Abstract
Mast cells are innate immune cells that function as regulatory or effector cells and serve to amplify adaptive immunity. These cells also function in adaptive immunity primarily through cell surface Fc receptors that bind immunoglobulin antibodies. The dysregulation of their adaptive role makes them central players in allergy and asthma. Upon encountering an allergen (antigen), which is recognized by immunoglobulin E (IgE) antibodies bound to the high affinity IgE receptor (FcεRI) expressed on their cell surface, mast cells secrete both preformed and newly synthesized mediators of the allergic response. Blocking of these responses is an objective in therapeutic intervention of allergic diseases. Thus, understanding the mechanisms by which antigens elicit mast cell activation (via FcεRI) holds promise towards identifying therapeutic targets. Here we review the most recent advances in understanding antigen-dependent mast cell activation. Specifically, we focus on the requirements for FcεRI activation; the regulation of calcium responses; co-stimulatory signals in FcεRI-mediated mast cell activation and function; and how genetics influences mast cell signaling and responses. These recent discoveries open new avenues of investigation with therapeutic potential.
Keywords: Calcium, FcεRI, IgE, Kinase, Mast Cell
I. Introduction
Mast cell activation requires coordinated events that are able to discriminate how the cell responds when encountering a given allergen challenge (Blank and Rivera, 2004;Gilfillan and Tkaczyk, 2006)]. These events begin with the allergen-dependent aggregation of the IgE antibody-occupied high affinity receptor for IgE (FcεRI) and are propagated inside the cell to assemble a highly sophisticated network of signaling molecules that control the cells response to the particular challenge (Gilfillan and Tkaczyk, 2006). The assembled signaling network may differ depending on the type and strength of a stimulus (Gonzalez-Espinosa et al., 2003). Regulation of network assembly exists at multiple levels and employs proteins and lipids that may have positive, negative, or dual roles in coordinating and regulating the signaling response (Rivera and Gilfillan, 2006;Rivera and Olivera, 2007). This context or compartment specific function underlies many of the mysteries remaining to be uncovered on how signals are transduced.
In this review we provide a very brief and general review of the FcεRI signaling to acquaint the reader with the major players. More detailed reviews on this topic are available (Gilfillan and Tkaczyk, 2006;Kraft and Kinet, 2007;Rivera and Gilfillan, 2006). The major focus of this review is on recent advances in understanding the molecular events elicited by engagement of FcεRI. In particular, we focus on new information regarding FcεRI signaling and responses; the vital discovery of key components of the calcium apparatus in mast cells and other immune cells; the identification of important co-stimulatory components of FcεRI signaling; and the influence of genetics on mast cell responsiveness. The rapid advances of the last several years reveal increasing molecular complexity in how mast cells are activated upon engagement of FcεRI. However, the findings also reveal new areas of investigation with therapeutic potential in disease.
II. A general outline of FcεRI signaling
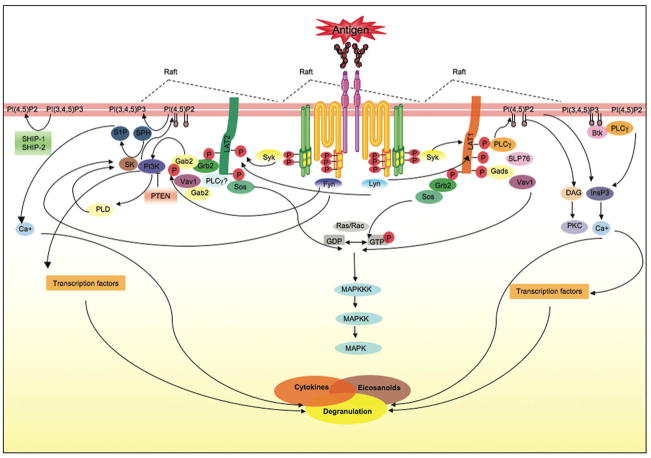
The FcεRI exists in two forms. It can be expressed as a trimer or tetramer(see Fig. 1) comprised of an IgE-binding α chain, a membrane tetraspanning β chain that is absent in the trimeric receptor, and a disulfide-linked homodimer of γ chains (Nadler et al., 2000). While the trimeric form can be expressed on a variety of immune cells (such as monocytes, eosinophils, Langerhan cells, etc.) the tetramer is expressed primarily on mast cells and basophils (Blank and Rivera, 2004;Nadleret al., 2000). Both the β and γ chains contain immunoreceptor tyrosine-based activation motifs (ITAMs), which are essential for the signaling competence of immunoreceptors. FcεRI lacks intrinsic tyrosine kinase activity and associates with the non-receptor Src family tyrosine kinase Lyn kinase, (Fig. 1) whose activity is key for phosphorylation of the tyrosine residues in its ITAM motifs through transphosphorylation (Pribluda, Pribluda and Metzger, 1994). A small fraction of Lyn can be found to weakly interact with the FcεRIβ ITAM prior to engagement of this receptor. This interaction is greatly enhanced by FcεRI stimulation as the Lyn SH2 domain binds the phosphorylated Y219 in the FcεRIβ ITAM (Furumoto et al., 2004;On et al., 2004). Efficient phosphorylation of the receptor also requires plasma membrane liquid-ordered phase domains (commonly referred to as lipid rafts; see Fig. 1) (Field, Holowka and Baird, 1997;Sheets, Holowka and Baird, 1999;Young, Holowka and Baird, 2003). These domains are enriched in cholesterol, sphingolipids and other saturated phospho-lipids, as well as with a variety of signaling proteins including Lyn kinase. Another Src family member, Fyn kinase, also appears to associate with the FcεRIβ ITAM (Fig. 1) and appears to be recruited similar to Lyn via SH2 domain-phosphotyrosine interaction (NF and JR, unpublished observation). However, although it does not appear to participate in the phosphorylation of FcεRI, it is required for normal mast cell degranulation and cytokine production (Gomez et al., 2005). The spatial and temporal association of Fyn with the FcεRIβ ITAM remains to be unraveled.
Figure 1. FcεRI signaling in mast cells.
Antigen-clustering of FcεRI through bound antigen-specific IgE initiates mutiple events required for mast cell activation. Clustering of FcεRI drives the coalesence of cholesterol-enriched membrane microdomains (rafts) that contain signaling molecules like the Src family kinase Lyn. Lyn transphosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) of the β and γ subunits of a neighboring FcεRI. This requires that these receptors have an appropriate distance/and or configuration within the antigen-induced receptor clusters. Lyn and Fyn can interact with the phosphorylated β subunits whereas Syk kinase interacts with the γ subunit. Fyn, Lyn, and Syk contribute to the formation of multi-molecular signaling complexes that are coordinated by adaptors, like LAT 1 and 2, Gab2, Grb2, Gads, among others. These signaling complexes provide docking sites for other signaling proteins including PLCγ, SLP76, Vav1, Sos and others. The activity of the molecules in these complexes is coordinated to initiate the production of lipid second messengers that are essential for calcium mobilization and PKC activation leading to degranulation, and the de novo synthesis and secretion of eicosanoids and cytokines. This process is tightly regulated a multiple levels. The signaling complexes provide an environment where a balance of positive signals with negative feedback signals can occur. Lipid enzymes (like PI3K) produce and/or remove lipid messengers (by converting PI(4,5)P2, the substrate of PLCγ, to PI(3,4,5)P3). This production or loss of lipid messengers is important in the targeting, activation and regulation of signaling protein function. Protein kinases can also phosphorylate protein and lipid enzymes modifying their activity. Protein phosphatases (like SHP-1) and lipid phosphatases (like SHIP and PTEN) will dephosphorylate phosphorylated proteins and lipids, respectively, disassembling the signaling complex or inactivating signaling proteins. This is a highly dynamic process that adjusts spatio-temporally to fine-tune mast cell responses.
Phosphorylated ITAMs bind a variety of proteins that are key for signal amplification (Rivera and Gilfillan, 2006). The tyrosine kinase Syk (Fig. 1), an essential kinase for the propagation of signals, is one of these proteins (Benhamou et al., 1993;Kihara and Siraganian, 1994). Other proteins thought to interact include the tyrosine phosphatases SHP-1 and 2 (Swieter, Berenstein and Siraganian, 1995). The temporal order of these interactions is not clear. Syk binds primarily to the phosphorylated γ chain ITAM, a step necessary for its activation. While weak interaction of Syk with FcεRIβ phospho-ITAM peptides can be observed in vitro there is no evidence of this interaction in vivo. Once activated Syk is essential in amplification of mast cell signaling and in driving normal mast cell effector responses (Costello et al., 1996;Zhang, Kimura and Siraganian, 1998). Its central role has made this kinase a prime therapeutic target in diseases where its activity is fundamental in immune responses. Syk function in mast cells and other immune cells has been recently reviewed (Berton, Mocsai and Lowell, 2005;Siraganian et al., 2002) so it will not be covered in detail herein. However, its important to note that Syk phosphorylates many signaling proteins, but key to its amplification function is the phosphorylation of the adaptor molecules required in the assembly of membrane localized signaling networks.
Among these adaptors, the linkers for activation of T cells (LAT)1 and 2 (2 was formerly known as NTAL or LAB) and the Grb2-associated binder 2 (Gab2) serve as essential scaffolds in organizing, coordinating, and regulating the generated signals (Fig. 1) (Rivera, 2002;Rivera, 2005;Tkaczyk et al., 2005). Characteristic to adaptor proteins are numerous tyrosine residues that are targets for Syk and other kinases (Rivera, 2005). Once phosphorylated, these adaptors bind a variety of signaling proteins such as lipases (PLC1 and 2), phosphatases (SHIP-1 and-2), protein and lipid kinases (Lyn, Fyn, Btk, PI3K, PIP5K, etc.). Adaptors serve to coordinate and localize signals that lead to the production of a number of lipid second messenger molecules. Some of these messengers like inositol 3,4,5-trisphosphate (IP3), phosphatidylinositol 4, 5-bisphosphate (PIP2), phosphatidylinositol 3, 4, 5-trisphosphate (PIP3), and sphingosine-1-phosphate (S1P) are implicated in regulating and/or eliciting calcium mobilization in mast cells (Fig. 1) (Rivera and Olivera, 2007). These lipid second messengers, as well as others like diacylgycerol (DAG), play key roles in regulating the membrane recruitment and/or activity of many proteins (such as PLC’s, PKC’s, and others) as well as serve as ligands interacting with intra-and extra-cellular receptors that elicit provide signals leading to effector responses.
