Abstract
Cholangiocarcinoma (CCA) is a fatal cancer of the biliary epithelium, arising either within the liver (intrahepatic, ICC) or in the extrahepatic bile ducts (extrahepatic ECC). Globally, CCA is the second most common primary hepatic malignancy. Several recent epidemiological studies have shown that the incidence and mortality rates of ICC are increasing. This review of the literature on the international epidemiological rates of CCA, both intra- and extrahepatic, explores possible explanations for the trends found. The possible role of epidemiological artifact in the findings is discussed and the known risk factors for CCA are summarized. These include primary sclerosing cholangitis, liver fluke infestation, congenital fibropolycystic liver, bile duct adenomas, and biliary papillomatosis, hepatolithiasis, chemical carcinogens such as nitrosamines, Thorotrast, chronic viral hepatitis, cirrhosis, chronic non-alcoholic liver disease and obesity. Potential pathways involved in the molecular pathogenesis of CCA are also summarized.
Keywords: Cholangiocarcinoma
Introduction
Cholangiocarcinoma (CCA) is a fatal cancer of the biliary epithelium, arising either within the liver (intrahepatic) or in the extrahepatic bile ducts (extrahepatic). Globally, CCA is the second most common primary hepatic malignancy. Several recent epidemiological studies have shown that incidence and mortality rates of intrahepatic CCA are increasing 1,2,3,4,5,6,7,8.
Most time-trend studies have covered periods when mortality or incidence data were coded to the 8th or 9th revisions of the International Classification of Diseases (ICD). In these revisions, tumors with histology ‘cholangiocarcinoma’ (‘CCA’) were not coded to any specific topography. Hence, the data analyzed in these studies actually reflect time trends in the topographical codes for ‘intrahepatic bile duct tumors’ (IHBT). These tumors will not all have ‘CCA’ histology and, moreover, not all intrahepatic CCAs will be coded topographically as IHBT, but, rather, in ICD8 and 9 were also classified topographically as ‘primary liver tumors’. Since the 10th revision of the ICD was introduced into mortality statistics (in 2001 in the UK), all intrahepatic CCAs are specifically coded to the IHBT (C22.1) topographical code.
Changing epidemiology of cholangiocarcinoma
The peak age for CCA is the seventh decade, with a slightly higher incidence in men 1. Given the poor prognosis of CCA, mortality and incidence rates are similar. CCA incidence rates vary markedly worldwide, presumably reflecting differences in local risk factors and genetics. The highest rates are in northeast Thailand (96 per 100,000 men) and are about 100 times greater than in the West 1. The rise in IHBTs was first reported in the United Kingdom, where, in the mid-1990s, they overtook hepatocellular carcinoma as the leading cause of death for primary liver cancer 2,5,7. From 1968 to 2001, age-standardized mortality rates (ASMR per 100,000 population) for IHBT increased from 0.10 to 1.49 in males (M) and from 0.05 to 1.24 in females (F). The annual total number of deaths rose almost 30-fold, from 36 in 1968 to 1003 in 2004. Between 1979 and 2000, age standardized incidence rates (ASIR/100,000 population) increased from 0.11 to 1.2 (F) and from 0.13 to 1.36 (M). Age-specific incidence rates were highest in those over 75 years of age 6. Between 1971 and 2001 the incidence of gallbladder cancer halved and extrahepatic biliary tumors (EHBT) fell by a third in the UK 7.
