Abstract
Type II diabetes mellitus (DM) and metabolic syndrome are associated with accelerated restenosis following vascular interventions due to neointimal hyperplasia. The efficacy of nitric oxide (NO)-based therapies is unknown in these environments. Therefore, the aim of this study is to examine the efficacy of NO in preventing neointimal hyperplasia in animal models of type II DM and metabolic syndrome and examine possible mechanisms for differences in outcomes. Aortic vascular smooth muscle cells (VSMC) were harvested from rodent models of type II DM (Zucker diabetic fatty), metabolic syndrome (obese Zucker), and their genetic control (lean Zucker). Interestingly, NO inhibited proliferation and induced G0/G1 cell cycle arrest to the greatest extent in VSMC from rodent models of metabolic syndrome and type II DM compared with controls. This heightened efficacy was associated with increased expression of cyclin-dependent kinase inhibitor p21, but not p27. Using the rat carotid artery injury model to assess the efficacy of NO in vivo, we found that the NO donor PROLI/NO inhibited neointimal hyperplasia to the greatest extent in type II DM rodents, followed by metabolic syndrome, then controls. Increased neointimal hyperplasia correlated with increased reactive oxygen species (ROS) production, as demonstrated by dihydroethidium staining, and NO inhibited this increase most in metabolic syndrome and DM. In conclusion, NO was surprisingly a more effective inhibitor of neointimal hyperplasia following arterial injury in type II DM and metabolic syndrome vs. control. This heightened efficacy may be secondary to greater inhibition of VSMC proliferation through cell cycle arrest and regulation of ROS expression, in addition to other possible unidentified mechanisms that deserve further exploration.
Keywords: proliferation, neointimal hyperplasia, cell cycle, reactive oxygen species, vascular smooth muscle cells
the national institutes of Health (NIH) in 2005 estimated the prevalence of diagnosed diabetes mellitus (DM) in the United States to be greater than 14.6 million (45). Similarly, the prevalence of metabolic syndrome has become increasingly common in the United States, as awareness of the disease has become more widespread. It has been well established that patients with DM and metabolic syndrome have aggressive forms of vascular disease (24, 44). In addition, these patients have significantly worse outcomes following vascular interventions. Studies have demonstrated an accelerated and more aggressive course of restenosis in patients with DM following angioplasty of the coronary (40) and peripheral arteries (8) and increased morbidity and mortality following angioplasty (38), endarterectomy (1), and bypass grafting (26). This accelerated restenosis and increased morbidity and mortality can be attributed to an exaggerated arterial injury response, as demonstrated in animal models of type II DM and metabolic syndrome (13).
The enhanced vascular disease and aggressive response to injury is, in part, due to the proinflammatory and proliferative environment potentiated by increased serum levels of insulin and glucose and other biochemical and metabolic derangements (11). Investigators have demonstrated that some of the changes that occur as a result of the diabetic environment include reduced bioavailability of nitric oxide (NO) (12), increased reactive oxygen species (ROS) production (30, 46), a proinflammatory milieu (3), MAPK activation (52), protein kinase C activation (30), and activation of the receptor for advanced glycation end products (4).
In contrast, NO has been shown to possess many different vasoprotective properties (5), including inhibition of platelet aggregation (48), leukocyte chemotaxis (41), vascular smooth muscle cell (VSMC) proliferation and migration (16, 19), and endothelial cell apoptosis (51). Additionally, NO stimulates endothelial cell proliferation (56) and is a potent vasodilator (29). In sum, NO inhibits the components that lead to the development of neointimal hyperplasia in addition to reestablishing native arterial architecture. In fact, numerous investigators have verified the efficacy of NO-based therapies in small and large animal models (9, 10, 18, 25, 34, 42, 43, 49). Yet these therapies aimed at the prevention of neointimal hyperplasia have largely been studied in animal models, where normal blood vessels are subjected to injury, followed by various treatment modalities. It is unclear how the reduced bioavailability of NO, proinflammatory milieu, and increased oxidative stress associated with insulin resistance and hyperglycemia would impact these NO-based therapies. Our hypothesis is that NO will be less effective in an insulin-resistant, hyperglycemic environment. Therefore, in this paper, we sought to determine the efficacy of NO, a well-established inhibitor of neointimal hyperplasia, in the environments of type II DM and metabolic syndrome following arterial injury and study the mechanism by which NO exerts its effects in these hostile environments.
MATERIALS AND METHODS
Animal models.
Eleven-week-old male Zucker diabetic fatty (ZDF) rats and obese Zucker (OZ) rats [both with a mutation for the leptin receptor (57)] and their genetic control lean Zucker (LZ) rats were obtained from Charles River Laboratories (Wilmington, MA). When fed the Purina 5008 diet, the inbred ZDF male rats exhibited the diabetic phenotype characterized by hyperinsulinemia, hyperglycemia, hypercholesterolemia, and hypertriglyceridemia. The outbred OZ rats when fed normal chow represented metabolic syndrome characterized by obesity, hyperinsulinemia, hypercholesterolemia, and hypertriglyceridemia. The LZ rats represented a genetic control with normal metabolic parameters.
Cell culture.
Abdominal aortic VSMC were isolated and cultured from LZ, OZ, and ZDF rodent strains using the collagenase method, described by Gunther et al. (22). Cells were maintained in media containing equal volumes of DMEM-low glucose (SAFC Biosciences, Lenexa, KS) and Ham's F12 (JRH, Lenexa, KS), supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 U/ml penicillin (Invitrogen), 100 μg/ml streptomycin (Invitrogen), and 4 mM l-glutamine (VWR, West Chester, PA) and incubated at 37°C, 95% air and 5% CO2. VSMC were used between passages 4 and 8 for all experiments.
Tritiated [3H]thymidine incorporation assay.