It is the cooperativity of proteins and lipids that in mast cells, like in many other immune cells, is key in eliciting full functional responses. For example, the cooperativity of increased cytosolic calcium with PKC’s has been demonstrated as essential for mast cell degranulation (Blank and Rivera, 2004;Rivera and Beaven, 1997). However phorbol esters alone, which mimic the function of DAG in activating PKC, do not induce mast cell degranulation but instead activate the production of some cytokines. This cooperativity is also supported by the plethora of studies where genetic deletion has led to a partial effect on mast cell signaling and responses. Moreover, redundancy in the role of signaling proteins is common and thus the presence of several isoforms of a protein or the expression of a protein with overlapping function circumvents the genetic defect (Kawakami et al., 2000). For mast cells, this redundancy may be further complicated because of their well-recognized tissue heterogeneity (Galli, 1997b), reflecting differences in gene expression. Thus, it is important to keep in mind that variations of this general view of mast cell FcεRI signaling are likely to exist.
III. Recent advances in FcεRI proximal signaling
A. Spatial organization in FcεRI phosphorylation
i. Spatial requirement for transphosphorylation
It has long been recognized that aggregation of FcεRI (Fig. 1), like for other antigen receptors, is necessary for its activation (Metzger, 1978). This requirement has been proposed to facilitate the transphosphorylation of FcεRI by receptor-associated Lyn kinase (Pribluda, Pribluda and Metzger, 1994). However, direct evidence of the spatial requirements for transphosphorylation was lacking until recently. Studies using trivalent ligands with rigid DNA spacers of varying lengths (16, 26, 36, or 46 bases) have revealed length dependent stimulation of FcεRI phosphorylation (Sil et al., 2007). With average distances ranging from 5 to 13 nm, the findings showed an inverse relationship between length and progressive enhancement of FcεRI phosphorylation and mast cell degranulation. These findings revealed structural constraints in FcεRI phosphorylation and demonstrate that the appropriate spatial organization of an FcεRI cluster is important for its phopshorylation. Given that the ligand with the longest length (~13 nm) is likely to prevent direct contact of aggregated receptor complexes, the findings demonstrate that the most effective ligands for FcεRI phosphorylation are those that allow proximity of aggregated FcεRI complexes thus supporting a transphosphorylation model. However, it should be noted that PLCγ phosphorylation and Ca2+ release from intracellular stores was not inversely correlated with ligand length suggesting that the threshold for these responses might be met under conditions that lead to minimal FcεRI phosphorylation.
ii. New insights on liquid-ordered domain (lipid raft) involvement in FcεRI phosphorylation and function
Plasma membrane-localized cholesterol-enriched microdomains (lipid rafts) are well known participants in cellular signaling and other cell functions (Simons and Toomre, 2000). Lipid rafts have been implicated as initiators of FcεRI phosphorylation (Sheets, Holowka and Baird, 1999), as domains that may facilitate exocytosis (Pombo, Rivera and Blank, 2003;Puri and Roche, 2006), and as essential for Kit-mediated survival and proliferation (Jahn et al., 2007). Considerable evidence supports the role of lipid rafts in FcεRI phosphorylation. Nonetheless, there is still substantial uncertainty as to whether lipid rafts are necessary for initiating FcεRI phosphorylation or if they are simply participants in this process, due to the concentration of Lyn kinase in these domains and the recruitment of FcεRI into these domains upon its engagement (Kovarova et al., 2001;Rivera et al., 2002;Sheets, Holowka and Baird, 1999). A recent study using a mouse model of Smith-Lemli-Opitz disease, a disease of cholesterol-deficiency due to a mutation in 7-dehyrochloesterol reductase, revealed that partial loss of cholesterol from lipid rafts led to considerable loss (>80%) of Lyn kinase from these domains and partly diminished (40%) FcεRI phosphorylation with the most significant effect seen at late times post-receptor stimulation (Kovarova et al., 2006). This suggests that Lyn in lipid rafts may participate in sustaining FcεRI phosphorylation and argues that initiation of receptor phosphorylation may not require the Lyn found in lipid raft domains. However, this may be an overly simplistic view since the methods used to biochemically define a lipid raft are unlikely to detect small microdomains.
Studies using high resolution electron microscopy (Wilson, Pfeiffer and Oliver, 2000;Wilson et al., 2001) partly address this issue. These studies demonstrated the compartmentation of FcεRI and Lyn in small electron dense microdomains prior to FcεRI engagement. Aggregation of FcεRI enhances the size of these microdomains increasing the numbers of receptors and Lyn within. The findings also demonstrated that the adaptor molecule LAT is found in microdomains that are distinct from those that contain FcεRI. Since biochemical and genetic evidence ascribes the residence of molecules like Lyn and LAT as lipid raft microdomain residents, this would suggest that there are distinct (heterogeneous) cholesterol-enriched microdomains on the plasma membrane. This view is further supported by studies on the distribution of endogenous and transfected Thy 1 (CD90) isoforms in the plasma membrane of RBL-2H3 cells (Heneberg et al., 2006). Thy-1 is a GPI-anchored membrane protein that distributes into cholesterol-enriched membranes and its aggregation can cause cellular signaling and responses. Using primarily biophysical approaches the authors studied Thy-1 isoform distribution on the membrane and its cross-talk with FcεRI. The two Thy-1 isoforms studied were found in small autonomous clusters in resting cells whereas aggregation of one isoform led to significant co-localization of the two isoforms as detected by electron microscopic and flouresence resonance energy transfer (FRET) analysis. The adaptor proteins LAT 1 and LAT 2 were also found in autonomous domains with occasional co-localization of Thy-1. This co-localization was greatly enhanced by Thy-1 aggregation. FRET also detected Thy-1 cross-talk with FcεRI upon the latters enagement. Thus, the findings revealed the presence of distinct microdomains in resting cells that are able to coalesce upon aggregation of constituent proteins or of FcεRI.
The use of two-photon fluorescence lifetime imaging microscopy and fluorescence polarization anisotropy imaging has now allowed the real time monitoring of membrane structure and organization. Studies in which the cholesterol-rich membrane domains are labeled with the lipid analog dil-C18, and FcεRI is labeled with Alexa 488-IgE, demonstrated that upon FcεRI aggregation dil-C18-labeled membrane domains coalesce and redistribute to membrane patches containing FcεRI (Davey et al., 2007). This increases the fluorescence lifetime of both dil-C18 and Alexa 488-IgE, an increase in dil-C18 fluoresence lifetime was previously shown to reflect increased lipid order. In addition, upon FcεRI engagement FRET can be demonstrated to occur between the Alexa 488-labeled receptor and dil-C18-labeled domains (Davey et al., 2008), suggesting the reordering of the cholesterol-enriched membrane and the aggregated FcεRI to allow FRET signals. Thus, membrane nanostructure appears to be altered and the rotational diffusion of components within these domains is decreased, suggesting increased interactions and ordered structure.
Collectively, these findings reveal important insights on the role of “lipid rafts” in FcεRI signaling. The studies begin to define these cholesterol-rich membrane microdomains as a lipid environment that is dynamic, small, heterogeneous in protein content, and that is likely to contain limited numbers of proteins prior to cell stimulation. Thus, a unifying hypothesis for the differing views on the role of lipid rafts in FcεRI phosphorylation is emerging. From these and earlier studies one might postulate that the FcεRI may be found, at any given time, within these dynamic cholesterol-rich microdomains, an environment that enhances its proximity to Lyn kinase. The aggregation of FcεRI could induce transphosphorylation with a neighboring receptor as it might bring together receptors with or without Lyn kinase. Coalescence of these dynamic cholesterol-rich domains (some of which might include Lyn) would stabilize or increase this transphosphorylation and cause assembly of a stable signaling complex (Fig. 1). This view of a need for stable clustering of FcεRI to form stable signaling complexes is supported by our finding that a antigen of low affinity fails to assemble a stable signaling complex even under conditions where it is able to elicit FcεRI phosphorylation to a similar extent as a high affinity antigen (RS and JR, unpublished observation). These findings are in agreement with the view that FcεRI aggregation induces membrane changes that allow coalesence of proteins and lipids required for efficient propagation of signals.
B. Lyn kinase as a negative regulator
As detailed above, the positive role of Lyn kinase in mast cell activation through its control of FcεRI phosphorylation is clearly recognized. However, Lyn also plays an important role as a negative regulator of mast cell effector responses (Hernandez-Hansen et al., 2004;Odom et al., 2004) (Xiao et al., 2005). This was evident from in vivo studies where Lyn-deficient mice were found to develop atopic-like allergic disease and mast cells derived from these mice were hyperresponsive relative to cells from wild type mice (Odomet al., 2004). One possible cause for this hyperresponsive phenotype is that Lyn is required for phosphorylation of the lipid raft-localized Csk-binding protein (Cbp) and thus for membrane targeting of a regulatory kinase, C-terminal Src kinase (Csk). Csk negatively regulates Src family kinases by phosphorylation of a negative regulatory tyrosine in the C-terminus that causes intra-molecular interaction with its own SH2 domain. We now know that this regulatory step is dependent on Lyn localization to lipid rafts and is required to downregulate Fyn kinase activity (Kovarovaet al., 2006). This negative role of Lyn also appears to be independent of its association with FcεRI. Another possible mechanism by which Lyn exerts negative control on mast cell effector responses is through the inositol phosphatase, SHIP. Lyn-deficiency causes the loss of SHIP activity (Hernandez-Hansenet al., 2004). This inositol phosphatase regulates the intracellular levels of PIP3 upon cell activation and thus loss of its activity increases the concentration of intracellular PIP3. As mentioned above this key lipid second messenger is important for recruitment and formation of membrane-localized signaling networks and thus increasing its intracellular concentration would likely enhance signaling leading to enhanced cellular responses (this will be further detailed in the next section). Lyn-deficient mast cells were shown to have increased levels of intracellular PIP3 (Odom et al., 2004).
How Lyn kinase plays both a positive and negative role in mast cell activation is not completely clear. However, a recent study (Xiao et al., 2005) sheds light on this apparent paradox. This study demonstrates that low or high strength stimulation of wild type and Lyn-deficient mast cells distinguishes the positive versus negative role of Lyn, respectively. Thus, the findings showed that Lyn activity is required for mast cell degranulation and cytokine production when encountering a low strength stimulus whereas under high strength stimulation enhanced degranulation and cytokine production was seen when Lyn was absent (Xiao et al., 2005). Given that the expression of FcεRIβ (but not the ITAM-tyrosine mutated FcεRIβ) in Lyn and FcεRIβ double-deficient mast cells gave a similar profile of functional responses to high strength stimulation in Lyn-deficient cells, the authors conclude that negative regulation by Lyn is mediated through its interaction with the FcεRIβ. Evidence that much of Lyn’s negative role is predominantly mediated by the pool of Lyn in lipid rafts (Kovarova et al., 2006) suggests that this interaction with FcεRIβ takes place upon coalesence of these domains. Thus, this fits well with the concept that a strong stimulus, which leads to extensive coalesence of microdomains (Davey et al., 2007a;Daveyet al., 2007b), would serve to promote the negative role of Lyn kinase in order to control the extent of the inflammatory response.