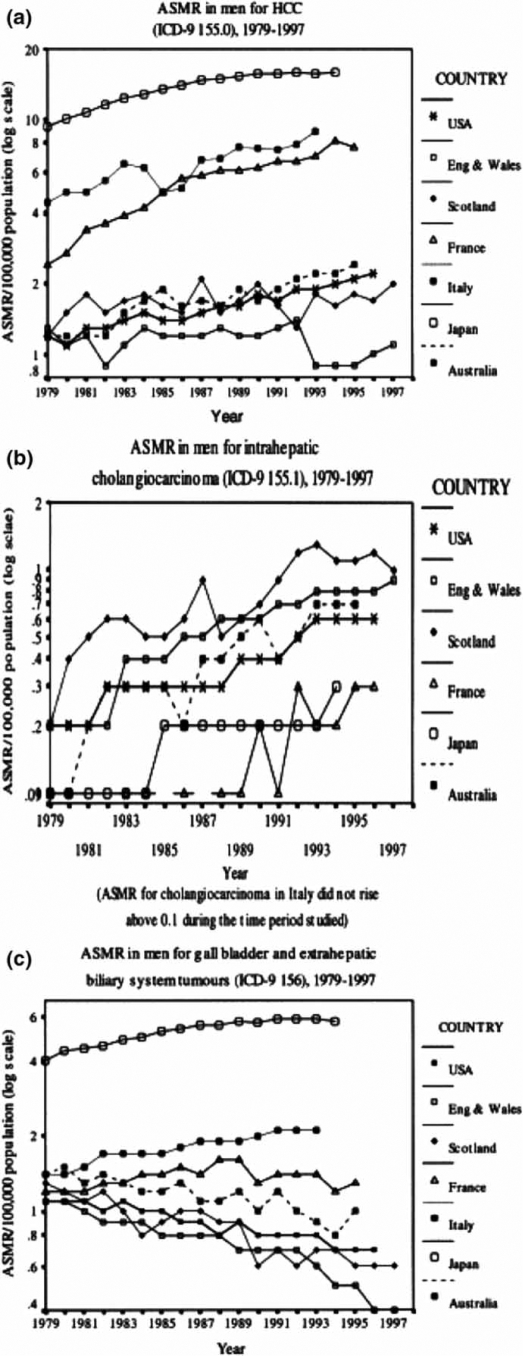
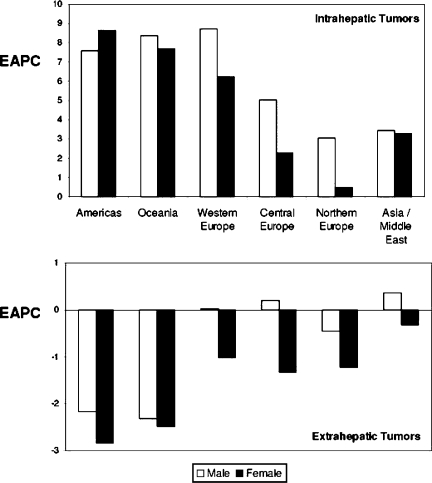
Further epidemiological studies have shown that mortality rates of IHBT are rising, while those of EHBT are falling, globally. World Health Organization mortality data were examined for the US, UK, France, Italy, Japan and Australia, 1979–1998 2. ASMR for IHBT increased in both sexes in all countries (Figure 1b), except in Japanese women. The highest increases were in Australia (M, 0.1 to 0.7) and the UK (0.2 to 0.83). Conversely, ASMR for EHBT decreased in most countries (such as the USA: 1.1 to 0.7), the exceptions being Italy and Japan (Figure 1c). Another study of the WHO databank calculated estimated annual percentage changes (EAPC) in age-adjusted mortality rates from 22 countries (Table I) 4. All major world regions were represented, other than Africa, where consecutive data were unavailable. EAPC for IHBT increased in both sexes in all countries except two: Norway and the Czech Republic, which had data available for the smallest time period (less than 8 years). The range of EAPC increases was 1.5 (Hungarian men) to 22 per 100,000 population (Slovenian men). There was an increase in IHBT mortality in all geographical regions: the Americas, Oceania, and across Europe, particularly western compared with central and northern Europe. In all countries, with the exception of Japan and Greece, EAPC for EHBT had fallen or remained stable (Table I, Figure 2) 4. A US study examined trends in IHBT incidence using data from the Surveillance, Epidemiology, and End Results (SEER) program, which represents over 10% of the total US population. Data from 1976–2000 were analysed by age, gender, and ethnicity. Intrahepatic CCA incidence increased in all groups, but was highest in black men (139%), followed by white men (124%), white women (111%), and black women (86%) 8.
