Abstract
In this review we integrate recent human and animal studies from the viewpoint of chronic pain. First, we briefly review the impact of chronic pain on society and address current pitfalls of its definition and clinical management. Second, we examine pain mechanisms via nociceptive information transmission cephalad and its impact and interaction with the cortex. Third, we present recent discoveries on the active role of the cortex in chronic pain, with findings indicating that the human cortex continuously reorganizes as it lives in chronic pain. We also introduce data emphasizing that distinct chronic pain conditions impact on the cortex in unique patterns. Fourth, animal studies regarding nociceptive transmission, recent evidence for supraspinal reorganization during pain, the necessity of descending modulation for maintenance of neuropathic behavior, and the impact of cortical manipulations on neuropathic pain is also reviewed. We further expound on the notion that chronic pain can be reformulated within the context of learning and memory, and demonstrate the relevance of the idea in the design of novel pharmacotherapies. Lastly, we integrate the human and animal data into a unified working model outlining the mechanism by which acute pain transitions into a chronic state. It incorporates knowledge of underlying brain structures and their reorganization, and also includes specific variations as a function of pain persistence and injury type, thereby providing mechanistic descriptions of several unique chronic pain conditions within a single model.
Keywords: Human brain imaging, chronic pain, neuropathy, reorganization, pharmacotherapy, animal models, cortex, learning and memory
1. Introduction
Chronic pain is a controversial topic, and doubts have been raised regarding its diagnosis and treatment as a clinical condition (e.g., CRPS, fibromyalgia). During the past twenty years or so, however, tremendous advances have been made in our understanding of the peripheral and central processes involved in chronic pain conditions. The present chapter is an attempt to develop from these recent studies ideas that may lead toward a theory of chronic pain capable of accounting for its various clinical manifestations. We examine current definitions and clinical subtypes of chronic pain, assess data from human and animal studies, and expound upon the correspondence or lack thereof between proposed models. We conclude by presenting a speculative theory of chronic pain rooted in the neural mechanisms underlying this phenomenon that is consistent with both the human and animal research.
2. What is chronic pain?
2.1 A definition
The standard definition of chronic pain endorsed by the International Association for the Study of Pain states that it is pain that persists past the healing phase following an injury (Merskey and Bogduk, 1994). Determining the end of the healing phase is difficult, however, and instead the common clinical definition is a fixed time of persistent pain following its initial onset. For chronic back pain the usual time is 6 months, whereas in post-herpetic neuralgia 3 months of persisting pain is the more common time point at which the condition is dubbed chronic. These are purely functional and relatively arbitrary time posts that have little relation to underlying mechanisms.
Animal models of neuropathic and/or inflammatory injuries that cause a persistent pain condition suggest that peripheral and spinal cord circuitry transmitting nociceptive signals cephalad undergoes dramatic reorganization (Woolf and Salter, 2000; Woolf and Salter, 2006). The details of these plastic changes are beyond the current review, and will therefore only be summarized in relation to their relevance to clinical chronic pain. These models, especially the variety of peripheral nerve injuries that give rise to neuropathic pain behavior, indicate that subtle differences in the type of injury giving rise to pain behavior stabilize at different time delays from the initial injury. For example, the Seltzer partial sciatic nerve injury model (Seltzer et al., 1990) proposes that full blown thermal hyperalgesia and mechanical allodynia begin within hours of the injury and these abnormal pain behaviors are then maintained for weeks thereafter. In contrast, in the spared nerve injury model (Decosterd and Woolf, 2000) where the tibial and peroneal branches of the sciatic nerve are cut, stable and peak thermal hyperalgesia and mechanical allodynia are only observed at about two weeks after the initial injury. While the difference between these two neuropathic pain models may seem trivial, the point is that the ensuing pain behaviors require very different time delays to reach their full expression. To our knowledge there are no studies as to the mechanisms underlying these time delays. If one assumes that the peak pain is an indication for the longer lasting stable pain behavior that we equate to chronic pain, then the delay to reaching this behavior is clinically relevant. The processes underlying this delay, therefore, would be largely unrelated to the duration of the healing phase because the extent of injury and its related healing are very similar in both cases. These animal behavioral results cast doubt even regarding the validity of the standard definition for chronic pain. Moreover, they point to the notion that the definition of chronicity cannot be independent of the type of chronic pain in question and thus should be functionally determined for each type separately, as well as be based on the peripheral and central mechanisms undergoing reorganization in each condition.
2.2 Types of chronic pain
2.2.1 Human clinical conditions
There is a long list of chronic clinical pain conditions. These are generally labeled by their site of injury (e.g., back, head, viscera) and type of injury (e.g., neuropathic, arthritic, cancer, myofascial, diabetic). Clinical manifestations are often a combination of multiple pain conditions; even in a single condition several diverse tissue types are observed to contribute. Perhaps the most notorious example is chronic back pain, where it is very difficult to ascertain the type of tissue injured, and with the extent of joint degeneration, muscle, and nerve injuries varying broadly across patients the relative involvement of each remains obscure and undeterminable.
Clinical scientists continue to argue over the validity of diverse chronic pain conditions, such as CRPS and fibromyalgia, or whether they are not the creation of the patient in complicity with consenting physicians. This frustration seems to emanate mainly from an inability to localize peripheral parameters that can define the condition coupled with the lack of efficacious treatments. Physicians dealing with complicated chronic pain patients complain that regardless what they do the patients continue to complain and continue to report suffering from pain. However, this grim picture is beginning to change. The animal models developed over the last 20 years or so along with our ability to peer into the brains of chronic pain patients are rapidly changing our notions regarding chronic pain.
This review will use the example of chronic back pain to illustrate the new advances in understanding the mechanisms of chronic pain. The significance of this choice is evidenced by the impact of this particular condition on society. Moreover, this is the condition that we have studied for over ten years from the viewpoint of central nervous system mechanisms. Low back pain is a public health problem affecting between 70–85% of adults at some time in their life (Frymoyer, 1988). The annual prevalence of chronic low back pain ranges from 15% to 45%, with point prevalences averaging 30% (Andersson, 1997). In the USA, chronic and acute back pain is the most common cause of activity limitation in people younger than 45 years and the second most frequent reason for visits to physicians (Hart et al., 1995; Praemer et al., 1992). Data from other Western countries are similar. Estimates from the UK show low back pain as the largest single cause of absence from work, accounting for 12.5% of all sick days and over £11 billion in direct and indirect costs in 2000 (Frank, 1993; Maniadakis and Gray, 2000). An article by Deyo (Deyo, 1998) states that:
“Up to 80% of all adults will eventually experience back pain, and it is a leading reason for physician office visits, for hospitalization and surgery, and for work disability…. Clearly, back pain is one of society’s most significant non-lethal medical conditions. And yet the prevalence of back pain is perhaps matched in degree only by the lingering mystery accompanying it.”
Defining low back pain is difficult. In general, acute low back pain refers to 0–7 days of pain (pain free at onset), subacute low back pain is classified as pain between 7 days and 3 months of duration, and chronic low back pain is defined as pain lasting for more than 3 months (Frank, 1993). Furthermore, back pain can arise due to either mechanical or non-mechanical causes, the latter being associated with other underlying diseases. For instances in which such other processes are not identified, back pain is assumed to occur on a mechanical basis, even when an exact underlying anatomic abnormality cannot be clearly detected. Many people with mechanical back pain also show a neuropathic presentation (traditionally classified as radiculopathy) manifest by tingling, burning, or numbness (Audette et al., 2005), or as pain ‘shooting’ into the distal affected part. Though commonly associated with acute disc herniation these neuropathic symptoms are also now commonly being identified in people with chronic low back pain.
2.2.2 Animal models approximating clinical chronic pain conditions
Animal models for chronic pain have become a fundamental tool with which underlying mechanisms can be systematically studied. Wall’s autotomy model (Wall et al., 1979), dorsal root rhizotomy model (Lombard et al., 1979), and Bennett’s chronic constriction injury model (Bennett and Xie, 1988) constituted the early pioneering work in the field. Since then new models are continually being proposed that are becoming more sophisticated in their ability to more closely model real clinical conditions. Animal models for cancer pain (Mantyh, 2006), for spinal cord injury pain (Yezierski, 2005), and for migraine (Yamamura et al., 1999) are some of the latest additions. At minimum, these animal models confirm that chronic pain states are biological entities and not just the imagination of patients. Moreover, they allow for a mechanistic study of pathophysiology, and this has been a fantastic boon to understanding the peripheral and spinal cord mechanisms underlying various types of chronic pain. Where these models fall short, however, is in many clinical conditions where the actual correspondence between the purported model and the clinical manifestation remains to be directly tested. As a result we are often unsure if these models are providing specific mechanistic information or general hints as to the possible list of mechanisms that may underlie the true clinical condition. In chronic back pain, for example, do models of peripheral nerve injury provide insights into symptoms of back pain? What about skin or muscle inflammation? Another shortcoming is their inability to dispel suspicion regarding more complex conditions, such as CRPS and fibromyalgia, for which we do not even know how to begin building animal models. Thus in many respects the initial excitement that these models provided regarding the opportunity for designing new therapies for clinical pain conditions has already waned. It is now almost 20 years since the Bennett CCI model, and despite over a hundred peripheral and central molecular targets having been generated from these models and large sums of research dollars invested by pharmaceutical companies we have yet to identify any new therapy based on an animal model for neuropathic pain. The reasons behind this failure may be complex and multifactorial. Nonetheless, it underscores the necessity of translational studies where information generated in animal models are tested in humans and vice versa, allowing mechanistic notions to guide human research and hints from human studies tested thoroughly in animal models.
2.2.3 Management of chronic pain: Current management of low back pain remains insufficient
The majority (>90%) of individuals with acute low back pain recover full function in days or weeks with little or no lingering pain. Recent studies in this population have highlighted the importance of early mobilization and the early use of effective analgesic agents (Hagen et al., 2005). Yet a small number of individuals with acute low back pain (approximately 5% or less) go on to develop subacute and then chronic low back pain. Of this group, a high percentage will fail treatment and are often referred to pain clinics and centers, where multi-disciplinary techniques utilizing non-pharmacologic, pharmacologic and anesthesiologic interventions are variably beneficial. Most chronic low back pain patients, however, continue to have significant degrees of pain, are significantly limited in their functional capacity, and become emotionally altered by their chronic pain condition.
