122. [PubMed] [Google Scholar]
- 5.Tandon V, Williamson JB, Cowie RA, Wraith JE. Spinal problems in mucopolysaccharidosis I (Hurler syndrome) J Bone Joint Surg Br. 1996;78:938–44. doi: 10.1302/0301-620x78b6.1279. [DOI] [PubMed] [Google Scholar]
- 6.Khan SA, Sehat K, Calthorpe D. Cervical cord compression in an elderly patient with Hurler’s syndrome: a case report. Spine. 2003;28:E313–E315. doi: 10.1097/01.BRS.0000084663.17968.3A. [DOI] [PubMed] [Google Scholar]
- 7.Bill CB, Rose JS, Godmilow L, Sklower S, Willner J, Hirschhorn K. Spastic quadriparesis due to C1 C2 subluxation in Hurler Syndrome. J Pediatr. 1978;92:441–443. doi: 10.1016/s0022-3476(78)80442-9. [DOI] [PubMed] [Google Scholar]
- 8.Field RE, Buchanan JAF, Copplemans MGJ, Aichroth PM. Bone-marrow transplantation in Hurler’s syndrome. J Bone Joint Surg Br. 1994;76:975–981. [PubMed] [Google Scholar]
- 9.Shull RM, Walker MA. Radiographic findings in a canine model of mucopolysaccharidosis I. Invest Radiol. 1988;23:124–30. doi: 10.1097/00004424-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Breider MA, Shull RM, Constantopoulos G. Long-term effects of bone marrow transplantation in dogs with mucopolysaccharidosis I. Amer J Path. 1989;134:677–692. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Xu L, Hennig AK, Kovacs A, Fu A, Chung S, Lee D, Wang B, Herati RS, Mosinger Ogilvie J, Cai SR, Parker Ponder K. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, Toone J, Jirik FR. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6:503–11. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- 13.Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: Report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Ped. 1973;82:249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- 14.Dickerman RD, Colle KO, Bruno CA, Jr, Schneider SJ. Craniovertebral instability with spinal cord compression in a 17-month-old boy with Sly syndrome (mucopolysaccharidosis type VII): a surgical dilemma. Spine. 2004;29:E92–E94. doi: 10.1097/01.brs.0000112074.48566.fa. [DOI] [PubMed] [Google Scholar]
- 15.Pizzutillo PD, Osterkamp JA, Scott CI, Lee MS. Atlantoaxial instability in mucopolysaccharidosis type VII. J Ped Ortho. 1989;9:76–78. doi: 10.1097/01241398-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Ponder KP, Melniczek JR, Xu L, Weil MA, O’Malley TM, O’Donnell PA, Knox VW, Aguirre GD, Mazrier H, Ellinwood NM, Sleeper M, Maguire AM, Volk SW, Mango RL, Zweigle J, Wolfe JH, Haskins ME. Therapeutic neonatal gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA. 2002;99:13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sands MS, Vogler C, Kyle JW, Grubb JH, Levy B, Galvin N, Sly WS, Birkenmeier EH. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mango RL, Xu L, Sands MS, Vogler C, Seiler G, Schwarz T, Haskins ME, Ponder KP. Neonatal retroviral vector-mediated hepatic gene therapy reduces bone, joint, and cartilage disease in mucopolysaccharidosis VII mice and dogs. Mol Genet Metab. 2004;82:4–19. doi: 10.1016/j.ymgme.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Russell C, Hendson G, Jevon G, Matlock T, Yu J, Aklujkar M, Ng K-Y, Clarke LA. Murine MPS I: insights into the pathogenesis of Hurler syndrome. Clin Genet. 1998;53:349–361. doi: 10.1111/j.1399-0004.1998.tb02745.x. [DOI] [PubMed] [Google Scholar]
- 21.Simonaro CM, D’Angelo M, Haskins ME, Schuchman EH. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Ped Res. 2005;57:701–7. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 22.Simonaro CM, D’Angelo M, He X, Eliyahu E, Shtraizent N, Haskins ME, Schuchman EH. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172:112–22. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staba SL, Escolar ML, Poe M, Kim Y, Martin PL, Szabolcs P, Allison-Thacker J, Wood W, Wenger DA, Rubinstein P, Hopwood JJ, Krivit W, Kurtzberg J, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–9. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 24.Shull RM, Kakkis ED, McEntee MF, Kania SA, Jonas AJ, Neufeld EF. Enzyme replacement in a canine model of Hurler syndrome. Proc Natl Acad Sci USA. 1994;91:12937–12941. doi: 10.1073/pnas.91.26.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakkis ED, Muenzer J, Tiller GE, Waber L, Belmont J, Passage M, Izykowski B, Phillips J, Doroshow R, Walot I, Hoft R, Neufeld EF. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–8. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Kato K, Sukegawa K, Tomatsu S, Fukuda S, Emura S, Kojima S, Matsuyama T, Sly WS, Kondo N, Orii T. Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplantation. 1998;21:629–634. doi: 10.1038/sj.bmt.1701141. [DOI] [PubMed] [Google Scholar]
- 27.Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N, Sly WS, Birkenmeier E. Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest. 1993;68:676–686. [PubMed] [Google Scholar]