Figure 1. .
Mortality trends in men from 1979 onwards for: (a) ICD-9 155.0 (primary hepatic parenchymal cancer, including hepatocellular carcinoma, HCC), (b) 155.1 (ICC) and (c) 156 (tumors of the gallbladder and ECC). Source: Khan et al. J Hepatol 2002;37:806–13.
Table I. Trends in mortality from intrahepatic and extrahepatic biliary tract cancers, WHO Databank (EAPC = estimated annual percent change in age-adjusted mortality rates). Source: Patel. BMC Cancer 2002;2:10.
| Extrahepatic tumors |
Intrahepatic tumors |
||||||
|---|---|---|---|---|---|---|---|
| Region | Country | EAPC |
EAPC |
||||
| Years | Male | Female | Years | Male | Female | ||
| Americas | Canada | 1970–1997 | −1.9 | −2.8 | 1979–1997 | 7.9 | 8.8 |
| USA | 1970–1996 | −2.5 | −2.8 | 1979–1997 | 7.3 | 8.5 | |
| Oceania | Australia | 1970–1995 | −1.9 | −1.9 | 1979–1995 | 12.3 | 12.0 |
| New Zealand | 1970–1996 | −2.7 | −3.1 | 1979–1996 | 4.4 | 3.4 | |
| Western Europe | Austria | 1970–1998 | −1.5 | −2.7 | 1980–1998 | 6.0 | 5.2 |
| Belgium | 1970–1994 | −1.5 | −2.5 | ||||
| Former FDR | 1970–1990 | −0.9 | −1.8 | ||||
| France | 1970–1996 | 1.2 | −0.1 | 1983–1996 | 10.0 | 5.2 | |
| Germany | 1990–1997 | −1.2 | −3.2 | 1991–1997 | 13.2 | 2.5 | |
| Greece | 1970–1997 | 3.2 | 2.2 | 1979–1997 | 1.8 | 2.4 | |
| Ireland | 1970–1996 | −0.5 | −2.5 | 1979–1996 | 10.9 | 8.5 | |
| Italy | 1970–1995 | 2.9 | 2.3 | 1986–1995 | 4.2 | 5.9 | |
| Netherlands | 1970–1995 | −2.3 | −3.9 | 1979–1995 | 3.5 | 0.2 | |
| Portugal | 1984–1998 | 1.7 | −0.1 | 1984–1996 | 10.4 | 10.3 | |
| Spain | 1970–1994 | 2.9 | 3.0 | 1982–1995 | 18.3 | 12.7 | |
| UK | 1970–1996 | −3.7 | −3.1 | 1979–1997 | 8.8 | 9.6 | |
| Central Europe | Czech | 1986–1993 | 0.3 | −2.2 | 1986–1993 | –8.5 | –5.6 |
| Former Czech | 1970–1991 | 1.4 | 0.6 | ||||
| Former GDR | 1976–1990 | −0.5 | −1.9 | ||||
| Hungary | 1970–1995 | 1.1 | −0.6 | 1979–1995 | 1.5 | 2.1 | |
| Slovenia | 1985–1996 | −1.3 | −2.5 | 1988–1996 | 22.1 | 10.3 | |
| Northern Europe | Iceland | 1971–1995 | −2.1 | −2.3 | |||
| Norway | 1970–1995 | 0.1 | −0.9 | 1989–1995 | –3.0 | –2.5 | |
| Sweden | 1970–1995 | 0.6 | −0.5 | 1987–1996 | 9.1 | 3.5 | |
| Asia/Middle East | Israel | 1970–1991 | −3.9 | −4.4 | 1987–1996 | –3.1 | 2.9 |
| Japan | 1970–1994 | 3.2 | 2.4 | 1979–1994 | 7.4 | 0.9 | |
| Singapore | 1970–1996 | 1.7 | 1.0 | 1979–1998 | 6.1 | 6.0 | |
Figure 2. .