The goal of treating low back pain has been relief of pain, restoration of physical function, and the successful maintenance and/or reintegration of the patient into the workplace and society. A broad range of management options have been advocated including oral, topical and injectable medications, devices, surgical approaches, physical therapy, educational and psychological interventions, and others. Yet remarkably there remain a lack of well-designed and appropriately conducted clinical trials to evaluate the efficacy of these treatments (Schnitzer et al., 2004). This is particularly true in the case of chronic low back pain, in which no long-term trials (> 3 months) have been conducted with any therapeutic agent.
Furthermore, while the few short-term (only weeks in length) placebo-controlled trials conducted to date have found some support for the use of non-steroidal anti-inflammatory drugs (NSAIDs) and antidepressants in treating lower back pain, their results are usually not significant enough to reach the level of clinical effectiveness. With efficacy requiring a minimum 25% decrease in pain from placebo, a 2000 meta-analysis of NSAID studies found no evidence that these drugs were effective in treating chronic low back pain (van Tulder et al., 2000). More recent treatment studies have also failed to reach clinical effectiveness, with most only finding about a 10% decrease in pain (e.g. (Coats et al., 2004; Pallay et al., 2004)). A systematic review of antidepressants treatment for chronic back pain also concluded that they produce only a moderate symptom reduction (Staiger et al., 2003), and another recent review concluded that: “Many drugs used for back pain are no more, or only slightly more, effective than placebos. Others have side effects that outweigh their usefulness in relieving pain. On the basis of the evidence, no drug regimen can be legitimately recommended for back pain” (Bogduk, 2004). The World Health Organization Advisory Panel likewise concluded that there is no single treatment superior to others for relieving chronic back pain (Ehrlich, 2003).
The lack of long-term and failure of short-term clinical trails on chronic low back pain is worsened by the fact that few if any studies have evaluated patients with a neuropathic component to their pain. Indeed, in many studies evidence of neuropathy is an exclusion criterion. Nevertheless, a significant number of individuals display a neuropathic component to their chronic back pain. This is of significant clinical importance considering that neuropathy has traditionally been unresponsive to treatment with NSAIDs, anti-depressants, and other pharmacologic agents.
2.2.4. Existing models of chronic pain have low predictive power
2.2.4.1 Anatomical, biochemical and genetic risk factors for back pain
The probability that the particular cause of back pain can be identified by radiographs is less than 1% (van den Bosch et al., 2004). Nevertheless, disc space narrowing appears to be more strongly associated with back pain than other radiographic features (Pye et al., 2004). Moreover, the histological composition of herniated disc material seems to correlate with clinical symptoms such as reported pain (correlation coefficient 0.7) (Willburger et al., 2004). Another cause of pain and radicular symptoms seems to be due to pressure on the nerve tissue from ligamentum flavum and facet joints (Okuda et al., 2004).
Findings from two adult female twin studies indicate that 50–70% of the variation of cervical and lumbar disc degenerative processes are due to genetic factors (MacGregor et al., 2004; Sambrook et al., 1999). A similar Danish study also showed a genetic influence, albeit with more modest results (Hartvigsen et al., 2004). Other experiments have identified a number of candidate genes that underlie disc degeneration and pain, see (Manek and MacGregor, 2005), suggesting that genetic factors may also have an important role.
2.2.4.2 Psychosocial factors
As peripheral physical factors have failed to show a relationship with back pain, a long list of psychosocial and demographic factors have been studied. Cumulatively, however, these factors provide poor predictions regarding chronic pain.
Depression is ranked as one of the strongest predictors for low back pain. This association is observed by multiple studies. One of the latest studies involved a national survey (n = 91,347) and 2-year follow-up survey (n = 55,690) utilizing both cross-sectional and cohort-study models. The results indicate that depression and low back pain are interrelated (correlation coefficient of about 0.4), with associational odds ratios increasing with intensity of back pain and severity of depression (Meyer et al., 2007). Similar results have been reported earlier (Reid et al., 2003).
In order to investigate the predictive power of baseline depression on the transition from acute to chronic pain (3 months post-acute back pain), a recent prospective model evaluating the direct and indirect effects of cumulative trauma exposure, acute pain severity and disability, baseline depressive symptomology, and pain beliefs on chronic pain severity and disability (Young et al., 2007) was recently tested. The model only accounted for 26% of the variance in chronic pain and 58% of the disability. Acute pain intensity did not directly predict pain three months later and baseline pain beliefs failed to predict chronic pain. Despite these relatively weak relationships to chronic pain, the authors argued that their findings support the growing literature contending that progression to chronic pain is more dependent on psychosocial and occupational factors than on medical characteristics of the spinal condition.
The prognostic value of factors influencing the course of low back pain and return to work in occupational health care was studied in a cohort of 299 workers on sick leave between 3 and 6 weeks due to low back pain (Heymans et al., 2006). The authors investigated the possible associations between a broad set of prognostic indicators related to characteristics of worker, job, low back pain, and psychosocial issues upon return to work lasting for a follow-up period of 12 months. The explained variation of the models was also calculated. Median time to return to work was 75 days. The explained variance of the multivariate model for return to work was only 18%. Another study focusing on the psychosocial factors that might predict return to work in 253 subacute and chronic pain injured workers showed that the best predictors were intensity and duration of pain, where the outcome model could account for about 40% of the variance (Schultz et al., 2004). An earlier study examining workers with acute back pain found that 204 of the recruited 854 claimants (23.9%) were still receiving compensation payments up to three months later. Their model demonstrated that severe leg pain, obesity, high scores on a disability index and health questionnaire, and physical requirements of the workplace to which subjects would return were all significant, independent risk factors for chronicity (Fransen et al., 2002). Smoking is also reported to increase risk for low back pain (Feldman et al., 1999).
In general, a long series of studies now describe psychosocial and psychological factors in predicting functional and social disability, where the interrelationship between ratings of catastrophizing, pain-related fear of (re-) injury, depression, disability, and pain severity are studied and modeled in combination with demographics in various chronic pain conditions. Yet while these factors may be associated with pain in certain individuals, attempts to create models of chronic back pain based upon them have been unproductive. For example, one model - the fear-avoidance model (Vlaeyen and Linton, 2000) - suggests that fear of pain and related pain behaviors can be relieved by exposing individuals to movements and tasks they have avoided due to fear of (re-) injury, predicting that such exposure should then result in reducing the intensity of chronic pain. To test this, a recent randomized controlled trial investigation (Woods and Asmundson, 2007) assessed effectiveness of exposure relative to other conditions in 45 chronic low back pain patients. Although the exposed patients improved on a long list of measures related to fear, the primary outcome measure regarding their disability showed no improvement. Further, if psychological and social factors had strong power in predicting chronic back pain, then quality of life and health care utilization, which have been shown to be dependent upon such factors (Keeley et al., 2007) should also show a relationship with a developing a chronic pain condition. Yet even when back pain is caused by a major physical trauma (Harris et al., 2007a), they remain only weakly related to chronic pain. It is now being recognized that psychosocial factors constitute “non-negligible risks” for the development of low back pain (Clays et al., 2007), and cannot account for how or why a patient transitions into the chronic pain state. A recent article titled “Why is a treatment aimed at psychosocial factors not effective in patients with (sub) acute low back pain?” (Jellema et al., 2005) concisely articulates the need to direct studies of chronic pain elsewhere.
In summary, there is a long list of risk factors but no dominant physical or psychosocial parameter that can substantially explain chronic pain. This clinical data (e.g. (Heneweer et al., 2007), and many others) does show, however, that the more severe the back pain (together with previous history of back pain), the longer it is sustained, and when it is accompanied with sciatic nerve damage there is increased likelihood that it will turn chronic, especially when coupled with high depression. Importantly, the emerging evidence for a genetic component also implies that certain brain-derived parameters (described below) may be part of a predisposition for chronic pain rather than its consequence.
2.2.5 Brain derived markers strongly correlate with clinical parameters of chronic pain
The primary objective of research in our lab over the last ten years or so has been to develop brain markers discriminating chronic pain patients from healthy subjects. Here we review examples of these measures’ predictive power with respect to the clinical properties of chronic pain, and attempt to integrate this information into a model of the human brain in chronic pain.
2.2.5.1 Brain chemistry
We published the first study showing that brain chemistry is abnormal in chronic back pain patients as compared to matched healthy controls, using magnetic resonance spectroscopy (Grachev et al., 2000). Our study revealed correlations between brain regional chemistry and clinical parameters of pain duration, intensity, and McGill Questionnaire dimensions. We found that the relative concentrations of chemicals in the cingulate cortex and thalamus reflected pain duration (in opposite directions). Moreover, chemical concentrations were found to positively correlate with sensory, affective, and intensity ratings of chronic back pain. In the study we reported: “Highly significant empirical relationships were seen between perceptual predictors and regional chemicals: (1) the combination of sharp pain, stabbing pain, pain duration, and trait anxiety predict the concentration of DLPFC N-acetyl aspartate (R2 = 0.98, P < 0.001); (2) the combination of pain duration, and state and trait anxiety predict the concentration of thalamic glucose (R2 = 0.97, P < 0.0004).” A small number of similar studies have been published since, in a number of chronic pain conditions (Fukui et al., 2006; Pattany et al., 2002; Sorensen et al., 2008). Yet, the topic remains in its infancy. It is likely that the method would provide clinically important information regarding various chronic pain conditions, especially as it is becoming an important biomarker in neurodegenerative conditions. For example metabolic changes are observed in presymptomatic mutation carriers years before onset of Alzheimer disease (Godbolt et al., 2006), suggesting that metabolic markers may also be useful in predicting predisposition to chronic pain.
An alternative approach is the use of positron emission tomography (PET) to examine binding changes for various ligands in chronic pain. With this approach a recent study identified mu-opiate binding decreases in fibromyalgia, with the decrease being related to the pain characteristics in a number of brain regions (Harris et al., 2007b). Dopamine release in the basal ganglia is also disrupted in fibromyalgia patients (Wood et al., 2007), and in burning mouth syndrome and atypical facial pain (Hagelberg et al., 2003a; Hagelberg et al., 2003b), and D2 binding in the basal ganglia has been proposed as a marker for diagnosis and treatment of chronic pain (Hagelberg et al., 2004).
