Abstract
The density of native (pre-existing) collaterals and their capacity to enlarge into large conduit arteries in ischemia (arteriogenesis) are major determinants of the severity of tissue injury in occlusive disease. Mechanisms directing arteriogenesis remain unclear. Moreover, nothing is known about how native collaterals form in healthy tissue. Evidence suggests VEGF, which is important in embryonic vascular patterning and ischemic angiogenesis, may contribute to native collateral formation and arteriogenesis. Therefore, we examined mice heterozygous for VEGF receptor-1 (VEGFR-1+/-), VEGF receptor-2 (VEGFR-2+/-), and over-expressing (VEGFhi/+) and under-expressing VEGF-A (VEGFlo/+). Recovery from hindlimb ischemia was followed for 21 days after femoral artery ligation. All statements below are p<0.05. Compared to wild-type mice, VEGFR-2+/- showed similar: ischemic scores, recovery of hindlimb perfusion, peri-collateral leukocytes, collateral enlargement and angiogenesis. In contrast, VEGFR-1+/- showed impaired: perfusion recovery, peri-collateral leukocytes and collateral enlargement, worse ischemic scores, and comparable angiogenesis. Compared to wild-type mice, VEGFlo/+ had 2-fold lower perfusion immediately after ligation (suggesting fewer native collaterals which was confirmed by angiography) and blunted recovery of perfusion. VEGFhi/+ mice had 3-fold greater perfusion immediately after ligation, more native collaterals, and improved recovery of perfusion. These differences were confirmed in the cerebral pial cortical circulation where, compared to VEGFhi/+ mice, VEGFlo/+ formed fewer collaterals during the perinatal period when adult density was established, and had 2-fold larger infarctions after middle cerebral artery ligation. Our findings indicate VEGF and VEGFR-1 are determinants of arteriogenesis. Moreover, we describe the first signaling molecule, VEGF-A, that specifies formation of native collaterals in healthy tissues.
Keywords: Arteriogenesis, angiogenesis, cerebral circulation, cerebral infarction, vascular development
Introduction
Ischemic vascular disease is the leading cause of morbidity and mortality in the developed world.1 The number and diameter of native (pre-existing) collaterals in healthy tissue and their capacity to enlarge (remodel) are critical determinants of the severity of ischemic injury following arterial obstruction. While molecules regulating collateral remodeling in ischemia are beginning to be understood,2 nothing is known regarding when native collaterals form and the responsible signaling mechanisms.3
Collateral enlargement in ischemia requires proliferation of endothelial and mural cells, leukocyte recruitment and remodeling of extracellular matrix. Evidence suggests VEGF participates in these processes.4 However, trials testing whether exogenous VEGF can augment collateral enlargement have met with mixed results.1 The multiple VEGF isoforms, receptors and intracellular pathways, plus the inaccessibility of the collateral circulation, make it difficult to determine a direct effect on collateral remodeling. Most studies have administered a single VEGF isoform with limited control of concentration, leading to defective neovascularization, tissue edema and impaired vessel growth.5 Recent studies have shown that multiple gradient-forming isoforms are expressed within narrow concentration windows that, if exceeded too greatly in either direction, lead to aberrant vessel formation, embryonic lethality or disturbed vessel maintenance and growth in the adult.6-10 Interestingly, Yu et al and Xie and co-workers have shown that administration of an engineered zinc-finger transcription factor that drives expression of multiple VEGF isoforms improved recovery of limb perfusion following femoral artery ligation.11,12
Studies by Jacobi and colleagues, Lloyd et al and Toyota and co-workers using VEGF antagonists given systemically suggest that endogenous VEGF may contribute to ischemic collateral remodeling.13-15 However, no study has examined this question with a genetic approach or identified the responsible VEGF receptor type. VEGF acts primarily through VEGFR-1 (flt-1) and VEGFR-2 (flk-1/KDR). VEGF-induced angiogenesis is mediated predominantly by VEGFR-2. Experiments in adult mice using VEGFR-1 specific agonists, PIGF and VEGF-B, and targeted mice lacking the kinase domain of VEGFR-1 demonstrate a positive role for VEGFR-1.17,18 However, during embryonic development alternately transcribed soluble VEGFR-1 (sFlt-1) participates as a negative regulator of VEGF signaling.4,16 In this study, we examined the effect of targeted over- and under-expression of VEGF, VEGFR-1 and VEGFR-2 on collateral remodeling in ischemia.
It is not known when the native collateral circulation develops or what pathways specify formation of these rare arteriole-to-arteriole anastomoses that interconnect adjacent arterial trees. We previously found that compared to C57BL/6 mice, the BALB/c strain is markedly deficient in collateral density, and identified an expression quantitative trait locus at or near Vegfa that positively correlated with low VEGF expression at the BALB/c allele.19 Thus in the present study, we tested the hypothesis that VEGF expression is a determinant of collateral formation.
