Abstract
Lafora disease is a progressive myoclonus epilepsy with onset in the teenage years followed by neurodegeneration and death within 10 years. A characteristic is the widespread formation of poorly branched, insoluble glycogen-like polymers (polyglucosan) known as Lafora bodies, which accumulate in neurons, muscle, liver, and other tissues. Approximately half of the cases of Lafora disease result from mutations in the EPM2A gene, which encodes laforin, a member of the dual specificity protein phosphatase family that is able to release the small amount of covalent phosphate normally present in glycogen. In studies of Epm2a–/– mice that lack laforin, we observed a progressive change in the properties and structure of glycogen that paralleled the formation of Lafora bodies. At three months, glycogen metabolism remained essentially normal, even though the phosphorylation of glycogen has increased 4-fold and causes altered physical properties of the polysaccharide. By 9 months, the glycogen has overaccumulated by 3-fold, has become somewhat more phosphorylated, but, more notably, is now poorly branched, is insoluble in water, and has acquired an abnormal morphology visible by electron microscopy. These glycogen molecules have a tendency to aggregate and can be recovered in the pellet after low speed centrifugation of tissue extracts. The aggregation requires the phosphorylation of glycogen. The aggregrated glycogen sequesters glycogen synthase but not other glycogen metabolizing enzymes. We propose that laforin functions to suppress excessive glycogen phosphorylation and is an essential component of the metabolism of normally structured glycogen.
Glycogen is a branched polymer of glucose that acts as a repository of energy used in times of need (1). Liver and skeletal muscle contain the two largest deposits in mammals, but heart, brain, adipose, as well as many other tissues synthesize the polymer. Polymerization occurs via α-1,4-glycosidic linkages between glucose residues, with branch points introduced by α-1,6-glycosidic linkages. The frequency of branching determines the topology of glycogen and distinguishes it from the carbohydrate moiety of starch (2). A unique three-dimensional structure of glycogen cannot be determined experimentally because of the polydispersity of the molecule, but a widely accepted model of its structure has been proposed (3). Glycogen is composed of successive layers, or tiers, of glucose residues; a full size molecule consists of 12 tiers, with Mr = ∼107 and a diameter of ∼40 nm (4). Glycogen has been reported to contain small amounts of covalently linked phosphate, on the order of 0.064% by weight or 0.121% mol/mol (5–7). The mechanism by which the phosphate is introduced remains unclear. One suggestion (5) has been the existence of a glucose-1-phosphate transferase that would form C1–C6 bridging phosphodiesters, but this enzyme has not been further defined at the molecular level. In plant amylopectin, C1 and C3 phosphomonoesters have been shown to be formed by dikinase enzymes (8), but extensive bioinformatic analysis of mammalian genomes has failed to reveal any analogous enzyme.
Reduced branching of glycogen is associated with the accumulation of insoluble polysaccharide or polyglucosan in several disease states (9–11). In Andersen disease and Tarui disease, it is thought that an imbalance between elongating and branching enzymatic activities explains the formation of polyglucosan. Lafora disease (LD)2 (OMIM254780) is an autosomal recessive form of juvenile onset progressive myoclonus epilepsy. Patients appear relatively normal until early adolescence when seizures and rapid mental deterioration ensue resulting in death within 10 years (12–14). The hallmark of the disease is the presence of intracellular inclusions known as Lafora bodies (LB), the main constituent of which is polyglucosan. LB are found in many tissues, including skeletal muscle and brain. To date, mutations in two genes have been shown to account for ∼90% of LD cases. Approximately 60% of LD cases can be attributed to mutations in the EPM2A (epilepsy progressive myoclonus type 2A) gene, which encodes, by sequence, a dual specificity protein phosphatase named laforin (12, 15, 16). Laforin also contains a highly conserved carbohydrate-binding module subtype 20 (17) that interacts with glycogen and the polyglucosan found in patients with LD (18, 19). The second gene, mutated in ∼30% of LD patients is EPM2B (NHLRC1), which encodes malin, an E3 ubiquitin ligase (20).
Two mouse models of LD have been developed (18, 21). Disruption of the mouse Epm2a gene resulted in viable homozygous null mice that had many, but not all, of the features of the human disease (21). LB begin to appear at 4 months of age, followed by a much more robust accumulation of polyglucosan after 9 months when neurological defects were detectable. The second mouse model utilized transgenic overexpression of a dominant negative form of laforin generated by mutating the catalytic Cys266 to Ser (18). The mice developed LB in muscle, liver, and neurons, and by immunogold electron microscopy, laforin was shown to be in the proximity of the polyglucosan deposits.
Since the identification of laforin and malin, significant effort has been directed at identifying their physiological targets in an attempt to define the molecular basis for the disease. Several potential targets for malin have been proposed, including laforin (22), glycogen synthase (GS) (23), the glycogen debranching enzyme, amylo-1,6-glucosidase,4-α-glucanotransferase (AGL) (24), and the type 1 phosphatase regulatory subunit protein targeting to glycogen (PTG) (23, 25) (see “Discussion”). Candidate substrates for laforin have been more elusive. We recently demonstrated that in vitro laforin could release the covalent phosphate present in glycogen (26). Furthermore, we showed that glycogen isolated from Epm2a–/– mice contained elevated levels of covalent phosphate, supporting the argument that laforin acts as a glycogen phosphatase in vivo. In the present study, we have extended this work to demonstrate that the absence of laforin leads to hyperphosphorylated glycogen that has abnormal physical properties and that over time develops into a poorly branched, insoluble polysaccharide that tends to aggregate, paralleling the formation of LB in LD.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents—Potato amylopectin, malachite green oxalate, and α-amylase (Bacillus species) were from Sigma-Aldrich. Amyloglucosidase (Aspergillus niger) was from Fluka. Anti-GS antibody was from Cell Signaling Technology; anti-laforin and anti-branching enzyme antibodies were from Abnova; anti-glyceraldehyde-3-phosphate dehydrogenase antibody was from Biodesign; anti-AGL antibody was from Abgent; anti-glycogenin-1 antibody was as previously described (27); and anti-PTG antibody was a generous gift from Dr. Alan Salteil (University of Michigan, Ann Arbor, MI).
Animals—The Epm2a–/– mice have been described (21). Animals 3 months or 9–12 months of age were killed by cervical dislocation, the heads were decapitated directly into liquid nitrogen, and the other tissues were rapidly excised, immersed in liquid nitrogen, and stored at –80 °C until use.