[3H]thymidine incorporation was assessed as a surrogate for cellular proliferation. Of note, cell counting was performed, and the results of cell counting correlated with [3H]thymidine incorporation. Aortic VSMC plated in 12-well plates (4 × 104 cells/well) were growth arrested for 24 h, after which they were exposed to media, with or without the NO donor S-nitroso-N-acetylpenicillamine (SNAP; 250–1,000 μM), with or without the NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino]diazen-1-ium-1,2-diolate (DETA/NO; 500–1,000 μM), with or without glucose (25 mM), or with and without insulin (200 nM) in the presence of [3H]thymidine (5 μCi/ml, PerkinElmer, Wellesley, MA) for an additional 24 h. [3H]thymidine incorporation into trichloroacetic acid-precipitated DNA was quantified by scintillation counting. SNAP releases 1 mol of NO per mole of SNAP. DETA/NO releases 2 mol of NO per mole of DETA/NO.
Cell death assay.
Aortic VSMC plated in six-well plates (1 × 105 cells/well) were growth arrested for 24 h, after which they were exposed to media with or without the NO donor SNAP (250–1,000 μM) in 10% FBS for 24 h. Cells were trypsinized, collected, pelleted, and then resuspended in 250-μl phosphate-buffered saline (PBS). Forty microliters of this suspension were added to 160 μl of Guava ViaCount Reagent (Guava Technologies, Hayward, CA), and cell death was assessed using Guava PCA (Guava Technologies).
Flow cytometry.
Aortic VSMC plated in six-well plates (1 × 105 cells/well) were growth arrested for 24 h, after which they were exposed to media with or without the NO donor DETA/NO (0–1,000 μM) for 24 h. Trypsinized cells were resuspended in 50-μl PBS and fixed with 450 μl of ice-cold 70% ethanol, followed by resuspension in a propidium iodide staining solution [1× PBS (pH 7.4), 50 μg/ml propidium iodide (Invitrogen), 204 μg/ml RNase A (Sigma, St. Louis, MO), 0.1% Triton X-100 (Fisher Biotech, Fair Lakes, NJ)]. Samples were analyzed on a Coulter Epic XL flow cytometer. Analysis was performed using ModFit 3.1 LT (Verity, Topsham, ME).
Western blot analysis.
VSMC were collected after 24 h of exposure to the different treatment groups by scraping and were resuspended in 20 mM Tris with 100 μM phenylmethylsulfonylflouride (Sigma), 1 μM leupeptin (Sigma), and 1 μM sodium orthovanadate (Sigma). Protein was quantified with the bicinchoninic acid protein assay, according to manufacturer's instructions (Pierce, Rockford, IL). Whole cell samples (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 13% gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Membranes were hybridized with a rabbit polyclonal anti-p21 or anti-p27 antibody (1:1,000; Santa Cruz, Santa Cruz, CA), followed by horseradish peroxidase-linked goat anti-rabbit immunoglobulin (1:10,000; Pierce). Proteins were visualized using chemiluminescent reagents, according to the manufacturer's instructions (Supersignal Substrate; Pierce), and the membranes were exposed to film and developed. Western blot films were scanned to JPEG images, and densitometry was performed on representative images using ImageJ (NIH, Bethesda, MD).
NO-eluting gel.
The NO-eluting gel was made by combining 100 mg of disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO) powder with 0.6 g of polaxamer 407 (BASF, Mt. Olive, NJ), as previously described (47). This mixture was then combined with 2 ml of cold PBS and placed on ice in the 4°C refrigerator. The mixture was agitated every 5 min for ∼30 min until a gel formed. To measure NO release from the gel, 200 μl of the gel (the same amount placed in the neck of each rat) were place into 500 μl of PBS, with dedicated tubes set up for each time point. At the appropriate time point, the supernatant was removed, and nitrite release was measured using the Greiss reaction, as previously described (36).
Animal surgery.
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication 85-23, 1996) and approved by the Northwestern University Animal Care and Use Committee. Rats were fasted overnight before the procedure and blood draw. Rats were anesthetized with inhaled isoflurane (0.5–3%). Atropine was administered subcutaneously (0.1 mg/kg) to decrease airway secretions. Weight was documented, and blood glucose was measured. Following a midline neck incision, the rat carotid artery balloon injury model was performed using a 2F Fogarty catheter, as previously described (36, 50). After injury and restoration of blood flow, 200 μl of the NO-eluting gel (containing 10 mg PROLI/NO) were applied evenly to the external surface of the injured common carotid artery of rats in the treatment group, and the neck incision was closed. The control group received polaxamer gel alone. Rats were killed at 2 wk (LZ n = 11, OZ n = 12, ZDF n = 13), and blood was collected to measure insulin, glucose, cholesterol, and triglyceride levels. Again, the rats were fasted overnight before this blood draw.
Tissue processing.
Carotid arteries were harvested following in situ perfusion fixation with cold PBS (250 ml) and 2% paraformaldehyde (500 ml). Vessels were placed in paraformaldehyde at 4°C for 1 h and then 30% sucrose in PBS at 4°C overnight. The tissue was quick-frozen in Optimal Cutting Temperature compound (Tissue Tek, Hatfield, PA), and 5-μm sections were cut throughout the entire injured segment of the common carotid artery.
Morphometric analysis.
Carotid arteries harvested at 2 wk were examined histologically for evidence of neointimal hyperplasia with hematoxylin-eosin-stained sections using a standardized methodology, as previously described (33, 47, 50). Briefly, digital images of stained sections were collected with light microscopy using an Olympus BHT microscope (Melville, NY) with ×4, ×10, and ×40 objectives. For the morphometric analysis, six evenly spaced sections through each injured carotid artery were analyzed using ImageJ (NIH). With this software, units are measured in pixels (arbitrary units), but are uniformly consistent throughout all sections. Intimal area was measured as the area between the lumen and the internal elastic lamina. Medial area was measured as the area between the internal and external elastic lamina.
Apoptosis.
Apoptosis was evaluated in sectioned carotid arteries harvested at 2 wk by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) using a commercially available system, according to the manufacturer's instructions (DeadEnd Colorimetric TUNEL system; Promega, Madison, WI). The positive control consisted of a sample treated with DNase I to cause DNA fragmentation. The negative control consisted of a sample treated without the rTdT enzyme.