Materials and Methods
Twelve-to-16 week old mice were randomized, and procedures were conducted blindly. Femoral artery ligation was distal to the lateral caudal femoral and superficial epigastric arteries. Laser Doppler perfusion imaging and arterial pressure (cannulated femoral artery) determinations were obtained under light isoflurane (1.125%) and oxygen anesthesia. Histology, x-ray angiography and cerebral arteriography were done after pressure-perfused maximal dilation and fixation. RNA was extracted after perfusion with RNA-later. Data are reported as the mean ± SEM, and were subjected to ANOVA followed by Dunn-Bonferonni Corrected t-tests or Student's t-tests where appropriate, or by Mann Whitney U tests for capillary number-to-muscle fiber ratios and appearance scores. For additional details, see the online data supplement at http://circres.ahajournals.org.
Results
Reduced recovery after femoral ligation in VEGFR-1 heterozygous mice
Although VEGFR-1-/- or VEGFR-2-/- mice are embryonic lethal, heterozygous mice appear normal but have defects in cardiac preconditioning.20 Compared to wild-type, VEGFR-1+/- had larger paw appearance scores indicating greater ischemia19 (Fig. 1a). In contrast, VEGFR-2+/- showed no differences (Fig. 1b). In agreement, recovery of plantar perfusion (Fig. 1c), which correlates with overall hindlimb blood flow,21 was attenuated in VEGFR-1+/- but not in VEGFR-2+/- mice (Figs. 1d,e).
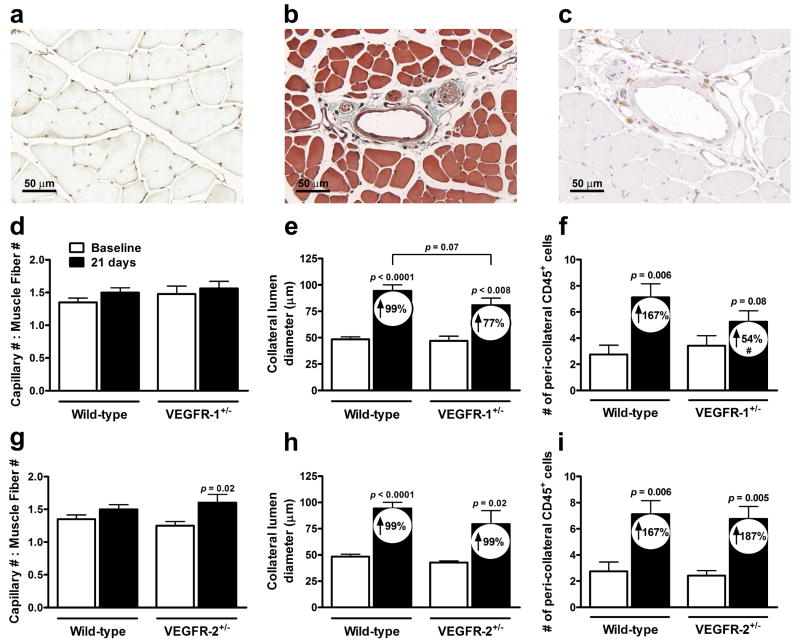
Figure 1.
Impaired recovery of perfusion in VEGFR-1+/- mice after femoral ligation. (a,b) VEGFR-1+/- mice have greater hindlimb ischemic appearance score. Scale: 0=normal, 1=cyanosis or loss of nail(s), 2=partial or complete atrophy of digit(s), 3=partial atrophy of fore-foot; n=6-20 per data-point. (c) Laser Doppler perfusion images of plantar foot with region of interest (ROI). (d,e) Quantification of plantar perfusion measured over ROI. Data are mean±SEM for this and other figures. Two-way ANOVA followed by Dunn-Bonferonni t-Test; *p<0.05 vs. wild-type CD-1; n=6-16 mice per data-point.
To investigate mechanism, we measured capillary density in gastrocnemius. While capillary-to-muscle fiber number ratios at baseline were comparable among groups, ratios significantly increased at day-21 in VEGFR-2+/-, and trended similarly in VEGFR-1+/- mice. (Figs. 2a,d,g). The increase in VEGFR-2+/- was associated with slightly greater capillary density and muscle atrophy (Online Figure I b,f). Capillary density and muscle atrophy were not different in VEGFR-1+/- (Online Figure I a,e). We next examined collateral diameters in the anterior and posterior gracilis muscles and perfusion in the superficial adductor region containing these collaterals. Lumen diameter was not different at baseline. However, outward remodeling at day-21 was less in VEGFR-1+/- (p=0.07, 2-tailed) (Figs. 2b,e,h). In addition, VEGFR-1+/- had reduced adductor perfusion (Online Figure II d). Collateral remodeling and perfusion were not different in VEGFR-2+/- mice (Fig. 2h, Online Figure II e). Leukocytes contribute importantly to collateral artery growth.1-3 Peri-collateral CD45+ leukocytes were not different in VEGFR-2+/-, but were reduced in VEGFR-1+/- mice (Figs. 2c,f,i), consistent with their attenuated collateral remodeling. In addition, circulating leukocytes, lymphocytes, and monocytes were lower three days after ligation in VEGFR-1+/- (Online Table I). These findings indicate that reduced expression of VEGFR-1 results, after femoral artery occlusion, in fewer circulating and peri-collateral leukocytes, reduced collateral enlargement and flow, reduced recovery of hindlimb flow, and greater tissue ischemic injury suggesting VEGFR-1 is important in collateral remodeling.
