Abstract
Background:
Imatinib mesylate is used in combination with hydroxyurea (HU) in ongoing clinical phase II studies in recurrent glioblastoma multiforme (GBM). CYP3A4 enzyme-inducing antiepileptic drugs (EIAEDs) like carbamazepine, phenytoin, and oxcarbazepine - as well as non-EIAEDs like valproic acid, levetiracetam, and lamotrigine - are frequently used in patients with GBM. Since CYP3A4 is the major isozyme involved in the metabolism of imatinib, we investigated the influence of EIAEDs on imatinib pharmacokinetics (pk).
Methods:
GBM patients received 600 mg imatinib p.o./o.d. in combination with 1.0 g HU p.o./o.d..together with either EIAEDs, non-EIAEDs, or no antiepileptic drug (non-AEDs) comedication. Trough plasma levels of imatinib and its active main metabolite N-desmethyl-imatinib (CGP74588) were determined biweekly in these patients, total 543 samples being collected from 224 patients (up to 6 times / patient). All three groups were compared to each other and with historical pharmacokinetic data obtained from patients with chronic myeloid leukemia (CML).
Results:
Mean imatinib trough levels in patients not receiving AEDs ( 1404 ng/ml, CV 64%) and on non-EIAEDs (1374 ng/ml, CV 46%) were comparable with mean imatinib trough levels of the historical control group of CML patients (1400 ng/ml, CV 50%). Mean trough levels of imatinib were reduced up to 2.9-fold (477 ng/ml, CV 70%) in patients treated with EIAEDs. Only slight, but although significant differences were observed in the mean trough level of the metabolite CGP74588 between EIAED-, non-EIAED and no-AED patients, 240 ng/ml (CV 57%) , 351 ng/ml (CV 34%) and 356 ng/ml (CV 52%), respectively. The corresponding mean level for CML patients was 300 ng/ml (CV 50%).
Conclusion:
Significant decreases of imatinib and CGP74588 trough levels were observed for patients receiving EIAEDs. The EIAED-induced reduction in trough imatinib levels can be avoided by switching to non-EIAEDs comedication or compensated by administering higher imatinib doses. In addition these data demonstrate that there is no significant difference in the pharmacokinetics of imatinib between patients with glioblastoma and CML.
Key Words: Imatinib, STI571, CGP74588, EIAED, phenytoin, valproate, carbamazepine, oxcarbazepine, topiramate, lamotrigine, levetiracetam, pharmacokinetics, main metabolite, cytochrome P450, CYP3A4, glioblastoma multiforme, CML.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most frequent primary malignant intracranial tumor in adults. Despite advances in multidisciplinary treatment approaches like neurosurgery, radiation and chemotherapy the overall prognosis remains poor with a median survival between 12 and 18 months after diagnosis. Recurrence under optimal treatment including chemotherapy with temozolomide seems to be nearly unavoidable. Following progression the available therapies like surgical reintervention, external beam radiotherapy and salvage chemotherapy provide a limited survival benefit [1-6].
In search of new treatment strategies preclinical and clinical research focussed on novel agents targeting distinct signaling pathways. CNS malignancies including GBM were found to express epithelial growth factor-receptor (EGF-R) and platelet derived growth factor-receptor (PDGF-R). Imatinib mesylate (STI571, Gleevec) is a selective inhibitor of the tyrosine kinases BCR-ABL, c-KIT and PDGF-R and has been shown to be highly active in chronic myeloid leukemia (CML) and has significant antitumor efficacy against gastrointestinal stroma tumors (GIST). In GBM only modest responses could be reached if imatinib is used as a single agent. In contrast, a combination of 600mg imatinib with 1000mg Hydroxyurea (HU) daily leads to an increased response rate in patients with progressive GBM. Currently, the mechanism underlying the enhanced activity of this combination regime is not well understood [7-14].