ROS.
The in situ presence of superoxide was evaluated in carotid artery sections harvested at 2 wk using the oxidative fluorescent dye dihydroethidium (DHE), as described previously (27). Five hundred microliters of DHE (2 μM) were applied to the frozen 5-μm carotid sections, cover slipped, and incubated for 30 min. Positive control consisted of sections exposed to hydrogen peroxide (200 μM). Fluorescent images of DHE staining throughout the entire cross section of the artery were obtained with the Nikon inverted microscope eclipse TE2000-U (Melville, NY) using excitation and emission wavelengths of 488 and 585 nm, respectively. DHE staining was quantified by 10 independent blinded observers using a standardized grading scale from 0 to 3 as follows: 0 = no positive staining, 1 = minimal positive staining, 2 = moderate positive staining, and 3 = intense positive staining.
Nitrotyrosine staining.
Carotid arteries were examined for evidence of nitrotyrosine expression using immunoperoxidase staining. Briefly, immunohistochemistry was carried out using the HRP Polymer & DAB Plus Chromogen UltraVision LP Value Detection System (Thermo Scientific, Fremont, CA). Endogenous peroxidase activity was blocked by incubating the slides in a hydrogen peroxide blocking reagent (provided in kit) for 15 min. To block nonspecific background staining, specimens were incubated in Ultra V Block (provided in kit) for 10 min. Nitrotyrosine expression was detected using a monoclonal anti-nitrotyrosine antibody (1:200, Cayman Chemical, Ann Arbor, MI) for 1 h at room temperature. Specimens were then incubated in the Value Primary Antibody Enhancer (provided in kit) for 20 min, rinsed, and incubated in Value HRP Polymer (provided in kit) for 30 min. The specimens were incubated in DAB Plus Chromogen and DAB Plus Substrate (provided in kit) for 3 min. Counterstaining was performed with Gill 2× hematoxylin (Protocol, Fisher Scientific, Waltham, MA). For negative controls, the primary antibody was omitted.
Statistical analysis.
Results are expressed as means ± SE. Differences between multiple groups for the in vitro studies were analyzed using the one-way repeated-measures ANOVA with the Student-Newman-Keuls post hoc test for all pairwise comparisons (SigmaStat; SPSS, Chicago, IL). The one-way ANOVA was used to assess significance for the in vivo animal studies. Statistical significance was assumed when P < 0.05.
RESULTS
NO inhibits [3H]thymidine incorporation in vitro to a greater degree in metabolic syndrome and type II DM.
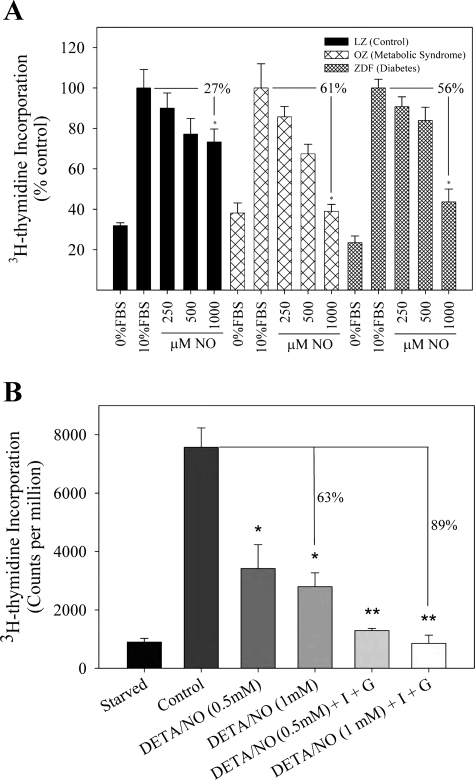
To better characterize the effects of NO on proliferation of the three strains of VSMC (LZ, OZ, ZDF), an in vitro assay to assess proliferation was conducted using [3H]thymidine incorporation. Of note, the results of these experiments on [3H]thymidine incorporation correlated with experiments on cell counting. The addition of the highest concentration of SNAP (1,000 μM) significantly inhibited [3H]thymidine incorporation in VSMC in all three rat strains compared with baseline levels and LZ controls (Fig. 1A, P < 0.05). The percent reduction between the control group and the 1,000 μM SNAP treatment group was 61 and 56% for OZ (metabolic syndrome) and ZDF (type II DM) VSMC, respectively, which was statistically greater than the 27% reduction observed in the LZ (control) VSMC (P < 0.05). Thus, while the control rats did demonstrate a reduction in proliferation with the addition of SNAP, the inhibition of VSMC proliferation was most dramatic in VSMC harvested from rats demonstrating metabolic syndrome and type II DM.
Fig. 1.
Nitric oxide (NO) inhibits [3H]thymidine incorporation to a greater extent in type II diabetes mellitus (DM) and metabolic syndrome compared with control. A: rat aortic vascular smooth muscle cells (VSMC) harvested from lean Zucker (LZ; control), obese Zucker (OZ; metabolic syndrome), and Zucker diabetic fatty (ZDF; type II DM) rodents were exposed to starvation media [0% fetal bovine serum (FBS)], control media (10% FBS), or varying concentrations of NO donor S-nitroso-N-acetylpenicillamine (SNAP; 0–1,000 μM) for 24 h and [3H]thymidine incorporation was assessed. *P < 0.05 compared with 10% FBS for each group. B: LZ cells were exposed to control media or hyperglycemic (G, 25 mM) or hyperinsulinemic (I, 200 nM) environments and then treated with the NO donor (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA/NO). [3H]thymidine incorporation was assessed at 24 h. *P < 0.001 vs. control; **P < 0.007 vs. DETA/NO, 0.5–1.0 mM. Each group was assessed in triplicate. Values are representative of 5 independent experiments.