Figure 2.
Reduced collateral remodeling (lumen expansion) and leukocyte recruitment in VEGFR-1+/- mice. (a) Lectin-stained capillaries in gastrocnemius. (d,g) Capillary number-to-muscle fiber number ratios at baseline (before) and 21 days after femoral ligation. (b) Cyano-Massons-elastin-staining of collateral in gracilis. (e,h) Collateral lumen diameter at baseline and 21 days after ligation. Numbers inside bars here and elsewhere are percent increase from baseline. (c,f,i) CD45+ leukocytes in a 1-diameter area around anterior gracilis collaterals. Paired t-Test vs. baseline; Un-paired t-Test vs. wild-type; #p<0.05 vs. percentage change from wild-type; n=6-11 mice per data-point.
The VEGF hypermorphic allele augments —and hypomorphic allele attenuates— recovery after femoral ligation
Nagy and co-workers have constructed unique VEGF-A lacZ reporter mice with hypermorphic or hypomorphic VEGF-A alleles.8,9,22 Homozygosity of either allele results in embryonic lethality. However, VEGFhi/+ and VEGFlo/+ are viable and appear normal despite expressing VEGF at intermediate levels to homozygous and wild-type counterparts.8,9 Since data are lacking, we assessed expression of the VEGF isoforms, VEGF120, VEGF164, and VEGF188, by qRT-PCR in calf and collateral zone adductor muscles.
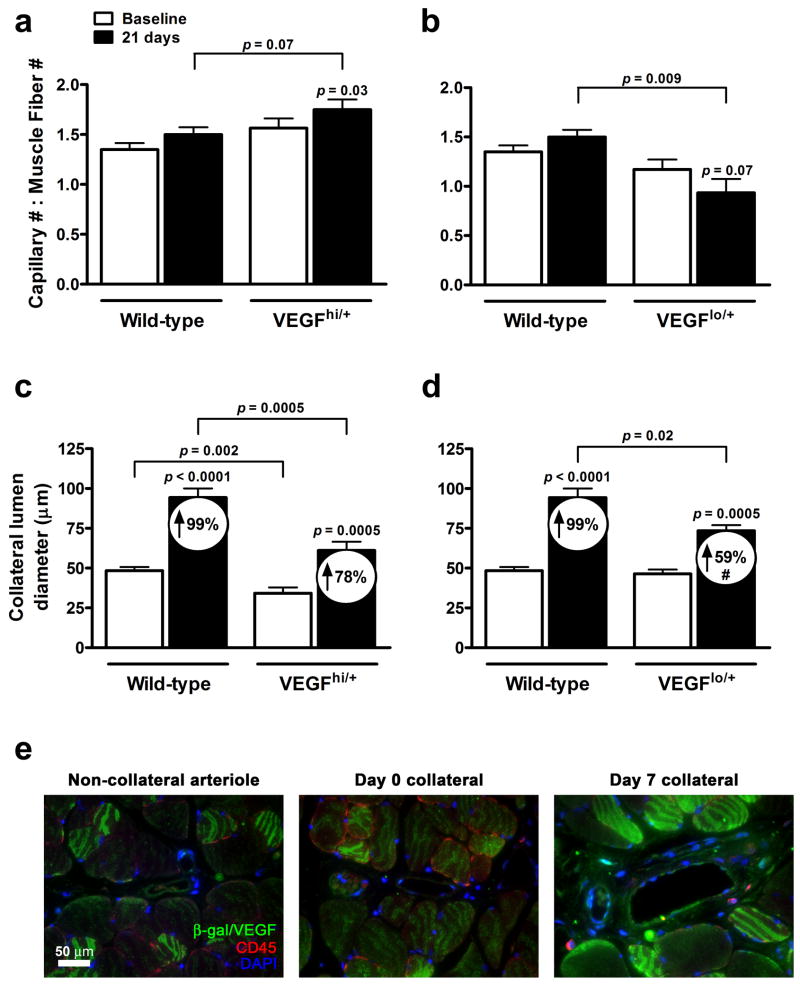
Baseline VEGF expression in calf from the non-ligated leg was 2-fold higher in VEGFhi/+ and 25-75% lower in VEGFlo/+ compared to wild-type (Online Figure III a). Thirty-six hours after femoral ligation, expression in calf increased in all three strains for VEGF120 (Figs. 3a-c). In contrast, expression in the adductor of the ligated leg, which does not experience detectable ischemia in this model,23 showed upregulation of the high-molecular weight isoforms, VEGF-164 and -188, in wild-type and VEGFhi/+ mice (Figs. 3d,e). Interestingly, all VEGF isoforms decreased in the VEGFlo/+ mice (Fig. 3f). Immunofluorescence was performed against β-galactosidase in adductor sections of VEGFhi/+ mice to localize VEGF expression (Fig. 5e). Low-level basal expression of VEGF was evident in skeletal muscle surrounding non-collateral arterioles and un-remodeled collaterals (Fig. 5e, left and middle panels), consistent with data from Maharaj and colleagues, describing VEGF expression pattern in these mice.24 However, 7 days after ligation, VEGF expression increased in peri-collateral skeletal muscle (Fig. 5e, right panel). Co-staining with CD45 revealed a small population of recruited leukocytes that also expressed VEGF (Fig. 5e, right panel, Online Figure IV ).