Glycogen Purification—Glycogen for use in branching assays, electron microscopy, ethanol solubility assays, and covalent phosphate determination was purified as described previously (26). Because of the lack of water solubility of the 9–12-month-old Epm2a–/– glycogen samples, both knock-out and wild type samples were boiled for 3–5 min in a water bath to fully redissolve the glycogen pellets. 6.7 mm LiCl was used to aid the precipitation of glycogen in ethanol after dialysis.
Glycogen Determination—Glycogen was quantitated in total tissue or in the low speed supernatant (LSS) and the low speed pellet (LSP) after low speed centrifugation (8000 × g) by measuring glucose equivalents after digestion with amyloglucosidase (28, 29).
Branching Determination—The degree of branching of polysaccharides was analyzed by the protocol of Krisman (30).
Electron Microscopy—Purified glycogen (15–25 μg), whether from whole tissue or after separation by low speed centrifugation and subsequent purification, was spotted on a Formvar-coated grid and allowed to settle for 30–60 s, at which time a drop of NanoVan (Nanoprobes) was added and wicked off 30 s later. The glycogen was viewed with a Technia G12 Biotwin transmission electron microscope (FEI, Hillsboro, OR) equipped with an AMT CCD camera (Advanced Microscopy Techniques, Danvers, MA) at 80 kEV and 150,000× magnification at the Indiana University Electron Microscopy Center. Particle diameters were measured, and a histogram was constructed depicting the size distribution of the particles for different age groups and genotypes.
Ethanol Solubility Assay—Purified skeletal muscle glycogen was subjected to treatment with wild type laforin and C266S laforin as described previously (26). The reaction was terminated by boiling for 10 min in a water bath, cooled, and then centrifuged to remove denatured protein. The supernatant was dialyzed overnight against water, and the glycogen was recovered by ethanol precipitation, at which time we noticed that the glycogen treated with active laforin precipitated like wild type glycogen, without the need for added LiCl. This glycogen was also used for electron microscopy studies as described above.
Western Blotting and Glycogen Synthase Activity Assays—Skeletal muscle or brain was homogenized in 10 volumes of buffer (31) and subjected to low speed centrifugation at 8000 × g for 10 min. The LSP was resuspended in the initial volume of buffer. GS activity in LSS and LSP was measured as previously described (32). Comparable amounts of the LSS and the LSP samples were used for Western blot analysis. The remainder of the LSS was subjected to high speed centrifugation at 100,000 × g for 90 min. The high speed pellet was resuspended by sonication in one-fifth of the original homogenization volume and subjected to Western blot analysis for PTG. Glycogenin was detected after incubation of both the LSP and the high speed pellet with or without ∼100 μg/ml α-amylase for 30 min and subjected to Western blot analysis.
Statistics—The data are displayed as the means ± S.E. Statistical significance was evaluated using an unpaired Student t test and considered significant at p < 0.05.
RESULTS
Glycogen and Glycogen Phosphate Levels Increase with Age in the Absence of Laforin—Previous analyses of 3–4-month-old Epm2a–/– mice (26) revealed no major changes in glycogen level or glycogen metabolizing enzyme activities but a clear increase in the glycogen phosphate content. However, LD is a progressive disorder, and in the Epm2a–/– mice neurological symptoms were only apparent in older, 9-month-old animals (21). We therefore compared young animals with mice 9–12 months of age. Analysis of muscle glycogen isolated from 3-month-old animals reproduced our earlier findings (26), with no difference in glycogen levels between wild type and Epm2a–/– animals and an ∼4-fold increase in the phosphate content (Fig. 1, A and B). In the older animals, there was a 3-fold elevation in total glycogen content and a further increase in phosphate to approximately six times wild type. Phosphate content of glycogen was unchanged as wild type animals aged from 3 to 9–12 months. Brain glycogen levels were already increased at 3 months and progressed to a 5-fold elevation in the old animals (Fig. 1C). Because of the much lower amounts of glycogen recoverable from brain, it was not feasible to measure phosphate content. The greater sensitivity of brain glycogen accumulation to the absence of laforin could reflect differences in brain metabolism and explain the more serious consequences in this tissue.
FIGURE 1.
Glycogen and glycogen phosphate levels increase with age in the absence of laforin. A, glycogen phosphate levels in muscle glycogen from wild type (WT) and Epm2a–/– mice at 3 months (open bars) and 9–12 months (filled bars). *, p < 0.001 versus wild type; #, p < 0.01 versus 3-month-old Epm2a–/– (n = 7–8). B, skeletal muscle glycogen content levels in wild type and Epm2a–/– mice. *, p < 0.001 versus WT and 3-month-old Epm2a–/– (n = 11–15). C, brain glycogen content in WT and Epm2a–/– mice. *, p = 0.0001 versus WT; #, p < 0.002 versus 3-month-old Epm2a–/– (n = 3–4).
Age-dependent Changes in Chemical and Physical Properties of Glycogen in Epm2a–/– Mice—LD is characterized by poorly branched glycogen-like polymers in the LB (12–14). We therefore monitored the degree of branching of glycogen purified from laforin-deficient mice by recording the visible absorption spectrum in the presence of iodine. Iodine intercalates into polysaccharide helices to give a characteristic spectrum whose absorption maximum is influenced by the degree of branching and hence the length of uninterrupted helical segments (30). This technique readily distinguishes wild type mouse glycogen from the amylopectin of plant starch (average branching one in ∼30 glucoses) whose absorption maximum is at a much higher wavelength (Fig. 2A). Mammalian glycogen typically has branches every ∼12 glucose residues. The iodine spectra of muscle glycogen from 3- or 9–12-month-old wild type mice are superimposable (triangles and diamonds), indicating no change in branching with age in normal animals. Glycogen from 3-month-old Epm2a–/– mice had an iodine spectrum slightly but significantly shifted to longer wavelength (Fig. 2, A and B). However, the corresponding spectrum for 9–12-month-old knock-out animals was greatly altered, approaching that of amylopectin and indicative of a substantial reduction in the number of branches. Removal of the phosphate by laforin treatment did not alter the iodine spectrum (data not shown). Poorly branched glucose polymers, like amylopectin, are less soluble in water than more branched polysaccharides. Indeed, during isolation (see “Experimental Procedures”), it was more difficult to dissolve precipitated glycogen from the 9–12-month-old Epm2a–/– mice than glycogen from any of the other mouse groups analyzed. Likewise, after storage of 9–12-month-old mouse glycogen solutions at –20 °C, thawing resulted in a precipitate that required heating to redissolve. Similar treatment of glycogen from wild type or 3-month-old Epm2a–/– mice did not result in precipitated material.