2.2.5.2 Cognition
We reported that, in contrast to age, sex, and education matched healthy controls, chronic back pain and CRPS patients are significantly impaired on an emotional decision-making task (Apkarian et al., 2004a). Moreover, the performance of chronic back pain patients was highly correlated with their verbal report of pain at the time of performing the task (R2 = 0.64, p < 0.003). Thus we found that emotional decision-making abilities can explain more than 60% of the variance in chronic back pain intensity. In contrast to chronic back pain, CRPS patients’ performance was not modified when their pain was manipulated using a sympathetic block. The latter implies that the brain mechanisms underlying the two types of chronic pain may be distinct and thus also distinctly modulate emotional states. A long list of cognitive abnormalities has been described in chronic pain patients. The most noteworthy are attentional and memory deficits (Dick and Rashiq, 2007; Sjogren et al., 2005). However, little effort has been placed in differentiating such deficits based on chronic pain type, and only the results we describe on emotional decision-making has been related to the brain (see below).
2.2.5.3 Brain morphometry
We concluded the brain chemistry study by stating: “The tantalizing conclusion that chronic pain may be associated with neural degeneration needs to be re-examined with morphometric analysis.” (Grachev et al., 2000), and in 2004 published the first brain morphometric study showing anatomical evidence for brain atrophy in chronic back pain patients (Apkarian et al., 2004b). This result has now been replicated in chronic back pain and other types of chronic pain conditions (Kuchinad et al., 2007; Schmidt-Wilcke et al., 2005; Schmidt-Wilcke et al., 2006). Notably we were again able to show that these morphological changes are correlated with the clinical parameters of the condition. Neocortical gray matter volume, after correcting for intracranial volume, age and sex, was significantly less in chronic back pain patients than in matched controls. Moreover, this parameter showed dependence on pain duration, with similar slopes for patients with and without neuropathic (radicular) back pain, but only significantly for the neuropathic back pain group. When the same data was analyzed to directly compare regional gray matter differences between CBP patients and controls, two brain areas showed the most robust difference: bilateral DLPFC and right Thalamus. When we studied the DLPFC further in relationship to clinical parameters, gray matter density was found to be highly significantly dependent on the presence and type (neuropathic or non-neuropathic) of chronic back pain (p < 10−6). Moreover, to differentiate the relationship between regional gray matter and pain characteristics, we derived an index of change in DLPFC gray matter, corrected for age and gender confounds, and regressed it with pain characteristics. Across all CBP patients, the combination of sensory and negative-affective dimensions of CBP predicted DLPFC gray matter change (p < 0.007). Furthermore, when CBP subgroups were analyzed separately, we discovered distinct relationships. For nuCBP subjects (chronic back pain patients with radiculopathy), pain intensity, duration, and negative affect predicted DLPFC gray matter change (p < 0.002), whereas in non-nuCBP (non-radicular) subjects pain intensity, duration, and both sensory and affective dimensions contributed to DLPFC gray matter change (p < 0.0002). Thus, regional gray matter changes are strongly related to pain characteristics, and this pattern is different for neuropathic compared with non-neuropathic types. This dissociation is consistent with extensive clinical data showing that neuropathic pain conditions are more debilitating and have a stronger negative affect (Dworkin, 2002), and we suggested that this difference is directly attributable to the larger decrease in gray matter density in the DLPFC of neuropathic CBP patients. In the discussion of this study we suggested “regional atrophy dictates the brain activity observed in chronic pain, and it may explain the transition from acute to chronic pain” (Apkarian et al., 2004b).
2.2.5.4 Spontaneous fluctuations of pain
We recently revealed that the spontaneous pain of chronic pain patients fluctuates in the scale of seconds to minutes, that these fluctuations are distinct for various chronic pain conditions, and that normal healthy subjects are unable to mimic them (Foss et al., 2006). Participants were instructed to continuously rate their subjective assessment of the intensity of pain. We observed that the fluctuations of spontaneous pain do not possess stable mean or variance, implying that these time series can be better characterized by fractal analysis.
To this end, we applied time and frequency domain techniques to characterize variability of pain ratings with a single parameter: fractal dimension, D. We demonstrated that D is distinct between types of chronic pain, and from ratings of thermal stimulation and of imagined pain; and that there is a correspondence between D for pain ratings and D for brain activity in chronic back pain patients using fMRI. In back pain patients, average fractal dimension D = 1.55 ± 0.08 (mean & SD, n = 23); whereas for postherpetic neuropathy (PHN) patients D = 1.42 ± 0.11 (n = 58). These measures are highly significantly different from each other (p < 10−6), and have distinct properties. Back pain patients mainly show anti-persistence; meaning that on the average more intense pain is followed by weaker pain. By contrast, PHN patients show both anti-persistent and persistent time series. We concluded the study by stating: “We demonstrate that temporal properties of pain can reveal novel information with potential mechanistic and clinical significance” (Foss et al., 2006). This study remains the only one where spontaneous pain fluctuations at such time scales have been characterized.
2.2.5.5 Brain activity
Using non-invasive brain imaging (fMRI) in combination with online ratings of fluctuations of spontaneous pain we identified the brain activity idiosyncratic to chronic back pain (Baliki et al., 2006). Our data was analyzed using two different vectors: a) when ratings of spontaneous pain were high in contrast to low, and b) when ratings of spontaneous pain were rapidly increasing in contrast to all other times. The brain activity obtained, after subtracting a visual rating task that corrects for the cognitive, evaluative and motor confounds, differed greatly for the two conditions. During epochs when pain was high, activation of the medial prefrontal cortex (mPFC) was most robust, with less activity seen in the amygdala and the ventral striatum. For periods when pain was rapidly increasing, however, the insula, anterior cingulate cortex (ACC), multiple cortical parietal regions, and the cerebellum became activated. In the same study, using the same procedures (continuous ratings of perceived pain and subtraction of a visual control), we identified brain activity in back pain patients and healthy controls for an acute thermal stimulus applied to the back. The results showed no difference between patients and healthy controls for brain regions activated during acute thermal pain stimulation of the back. This activity pattern closely matched brain activity observed in earlier studies regarding acute pain in healthy subjects (Apkarian et al., 2005), and was similar also to the activity we observed for spontaneous pain for the contrast of rapidly increasing pain.
We also studied two separate groups of CBP patients using 2 MRI magnets, and in both groups identified the mPFC as the primary region activated for high pain. Moreover, in both groups mPFC activity was strongly correlated with pain intensity at the time of scan. In both groups we can assert that more than 80% of the variance of patient’s back pain intensity is accounted for by mPFC activity. Moreover, the insula activity, during the increasing phase of pain, seems to predict the duration of pain in years at a confidence level of 80%. In contrast, when levels of anxiety or depression were examined, none of the brain regions identified showed any relationship to these parameters. These results indicate that spontaneous CBP engages the emotional-mentalizing region of the brain into a state of continued negative emotions (suffering) regarding the self, punctuated by occasional nociceptive inputs that perpetuate the state. The sustained prefrontal activity is most likely related to the maladaptive psychological and behavioral cost associated with chronic pain. In the discussion of this paper we pose the question: What is chronic back pain? The answer we offered is particularly relevant to the present review:
“The common clinical approach to [studying] CBP is to relate its behavioral manifestations to the site of injury. Although some CBP patients have identifiable structural or mechanical cause for their pain, most do not … Given the poor association between structural abnormalities to pain, other nonspecific variables have been proposed as predictors of clinical outcome, like demographics including age, gender, and education …, psychosocial factors such as level of depression, anxiety, pain catastrophizing, fear and/or helplessness, job satisfaction, and environmental reinforcers such as compensation and litigation … Despite this long list, incorporating these parameters accounts for a relatively small portion of the variance of CBP … In contrast, examining CBP from the viewpoint of the brain indicates that CBP, regardless of whether it is secondary to fracture, inflammatory joint disease, postsurgical, combinations of these, or idiopathic … presents a well defined set of abnormalities. It is associated with a specific pattern of brain chemical changes [see (2.2.5.1) above] … that are consistent with decreased gray matter density in DLPFC [see (2.2.5.3) above], … which in turn may account for heightened mPFC activity and decreased ability in emotional-decision making [see (2.2.5.2) above]. These brain parameters account for over 70–80% of the variance for intensity and duration of CBP. Therefore, they must be considered an integral part of the clinical state of CBP.” (Baliki et al., 2006)
A recent review of advances regarding mechanisms of back pain that heavily relies on our findings concludes:
“[Chronic back pain] patients have back pain yet no conservative or surgical pain relieving measures directed at the back appear effective. They display a number of biomechanical abnormalities, however treatment directed at normalising lumbar biomechanics has little effect and there is no relationship between changes in outcome and changes in spinal mechanics. Finally, these patients demonstrate some psychological problems but psychologically based treatments offer only partial solution to the problem. A possible explanation for these findings is that they are epiphenomena, features that are incidental to a problem of neurological reorganisation and degeneration.” (Wand and O’Connell, 2007)
Wand et al.’s conclusion is perhaps more extreme than our position, though we certainly concur that a large component of chronic pain must include neurological abnormalities. We do not, however, completely discount the contribution of peripheral injury related signals that may be critical in the final neurological outcome. In fact we continue to search for approaches that may provide information regarding spinal cord processes that we can relate to brain abnormalities. For example, we continue to experiment with MRI scan sequences (T1, MRS, DTI) that may enable studying the lumbar spinal cord in chronic pain.
2.2.5.6 Overview
Chronic pain (especially chronic back pain) remains intractable and minimally understood.
Our recent research has demonstrated that brain-derived biomarkers relate far better to the clinical characteristics of chronic back pain than do physical factors or psychosocial approaches used in the past.
Therefore, future studies where changes of these biomarkers are tracked in time should provide insights into underlying mechanisms and identify causal relationships.