An important caveat in defining a negative role for Lyn kinase has been the exclusive use of Lyn-deficient mice, as some of the observed effects might be attributed to the possible importance of Lyn in development. However, several new models (Hong et al., 2007;Kitaura et al., 2007) have recently emerged that strongly support the concept of Lyn as a negative regulator of mast cell responses. EL mice have been used as a model of epilepsy with the susceptibility locus mapped to chromosomes 2 and 9. ASK mice are spontaneous variants derived from the EL strain that are epilepsy resistant. ASK mice have been shown to be highly susceptible to anaphylaxis whereas EL are resistant. Studies exploring the mechanism for this susceptibility demonstrate that mast cells from ASK mice exhibited a similar hyperresponsive phenotype as Lyn-deficient mast cells. ASK mast cells showed enhanced cytokine production, although degranulation was not elevated relative to EL mice. Exploration of Lyn function in mast cells from EL and ASK mice showed reduced Lyn kinase activity in ASK mast cells that was coupled to reduced Cbp phosphorylation and increased Fyn and Src kinase activities (Kitaura et al., 2007). This of course differs from Lyn-deficiency in that Lyn protein is still present in ASK cells, Src kinase activity was increased in ASK mast cells but normal in Lyn-deficient cells, and degranulation was not enhanced in ASK mast cells. Regardless, the consequence of Lyn inactivity is highly similar to the effects of Lyn-deficiency on mast cell responsiveness. Moreover, these findings clearly confirm the negative role of Lyn in cytokine production. Additional evidence is also afforded by studies on Hck-deficient mast cells (Hong et al., 2007). In this study the findings point to Hck-mediated negative regulatory control on Lyn activation. Thus, in the absence of Hck, Lyn activity is substantially increased suppressing degranulation and cytokine production when cells encounter a strong stimulus. The mechanism for this effect is less clear since Cbp phosphorylation was enhanced and Fyn activity was normal. Nonetheless, Hck appears to dampen the negative function of Lyn kinase. Collectively these studies argue that, under optimal (strong) conditions of stimulation, Lyn kinase plays a dominant role in controlling the extent of mast cell responses. Thus, the concept of therapeutic targeting of Lyn kinase in allergic disease (Metzger, 1999) should be reconsidered.
C. Fyn kinase and PIP3 in mast cell responsiveness
In contrast to Lyn, low strength and high strength stimulation of mast cells demonstrate that Fyn functions to positively regulate mast cell responsiveness through its role in regulating the activation of phosphatidylinositol 3-OH kinase (PI3K) through the adaptor Gab2 (Gu et al., 2001;Parravicini et al., 2002) and thus its product, PIP3 (Fig. 1). PIP3 is essential for mast cell responses as reflected by the inhibitory effect of the PI3K inhibitors or genetic manipulation of PI3K activity, on mast cell degranulation and cytokine production (Barker et al., 1995;Parravicini et al., 2002);(Ali et al., 2004). As mentioned above, Lyn-deficient mast cells have high levels of PIP3. In contrast, Fyn-deficient mast cells show a substantial reduction in PIP3, which correlates with the reduced degranulation response of these cells.
The importance of intracellular PIP3 levels is further highlighted by studies performed on mast cells derived from the lipid phosphatase SHIP-1-deficient mouse or where SHIP-2 or another lipid phosphatase PTEN were downregulated by silencing RNA strategies in mouse or human mast cells, respectively (Furumoto et al., 2006;Huber et al., 1998a;Leung and Bolland, 2007). In all cases decreased expression of these phosphatases led to increased PIP3 levels and enhanced mast cell responses. SHIP-1 and SHIP-2 regulate the production of PIP3 by dephosphorylating the 5′ position to generate PI (3,4)-P2 (Fig. 1). PTEN directly opposes PI3K function as it dephosphorylates the 3′ position of PIP3, yielding PI (4, 5)-P2 (Fig. 1). The increased degranulation and cytokine production seen in SHIP-1-and PTEN-deficient mast cells was associated with increased FcεRI-dependent calcium mobilization (Huber et al., 1998b;Huber et al., 2002). In contrast, SHIP-2-deficient mast cells showed normal calcium levels relative to wild type cells but had enhanced mast cell degranulation and cytokine production (Leung and Bolland, 2007). This enhanced response was associated with increased microtubule polymerization, which is thought to be important in movement of exocytotic vesicles (Martin-Verdeaux et al., 2003;Nishida et al., 2005), and enhanced Rac 1 activity.
Some additional divergence, which may reflect species differences or distinct roles of PTEN and SHIP, are noteworthy. PTEN-deficiency caused a constitutive phosphorylation of Akt and MAP kinases in HuMC (Furumoto et al., 2006) whereas this was not readily apparent in SHIP-1 or SHIP-2 deficient mast cells (Huber et al., 2002). In all cases, Akt and some of the MAP kinases showed increased phosphorylation upon FcεRI stimulation. Cytokine secretion was constitutive in HuMC as both IL-8 and GM-CSF secretion was observed prior to stimulation and was further enhanced after FcεRI engagement (Furumoto et al., 2006). In contrast, SHIP-1 and SHIP-2-deficient cells seemed to require FcεRI stimulation to elicit an enhanced cytokine production (Huber et al., 2002;Leung and Bolland, 2007). This suggests that in mouse mast cells, while SHIP-1 and-2 also increased PIP3 levels, these levels may not be sufficient to drive a constitutive response in these cells or PTEN function may diverge from SHIP function in this respect. Regardless, the findings promote the view that PTEN is required for control of PI3K in mast cell homeostasis and activation whereas SHIP-1 and SHIP-2 are primarily active when mast cells are stimulated via FcεRI. Thus, it seems reasonable to conclude that the dominant role of Fyn kinase in promoting PI3K activity places it in control of a key pathway for mast cell responsiveness.
IV. Regulation of Calcium Mobilization in Mast Cells
A. The kinases
Fyn, Lyn, and Syk are all contributors to calcium responses in mast cells. It is well known that Lyn plays an important role in propagating calcium responses through its ability to phosphorylate the FcεRIγ ITAM leading to Syk activation (reviewed in (Rivera and Gilfillan, 2006). Once activated Syk phosphorylates multiple proteins (such as the adaptor LAT and phospholipase Cγ) that are involved in the regulation of calcium responses (Rivera, 2002). Thus, both Lyn-and Syk-deficient mast cells showed marked decreases in calcium responses (Costelloet al., 1996;Kawakami et al., 2000;Odomet al., 2004;Zhang et al., 1996). However, it is important to note that while Lyn-deficient mast cells showed a prolonged delay in calcium responses there is a modest increase in intracellular calcium with time. In contrast, Syk-deficient mast cells did not show a calcium response. Thus, it seems that, unlike Lyn, Syk is critical for calcium responses. Consistent with this view, Syk activation occurs in the absence of Lyn although it is delayed and reduced relative to wild type cells.
Recently, we’ve come to appreciate that Fyn kinase also plays a role in the calcium response (RS and JR, unpublished observation). In an early study of Fyn function in mast cells a slightly more transient calcium response was noted (Parravicini et al., 2002). Using a more sensitive fluorometric analysis, Fyn kinase has now been found to play an important role in the regulation of calcium responses. Loss of Fyn did not affect the mobilization of calcium from intracellular stores but instead reduced the influx of calcium into the cell. This loss of calcium influx is accompanied by a considerable defect in mast cell degranulation. The exact mechanism by which Fyn contributes to calcium influx remains to be determined, however, several possible options exist. One possible mechanism is through Fyn-dependent phosphorylation of plasma membrane calcium channels. Some transient receptor potential channel (TRPC) family members are substrates for Src family kinase, like Fyn (Hisatsune et al., 2004;Vazquez et al., 2004) and the recent finding (Ma et al., 2008) in the mast cell line (RBL-2H3) showing that TRPC channels can contribute to mast cell calcium influx makes this a plausible scenario. Another possible mechanism could be through the generation of sphingosine-1-phosphate (S1P) by sphingosine kinase 2 (SphK2). Sphingosine kinases 1 and 2 convert sphingosine (Sph) into S1P (Fig. 1), a highly biologically active molecule that functions as an intracellular lipid second messenger (LSM) as well as a ligand for a family of G protein-coupled receptors (GPCRs) expressed on the cell surface of many cell types. The genetic deletion of SphK2 was found to cause a defect in calcium influx (Olivera et al., 2007) independent of the S1P effects on S1P receptors. Additionally, activation of SphK2 was found to be highly dependent on Fyn kinase, as loss Fyn expression caused a marked defect in SphK2 activation (Olivera et al., 2006). Thus, Fyn kinase might mediate its effect on calcium influx through its role in SphK2 activation. However, what intracellular targets might be modified by SphK2 activity in mast cells are yet to be determined.
B. The adaptors
The lipid raft resident transmembrane adaptor molecules LAT1 and LAT2 are known participants in the regulation of mast cell calcium responses (Draberova et al., 2007;Iwaki et al., 2008;Saitoh et al., 2000). How LAT1 regulates calcium in mast cells has been worked out in great detail (Saitoh et al., 2000;Saitoh et al., 2003). Briefly, once phosphorylated by Syk, LAT1 recruits phospholipase Cγ (PLCγ) leading to its membrane localization and activation (Fig. 1). This interaction is stabilized by cooperative binding of the SH2-containing leukocyte protein of 76 kDa (SLP-76), an adaptor protein that links PLCγ1 with LAT1-bound adaptors called Gads (Houtman, Barda-Saad and Samelson, 2005). PLCγ1 and PLCγ2 produce diacylglycerol (DAG) and IP3 from PI(4,5)P2. IP3 is essential for calcium mobilization from intracellular stores as it binds to receptors in the endoplasmic reticulum (ER) elicting the release of calcium from these stores. The key role of PLC’s is manifest through the requirement of calcium and DAG for the activation of classical PKCs, like PKCβ, whose activity has been demonstrated as essential for mast cell degranulation and cytokine production (Fig. 1) (Nechushtan et al., 2000;Ozawa et al., 1993). Thus, adaptors such as LAT1 play an important role in regulating the calcium response and the activation of PKC through PLCγ binding and activation. As might be imagined the binding and activation of PLCγ must be tightly controlled and one mechanism appears to be through the required cooperativity in protein binding and function by LAT1 (Saitoh et al., 2003). This view of cooperativity in protein binding for LAT1-mediated calcium responses is supported by the markedly reduced calcium responses observed in LAT1-and SLP-76-deficient mast cells as well as tyrosine mutants of these adaptors (Pivniouk et al., 1999;Saitoh et al., 2003;Silverman et al., 2006).