Given that the above experiment examined the effect of NO on VSMC harvested from each animal model and not the effect in the diabetic milieu of type II DM, we wanted to evaluate the effect of NO on VSMC in a hyperinsulinemic and hyperglycemic environment. In support of the data above, NO was found to inhibit [3H]thymidine incorporation to a greater extent in LZ cells in a hyperinsulinemic (200 nM) and hyperglycemic (25 mM) environment (89% inhibition) compared with VSMC in control media with no added glucose or insulin (only 63% inhibition, P < 0.007; Fig. 1B). Thus NO is more effective at inhibiting proliferation in VSMC harvested from the animal models of type II DM, as well as in VSMC exposed to the metabolic conditions of type II DM.
NO induces minimal VSMC cell death in vitro.
To determine whether NO induces VSMC death in vitro, death was assessed using the Guava PCA. As expected, VSMC maintained in starvation media (0% FBS) did induce a statistically significant increase in VSMC death in all three cell lines compared with VSMC maintained in regular growth media (LZ 39 ± 3.4%, OZ 36 ± 1.4%, ZDF 48 ± 0.9%, P < 0.05). The addition of varying concentrations of SNAP (0–1,000 μM) to regular growth media resulted in no statistically significant impact on cell death (LZ 9 ± 1.3%, OZ 14 ± 2.3%, ZDF 14 ± 1.1%, P = nonsignificant) compared with VSMC maintained in growth media alone, as measured in the three cell lines (LZ 16 ± 3.0%, OZ 11 ± 1.5%, ZDF 9 ± 1.6%, P = nonsignificant). These data are representative of five independent experiments.
NO causes a G0/G1 cell cycle arrest.
To further investigate the heightened efficacy of inhibition of VSMC proliferation by NO, the percentage of cells in the various stages of the cell cycle was analyzed. For all three animal models, the majority of VSMC at baseline was found in G0/G1 phase (Figs. 2). The addition of the NO donor DETA/NO resulted in an increase in the percentage of VSMC found in the G0/G1 phase, with a corresponding decrease in the percentage of cells in the G2/M and S phases. However, following exposure to DETA/NO, a larger percentage of cells was arrested in G0/G1 in OZ (80%) and ZDF (87%) vs. LZ VSMC (72%, P < 0.05). Accordingly, the addition of DETA/NO increased the expression of the cyclin-dependent kinase cell cycle inhibitory proteins p21 in all three cell lines, but resulted in the greatest increase in p21 from baseline in ZDF cells (Fig. 3). DETA/NO increased p27 expression in all three cell lines, but no significant difference was noted between the cell types.
Fig. 2.
NO causes a greater G0/G1 cell cycle arrest in type II DM and metabolic syndrome compared with controls. Rat aortic smooth muscle cells harvested from LZ (control), OZ (metabolic syndrome), and ZDF (type II DM) rats were exposed to control media (10% FBS) or NO donor DETA/NO (1,000 μM) for 24 h. From left to right: representative flow cytometry analysis of control cells, then DETA/NO treated cells, followed by Modfit quantification of the percentage of cells in each stage of the cell cycle for LZ (control) (A), OZ (metabolic syndrome) (B), and ZDF (type II DM) (C) cells. *P < 0.05 compared with uninjured. Each group was assessed in triplicate. Values are representative of 4 independent experiments.
Fig. 3.
NO increases expression of the cyclin-dependent kinase inhibitor p21 more in type II DM vs. controls. Western blot analysis is shown of the cyclin-dependent kinase inhibitors p21 and p27 for LZ (control), OZ (metabolic syndrome), and ZDF (type II DM) VSMC. The p21 Western blot is representative of 7 independent experiments, whereas the p27 Western blot is representative of 5 independent experiments. The graphs represent densitometry of the Western blots shown, adjusted according to the control within each cell type.
NO inhibits neointimal hyperplasia more effectively in metabolic syndrome and type II DM.
We then examined the effect of NO on neointimal hyperplasia following rat carotid artery balloon injury in animal models of type II DM (ZDF, n = 13) and metabolic syndrome (OZ, n = 12), as well as control animals (LZ, n = 11). Animal weights, serum glucose, insulin, cholesterol, and triglycerides from the animals are summarized in Table 1. These data confirm that the rat models exhibited a biochemical profile in accordance with their intended disease. The OZ rats, which represent metabolic syndrome, demonstrated hyperlipidemia, obesity, and insulin resistance without hyperglycemia. The ZDF rats, which represent type II diabetes, demonstrated hyperlipidemia, insulin resistance, and hyperglycemia. Due to glucose spilling and the nature of the ZDF model, these rats often are of normal weight. As expected, the addition of the NO donors did not demonstrate any significant effect on these parameters. While there was a trend toward increased insulin levels following treatment with PROLI/NO, this did not reach statistical significance.
Table 1.
Baseline characteristics of LZ (control), OZ (metabolic syndrome), and ZDF (type II diabetic) rats
| LZ Injury Alone | LZ Injury + PROLI/NO | OZ Injury Alone | OZ Injury + PROLI/NO | ZDF Injury Alone | ZDF Injury + PROLI/NO | |
|---|---|---|---|---|---|---|
| n | 5 | 6 | 6 | 6 | 6 | 7 |
| Weight (OD), g | 323±12 | 318±7 | 496±1* | 502±11* | 356±7† | 359±11† |
| Weight (Sac), g | 336±11 | 346±7 | 542±4* | 543±11* | 356±20† | 389±18† |
| Glucose (OD), mg/dl | NR | 122±5 | NR | 129±1 | 459±30*† | 416±24*† |
| Glucose (Sac), mg/dl | 108±7 | 111±3 | 102±6 | 96±5 | 467±27*† | 345±56*† |
| Insulin, pM | 41±10 | 30±0 | 88±29* | 113±26* | 87±15* | 101±19* |
| Cholesterol, mg/dl | 74±4 | 71±3 | 116±4* | 117±5.1* | 158±4* | 148±12* |
| Triglycerides, mg/dl | 85±8 | 69±7 | 369±13* | 370±18* | 333±20* | 366±45* |
Values are means ± SE; n, no. of rats. LZ, lean Zucker; OZ, obese Zucker; ZDF, Zucker diabetic fatty; PROLI/NO, disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate; OD, operative date; Sac, sacrifice date; NR, not recorded.