Regional differences in the mean estimated annual percentage change in age-adjusted (1970 World Standard population) gender-specific mortality rates from intrahepatic biliary tract tumors (top) and gallbladder and extrahepatic biliary tract tumors (bottom). Source: Patel BMC Cancer 2002;2:10.
In contrast to the above investigations, none of which included Denmark, a recent study of Danish Cancer Registry data between 1978 and 2002 showed a fall in incidence rates of both IHBT (1.27 to 0.46 per 100,000 population) and EHBT (1.05 to 0.74). This occurred across all age groups and in both sexes 9.
Potential epidemiological artifact?
Does the rise in intrahepatic CCA incidence rates represent a real increase in this tumor? In a study of the SEER database it was found that age-adjusted incidence rates of IHBT increased by 165%, i.e. from 0.32/100,000 in 1975–1979 to 0.85/100,00 in 1995–1999 10. Increased detection of a tumor is usually associated with an increase in the proportion of patients with early stage disease or smaller sized lesions. The rise in IHBT was not associated with either. Furthermore, the increase in IHBT incidence has not levelled off, as would be expected if it was secondary to improvement in diagnostic techniques, such as ERCP, MRI, or CT, which have become established practice for several years 2,5,11. The data on EHBT may be more difficult to determine, as gallbladder cancers have previously been combined with EHBT for ICD coding purposes, which is revised on a regular basis. Gallbladder cancers are known to be decreasing, probably as a result of increasing cholecystectomy rates over the past few decades 2,5,11. This may partly explain falling rates of tumors coded as EHBT.
Another potential source of artifact is misclassification of hilar (Klatskin) tumors. Although these are topographically extrahepatic, the second edition of ICD-0-2 assigned them a histology code “8162/3, Klatskin”, which was cross-referenced to intrahepatic CCA. A recent study examined the impact of this misclassification of hilar (Klatskin) CCA on reported incidence rates 12. Annual percentage changes (APCs) were calculated with data from the SEER program. During 1992–2000, when SEER used ICD-O-2, 91% of hilar CCAs were incorrectly coded as intrahepatic CCA, resulting in an overestimation of intrahepatic CCA incidence by 13% and underestimation of extrahepatic CCA incidence by 15%. However, even after the exclusion of tumors that were coded as Klatskin, age-adjusted annual intrahepatic CCA incidence still increased (APC = 4%; p<0.001) 12.
Overall, incidence rates of IHBTs appear to be rising in most countries studied. This is not explained by any observed change in the incidence of known risk factors. Although the reason for the rise in IHBT remains unclear, the heterogeneity in rates with respect to regions, gender, and ethnicity suggests that the rise is likely, at least in part, to reflect a genuine increase in these tumors. The heterogeneity in rates between different regions, sexes, and ethnic groups suggests the increase in CCA is genuine.
Most cases of CCA are sporadic, and recognized risk factors account for a minority of cases. Given that CCA has risen in a relatively short period of time, an environmental factor is likely to play a role in carcinogenesis. Environmental carcinogens are unlikely to be evenly distributed geographically and would be expected to give rise to spatial patterns, or clustering, of CCA risk. Preliminary data from the UK support this. A spatio-temporal analysis of intrahepatic biliary tract mortality, at the District level, between 1981 and 2004, showed strong evidence of spatial clustering, particularly in rural areas with a high proportion of agricultural land use 13.
Risk factors and pathogenesis for cholangiocarcinoma 11,14,15
Bile duct factors
Primary sclerosing cholangitis (PSC) is the commonest known predisposing factor for CCA in the West. CCA rates of up to 40% have been reported in PSC patients 1. In East Asia, where the disease is common, CCA has been pathogenically associated with liver fluke infestation, particularly the endemic Opisthorcis viverrini1,16. Malignant change in the biliary epithelium of Syrian hamsters occurs following infection with O. viverrini, particularly if fed nitrosamines, which are known carcinogens produced by bacteria in certain foodstuffs 17. Congenital fibropolycystic liver disease carries a 15% risk of malignant change, the average age being 34 years 18. Bile duct adenomas and biliary papillomatosis are also associated with CCA risk.