In contrast, the mechanism by which LAT2 regulates calcium responses is less clear. Unlike LAT1, LAT2 does not have the YLVV motif to directly bind PLCγ. Nonetheless, it might associate with PLCγ through binding of Grb2, an adaptor molecule that can bind to both LAT2 and PLCγ (Fig. 1)(Iwaki et al., 2008). There is still considerable debate on the role of LAT2 in mast cells. LAT2 was found to be required for mast cell degranulation in studies silencing LAT2 expression with RNAi (Tkaczyk et al., 2004). In contrast, genetic deletion of LAT2 caused enhanced mast cell degranulation (Volna et al., 2004;Zhu et al., 2004). Similarly, while LAT2 is not expressed in resting T cells, in activated T cells genetic loss of its induced expression resulted in enhanced calcium responses and increased cytokine production (Zhu et al., 2006). Thus, LAT2 appears to have a dual role as a positive and negative regulator of calcium that may selectively manifest given the difference in the employed strategies for these studies. One might propose a possible competition of LAT1 and LAT2 for lipid raft occupancy or for limiting amounts of key signaling proteins as an explanation for dual roles (Rivera, 2005).
Recent studies help to shed additional light on this matter. While previous studies had demonstrated that LAT2 is a target of Lyn activity (Tkaczyk et al., 2004), recent analysis of the phosphorylation of the individual LAT2 tyrosines revealed their differential phosphorylation depending on the stimulus (Iwaki et al., 2008). In vitro experiments have shown that Lyn, Syk, and Kit kinases can phosphorylate overlapping and distinct tyrosine residues in LAT2 (Iwaki et al., 2008). For example, upon phosphorylation of LAT2 by Syk, PLCγ binding could be detected whereas this interaction was not readily detected upon Lyn or Kit phosphorylation of LAT2. The findings showed that the putative Grb2 binding sites at Y193 and Y233 (which are most effectively phosphorylated by Syk) were important for PLCγ association. This suggests that LAT2 may have different roles depending on the stimulus and thus the complete absence of LAT2 (upon genetic deletion) could dominantly manifest an otherwise passive negative role whereas its downregulation (upon RNAi inhibition) may reveal only its positive role as some LAT2 is still available in these cells. This view has recently received additional support from the study of Ca2+ regulation by phosphorylated and unphosphorylated LAT2 in mast cells (Draberova et al., 2007). The findings demonstrate that the overexpression as well as the downregulation (by RNAi) of LAT2 expression in the RBL-2H3 mast cell line causes decreased mast cell degranulation. Interestingly, the overexpression of LAT2 was inhibitory for phosphorylation of FcεRI, Syk, and LAT1 whereas its underexpression did not alter the phosphorylation of these proteins suggesting that the mechanisms by which LAT2 decreased mast cell degranulation when over-or under-expressed was different. Most strikingly, Lyn kinase activity was greatly enhanced when LAT2 was overexpressed. This observation appears at odds with the state of decreased FcεRI and Syk phosphorylation. However, since LAT2 phosphorylation was also greatly enhanced by its overexpression, the findings suggest a possible sequestration of Lyn by LAT2 thus effectively blocking its role in FcεRI phosphorylation. Importantly, the study defines a primary role for LAT2 in regulating calcium influx in a manner that is not dependent on its increased tyrosine phosphorylation. While defects in PLCγ1 phosphorylation and in IP3 production where observed when LAT2 is either over-or under-expressed, the amount of LAT2 expressed showed a direct correlation to calcium influx rather than to efflux of calcium from intracellular stores (Draberova et al., 2007). Thus, the findings put forward an interesting hypothesis; namely, that LAT1 may be essential for intracellular calcium mobilization through its role in the activation of PLCγ and IP3 production whereas LAT2 may principally function to regulate calcium influx. This could be feasible regardless of the observation that PLCγ can associate with LAT2 indirectly, since PLCγ2 was shown to regulate calcium influx independent of its catalytic activity (Patterson et al., 2002;Wang et al., 2000). This of course remains to be directly demonstrated, however, increasing evidence suggests that calcium influx requires its own network of signaling molecules comprised of proteins found in the plasma and endoplasmic reticular membranes that communicate to elicit the inward flow of calcium (Smyth et al., 2006).
B. The Calcium Apparatus
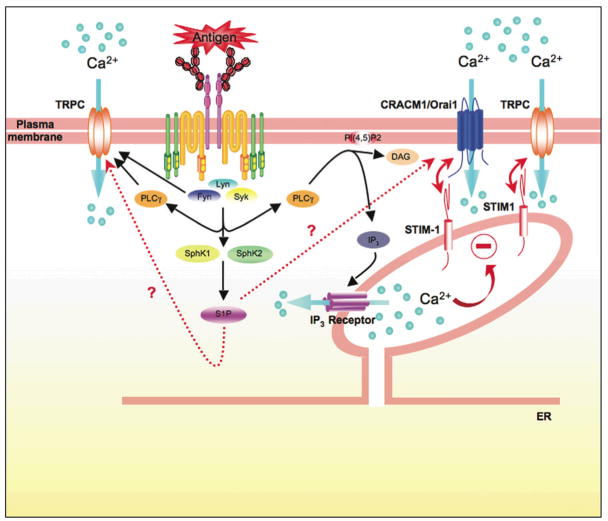
Increases in the intracellular concentration of free Ca2+ is essential for mast cell degranulation and cytokine responses (reviewed in (Blank and Rivera, 2004). Store-operated Ca2+ entry requires the depletion of intracellular calcium stores as the trigger to induce Ca2+ influx across the plasma membrane through store-operated channels (SOC) (Putney, 1986) (Fig. 2). The demonstration by Hoth and Penner of a calcium release-activated calcium (CRAC) channels in mast cells (Hoth and Penner, 1992) more than 15 years ago has led to an extensive effort to identify these channels and their mechanism of activation (Vig and Kinet, 2007). Major advances have recently been made towards identifying components of this apparatus. The discovery of STIM-1 as an ER-localized calcium sensor (Liou et al., 2005;Roos et al., 2005) and the subsequent discovery of Orai1/CRACM1 (Feske et al., 2006;Vig et al., 2006b) as a key component for calcium entry has rapidly advanced our mechanistic understanding of how store operated channels (SOC) work in response to a receptor stimulus (Fig. 2). In this section, we briefly review some of these advances in the context of mast cell signaling and function. More extensive and recent reviews on the topic of calcium regulation are available (Smyth et al., 2006;Vig and Kinet, 2007).
Figure 2. Calcium Regulation in mast cells.
Calcium is an important second messenger whose mobilization is precisely controlled in mast cells. Antigen-aggregation of FcεRI leads to the activation of Lyn, Fyn, and Syk kinases. These kinases contribute to the calcium response by initiating and/or supporting PLCγ activity. PLCγ catalyzes the hydrolysis of membrane-localized phosphatidylinositol 4,5-bisphosphate (PI 4,5-P2) to inositol 3,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds IP3 receptors in the ER membrane, resulting in the release of Ca2+ from intracellular Ca2+ stores. DAG regulates the activity of various proteins, such as members of the protein kinase C family. The decrease in the Ca2+ concentration in the ER stores is sensed by STIM-1 (a calcium sensor) causing a change in its conformation that allows its translocation to the plasma membrane where it can interact with CRACM1/Orai1. STIM-1 and CRACM1/Orai1 synergize to elicit the influx of calcium. CRACM1/Orai1 appears to encode the icrac current channel in mast cells originally described by Hoth and Penner. Recent evidence also demonstrates that STIM-1 and Orai1 cooperate with transient receptor potential channels (TRPC) in mast cells to stimulate non-selective entry of Ca2+. These findings demonstrate an increasing complexity in the regulation of Ca2+ entry in mast cells. In other cell types some TRPC’s have also been demonstrated to be targets of Src family kinases, like Fyn, and to be directly regulated by PLCγ. The activation of FcεRI also causes the activation of sphingosine kinases 1 and 2 (SphK1 and SphK2) and the production of sphingosine-1-phosphate (S1P). This sphingolipid regulates Ca2+ influx within the cell, but the intracellular target of S1P is unknown. Moreover the relationship between S1P, STIM-1 and CRACM1/Orai1, if any, remains to be determined.
As outlined above IP3 is a key second messenger in the release of calcium from ER stores of mast cells through its engagement of IP3 receptors which are clustered upon IP3 binding resulting in a receptor/channel opening and release of stored free Ca2+ into the cytosol (Putney et al., 1989) (Fig. 2). We now know that this emptying of stores and increase in cytosolic free Ca2+ concentration triggers the conformational change in the calcium sensor ER single transmembrane spanning protein (STIM-1, which has a molecular mass of 77 kDa) that causes it to localize proximal to the plasma membrane (although whether it integrates in the plasma membrane is still uncertain) and functions to enhance plasma membrane calcium channel activity (Liou et al., 2005;Peinelt et al., 2006). More recent studies have demonstrated that deficiency of STIM-1 expression in mast cells caused marked loss of FcεRI-mediated degranulation and cytokine production and mice with low levels of STIM-1 showed a reduced anaphylactic response (Baba et al., 2008). The activation of transcription factors such as NFκB and NFAT was markedly reduced whereas early signaling (Syk, LAT, etc.) upon FcεRI stimulation (including IP3 production) appeared normal. As might be expected, a marked defect in the calcium response was observed in these cells. Strikingly, some residual influx of calcium was observed suggesting a possible STIM-1-independent mechanism contributing to calcium influx in mast cells. Regardless, the findings provide convincing evidence of a key role for STIM-1 in the regulation of mast cell calcium fluxes.
The combined approach of an RNAi screen in Drosophila S2 cells for inhibition of thapsigargin-induced calcium responses or for disruption of an indicator of NFAT nuclear translocation led two groups (Feske et al., 2006;Prakriya et al., 2006;Vig et al., 2006b) to the almost simultaneous discovery of another key component of the calcium apparatus termed CRACM1 or Orai1 (Fig. 2). This is a small protein with a molecular mass of 32.7 kDa predicted to span the membrane four times and the presence of both its N- and C-terminal domains in the cytosol has been demonstrated. Mutation of this protein was found in some severe combined immunodeficiency (SCID) patients (Feske et al., 2006) and it was shown to be crucial for calcium entry (Peinelt et al., 2006;Prakriya et al., 2006;Vig et al., 2006b). However, whether CRACM1/Orai1 is itself a calcium channel is still not clear. Nonetheless, a strong functional synergy in calcium entry was found by co-expression of STIM-1 with CRACM1/Orai1 (Peinelt et al., 2006) and it was also found to multimerize (Vig et al., 2006a), which is characteristic of how many ion channels form a functional pore (Fig. 2). Recent studies have found that mast cells deficient in CRACM1/Orai1 showed a marked defect in degranulation and cytokine production (Vig et al., 2008). Reconstitution of these deficient cells with CRACM1/Orai1 caused a partial restoration of function. While the deficiency in expression of CRACM1/Orai1 showed a marked defect on mast cell function in vitro and in vivo (in passive cutaneous anaphylaxis), it did not completely ablate calcium responses. This is consistent with the idea that icrac-independent calcium mobilization may contribute to the increased levels of intracellular calcium upon FcεRI stimulation. Regardless, the findings demonstrate that CRACM1/Orai1 is a key component in FcεRI calcium influx and thus makes this protein a potential therapeutic target for intervention in allergic disease.
