Abstract
The mechanism of isoproterenol-induced myocardial damage is unknown, but a mismatch of oxygen supply vs. demand following coronary hypotension and myocardial hyperactivity is the best explanation for the complex morphological alterations observed. Severe alterations in the structural integrity of the sarcolemma of cardiomyocytes have been demonstrated to be caused by isoproterenol. Taking into account that the sarcolemmal integrity is stabilized by the dystrophin-glycoprotein complex (DGC) that connects actin and laminin in contractile machinery and extracellular matrix and by integrins, this study tests the hypothesis that isoproterenol affects sarcolemmal stability through changes in the DGC and integrins. We found different sensitivity of the DGC and integrin to isoproterenol subcutaneous administration. Immunofluorescent staining revealed that dystrophin is the most sensitive among the structures connecting the actin in the cardiomyocyte cytoskeleton and the extracellular matrix. The sarcomeric actin dissolution occurred after the reduction or loss of dystrophin. Subsequently, after lysis of myofilaments, γ-sarcoglycan, β-dystroglycan, β1-integrin, and laminin α-2 expressions were reduced followed by their breakdown, as epiphenomena of the myocytolytic process. In conclusion, administration of isoproterenol to rats results in primary loss of dystrophin, the most sensitive among the structural proteins that form the DGC that connects the extracellular matrix and the cytoskeleton in cardiomyocyte. These changes, related to ischaemic injury, explain the severe alterations in the structural integrity of the sarcolemma of cardiomyocytes and hence severe and irreversible injury induced by isoproterenol.
Keywords: dystrophin, dystrophin-glycoprotein complex, ischaemia, isoproterenol, myocytolysis, myofibrilar degeneration
Isoproterenol has been used as an infarct-like myocardial necrosis-inducing drug in experimental animals since 1959 (Rona et al. 1959). The morphological changes have been claimed as comparable to those taking place in human myocardial infarction (Ferrans et al. 1964). The cardiac lesions are generally localized in the apex, lower aspect of the left ventricle, interventricular septum, and, occasionally, in the right ventricle. These lesions are focal, many coalescent, and characterized by capillary dilatation, interstitial and intracellular oedema, lysis of myofibrils and granular disintegration or hyaline necrosis of myofibres (Chappel et al. 1959). The lysis of myofibrils is known as myocytolysis (Schlesinger & Reiner 1955) or myofibrilar degeneration (Reichenbach & Benditt 1970).
Isoproterenol stimulates cardiac muscle cells due to its positive inotropic and chronotropic effect (Csapóet al. 1972) and causes complex changes leading to alterations in membrane permeability (Handforth 1962; Rona 1985). The exact mechanism of isoproterenol-induced myocardial damage is still unknown, but a mismatch of oxygen supply vs. demand following coronary hypotension and myocardial hyperactivity is the best explanation for the complex morphological alterations observed in the presence of a patent vasculature (Grimm et al. 1998).
Severe alterations in the structural integrity of the sarcolemma of cardiomyocytes have been demonstrated to be caused by isoproterenol (Yunge et al. 1989). Taking into account that the sarcolemmal integrity is stabilized by the dystrophin-glycoprotein complex (DGC) that connects actin and laminin in contractile machinery and extracellular matrix (McNally et al. 2003) and by integrins that also are essential to define cellular–extracellular interaction, this study was undertaken to test the hypothesis that isoproterenol affects sarcolemmal stability through changes in the DGC and integrins.
Materials and methods
Animals
Male Wistar rats, weighing 150 g on average were obtained from the breeding colony of the Faculty of Medicine of Ribeirão Preto. They were housed under controlled conditions of temperature and fed a commercial rat food and water ad libitum on a 12:12 h’s light-dark cycle. The experiments were conducted according to the guidelines of the Animal Care Committee of the Faculty of Medicine of Ribeirão Preto, and the experimental protocols were approved by the Committee.
Experimental protocol
The animals were divided into two groups: control group and isoproterenol-treated group. The animals from isoproterenol-treated group received two subcutaneous injections of DL-Isoproterenol hydrochloride (Sigma Co., St Louis, MO, USA), separated by a 24-h interval, at a dose of 85 mg/kg of body weight. With this dosage the severest infarct-like cardiac changes can be observed non-associated with marked changes outside the heart except for congestion of the lungs and abdominal viscera (Rona et al. 1959).
Isoproterenol solution was prepared with distilled water under sterile condition immediately before injection. Control group received two subcutaneous injections of physiological saline. These rats were killed 24 h after the second injection of isoproterenol or physiological saline.
Harvesting and preparation of hearts
The hearts were rapidly removed, rinsed in ice-cold 0.9% saline solution, weighed, and fixed as a whole by immersion in phosphate-buffered 10% formalin for 24 h at 4 °C for histological study. Both ventricles from each heart were isolated and cut into two fragments by a midventricular coronal section. Each block was serially cut in the same direction, and sections were stained with haematoxylin and eosin. The absolute thicknesses of the septum and left and right ventricular wall and the areas of each ventricular chamber were measured (n = 7). For this morphometric study, the public-domain software NIH Image J (developed at the U.S. National Institute of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/) was used.
High resolution light microscopy
The hearts were removed as described above. For plastic embedding, fixed hearts were frontally cut into anterior and posterior halves, dehydrated in ascending concentrations of alcohol and embedded in Historesin (Leica Instruments, Heidelberg, Germany). Sections 2.5 μm-thick were stained with toluidine blue and examined in a Leica DMR microscope (Leica Mycrosystems Wetzlar GmbH, Wetzlar, Switzerland) (n = 6). Areas of the left and right ventricles and septum with myocytolysis were evaluated in the hearts of rats treated with isoproterenol using Leica Qwin 3.2.0 software (Leica Imaging Systems Ltd., Cambridge, UK). The values of the areas with myocytolysis were expressed in percentage of the total area of the left and right ventricles and septum.