P < 0.05 vs. LZ;
P < 0.05 vs. OZ.
Balloon injury produced reproducible neointimal lesions at 2 wk following injury in the rat carotid artery (Fig. 4A). Injury increased the intimal area to the greatest degree in OZ rats compared with LZ and ZDF rats (P < 0.05). The application of PROLI/NO significantly reduced intimal area and intima-to-media area ratio (I/M) in all three rat strains compared with injury alone (P < 0.05) (Fig. 4, B and D). Treatment with PROLI/NO resulted in a trend toward decreased medial area in all of the rat strains. However, these results were not statistically significant (Fig. 4C). The NO-based therapy showed a dramatic 93% reduction in the I/M in type II DM rats (0.42 ± 0.09 vs. injury alone, P < 0.05) and a 69% reduction in the I/M in metabolic syndrome rats (0.08 ± 0.03 vs. injury alone, P < 0.05). These reductions are significantly greater than the 46% reduction in I/M seen in the control rats (0.43 ± 0.07, P < 0.05). Thus NO robustly inhibited neointimal hyperplasia in the rat models of metabolic syndrome and type II DM compared with control rats.
Fig. 4.
NO inhibits neointima formation following arterial injury more effectively in type II DM and metabolic syndrome. NO treatment groups received a topical application of NO donor disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO); arteries were harvested 2 wk after injury and analyzed morphometrically. A: representative hematoxylin-eosin-stained sections from LZ (control, n = 11), OZ (metabolic syndrome, n = 12), and ZDF (type II DM, n = 13) rats: uninjured, injury alone, or injury plus application of PROLI/NO gel. Intimal area (B), medial area (C), and intima-to-media area ratios (I/M) (D) are shown for rodents subjected to vascular injury alone and vascular injury followed by application of PROLI/NO gel. *P < 0.05 compared with injury alone; **P < 0.05 compared with LZ injury alone.
NO release was measured from the PROLI/NO and control gels using the Greiss reaction, which measures nitrite. The control gel contained no PROLI/NO. Similar to our laboratory's prior publications with NO-eluting gels (33), the PROLI/NO gel released a high amount of nitrite initially, with continued release out to 6 days (Fig. 5).
Fig. 5.
NO release from the PROLI/NO gel. NO release from the PROLI/NO and control gels was assessed using the Greiss reaction, which measures nitrite. The PROLI/NO gel released a large amount of nitrite within the first 24 h and continued to release nitrite for 6 days.
NO induces minimal VSMC apoptosis in vivo.
Similar to the in vitro studies, we sought to determine whether NO limited the formation of neointimal hyperplasia by inducing VSMC cell death in vivo. Sections stained using TUNEL did not reveal a distinct pattern of apoptosis among the different treatment groups (data not shown). These data are consistent with the in vitro data in which NO did not induce significant VSMC death.
NO reduces ROS more in rodent models of type II diabetes and metabolic syndrome in vivo compared with controls.
To investigate the heightened efficacy of NO-mediated inhibition of VSMC proliferation, we utilized the ROS stain DHE, which predominantly detects superoxide. Balloon injury produced a reproducible increase in ROS that was inhibited by the application of NO-eluting PROLI/NO gel (Fig. 6). Injury increased the amount of ROS more in OZ and ZDF rats compared with LZ rats (P < 0.05), and the application of PROLI/NO gel significantly reduced the amount of ROS in OZ (43% reduction) and ZDF (30% reduction) rats compared with LZ rats (23% reduction) (Fig. 6B).
Fig. 6.
NO causes a greater inhibition of reactive oxygen species following injury in type II DM and metabolic syndrome. A: representative dihydroethidium-stained rat carotid artery sections from LZ (control), OZ (metabolic syndrome), and ZDF (type II diabetes) rats: uninjured, injury alone, or injury plus application of PROLI/NO gel. B: quantification of dihydroethidium stain throughout all layers of the arterial wall for LZ (control), OZ (metabolic syndrome), and ZDF (type II diabetes) rats subjected to no injury, vascular injury alone, and vascular injury followed by application of PROLI/NO gel. Blinded grading was performed on a 0–3 scale. Positive control consisted of sections exposed to hydrogen peroxide (200 μM). *P < 0.05 compared with uninjured; **P < 0.05 compared with injury alone.
Treatment with NO results in greater nitrotyrosine expression.
To assess overall nitrosative stress in the vessel wall at baseline, following injury, and after treatment with NO, immunohistochemical staining was performed for nitrotyrosine staining. At baseline, OZ and ZDF arteries demonstrated more nitrotyrosine staining, mostly in the adventitia (Fig. 7). Following injury, nitrotyrosine expression increased in all groups throughout all layers of the arterial wall. Treatment with NO resulted in further increases in nitrotyrosine staining, as would be expected. These findings were greatest in OZ and ZDF animals.
Fig. 7.
NO increases nitrotyrosine expression. Representative immunohistochemical staining is shown for nitrotyrosine expression of rat carotid artery sections from LZ (control), OZ (metabolic syndrome), and ZDF (type II diabetes) rats: uninjured, injury alone, or injury plus application of PROLI/NO gel.
DISCUSSION
We examined the effect of NO on VSMC proliferation, cell cycle regulation, and cell death in vitro and neointimal hyperplasia and ROS production in vivo. In summary, we found that NO was a more effective therapy in the altered milieu of type II DM and metabolic syndrome compared with control animals, thereby disproving our hypothesis. This is important because DM and metabolic syndrome predispose patients to vascular disease. Consequently, many of these patients undergo treatment of severe vascular disease with interventions such as angioplasty, endarterectomy, and bypass grafting. Unfortunately, the vasculopathy of DM and metabolic syndrome results in greater rates of failure and restenosis after these interventions. As mentioned previously, NO is vasoprotective and inhibits many of the events that lead to the development of neointimal hyperplasia. The decreased bioavailability of NO in DM may leave these patients without physiological protection, not only for the normal day-to-day rigors of vascular activity, but also for derangements inherent to vascular interventions (3). This study demonstrates that a NO-based therapy has enhanced potency in the environments of DM and metabolic syndrome, and these data suggest a clinical role for NO-eluting vascular therapies in these disease states.