Up to 10% of patients with intrahepatic biliary stones develop CCA 19. Relatively rare in the West, hepatolithiasis is common in parts of Asia and is associated with intrahepatic CCA 1. A Taiwanese study found that up to 70% of patients undergoing resection for CCA had hepatolithiasis 20,21. Choledocholithiasis (odds ratio, OR, 24) and cholecystolithiasis (OR 4.0) were associated with an increased risk of CCA in a recent Danish case-control study 22.
Other factors
Various chemicals have been linked to CCA. The banned carcinogenic contrast agent Thorotrast has been strongly associated with CCA, in some cases many years after exposure 23. Epidemiological associations have been made to industrial toxins such as dioxins and nitrosamines 24. Excess alcohol was higher in intrahepatic CCA (22%) compared to controls (4%) in a recent US case-control study 25. This study found no difference in the prevalence of diabetes and smoking. In another US case-control study, significantly higher rates of thyrotoxicosis, chronic pancreatitis, non-specific liver cirrhosis, and alcohol were found in both extrahepatic CCA and intrahepatic CCA compared to controls. Higher rates of HCV infection, chronic non-alcoholic liver disease, and obesity (all of which are increasing in incidence) were associated with intrahepatic CCA only, suggesting that different conditions might explain the divergent incidence trends of intrahepatic CCA and extrahepatic CCA 26. This study found that smoking was associated with intrahepatic CCA.
Cirrhosis from any cause is associated with an increased risk of CCA 26,27. In a cohort study of over 11,000 patients with cirrhosis followed up over 6 years, a 10-fold excess risk of CCA was found compared to the general population 27. Hepatitis B (HBV) and hepatitis C (HCV) are established risk factors for hepatocellular carcinoma and may have a role in cholangiocarcinogenesis. In a Korean case-control study, 13% of CCA cases were found positive for anti-HCV and 14% for HBV surface antigen (HBsAg), compared to 4% and 2% of controls, respectively 28. In a study from Italy, 23% of CCA patients were found anti-HCV positive and 11.5% HBsAg positive compared with 6% and 5.5% of controls 29. A Japanese prospective controlled study found the risk of CCA in HCV-related cirrhosis to be 3.5% after 10 years, 1000 times greater than in the general population 30. A case-control study from the US reported adjusted OR of 6 for HCV infection, and similarly for HIV 31. In another US study, a higher prevalence of anti-HCV antibodies and antibodies to Hepatitis B core antigen (anti-HBc) was found in intrahepatic CCA cases (6% and 9.6%, respectively) compared to controls (0.8% and 0%) 25.
Molecular pathogenesis of cholangiocarcinogenesis
Differences in geographical rates, gender, race, and risk factors suggest diverse mechanisms are likely to be involved in the multi-step development of CCA 11,14. Chemically associated carcinogenesis may involve the induction of promutagenic DNA adducts, which occur more commonly in CCA tissue compared to controls 32. The main hepatobiliary risk factors all tend to cause chronic inflammation and/or cholestasis in the bile duct. At the molecular level, cholangiocarcinogenesis is probably triggered by changes in the bile duct microenvironment secondary to these events (Table II) 14.
Table II. Proposed steps in the molecular pathogenesis of cholangiocarcinoma 14,33.