Immunofluorescence microscopy
Hearts from isoproterenol-treated and control rats were obtained as described previously. The hearts were frontally cut into anterior and posterior halves, immediately frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C for sectioning. Frozen 5 μm-thick sections were transferred to sylane-coated slides. Sections for immunolabelling against the primary antibodies dystrophin (rabbit polyclonal antibody anti-dystrophin; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA, dilution 1:100 and 1:50), albumin (mouse monoclonal antibody anti-albumin-FITC conjugated, Bethyl Laboratories Inc.; Montgomery, TX, USA, dilution 1:600), α-sarcomeric actin (mouse monoclonal antibody anti-α-sarcomeric actin; Sigma Co., dilution 1:400), CD-68 (mouse monoclonal antibody anti-CD68; Serotec Ltd., Kidlington, Oxford, UK, dilution 1:200), eNOS (rabbit polyclonal antibody anti-endothelial nitric oxide synthase; Labvision Co., Freemont, CA, USA, dilution 1:150), CD4 (mouse monoclonal antibody anti-CD4; Biolegend, San Diego, CA, USA), and CD-45 (mouse monoclonal antibody anti-CD45, leucocyte common antigen; BD Biosciences, San Jose, CA, USA, dilution 1:100) were fixed in cold acetone for 10 min. Sections for immunostaining against merosin laminin α-2 chain (mouse monoclonal antibody anti-merosin laminin α-2 chain; Vector Laboratories Inc., Burlingame, CA, USA, dilution 1:100) were fixed with 2% paraformaldehyde after the secondary antibody. The sections for the reactions with the primary antibodies against γ-sarcoglycan (mouse monoclonal antibody anti-γ-sarcoglycan; Vector Laboratories, dilution 1:400), β-dystroglycan (mouse monoclonal antibody anti-β-dystroglycan, Vector Laboratories, dilution 1:400) and β-1 integrin (mouse monoclonal antibody anti-β-1 integrin; Chemicon International Inc., Billerica, MA, USA, dilution 1:200) were not fixed. The antibodies were diluted in 1% BSA and incubated overnight at 4 °C. Immunolabelling was performed using secondary antibodies against fluorocrome-conjugated anti-mouse immunoglobulin G/FITC, anti-mouse immunoglobulin G/AMCA and anti-rabbit immunoglobulin G/Texas red (Vector Laboratories) were diluted 1:200 in HEPES 0.01 M, incubated for 1 h at room temperature. Sections immunostainning with antibodies against γ-sarcoglycan, laminin, CD68 and eNOS were also labelled with phalloidin complexed to Alexa Fluor 594 (Molecular Probes, Eugene, OR, USA, dilution 1:1000) diluted in 0.1% BSA at 37 °C for 30 min. The nuclei were labelled with DAPI (Molecular Probes).
Assessment of apoptosis
For the recognition of apoptotic cells, we used the TUNEL, method (TdT-mediated dUTP-biotin nick end-labelling, which identifies early DNA fragmentation in the nucleus. A commercially available staining kit (Promega Corporation, Madison, WI, USA) was used for this purpose according to manufacturer's instructions. Sections 5 μm-thick were obtained from paraffin-embedded blocks and mounted on poly-l-lysine-coated slides. Following terminal deoxynucleotidyl transferase reaction, sections were slightly stained with Harris' haematoxylin.
Echocardiography
To evaluate the cardiac function in animals given isoproterenol, we assessed echocardiographic parameters of control and experimental hearts were assessed. Because of the transducer accuracy limitations of the clinical echocardiographic equipment when used for small animals and the short course of the experiment, we used animals weighing more than 300 g and two different doses of isoproterenol, 85 and 150 mg/kg. The animals were anaesthetized intraperitoneally with ketamine (74 mg/kg) and xylazine (8 mg/kg). While anaesthetized, rats were allowed to breathe spontaneously and before echocardiography, the anterior chest hair was shaved and the rats were positioned in the in left lateral decubitus. The global left ventricular (LV) systolic function was evaluated with a high-resolution two-dimensional echocardiographic system Sonos HP 5500 Philips (Andover, MA, USA) equipped with a S12 MHz frequency transducer. Left ventricular M-mode measurements at the level of papillary muscles were used to define the internal diameters of left and right ventricles at systole and diastole. The fractional shortening (%FS) was used as an index of systolic function. Wall motion was analysed with a 16-segments model based a three short-axis views, the scores separately graded for the apical (ratio of four segments), middle (ratio of six segments) and basal (ratio of six segments) regions of the left ventricle. Wall motion scored 1 for normal, 2 for hypokinetic, 3 for akinetic, 4 for dyskinetic, and 5 for aneurismal, according to the Guidelines of the American Society of Echocardiography (Schiller et al. 1989).
Statistical analysis
Data were analysed using a GraphPad Prism 4 statistic program (GraphPad Software Inc., San Diego, CA, USA). For analysis of differences between the two groups the Student's t-test was performed. The data are reported as the mean ± standard error. The Kruskal–Wallis non-parametric test, followed by Dunn's post test, was used to determine differences between the mean scores estimated for each region of the left ventricles in rats given isoproterenol, 85 or 150 mg/kg in comparison with the values estimated in the corresponding regions of control hearts. A level of significance of 5% was chosen to denote difference between means.
Results
Body and heart weight
The dose of 85 mg/kg of isoproterenol caused death of two animals (7.69%) before the end of the experimental period and this material was disregarded. Similarly, a mortality rate of 10% was observed when this dose was administered to rats weighing 260 g on average (Rona et al. 1959). The animals that received isoproterenol, a well-known bio-behavioural stress mediator, presented characteristic symptoms after injection such as unusual postures, open mouth breathing, less eating. No foamy exudate from the nostrils or subcutaneous oedema was observed. As a consequence of less food intake, a 4% retardation of the mean body weight gain was experienced by the animals in the isoproterenol-treated group (169.2 ± 1.97 g) in comparison with that of animals in the control group (175.0 ± 1.51 g). The mean heart weight in isoproterenol-treated rats (0.89 ± 0.01 mg) was 21% higher compared with that of controls (0.74 ± 0.01 mg). The heart ratio in the isoproterenol-treated group (5.35 ± 0.13 g/kg) was 26% higher in comparison with that of the control group (4.23 ± 0.09 g/kg).
Light and high resolution microscopy
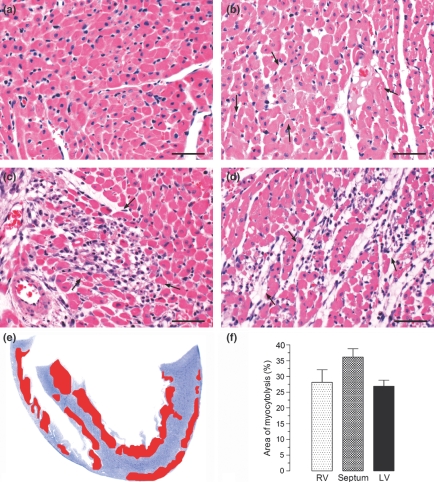
The hearts embedded in paraffin and plastic from both groups were examined. The use of plastic embedding allowed thin sections with adequate resolution of structural details. The microscopic study of the myocardium revealed that the control hearts did not present any pathological change. The histological findings in the hearts from isoproterenol-treated rats were graded in three degrees according to the severity of the lesions: grade 1, foci of myocytes with intracellular oedema (tumefaction) and vacuolar degeneration; grade 2, foci of myocytolysis, many of them coalescent, associated with an inflammatory infiltrated composed of mononuclear cells; and grade 3, foci showing remnants of cardiomyocytes, empty spaces and very few inflammatory cells (Figures 1a–d and 3a,c,e,g).