Figure 3.
Quantitative RT-PCR of VEGF isoforms (a-f) and HIF subunits (g-i) in calf (a-c) and adductor (d-i) of wild-type (a,d,g), VEGFhi/+ (b,e,h), and VEGFlo/+ (c,f,i) mice. Data for muscle taken from ligated leg are relative to muscle from non-ligated leg and normalized to 18S rRNA. Non-parametric t-Test vs. non-ligated; n=5-6 mice per data-point.
Figure 5.
Collateral remodeling and angiogenesis are attenuated in VEGFlo/+ mice. Capillary number-to-muscle fiber number ratios (a,b) and collateral lumen diameters (c,d) at baseline and day-21 post-ligation. Paired t-Test vs. Baseline; Unpaired t-test vs. wild-type; #p<0.05 vs. percentage change from wild-type; n=7-11 per data-point. (e) Immunofluorescent localization of VEGF in VEGFhi/+ mice. Non-collateral arteriole (left panel) and gracilis collateral at day-0 (middle panel) and day-7 post-ligation (right panel).
To determine involvement of HIF signaling in this unique pattern of upregulation in the adductor collateral zone, we performed qRT-PCR for Hif-1α, Hif-1β and Hif-2α. Whereas expression was similar in all strains in the non-ligated leg's adductor (Online Figure III b), Hif-1α increased 2-fold while Hif-1β was unaffected in the ligated leg (Figs. 3g-i). Interestingly, Hif-2α decreased, suggesting an alternative role for this subunit. These data suggest that ischemia in calf promotes alternative splicing of VEGF favoring the soluble isoforms, whereas shear-induced collateral remodeling in adductor is associated with expression of matrix-bound isoforms.
Compared to wild-type mice, VEGFhi/+ exhibited less (Fig. 4a) whereas VEGFlo/+ experienced greater (Fig. 4b) ischemic appearance scores after femoral ligation. Likewise, VEGFhi/+ mice exhibited less reduction in perfusion immediately after ligation and better recovery thereafter (Figs. 4c,e,f), whereas VEGFlo/+ experienced greater reduction and worse recovery (Figs. 4d,e,f); however, only the indicated time-points differed significantly by Bonferroni tests. The milder ischemia in VEGFhi/+ is consistent with their trend toward a smaller increase in VEGF120 in the calf, compared to wild-type (Fig. 3b). The slight reduction of VEGF120 in VEGFlo/+, compared to wild-type, and the decrease of VEGF164 (Fig. 3c) may reflect the reduced stability of VEGF transcripts in the VEGFlo/+ strain.9
Figure 4.
VEGFhi/+ mice have better and VEGFlo/+ worse recovery after femoral ligation. (a,b) Lower ischemic appearance score in VEGFhi/+ and more ischemia in VEGFlo/+ (see Fig. 1 for scale); n=6-20 per data-point. (c,d,e) Quantification of perfusion measured over plantar ROI shows less reduction immediately after ligation (“Post-Op”) and better recovery in VEGFhi/+, and opposite effects in VEGFlo/+. (f) Representative Doppler images of Pre- and Post-Op time points. ANOVA followed by Dunn-Bonferonni t-Test; *p<0.05, **p<0.01, ***p<0.001 vs. wild-type; n=6-16 per data-point. (g) Mean arterial pressure (MAP) was measured under anesthetic conditions used for Doppler measurements.
Because differences in arterial pressure could cause differences in Doppler perfusion measurements, we measured arterial pressure under the same conditions of anesthesia used for Doppler imaging. Mean arterial pressure did not differ in VEGFhi/+ versus VEGFlo/+ (Fig. 4g). Moreover, since VEGF is a vasodilator, increased VEGF in VEGFhi/+ (versus decreased VEGF in VEGFlo/+) favors lower (versus higher) arterial pressure and less (versus more) perfusion immediately after ligation (and thereafter) – which is the opposite of what we observed (Fig. 4c,d).
Measurement of plantar perfusion immediately after ligation largely reflects anatomical differences in pre-existing collateral density and/or diameter, and to a lesser extent, capillary density, since vascular tone is strongly inhibited in the ischemic limb.25 Thereafter, recovery of perfusion is primarily determined by collateral remodeling, with a smaller contribution of angiogenesis. To distinguish VEGF's potential effect on these determinants, we first examined capillary-to-muscle fiber ratio in gastrocnemius at baseline (Figs. 5a,b). There was no difference between wild-type and VEGFhi/+ or VEGFlo/+ mice, suggesting that differences in collateral number and/or diameter underlie the differences in perfusion immediately after ligation. Capillary-to-fiber ratio increased at day-21 in VEGFhi/+. Interestingly, baseline fiber size was greater in VEGFhi/+, consistent with VEGF's role in skeletal muscle development. (Online Figure I c,g).26 Capillary-to-fiber ratio decreased at day-21 in VEGFlo/+ mice. While capillary number-per-tissue-area of VEGFlo/+ did not differ from wild-type, greater atrophy occurred (Online Figure I d,h), resulting in reduced capillary-to-muscle fiber ratio.