FIGURE 2.
Age-dependent changes in chemical and physical properties of glycogen in Epm2a–/– mice. A, iodine spectra of skeletal muscle glycogen (50–75 μg/ml) purified from wild type (WT) and Epm2a–/– mice. Amylopectin is shown for reference. B, quantitation of A.*, p < 0.002 versus wild type; #, p < 0.0001 versus 3-month-old Epm2a–/– (n = 3–8). C, skeletal muscle glycogen (5 mg/ml) purified from a 9-month-old Epm2a–/– mice that was treated with catalytically inactive laforin (C266S) (tube i) or active laforin (tube ii), and brought to 66% (v/v) ethanol. Tube iii, addition of LiCl to tube i. Tube iv, addition of LiCl to tube ii. Tube v, addition of LiCl to a 66% ethanol solution without glycogen.
Polysaccharides like glycogen are insoluble in ethanol, a property that is useful for their separation from ethanol soluble constituents. Thus, our normal isolation protocol for glycogen from tissues (see “Experimental Procedures”) utilizes precipitation in 66% (v/v) ethanol. We observed that glycogen from Epm2a–/– mice, regardless of age, was much more soluble in 66% ethanol than wild type glycogen and did not come readily out of solution (data not shown). The addition of traces of a salt, like LiCl, has traditionally been used to ensure complete precipitation of polysaccharides from ethanol, and this was effective for the glycogen from both the 3-month-old and the 9–12-month-old Epm2a–/– mice. Glycogen from 9–12-month-old Epm2a–/– mice was exposed to either the catalytically inactive C266S mutant laforin or wild type laforin followed by the addition of ethanol to 66%. Treatment with inactive laforin had no effect, with the glycogen remaining in solution (Fig. 2C, tube i). Treatment with active laforin, which removes most of the phosphate, resulted in a polysaccharide that precipitated normally (Fig. 2C, tube ii). The addition of LiCl to both samples hastened precipitation of glycogen, although the phosphorylated glycogen formed more flocculent material that, under gravity, was pelleted at a slower rate (Fig. 2C, tubes iii and iv). The visible material was not salt because it was absent in the control sample lacking any glycogen (Fig. 2C, tube v).
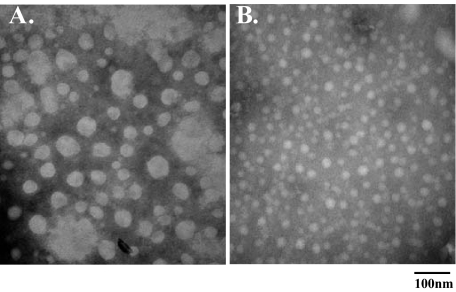
Age-dependent Changes in Glycogen Structure in Epm2a–/– Mice—The high molecular weight of glycogen makes it large enough to be analyzed by electron microscopy, and the literature contains numerous examples of such visualization (33, 34). For glycogen in skeletal muscle, the particles have been described as rosettes that have a characteristic granular appearance. Analysis of the glycogen purified from the muscle of wild type mice by transmission electron microscopy indicated particles reminiscent of these structures, whether from old or young animals (Fig. 3, A and B). Glycogen from 3-month-old Epm2a–/– mice had a generally similar appearance, (Fig. 3C). In contrast, glycogen from the 9–12-month-old mice had a strikingly distinct appearance, with a more defined boundary, less granularity, and a more even density (Fig. 3D). Furthermore, many fields had large regions where particles appear to have coalesced or aggregated, either before or during preparation of the grids. The size distribution of the particles was estimated by measuring apparent particle diameter. Glycogen particles from 3-month-old wild type mice had an average diameter of 27.1 nm, in the range reported for native muscle glycogen particles (Fig. 3E) (4). With age, there appeared to be a shift in the population to a slightly larger size (average 35.1 nm) but retaining the approximately Gaussian distribution seen in the younger animals. Glycogen particles from the 3-month-old knock-out animals were a little smaller (average diameter, 24.2 nm) but still normally distributed. The comparable analysis of the particles from old Epm2a–/– mice was hampered by the presence of the large conglomerates, within which only hints of individual particles were visible. Particles in regions outside of the conglomerates had much greater variability in size, with many particles of diameter well beyond that anticipated for a normal glycogen particle (Fig. 3D). This could be due to a different density of the polysaccharide or more likely an aggregation of individual molecules that cannot be resolved by this analysis.
FIGURE 3.
Age-dependent changes in glycogen structure in Epm2a–/– mice. Electron micrographs of skeletal muscle glycogen purified from: 3-month-old wild type (WT)(A); 9–12-month-old wild type (B); 3-month-old Epm2a–/– (C); 9–12-month-old Epm2a–/– (D); and size distribution of skeletal muscle glycogen particles purified from wild type and Epm2a–/– mice (E).
The glycogen purified from the old knock-out mice was characterized by having the highest phosphate content and much reduced branching. This glycogen was subjected to treatment with laforin to remove phosphate or the catalytically inactive laforin mutant C266S. After removing protein and buffer, the glycogen was analyzed by electron microscopy. Glycogen from the control sample had an appearance comparable with that shown in Fig. 3D (Fig. 4A). Treatment with active laforin caused a significant change in appearance, leading to a population of particles with much reduced size and individual appearance tending more to that of wild type. However, the granularity of the normal particles was not completely restored. Because the removal of phosphate by laforin did not affect branching (previous section), we conclude that the phosphate is primarily responsible for the abnormal morphology of the glycogen from the 9–12-month-old animals, in particular the presence of larger particles and aggregates.
FIGURE 4.
Effect of phosphate removal on skeletal muscle glycogen from 9–12-month-old Epm2a–/– mice. Electron micrographs of glycogen treated with the catalytically inactive laforin (C266S) (A) or active wild type laforin (B).