2.2.5.7 Implications
There seems to be a tight relationship between impairments in emotional decision-making and activity in brain regions involved in the perception of ongoing chronic back pain, suggesting that the two may be causally related. As the extent of impairment on emotional decision making in chronic back pain is directly related on the magnitude of pain suffering by the patient at the time the task is performed, and as the extent of mPFC activity is also tightly correlated to the magnitude of pain perceived by these patients, one can conclude that these events are in fact inter-related.
There is also a tight relationship between brain regional atrophy and brain chemistry abnormalities. They both indicate neuronal damage to the DLPFC in chronic back pain patients. As the primary chemical observed to decrease in DLPFC was N-acetyl-aspartate and as this chemical is mainly found within the soma of neurons, one is tempted to conclude that there is in fact a neuronal density decrease in the DLPFC that is more severe in patients that have had the condition for longer times. The latter suggests that the neuronal loss is a continuous ongoing process that is at least partially irreversible. Nevertheless, these implications need to be directly tested. The question regarding the extent to which brain atrophy may be reversed with successful and aggressive therapy is one that requires urgent determination. It should also be noted that genetic predispositions cannot be excluded regarding both abnormal brain chemistry and decreased regional gray matter density. In fact, the published results regarding the relationship between whole neocortical gray matter volume and its decrease with pain duration indicates that if one extrapolates the data to time zero (i.e. at the time when the presumed initial injury occurred) the patients’ estimated neocortical gray matter volume would still be smaller than that of healthy subjects, which would indicate the presence of a genetic predisposition.
The observation that spontaneous pain in chronic pain fluctuates over the scale of seconds to minutes is novel and potentially provides a simple objective tool for determining the presence and magnitude of chronic pain in the clinic. Considering that normal healthy subjects are unable to mimic these fluctuations, and report fluctuations in response to applied thermal painful stimuli that do not even remotely resemble those of chronic pain patients, this method would be particularly useful in identifying chronic pain patients. Furthermore, given that distinct chronic clinical pain conditions seem to result in distinct patterns of fluctuations, it could also serve to clinically discriminate between patient groups. Since fluctuations likely reflect the integration of pain signaling mechanisms with pain coping mechanisms, the presence of distinct fluctuation patterns suggests that the underlying mechanisms for various clinical chronic pain conditions must also be distinct. This point was recently directly tested by us and is further elaborated below.
Given that distinct chronic pain conditions show unique fluctuations of spontaneous pain, fluctuation patterns provide a unique signature with which one can interrogate the brain regarding related neuronal activity. By utilizing this approach our fMRI study indicated that when spontaneous chronic back pain was increasing, it activated brain regions closely resembling those found to be active during acute pain. By contrast, during time periods when spontaneous pain was sustained at a high level, the mPFC was the only primary area activated. Thus it appears that in these patients an initial nociceptive signal invades several brain pain regions, and then goes on to be sustained in the mPFC. Our study also demonstrated that this mPFC activity is negatively correlated with DLPFC activity. Given the decreased levels of NAA and gray matter density in the DLPFC of these patients, together the results evidence a tight interplay between brain activity, neuronal death, and cognitive abnormalities in chronic back pain. The causal interrelationship between these factors remains to be demonstrated, and the temporal evolution of these changes in relation to the initial injury and relative to each other needs to be studied. Still, it is remarkable that inter-relationships between brain derived activity, atrophy, chemistry, and cognitive parameters can all be found by examination of only a single clinical chronic pain condition. Moreover, such studies provide a new means of mapping clinical parameters to brain parameters. The brain biomarkers discussed above, for example, all show tight relationships (albeit to different extents) with certain clinical characteristics, particularly back pain intensity and duration. They do not, however, relate to patient increases in anxiety or depression, suggesting that these psychosocial factors are not directly related to the chronic pain condition and are instead represented by separate brain mechanisms. In sum, investigating chronic pain in terms of its underlying neural mechanisms not only sheds insight on how brain-derived biomarkers relate to one another, but also provides a means for mapping clinical parameters in the brain and reevaluating prior speculations of their role in the condition.
2.3 Distinct brain pain states
2.3.1 Brain activity for pain is distinct between clinical populations and stimulus conditions
An additional thesis of this review is that different chronic pain conditions involve distinct brain activity. We argue that at least two parameters control the diversity of these patterns: 1) type of injury, and 2) duration of the condition from the time of injury.
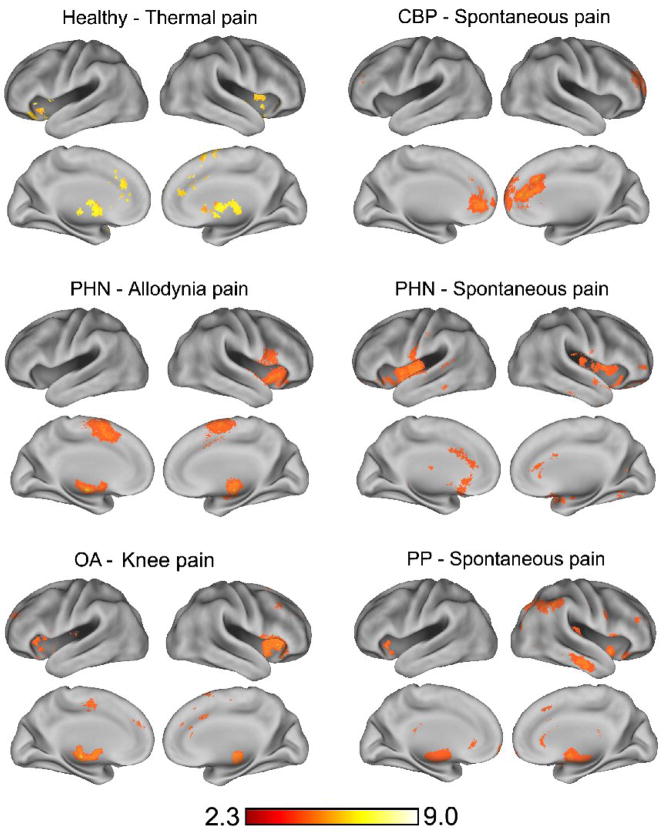
Acute pain appears to activate a fairly constant set of brain regions, as demonstrated in a meta-analysis (Apkarian et al., 2005) and shown in a recent study contrasting brain activity between rating thermal pain and rating magnitude of a visual bar (Baliki et al., 2008a). In contrast with acute pain, figure 1 shows that distinct chronic pain conditions such as CBP, post-herpetic neuralgia (PHN), osteoarthritis of the knee (OA), and pelvic pain (PP) all show different patterns of brain activity when ratings of either evoked pain or fluctuating spontaneous pain are contrasted with visual control task. There is now growing data regarding brain activity in various clinical pain conditions. However, direct comparison of reported brain activations are complicated by the difficulty of comparing results from diverse labs, distinct paradigms, and a variety of unique manipulations performed. Nevertheless, despite these complications a meta-analysis confirms that overall brain activity patterns in chronic pain patients generally diverge from that seen in acute pain, particularly with respect to the emerging prominence of prefrontal cortex activations (Apkarian et al., 2005). The data shown in figure 1 are more reliably comparable in that all the results were generated within our lab using the same fMRI magnet, the same data analysis techniques, and the same general experimental approach, all of which provides more confidence that observed differences are less likely to be due to non-interesting confounds. In all cases presented the activations are taken from a contrast between the pain state and a visual rating control task, which has allowed us to ascertain brain activity for spontaneous pain in three distinct clinical conditions. Consistent with the discussion above, where distinct temporal properties of spontaneous pain were seen for CBP versus PHN, brain activity related to spontaneous pain appears to also be distinct between these two groups, see (Geha et al., 2007). The PHN patients studied all also suffered from touch-evoked pain (tactile allodynia), and were further tested while they rated pain evoked by stroking the allodynic skin in contrast to visual rating (Geha et al., 2008). Although very similar brain regions were activated during spontaneous pain and allodynia pain, the activations of each were always distinct with only 3% of activity overlapping between the two tasks. We also conducted a study on knee OA examining pain evoked by mechanical stimulation of the painful joint. While we were unable to separate spontaneous pain from evoked pain in these patients, the brain activity elicited by mechanical stimulation of the painful joint in knee OA is in itself distinct from acute pain and other chronic painful conditions. Lastly, while our studies with chronic pelvic pain patients are still in the initial stages, preliminary data suggests that this spontaneous visceral type of pain can also be uniquely differentiated from other types of pain in the brain.
Figure 1.
Group average brain activity for pain in 6 different chronic pain populations or conditions. In all cases activity for continuous rating of pain contrasted with rating equivalent visual bar magnitudes is shown. Thermal pain activity is for n=16 healthy controls. CBP spontaneous pain is for n=13 Chronic Back Pain patients. In Post Herpetic Neuralgia (PHN), n=11, allodynia pain is for tactile stimuli that evoke pain, and for spontaneous pain. In osteoarthritis (OA, n=14) activity is shown for mechanical stimulation of the painful knee joint. In pelvic pain (PP, n=3) activity is shown for spontaneous pain.
2.3.2 Modulation of brain activity with pain intensity is also distinct by chronic pain conditions
Figure 2 reveals in some of the same groups and conditions as in figure 1 that the brain regions correlating with pain ratings are more circumscribed and again distinct for each condition and group. In healthy subjects, pain ratings during the application of thermal painful stimuli are encoded in the insula, anterior cingulate, DLPFC, thalamus and basal ganglia. Allodynia pain ratings in PHN patients, on the other hand, appear to be represented in the insula, S2 and basal ganglia. Only the mPFC codes pain ratings in CBP, and OA knee stimulation pain ratings are coded mainly by insula. We attribute these differences to the type of peripheral injury (neuropathic, inflammatory or both), to the duration that each group of patients have had the condition, as well as to the type of stimulus used (thermal, allodynia, knee OA). The brain regions identified in figures 1 and 2 are important nodes within circuitry that might undergo reorganization during the transition to chronic pain as a function of the type of initiating injury, perhaps coupled with genetic predispositions, where predisposing factors interact with peripheral, spinal cord, and cortical reorganization that seems to ensue soon after the injury and where the peculiarities of the central reorganization may be the critical factor leading to either chronic pain or cessation of pain and resumption of normal life. The memory traces of pain both prior to injury as well as those accumulated since injury – which would continue to be stored as long as the pain persists and as such the duration of chronic pain would impart a specific brain anatomical and physiological reorganization signature -may also be critical in the transition from acute to chronic pain and in the persistence of chronic pain (see below). While resolving the relationships between all these parameters will require many years of study, the identification of distinct activity patterns and their links to pain intensity provides an important initial step in connecting clinical characteristics with brain parameters in various chronic pain conditions.