Figure 1.
Representative histological features of control myocardium (a) compared with those of myocardium from rats given isoproterenol (85 mg/kg) arranged in an apparent sequence of events : (b) in the first stage, foci of myocytes with tumefaction (arrows), (c) in the second stage, focus of myocytolysis with an inflammatory infiltrated composed of mononuclear cells, and (d) in the third stage, focus showing remnants of cardiomyocytes, empty spaces and a few mononuclear cells. Haematoxylin and eosin. Bar = 50 μm. (e) Schematic drawing showing the mean distribution of necrotic areas in predominantly in the cardiac apex, subendocardium and subepicardium of the left ventricle, in the septum and in the subendocardium of the right ventricle. (f) Mean distribution of the myocytolytic areas in the myocardium of isoproterenol-treated rats.
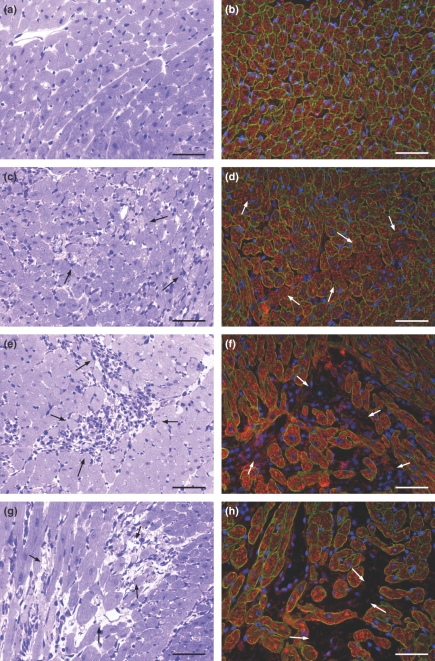
Figure 3.
Representative histological features of control myocardium (a) in comparison with the myocardium of rats given isoproterenol arranged in a sequence of events: (c) foci of myocytes with tumefaction (arrows); (e) focus of myocytolytic necrosis with an inflammatory infiltrate composed of mononuclear cells (arrows); (g) focus showing remnants of myocytes, empty spaces and a few mononuclear cells (arrows). High resolution light microscopy, toluidine blue. Immunofluorescence analysis of dystrophin expression (green fluorescence) in control (b) and isoproterenol-treated myocardium (d, f, h). (d) The immunofluorescent signal is markedly and focally reduced in myocytes of rats given isoproterenol (arrows). (f, h) The foci of myocytolytic necrosis show complete loss of dystrophin immunofluorescent signal with increased number of inflammatory cells (revealed by the blue fluorescence of DAPI) in f (arrows) and a few inflammatory cells in h (arrows). The red fluorescent signal corresponds to actin as stained by phalloidin complexed to Alexa Fluor. Bar = 50 μm.
For analysis of distribution of myocytolytic necrosis areas we used hearts sectioned frontally and embedded in plastic. The myocardium of isoproterenol-treated rats showed focal and coalescent myocytolytic focal lesions predominantly at the cardiac apex, subendocardium and subepicardium of the left ventricle, in the septum and in the subendocardium of the right ventricle. The mean distribution of myocytolytic foci in the myocardium from isoproterenol-treated rats was 26.89 ± 1.88% in the left ventricle, 36.12 ± 2.7% in the septum and 28.15 ± 3.96% in the right ventricle (Figure 1e,f).
Morphometry
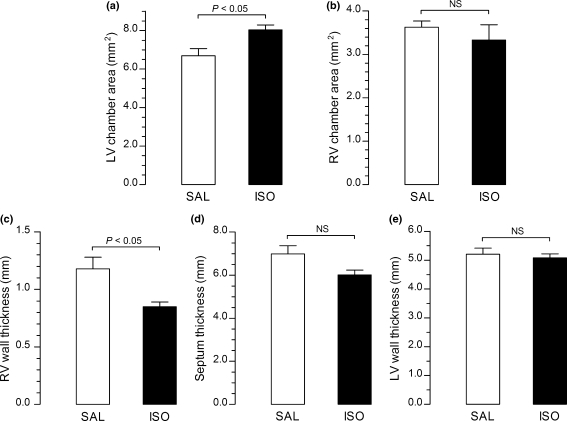
Hearts coronally sectioned and embedded in paraffin were used for morphometric analysis. The left and right ventricular chamber areas and wall thicknesses of right and left ventricles and interventricular septum were measured. The data showed that the mean area of the left ventricular chamber of the hearts from isoproterenol-treated rats (8.03 ± 0.26 mm2) increased 20% compared with that of controls (6.69 ± 0.37 mm2) (Figure 2a). No significant differences were found in the mean area of the right ventricular chambers of isoproterenol-treated rats (3.32 ± 0.35 mm2) compared with that of controls (3.63 ± 0.14 mm2) (Figure 2b). The mean septum and left ventricular wall thicknesses were similar in both isoproterenol-treated group (6.01 ± 0.22 and 5.08 ± 0.14 mm respectively) and control group (6.98 ± 0.38 and 5.20 ± 0.21 mm respectively), although these values tended to be lower in experimental animals (Figure 2d,e). In addition, the mean right ventricular wall thickness was 26% lower in the isoproterenol-treated rats (0.85 ± 0.04 mm) in comparison with that in controls (1.17 ± 0.09 mm) (Figure 2c).
Figure 2.
Graphs showing the mean left and right ventricular chamber areas and right, left and septum thicknesses of hearts from isoproterenol-treated rats (ISO) compared with control values (SAL). The mean area of the left ventricular chamber of the hearts from isoproterenol-treated rats is 20% increased compared with that of controls (a). No significant differences can be seen in the mean area of the right ventricular chamber of isoproterenol-treated rats compared with that of controls (b). The mean right ventricular wall thickness is 26% lower in the isoproterenol-treated rats in comparison with that in controls (c). The mean septum (d) and left ventricular wall thicknesses (e) are similar in both isoproterenol-treated and control groups, although these values tended to be lower in experimental animals.
Immunofluorescence microscopy
The immunofluorescent study of the DGC revealed that dystrophin immunolabelling was focally reduced or completely lost in cardiac myocytes of isoproterenol-treated rat hearts. The change in dystrophin expression was detected before the loss of actin. In transverse sections, double immunolabelling for dystrophin and sarcomeric actin clearly indicated three different patterns in accordance with the severity of the myocardial lesions: (i) corresponding to grade 1 myocardial lesions, loss of dystrophin and presence of sarcomeric actin associated with no inflammatory cells, (ii) corresponding to grade 2 myocardial lesions, loss of dystrophin and variable degree of sarcomeric actin dissolution associated with great number of inflammatory mononuclear cells, and (iii) corresponding to grade 3 myocardial lesions, loss of dystrophin and sarcomeric actin associated with scant mononuclear cells (Figure 3d,f,h). The control group showed consistent pattern of distribution of dystrophin and sarcomeric actin (Figure 3b).