| Step | Postulated mechanisms |
|---|---|
| Biliary epithelial cell proliferation signaling | Generation of cytokines, growth factors, e.g. IL-6, hepatocyte growth factor; upregulation of c-met and oncogenes, e.g. c-erb-2 |
| Cholangiocyte DNA damage | Nitric oxide; chemical genotoxins |
| Augmented cell cycle progression | Mutations in key cell cycle genes, e.g. k-ras, p53 |
| Dysregulation of apoptosis | Disabling Fas-R signaling through the expression of FLICE inhibitor; p53 mutation; nitric oxide |
| Loss of telomere shortening | High levels of telomerase mRNA |
| Stimulation of angiogenesis | Increased vascular endothelial growth factor |
| Loss of heterozygosity and microsatellite instability | DNA damage, e.g. due to defective DNA repair |
| Increased exposure of cholangiocyte to xenotoxins in bile | Polymorphisms in metabolizing enzymes, e.g. CYP1A2, UGT1A7 |
| Disrupted bile flow, bile acid activation, and cholangiocyte exposure to endogenous toxins | Polymorphisms in biliary transporter genes, e.g. BSEP, MDR, FIC1 |
Consensus statements
Incidence rates of intrahepatic bile duct tumors appear to be rising in most countries studied. The heterogeneity in rates with respect to regions, gender, and ethnicity suggests that the rise is likely, at least in part, to reflect a genuine increase in this tumor.
There should be international consistency in the diagnosis and topographical and morphological classification of all bile duct tumors to facilitate accurate surveillance of future epidemiological trends.
Further studies are required into the etiopathogenesis of CCA.
References
- 1.Shaib , et al. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 2.Khan , et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor-Robinson , et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–20. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandeville. et al. Update of mortality and incidence rates for intrahepatic cholangiocarcinoma and other hepatobiliary tumours in England and Wales. J Hepatol 2005;42 Suppl 2. [Google Scholar]
- 7.West , et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94:1751–8. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGlynn , et al. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 9.Jepsen , et al. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–7. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 10.Shaib , et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–7. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Khan , et al. Cholangiocarcinoma: Seminar. Lancet. 2005;366:1303–14. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 12.Welzel , et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–5. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 13.Toledano, et al. Spatial epidemiology of biliary tract tumours. British Association for the Study of the Liver (BASL), Imperial College London. 2007 (Abstract). [Google Scholar]
- 14.Berthiaume , et al. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127–37. doi: 10.1055/s-2004-828890. [DOI] [PubMed] [Google Scholar]
- 15.Malhi , et al. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006;45:856–67. doi: 10.1016/j.jhep.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanapa Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002;89:962–70. doi: 10.1046/j.1365-2168.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 17.Thamavit , et al. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–9. [PubMed] [Google Scholar]
- 18.Simeone Yamada Lippincott, Williams & Wilkins; Philadelphia: 1999. Gallbladder and biliary tree: anatomy and structural anomalies, Textbook of gastroenterology; pp. 2244–57. [Google Scholar]
- 19.Kubo , et al. Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995;19:637–41. doi: 10.1007/BF00294744. [DOI] [PubMed] [Google Scholar]
- 20.Chen Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol. 1999;14:1144–9. doi: 10.1046/j.1440-1746.1999.01983.x. [DOI] [PubMed] [Google Scholar]
- 21.Okuda , et al. Cholangiocarcinoma: recent progress. Part 1: Epidemiology and etiology. J Gastroenterol Hepatol. 2002;17:1049–55. doi: 10.1046/j.1440-1746.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 22.Welzel , et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–41. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 23.Sahani , et al. Thorotrast-induced cholangiocarcinoma. Abdom Imaging. 2003;28:72–4. doi: 10.1007/s00261-001-0148-y. [DOI] [PubMed] [Google Scholar]
- 24.Hardell , et al. Aetiological aspects on primary liver cancer with special regard to alcohol, organic solvents and acute intermittent porphyria. Br J Cancer. 1984;50:389–97. doi: 10.1038/bjc.1984.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaib , et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–21. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 26.Welzel , et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States. Clin Gastroenterol Hepatol. 2007;5:1221–8. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorensen , et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–5. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 28.Shin , et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–40. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 29.Donato , et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–64. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi , et al. Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–7. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 31.Shaib , et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–6. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Khan , et al. DNA adducts, detected by 32P postlabelling, in human cholangiocarcinoma. Gut. 2003;52:586–91. doi: 10.1136/gut.52.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan, et al. Histopathology and molecular pathogenesis of cholangiocarcinoma, Biliary tract cancer: a multidisciplinary approach. In:. New York: Demos Medical Publishing; 2008. In press. [Google Scholar]