Concomitant administration of enzyme-inducing antiepileptic drugs (EIAEDs) as well as non enzyme-inducing antiepileptic drugs (non-EIAEDs) are a common therapy in patients with brain tumors. The EIAEDs carbamazepine and phenytoin are potent inducers of cytochrome P450 isoenzyme CYP3A4, whereas oxcarbazepine and topiramate are known as weak inducers of the enzyme. The non-EIAEDs levetiracetam and maybe lamotrigine are most likely not involved in drug interactions. Valproic acid slightly inhibits CYP3A4 activity and is able to significantly displace drugs from plasma albumin. Imatinib is metabolised mainly by cytochrome P450 CYP3A4 to its main metabolite CGP74588 that has similar in vitro activity to the parent compound. Therefore significant interactions may occur between imatinib and EIAEDs leading to changes in plasma concentration of imatinib, CGP74588 as well as coadministered drugs. Some studies have investigated the effects of EIAEDs on the pharmacokinetics of imatinib and CGP74588 [15-21, 25]. They demonstrated that EIAED’s could lead to a substantially decreased plasma exposure of imatinib. In patients receiving imatinib for chronic myelogenous leukemia and gastrointestinal stromal tumors they should be avoided if possible. On the other hand, imatinib is still the most important drug for the therapy of CML and GIST, and moreover the pk of alternative tyrosine kinase inhibitors is presumably also altered by EIAEDs.
To improve the knowledge about the influence of the various EIAEDs on the imatinib pk, we determined trough levels of imatinib and CGP74588 in a collective of 224 GBM patients. The analysed imatinib trough level confirm the results that were found in earlier studies and in addition demonstrate, that the imatinib pk from GBM patients without EIAED application is not different from the pk in CML patients.
PATIENTS AND METHODS
Patients and Sample Collection
We analyzed trough levels of imatinib and CGP74588 in a total of 224 patients (age 19 to 69 years, median 51 years) with histologically confirmed diagnosis of glioblastoma multiforme/ astrocytome WHO grade IV who received 300 - 600mg imatinib o.d. (once daily) and 2 - 3 x 500mg Hydroxyurea p.o. daily. The differences of the median age and gender in the compared trough level groups were not significant. All presented trough levels were linearly calculated for a 600mg / o.d. dose of imatinib. Group A consists of 111 patients treated with imatinib and HU without antiepileptic co-medication (non-AED), Group B consists of 28 patients treated with imatinib and HU in combination with non-EIAEDs, Group Cconsists of 85 patients treated with imatinib and HU in combination with EIAEDs. In group B (non-EIAED) 4 patients received lamotrigine, 15 valproic acid and 9 levetiracetam. In group C (EIAED) 15 patients received phenytoin, 63 carbamazepine, 6 oxcarbazepine and 1 topiramate. Patients who were concomitantly treated with other CYP3A4 inducing drug compounds were excluded. At the beginning of treatment all patients had adequate renal (serum creatinine ≤ 1.5 x ULN), hepatic (SGOT and SGPT ≤ 2.5 x ULN, total bilirubin ≤ 1,5 x ULN) and bone marrow (ANC ≥1.5 x 109/L, platelets ≥ 100 x 109/L and Hgb >10g/dL) function.
Blood samples were scheduled on week 2, 4, 6, 18, 30 and 42 of the study prior to the morning dosage administration, but only in a small portion of patients all scheduled samples were drawn in this multicenter study (543 samples from 224 patients). Samples were centrifuged at 1000g for 10min, 1ml of plasma was separated, stored at –20°C and sent by express mail to our laboratory for analysis.
HPLC Measurement
The concentration of Imatinib and CGP74588 was determined using a single high performance liquid chromatograph method with ultraviolet detection. After protein precipitation samples were prepared and both substances were online enriched on a Zirchrom-PBD (ZirChrom Separations, USA, Anoka) guard column. Analysis was performed with a Zirchrom-PBD analytical column followed by ultraviolett detection at 260nm. Lower limit of quantification was 10ng/ml for both Imatinib and CGP74588. The intra-day precision in plasma samples, as expressed by the coefficient of variation, ranges between 1.74% and 8.60% for imatinib and 1.45% and 8.87% for CGP74588, depending on the concentration. The inter-day precision for a plasma concentration of 1000 ng/ml analyzed over a 7-month time period was 8.31% for imatinib and 6.88% for CGP74588.
The method was validated and is established for routine use as described previously [22-24]. The GINA star software (Raytest, Straubenhardt, Germany) was used for data acquisition, evaluation and integration of chromatograms.