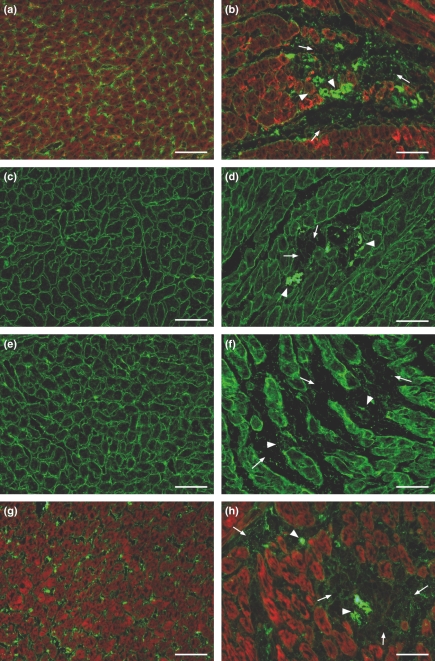
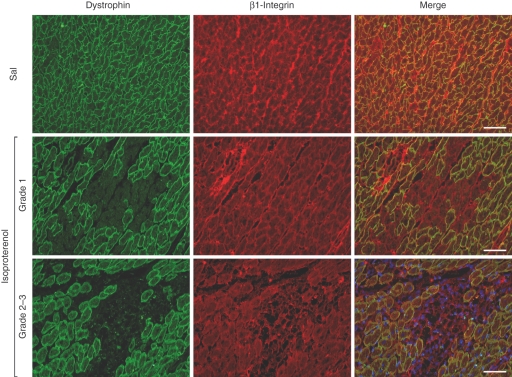
The evaluation of the expression of the other components of the DGC, γ-sarcoglycan, β-dystroglycan, and of β-1 integrin and merosin laminin α-2 showed that they have taken a secondary part in the process of breakdown of myocytes in myocytolytic areas. The foci of myocytolytic necrosis showed slight attenuation of γ-sarcoglycan, β-dystroglycan, β-1 integrin and merosin laminin α-2 chain, associated with complete loss of actin in the same areas. Subsequent to the lysis of myofilaments, corresponding to grade 3 myocardial lesions, these structures also could be seen collapsed associated with a few inflammatory cells (Figure 4b,d,f,h). No pathological changes were found in the myocardium from control animals (Figure 4a,c,e,f) To distinguish exactly the sequence of expression reduction of the components of the DGC in the same field, we employed double staining of dystrophin and γ-sarcoglycan or β-dystroglycan or β-1 integrin or merosin laminin α-2 chain. We noted that in the foci corresponding to myocardial lesion grade 1 the loss of dystrophin is clearly seen with the persistence of the remainder of the DGC elements, β-1 integrin, and merosin laminin α-2 chain; although attenuated while in foci corresponding to myocardial lesions grade 2 and 3, the loss of dystrophin is clearly noted with attenuated expression or breakdown of the remainder DGC elements, β-1 integrin, and merosin laminin α-2 chain. Figure 5 regarding double staining of dystrophin and β-1 integrin clearly illustrates the phenomenon (data on γ-sarcoglycan, β-dystroglycan and merosin laminin α-2 chain are not shown).
Figure 4.
Representative images of immunofluorescence analysis of γ-sarcoglycan, β-dystroglycan, β-1 integrin and merosin laminin α-2 (green fluorescence) in control (a, c, e, g) and isoproterenol-treated (b, d, f, h) rat hearts. The foci of myocytolytic necrosis show attenuation (arrows) and collapse (arrow heads) of these glycoproteins associated with complete loss of actin (red fluorescence of phalloidin complexed with Alexa Fluor) in the same areas (b, h). Bar = 50 μm.
Figure 5.
Sequence of dystrophin and β-1 integrin expression reduction in the same field in saline control and isoproterenol-treated hearts. In the foci corresponding to myocardial lesions type 1, loss of dystrophin expression is clearly seen with the persistence of β-1 integrin. In the foci corresponding to myocardial lesion types 2–3, the loss of dystrophin is associated with attenuation or breakdown of β-1 integrin. Bar = 50 μm.
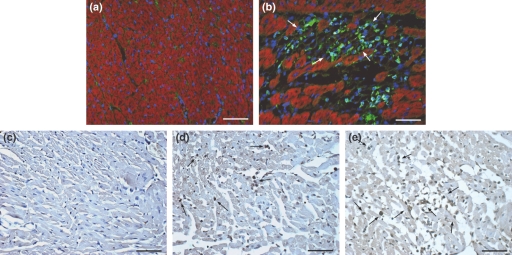
The inflammatory infiltrate observed on light microscopy was characterized using primary antibodies against macrophages (CD68), CD4 lymphocytes, and against leucocyte common antigen (CD45). In myocardium from control rats, we found no inflammatory cells. The myocardium from isoproterenol-treated rats showed an inflammatory infiltrate mainly characterized by positive CD68+ cells in the myocytolytic foci (Figure 6a,b). The CD45 immunolabelling reproduced the immunostaining with anti-CD68 (data not shown). The CD4 immunolabelling was negative in the hearts from isoproterenol-treated rats.
Figure 6.
(a, b) Representative images of the immunofluorescence analysis of macrophages in control and isoproterenol-treated rat hearts (green fluorescence of cytoplasm and blue fluorescence of nuclei). The control myocardium (a) shows no macrophages. The myocardium from isoproterenol-treated rats (b) shows a focal inflammatory infiltrate in the myocytolytic foci characterized by CD-68+ cells. C-E. Representative images of apoptosis assessment. In the control myocardium, the TUNEL reaction was constantly negative (c). In the myocytolytic foci grades 1–3, a positive TUNEL reaction was constantly and clearly noted (arrows) in cardiomyocytes and macrophages (mononuclear cell infiltrate) (d, e). Bar = 50 μm.