Additional complexity in the calcium apparatus has been recently recognized by the demonstration that both STIM-1 and CRACM1/Orai1 appear to work synergistically (Peinelt et al., 2006) and associate with other calcium channels, particularly members of the transient receptor potential channels (TRPC) (Fig. 2). Recently, the use of silencing RNAi strategy and ectopic expression experiments has revealed that TRPC5 and STIM-1 cooperate to elicit both Ca2+ and Sr2+ influx in the mast cell line (RBL-2H3) (Ma et al., 2008), and this contributes to the enhanced calcium response needed for degranulation. This cooperation was easily distinguished from selective Ca2+ entry induced by co-expression of STIM-1 and CRACM1/Orai1. Thus, these findings suggest that calcium channels other than icrac are likely contributors to mast cell calcium influx and functional responses. As a tissue resident cell that is found in considerably different microenvironments, one might speculate that the type of calcium channel expressed may differ depending on the microenvironment. This adaptability would afford flexibility in eliciting functional responses in innate and adaptive immunity (Galli, 1997b).
V. Sphingosine-1-Phosphate: A co-stimulatory signal in FcεRI-mediated mast cell activation
As described in the section “Regulation of Calcium Mobilization in Mast Cells”, S1P has an intrinsic effect on mast cell activation through regulation of calcium responses, but the metabolic breakdown of sphingomyelin generates multiple bioactive lipids, including ceramide (Cer), Sph, and S1P. These lipids have diverse functions and can mediate cell growth, survival, differentiation, calcium homeostasis and chemotactic motility and show counteracting properties in regulating cell function (Olivera and Rivera, 2005;Spiegel and Milstien, 2003). In mast cells, S1P has been demonstrated to positively regulate responses whereas Sph and Cer inhibit mast cell responsiveness (Olivera and Rivera, 2005;Prieschl et al., 1999). Engagement of FcεRI on mast cells activates the two known mammalian sphingosine kinases (SphK1 and SphK2) resulting in the regulation of Sph levels and the production and secretion of S1P by these cells (Olivera et al., 2006). S1P also mediates its functional effect on mast cells as a ligand for a family of S1P receptors (S1P1–5), two of which (S1P1 and S1P2) are expressed on mast cells (Fig. 3). Thus, the role of FcεRI-mediated production of S1P in mast cells is of considerable interest and recent studies delineate an important role for S1P and its receptors in mast cell function and allergic responses.
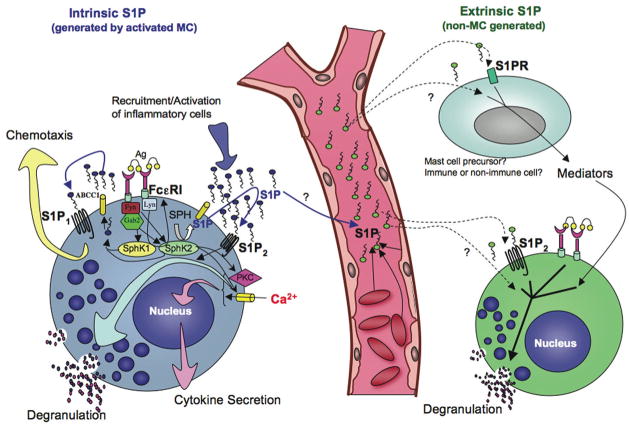
Figure 3. Role of Sphingosine kinases, S1P and its receptors in mast cell functions.
Mast cell function in its physiological environment is affected by “extrinsic S1P” generated by cells other than mast cells (right side, dashed lines with arrows), and “intrinsic S1P” (S1P generated by stimulated mast cells; left side blue arrows). Crosslinking of the FcεRI by IgE/antigen in mast cells results in the rapid activation and translocation of SphK to the plasma membrane and the generation of S1P. This is mediated by the Src kinases Lyn and Fyn. Fyn is required for SphK1 and SphK2 activation, whereas Lyn is required for the early phase of activity and membrane translocation (left panel). Fyn-dependent Gab2/PI3K activation, followed by PLD activation is required for SphK1 stimulation, while a not yet determined Fyn-dependent but Gab2-independent pathway is needed for full SphK2 activation. Our recent studies implicate SphK2, and not SphK1, in the influx of calcium following IgE receptor independently of IP3 generation, and thus affecting mast cell functions. S1P is secreted by activated mast cells to the extracellular media independently of their degranulation via an ATP binding cassette (ABC) transporter (Mitra et al., 2006). Furthermore, S1P is able to rapidly bind and activate its receptors S1P1 and S1P2 on the plasma membrane. S1P1 induces cytoskeletal rearrangements leading to the movement of mast cells towards an antigen gradient, while transactivation of S1P2 enhances the degranulation response. Mast cell secreted S1P can also promote inflammation by activating and recruiting other immune cells involved in allergic and inflammatory responses. Mast cell granules are illustrated as blue circles, and the process of degranulation as granules in contact with the plasma membrane emptying their content (smaller pink and blue dots). Mast cells may be affected by changes in circulating S1P by different mechanisms. It is possible that changes in S1P in the circulation affect the priming of S1P2 in mast cells, which are found in close proximity to blood vessels, enhancing degranulation upon cell activation. Increases or decreases in extrinsic S1P levels might also induce the differentiation of mast cell precursors towards a more or less responsive phenotype, respectively. Constant exposure to higher or lower levels of S1P might also indirectly influence mast cell differentiation or function via mediators derived from other immune or non-immune cells that respond to the fluctuation in S1P levels.
A. Linking FcεRI to Sphingosine Kinase Activation
How SphKs are activated is not completely understood. However, the evidence gathered to date suggests that a complex interplay of protein kinase- and lipid-derived signals are required in an apparent two step process in which SphKs translocate rapidly to plasma membranes and become active following FcεRI engagement (Olivera and Rivera, 2005;Olivera et al., 2006;Urtz et al., 2004). At the moment it has been difficult to resolve whether the translocation of SphKs to the plasma membrane is essential for activation or whether it is a consequence of activation. Regardless, under circumstances where the stimulation of SphK by FcεRI is impaired there is also no translocation of SphK to membranes (Olivera et al., 2006;Urtz et al., 2004). We now know that the FcεRI-mediated translocation and activation of SphKs requires both Fyn and Lyn (Fig. 3). While there is an absolute requirement for Fyn activity in activating SphKs, Lyn activity is not essential (Olivera et al., 2006;Urtz et al., 2004). The requirement for Fyn-dependent activation of SphK1 includes a role for Gab2/PI3K/PLD activities (Olivera et al., 2006). The adaptor Gab2 is required for the activation of PI3K in mast cells and the production of phosphatidylinositols (including PIP3) are needed for the activation and function of PLD, which in turn produces phosphatidic acid (PA). Both of these lipid messengers can either bind, activate or induce the translocation of SphKs (Delon et al., 2004;Melendez and Khaw, 2002;Olivera, Rosenthal and Spiegel, 1996). In contrast to SphK1, the activation of SphK2 requires Fyn, but seems to be less dependent on the activity of Gab2/PI3K (Olivera et al., 2006). Fyn and Lyn also interact with SphKs and this interaction appears to be required for the translocation and activation of SphKs (Olivera et al., 2006;Urtz et al., 2004).
B. Linking FcεRI to Sphingosine-1-phosphate receptors
Fyn-deficent mast cells are defective in FcεRI-induced degranulation, but degranulation can be partially corrected by the addition of exogenous S1P upon Ag stimulation (Olivera et al., 2006). This suggests that S1P production in mast cells partially contributes to their FcεRI-mediated degranulation (Fig. 3). Inhibition of SphK activation, and thus S1P generation, by either competitive analogs of Sph in the RBL tumor mast cell line (Choi, Kim and Kinet, 1996) or by antisense SphK mRNA in human mast cells (Melendez and Khaw, 2002) prevented IgE-triggered calcium responses and inhibited degranulation. Thus, the S1P produced by SphK activation in mast cells has both an intracellular and extracellular regulatory role in mast cell degranulation. The role of S1P in regulating calcium was discussed above, here we now summarize what is known about its S1P receptor-mediated role in mast cell degranulation.
As previously mentioned, mast cells express two of the five receptors for S1P, S1P1 and S1P2 (Fig. 3) (Jolly et al., 2004). The first evidence of a link between the FcεRI and S1P receptors on mast cells was the demonstration that FcεRI-induced S1P formation resulted in the transactivation of these two receptors (Jolly et al., 2004). The S1P receptors couple to different subunits of heterotrimeric G proteins (αi, αq, and α12/13), and therefore they can trigger diverse signals, including activation of Src kinases, small GTPases, MAPK cascades, phopholipases, PKC and calcium mobilization (Pyne and Pyne, 2000). S1P1 has been well defined as a chemotatic receptor and in the immune system it is known to be required for thymocyte emigration and lymphocyte recirculation (Matloubian et al., 2004) and outside of the immune system it is required for vascular morphogeneseis (Allende and Proia, 2002) among a host of other functions (Spiegel, Foster and Kolesnick, 1996). For mast cells, it was demonstrated that S1P1 is required for their migration towards low concentrations of antigen (Fig. 3). In contrast, S1P2 was required for normal degranulation (Fig. 3), as downregulation of its expression or deletion of the S1P2 gene in mast cells led to a marked loss (a 50% inhibition) of degranulation (Jolly et al., 2004). Interestingly, S1P2 also appears to exert some control in the function of S1P1, since overexpression of S1P2 inhibited mast cell chemotactic motility. This is consistent with the finding that S1P2 mRNA expression is enhanced as a late consequence of FcεRI engagement, while S1P1 mRNA expression is constitutive. Thus, a gradient of antigen might attract mast cells to their site of action via S1P1. Subsequently, as the mast cells approach higher concentrations of antigen, enhanced S1P2 expression would inhibit migration while promoting degranulation. This is consistent with the requirement of low concentrations of antigen in eliciting the production of chemokines whereas high antigen concentrations are required for mast cell degranulation (Gonzalez-Espinosa et al., 2003). How S1P2 collaborates with FcεRI to elicit a full degranulation of mast cells is not well understood but the findings suggest that the transactivation of this receptor is important for mast cell degranulation. Thus, S1P engagement of S1P2 can be defined as a co-stimulatory signal for FcεRI-mediated mast cell degranulation. The findings also suggest that the extracellular levels of S1P may influence the degranulation of mast cells, a phenomena for which there is now in vivo evidence.