To understand the role of NO in vessels from DM and metabolic syndrome, a better understanding of the pathophysiology of diabetic vascular disease must be gained. How insulin resistance and hyperglycemia create a pathological vascular environment in DM is not completely understood, but extensive research has elucidated several factors that contribute to the vasculopathy seen in DM. The bioavailability of NO represents a key marker in vascular health. Endothelial cells constitutively produce NO, and NO has many beneficial effects on the blood vessel. However, endothelial dysfunction, which is associated with impaired endothelium-dependent NO production, has been demonstrated in experimental models of diabetes, as well as in patients (17, 54). This dysfunction results from multiple derangements, but ROS play a predominant role. The diabetic environment activates systems like the MAPK pathway, protein kinase C, receptor for advanced glycation end products, and nuclear factor-κB in VSMC that augment the production of superoxide (6). Furthermore, insulin directly stimulates superoxide production through activation of NADPH oxidase, and glucose directly blocks electron transfer in the mitochondria, both of which result in further superoxide production (23, 46, 55). The increased superoxide, in turn, results in further decreases of NO production by uncoupling endothelial NO synthase (eNOS); uncoupled eNOS in turn produces more superoxide (2). Lastly, hyperinsulinemia stimulates angiotensin II, which results in inhibition of Akt (39). This further diminishes production of NO by preventing phosphorylation of eNOS by Akt (15). Thus this hyperinsulinemic and hyperglycemic environment is fraught with a decreased bioavailability of NO and a heightened ROS presence, both of which contribute to the altered response to vascular injury (7).
Oxidative stress has been shown to stimulate VSMC proliferation and lead to greater neointimal hyperplasia (20, 21). Jacobson et al. (31) delivered a NADPH oxidase inhibitor to rodents systemically following arterial injury. They observed decreased superoxide levels in the arterial wall, as well as diminished neointimal hyperplasia. Dourron et al. (14) delivered the NADPH oxidase inhibitor to the periadventitial surface of the artery, similar to our present study, and also demonstrated reduced superoxide levels in the arterial wall and decreased neointimal hyperplasia at 2 wk. Tempol, a ROS scavenger, has also reduced neointimal hyperplasia in diabetic rodents (32). Thus it is clear that ROS play an important role in the arterial injury response. Since hyperinsulinemia and hyperglycemia are known to stimulate ROS production directly and indirectly, and less NO is present, the arterial wall is at a significantly disadvantaged state. Small changes in the arterial injury response, especially in the early phase, may result in significant changes in the end biological response. Thus the provision of excess NO, which decreases production and increases degradation of ROS, to the arterial wall following injury can throw off the negative balance and shift the environment to a more vasoprotective state. However, the effect of NO on ROS is unlikely to be the only mechanism by which NO is more effective in the diabetic milieu, since NO is known to affect many different aspects of the arterial injury response, including platelets, leukocytes, VSMC, endothelial cells, and fibroblasts. Further study is warranted to delineate these different regulatory pathways.
While we are unaware of any studies that have investigated the efficacy of a NO-based therapy to inhibit neointimal hyperplasia in a diabetic environment, Barbato et al. (3) examined the effect of inducible NO synthase (iNOS) gene transfer in rodents exhibiting metabolic syndrome. They demonstrated that NO inhibited neointimal hyperplasia to a greater extent in metabolic syndrome rats, which showed a 66% reduction in the I/M after infection with an adenovirus carrying the iNOS gene, while control rats demonstrated a 50% reduction with NO (3). The vascular injury response in the face of metabolic syndrome was associated with increased adhesion molecule expression, inflammatory cell infiltration, and proliferation (3). In their study, iNOS was able to inhibit this heightened injury response and reduce neointimal formation in this proinflammatory environment (3). In fact, our reduction in neointimal hyperplasia with the application of NO in the environment of metabolic syndrome (69%) is consistent with that observed by Barbato et al. (3) (66%).
Our present study is not without limitations. Our results verify the efficacy of NO in inhibiting neointimal hyperplasia in the type II DM rodent arterial injury model. However, it remains to be seen what effect NO would have in a large-animal model of type II diabetes and metabolic syndrome. Another limitation to our study is that we used two different classes of NO donors: S-nitrosothiols (i.e., SNAP) and diazeniumdiolates (i.e., PROLI/NO and DETA/NO). However, all three NO donors reliably release NO, and our aim is to examine the effect of NO on neointimal hyperplasia in the midst of insulin resistance and hyperglycemia. One mole of NO is released per 1 mol of SNAP, while 2 mol of NO are released per 1 mol of DETA/NO. We have experience using both S-nitrosothiols and diazeniumdiolates for in vitro and in vivo experiments; both inhibit VSMC proliferation and induce G0/G1 cell cycle arrest, with no significant differences between them (33, 35, 37, 47). Furthermore, diazeniumdiolates were utilized for the present in vivo experiments due to our laboratory's previously published studies and, therefore, our familiarity with their in vivo characteristics (35, 51). DETA/NO was chosen instead of PROLI/NO for the in vitro studies simply because of its longer half-life in solution at 37°C and pH 7.4 (half-time = 20 h vs. 2 s, respectively). PROLI/NO would be an inappropriate choice for a 24-h in vitro assay. Lastly, while our gel produced significant results in the short term, the long-term results of NO application and the use of improved vehicles of NO delivery (like NO-eluting stents) should be researched further.