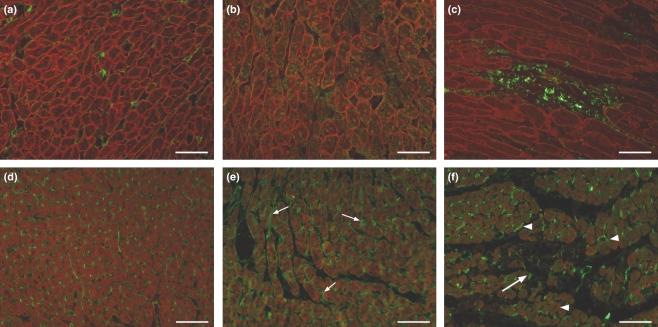
Considering that the damage of the sarcolemma could lead to increased membrane permeability, the sarcolemmal integrity was evaluated through an immunofluorescence assay using dystrophin-albumin labelling. The control myocardium showed uniform dystrophin staining and the presence of albumin in the interstitium just marking the small vessels. The myocardium of isoproterenol-treated rats showed either focal areas of cells with loss of dystrophin and intense accumulation of albumin in the intracellular space as revealed by green and red fluorescence of albumin and dystrophin, respectively, or areas with no dystrophin immunolabelling but showing green albumin fluorescence irregularly revealing the cells, clearly demonstrating alterations in myocytes membrane permeability (Figure 7a–c).
Figure 7.
(a–c) Representative images of the fluorescence analysis of dystrophin (red fluorescence) and albumin (green fluorescence) in control (a) and isoproterenol-treated rat hearts (b, c). The control myocardium shows uniform dystrophin staining and the albumin restricted to the interstitium just marking the small vessels. The myocardium of rats given isoproterenol shows areas of red and green fluorescence (b) and areas of no dystrophin labelling showing green fluorescence irregularly revealing the cardiomyocytes (c). (d–f) Representative images of fluorescent signals of eNOS (green fluorescence) and actin (red fluorescence) in control (d) and in rats given isoproterenol (e, f). In the myocardium of control animals, the eNOS staining marks the small vessels endothelium. The myocardium from isoproterenol-treated rats shows increased eNOS expression in the small vessels (small arrows in e and arrowheads in f), markedly around the myocytolytic areas and also in the adjacent cytoplasm of myocytes. eNOS expression was practically absent in the myocytolytic areas except for remnants of interstitial small vessels (f, arrows). Bar = 50 μm.
Taking into account that compensatory mechanisms to the ischaemic insult elicited by isoproterenol could be acting and eNOS is known to oppose directly the inotropic effects of catecholamines (Massion et al. 2005), eNOS expression was evaluated. For this study, the eNOS-phalloidin immunolabelling was performed. In the myocardium of control animals, the eNOS staining was positive and specifically marked the small vessels endothelium. The myocardium from isoproterenol-treated rats showed increased eNOS expression in the small vessels, markedly around the myocytolytic areas and also in the adjacent cytoplasm of myocytes. eNOS expression was practically absent in the myocytolytic areas except for remnants of interstitial small vessels (Figure 7d–f).
Assessment of apoptosis
Considering the absolute predominance of macrophages in the myocytolytic foci, the occurrence of apoptotic cells was evaluated using the TUNEL method. In the myocytolytic foci grade 1, a positive reaction was constantly and clearly noted in cardiomyocytes and in myocytolytic foci grades 2–3 cardiomyocytes and macrophages (mononuclear cell infiltrate) were both clearly and constantly marked (Figure 6c–e).
Echocardiography
The mean heart rate after the sedation during echocardiography was not significantly different in control (311 ± 13.6 bpm) and isoproterenol-treated (341 ± 14.2 bpm) rats. Two-dimensional and the M-mode images taken at the midpapillary level showed significant differences in the end-diastolic and the end-systolic dimensions between control (0.600 ± 0.01 and 0.271 ± 0.02 cm respectively) and isoproterenol-treated (0.709 ± 0.02 and 0.368 ± 0.03 cm respectively) rats. The mean fractional shortening (FS) was not different in control (48.5 ± 1.96%) and isoproterenol-treated (51.3 ± 3.06%) rats. The wall motion segmented scoring was used in this study taking into account that it provides an accurate semiquantitative index of left ventricular function in human and small animals (Morgan et al. 2004; Zhang et al. 2007). The mean wall motion basal and middle scores in isoproterenol rat hearts (1 and 1.04 ± 0.04 respectively) were similar to the values in corresponding segments in the control hearts (1 for both segments). Importantly, the mean wall motion apical scores in isoproterenol hearts (2.21 ± 0.34) were significantly higher compared with the corresponding value in the control hearts (1).
Discussion
The administration of isoproterenol was efficient in inducing infarct-like myocardial lesions, as extensively described (Rona et al. 1959; Ferrans et al. 1964). Conventional and high resolution light microscopy revealed that the myocardium of animals treated with isoproterenol presented foci of myocytolysis classifiable into three distinct and different degrees of severity that can be arranged in a sequence of events: first, foci of myocardial cell tumefaction and vacuolar degeneration, second, foci of myocytolysis associated with an inflammatory infiltrate composed predominantly of macrophages, and third, focal areas of myofibre loss associated with scant macrophages. At this very early acute phase after isoproterenol administration, the central role of the macrophages is the phagocytosis of cell debris, their presence becoming less common after removal of the phagocytosed material, which is followed by the incoming fibroblasts to the foci (data not shown). Many apoptotic macrophages and cardiomyocytes were found in the hearts of rats given isoproterenol. Thus, apoptosis plays an important role in the disappearance of the infiltrated mononuclear cells and myocardial cells after isoproterenol administration. This agrees with previous observations showing that β-adrenergic activation causes necrotic as well as apoptotic death of cardiac myocytes (Remondino et al. 2003). The lesions were predominant in the cardiac apex, subendocardium of left and right ventricles and represented 26.89%, 36.12% and 28.15% of the total areas of the left ventricle, septum and right ventricle respectively.
We found different sensitivity of the components of the DGC to isoproterenol subcutaneous administration. Particularly, immunofluorescent staining revealed that dystrophin is the most sensitive among the structures connecting the actin in the cardiomyocyte cytoskeleton and the extracellular matrix represented by laminin in this study. The sarcomeric actin dissolution, as part of the process of myocytolysis or myofibrillar degeneration, occurred after the reduction or loss of dystrophin. Subsequent to the lysis of myofilaments, the expression of γ-sarcoglycan, β-dystroglycan, β1-integrin and laminin α-2 was reduced followed by their breakdown, appearing as epiphenomena of the myocytolytic process. Double staining clearly confirmed that dystrophin loss occurred prior to the reduction in expression of the remainder of the DGC elements. It is known that dystrophin loss leads to secondary instability of the other components of the DGC (Ervasti et al. 1990). The DGC comprises glycoproteins, such as dystroglycans, sarcoglycans, tightly associated with dystrophin that adheres to laminin, the major component of the basement membrane. This complex contributes to cell shape, mechanical resistance and signal transduction to cardiomyocytes (Hein et al. 2000; Kostin et al. 2000). Dystrophin is localized beneath the sarcolemma and links actin to the extracellular matrix through the membrane-spanning glycoproteins. Dystroglycan binds to laminin in the extracellular matrix (McNally et al. 2003) and sarcoglycan is a transmembrane subcomplex and stabilizes the interaction between α- and β-dystroglycans (Straub et al. 1998). Integrins are also essential in defining appropriate cellular–extracellular interaction (Ross & Borg 2001). Several human studies and animal models have indicated that the disruption of the DGC may lead to cardiomyopathy (Lapidos et al. 2004; Heydemann & McNally 2007). Cardiomyocytes lacking dystrophin are abnormally vulnerable to mechanical stress-induced injury, with loss of sarcolemmal integrity and myofibrilar degeneration (myocytolysis), and consequent contractile dysfunction (Danialou et al. 2001; Lapidos et al. 2004).