With the possibility that abnormal glycogen phosphorylation might affect the aggregation of glycogen, we wondered whether our standard sample preparation for enzyme analysis, in which low speed centrifugation removes gross cellular debris from tissue extracts, may have removed some of the glycogen. This separation is not an issue for samples directly processed for measurement of glycogen. We found that in extracts from the muscle of 9–12-month-old Epm2a–/– mice, ∼60% of the glycogen was recovered in the LSP, compared with ∼25% for wild type muscle (Fig. 5A). In brain extracts, over 75% of the glycogen was in the pellet from knock-out mouse extracts (Fig. 5B). The increased glycogen in the old knock-out mice was associated with the LSP because the glycogen level in the LSS was the same as wild type. Glycogen purified from the LSP and LSS fractions of muscle extracts of the 9–12-month-old Epm2a–/– animals was analyzed by electron microscopy (Fig. 5, C and D). During this procedure, it was noted that the LSP glycogen was significantly harder to dissolve in water than the LSS fraction. The pellet fraction was highly enriched for the morphologically abnormal structures described in Fig. 3, whereas in the supernatant the particles were predominantly of more wild type appearance. The glycogen in the pellet also had significantly higher glycogen phosphate content (Fig. 5E). The phosphorylation of the LSS glycogen was, in absolute terms, comparable with that of the whole glycogen fraction of 3-month-old Epm2a–/– mice (Fig. 1). Analysis of glycogen branching by iodine spectra indicated that the pellet was enriched for poorly branched glycogen, with an absorption maximum close to that of amylopectin (Fig. 5F). In these old knock-out mice, it appears that low speed centrifugation can effectively separate a polyglucosan fraction from normal glycogen that remains in the supernatant.
FIGURE 5.
Fractionation of glycogen from 9–12-month-old Epm2a–/– mice. Total skeletal muscle or brain extracts from 9–12-month-old wild type (WT) and Epm2a–/– mice were subjected to low speed centrifugation to generate supernatant (LSS) and pellet (LSP). A, percentage of total muscle glycogen, in the LSP. *, p < 0.05 (n = 3). B, percentage of total brain glycogen in the LSP. *, p < 0.005 (n = 3). C, electron micrograph of glycogen purified from the LSS of a muscle extract. D, electron micrograph of glycogen purified from the LSP of a muscle extract. E, skeletal muscle glycogen phosphate levels in LSS and the LSP. #, p = 0.002 (n = 6). F, iodine spectra of muscle glycogen (50–75 μg/ml) (purified from the LSS and the LSP; n = 6). Amylopectin is shown for reference.
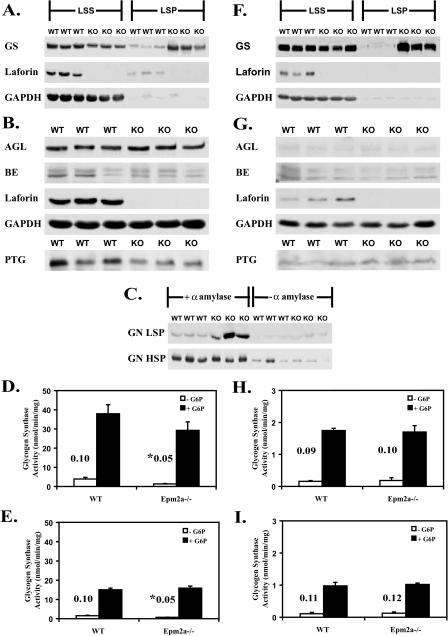
Analysis of Glycogen Metabolizing Enzymes and Related Proteins in Old Epm2a–/– Mice—In previous work, we had found little difference in the activities of GS and branching enzyme in young Epm2a–/– mice (26, 35). We extended the analysis to the 9–12-month-old Epm2a–/– animals, adding quantitation of some other potentially relevant proteins and also taking into account the partitioning of the glycogen between the LSS and LSP. In wild type mice, most laforin is present in the LSS (Fig. 6, A and F). In this, and other studies (36), we did not observe any smaller, immunoreactive fragments of laforin. As judged by Western analysis, the total GS protein level (LSS plus LSP) was increased by ∼50% in the muscle (Fig. 6A) and ∼2.5-fold in brain (Fig. 6F) of knock-out animals, and this elevation could be attributed to an increase in the protein associated with the LSP. There was in fact a slight decrease in the soluble GS protein. GS activity measured in the presence of glucose-6-phosphate (G6P), usually taken as a measure of total GS (37), however, was statistically unchanged between genotypes in either the LSS or LSP fractions. In Epm2a–/– extracts, in either muscle (Fig. 6, D and E) or brain (Fig. 6, H and I), the activity in the LSP fractions was lower than in the LSS, despite the presence of as much or more GS protein in the LSP. In other words, the measured GS specific activity was reduced in the LSP. In contrast, the activity associated with the LSP fraction of wild type mice was commensurate with the relatively small amount of GS protein present. The reason for the unexpectedly low specific activity in the LSP fractions, muscle or brain, of the GS of the knock-out mice is not immediately obvious. One possibility would be the occurrence of some stable and novel modification of the GS that reduces activity even in the presence of G6P. Alternatively, the enzyme assays, for some reason, may not reflect the true activity of the GS in the LSP of the Epm2a–/– animals. One idea would be that association of GS with the insoluble polyglucosan effectively removes the enzyme from the soluble phase, limiting our ability to measure activity by traditional assays, but in vivo allowing for effective elongation and synthesis of glycogen. The –/+ G6P activity ratio has been used as a kinetic index of GS inactivation by reversible phosphorylation. In muscle, but not brain, there was a significant reduction in the activity ratio in the Epm2a–/– samples (Fig. 6, D, E, H, and I).
FIGURE 6.
Analysis of glycogen metabolizing enzymes and related proteins in old Epm2a–/– mice. Muscle or brain extracts from 9–12-month-old wild type (WT) and Epm2a–/– mice were analyzed. A, GS protein levels in the LSS and the LSP of muscle extracts. B, glycogen debranching enzyme (AGL) and branching enzyme (BE) protein levels in muscle extracts, and muscle PTG protein levels in the high speed centrifugation glycogen pellet. C, glycogenin protein levels in the LSP and the high speed pellets (HSP) of muscle treated or not with α-amylase. D, GS activity in LSS from muscle in the absence (white bars) or presence (black bars) of G6P. The corresponding activity ratio is shown. *, p < 0.05 (n = 3). E, GS activity in the LSP from muscle in the absence or presence of G6P. The corresponding activity ratio is shown. *, p < 0.05 (n = 3). F, GS protein levels in the LSS and the LSP of brain extracts. G, AGL and branching enzyme protein levels in brain extracts, and PTG protein levels in the high speed centrifugation glycogen pellet of brain extracts. H, GS activity in the LSS from brain. The activity ratio is shown (n = 3). I, GS activity in the LSP from brain. The activity ratio is shown (n = 3). KO, knock-out.