Given the importance of collateral conductance following femoral ligation, we determined collateral diameter at baseline and day-21 post-ligation. Lumen diameters in VEGFhi/+ mice were significantly smaller than wild-type at both time-points; however percent remodeling and adductor perfusion in VEGFhi/+ were not different (Fig. 5c, Online Figure II f). In contrast, baseline diameters in VEGFlo/+ mice did not differ from wild-type, however remodeling and adductor perfusion were reduced (Fig. 5d, Online Figure II g). The baseline diameter data suggest (since baseline capillarity do not differ) that differences in collateral density may underlie the differences in perfusion immediately after ligation, ie, greater number of smaller diameter collaterals in VEGFhi/+, versus fewer in VEGFlo/+. In addition, less remodeling in VEGFlo/+ mice suggests VEGF contributes to collateral remodeling.
VEGF genotype determines the number of pre-existing collaterals
To determine if VEGF expression influences native collateral density, as suggested by the above findings, we performed X-ray arteriography immediately and 7 days after ligation (the latter to improve detection of smaller collaterals after onset of remodeling). Analogous to Rentrop analysis, we counted vessels crossing a line drawn through the middle of the adductor collateral zone (Fig. 6a); this method agrees with other methods of quantifying pre-existing collateral capacity in the hindlimb.19 An apparent VEGF gene dose-relationship was detected, wherein VEGFhi/+ had more and VEGFlo/+ had fewer collaterals than wild-type mice (Fig. 6b).
Figure 6.
Collateral density in skeletal muscle and pial circulations correlates with VEGF genotype. (a) Post-mortem X-ray arteriogram of thigh. Vessels were counted (data given in (b)) that crossed a line drawn from the mid-point between femoral ligations through the thigh collateral zone at baseline and day-7 post-ligation. (c) Post-mortem arteriogram of pial circulation. Collaterals were counted (data given in (d)) that interconnected middle and anterior cerebral artery trees at post-natal day-1, day-21 and 12 weeks. Brackets, 1-way ANOVA followed by Dunn-Bonferonni t-Test; *p<0.05 vs. P1; #p<0.05, ###p<0.001 vs. corresponding wild-type time-point; n=8-11 per data-point. (e) TTC staining 24 hours after MCAO. (f) Infarction volumes reported as % cortex volume. (g) Correlation of infarction volume and collateral number.
We next sought to determine if collaterals are present at birth and if VEGF expression correlates with newborn and adult density in another tissue besides skeletal muscle. We recently reported that differences in collateral density among mouse strains exist across multiple tissues.19 This includes collaterals of the cerebral cortical circulation, which are confined to the pial (leptomeningeal) membrane and thus readily quantified.19 We therefore counted pial collaterals in VEGFhi/+, VEGFlo/+ and wild-type mice using a polyurethane casting agent (Fig. 6c; resolution of x-ray and polyurethane arteriography given in Suppl).27 Interestingly, collaterals were present at birth (P1; Fig. 6d). As in the hindlimb (Figs. 6b) an apparent VEGF gene dose-relationship was detected at P1, P21 and 3-months of age, with VEGFhi/+ mice having more and VEGFlo/+ having fewer collaterals (Figs. 6c,d). Wild-type and VEGFhi/+ mice were born with the same number of collaterals while VEGFlo/+ were born with fewer, suggesting VEGF levels specify the density of nascent collaterals formed during embryogenesis. In VEGFhi/+, pial collateral number remained constant throughout post-natal development. In contrast, wild-type mice experienced a decline in collateral density by P21 that remained unchanged in adults. VEGFlo/+ mice also lost collaterals with age.
To determine if an increase in collateral density offered protection, we performed middle cerebral artery occlusion (MCAO) in wild-type, VEGFlo/+ and VEGFhi/+ mice. Twenty-four hours after MCAO, a relationship between VEGF genotype and infarct volume was evident (Fig. 6e,f) with VEGFhi/+ mice having ∼2-fold smaller infarcts. Furthermore, infarct volume negatively correlated with collateral number (Fig. 6g). These data suggest that collaterals form during the perinatal period, that VEGF is important in their formation, post-natal maturation and establishment of the adult collateral density, and that the latter may be an important determinant of the severity of stroke.
Discussion
Our results suggest that VEGFR-1 positively regulates arteriogenesis through recruitment of leukocytes to the peri-collateral region. We also provide the first examination of when the native collateral circulation becomes established, finding that pial collaterals are already present at birth and increase over the first three weeks of life to achieve the density present in the adult. In addition, we identify the first gene, VEGF-A, whose expression impacts the density of pre-existing collaterals in healthy tissue.