Figure 2.
Brain regions modulated by rating perceived pain for some of the conditions shown in Figure 1. Abbreviations are the same as in Figure 1.
2.3.3 Modulation of brain activity with a peripheral Na-channel blocker
Applying Lidocaine patches to painful skin is now an FDA approved therapeutic procedure for PHN pain. In a recent study we used this manipulation to examine the relationship between brain activity and both spontaneous and touch-evoked pain in PHN patients (Geha et al., 2007; Geha et al., 2008). The general concept is simple. If Lidocaine treatment modulates pain, then subjects scanned before and after the treatment should show a modulation of brain regions related to their subjective reports of changes in pain. Clinically this procedure has been shown to result in an initial decrease in pain that is reported maximum at 6 hours after application of the patch, even though patients report further benefits with continued use. Therefore, these studies were also designed to test whether fMRI can be used to differentiate short-term from long-term effects at the level of brain activity. These studies were the first to test the notion of using serial imaging of brain activity before and after a therapeutic manipulation and test the technology’s usefulness in studying pharmacological manipulations. The general logic was that the specific therapy has the advantage that it acts locally by modulating Na channel excitability. Thus, it cannot confound the effects of subjective perceptual changes by direct action of the drug on the brain. Moreover, just as studies have begun to identify those brain regions activated for spontaneous versus evoked pain, this therapeutic manipulation afforded the opportunity to make further discriminations between regions that respond to treatment acutely versus those modulated in the longer run.
For spontaneous pain of PHN (Geha et al., 2007), lidocaine patch therapy did decrease its magnitude after 6 hours of use and further decreased its magnitude with continued use for two weeks. In addition, the brain regions responding to the therapy in the short tem were in fact distinct from those responding in the longer term. Seventeen distinct brain regions were identified activated for spontaneous pain. Only twelve of these regions, however, were modulated by the lidocaine therapy. Of those, only two regions decreased in activity in the short term (left thalamus and ACC). Another two decreased in activity in the longer term (left vental striatum and left amygdala). These four areas, therefore, were the most specific responses to the therapy and distinguished between acute effects of the therapy from the longer-term effects. The mechanisms underlying this brain response shift between short and long-term therapy remains unclear. It may underlie differential sensitivity of peripheral fibers as well as differential reorganization of central circuitry. More importantly the effects suggest functional segregation of brain circuitry where the short term effects appear to be mediated more by the spinothalamic tract while the longer effects more through pathways outside of the spinothalamic tract, especially those with projections from non-peptidergic IB4 neurons terminating in spinal cord lamina II (Braz et al., 2005).
In contrast with spontaneous pain, touch evoked pain (dynamical mechanical allodynia) was not modulated by lidocaine therapy (Geha et al., 2008). Therefore therapy effects could not be distinguished between brain regions. Across the three scan sessions, however, bilateral ventral striatum and left medial temporal gyrus (including amygdala and extended amygdala) were the regions best correlated to the change in allodynia pain. These same regions were also the ones that best coded the change in spontaneous pain with lidocaine therapy, although there was minimal overlap in the subdivisions of the ventral striatum and medial temporal gyrus for coding the two types of pain.
In an earlier study we showed that brain responses to an analgesic can be studied in individual subjects (Baliki et al., 2005) by demonstrating that the effects of a single dose of a cyclo-oxygenase-2 (COX2) inhibitor could be determined with fMRI. Studying a psoriatic arthritis patient we found that decreases in activity in S2 and anterior insula were tightly correlated with the change in pain perception following ingesting the drug. Statistical power in this case was obtained by performing repeated fMRI scans. Repeat scan fMRI study in an experimental sensitization paradigm in healthy subjects has also been studied for gabapentin (Iannetti et al., 2005). In this case the main outcome was a reduction in deactivations during sensitization. While these studies show that fMRI can be used to study analgesics, they are limited to drugs that show minimal central, especially cortical, effects. It is not clear how drugs that bind to receptors in the cortex would be studied with fMRI, as a long list of pitfalls may distort such studies. In the context of understanding functional specialization of brain activity in chronic pain, the use of analgesics and their relationship to regional brain modulation provides a powerful tool for further parcellating the functional specialization of various brain activities. Such an approach has been used recently, for example, to differentiate brain activations in acute pain in relation to modulation with opiate analgesia using PET studies (Zubieta et al., 2001; Zubieta et al., 2002). However, whether fMRI may eventually be used for identifying new therapeutic drugs remains to be demonstrated, for a discussion on these issues see (Borsook et al., 2006; Schweinhardt et al., 2006).
2.3.4 Brain activity during a simple visual magnitude-rating task is modulated by intensity of chronic back pain
In PHN we now have evidence that brain activity during a visual attentional task is modulated by the intensity of ongoing pain at the time of performing the task (Geha et al., 2007). The finding provides insight into the adjustments the brain in chronic pain undergoes to compensate for the condition. For instance, the result shows that even in a simple, clearly non-emotional task, activity in mPFC is increased, while motor and posterior parietal cortical activity is decreased, in proportion to the intensity of the chronic PHN pain. The actual parameters that control this compensation remain to be identified. The observation is has two important implications. First, it indicates the pitfall of studying brain activity in chronic pain patients in general by indicating that any task that such subjects perform is distorted by the ongoing presence of the chronic pain. Therefore, observed brain activity differences in contrasts between healthy subjects and chronic pain patients on a given task may be due to this distortion rather than actual differences in neuronal processing. This would explain the inconsistent results of a long list of studies performed using acute painful stimuli to understand distinctions in pain processing for various clinical pain conditions, see (Apkarian et al., 2005). Second, it suggests that while the effects of chronic pain can in fact be demonstrated and studied by using simple everyday non-painful tasks, comparisons based on simple subtractions leading to contrast maps may be misleading. On the other hand, brain activity comparisons between chronic pain patients and normal controls for peripheral stimuli can be more meaningful and result in important new information if brain activity is compared after equating pain perception magnitudes, rather than just comparing stimulus evoked activity differences. This approach has been adopted to compare brain activity in chronic back pain and fibromyalgia to mechanical painful stimuli, and in both patient groups brain activity seems exaggerated relative to healthy subjects, after equating pain perception (Giesecke et al., 2004; Gracely et al., 2002).
2.3.5 Impact of chronic pain on resting state brain activity
Although it is difficult to think that the brain has a “resting” state, for operational purposes, one can define it as the state of the brain of a subject awake and not engaged in any demanding sensory, motor, or intellectual activity. Characterization of this state is fundamental in neuroimaging studies, because it defines the baseline or control against which other task-related conditions can be compared. The notion of a specific network of brain regions active in rest-states (RSN) came from the observation of a consistent pattern of deactivations seen across many goal-oriented tasks (Shulman et al., 1997). This view attributes signal decreases during cognitive tasks in PET studies in the RSNs present at baseline and attenuated during specific goal-oriented tasks (Raichle et al., 2001). In other words, what was activated in resting state is inferred by identifying what is being deactivated during a task. The mPFC, precuneus, and hippocampus are commonly observed in RSNs, which seem to be particularly sensitive to cognitive states in self-referential tasks (Simpson, Jr. et al., 2001). Thus, RSNs are proposed to be involved in attending to environmental stimuli, both internally and externally generated (Raichle et al., 2001), in reviewing past knowledge to prepare for future actions (Binder et al., 1999), in episodic memory processing (Greicius et al., 2004), and is proposed to be the neural correlate of James’s (1890) stream of consciousness (Greicius and Menon, 2004), and in daydreaming.
We recently demonstrated the impact of chronic pain on resting state brain activity (Baliki et al., 2008b). In a group of CBP patients and healthy subjects, participants tracked the variability of a bar presented on a screen. Performance of this task was the same between the two groups, as was the brain activity positively engaged in the task. However, deactivations in CBP patients were less. As the deactivations in this task involve the mPFC and precuneus, and given that in CBP the mPFC is continuously overactive due to the presence of spontaneous pain, we propose that the decreased deactivation is a direct consequence of the chronic pain. There is a growing literature regarding the role of deactivation in brain function in general (Greicius et al., 2004; Greicius et al., 2007; Greicius and Menon, 2004), showing that in simple tasks it corresponds to resting state activity, and also demonstrating that this activity is reduced or is abnormal in patients with major depression or cognitive deficits such as in Alzheimer disease. Abnormal deactivations therefore imply that the RSN itself may be abnormal. When RSN was identified in CBP and healthy controls for the visual tracking task (and correlations between a seed regional activity and the rest of the brain were mapped), we observed highly distorted correlated/anticorrelated functional networks in CBP. When distinct brain regional activity were used as seeds, as well as the conjunction between all networks identified with six seeds, in CBP and in controls, we observed that these networks were not comparable between the two groups and that some elements had shrunk in space while others had enlarged. These results demonstrate that the impact of back pain on the brain can be studied with this simple task and that specific patterns of RSN activity may be related to the temporal evolution of chronic pain. A similar approach was recently used to study RSN changes during acute pain in healthy subjects for a cognitive task (Seminowicz and Davis, 2007). The study shows that the RSN changed in a pattern opposite to our observation in CBP, namely, the positive RSN component was enhanced while the negative component remained unchanged or further enhanced. The observation is important in again highlighting the fact that the effects of acute pain on brain dynamics seem opposite/different to those observed for chronic pain. The latter at least demonstrates yet again that studying the brain in acute pain may in fact provide the wrong clues, as to the impact of chronic pain on the brain.