Pharmacokinetics
All trough concentrations of imatinib and CGP74588 were determined after the patients had received imatinib for at least two weeks. In consideration of the half- life of imatinib (16.5- 26h) and CGP74588 (29,5- 73,5h) within this period all patients should have reached pseudo steady state conditions [23-25]. The obtained data were compared between treatment groups, and with historical concentrations and simulations from CML patients (Reference). The mean, median, maximum (max), minimum (min), standard deviation (SD) and coefficients of variation (CV) were calculated for the trough concentrations using the Microsoft excel software.
Statistical Analysis
The statistical analysis was performed using Excel and SPSS for Windows. Comparisons between groups were perfomed using a t-test for independent samples. The resulting trough levels are presented as means, standard deviation (±SD) and coefficient of variation (CV), if not otherwise indicated. Differences were considered as statistically significant if a P-value < 0.05 was achieved.
RESULTS
The results of the measured imatinib and CGP74588 steady-state trough concentrations in 543 plasma samples from the enrolled 224 GBM patients are summarized in tables 1 and 2 for a dose of 600mg imatinib o.d. No significant differences in trough level of imatinib were observed between group A (non-AED) Ctrough = 1404 ng/ml (CV 64%) and group B (non-EIAED) Ctrough = 1374 ng/ml (CV 46%). Analogous results were found for CGP74588, group A Ctrough = 356 ng/ml (CV 52%) and group B (non-EIAED) Ctrough = 351 ng/ml (CV 34%). The parameters which were obtained previously from hematological patients, Ctrough = 1400ng/ml (CV 50%) for imatinib and Ctrough = 300ng/ml (CV 50%) for CGP74588 were quite similar to those seen in groups A and B. Even within group B the comparison of separate drug compounds levetiracetam (imatinib: Ctrough = 1369 ng/ml,CV 47%; CGP74588: Ctrough = 347 ng/ml, CV 36%), lamotrigine (imatinib: Ctrough = 1466ng/ml, CV 28%; CGP74588: Ctrough = 431ng/ml, CV 25%) and valproic acid (imatinib: Ctrough = 1399ng/ml, CV 47%; CGP74588: Ctrough = 355ng/ ml, CV 33%) showed no significant difference.
Table 1.
Mean Trough Levels and Statistical Parameters for Imatinib Mesylate and CPG74588 in GBM Patients (All Data refer to a 600mg Imatinib o.d. Application)
| Group A no Antiepileptics |
Group B Non EIAED Antiepileptics |
Group C EIAED Antiepileptics |
not Clearly Classified | |||||
|---|---|---|---|---|---|---|---|---|
| Valproic Acid | Levetiracetam | Phenytoin | Carbamazepine | Oxcarbazepine | Topiramate | Lamotrigine | ||
| n samples | 224 | 28 | 24 | 22 | 199 | 33 | 4 | 9 |
| n patients | 111 | 15 | 9 | 15 | 63 | 6 | 1 | 4 |
| imatinib trough level in ng/ml | ||||||||
| Mean ng/ml | 1404 | 1399 | 1369 | 380 | 473 | 534 | 722 | 1466 |
| SD ng/ml | 899 | 664 | 640 | 266 | 358 | 193 | 199 | 405 |
| CV in % | 64 | 47 | 47 | 70 | 76 | 36 | 28 | 28 |
| Median in ng/ml | 1245 | 1264 | 1206 | 390 | 363 | 569 | 724 | 1249 |
| Max in ng/ml | 6881 | 3368 | 2678 | 1001 | 1952 | 868 | 960 | 2019 |
| Min in ng/ml | 38 | 235 | 383 | 22 | 14 | 127 | 481 | 1050 |
| CGP74588 trough level in ng/ml | ||||||||
| Mean ng/ml | 356 | 355 | 347 | 268 | 240 | 216 | 291 | 431 |
| SD ng/ml | 186 | 117 | 123 | 196 | 137 | 86 | 140 | 107 |
| CV in % | 52 | 33 | 36 | 73 | 57 | 40 | 48 | 25 |
| Median in ng/ml | 323 | 311 | 338 | 205 | 209 | 204 | 297 | 420 |
| Max in ng/ml | 1482 | 616 | 663 | 768 | 724 | 464 | 455 | 577 |
| Min in ng/ml | 27 | 192 | 170 | 45 | 40 | 50 | 114 | 278 |
Table 2.