C. S1P is an in vivo effector of mast cell function
S1P is secreted by mast cells upon FcεRI engagement (reviewed in (Olivera and Rivera, 2005)). Unlike T cells, B cells, etc., mast cells secrete a substantial amount of S1P suggesting it is important for mast cell effector functions. S1P is also highly elevated in the airways of asthmatic individuals (Jolly et al., 2002), and in the joints of arthritic individuals (Kitano et al., 2006). Both asthma and rheumathoid arthritis are inflammatory conditions in which mast cells have been demonstrated to be important effector cells, the latter primarily in a mouse model (Galli, 1997a;Lee et al., 2002). This raises the possibility that S1P is a autocrine and/or paracrine mediator involved in the pathophysiology of asthma or other allergic and/or inflammatory diseases.
In recent studies we have found a close correlation between circulating levels of S1P and the histamine levels in the plasma following anaphylactic challenge (Olivera et al., 2007). Wild type mice having high circulating S1P showed the highest levels of histamine in the plasma, whereas those with low levels of S1P had reduced plasma histamine. This correlation is further observed in the in vivo anaphylactic responses of wild type mice or mice with a genetic deletion of SphK1 or SphK2. SphK1-null mice had reduced levels of circulating S1P (Allende et al., 2004;Zemann et al., 2006) and these mice were highly resistant to anaphylaxis, suggesting that the level of circulating S1P was a determinant of mast cell responsiveness. In contrast, SphK2-null mice had enhanced levels of circulating S1P relative to wild type mice (Zemann et al., 2006) and were found to respond normally to an anaphylactic challenge. These findings pointed to a dominant extrinsic role for S1P in regulating mast cell responsiveness (Fig. 3). This dominant extrinsic role of S1P could overcome the intrinsic defect of SphK2-null mast cells, which showed defective degranulation in vitro. Moreover, the low circulating levels of S1P in SphK1-null mice was dominant over the observed normal in vitro degranulation of SphK1-null mast cells manifesting as defective in vivo degranulation. This dominance of circulating S1P levels was further explored by generating mice that were null for SphK2 with one functional allele for SphK1. This returned the levels of circulating S1P to normal, as compared to the SphK2-null mouse, and these mice were now resistant to an anaphylactic challenge. This relationship between increased circulating levels of S1P and increased in vivo mast cell responsiveness is also manifest in mice differing in genetic backgrounds (see below), or mice where S1P levels were artificially increased by use of the sphingosine lyase inhibitor, 2-acetyl-4-tetrahydroxybutylimidazole (THI). In both cases increased circulating S1P was associated with increased in vivo mast cell responsiveness (Fig. 3) (AO and JR, unpublished observation). These findings demonstrate a dominant extrinsic role for S1P, but it should be noted that S1P generated within the mast cell is also important in regulating mast cell responsiveness as mast cells that do not produce S1P, such as Fyn-null mast cells (Olivera et al., 2006), are considerably more defective in degranulation than S1P2-null mast cells (Jolly et al., 2004). Both intrinsic and extrinsic regulation of mast cell responses by S1P appears to participate in FcεRI-induced mast cell responses. However, the intracellular target(s) of S1P in these cells remains to be defined.
VI. The role of genetics in mast cell responsiveness
The study of the physiological or pathophysiological relevance of mast cell signaling and function has required the use of mouse models. Mice have become the primary mammalian model organism because of their close genetic relationship to humans and the ease by which alterations in their genome allow the study of fundamental biological processes and disease. It has been well established that theses two species share many of the genetic pathways regulating normal and pathological conditions regardless of the obvious physiological and anatomical differences. Since the first use of the mouse as a model for physiological studies it became clear that not all isogenic backgrounds are appropriate for a given study. Indeed, certain isogenic strains can be poor mimics of human disease or even eclipse the effects of a targeted mutation, which is manifest in another strain (Rivera and Tessarollo, 2008). For example, there is a contrasting degree of airway inflammation seen in the ovalbumin (OVA) model of asthma depending on the mouse strain (Herz, Renz and Wiedermann, 2004). When sensitized to OVA, an aerosol challenge of BALB/c mice with OVA causes a marked increase in lymphocytes, eosinophils, and neutrophils in the bronchoaveolar fluids. This is paralleled by highly elevated amounts of IL-4, IL-5, and TNF and easily measurable airway hyperreactivty. This same model in a different strain, such as C57BL/6, mice showed a much weaker lung inflammation, a lesser cytokine response, and a more modest airway hyperreactivity (Herz, Renz and Wiedermann, 2004). These types of studies argue that, like in humans, the genetic environment is a determinant in allergic responses. However, whether the genetic makeup could directly affect the responsiveness of the mast cell was not known. Here we discuss new evidence supporting the view that differences in genetic makeup contribute to the degree of mast cell responsiveness.
A. An apparent contradiction in Lyn function in mast cells is manifest through genetic differences in mouse strains
Various experiments conducted to study the role of Lyn kinase in mast cell activation were apparently contradictory. Thus, the early demonstration that Lyn-null mice were resistant to IgE/Ag-induced passive cutaneous anaphylaxis (Hibbs et al., 1995) appeared to contradict experiments showing that mast cells from another independently generated Lyn-null mouse showed normal mast cell degranulation (Nishizumi and Yamamoto, 1997). Moreover, several later reports showed either normal or enhanced degranulation in Lyn-null mast cells (Hernandez-Hansen et al., 2004;Kawakami et al., 2000;Odom et al., 2004), including an enhanced passive systemic anaphylactic response and increased airway hypersensitivity in Lyn-null mice (Beavitt et al., 2005;Odom et al., 2004), whereas the loss of Lyn function in the mast cell line RBL-2H3 caused loss of mast cell degranulation (Vonakis et al., 2005). While a number of variables differed in these studies, the most identifiable difference in experiments using mouse cells was that they were derived from mice either of the C57BL/6 or mice with a mixed background of 129/SvJ X C57BL/6.
To determine the relative contribution of the difference in genetic background to the published observation an analysis of the two pure genetic backgrounds (C57BL/6 versus 129/SvJ) was undertaken. Analysis of mast cells derived from C57BL/6 mice carrying a null mutation for Lyn showed a dramatic inhibition of FcεRI-mediated degranulation relative to their wild-type counterparts (Yamashita et al., 2007). In contrast, mast cells from 129/SvJ mice carrying the same mutation showed an enhanced degranulation response when compared to their wild-type counterparts and to all C57BL/6-derived mast cells. These studies identified that in mast cell derived from C57BL/6 mice Fyn kinase expression was two fold less than that seen in 129/SvJ-derived mast cells. Since Fyn functions to promote degranulation (Parravicini et al., 2002), ectopic expression in C57BL/6 cells was explored in the context of Lyn-deficiency. The increased expression of Fyn caused conversion of the poor responsive C57BL/6 Lyn-null phenotype to a highly responsive 129/SvJ Lyn-null phenotype. Thus, the findings demonstrated that relatively modest differences in gene expression (a 50% decrease) could cause a marked phenotypic change. Interestingly, the role of Fyn and Lyn in human mast cells was also investigated in this study. The silencing of Fyn or Lyn expression in human mast cells caused the inhibition or augmentation of degranulation, respectively (Yamashita et al., 2007). Thus, it appears that human mast cells show a phenotype that is most similar to that of the 129/SvJ mouse rather than the C57BL/6 mouse.
B. Genetics influences the in vivo environment altering mast cell responsiveness
129 mouse strains do not breed well, and are reported to have abnormal anatomy and behavior, thus, the backcross newly generated mutant 129 mice into the C57BL/6 background is widely practiced. C57BL/6 are long-lived and are permissive for expression of most mutations (http://jaxmice.jax.org/strain/000664.html) and breed relatively well. However, 129 mouse strains and C57BL/6 mice demonstrate a skewing towards T helper 2 (Th2) and T helper1 (Th1) responses, respectively (O’Neill et al., 2000) and C57BL/6 mice are relatively resistant to tumor development. This limits the use of these strains for certain immunological questions. In vivo interrogation of allergic responses must take into consideration the Th1 or Th2 environment present in these mice. Thus, for example, 129/SvJ mice have considerably higher levels of circulating IgE than that found in C57BL/6 mice. Relative to the C57BL/6 mouse, increased levels of IgE in the 129/SvJ mouse enhances the onset and extent of allergic responses because high IgE levels cause increased expression of FcεRI and also cause full occupancy of this receptor, increasing the sensitivity to an allergenic stimulus (Yamashita et al., 2007). More recently, we’ve found that the 129/SvJ mice also have higher levels of circulating S1P relative to the C57BL/6 mouse (AO and JR, unpublished observation). As outlined above the increase in S1P in 129/SvJ mice may manifest in increased mast cell responsiveness. Indeed, in vivo passive systemic anaphylaxis challenge of 129/SvJ mice showed increased circulating histamine levels when compared to C57BL/6 mice. Thus, while many questions that surround the issue of how genetics influence mast cell responsiveness are still unanswered, the experiments to date reveal the importance of genetics to the mast cell and its environment. In addition, these studies also caution against the extrapolation of experimental results in mice (based on experiments conducted on mast cells or mice from a single genetic strain) as relevant to human disease.
VII. Summary and Perspectives
A. On early signals in the regulation of mast cell activation
The recent advances in our understanding of FcεRI-mediated activation of mast cells have clearly shown an increasing complexity that was previously unappreciated. These findings define a complex relationship of proteins and lipids that serve to target, activate, and regulate the molecular steps required for full activation of a mast cell (Fig. 1). At the earliest step in mast cell activation, namely the clustering of FcεRI, one finds that proximity of FcεRI in these clusters is essential in determining if FcεRI becomes phosphorylated and whether the mast cell will become fully activated or not (Sil et al., 2007). This suggests an intrinsic mechanism at the very first step of activation that screens a productive engagement of FcεRI from an unproductive one. Given that the FcεRI is likely to be occupied by IgE of varying antigenic specificities in vivo, the requirement for close proximity of FcεRI for its activation would likely avoid the spurious activation of these cells by weak antigens since the proximity of any two receptors is unlikely to be sustained by weak antigens due to their more rapid dissociation (Torigoe, Inman and Metzger, 1998).