- 28.Sifuentes M, Doroshow R, Hoft R, Mason G, Walot I, Diament M, Okazaki S, Huff K, Cox GF, Swiedler SJ, Kakkis ED. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–80. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Haskins ME, Melniczek JR, Gao C, Weil MA, O’Malley TM, O’Donnell PA, Mazrier H, Ellinwood NM, Zweigle J, Wolfe JH, Ponder KP. Transduction of hepatocytes after neonatal delivery of a Moloney murine leukemia virus based retroviral vector results in long-term expression of beta-glucuronidase in mucopolysaccharidosis VII dogs. Mol Ther. 2002;5:141–53. doi: 10.1006/mthe.2002.0527. [DOI] [PubMed] [Google Scholar]
- 30.Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O’donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins ME, Ponder KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–31. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 31.Wang B, O’Malley TM, Xu L, Vite C, Wang P, O’Donnell PA, Ellinwood NM, Haskins ME, Ponder KP. Expression in blood cells may contribute to biochemical and pathological improvements after neonatal intravenous gene therapy for mucopolysaccharidosis VII in dogs. Mol Genet Metab. 2006;87:8–21. doi: 10.1016/j.ymgme.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Swischuk LE. The beaked, notched, or hooked vertebra: its significance in infants and young children. Radiology. 1970;95:661–664. doi: 10.1148/95.3.661. [DOI] [PubMed] [Google Scholar]
- 33.Menon KP, Tieu PT, Neufeld EF. Architecture of the canine IDUA gene and mutation underlying canine mucopolysaccharidosis I. Genomics. 1992;14:763–68. doi: 10.1016/s0888-7543(05)80182-x. [DOI] [PubMed] [Google Scholar]
- 34.He X, Li CM, Simonaro CM, Wan Q, Haskins ME, Desnick RJ, Schuchman EH. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab. 1999;67:106–12. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 35.Ray J, Bouvet A, DeSanto C, Fyfe JC, Xu D, Wolfe JH, Aguirre GD, Patterson DF, Haskins ME, Henthorn PS. Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics. 1998;48:248–53. doi: 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- 36.Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR. Isolation and identification of the major heparan sulfate proteoglycans in the developing bovine rib growth plate. J Biol Chem. 2002;277:19461–19469. doi: 10.1074/jbc.M200786200. [DOI] [PubMed] [Google Scholar]
- 37.Byers A, Caterson B, Hopwood JJ, Foster BK. Immunolocation analysis of glycosaminoglycans in the human growth plate. J Histochem Cytochem. 1992;40:275–282. doi: 10.1177/40.2.1372629. [DOI] [PubMed] [Google Scholar]
- 38.Hansen AL, Foster BK, Gibson GJ, Binns GF, Wiebkin OW, Hopwood JJ. Growth-plate chondrocyte cultures for reimplantation into growth-plate defects in sheep. Characterization of cultures. Clin Orthop Relat Res. 1990;256:286–298. [PubMed] [Google Scholar]
- 39.Poole AR, Webber C, Pidoux I, Choi H, Rosenberg LC. Localization of a dermatan sulfate proteoglycan (DS-PGII) in cartilage and the presence of an immunologically related species in other tissues. J Histochem Cytochem. 1986;34:619–625. doi: 10.1177/34.5.3701029. [DOI] [PubMed] [Google Scholar]
- 40.Nakano T, Sim JS. A study of the chemical composition of the proximal tibial articular cartilage and growth plate of broiler chickens. Poult Sci. 1995;74:538–50. doi: 10.3382/ps.0740538. [DOI] [PubMed] [Google Scholar]
- 41.Sands MS, Birkenmeier EH. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A. 1993;90:6567–71. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birkenmeier EH, Davisson MT, Beamer WG, Ganschow RE, Vogler CA, Gwynn B, Lyford KA, Maltais LM, Wawrzyniak CJ. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989;83:1258–66. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902–7. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evers M, Saftig P, Schmidt P, Hafner A, McLoghlin DB, Schmahl W, Hess B, von Figura K, Peters C. Targeted disruption of the arylsulfatase B gene results in mice resembling the phenotype of mucopolysaccharidosis VI. Proc Natl Acad Sci U S A. 1996;93(16):8214–9. doi: 10.1073/pnas.93.16.8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia AR, Pan J, Lamsa JC, Muenzer J. The characterization of a murine model of mucopolysaccharidosis II (Hunter syndrome) J Inherit Metab Dis. 2007;30:924–34. doi: 10.1007/s10545-007-0641-8. [DOI] [PubMed] [Google Scholar]
- 46.Bhaumik M, Mulle VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, Wurzelmann S, Finamore P, Hopwood JJ, Walkley SU, Stanley P. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology. 1999;9:1389–1396. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- 47.Li HH, Yu WH, Rozengurt N, Zhao HZ, Lyons KM, Anagnostaras S, Fanselow MS, Suzuki K, Vanier MT, Neufeld EF. Mouse model of Sanfilippo syndrome type B produced by targeted disruption of the gene encoding alpha-N-acetylglucosaminidase. Proc Natl Acad Sci U S A. 1999;96:14505–10. doi: 10.1073/pnas.96.25.14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomatsu S, Vogler C, Montano AM, Gutierrez M, Oikawa H, Dung VC, Orii T, Noguchi A, Sly WS. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol Genet Metab. 2007;91:251–8. doi: 10.1016/j.ymgme.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Mourao PA, Dietrich CP. Chondroitin sulfates of the epiphysial cartilages of different mammals. Comp Biochem Physiol B. 1979;62:115–117. doi: 10.1016/0305-0491(79)90023-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.