Based on Western blotting, the levels of branching enzyme and debranching enzyme (AGL) were not greatly changed in extracts of muscle (Fig. 6B) or brain (Fig. 6G) from Epm2a–/– mice. Small amounts of both proteins were detectable in the LSP with a small increase in the knock-out animals tracking with the elevated levels of glycogen in this fraction. However quantitation of total protein (LSS+LSP) showed no differences between wild type and knock-out mice (not shown). PTG is a type 1 protein phosphatase regulatory subunit (38) that has been implicated in laforin action, first as a protein capable of interacting with laforin (39) and second as a potential target for a malin-laforin ubiquitylating complex, leading to its proteasomal degradation (23, 25). PTG associates with glycogen, and with available antibodies, we can only detect PTG when enriched in high speed glycogen pellets. As shown in Fig. 6 (B and G), there was, if anything, a decrease in PTG protein in the muscle of Epm2a–/– mice and no change in the brain.
Glycogenin is the self-glucosylating initiator protein of glycogen synthesis (40–42) that is thought to remain covalently attached to glycogen. As such, its level will index the number of glycogen molecules present. Glycogenin can only be detected by Western blotting once glycogen has been degraded, in these experiments by treatment with α-amylase (Fig. 6C). Consistent with this idea, the large majority of the glycogenin is only detected after α-amylase digestion. The glycogenin level essentially correlated with the amount of glycogen present, being elevated in the LSP of the knock-out mice where there is more glycogen (Fig. 6C). The high speed supernatant fraction harvests and concentrates the glycogen present in the LSS, and in this fraction there is no change in glycogenin protein level. If the measured glycogen level in the different fractions (Fig. 6C) is normalized to the amount of glycogenin, there is no great variation. This ratio is increased in the LSP fraction from the Epm2a–/– mice, but only by ∼60%. The main conclusion from this analysis is that the morphologically abnormal glycogen in the old knock-out mice consists of glycogen molecules whose molecular masses are not greatly different from that of wild type.
DISCUSSION
LD is a progressive disorder, and patients appear normal until they develop symptoms in their teenage years (12–14). Any molecular explanation for LD must account for the gradual nature of its onset, despite the fact that genetic defects in proteins will be present from birth. Currently, a prevailing hypothesis is that the symptoms of the disease accompany the accumulation of the LB, which in neurons leads to cell death and the associated epilepsy, myoclonus, ataxia, and other symptoms. LB contain abnormally branched deposits of the normal cellular storage compound, glycogen (43, 44). We describe an age-dependent change in the properties of glycogen in a mouse model of LD, the Epm2a–/– mouse, that is commensurate with the course of the disease and that can explain its progressive nature. From the analysis of glycogen from young and older animals, we propose a mechanism (Fig. 7) whereby initial hyperphosphorylation of glycogen in Epm2a–/– animals leads to altered physico-chemical properties that, however, do not grossly affect the normal synthesis and degradation cycle of the polysaccharide. With time, however, the glycogen develops into a polysaccharide whose branching structure is substantially altered to form an insoluble polymer, with the characteristics of polyglucosan. We envisage a threshold in glycogen phosphorylation beyond which the glycogen is destined to become the insoluble polyglucosan that results in the pathology of the disease.
FIGURE 7.
Possible roles for laforin in malin in the formation of polyglucosan. We propose that laforin is a physiological glycogen phosphatase whose impairment leads to the structural abnormalities in glycogen described in this work and to Lafora body formation. The role of malin remains a matter of active investigation. Malin could act together with or upstream of laforin in the removal of phosphate from glycogen (a). Malin could function independently of laforin to reduce glycogen phosphorylation, such as by down-regulating the enzyme(s) responsible for glycogen phosphorylation (b). Alternatively, malin could suppress polyglucosan formation independently of glycogen phosphorylation by controlling glycogen metabolic enzymes (c), as has been proposed.
The frequency of phosphorylation measured for muscle glycogen from wild type mice, regardless of age, was approximately one in 1500 glucose residues. The value we reported for rabbit muscle glycogen was one in 650 (26), similar to previous reports (5, 6). Thus, phosphorylation is relatively rare and, in the 3-month-old Epm2a–/– samples, is only increased to one in ∼375 glucose residues. It is difficult to conceive how the addition of such a relatively small number of charged groups could alone cause a dramatic change in ethanol solubility. One might postulate that increased phosphorylation would actually make the polymer less soluble in ethanol. Phosphorylation is clearly responsible for the increased ethanol solubility because its removal with laforin reverts the solubility properties to that of wild type glycogen (Fig. 2C). We propose that the phosphates elicit a global change in glycogen structure. Glycogen, like amylopectin, contains polyglucose helices. The crystal structure of a cycloamylose has been determined (45). This molecule is a 26-residue cyclic polymer of glucose in α-1,4-linkages (cyclomaltohexicosaose) that adopts a structure with two antiparallel polyglucose helices in which the glucose oxygen atoms are oriented toward the exterior surface and the carbon skeletons of the glucoses line the interior of the helix. Although this molecule maintains the helical structures by constraints not present in native glycogen or amylopectin, it is not unreasonable to propose that some of the structural features occur in the native polysaccharides. In the cycloamylose structure, the helices are stabilized by intrahelical hydrogen bonding mediated by the physically adjacent 6′, 2′ and 3′ hydroxyls. Introduction of phosphate groups to virtually any glucose hydroxyl group would disrupt the local hydrogen bonding, destabilize the secondary structure, and potentially expose the more hydrophobic faces of the glucose residues to solvent. We propose that the glycogen molecule can maintain its compact structure with a low number of phosphorylations but that, past some threshold, too many helices lose their regular structure, resulting in altered packing of the polyglucose chains and global disruption of the structure of the molecule. In this situation, the ethanol solubility is actually enhanced by the disruption of the secondary structure caused by phosphorylation.