Other points of regulation in FcεRI-mediated mast cell activation are also revealed in the recent studies. The positive and negative balance mediated through Src Family Kinases. Fyn and Lyn, as well as Hck and possibly others (Hernandez-Hansen et al., 2004;Hong et al., 2007;Kawakami et al., 2000;Kitaura et al., 2007;Odom et al., 2004;Xiao et al., 2005), appear to be key components of a network that fine tunes the extent of a mast cells response when FcεRI is engaged (Fig. 1). In some cases, this appears to be mediated indirectly through regulatory proteins that inactivate Src kinases, like the Cbp/Csk complex (Kitaura et al., 2007;Odom et al., 2004), but we cannot exclude a more direct control through crosstalk of Src family kinases. There is also an increasing body of literature that argues that the cholesterol-enriched plasma membrane microdomains, which are heterogeneous signaling module (Heneberg et al., 2006;Wilson, Pfeiffer and Oliver, 2000;Wilson et al., 2001), also play an important regulatory role (Fig. 1). Thus, loss of Lyn from these domains or the failure to cause effective coalesence of these domains and their signaling constituents has marked consequences on the ability of a mast cell to become fully activated and/or may result in selective reponses (Heneberg et al., 2006;Kovarova et al., 2006;Young, Holowka and Baird, 2003). It is important to note, that selective signaling and responses have been shown to occur, such as upon weak stimulation of FcεRI (Gonzalez-Espinosa et al., 2003). This is important as weak stimuli are less effective in coalescing cholesterol-enriched membrane microdomains (Davey et al., 2008;Davey et al., 2007;Heneberg et al., 2006). This is also likely to affect signaling and the function of adaptor molecules, like LAT1 and LAT2, which participate in amplification of various signaling pathways, including calcium mobilization (Draberova et al., 2007;Iwaki et al., 2008;Saitoh et al., 2000) as they are localized within these membrane microdomains. However, how the regulatory role of LAT1 and LAT2 is partitioned in FcεRI-induced signals and mast cell responses remains to be defined. It should be noted that regulation of responses also exist downstream of LAT1 (Torigoe et al., 2007), however, the molecular events involved in the downstream regulatory checkpoint(s) are unknown. Possible candidates for such a role may include the generation of lipid mediators as they can contribute to the overall activation of mast cells by their effects on recruitment and activation of many signaling molecules but can also be dominant in certain pathways leading to selective responses.
B. Marrying of lipids in the regulation of calcium and mast cell effector responses
The generation of lipid second messengers has long been recognized to be important in the effector function of mast cells as well as in other cell types (Becker and Hannun, 2005;Rivera and Olivera, 2007;Spiegel, Foster and Kolesnick, 1996). The recent advances have clearly demonstrated that lipid messengers can be dominant in certain signaling pathways and cause cellular responses independent of many of the additional events initiated by FcεRI activation. A superb example is the finding that downregulation of PTEN expression in human mast cell (by shRNA silencing), which caused increased PIP3 production, resulted in the activation of Akt and MAP kinases and in mast cell cytokine secretion independently of FcεRI engagement (Furumoto et al., 2006). These findings show that selective mast cell responses depend almost entirely on lipid signals and do not require the additional signals that are elicited by FcεRI engagement. It should be noted that increased or decreased PIP3 levels (which can be regulated by PTEN) in mast cells is associated with increased or decreased mast cell responsiveness, respectively (Ali et al., 2004;Barker, Lujan and Wilson, 1999;Furumoto et al., 2006;Gomez et al., 2005;Odom et al., 2004), in many cases similarly affecting calcium responses. How PIP3 levels might affect calcium responses is not well defined but it is known that many of the proteins, such as PLCγ or SphKs, that regulate calcium also require PIP3 for their translocation and function. One can imagine that this must be finely tuned particularly since the same substrate, namely PI-4, 5-bisphosphate, is used by both PLCγ and PI3K. However, it does not always follow that alterations of PIP3 levels affect calcium responses (Leung and Bolland, 2007). Regardless, the accumulated data makes a convincing argument for PIP3 as an essential lipid messenger in FcεRI-mediated mast cell activation (Fig. 1) (Ali et al., 2004;Furumoto et al., 2006;Leung and Bolland, 2007).
Mast cell effector functions are not only regulated by lipids, through the modulation of intracellular signaling pathways, but also through autocrine/paracrine mechanisms involving engagement of cell surface receptors on these cells. S1P is such a lipid, whose production and function in mast cells causes intracellular effects (such as calcium regulation) but also modulates mast cell chemotaxis and degranulation through the S1P receptors expressed on these cells (Fig. 2) (Jolly et al., 2004;Olivera et al., 2007;Olivera et al., 2006;Urtz et al., 2004). At the moment, how the intracellular and extracellular effects of S1P cooperate in FcεRI-mediated mast cell activation is a key missing piece in this puzzle. This may be revealed by the identification of the intracellular targets for S1P and its possible partnership in calcium regulation. Now that the molecular elements of the calcium apparatus are rapidly being defined (Smyth et al., 2006;Vig and Kinet, 2007), it will be of particular interest to determine if these are targets of S1P. Given that a previous study suggests that sphingosine is an inhibitor of icrac-current (Mathes, Fleig and Penner, 1998) and that loss of S1P production selectively affects calcium influx (Olivera et al., 2007), it is possible that SphKs or S1P may play a direct role in regulating calcium channels (Figs. 2 and 3). However, as the recent studies are demonstrating, an intrinsic defect in calcium responses leading to decreased in vitro responsiveness of a mast cell may be overcome by the in vivo environment in which they are found (Olivera et al., 2007).
C. Translating molecular mechanisms to an in vivo environment
Recent studies affirm that in vitro analysis of mast cell activation and function (a reductionist approach) does not always translate in vivo (Olivera et al., 2007;Yamashita et al., 2007). Multiple factors may be responsible for this failure of translation. These include genetics, the in vivo environment, and the heterogeneity of mast cells in vivo. It is not surprising that these would be important determinants of in vivo mast cell responsiveness and function as this type of plasticity is required of a cell that transcends both innate and adaptive immunity (Galli et al., 2005;Williams and Galli, 2000). What is unexpected, however, is that S1P as an extrinsic regulator of mast cell responsiveness seems to be dominant in the in vivo environment and that genetics may contribute to the overall circulating S1P levels, which are closely associated with mast cell responsiveness (Fig. 3). Obviously, we need to understand how circulating S1P levels are regulated in vivo and what is the source of this S1P, as the data to date suggests a non-mast cell source for circulating S1P (Olivera et al., 2007). Of particular interest is whether one might be able to convert a low mast cell responder mouse (such as a C57BL/6) to a high mast cell responder mouse (129/SvJ or Balb/c) by increasing the circulating S1P levels in the low responder mouse. The importance of such a shift may well relate to human disease given the close association between the circulating levels of S1P and the extent of the response observed during a passive systemic challenge (Olivera et al., 2007). The demonstration that allergen-challenged asthmatics showed high levels of S1P in their lungs relative to controls (Ammit et al., 2001) also argues for a close link to mast cell responsiveness in disease. Further studies are clearly required to define a causal relationship and how genetics may influence circulating S1P levels. Nonetheless, it is clear that the recent advances in our knowledge of the mechanisms underlying FcεRI-mediated mast cell activation have uncovered new complexities. More importantly, they also reveal new areas of investigation with therapeutic potential in disease.
Footnotes
The research of the authors reported herein was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
References
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- Allende ML, Sasaki T, Kawai H, Olivera A, Mi Y, van Echten-Deckert G, Hajdu R, Rosenbach M, Keohane CA, Mandala S, Spiegel S, Proia RL. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem. 2004;279:52487–52492. doi: 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. Faseb J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- Barker SA, Caldwell KK, Hall A, Martinez AM, Pfeiffer JR, Oliver JM, Wilson BS. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by FcεRI cross-linking. Mol Biol Cell. 1995;6:1145–1158. doi: 10.1091/mbc.6.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker SA, Lujan D, Wilson BS. Multiple roles for PI 3-kinase in the regulation of PLCγ activity and Ca2+ mobilization in antigen-stimulated mast cells. J Leukocyte Biol. 1999;65:321–329. doi: 10.1002/jlb.65.3.321. [DOI] [PubMed] [Google Scholar]
- Beavitt SJ, Harder KW, Kemp JM, Jones J, Quilici C, Casagranda F, Lam E, Turner D, Brennan S, Sly PD, Tarlinton DM, Anderson GP, Hibbs ML. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J Immunol. 2005;175:1867–1875. doi: 10.4049/jimmunol.175.3.1867. [DOI] [PubMed] [Google Scholar]
- Becker KP, Hannun YA. Protein kinase C and phospholipase D: intimate interactions in intracellular signaling. Cell Mol Life Sci. 2005;62:1448–1461. doi: 10.1007/s00018-005-4531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M, Ryba NJP, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity receptor signaling. Identification as a component of pp72 and associaition with the receptor γ chain after receptor aggregation. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Blank U, Rivera J. The Ins and Outs of IgE-dependent mast cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VLJ. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- Davey AM, Krise KM, Sheets ED, Heikal AA. Molecular perspective of antigen-mediated mast cell signaling. J Biol Chem. 2008;283:7117–7127. doi: 10.1074/jbc.M708879200. [DOI] [PubMed] [Google Scholar]