Mean Trough Levels in Three GBM Patient Groups and in CML Patients. All Data refer to a 600mg Imatinib o.d. Application
| Group A no-AED |
Group B non-EIAED |
Group C EIAED |
CML Patients | |
|---|---|---|---|---|
| imatinib trough level in ng/ml | ||||
| mean in ng/ml | 1404 | 1374 | 477 | 1400 |
| SD in ng/ml | 899 | 631 | 335 | 700 |
| CV in % | 64 | 46 | 70 | 50 |
| CGP74588 trough level in ng/ml | ||||
| mean in ng/ml | 356 | 351 | 240 | 300 |
| SD in ng/ml | 186 | 121 | 137 | 150 |
| CV in % | 52 | 34 | 57 | 50 |
| Ratio CGP74588/imatinib in % | ||||
| mean in % | 25 | 26 | 46 | 21 |
The patients treated with EIAEDs (group C) showed a significant decrease of about 68% in the steady-state level of Imatinib Ctrough = 477 ng/ml (CV 70%, P<0.05) compared to the other groups. Furthermore the values within group C had a greater variability as compared to groups A and B. Patients receiving phenytoin showed the lowest concentrations of imatinib: Ctrough = 380 ng/ml (CV 70%), followed by Ctrough = 473ng/ml (CV 76%) for patients on carbamazepine. In the oxcarbazepine group we obtained a Ctrough = 534 ng/ml (CV 36%) and from one patient taking topiramate four measured plasma samples gave Ctrough = 722ng/ml (CV 28%). Thus the decrease in imatinib trough level in group C ranged from about 73% to about 48% compared to groups A and B. In contrast the influence of EIAEDs on metabolite were less remarkable but although significant. For CGP74588 a mean Ctrough = 240 ng/ml (CV 59%, P<0.05) was found corresponding to a decrease of about 32% compared to group A and B. Within group C for phenytoin (Ctrough = 268 ng/ml CV 73%), carbamazepine (Ctrough = 240 ng/ml CV 57%) and oxcarbazepine (Ctrough = 216 ng/ml CV 40%) quite similar values were found. Only the patient on topiramate showed a slightly higher CGP74588 level Ctrough = 291ng/ml CV 48%.
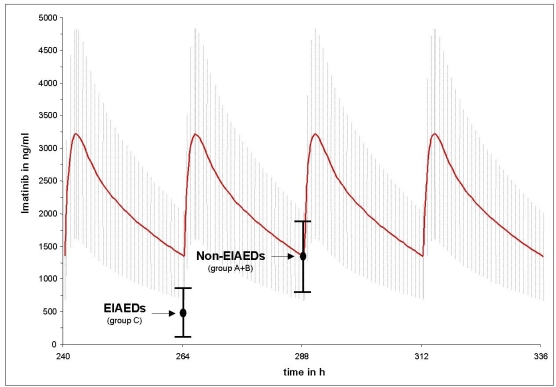
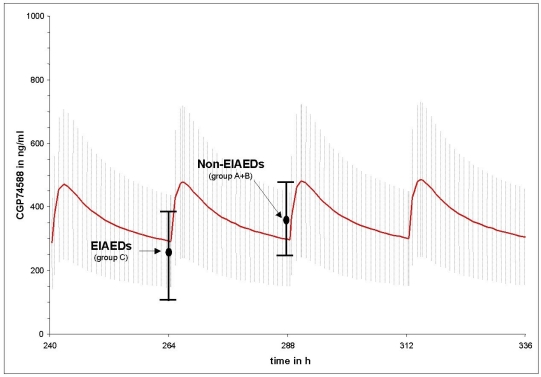
Figs. (1 and 2) compares the mean imatinib and CGP74588 trough level results of group A (non-AED) plus B (non-EIAEDs) to group C (EIAEDs), depicted on the calculated plasma curves from CML patients. They show that the mean trough level of group A plus B fits nearly perfectly to the the CML curves, while for group C significant decreases were observed.
Fig. (1).
Differences between the mean trough level of imatinib in patients on EIAEDs and without EIAEDs compared to the mean imatinib trough level in CML patients without antiepileptic drugs. Points indicate mean measured trough level of imatinib and black vertical bars indicate the standard deviation. The red curve represents the calculated mean imatinib plasma decay curve under pseudo steady-state conditions from CML patients taking 600mg imatinib o.d.. The grey hutching represents the related standard deviation.