To our knowledge, this is the first report to demonstrate the pattern of sensitivity to isoproterenol of these linkage proteins in one experiment. Recent investigation demonstrated different sensitivity of the membrane-related proteins to experimentally induced myocardial ischaemia in dogs, when, similarly, dystrophin was more affected than the transmembrane proteins dystroglycans, sarcoglycans, sarcospan, dystrobrevin and syntrophin (Rodríguez et al. 2005).
Abnormal immunohistochemical staining for dystrophin in isoproterenol-induced myocardial injury (subcutaneous administration, 100 mg/kg) in rats was described previously (Miyazato et al. 1997). In contrast to our findings, the authors reported that cardiomyocytes presented three different patterns: normal for actin but abnormal for dystrophin, abnormal for actin but normal for dystrophin, and abnormal for both actin and dystrophin. The authors stressed that the two latter patterns could be probably because of the low sensitivity of the immunohistochemical method employed; otherwise, there would be more than one mechanism of isoproterenol-induced myocyte injury. Subsequently, the association of intraperitoneal administration of isoproterenol (10 mg/kg) and dystrophin disruption has been demonstrated in damaged myocardium (Xi et al. 2000; Toyo-oka et al. 2004; Kawada et al. 2005).
Taking into account our findings, it is attempting to hypothesize on the molecular mechanism of isoproterenol-induced myocardial injury: reduction and loss of dystrophin may be the primary event that precedes cardiomyocyte myofilamentar degeneration and lysis. Observations either in human Duchenne muscular dystrophy produced by mutation in the dystrophin gene on the X chromosome or in murine models of dystrophin mutation gene support this hypothesis. In each of these situations, the loss of dystrophin destabilizes the complex of associated proteins at the sarcolemma leading to myocyte destruction. Sarcolemmal destabilization has been demonstrated to alter membrane permeability (Straub et al. 1997) leading to increased intracellular calcium content that stimulates calcium-activated proteases and contributes to muscle destruction. Subcutaneous isoproterenol administration has been shown to cause increased sarcolemmal permeability (Yunge et al. 1989) with intracellular calcium overload and severe myocardial fibre injury and death (Milei et al. 1978; Fleckenstein et al. 1983; Tappia et al. 2001). The correlation between loss of dystrophin and damage of sarcolemma with increased permeability of the membrane is provided here through the dystrophin-albumin immunofluorescent staining. The myocardium of isoproterenol-treated rats showed either focal areas of cells with loss of dystrophin and accumulation of albumin in the intracellular space or areas of actin breakdown and albumin accumulation in the place of the cells. This indicates severe or irreversible injury of the cardiac myocytes. Previous studies have shown that loss of dystrophin correlates with increased sarcolemmal permeability in ischaemic myocardium (Armstrong et al. 2001; Rodríguez et al. 2005).
An important question is ‘what is the mechanism of the reduction of dystrophin expression in the hearts of isoproterenol-treated rats?’ First, ischaemic stress induced by isoproterenol can lead to loss of sarcolemmal dystrophin. Acute ischaemia can cause loss of immunostained dystrophin and corresponding depletion of dystrophin in cardiomyocytes in vitro (Armstrong et al. 2001). Second, mechanical stress can induce a state of fragility of the dystrophin-actin binding domain by inducing structural alterations in dystrophin. Alterations of gene expression have been shown to lead to alterations in dystrophin expression in vivo (Sadoshima & Izumo 1997). Third, endogenous or exogenous proteases can cause dystrophin expression loss. It has been demonstrated that protease 2A directly cleaves dystrophin during Coxsackievirus infection of cultured cardiac ventricular myocytes and in infected mice hearts leading to impaired dystrophin function (Xiong et al. 2007).
The DGC has both mechanical and signalling roles and interacts with known signalling components including nitric oxide synthases (Heydemann et al. 2004). The pool of eNOS is expressed in sarcolemma and T-tubular caveolae, in the same place as beta 3-adrenoceptors, and in endothelial cells (Massion et al. 2005). It is known to attenuate the beta1/beta2-adrenergic increase in inotropy and chronotropy and reinforce the vagal control of cardiac contraction, thereby preventing the excessive stimulation by catecholamines through NO vasodilating effect. eNOS expression was enhanced in blood vessels and in cytoplasm of cardiomyocytes through the entire myocardium of rats given isoproterenol. However, eNOS expression was almost completely absent in the myocytolytic foci. This is probably a compensatory response to the ischaemic insult elicited by isoproterenol administration. The same way, recent study has demonstrated a lasting upregulation of eNOS in blood vessels and cardiomyocytes after experimental induction of myocardial infarction in rats (Berges et al. 2007).
In this study, laminin α-2 expression was still present in areas of myocytolysis, collapsing thereafter. Laminin-2 is a major component of the basal lamina and connects the actin to the extracellular matrix (McNally et al. 2003). Loss of this linkage has been suggested as a determining factor for myofibre necrosis (Ohlendieck 1996; Kido et al. 2004; Allikian & McNally 2007). In addition, laminin expression has been shown to decrease during the transition to heart failure and predisposes cardiomyocytes to apoptosis (Berk et al. 2007).
The morphometric study of the hearts showed an increase of 20% in the left ventricular chamber area and a decrease of 26% in right ventricular wall thickness in the hearts of isoproterenol-treated rats. The mean left ventricular wall and septum thicknesses were not significantly different in both experimental groups, but tended to be lower in isoproterenol-treated myocardium. The occurrence of left ventricular chamber enlargement, reduction in right ventricular wall thickness and tendency towards reduction of left ventricular and septum thicknesses in isoproterenol-treated animals suggest myocardial depression. Cardiac function of control and isoproterenol-treated rats was evaluated through echocardiographic parameters. Transthoracic echocardiography clinical equipments have been used in cardiovascular research with small animals, even with limitations on the assessment of ejection fraction and cardiac output parameters (Watson et al. 2004; Weytjens et al. 2006; Wasmeier et al. 2007). The echocardiographic study showed differences in left ventricular dimensions in isoproterenol-treated animals compared with controls. The fractional shortening was not statistically significant compared with control and isoproterenol-treated animals. Compensatory mechanisms in the short time of our experiment could maintain the normal cardiac function in spite of severe myocardial morphological changes. A normal fractional shortening in spite of myocardium damage could be because of marked increase in myocardial norepinephrine release and α-adrenoceptors inotropic upregulation counterbalancing the negative inotropic actions of myocardial damage and β-adrenoceptors downregulation (Osadchii et al. 2007). For this reason, the wall motion score was used as additional index to provide an accurate semiquantitative index of regional function (Morgan et al. 2004). Hypokinesis or akinesis in the apical segments could be seen in the hearts of rats given isoproterenol compared with controls. This agrees with the long-known observation that the cardiac apex is the main location of isoproterenol-induced lesions (Rona et al. 1959).