2.3.6 Temporal evolution of the brain network engaged in perception of chronic pain
There has been a historic divide between scientists identifying themselves as localizationists and those who considered the brain to be a connected network, where the dynamics of the connected network characterize brain states. This is now changing, and it is now more widely accepted that both localization and integration are present in the central nervous system at almost every level examined. This view is particularly supported by functional brain imaging research, which can examine regional brain activity and integrate such activity into networks whose properties may be studied using a long list of recently developed tools. That the brain’s intrinsic dynamics can be studied using functional brain imaging was first demonstrated in 1995 (Biswal et al., 1995). We have used a similar approach to examine the topological properties of the connectivity of the functional brain when network connectivity for all gray matter voxels together is examined for a variety of tasks (Eguiluz et al., 2005). These results indicate that the healthy brain can be regarded as a network with fast signal processing, able to synchronize and manifests well-defined properties that make it resistant to failure. The resting brain state and its properties in fact are more recent extensions in the topic and perhaps some of the most exciting new developments in our understanding of the functional human brain as a dynamical network.
The earlier sections of this review have been dedicated more to the issue of which brain regions are specifically engaged in various types of chronic pain. Taken more generally, our position is that subjective states can only really be understood in the context of the dynamics of the brain when it is viewed as a connected network. Demonstrating abnormal resting state network in CBP is the most concrete step towards this effort. We have also recently shown that the perception of tactile allodynia pain in PHN can be understood as a temporal evolution of distinct brain states, as characterized by network connectivity (Geha et al., 2007). While tactile allodynia activity in the putamen was seen as best correlated to perception, this activity preceded perception of allodynia by a few seconds and lagged behind the touch stimulus that gave rise to the pain perception. We reasoned therefore that studying brain connectivity in relation to this signal should indicate brain connectivity properties relative to perception of allodynia. By extracting the activity in putamen and delaying it or advancing it so that it would be better correlated to the stimulus or perception, we could identify all the brain regions related to this signal within a correlated/anticorrelated network. This approach revealed two highly distinct maps. When the signal was delayed to better match the stimulus, large parts of the parietal cortex were anti-correlated, while the brainstem, cerebellar, and inferior temporal areas were positively correlated with the delayed seed signal. Alternatively, when the signal was advanced to better match with perception, thalamus and large parts of parietal cortex were positively correlated, while medial prefrontal cortex was anti-correlated with the delayed seed signal. As correlations are transitive, and given that putamen activity was correlated to perceived magnitude of allodynia, the networks identified were also correlated to the magnitude of allodynia. Therefore, the observed spatial-temporal change in brain connectivity reflects transmission of allodynia-related information within the brain. This demonstration poses many novel questions that need to be investigated in the future. For example, can we capture the perceptual subjective properties of chronic pain within the architecture of the brain network? More generally, can we relate network properties to clinical properties? Moreover, can we chart the relationship between regional activity and global brain connectivity, and relate these parameters to chronic pain, both as a function of time, severity and duration of chronic pain, as well as a function of therapies?
The disadvantage of fMRI, from a dynamical network perspective, is its low temporal resolution. Thus, fast events that may underlie network properties may be missed. Electroencephalographic (EEG) studies are better suited to capture the fast populational synchronization/desynchronization events related to pain perception. In this respect an elegant study recently identified in awake humans the dynamical states of acute laser evoked pain while recording from dozens of subdural electrodes that covered the surface of primary somatosensory cortex (SI), parasylvian cortex and medial frontal (MF) cortex (Ohara et al., 2006). Their results show a specific pattern of high frequency synchrony (across many electrodes) preceding the stimulus only if the subject paid attention to the stimulus. The stimulus arrival destroyed this coherent pattern and was replaced by a low frequency oscillation with strong synchrony between MF and SI, only when the subject was paying attention to the stimulus. This lower frequency synchrony was weakened when the subject was distracted. These observations are the first demonstration of populational shifts in frequency and coherence in relation to pain perception, for further comments see (Apkarian and Chialvo, 2006). There is growing literature of the use of EEG and magnetoencephalographic (MEG) studies to delineate electrical sources of activity for pain perception and the relative timings of such events. However, this work remains mainly focused on brain circuitry for acute pain (Apkarian et al., 2005; Kakigi et al., 2005).
2.4 Animal studies regarding the role of supraspinal circuitry in chronic pain
2.4.1 Ascending pathways
The spinothalamic pathway has classically been assumed to be the primary thruway for imparting nociceptive information to supraspinal targets, even though multiple other pathways have repeatedly been shown to emanate from nociceptive neurons in the spinal cord and terminate in many diverse targets. The spinoreticular, spinomesencephalic, spinoparabrachial, and spinohypothalamic pathways all transmit nociceptive information cephalad (Bernard et al., 1996; Gauriau and Bernard, 2004; Willis and Westlund, 1997). Direct spinal projections to the frontal cortex and basal ganglia have also been identified (Newman et al., 1996). More recent data indicates that the spinal basal ganglia projection is from a unique population of spinal cord neurons transmitting IB4, lamina II to lamina V pathway nociceptive information cephalad (Braz et al., 2005).
The spinothalamic tract has been subdivided to functionally distinct lateral and medial components, with the former associated with intensity and localization and the latter in the affective component of pain. This functional separation has been repeatedly used in human brain imaging studies to explain potential functional segregations for brain regions seen activated, especially on acute pain, stemming from anatomical findings that medial spinothalamic terminations project to prefrontal cortical targets, particularly the anterior cingulate (Apkarian et al., 2005), and also based on differential response properties of nociceptive neurons in the lateral versus medial thalamus. Our studies regarding chronic pain conditions cast doubt regarding the preeminence of the spinothalamic pathway in chronic pain conditions, and instead imply that other pathways may become more important as pain persists over months and years, especially in more neuropathic conditions.
Although nociceptive-responsive neurons have been found in the dorsal column pathway, at least in monkeys and rodents (Al Chaer et al., 1997), their role in acute nociception has not been considered substantial, perhaps because dorsal column lesions in humans spare pain perception, instead disrupting only touch and proprioception (Aminoff, 1996). Moreover, When the anterolateral quadrant is transected, the patient is unable to feel pain of any pathological condition on the opposite side of the body and cannot feel pain or warmth or cold when suitable stimuli are applied (Nathan and Smith, 1979; White and Sweet, 1969). As shown by Peter Nathan’s group, the clinical effects of lesions of the dorsal columns are much more complicated than those of lesions of the anterolateral quadrant. It may well be that transections confined to the dorsal columns not only disrupt an ascending pathway but also interfere with descending modulatory mechanisms. Perhaps an improved knowledge of these influences might help us understand the complex modifications of sensory perception that have been reported to follow lesions of the dorsal columns in human beings - including changes in tactile sensations, tactile and postural hallucinations and increases in sensations of pain, tickle, warmth and cold. The role of the dorsal columns in neuropathic pain is emphasized primarily in the case of tactile allodynia. Evidence indicates that tactile allodynia and thermal hyperalgesia may involve separate pathways, since complete and partial spinal cord lesions block allodynia, but not hyperalgesia, in neuropathic injured rats. Furthermore, lesions of the dorsal column and microinjecting lidocaine into dorsal column nuclei block only tactile allodynia. Conversely, thermal hyperalgesia is blocked by desensitization of C-fibers. It has therefore been proposed that tactile allodynia is mediated by large diameter A beta fibers, whereas hyperalgesia is mediated by unmyelinated C-fibers (Ossipov et al., 2000). Recent studies, however, cast doubt on this clean functional segregation. It seems that dorsal column lesions in two separate models of neuropathic pain decrease tactile sensitivity (a behavioral marker for decreased touch allodynia), as well as cold and heat hyperalgesia. Moreover, these decreases seem to be reversible, as two to three weeks following the dorsal column lesions, all neuropathic signs return back to their initial, post-peripheral nerve injury induced and prior to dorsal column lesion, heightened sensitivity (El Khoury et al., 2002; Saade et al., 2002). Thus, besides a lack of functional segregation, the latter animal studies also demonstrate the continuous reorganization of nociceptive information transmission, even weeks after the initial peripheral injury. In a sense the dorsal column lesion simply becomes another neuropathic injury that then reorganizes spinocephalad nociceptive processing.
2.4.2 Descending modulation
The role of descending modulation on acute pain has been extensively studied and reviewed (Basbaum and Fields, 1984). Recent research indicates that descending anti-nociceptive pathways projecting from the periaqueductal gray (PAG) through multiple brainstem links is complemented by additional pathways that act in a pro-nociceptive fashion as well (Lima and Almeida, 2002). Moreover, there is now good evidence that this descending modulatory circuitry may be involved in more general tasks than just adjustments to pain and injury, such as micturition and sleep (Mason, 2005). Still, two recent studies emphasize the critical role that descending projections from the brainstem may play in maintenance of neuropathic pain states. The possibility that descending facilitation from the rostral ventromedial medulla may be required for maintenance of central sensitization was examined following ablation of mu-opioid receptor-expressing cells within the region. In contrast with controls, neuropathic injured rats whose mu-opioid cells were ablated showed enhanced behaviors and touch-evoked neural activity (FOS expression) in the spinal dorsal horn at day 3, but not at days 5 and 10, post-spinal nerve ligation, indicating that once initiated, maintenance of nerve injury-induced central sensitization in the spinal dorsal horn requires descending facilitatory mechanisms arising from the rostral ventromedial medulla (Vera-Portocarrero et al., 2006). A complimentary study also showed that cells in the rostral ventromedial medulla involved in descending nociceptive modulation (on-cells, shown to be pro-nociceptive, and off-cells shown to be anti-nociceptive) exhibited enhanced activity to innocuous and noxious stimuli, consistent with the notion that these cells are involved in the maintenance of spinal cord circuitry involved in sensitization (Carlson et al., 2007). Regarding mechanisms that induce this brainstem reorganization one has to hypothesize that both ascending pathways from the spinal to the brainstem, as well as descending projections from various cortical and sub-cortical sources (Zhang et al., 2005), convergently participate in shifting the system to this new state. How and to what extent different sources of inputs affect the descending modulatory system after neuropathic injury remains to be identified. Another difficulty in attempting to correlate the activity of bulbo-spinal modulatory systems with behavioural analgesia, is that several pain descending modulation networks operate in parallel and so the net effect cannot be attributed exclusively to activity in any single network (Bee and Dickenson, 2007).