Few studies have addressed whether endogenous VEGF mediates collateral growth. Supportive evidence exists in hindlimb13,14 and heart15 ligation models, where systemic administration of sFlt1/sFlk1 adenovirus,13 VEGF receptor inhibitor14 or neutralizing antibody15 impaired recovery of perfusion and angiographic collateral growth. Also, Matsunaga, Chilian and co-workers have shown that inhibition of eNOS with L-NAME, which also interferes with VEGF signaling, reduced ischemic coronary collateral growth but did not affect angiogenesis.28 Our findings that VEGFR-1+/- mice show reduced recovery of plantar and adductor perfusion, greater ischemia, reduced peri-collateral leukocyte and reduced collateral enlargement —but normal angiogenesis— suggest that VEGF acting through VEGFR-1 mediates collateral remodeling. This is in contrast to VEGFR-1's role as a VEGF inhibitor during development.4,16 It is difficult to measure VEGFR-1 signaling due to its weak kinase activity.18 However, studies using the VEGFR-1 specific agonists, PlGF and VEGF-B, and kinase-dead mutants find that VEGFR-1 possesses signaling functions in the adult.4,16 Our data add collateral growth to these functions. They also agree with evidence by Pipp and colleagues that mice lacking PlGF have impaired recovery from hindlimb ischemia.17 In addition, our findings of fewer leukocytes circulating and residing around remodeling collaterals, which suggest reduced leukocyte mobilization and transmigration, are consistent with their known role in arteriogenesis.17 These results are congruent with VEGFR-1's expression on leukocytes16 and its proposed role in the bone marrow niche,29 in homing,30 and chemotaxis.31 No deficits were evident in VEGFR-2+/- mice. However given VEGFR-2's strong kinase activity, a single allele may specify sufficient receptors to mediate VEGFR-2-dependent ischemic angiogenesis as well as any potential contribution of this receptor to arteriogenesis. This could explain why angiogenesis in the ischemic gastrocnemius was not impaired in VEGFR-2+/-. Conditional deletion of VEGFR-2 and VEGFR-1 will be required to confirm that VEGFR-2 does not participate in collateral growth, and to determine if the modest inhibition we observed in the VEGFR-1+/- reflects only partial reduction in receptor density.
Native collaterals are small in diameter and few in number. In addition, current antibodies cannot distinguish among VEGF isoforms and have limited resolution to detect secreted ligand. Thus, it is not known if VEGF isoforms increase around remodeling collaterals to contribute arteriogenesis. Deindl and colleagues23 did not detect increased VEGF in rabbit collaterals or in whole quadriceps muscle 3 days after femoral ligation. In contrast, we found that mRNAs for high-molecular weight isoforms of VEGF increased in the adductor collateral zone of wild-type mice 36 hours after ligation. Since VEGF transcripts agree with protein expression32 this discrepancy may be due to differences in duration of ligation, tissue analyzed or species studied. We assayed a 5mm wide mid-section of the adductor that contains hindlimb collaterals, including those in the gracilis muscles that we measured histologically. In contrast to the adductor, only VEGF120 was upregulated in the gastrocnemius. This differential expression where collaterals are remodeling versus where capillaries are sprouting, is consistent with the different physical properties and functions of VEGF isoforms. In the calf, free diffusion of VEGF120 would promote a wide area of angiogenesis. In addition, Hattori and co-workers have shown that VEGF120 released into the circulation from ischemic tissue aids mobilization of leukocytes from bone marrow.29 In contrast, collateral growth in the thigh is presumed to require temporal and spatial release of proteases, cytokines, and growth factors. Elaboration of the heparin-binding VEGF164 and VEGF188 isoforms around collaterals may establish VEGF gradients that stimulate diapedesis and proliferation of leukocytes, proliferation of endothelial cells, and migration of smooth muscle cells. Consistent with this hypothesis, immunofluorescence revealed VEGF upregulated in peri-collateral skeletal muscle and leukocytes in addition to collateral endothelium and smooth muscle. It will be important in future studies to determine the cell source(s), stimulus for VEGF release and isoform expression profile.
Deindl and colleagues23 also did not detect increased Hif-1α in the adductor, although differences in species, time after ligation and tissue sampled could be important. In contrast, we found a ∼two-fold increase in Hif-1α mRNA (and no increase in Hif-1β) in the adductor collateral zone. Jiang, Semenza and coworkers have shown that Hif-1α levels are regulated by tissue oxygen over a broad range,33 Thus, oxygen may decline sufficiently, though not to ischemic levels, to increase transcription and stabilization of Hif-1α transcripts. In addition, it is possible that other factors surrounding remodeling collaterals that regulate Hif-1α, such as reactive oxygen species, nitric oxide, growth factors, and increased shear stress itself34 may contribute to the increase in Hif-1α.