- Davey AM, Walvick RP, Liu Y, Heikal AA, Sheets ED. Membrane order and molecular dynamics associated with IgE receptor cross-linking in mast cells. Biophys J. 2007b;92:343–355. doi: 10.1529/biophysj.106.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J Biol Chem. 2004;279:44763–44774. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- Draberova L, Shaik GM, Volna P, Heneberg P, Tumova M, Lebduska P, Korb J, Draber P. Regulation of Ca2+ signaling in mast cells by tyrosine-phosphorylated and unphosphorylated non-T cell activation linker. J Immunol. 2007;179:5169–5180. doi: 10.4049/jimmunol.179.8.5169. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–4280. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral shRNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Nunomura S, Terada T, Rivera J, Ra C. The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J Biol Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- Galli SJ. Complexity and redundancy in the pathogenesis of asthma: reassessing the roles of mast cells and T cells. J Exp Med. 1997a;186:343–347. doi: 10.1084/jem.186.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ. The mast cell: A versatile effector cell for a challenging world. Int Arch Allergy Immunol. 1997b;113:14–22. doi: 10.1159/000237497. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–2330. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcεRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn-deficiency in mast cells. J Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Espinosa C, Odom S, Olivera A, Hobson JP, Martinez ME, Oliveira-Dos-Santos A, Barra L, Spiegel S, Penninger JM, Rivera J. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J Exp Med. 2003;197:1453–1465. doi: 10.1084/jem.20021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- Heneberg P, Lebduska P, Draberova L, Korb J, Draber P. Topography of plasma membrane microdomains and its consequences for mast cell signaling. Eur J Immunol. 2006;36:2795–2806. doi: 10.1002/eji.200636159. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, Oliver JM. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- Herz U, Renz H, Wiedermann U. Animal models of type I allergy using recombinant allergens. Methods. 2004;32:271–280. doi: 10.1016/j.ymeth.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- Hong H, Kitaura J, Xiao W, Horejsi V, Ra C, Lowell CA, Kawakami Y, Kawakami T. The Src family kinase Hck regulates mast cell activation by suppressing an inhibitory Src family kinase Lyn. Blood. 2007;110:2511–2519. doi: 10.1182/blood-2007-01-066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Houtman JC, Barda-Saad M, Samelson LE. Examining multiprotein signaling complexes from all angles. Febs J. 2005;272:5426–5435. doi: 10.1111/j.1742-4658.2005.04972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci USA. 1998a;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Helgason CD, Scheid MP, Duronio V, Humphries RK, Krystal G. Targeted disruption of SHIP leads to Steel factor-induced degranulation of mast cells. EMBO J. 1998b;17:7311–7319. doi: 10.1093/emboj/17.24.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Kalesnikoff J, Reth M, Krystal G. The role of SHIP in mast cell degranulation and IgE-induced mast cell survival. Immunol Lett. 2002;82:17–21. doi: 10.1016/s0165-2478(02)00012-3. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Spicka J, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, Kovarova M, Rivera J, Horejsi V, Metcalfe DD, Gilfillan AM. Kit- and FcεRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K. Lipid rafts are required for Kit survival and proliferation signals. Blood. 2007;110:1739–1747. doi: 10.1182/blood-2006-05-020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P, Rosenfeldt H, Milstien S, Spiegel S. The roles of sphingosine-1-phosphate in asthma. Mol Immunol. 2002;38:1239–1251. doi: 10.1016/s0161-5890(02)00070-6. [DOI] [PubMed] [Google Scholar]
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, Kawakami T. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- Kihara H, Siraganian RP. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]
- Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- Kitaura J, Kawakami Y, Maeda-Yamamoto M, Horejsi V, Kawakami T. Dysregulation of Src family kinases in mast cells from epilepsy-resistant ASK versus epilepsy-prone EL mice. J Immunol. 2007;178:455–462. doi: 10.4049/jimmunol.178.1.455. [DOI] [PubMed] [Google Scholar]
- Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcε receptor I aggregation. Mol Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarova M, Wassif CA, Odom S, Liao K, Porter FD, Rivera J. Cholesterol-deficiency in a murine model of Smith-Lemli-Opitz Syndrome reveals increased mast cell responsiveness. J Exp Med. 2006;203:1161–1171. doi: 10.1084/jem.20051701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Leung WH, Bolland S. The inositol 5′-phosphatase SHIP-2 negatively regulates IgE-induced mast cell degranulation and cytokine production. J Immunol. 2007;179:95–102. doi: 10.4049/jimmunol.179.1.95. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA. Canonical Transient Receptor Potential 5 Channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line. J Immunol. 2008;180:2233–2239. doi: 10.4049/jimmunol.180.4.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Verdeaux S, Pombo I, Iannascoli B, Roa M, Varin-Blank N, Rivera J, Blank U. Analysis of Munc18-2 compartmentation in mast cells reveals a role for microtubules in granule exocytosis. J Cell Sci. 2003;116:325–334. doi: 10.1242/jcs.00216. [DOI] [PubMed] [Google Scholar]
- Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–25030. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- Metzger H. The IgE-mast cell system as a paradigm for the study of antibody mechanisms. Immunol Rev. 1978;41:186–199. doi: 10.1111/j.1600-065x.1978.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Metzger H. It’s spring, and thoughts turn to… allergies. Cell. 1999;97:287–290. doi: 10.1016/s0092-8674(00)80738-2. [DOI] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immunoglobulin E receptor FcεRI: coupling form to function. Adv Immunol. 2000;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cβ. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in Lyn-deficient bone marrow-derived mast cells. J Immunol. 1997;158:2350–2355. [PubMed] [Google Scholar]
- O’Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH, Dalton JP. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 2000;22:147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenthal J, Spiegel S. Effect of acidic phospholipids on sphingosine kinase. J Cell Biochem. 1996;60:529–537. doi: 10.1002/(sici)1097-4644(19960315)60:4<529::aid-jcb9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- On M, Billingsley JM, Jouvin MH, Kinet JP. Molecular dissection of the FcRβ signaling amplifier. J Biol Chem. 2004;279:45782–45790. doi: 10.1074/jbc.M404890200. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Szallasi Z, Kazanietz M, Blumberg P, Mischak H, Mushinski J, Beaven M. Ca2+-dependent and Ca2+-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated Rat Basophilic RBL-2H3 cells. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunology. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, Kurosaki T, Snyder SH, Gill DL. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivniouk VI, Martin TR, Lu-Kuo JM, Katz HR, Oettgen HC, Geha RS. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J Clin Invest. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- Pombo I, Rivera J, Blank U. Munc18-2/syntaxin3 complexes are spatially separated from syntaxin3-containing SNARE complexes. FEBS Lett. 2003;550:144–148. doi: 10.1016/s0014-5793(03)00864-0. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fcε receptor I triggering. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri N, Roche PA. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic. 2006;7:1482–1494. doi: 10.1111/j.1600-0854.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW, Jr, Takemura H, Hughes AR, Horstman DA, Thastrup O. How do inositol phosphates regulate calcium signaling? FASEB Journal. 1989;3:1899–1905. doi: 10.1096/fasebj.3.8.2542110. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne N. Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol Ther. 2000;88:115–131. doi: 10.1016/s0163-7258(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Rivera J. Molecular adapters in FcεRI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. doi: 10.1016/s0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- Rivera J. NTAL/LAB and LAT: A balancing act in mast cell activation and function. Trends Immunol. 2005;26:119–122. doi: 10.1016/j.it.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Rivera J, Beaven MA. In: Protein Kinase C. Parker PJ, Dekker LV, editors. Landes Bioscience; Austin, TX: 1997. pp. 133–166. [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gonzalez-Espinosa C, Kovarova M, Parravicini V. The Architecture of IgE-dependent mast cell signaling. A complex story. Allergy Clin Immunol Int. 2002;14:25–36. [Google Scholar]
- Rivera J, Olivera A. Src family kinases and lipid mediators in control of allergic inflammation. Immunol Rev. 2007;217:255–268. doi: 10.1111/j.1600-065X.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- Rivera J, Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Odom S, Gomez G, Sommers CL, Young HA, Rivera J, Samelson LE. The four distal tyrosines are required for LAT-dependent signaling in FcεRI-mediated mast cell activation. J Exp Med. 2003;198:831–843. doi: 10.1084/jem.20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil D, Lee JB, Luo D, Holowka D, Baird B. Trivalent ligands with rigid DNA spacers reveal structural requirements for IgE receptor signaling in RBL mast cells. ACS Chem Biol. 2007;2:674–684. doi: 10.1021/cb7001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Shoag J, Wu J, Koretzky GA. Disruption of SLP-76 interaction with Gads inhibits dynamic clustering of SLP-76 and FcεRI signaling in mast cells. Mol Cell Biol. 2006;26:1826–1838. doi: 10.1128/MCB.26.5.1826-1838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2002;38:1229–1233. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW., Jr Emerging perspectives in store-operated Ca(2+) entry: Roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Curr Opin Cell Biol. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Swieter M, Berenstein EH, Siraganian RP. Protein tyrosine phosphatase activity associates with the high affinity IgE receptor and dephosphorylates the receptor subunits, but not Lyn or Syk. J Immunol. 1995;155:5330–5336. [PubMed] [Google Scholar]
- Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following KIT activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Iwaki S, Metcalfe DD, Gilfillan AM. Roles of adaptor molecules in mast cell activation. Chem Imunnol Allergy. 2005;87:43–58. doi: 10.1159/000087570. [DOI] [PubMed] [Google Scholar]
- Torigoe C, Faeder JR, Oliver JM, Goldstein B. Kinetic proofreading of ligand-FcεRI interactions may persist beyond LAT phosphorylation. J Immunol. 2007;178:3530–3535. doi: 10.4049/jimmunol.178.6.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science. 1998;281:568–572. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Early activation of sphingosine kinase in mast cells and recruitment to FcεRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez G, Wedel BJ, Kawasaki BT, Bird GS, Putney JW., Jr Obligatory role of Src kinase in the signaling mechanism for TRPC3 cation channels. J Biol Chem. 2004;279:40521–40528. doi: 10.1074/jbc.M405280200. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006a;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006b;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volna P, Lebduska P, Draberova L, Simova S, Heneberg P, Boubelik M, Bugajev V, Malissen B, Wilson BS, Horejsi V, Malissen M, Draber P. Negative regulation of mast cell signaling and function by the adaptor LAB/NTAL. J Exp Med. 2004;200:1001–1013. doi: 10.1084/jem.20041213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonakis BM, Gibbons SP, Jr, Rotte MJ, Brothers EA, Kim SC, Chichester K, MacDonald SM. Regulation of rat basophilic leukemia-2H3 mast cell secretion by a constitutive Lyn kinase interaction with the high affinity IgE receptor (FcεRI) J Immunol. 2005;175:4543–4554. doi: 10.4049/jimmunol.175.7.4543. [DOI] [PubMed] [Google Scholar]
- Wang D, Feng J, Wen R, Marine JC, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, Ihle JN. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. Observing FcεRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Surviladze Z, Gaudet EA, Oliver JM. High resolution mapping of mast cell membranes reveals primary and secondary domains of FcεRI and LAT. J Cell Biol. 2001;154:645–658. doi: 10.1083/jcb.200104049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J. Cutting Edge: Genetic variation influences FcεRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kimura T, Siraganian RP. Mutations in the activation loop tyrosines of protein tyrosine kinase Syk abrogate intracellular signaling but not kinase activity. J Immunol. 1998;161:4366–4374. [PubMed] [Google Scholar]
- Zhu M, Koonpaew S, Liu Y, Shen S, Denning T, Dzhagalov I, Rhee I, Zhang W. Negative regulation of T cell activation and autoimmunity by the transmembrane adaptor protein LAB. Immunity. 2006;25:757–768. doi: 10.1016/j.immuni.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Zhu M, Liu Y, Koonpaew S, Ganillo O, Zhang W. Positive and negative regulation of FcεRI-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]