The other principal finding of this study is that the glycogen purified from 9–12-month-old Epm2a–/– mice has properties consistent with the formation of LB. As the mutant mice age from 3 to 9–12 months, the increase in glycogen phosphorylation is relatively modest but is accompanied by a major decrease in branching, acquisition of water insolubility, development of gross morphological abnormalities as observed by electron microscopy, and a tendency to aggregate. Aggregation is inferred from the appearance of large conglomerated structures by electron microscopy and also the fact that low speed centrifugation of tissue extracts recovers the majority of the glycogen in the pellet fraction. Normal glycogen particles in tissue extracts require high speed centrifugation (for example, 50,000 × g for 2 h) to be removed from aqueous solution. Thus, the low speed centrifugation operationally defines two populations of glycogen particles. The glycogen particles present in the LSP are larger, have an altered iodine spectrum, are water-insoluble, and have an abnormal appearance by electron microscopy, as compared with the polysaccharide that remains in the supernatant. Based on the amount of covalently attached glycogenin, the actual sizes of the glycogen molecules in the two fractions are not greatly different, only some 60% larger in the LSP fraction. This relatively small increase cannot explain the large change in hydrodynamic properties allowing sedimentation by low speed centrifugation and argues for the presence of aggregates in the LSP. In other words, the glycogen particles in the LSP must be formed of multiple, individual glycogen molecules. Both aggregation and water insolubility likely contribute to the formation of LB. The insolubility can be attributed primarily to the reduced branching of the polysaccharide and is unaffected by dephosphorylation in vitro with laforin (data not shown). Aggregation requires the presence of the phosphate because its removal reverses much of the abnormal morphology seen by electron microscopy (Fig. 4). However, aggregation must also depend on the poorly branched structure because it is not observed with glycogen from the young Epm2a–/– animals. Thus, aberrant glycogen phosphorylation may be viewed as necessary but not sufficient for Lafora body formation in this model. How phosphorylation and reduced branching combine to favor aggregation of glycogen molecules in tissues is not clear at this time.
A major question in LD research is how the loss of function of laforin or malin causes LB (Fig. 7). The first reported laforin substrate was the protein kinase GSK-3 (46–48) that regulates GS activity. However, not all reports support this hypothesis (26, 35, 49), which is also inconsistent with the observation that GS –/+ G6P activity is not increased in Epm2a–/– animals in this and other studies (26). We favor the hypothesis that a primary function of laforin in vivo is as a glycogen phosphatase. It focuses on the idea that LD is essentially a metabolic disorder and that laforin is a glycogen metabolic enzyme. It could in fact be viewed as part of a repair or corrective mechanism, such as exists for the synthesis of other biopolymers, like nucleic acids. Normal glycogen contains low levels of covalent phosphate, and laforin will limit phosphorylation to a structurally tolerated level. Without functional laforin, as in the Epm2a–/– mice or LD patients, the usual cycles of glycogen synthesis and degradation initially proceed relatively normally, but the glycogen gradually accumulates structural defects that set it on the course to develop into polyglucosan.
Defects in malin also result in accumulation of polyglucosan (Fig. 7). Malin could function in concert or upstream of laforin in the control of glycogen phosphorylation, resulting in an essentially identical mechanism to that proposed for laforin. Alternatively, it could regulate glycogen phosphorylation independently of laforin, for example down-regulating enzyme(s) responsible for introducing phosphate into glycogen. Finally, it could function to limit the transition from normally branched glycogen to polyglucosan. Most work has addressed the last mentioned possibility. Lohi et al. (46) proposed that a malin·laforin·GS complex forms and is targeted for degradation. In studies of hepatocytes (50) and neurons (23), the groups of Guinovart and de Cordoba proposed that a malin·laforin complex ubiquitylated the type 1 phosphatase regulatory subunit PTG and, in neurons, also GS, leading to their degradation. Worby et al. (25) also reported that malin targets PTG for degradation in a laforin-dependent manner. Impaired degradation of either GS or PTG should result in increased glycogen-elongating activity, either by directly increasing GS levels or, in the case of PTG, by increased targeting of type 1 phosphatase to glycogen causing dephosphorylation and activation of GS. In the present study, PTG levels were not increased in the Epm2a–/– mice (Fig. 6B), arguing against laforin-dependent degradation in normal animals. If anything, there was a decrease in PTG in the muscle of knock-out mice. Consistent with this result, the –/+ G6P activity ratio of GS, normally viewed as an index of phosphorylation state, was decreased in the muscle of the knock-out mice and unchanged in brain.
The results with GS are more complex. In young Epm2a–/– mice, glycogen levels and GS protein were normal (26), but in old mice, the total glycogen levels were significantly elevated in muscle and brain, and the majority of this glycogen was recoverable in the LSP/polyglucosan fraction as abnormally structured polysaccharide. GS protein, judged by Western blot, was significantly elevated in this fraction. This result is consistent with the in vitro observation that purified GS binds more effectively to the abnormal glycogen isolated from old Epm2a–/– muscle (data not shown). This increase in GS could be consistent with laforin-dependent breakdown of GS by malin. However, it is not a unique explanation because in several mouse models, glycogen content alone tracks with GS protein level. Binding to glycogen appears to stabilize GS. For example, overexpression of another type 1 phosphatase regulatory subunit, RGL/GM, leads to increased glycogen GS protein in muscle (51). Decreased glycogen levels, as in RGL knock-out (29) or PTG knock-out3 animals, were associated with decreased GS. Is the elevated GS in the Epm2a–/– mice secondary to increased glycogen levels or the cause of glycogen overaccumulation? In any event, the GS recovered in the LSP of knock-out mice has low specific activity, so that the measured activity is not different from wild type.
An alternative mechanism for polyglucosan formation in Epm2a–/– mice would be a defect in the branching/debranching system. The debranching enzyme, AGL, was proposed to be a malin target by Cheng et al. (24) but the AGL protein level was unchanged in the muscle or brain of Epm2a–/– mice (Fig. 6, B and G). In any event, based on the two-stage degradation of glycogen by phosphorylase and debranching enzyme, excessive AGL activity should only reduce branching frequency if phosphorylase, normally an abundant enzyme, becomes limiting. During synthesis, reduced branching enzyme activity could also lead to impaired glycogen branching, but we have no supporting evidence from this and previous work (26). In summary, the current data do not support the enzymatic imbalance hypothesis as being the predominant initial defect in Epm2a–/– glycogen biosynthesis.