Fig. (2).
Differences between the mean trough level of CGP74588 in patients on EIAEDs and without EIAEDs compared to the mean of CGP74588 trough level in CML patients without antiepileptic drugs. Points indicate mean measured trough level of CGP74588 and black vertical bars indicate the standard deviation. The red curve represents the calculated mean CGP74588 plasma decay curve under pseudo steady-state conditions from CML patients taking 600mg imatinib o.d.. The grey hutching represents the related standard deviation.
The mean CGP74588 to imatinib concentration ratios for groups A, B and for patients with CML were 25%, 26% and 21%, respectively. Due to the higher decrease of imatinib concentrations compared to CGP74588 the ratio in patients on EIAEDs was high, 50%.
DISCUSSION
In the present investigation, EIAED- treated patients displayed a significantly reduced trough level of imatinib in comparison with patients who were on non-EIAEDs or did not take AEDs, respectively. The respective differences were less pronounced but although significant for CGP74588. In a recent publication, Wen et al. evaluated plasma concentrations of imatinib and CGP74588 in 11 patients that were concomitantly treated with EIAEDs and the same number of patients not receiving EIAEDs. Results demonstrate a decrease of imatinib plasma AUC of about 70% and of CGP74588 plasma AUC of about 10%, respectively. The overall AUC of imatinib and CGP74588 was reduced 2.7 fold whereas the calculated trough concentration Cmin showed a decrease of about 79% for imatinib and of about 40% for CGP74588 [20]. Table 3 compares these findings to our study. The results of this investigation are in line with our findings of 68% (about 2.9 fold) and 32% decline in trough levels of imatinib and CGP74588 in patients receciving EIAEDs. Additionally, our results are confirmed by the report of Reardon et al., where 17 patients with GBM were treated with imatinib and HU. The patients (n=8), who were not receiving EIAEDs had pharmacokinetic values comparable to those from patients with haematological malignancies and GIST. In contrast, patients (n=9) receiving EIAEDs showed a significant shorter t1/2, a lower AUC and higher Clapp than the other group. The influence of EIAEDs on the pharmacokinetics of CGP74588 was only moderate [15]. Similar effects were seen after pretreatment with the CYP3A4 inductor rifampicin in healthy volunteers with a 70% decrease of the AUC of imatinib. The slight increase in the concentration of CGP74588 observed in that report might be explained by the fact that patients had received only two single doses of imatinib within two weeks and therefore had not reached steady-state levels of imatinib and CGP74588 [21].
Table 3.
Dose-Normalized Trough Levels Published by Wen et al. Compared with the Data in the Present Study
Within the group of EIAEDs, the effects of phenytoin and carbamazepine on imatinib trough levels were comparable. As expected, thee effect of oxcarbazepine, a weaker CYP3A4 inducer on imatinib exposure was less profound. The patient receiving topiramate showed a less prominent decrease of imatinib concentrations (48%) compared to the rest of the EIAED group (68%) [17]. Since the data on topiramate-imatinib interaction are derived from one patient only, this result has to be interpreted with caution. But still, the observation would fit very well to the less pronounced enzyme-inducing activity reported for topiramat. These results suggest that a considerable increase of the individual imatinib dose may be considered in patients on EIAEDs. Reardon et al. reported that in patients with GBM on EIAEDs who received HU and 1000 mg imatinib, the imatinib exposure remained significantly lower compared to patients on non-EIAEDs. Surprisingly, patients with EIAEDs intake had an improved progression free survival. On the other hand, in spite of the lower AUC of imatinib the observed toxicity was significant which lead to a maximum tolerated dose of 500mg imatinib twice daily for patients with EIAED co-medication [15]. However, patients with CML or GIST who receive imatinib and EIAEDs will be at an increased risk of not being able to achieve the optimal clinical response due to a decreased imatinib exposure. On the other hand, patients whose dose is to be escalated should be carefully monitored for signs of toxicity or intolerance.