The report of stimulation-induced analgesia from the periaqueductal gray (Reynolds, 1969) unraveled the circuitry for descending modulation of nociception. This discovery also paved the way for uncovering the mechanisms underlying opiate analgesia and the description of endogenous opiate receptors and their interactions with descending modulation of nociception. This work has emphasized opiate inhibitory circuitry within the spinal cord and its modulation by opiate circuitry in the periaqueductal gray through descending pathways. However, opiate receptors have been described in many diverse brain regions as well as outside the central nervous system, and the nociceptive and anti-nociceptive function of opiates outside of descending modulation must also be recognized. Recent human brain imaging studies now provide convincing evidence that opiate receptor turnover within the thalamus, basal ganglia, and the cortex directly relate to pain and its various perceptual properties, both in acute pain (Zubieta et al., 2001; Zubieta et al., 2003) as well as in chronic pain conditions, such as fibromyalgia (Harris et al., 2007b).
2.4.3 Supraspinal reorganization with persistent pain: Evidence for an active role of the cortex in chronic pain
Animal models advanced over the last 15 years have revolutionized our understanding of chronic pain mechanisms. However, this work has for the most part concentrated on delineating abnormalities in afferent sensory inputs, spinal cord reorganization as a result of neuropathic or inflammatory injury, and changes in descending modulatory circuitry, all of which at least implicitly assume that the role of the cortex in such conditions is a passive reflection of events occurring in the spinal cord. With the advent of brain imaging studies, however, a new picture has emerged – one that clearly indicates an active role of the cortex in the processing of pain. In light of these new findings and consistent with them there is a growing literature of animal studies focusing on the full role of the central nervous system in chronic pain.
Recent animal studies show that cortical manipulations can modulate pain behavior (Baliki et al., 2003; Han and Neugebauer, 2005; Jasmin et al., 2003; Johansen and Fields, 2004; Senapati et al., 2005). Results emphasize the role of the insula, anterior cingulate, mPFC, and amygdala in pain, which are limbic structures with strong interconnectivity. Particularly relevant is a study by Johansen and Fields (Johansen and Fields, 2004) demonstrating that anterior cingulate activity is necessary and sufficient for noxious stimuli to produce an aversive memory, via a glutamate-mediated neuronal activation. Anatomically, the anterior cingulate and mPFC are in close proximity to one another and tightly interconnected. It is possible that the two structures are involved in different phases of acquisition and extinction of pain-related memory traces, and differences in brain activity patterns in clinical and acute pain conditions are consistent with this idea (Apkarian et al., 2005). Researchers have also demonstrated that the NR2B component of the NMDA receptor undergoes transient upregulation within the anterior cingulate in rats following an inflammatory injury, and administration of NR2B receptor-selective antagonists inhibit behavioral responses to peripheral inflammation (Wu et al., 2005). Two recent studies have further implicated anterior cingulate in contextual fear memory acquisition (Malin and McGaugh, 2006; Zhao et al., 2005), as well as the amygdala, hippocampus, and show NMDA in anterior cingulate is critical for fear acquisition. A similar study examined plasticity of amygdala central nucleus neurons following induction of arthritis and showed that pain-related synaptic plasticity is accompanied by protein kinase A (PKA)-mediated enhanced NMDA-receptor function and increased phosphorylation of NMDA-receptor 1 (NR1) subunits. Synaptic plasticity in the arthritis pain model, but not normal synaptic transmission in control neurons, was inhibited by a selective NMDA receptor antagonist (Bird et al., 2005). These results provide solid evidence that NMDA receptors undergo long-term plastic changes in the brain after injury, and contribute to persistent pain by changing neuronal activity. Consistent and complementary to these results we have evidence that rats with neuropathic injury show increased expression of cytokines in the prefrontal cortex and thalamus/striatum (Apkarian et al., 2006). The temporal relationship of these changes and differences between neuropathic and inflammatory conditions remain unknown and remain to be tackled. Nevertheless, it appears clear that the anterior cingulate is critical for fear acquisition, and investigation of the NMDA’s role in this region and others will be vital to understanding cortical mechanisms of pain.
2.5 Pain and memory
2.5.1 Pain and learning and memory are intimately related, and chronic pain can be defined in this context
Chronic pain is defined as a state of continued suffering, sustained long after the initial inciting injury has healed (Merskey and Bogduk, 1994). In terms of learning and memory one could recast this definition as: Chronic pain is a persistence of the memory of pain and/or the inability to extinguish the memory of pain evoked by an initial inciting injury. From this viewpoint the peripheral afferent barrage can be considered as part of the inciting event and the central representation/reorganization/sensitization can be viewed as the memory trace; relative contributions of each would then delineate types of pain conditions (acute, inflammatory, neuropathic) within the framework of mechanisms of memory of pain.
Part of the survival value of pain is its intimate association with learning. Pain induces single event learning, the memory of which can last for the rest of life; the saying that “one only burns the finger once” is literally true. This property is taken advantage of in many Pavlovian paradigms to study learning and memory, especially in fear conditioning where the more painful the unconditioned stimulus (US) the fewer trials it takes to establish an aversive negative emotional association to a conditioning stimulus (CS) that was originally affectively neutral (Schafe et al., 2001). The ability to extinguish aversive associations of fearful or painful events with repeated exposure to the unconditioned stimulus is also important for normal behavior; impaired ability to extinguish is clinically relevant (e.g., in people suffering from phobias, panic disorder, and post-traumatic stress disorder), and its mechanisms have recently become a major focus of research (Myers and Davis, 2002; Sotres-Bayon et al., 2004). The novel hypothesis that we advance is that chronic pain is a state of continuous learning, in which aversive emotional associations are continuously made with incidental events simply due to the persistent presence of pain. Simultaneously, continued presence of pain does not provide an opportunity for extinction because whenever the subject is re-exposed to the conditioned event he/she is still in pain. Failing to extinguish, therefore, makes the event become a reinforcement of aversive association. A concrete example might further clarify this idea: A person with chronic pain enters his/her bedroom. Depending on the intensity of the pain at that moment, the person establishes a negative association with the bedroom (place conditioned avoidance), and the stronger the pain the longer lasting will be the memory of this negative association. Given that the person has pain all the time, whenever he/she re-enters the bedroom, the negative association is reinforced, and thus there is no chance for extinction (erasing the negative association) by exposure to the space in the absence of pain. Of course, the person is rationally aware that the bedroom is not the source of the pain but his/her emotional system continues to provide contradictory cues. We propose that living with this conundrum and the effort of disentangling its associations underlies at least some of the suffering of chronic pain, and comprises an important component of the cortical negative emotional impact of neuropathic pain.
If one regards chronic pain as continuous presence of an unconditioned stimulus and as an inability to extinguish its associations with random events, then the brain circuitry underlying reward/punishment induced learning must be in a heightened state. Moreover, this state should interfere with or decrease the ability of associative learning for other events, especially for associations mediated through emotional cues.
Taking the discussion of learning and memory more broadly inevitably leads to the topic of nature vs. nurture in relation to pain. Learned associations that reinforce and sustain pain in the chronic state might also be coupled with genetic predispositions. Indeed, there is now data implicating specific genes in various pain phenotypes (Lacroix-Fralish et al., 2007). While recent genetics research on pain remains for the most part unclear, particularly regarding brain derived signals in human clinical conditions; it is an unavoidable topic that will need to be addressed in the near future.
2.5.2 Novel pharmacotherapeutic approaches can be advanced by accounting for the role of the cortex in chronic pain
If the above notions are true, then one should be able to demonstrate novel pharmacological therapies for chronic pain. We tested this by examining the effects of treatment of neuropathic injured rats with D-cycloserine (Millecamps et al., 2006). D-cycloserine (DCS) given systemically or centrally enhances cognitive processes (Stromme and Myhrer, 2002), improves attention and memory (Hughes, 2004; Vertes, 2006), and facilitates fear extinction (Richardson et al., 2004; Walker et al., 2002) through de novo memory trace formation involving n-methyl-D-aspartic acid (NMDA) plasticity (Falls et al., 1992; Milad and Quirk, 2002; Santini et al., 2004). We tested the effects of DCS on chronic neuropathic pain behavior, hypothesizing that it should enhance the extinction of pain-related memories and, thus, exhibit antinociceptive properties for neuropathic pain. The main finding was that repeated treatment with DCS, a partial agonist at the strychnine-insensitive glycine-recognition site on the NMDA receptor complex (Furukawa and Gouaux, 2003), reduces tactile sensitivity and protective paw posturing in rat models of neuropathic pain. This antinociception was dose-dependent and increased in efficacy for up to three weeks. Upon cessation of treatment, DCS effects on pain behavior persisted for a duration proportional to the length of treatment. When antinociception was assessed by measuring changes in mechanical sensitivity, the effect of the treatment was relatively small; however, a much larger effect of DCS treatment was revealed when antinociception was assessed by an operant stimulus avoidance task. Moreover, selective infusions of DCS into mPFC and amygdala (but not the spinal cord, thalamus, insula, or occipital cortices) were antinociceptive, and dependent on NMDA receptor availability. We presume that DCS-induced reinforcement of NMDA-receptor mediated transmission within mPFC works to disengage spontaneous pain from its associations previously formed in learning and memory. While there is as of yet no data as to the efficacy of this drug treatment in human chronic pain, we hope that initial human clinical trials will begin sometime this year.
2.6 A working model for chronic pain
Based on brain activity as well as changes in chemistry and morphometry of the human brain in chronic pain, as well as the recent evidence from animal studies, we propose a general working model for chronic pain (figure 3). It hinges on two fundamental hypotheses. The first is that chronic, particularly neuropathic, pain in contrast with acute, subacute, and inflammatory pain states involves distinct spinal cord nociceptive neurons with distinct supraspinal projections, resulting in distinct supraspinal modifications of pain and hedonic circuitry (figure 3, red = inflammatory, impinging mainly on sensory pathways for nociception; blue = neuropathic, impinging more on affective and hedonic pathways), and that the perception of chronic pain is an integrated sensory, emotional and hedonic construct, where threat value assessment and memory traces of pain directly modulate the extent to which a pain condition is rendered affective or sensory. The second hypothesis is that the transition from acute to chronic pain involves a time dependent neural reorganization that initiates a series of events that potentiate one pathway at the cost of the other. The temporal component should be interacting with the specific peripheral and central parameters where the injury related reorganization and experience related reorganization together shift the brain circuitry to distinct states in specific clinical chronic pain conditions. This model is an expansion of earlier models emphasizing the medial and lateral spinothalamic pathways, and takes into consideration many brain regions that have not been thought to be pain specific. The main conclusion of the model is that the transition from acute to chronic pain entails also a transition in the salience of pain, wherein the condition shifts from viewing a painful percept as a sign of external threat into an indication of an internalized disease state.