Consistent with deficient recovery of flow after femoral ligation in VEGFR-1+/-mice, a direct relationship was evident between VEGF genotype and recovery among VEGFlo/+, wild-type and VEGFhi/+ mice. Similar to VEGFR-1+/-, VEGFlo/+ had worse ischemic appearance scores and blunted recovery of plantar perfusion compared to wild-type. In addition, capillary-to-muscle fiber number decreased by day-21 in VEGFlo/+ as a result of greater muscle atrophy. In contrast, VEGFhi/+ mice showed enhanced recovery of perfusion and little ischemia. VEGF's role in the development and maintenance of striated muscle fiber size, as demonstrated by Bryan, D'Amore and colleagues,26 could contribute to the differences in atrophy and appearance scores. The variation in capillary-to-fiber ratio at day-21 that we observed in the hypomorphs, which is consistent with VEGF's role in ischemic angiogenesis, could also contribute to the differences in recovery of hindlimb perfusion and ischemic scores, although collateral remodeling is the primary determinant of recovery of hindlimb perfusion.
VEGFlo/+ mice exhibited attenuated adductor perfusion and impaired collateral remodeling like that seen in the VEGFR-1+/- mice. In contrast, collateral remodeling and adductor perfusion were not enhanced in VEGFhi/+. Although this appears discordant, several considerations are important. Immediately after ligation, perfusion decreased more in VEGFlo/+ and less in VEGFhi/+. The increase in shear stress in collaterals immediately after ligation favors inhibition of collateral smooth muscle tone. Moreover, studies by Yang, Terjung and colleagues have documented that ischemia and low pressure cause myogenic and metabolic inhibition of tone in the vasculature below the point of ligation.25 These considerations suggest that the differences in perfusion immediately after ligation arise from anatomical differences in pre-existing collateral number, collateral lumen size and/or, to a lesser degree, capillary density. Baseline capillary density was not different among VEGFhi/+, VEGFlo/+ and wild-type, and collateral lumen diameter was comparable in the latter two groups while being smaller in VEGFhi/+ mice. Collectively, these data suggest that density of native collaterals varies directly with level of VEGF expression. In VEGFhi/+ mice, a greater number of collaterals in parallel favors lower flow in individual collaterals and thus smaller baseline diameters, which is what we observed. Shear stress is the proximate stimulus of arteriogenesis.1,2 A greater number of collaterals favors less increase in shear in individual collaterals, which is consistent with our finding that remodeling was not greater in VEGFhi/+ mice than in wild-type despite increased VEGF expression. In VEGFlo/+ mice with fewer collaterals, the expected higher shear forces may not be able to overcome the deficit in VEGF expression. This could explain the reduced collateral remodeling and impaired perfusion that we observed.
We found that VEGF expression determines collateral density in a second tissue—the pial circulation. An additional intriguing finding was that VEGFlo/+ mice were born with fewer pial collaterals compared to wild-type and VEGFhi/+. These data suggest VEGF levels contribute to collateral formation in the embryo. We previously hypothesized that collaterals form during arterial tree formation and that reduced VEGF results in reduced collateral formation.19 Studies by the Bautch, Shima and Tomanek groups have shown that VEGF is important in branching morphogenesis,35-37 including formation of coronary artery stems by VEGF-B which activates VEGFR-1.37 Our data support the concept that collateral density and vascular branching are intimately related. In addition, collateral density was reduced by post-natal day-21 in wild-type and VEGFlo/+ mice, while density was maintained in VEGFhi/+. This is consistent with VEGF's role in stabilizing nascent blood vessels.4,5,16
We reported that native collateral density and VEGF expression differ strongly in two mouse strains.19 Inducible VEGF expression and collateral density in hindlimb, cerebral cortex and intestine were high in C57BL/6 mice and low in BALB/c. This association led us to hypothesize that VEGF is a determinant of native collateral formation. Our present studies using mice with a single targeted genetic difference, ie, altered VEGF expression,8,9,22 provide strong support for this hypothesis. We also suggest that genetic polymorphisms and environmental factors that reduce VEGF during embryonic or perinatal periods could reduce collateral formation, resulting in increased severity of stroke, myocardial infarction and peripheral artery disease. How VEGF affects collateral formation and stabilization are intriguing questions for future study. Our data suggest that murine pial collaterals form prior to birth and mature within a narrow three-week period. In the embryo, early vascular patterning involves genetically determined events and morphogenic factors including VEGF, whereas during later stages when flow becomes important, excess vessels are thought to be pruned leaving “hemodynamically favored” channels. It is possible that VEGF's role in collateral formation involves its known role in vascular patterning and/or hemodynamic patterns produced by local vasodilator actions of VEGF.
Other molecules in the VEGF signaling pathway may also contribute to collateral formation. Resar and coworkers recently identified a HIF-1α polymorphism that correlated with the presence of coronary collaterals in patients.38 The polymorphism results in an amino-acid substitution that confers increased HIF-1 activity. However, patients with the substituted allele did not have visible collaterals, which is at variance with our findings. Among mechanisms proposed by the authors to explain the apparent discrepancy between higher HIF-1 activity and lack of visible collaterals,38 we propose an additional hypothesis. Because clinical angiography only detects large vessels, collaterals in a person with a small native density would be more likely to experience larger increases in shear and thus enlarge to detectable diameters during progression of coronary artery disease. In contrast, collaterals in a person with high density would be expected to experience smaller increases in shear, resulting in less outward remodeling during disease progression and absence of angiographic detection.