Individual alterations in each component of the DGC and laminin are potentially involved in cardiac dysfunction. Dystrophin is the most sensitive component of the DGC in the myocardium of rats given isoproterenol subcutaneously. The reduced expression or breakdown of β-dystroglycan, γ-sarcoglycan, β-1 integrin and laminin α-2 are very likely epiphenomenal. Recent study using myocardial biopsy samples of patients with heart failure showed that changes in cytoskeletal proteins and, in particular, dystrophin might provide a final common pathway for contractile dysfunction in heart failure (Vatta et al. 2002). Reduction in contents of dystrophin and sarcoglycans was found in failing rat hearts after acute myocardial infarction (Yoshida et al. 2003). More recently, a study using hamster hearts with hereditary dilated cardiomyopathy and human hearts with dilated cardiomyopathy has shown a close correlation between loss of dystrophin and heart failure, dystrophin disruption clearly preceding the progression to advanced heart failure caused by degrading cardiomyocytes (Toyo-oka et al. 2004).
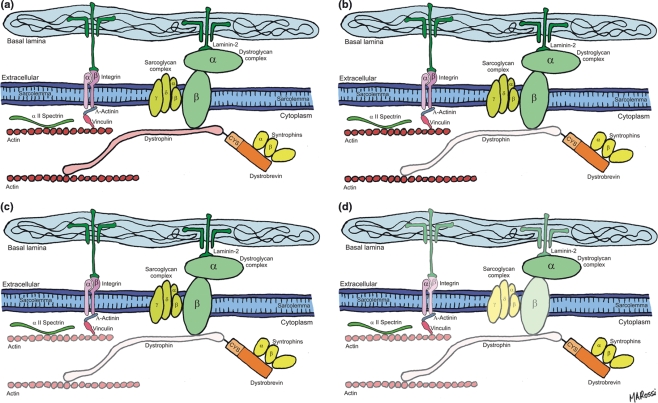
It can be concluded, therefore, that subcutaneous administration of isoproterenol to rats results in primary loss of dystrophin, the most sensitive among the structural proteins that form the DGC that connects the extracellular matrix and the cytoskeleton in cardiomyocyte. Figure 8 shows schematic diagrams representing the sequence of events observed: first, loss of dystrophin and presence of sarcomeric actin; second, loss of dystrophin and variable degree of sarcomeric actin dissolution; and third, loss of dystrophin, sarcomeric actin and reduction in the expression of γ-sarcoglycan, β-dystroglycan, β1-integrin and laminin α-2. These changes, related to ischaemic injury, can explain the severe alterations in the structural integrity of the sarcolemma of cardiomyocytes and hence severe and irreversible injury induced by isoproterenol.
Figure 8.
Schematic diagrams representing the sequence of events observed: (a) The DGC and integrins. Dystrophin links actin to the transmembrane proteins dystroglycan and sarcoglycan. Dystroglycan binds to laminin in the extracellular matrix. Integrins also link actin to laminin; (b–d) sequence of events observed: first, loss of dystrophin and presence of sarcomeric actin (b), second, loss of dystrophin and variable degree of sarcomeric actin dissolution (c), and third, loss of dystrophin, sarcomeric actin, and reduction in the expression of γ-sarcoglycan, β-dystroglycan, β1-integrin and laminin α-2 (d).
Acknowledgments
The authors thank Mônica A. Abreu, Maria E. Riul, and Lígia B. Santoro for excellent technical assistance. Prof. Rossi is Senior Investigator of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo e Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto (FAEPA). Dr Campos was recipient of a Master Science Degree from the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
References
- Allikian MJ, McNally EM. Processing and assembly of the dystrophin glycoprotein complex. Traffic. 2007;8:177–183. doi: 10.1111/j.1600-0854.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- Armstrong SC, Latham CA, Shivell CL, Ganote CE. Ischemic loss of sarcolemmal dystrophin and spectrin: correlation with myocardial injury. J. Mol. Cell. Cardiol. 2001;33:1165–1179. doi: 10.1006/jmcc.2001.1380. [DOI] [PubMed] [Google Scholar]
- Berges A, van Nassauw L, Timmermans JP, Vrints C. Time-dependent expression pattern of nitric oxide and superoxide after myocardial infarction in rats. Pharmacol. Res. 2007;55:72–79. doi: 10.1016/j.phrs.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J. Clin. Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel CI, Rona G, Balazs T, Gaudry R. Severe myocardial necrosis produced by isoproterenol in the rat. Arch. Int. Pharmacodyn. Ther. 1959;122:123–128. [PubMed] [Google Scholar]
- Csapó Z, Dusek J, Rona G. Early alterations of the cardiac muscle cells in isoproterenol-induced necrosis. Arch. Pathol. 1972;93:356–365. [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Dudley R, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Ohlendieck K, Kahl SD, et al. Deficiency of α-glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Ferrans VJ, Hibbs RG, Black WC, Weilbaecher DG. Isoproterenol-induced myocardial necrosis. A histochemical and electron microscopic study. Am. Heart J. 1964;68:71–90. doi: 10.1016/0002-8703(64)90242-x. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A, Frey M, Fleckenstein-Grün G. Consequences of uncontrolled calcium entry and its prevention with calcium antagonists. Eur. Heart J. 1983;4:43–50. doi: 10.1093/eurheartj/4.suppl_h.43. [DOI] [PubMed] [Google Scholar]
- Grimm D, Elsner D, Schunkert H, et al. Development of heart failure following isoproterenol administration in the rat: role of the renin-angiotensin system. Cardiovasc. Res. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- Handforth CP. Isoproterenol-induced myocardial infarction in animals. Arch. Pathol. 1962;73:161–165. [PubMed] [Google Scholar]
- Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc. Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc. Med. 2007;17:55–59. doi: 10.1016/j.tcm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Huber JM, Kakkar R, et al. Functional nitric oxide synthase mislocalization in cardiomyopathy. J. Mol. Cell. Cardiol. 2004;36:213–223. doi: 10.1016/j.yjmcc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Kawada T, Masui F, Tezuka A, et al. A novel scheme of dystrophin disruption for the progression of advanced heart failure. Biochim. Biophys. Acta. 2005;1751:73–81. doi: 10.1016/j.bbapap.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Kido M, Otani H, Kyoi S, et al. Ischemic preconditioning-mediated restoration of membrane dystrophin during reperfusion correlates with protection against contraction-induced myocardial injury. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H81–90. doi: 10.1152/ajpheart.01140.2003. [DOI] [PubMed] [Google Scholar]