In conclusion, our model for polyglucosan formation envisions a gradual transition from relatively normal glycogen metabolism to a state in which incremental damage to glycogen molecules, triggered by hyperphosphorylation, leads to abnormal glycogen structure that in the long term becomes insoluble, poorly branched aggregates of polyglucosan that can explain the formation of LB.
Acknowledgments
We thank Dr. Vincent Gattone and Caroline Miller of the Indiana University Electron Microscopy Center for help with electron microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grant DK27221 (to P. J. R.). This work was also supported by funds from the Canadian Institutes for Health Research (to J. M. G. and B. A. M.), a Canada Research Chair in Pediatric Neurogenetics (to B. A. M.), and an American Heart Association Predoctoral fellowship (to V. S. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: LD, Lafora disease; LB, Lafora bodies; GS, glycogen synthase; AGL, amylo-1,6-glucosidase, 4-α-glucanotransferase; PTG, protein targeting to glycogen; LSS, low speed supernatant; LSP, low speed pellet; G6P, glucose-6-phosphate; E3, ubiquitin-protein isopeptide ligase.
A. A. DePaoli-Roach, unpublished data.
References
- 1.Roach, P. J. (2002) Curr. Mol. Med. 2 101–120 [DOI] [PubMed] [Google Scholar]
- 2.Ball, S., Guan, H. P., James, M., Myers, A., Keeling, P., Mouille, G., Buleon, A., Colonna, P., and Preiss, J. (1996) Cell 86 349–352 [DOI] [PubMed] [Google Scholar]
- 3.Gunja-Smith, Z., Marshall, J. J., Mercier, C., Smith, E. E., and Whelan, W. J. (1970) FEBS Lett. 12 101–104 [DOI] [PubMed] [Google Scholar]
- 4.Shearer, J., and Graham, T. E. (2004) Exerc. Sport Sci. Rev. 32 120–126 [DOI] [PubMed] [Google Scholar]
- 5.Lomako, J., Lomako, W. M., Whelan, W. J., and Marchase, R. B. (1993) FEBS Lett. 329 263–267 [DOI] [PubMed] [Google Scholar]
- 6.Lomako, J., Lomako, W. M., Kirkman, B. R., and Whelan, W. J. (1994) Biofactors 4 167–171 [PubMed] [Google Scholar]
- 7.Fontana, J. D. (1980) FEBS Lett. 109 85–92 [DOI] [PubMed] [Google Scholar]
- 8.Ritte, G., Heydenreich, M., Mahlow, S., Haebel, S., Kotting, O., and Steup, M. (2006) FEBS Lett. 580 4872–4876 [DOI] [PubMed] [Google Scholar]
- 9.Cavanagh, J. B. (1999) Brain Res. 29 265–295 [DOI] [PubMed] [Google Scholar]
- 10.Nakajima, H., Raben, N., Hamaguchi, T., and Yamasaki, T. (2002) Curr. Mol. Med. 2 197–212 [DOI] [PubMed] [Google Scholar]
- 11.DiMauro, S., and Lamperti, C. (2001) Muscle Nerve 24 984–999 [DOI] [PubMed] [Google Scholar]
- 12.Ganesh, S., Puri, R., Singh, S., Mittal, S., and Dubey, D. (2006) J. Hum. Genet. 51 1–8 [DOI] [PubMed] [Google Scholar]
- 13.Chan, E. M., Andrade, D. M., Franceschetti, S., and Minassian, B. (2005) Adv. Neurol. 95 47–57 [PubMed] [Google Scholar]
- 14.Delgado-Escueta, A. V. (2007) Curr. Neurol. Neurosci. Rep. 7 428–433 [DOI] [PubMed] [Google Scholar]
- 15.Minassian, B. A., Lee, J. R., Herbrick, J. A., Huizenga, J., Soder, S., Mungall, A. J., Dunham, I., Gardner, R., Fong, C. Y., Carpenter, S., Jardim, L., Satishchandra, P., Andermann, E., Snead, O. C., 3rd, Lopes-Cendes, I., Tsui, L. C., Delgado-Escueta, A. V., Rouleau, G. A., and Scherer, S. W. (1998) Nat. Genet. 20 171–174 [DOI] [PubMed] [Google Scholar]
- 16.Ianzano, L., Zhang, J., Chan, E. M., Zhao, X. C., Lohi, H., Scherer, S. W., and Minassian, B. A. (2005) Hum. Mut. 26 397. [DOI] [PubMed] [Google Scholar]
- 17.Boraston, A. B., Bolam, D. N., Gilbert, H. J., and Davies, G. J. (2004) Biochem. J. 382 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, E. M., Ackerley, C. A., Lohi, H., Ianzano, L., Cortez, M. A., Shannon, P., Scherer, S. W., and Minassian, B. A. (2004) Hum. Mol. Genet. 13 1117–1129 [DOI] [PubMed] [Google Scholar]
- 19.Wang, J., Stuckey, J. A., Wishart, M. J., and Dixon, J. E. (2002) J. Biol. Chem. 277 2377–2380 [DOI] [PubMed] [Google Scholar]
- 20.Chan, E. M., Young, E. J., Ianzano, L., Munteanu, I., Zhao, X., Christopoulos, C. C., Avanzini, G., Elia, M., Ackerley, C. A., Jovic, N. J., Bohlega, S., Andermann, E., Rouleau, G. A., Delgado-Escueta, A. V., Minassian, B. A., and Scherer, S. W. (2003) Nat. Genet. 35 125–127 [DOI] [PubMed] [Google Scholar]
- 21.Ganesh, S., Delgado-Escueta, A. V., Sakamoto, T., Avila, M. R., Machado-Salas, J., Hoshii, Y., Akagi, T., Gomi, H., Suzuki, T., Amano, K., Agarwala, K. L., Hasegawa, Y., Bai, D. S., Ishihara, T., Hashikawa, T., Itohara, S., Cornford, E. M., Niki, H., and Yamakawa, K. (2002) Hum. Mol. Genet 11 1251–1262 [DOI] [PubMed] [Google Scholar]
- 22.Gentry, M. S., Worby, C. A., and Dixon, J. E. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 8501–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilchez, D., Ros, S., Cifuentes, D., Pujadas, L., Valles, J., Garcia-Fojeda, B., Criado-Garcia, O., Fernandez-Sanchez, E., Medrano-Fernandez, I., Dominguez, J., Garcia-Rocha, M., Soriano, E., Rodriguez de Cordoba, S., and Guinovart, J. J. (2007) Nat. Neurosci. 10 1407–1413 [DOI] [PubMed] [Google Scholar]