In conclusion, our results suggest that VEGFR-1 mediates collateral growth in ischemic disease by mobilizing leukocytes and recruiting them to the peri-collateral space. In addition, we show that collaterals form prior to birth and stabilize at their adult density by the third postnatal week. Lastly, we identify VEGF as the first molecule specifying pre-existing collateral density in normal tissue. We propose a model whereby collaterals form in a VEGF-dependent manner during embryogenesis. Further, these nascent collaterals require adequate VEGF during a critical period after birth to stabilize and mature sufficiently to achieve their full adult density. Further studies will be required to define when and how VEGF and other molecules contribute to collateral formation, as well as the downstream effectors that stabilize and maintain collaterals in tissues. An interesting recent study by Wustmann, Seiler and colleagues reported that coronary collateral flow in healthy patients varies widely.39 Moreover, several VEGF polymorphisms linked to altered expression have been described.40 Thus, it will be important to examine whether polymorphisms affecting the expression of VEGF and other genes are associated with altered collateral density. An understanding of pathways that specify collateral formation in normal tissues may lead to therapies to induce formation of new collaterals in patients with ischemic disease.
Acknowledgments
We thank Dr. Hua Zhang for performing middle cerebral artery occlusions, Kirk McNaughton and Carolyn Suitt for histology, Tim Xin and Erin Thompson for technical assistance, and Dr. Eric Meyer, University of Zurich for advice on use of PU4ii.27
Sources of Funding: NIH-NHLBI, HL62584 (JEF), T32-HL069768 (JAC), and F32-HL080847 (DC).
Footnotes
Disclosures: None
References
- 1.Grundmann S, Piek JJ, Pasterkamp G, Hoefer IE. Arteriogenesis: basic mechanisms and therapeutic stimulation. Eur J Clin Invest. 2007;37:755–66. doi: 10.1111/j.1365-2362.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 2.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–7. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. Faseb J. 2006;20:2657–9. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 8.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–6. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 9.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–92. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- 10.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–41. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y, Liu D, Yeh JL, Rebar E, Case C, Spratt K, Sessa WC, Giordano FJ. An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice. Faseb J. 2006;20:479–81. doi: 10.1096/fj.04-3670fje. [DOI] [PubMed] [Google Scholar]
- 12.Xie D, Li Y, Reed EA, Odronic SI, Kontos CD, Annex BH. An engineered vascular endothelial growth factor-activating transcription factor induces therapeutic angiogenesis in ApoE knockout mice with hindlimb ischemia. J Vasc Surg. 2006;44:166–75. doi: 10.1016/j.jvs.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–9. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol Heart Circ Physiol. 2005;288:H759–68. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- 15.Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian WM. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–13. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 16.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–94. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 17.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–85. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 18.Hiratsuka S, Maru Y, Okada A, Seiki M, Noda T, Shibuya M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–13. [PubMed] [Google Scholar]
- 19.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–91. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 20.Thirunavukkarasu M, Addya S, Juhasz B, Pant R, Zhan L, Surrey S, Maulik G, Menon VP, Maulik N. Heterozygous Disruption Of Flk-1 Receptor Leads To Myocardial Ischemia Reperfusion Injury In Mice: Application Of Affymetrix Gene Chip Analysis. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H947–59. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 22.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–22. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 23.Deindl E, Buschmann I, Hoefer IE, Podzuweit T, Boengler K, Vogel S, van Royen N, Fernandez B, Schaper W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779–86. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 24.Maharaj AS, Saint-Geniez M, Maldonado AE, D'Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–48. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang HT, Laughlin MH, Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2000;279:H1890–7. doi: 10.1152/ajpheart.2000.279.4.H1890. [DOI] [PubMed] [Google Scholar]
- 26.Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D'Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19:994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krucker T, Lang A, Meyer EP. New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc Res Tech. 2006;69:138–47. doi: 10.1002/jemt.20263. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 29.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–9. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol. 2008;28:322–8. doi: 10.1161/ATVBAHA.107.158022. [DOI] [PubMed] [Google Scholar]
- 32.Yuan A, Yu CJ, Chen WJ, Lin FY, Kuo SH, Luh KT, Yang PC. Correlation of total VEGF mRNA and protein expression with histologic type, tumor angiogenesis, patient survival and timing of relapse in non-small-cell lung cancer. Int J Cancer. 2000;89:475–83. doi: 10.1002/1097-0215(20001120)89:6<475::aid-ijc2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–80. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 34.Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002;17:581–8. doi: 10.3346/jkms.2002.17.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–35. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 36.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–98. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res. 2006;98:947–53. doi: 10.1161/01.RES.0000216974.75994.da. [DOI] [PubMed] [Google Scholar]
- 38.Resar JR, Roguin A, Voner J, Nasir K, Hennebry TA, Miller JM, Ingersoll R, Kasch LM, Semenza GL. Hypoxia-inducible factor 1alpha polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–91. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 39.Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–20. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- 40.Balasubramanian SP, Brown NJ, Reed MW. Role of genetic polymorphisms in tumour angiogenesis. Br J Cancer. 2002;87:1057–65. doi: 10.1038/sj.bjc.6600625. [DOI] [PMC free article] [PubMed] [Google Scholar]