In summary, we observed a significant decrease of imatinib and CGP74588 exposure in patients taking EIAEDs. No significant difference in imatinib trough concentrations was observed between GBM patients who did not receive AEDs or non-EIAEDs and CML patients. For the compounds levetiracetam, lamotrigine and even valproic acid, the later being considered as a weak inhibitor of CYP3A4 [19], we found no effect on imatinib trough levels. Thus, switching to non-EIAEDs if anticonvulsive therapy is needed, might be an option for patients receiving imatinib treatment. Application of Imatinib and EIAEDs should only done if absolutely necessary, and if so in combination with repetitively trough level controls.
REFERENCES
- 1.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 2.Hou LC, Veeravagu A, Hsu AR, Tse VCL. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:E5. doi: 10.3171/foc.2006.20.4.2. [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology and End Results (SEER) data 1973-1991. J NeuroSurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Dehdashti AR, Hegi ME, Regli L, Pica A, Stupp R. New trends in the medical management of glioblastoma multiforme: the role of temozolomide chemotherapy. Neurosurg Focus. 2006;20:E6. doi: 10.3171/foc.2006.20.4.3. [DOI] [PubMed] [Google Scholar]
- 5.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–3. [PubMed] [Google Scholar]
- 8.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–9. [PubMed] [Google Scholar]
- 9.Buchdunger E, O'Reilly T, Wood J. Pharmacology of imatinib (STI571) Eur J Cancer. 2002;38:28–36. doi: 10.1016/s0959-8049(02)80600-1. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 11.Kubota T. Gastrointestinal stromal tumor (GIST) and imatinib. Int J Clin Oncol. 2006;11:184–9. doi: 10.1007/s10147-006-0579-0. [DOI] [PubMed] [Google Scholar]
- 12.Wen PY, Yung WK, Lamborn K, et al. Phase I/II study of imatinib mesylate (ST1571) for patients with recurrent malignant gliomas (NABTC 99-08) Neuro-Oncol (abstr TA-63) 2004;6:384. [Google Scholar]
- 13.Van den Bent MJ, Brandes AA, van Oosterom A, et al. Multicentre phase II study of imatinib mesylate (Gleevec) in patients with recurrent glioblastoma: An EORTC NDDG/BTG intergroup study. Neuro-Oncol (abstr TA-57) 2004;6:383. [Google Scholar]
- 14.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol. 2005;16:1702–8. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 15.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–68. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 16.Vecht CJ, Wagner GL, Wilms EB. Treating seizures in patients with brain tumors: Drug interactions between antiepileptic and chemotherapeutic agents. Semin Oncol. 2003;30:49–52. doi: 10.1053/j.seminoncol.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Turnheim K. Arzneimittelwechselwirkungen mit Antiepileptika. Wien Klin Wochenschr. 2004;116:112–8. doi: 10.1007/BF03040747. [DOI] [PubMed] [Google Scholar]
- 18.Riva R, Albani F, Contin M, Baruzzi A. Pharmacokinetic interactions between antiepileptic drugs. Clinical considerations. Clin Pharmacokinet. 1996;31:470–93. doi: 10.2165/00003088-199631060-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wen X, Wang JS, Kivisto KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: preferential inhibition of cytochrome P450 2C9 (CYP2C9) Br J Clin Pharmacol. 2001;52:547–53. doi: 10.1046/j.0306-5251.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 21.Bolton AE, Peng B, Hubert M, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–6. doi: 10.1007/s00280-003-0722-9. [DOI] [PubMed] [Google Scholar]
- 22.Schleyer E, Pursche S, Kohne CH, et al. Liquid chromatographic method for detection and quantitation of STI-571 and its main metabolite N-desmethyl-STI in plasma, urine, cerebrospinal fluid, culture medium and cell preparations. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:23–36. doi: 10.1016/j.jchromb.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Le Coutre P, Kreuzer KA, Pursche S, et al. Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol. 2004;53:313–23. doi: 10.1007/s00280-003-0741-6. [DOI] [PubMed] [Google Scholar]
- 24.Bornhauser M, Pursche S, Bonin M, et al. Elimination of imatinib mesylate and its metabolite N-desmethyl-imatinib. J Clin Oncol. 2005;23:3855–6. doi: 10.1200/JCO.2005.05.246. [DOI] [PubMed] [Google Scholar]
- 25.Peng B, Hayes M, Resta D, et al. Clinical investigation of the pharmacokinetics and pharmacodynamics of imatinib in a phase 1 trial in chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–942. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]