Another aspect of our study that deserves discussion is the efficacy of periadventitial application of the NO-eluting gel to the surface of the artery. We, and others, have previously shown that periadventitial application of a NO donor is effective at inhibiting neointimal hyperplasia at 2 wk (9, 18, 33, 34, 43, 47). The efficacy of this approach is most likely due to the highly diffusible nature of NO, allowing this molecule to penetrate through the layers of the arterial wall, reaching even the inner endothelial cell layer. However, it has also been established that the adventitial fibroblasts contribute significantly to the development of neointimal hyperplasia. Thus periadventitial application has the added benefit of greatly affecting the adventitial fibroblasts, as a diffusion gradient is likely to exist throughout the wall. Lastly, we previously demonstrated that the short-acting PROLI/NO was more effective at inhibiting neointimal hyperplasia compared with the long-acting NO donor diazeniumdiolated poly(acrylonitrile) (47). We hypothesized that this was due to the larger release of NO initially from PROLI/NO compared with the long-acting NO donor. This large release would have an overall greater effect on the arterial injury cascade, given that the injury response begins within seconds to minutes following injury with platelet aggregation and leukocyte chemotaxis.
Lastly, how a short-acting NO donor such as PROLI/NO can have such a lasting effect on the arterial wall should be addressed. At physiological pH and temperature, low concentrations of PROLI/NO have been shown to have a very short half-life, on the order of seconds (53). However, we have shown in this paper, as well as prior publications, that, when shielded inside a gel at higher basic concentrations, PROLI/NO has an extended half-life of NO release (33). This is not surprising, given the kinetics of NO release from diazeniumdiolates. They are highly sensitive to pH, temperature, concentration, and the surrounding milieu of hydrogen ions (28). Furthermore, the gel acts to shield the PROLI/NO from hydrogen ions, thereby prolonging the half-life even further. Lastly, while it is difficult to equate the NO release from the PROLI/NO gel in vivo to the NO release from the NO donors in vitro, the highest concentration of NO delivered to the cells in vitro (i.e., 1 mM) is ∼1/20th as much PROLI/NO as is placed in the neck of the animals. Thus the amount of NO delivered to the carotid artery in vivo is reasonable and may even be on the low side, given the surface area of cells exposed to NO in vitro. Ultimately, however, our goal is the end biological effect, i.e., inhibition of neointimal hyperplasia, so that a therapy can be developed and used in all patients, regardless of their disease states, to prevent restenosis following vascular interventions.
In conclusion, periadventitial application of a NO-eluting gel onto the surface of an injured rat carotid artery inhibited neointimal hyperplasia more effectively in animal models of type II DM and metabolic syndrome compared with controls. While PROLI/NO inhibited neointimal hyperplasia in all three rodent models, the inhibition was nearly twice as great in type II DM vs. control. Before this therapy can be utilized in the clinical arena, it must be evaluated in the large-animal model, and long-term studies should be conducted. Overall, this therapy has promising clinical potential for an ever-growing population of patients with diabetic vascular disease.
GRANTS
This work was supported in part by funding from the NIH (1K08HL084203 to M. Kibbe); the American Vascular Association, the Department of Veterans Affairs (VA Merit Review Grant to M. Kibbe); the American Medical Association Foundation (2007 Seed Grant to S. S. Ahanchi); and through the generosity of Hilda Rosenbloom and Eleanor Baldwin. In addition, part of this research was supported with federal funds from the National Cancer Institute, NIH under contract NO1-CO-12400 with SAIC-Frederick, Inc. (L. K. Keefer), and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (L. K. Keefer).
Acknowledgments
The authors express thanks to the Northwestern University Institute for BioNanotechnology in Medicine and to Lynnette Dangerfield for administrative support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahari A, Bergqvist D, Troeng T, Elfstrom J, Hedberg B, Ljungstrom KG, Norgren L, Ortenwall P. Diabetes mellitus as a risk factor for early outcome after carotid endarterectomy–a population-based study. Eur J Vasc Endovasc Eur J Vasc Endovasc 18: 122–126, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barbato JE, Zuckerbraun BS, Overhaus M, Raman KG, Tzeng E. Nitric oxide modulates vascular inflammation and intimal hyperplasia in insulin resistance and the metabolic syndrome. Am J Physiol Heart Circ Physiol 289: H228–H236, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287: 2570–2581, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63: 175–195, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Busija DW, Miller AW, Katakam P, Simandle S, Erdos B. Mechanisms of vascular dysfunctionin insulin resistance. Curr Opin Investig Drugs 5: 929–935, 2004. [PubMed] [Google Scholar]
- 8.Capek P, Mclean GK, Berkowitz HD. Femoropopliteal angioplasty–factors influencing long-term success. Circulation 83: 70–80, 1991. [PubMed] [Google Scholar]
- 9.Chaux A, Ruan XM, Fishbein MC, Ouyang Y, Kaul S, Pass JA, Matloff JM. Perivascular delivery of a nitric oxide donor inhibits neointimal hyperplasia in vein grafts implanted in the arterial circulation. J Thorac Cardiovasc Surg 115: 604–612, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Hanson SR, Keefer LK, Saavedra JE, Davies KM, Hutsell TC, Hughes JD, Ku DN, Lumsden AB. Boundary layer infusion of nitric oxide reduces early smooth muscle cell proliferation in the endarterectomized canine artery. J Surg Res 67: 26–32, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease. I. Pathophysiology, clinical consequences, and medical therapy. Circulation 108: 1527–1532, 2003. [DOI] [PubMed] [Google Scholar]