- 24.Cheng, A., Zhang, M., Gentry, M. S., Worby, C. A., Dixon, J. E., and Saltiel, A. R. (2007) Gene Dev. 21 2399–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worby, C. A., Gentry, M. S., and Dixon, J. E. (2008) J. Biol. Chem. 283 4069–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tagliabracci, V. S., Turnbull, J., Wang, W., Girard, J. M., Zhao, X., Skurat, A. V., Delgado-Escueta, A. V., Minassian, B. A., Depaoli-Roach, A. A., and Roach, P. J. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 19262–19266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skurat, A. V., Cao, Y., and Roach, P. J. (1993) J. Biol. Chem. 268 14701–14707 [PubMed] [Google Scholar]
- 28.Bergmeyer, H. U. (1974) Methods of Enzymatic Analysis, 2nd English Ed., pp. 1196–1201, Academic Press, New York
- 29.Suzuki, Y., Lanner, C., Kim, J. H., Vilardo, P. G., Zhang, H., Yang, J., Cooper, L. D., Steele, M., Kennedy, A., Bock, C. B., Scrimgeour, A., Lawrence, J. C., Jr., and DePaoli-Roach, A. A. (2001) Mol. Cell. Biol. 21 2683–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krisman, C. R. (1962) Anal. Biochem. 4 17–23 [DOI] [PubMed] [Google Scholar]
- 31.Pederson, B. A., Csitkovits, A. G., Simon, R., Schroeder, J. M., Wang, W., Skurat, A. V., and Roach, P. J. (2003) Biochem. Biophys. Res. Commun. 305 826–830 [DOI] [PubMed] [Google Scholar]
- 32.Thomas, J. A., Schlender, K. K., and Larner, J. (1968) Anal. Biochem. 25 486–499 [DOI] [PubMed] [Google Scholar]
- 33.Drochmans, P. (1962) J. Ultra Res. 6 141–163 [DOI] [PubMed] [Google Scholar]
- 34.Rybicka, K. K. (1996) Tissue Cell 28 253–265 [DOI] [PubMed] [Google Scholar]
- 35.Wang, W., Lohi, H., Skurat, A. V., Depaoli-Roach, A. A., Minassian, B. A., and Roach, P. J. (2007) Arch Biochem. Biophys. 457 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, W., Parker, G. E., Skurat, A. V., Raben, N., DePaoli-Roach, A. A., and Roach, P. J. (2006) Biochem. Biophys. Res. Commun. 350 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roach, R. J., and Larner, J. (1977) Mol. Cell Biochem. 15 179–200 [DOI] [PubMed] [Google Scholar]
- 38.Brady, M. J., and Saltiel, A. R. (2001) Recent Prog. Horm. Res. 56 157–173 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Sanchez, M. E., Criado-Garcia, O., Heath, K. E., Garcia-Fojeda, B., Medrano-Fernandez, I., Gomez-Garre, P., Sanz, P., Serratosa, J. M., and Rodriguez de Cordoba, S. (2003) Hum. Mol. Genet. 12 3161–3171 [DOI] [PubMed] [Google Scholar]
- 40.Smythe, C., and Cohen, P. (1991) Eur. J. Biochem. 200 625–631 [DOI] [PubMed] [Google Scholar]
- 41.Lomako, J., Lomako, W. M., and Whelan, W. J. (2004) Biochim. Biophys. Acta 1673 45–55 [DOI] [PubMed] [Google Scholar]
- 42.Roach, P. J., and Skurat, A. V. (1997) Prog. Nucleic Acid Res. Mol. Biol. 57 289–316 [DOI] [PubMed] [Google Scholar]
- 43.Sakai, M., Austin, J., Witmer, F., and Trueb, L. (1970) Neurology 20 160–176 [DOI] [PubMed] [Google Scholar]
- 44.Yokoi, S., Austin, J., Witmer, F., and Sakai, M. (1968) Arch. Neurol. 19 15–33 [DOI] [PubMed] [Google Scholar]
- 45.Gessler, K., Uson, I., Takaha, T., Krauss, N., Smith, S. M., Okada, S., Sheldrick, G. M., and Saenger, W. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 4246–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohi, H., Ianzano, L., Zhao, X. C., Chan, E. M., Turnbull, J., Scherer, S. W., Ackerley, C. A., and Minassian, B. A. (2005) Hum. Mol. Genet. 14 2727–2736 [DOI] [PubMed] [Google Scholar]
- 47.Liu, Y., Wang, Y., Wu, C., Liu, Y., and Zheng, P. (2006) J. Biol. Chem. 281 34768–34774 [DOI] [PubMed] [Google Scholar]
- 48.Liu, R., Wang, L., Chen, C., Liu, Y., Zhou, P., Wang, Y., Wang, X., Turnbull, J., Minassian, B. A., and Zheng, P. (2008) Mol. Cell. Biol. in press [DOI] [PMC free article] [PubMed]
- 49.Worby, C. A., Gentry, M. S., and Dixon, J. E. (2006) J. Biol. Chem. 281 30412–30418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solaz-Fuster, M. C., Gimeno-Alcaniz, J. V., Ros, S., Fernandez-Sanchez, M. E., Garcia-Fojeda, B., Criado Garcia, O., Vilchez, D., Dominguez, J., Garcia-Rocha, M., Sanchez-Piris, M., Aguado, C., Knecht, E., Serratosa, J., Guinovart, J. J., Sanz, P., and Rodriguez de Cordoba, S. (2008) Hum. Mol. Genet 17 667–678 [DOI] [PubMed] [Google Scholar]
- 51.Aschenbach, W. G., Suzuki, Y., Breeden, K., Prats, C., Hirshman, M. F., Dufresne, S. D., Sakamoto, K., Vilardo, P. G., Steele, M., Kim, J. H., Jing, S. L., Goodyear, L. J., and DePaoli-Roach, A. A. (2001) J. Biol. Chem. 276 39959–39967 [DOI] [PubMed] [Google Scholar]