- Kostin S, Hein S, Arnon E, et al. The cytoskeleton and related proteins in the human failing heart. Heart Fail. Rev. 2000;5:271–280. doi: 10.1023/A:1009813621103. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ. Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Massion PB, Pelat M, Belge C, Balligand JL. Regulation of the mammalian heart function by nitric oxide. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005;142:144–150. doi: 10.1016/j.cbpb.2005.05.048. [DOI] [PubMed] [Google Scholar]
- McNally E, Allikian M, Wheeler MT, et al. Cytoskeletal defects in cardiomyopathy. J. Mol. Cell. Cardiol. 2003;35:231–241. doi: 10.1016/s0022-2828(03)00018-x. [DOI] [PubMed] [Google Scholar]
- Milei J, Nuñes RG, Rapaport M. Pathogenesis of isoproterenol-induced myocardial lesions: its relation to human ‘coagulative myocytolysis’. Cardiology. 1978;63:139–151. doi: 10.1159/000169891. [DOI] [PubMed] [Google Scholar]
- Miyazato H, Biro S, Setoguchi M, et al. Abnormal immunostainning for dystrophin in isoproterenol-induced acute myocardial injury in rats: evidence for change in dystrophin in the absence of genetic defect. J. Mol. Cell. Cardiol. 1997;29:1217–1223. doi: 10.1006/jmcc.1996.0357. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Faulx MD, McElfresh TA, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2049–2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K. Towards an understanding of dystrophin-glycoprotein complex: linkage between the extracellular matrix and the membrane cytoskeleton in muscle fibers. Eur. J. Cell Biol. 1996;69:1–10. [PubMed] [Google Scholar]
- Osadchii OE, Norton GR, Mckechnie R, et al. Cardiac dilatation and pump dysfunction without intrinsic myocardial systolic failure following chronic beta-adrenoceptor activation. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1898–1905. doi: 10.1152/ajpheart.00740.2006. [DOI] [PubMed] [Google Scholar]
- Reichenbach DD, Benditt EP. Catecholamines and cardiomyopathy: the pathogenesis and potential importance of myofibrilar degeneration. Hum. Pathol. 1970;1:125–150. [Google Scholar]
- Remondino A, Kwon SH, Communal C, et al. β-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-jun NH2 terminal kinase-dependent activation of mitochondrial pathway. Circ. Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- Rodríguez M, Cai WJ, Kostin S, et al. Ischemia depletes dystrophin and inhibits protein synthesis in the canine heart: mechanisms of myocardial ischemic injury. J. Mol. Cell. Cardiol. 2005;38:723–733. doi: 10.1016/j.yjmcc.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Rona G. Catecholamine cardiotoxicity. J. Mol. Cell. Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- Rona G, Chappel CL, Balazs T, Gaudry R. An infracted-like myocardial lesion and other toxic manifestations produced by Isoproterenol in the rat. Arch. Pathol. 1959;67:443–455. [PubMed] [Google Scholar]
- Ross RS, Borg TK. Integrins and the myocardium. Circ. Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography, Committee on Standards, Subcommittee on quantification of two-dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- Schlesinger MJ, Reiner L. Focal myocytolysis of the heart. Am. J. Pathol. 1955;31:443–459. [PMC free article] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V, Duclos F, Venzke DP, et al. Molecular pathogenesis of muscle degeneration in the delta-sarcoglycan-deficient hamster. Am. J. Pathol. 1998;153:1623–1630. doi: 10.1016/s0002-9440(10)65751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappia PS, Hata T, Hozaima L, et al. Role of oxidative stress in cathecolamine-induced changes in cardiac sarcolemmal Ca2+ transport. Arch. Biochem. Biophys. 2001;387:85–92. doi: 10.1006/abbi.2000.2234. [DOI] [PubMed] [Google Scholar]
- Toyo-oka T, Kawada T, Nakata J, et al. Translocation and cleavage of myocardial dystrophin as a common pathway to advanced heart failure: a scheme for the progression of cardiac dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7381–7385. doi: 10.1073/pnas.0401944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatta M, Stetson SJ, Perez-Verdia A, et al. Molecular remodeling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- Wasmeier GH, Melnychenko I, Voigt JU, et al. Reproducibility of transthoracic echocardiography in small animals using clinical equipment. Coron. Artery Dis. 2007;18:283–291. doi: 10.1097/MCA.0b013e3280d5a7e3. [DOI] [PubMed] [Google Scholar]
- Watson LE, Sheth M, Denyer RF, Dostal DE. Baseline echocardiographic values for adult male rats. J. Am. Soc. Echocardiogr. 2004;17:161–167. doi: 10.1016/j.echo.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Weytjens C, Cosyns B, D`hooge J, et al. Doppler myocardial imaging in adult male rats: reference values and reproducibility of velocity and deformation parameters. Eur. J. Echocardiogr. 2006;7:411–417. doi: 10.1016/j.euje.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Xi H, Shin WS, Suzuki J, et al. Dystrophin disruption might be related to myocardial cell apoptosis caused by isoproterenol. J. Cardiovasc. Pharmacol. 2000;36:S25–29. doi: 10.1097/00005344-200000006-00007. [DOI] [PubMed] [Google Scholar]
- Xiong D, Yajima T, Lim B-K, et al. Inducible cardiac-restricted expression of enteroviral protease 2A is sufficient to induce dilated cardiomyopathy. Circulation. 2007;115:94–102. doi: 10.1161/CIRCULATIONAHA.106.631093. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takahashi M, Koshimizu M, et al. Decrease in sarcoglycans and dystrophin in failing heart following acute myocardial infarction. Cardiovasc. Res. 2003;59:419–427. doi: 10.1016/s0008-6363(03)00385-7. [DOI] [PubMed] [Google Scholar]
- Yunge L, Bruneval P, Cokay MS, et al. Perturbation of the sarcolemmal membrane in isoproterenol-induced myocardial injury of the rat: permeability and freeze-fracture studies in vivo and in vitro. Am. J. Pathol. 1989;134:171–185. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Takagawa J, Sievers RE, et al. Validation of wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1187–1192. doi: 10.1152/ajpheart.00895.2006. [DOI] [PubMed] [Google Scholar]