- 12.De Vriese AS, Verbeuren TJ, Van d V, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desouza CV, Gerety M, Hamel FG. Neointimal hyperplasia and vascular endothelial growth factor expression are increased in normoglycemic, insulin resistant, obese fatty rats. Atherosclerosis 184: 283–289, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, Pagano PJ. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol 288: H946–H953, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108: 1341–1348, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubey RK, Jackson EK, Lüscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin 1 receptors. J Clin Invest 96: 141–149, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durante W, Sen AK, Sunahara FA. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br J Pharmacol 94: 463–468, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulton GJ, Davies MG, Barber L, Gray JL, Svendsen E, Hagen PO. Local effects of nitric oxide supplementation and suppression in the development of intimal hyperplasia in experimental vein grafts. Eur J Vasc Endovasc Surg 15: 279–289, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83: 1774–1777, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong KW, Zhu GY, Wang LH, Tang CS. Effect of active oxygen species on intimal proliferation in rat aorta after arterial injury. J Vasc Res 33: 42–46, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury. II. Animal and human studies. Circulation 108: 2034–2040, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA. Functional angiotensin-II receptors in cultured vascular smooth-muscle cells. J Cell Biol 92: 289–298, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656–1662, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New England Journal of Medicine 339: 229–234, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Harnek J, Zoucas E, Sjuve R, Arner A, Ekblad E, Schou H, De Sa VP, Stenram U. Local infusion of the nitric oxide donor SIN-1 after angioplasty–effects on intimal hyperplasia in porcine coronary arteries. Acta Radiologica 44: 395–402, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Herlitz J, Wognsen GB, Karlson BW, Sjoland H, Karlsson T, Caidahl K, Hartford M, Haglid M. Mortality, mode of death and risk indicators for death during 5 years after coronary artery bypass grafting among patients with and without a history of diabetes mellitus. Coron Artery Dis 11: 339–346, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hink U, Li HG, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RAK, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circulation Research 88: E14–E22, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev 102: 1135–1154, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14: S227–S232, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92: 637–643, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Jagadeesha DK, Lindley TE, Deleon J, Sharma RV, Miller F, Bhalla RC. Tempol therapy attenuates medial smooth muscle cell apoptosis and neointima formation after balloon catheter injury in carotid artery of diabetic rats. Am J Physiol Heart Circ Physiol 289: H1047–H1053, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg 47: 173–182, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaul S, Cercek B, Rengstrom J, Xu XP, Molloy MD, Dimayuga P, Parikh AK, Fishbein MC, Nilsson J, Rajavashisth TB, Shah PK. Polymeric-based perivascular delivery of a nitric oxide donor inhibits intimal thickening after balloon denudation arterial injury: role of nuclear factor-kappa B. J Am Coll Cardiol 35: 493–501, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Kibbe MR, Li J, Nie S, Choi BM, Kovesdi I, Lizonova A, Billiar TR, Tzeng E. Potentiation of nitric oxide-induced apoptosis in p53−/− vascular smooth muscle cells. Am J Physiol Cell Physiol 282: C625–C634, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, Simmons RL, Billiar TR, Tzeng E. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg 31: 1214–1228, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Kibbe MR, Nie S, Seol DW, Kovesdi I, Lizonova A, Makaroun M, Billiar TR, Tzeng E. Nitric oxide prevents p21 degradation with the ubiquitin-proteasome pathway in vascular smooth muscle cells. J Vasc Surg 31: 364–374, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Kip KE, Faxon DP, Detre KM, Yeh WL, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute percutaneous transluminal corollary angioplasty registry. Circulation 94: 1818–1825, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Ko Y, Sachinidis A, Wieczorek AJ, Appenheimer M, Dusing R, Vetter H. Insulin enhances angiotensin II induced DNA synthesis in vascular smooth muscle cells of the rat. Clin Investig 71: 379–382, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Kornowski R, Mintz GS, Kent KM, Pichard AD, Satler LF, Bucher TA, Hong MK, Pompa JJ, Leon MB. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation 95: 1366–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 88: 4651–4655, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks DS, Vita JA, Folts JD, Keaney JFJ, Welch GN, Loscalzo J. Inhibition of neointimal proliferation in rabbits after vascular injury by a single treatment with a protein adduct of nitric oxide. J Clin Invest 96: 2630–2638, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masters KS, Lipke EA, Rice EE, Liel MS, Myler HA, Zygourakis C, Tulis DA, West JL. Nitric oxide-generating hydrogels inhibit neointima formation. J Biomater Sci Polym Ed 16: 659–672, 2005. [DOI] [PubMed] [Google Scholar]
- 44.McNeill AM, Rosamond WD, Girman CJ, Heiss G, Golden SH, Duncan BB, East HE, Ballantyne C. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC study). American Journal of Cardiology 94: 1249–1254, 2004. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics, and Centers for Disease Control and Prevention. 1999–2003 National Health Interview Survey (NHIS) (Online). http://www.cdc.gov/nchs/nhis.htm [15 Oct 2008].
- 46.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, Aalami OO, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Kibbe MR. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med 44: 73–81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2: 1057–1058, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Schwarzacher SP, Lim TT, Wang BY, Kernoff RS, Niebauer J, Cooke JP, Yeung AC. Local intramural delivery of l-arginine enhances nitric oxide generation and inhibits lesion formation after balloon angioplasty. Circulation 95: 1863–1869, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Shears LL, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, Watkins SC, Tzeng E. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg 187: 295–306, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Tzeng E, Kim YM, Pitt BR, Lizonova A, Kovesdi I, Billiar TR. Adenoviral transfer of the inducible nitric oxide synthase gene blocks endothelial cell apoptosis. Surgery 122: 255–263, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Diabetes 52: 2562–2569, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Waterhouse DJ, Saavedra JE, Davies KM, Citro ML, Xu X, Powell DA, Grimes GJ, Potti GK, Keefer LK. Injectable formulation of disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), an ultrafast nitric oxide donor prodrug. J Pharm Sci 95: 108–115, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Yang M, Kahn AM. Insulin-stimulated NADH/NAD+ redox state increases NAD(P)H oxidase activity in cultured rat vascular smooth muscle cells. Am J Hypertens 19: 587–592, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94: 2036–2044, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zucker LM, Antoniades HN. Insulin and obesity in zucker genetically obese rat “fatty”. Endocrinology 90: 1320–1330, 1972. [DOI] [PubMed] [Google Scholar]