Figure 3.
A simplified working diagram of the main theoretical construct upon which this review is based. The afferent inputs and their segregation in the spinal cord, brainstem and thalamus is from (Braz et al., 2005); cortical connectivity is derived from Price (Price, 2000) and Apkarian et al. (Apkarian et al., 2005); and based on our fMRI studies of clinical pain patient populations. The cartoon is basically an expansion of a diagram originally proposed by (Melzack and Casey, 1968). The interaction between basal ganglia (Gl Pal), amygdala (Amyg), and medial prefrontal cortex (mPFC) constitutes the emotional, motivational and hedonic components that we hypothesize influence the quality of perceived pain and also modulate nociceptive processing at the spinal cord level through descending pathways. Blue pathways engage motivation, hedonics and affect. Red pathways are more involved in sensory coding, including inputs to the thalamus, primary and secondary somatosensory cortices (s1 and s2), and insula. The interaction between mPFC and dorsolateral prefrontal cortex (DLPFC) is mutually inhibitory. We hypothesize that blue pathways are strengthened in chronic neuropathic pain and red pathways are more involved in acute pain. The specific interactions between ascending and descending modulations would determine the specific brain activity patterns identified in distinct chronic pain conditions, which would reflect peripheral as well as learned central reorganization. The descending pathway indicated through the periaqueductal gray (PAG) in fact comprises of a multipliscity of descending projections.
3. Conclusions
If one assumes the simple notion that the brain is a dynamical network, wherein detailed connectivity is constantly modified by the instantaneous experience of the organism, then it should be evident that quantifying chronic pain as an outflow of spinal cord processing (and primarily focusing on spinothalamic pathway transmission), is simplistic and inadequate.
Observations of apoptosis in the spinal cord of neuropathic rats parallel recent findings of decreased brain regional gray matter density in human chronic pain conditions (Apkarian and Scholz, 2006). These findings indicate that the brain in chronic pain is a distinct state with properties that may not be reversible. The specific mechanisms underlying brain regional atrophy remain to be determined. However, part of this process may be initiated by the apoptotic events observed in the spinal cord.
The observation that brain activity for performing a trivial task is not the same between healthy subjects and chronic pain patients further reinforces the last point. Adding support are recent data indicating that medial thalamic nociceptive signals access large portions of the cortical mantle, particularly the prefrontal cortical superficial layers, allowing modification of cortical activity in a widespread manner (Monconduit and Villanueva, 2005). Taken together these findings substantiate the conclusion that the salience of pain in chronic and acute conditions has marked implications for the organism.
Integrating the evidence from human and animal studies, we presented an overall working model for the transition from acute to chronic pain. One strength of our model is that it can be adapted to specific clinical conditions, and hence mechanistically reflect differences between patients who live with pain to varying extents. The details of this model and its final state should be different for different clinical conditions and reflect the experience of a life lived with pain. Can we actually devise methods with which we can read past and present pain in individual patients by simply reading the mind? Research findings on the interrelationships and correlations between brain and clinical parameters (section 2.2.5), the advancing discoveries in animal research (section 2.4), and the ever-increasing sophistication of brain imaging techniques indicate this idea is a very real possibility. Finally we conclude by quoting Peter Nathan (Nathan, 1976) (provided by one of the reviewers of this manuscript): “Ideas need to be fruitful; they do not have to be right. And, curiously enough, the two do not necessarily go together.”
Acknowledgments
The authors thank all the patients and volunteers who have contributed to the science we have generated over the last decade or so. We also thank Elle Parks for commenting on earlier versions of this manuscript. This work was supported by NIH NINDS grants NS35115, NS53602, NS57704.
Abrreviation List
- CRPS
complex regional pain syndrome
- CCI
chronic constriction injury
- NSAIDs
non-steroidal anti-inflammatory drugs
- DLPFC
dorsolateral prefrontal cortex
- PET
positron emission tomography
- D2
dopamine receptor type 2
- CBP
chronic back pain
- D
fractal dimension
- fMRI
functional magnetic resonance imaging
- mPFC
medial prefrontal cortex
- ACC
anterior cingulate
- T1
MRI imaging for anatomical structures
- MRS
magnetic resonance spectroscopy
- DTI
diffusion tensor imaging
- NAA
N-acetyl aspartate
- PHN
post herpetic neuralgia
- OA
osteoarthritis
- PP
pelvic pain
- S2
secondary somatosensory cortex
- COX2
cyclo-oxygenase 2
- RSN
resting state network
- EEG
electroencephalography
- S1
primary somatosensory cortex
- MEG
magnetoencephalography
- NMDA
N-methyl-D-aspartate
- NR2B
a subunit of the NMDA receptor
- PKA
protein kinase A
- DCS
D-cycloserine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Chaer ED, Westlund KN, Willis WD. Nucleus gracilis: an integrator for visceral and somatic information. J Neurophysiol. 1997;78:521–527. doi: 10.1152/jn.1997.78.1.521. [DOI] [PubMed] [Google Scholar]
- Aminoff MJ. Historical perspective Brown-Sequard and his work on the spinal cord. Spine. 1996;21:133–140. doi: 10.1097/00007632-199601010-00029. [DOI] [PubMed] [Google Scholar]
- Andersson GBJ. The epidemiology of spinal disorders. In: Frymoyer JW, editor. The adult spine: principles and practice. Lippincott-Raven; Philadelphia: 1997. pp. 93–141. [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Chialvo DR. The shadows of pain. Pain. 2006;123:221–222. doi: 10.1016/j.pain.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Lavarello S, Randolf A, Berra HH, Chialvo DR, Besedovsky HO, Del Rey A. Expression of IL-1beta in supraspinal brain regions in rats with neuropathic pain. Neurosci Lett. 2006;407:176–181. doi: 10.1016/j.neulet.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Scholz J. Shared mechanisms between chronic pain and neurodegenerative disease. Drug Discovery Today: Disease Mechanisms. 2006;3:319–326. [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden R, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004a;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden R, Parrish T, Gitelman D. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004b;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audette JF, Emenike E, Meleger AL. Neuropathic low back pain. Curr Pain Headache Rep. 2005;9:168–177. doi: 10.1007/s11916-005-0058-8. [DOI] [PubMed] [Google Scholar]
- Baliki M, Al Amin HA, Atweh SF, Jaber M, Hawwa N, Jabbur SJ, Apkarian AV, Saade NE. Attenuation of neuropathic manifestations by local block of the activities of the ventrolateral orbito-frontal area in the rat. Neuroscience. 2003;120:1093–1104. doi: 10.1016/s0306-4522(03)00408-1. [DOI] [PubMed] [Google Scholar]
- Baliki M, Geha PY, Apkarian AV. The neural basis for magnitude perception. J Neurosci. 2008a submitted. [Google Scholar]
- Baliki M, Katz J, Chialvo DR, Apkarian AV. Single subject pharmacological-MRI (phMRI) study: Modulation of brain activity of psoriatic arthritis pain by cyclooxygenase-2 inhibitor. Mol Pain. 2005 doi: 10.1186/1744-8069-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008b;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neuroscience. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bester H, Besson JM. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog Brain Res. 1996;107:243–255. doi: 10.1016/s0079-6123(08)61868-3. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol. 2005;564:907–921. doi: 10.1113/jphysiol.2005.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bogduk N. Pharmacological alternatives for the alleviation of back pain 1. Expert Opin Pharmacother. 2004;5:2091–2098. doi: 10.1517/14656566.5.10.2091. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clays E, De Bacquer D, Leynen F, Kornitzer M, Kittel F, De Backer G. The impact of psychosocial factors on low back pain: longitudinal results from the Belstress study. Spine. 2007;32:262–268. doi: 10.1097/01.brs.0000251884.94821.c0. [DOI] [PubMed] [Google Scholar]
- Coats TL, Borenstein DG, Nangia NK, Brown MT. Effects of valdecoxib in the treatment of chronic low back pain: results of a randomized, placebo-controlled trial. Clin Ther. 2004;26:1249–1260. doi: 10.1016/s0149-2918(04)80081-x. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Deyo RA. Low-back pain. Sci Am. 1998;279:48–53. doi: 10.1038/scientificamerican0898-48. [DOI] [PubMed] [Google Scholar]
- Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007;104:1223–9. doi: 10.1213/01.ane.0000263280.49786.f5. tables. [DOI] [PubMed] [Google Scholar]
- Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain. 2002;18:343–349. doi: 10.1097/00002508-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Eguiluz VM, Chialvo DR, Cecchi GA, Baliki M, Apkarian AV. Scale-free brain functional networks. Phys Rev Lett. 2005;94:018102. doi: 10.1103/PhysRevLett.94.018102. [DOI] [PubMed] [Google Scholar]
- Ehrlich GE. Low back pain. Bull World Health Organ. 2003;81:671–676. [PMC free article] [PubMed] [Google Scholar]
- El Khoury C, Hawwa N, Baliki M, Atweh SF, Jabbur SJ, Saade NE. Attenuation of neuropathic pain by segmental and supraspinal activation of the dorsal column system in awake rats. Neuroscience. 2002;112:541–553. doi: 10.1016/s0306-4522(02)00111-2. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Rossignol M, Shrier I, Abenhaim L. Smoking. A risk factor for development of low back pain in adolescents. Spine. 1999;24:2492–2496. doi: 10.1097/00007632-199912010-00011. [DOI] [PubMed] [Google Scholar]
- Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006;95:730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- Frank A. Low back pain. BMJ. 1993;306:901–909. doi: 10.1136/bmj.306.6882.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen M, Woodward M, Norton R, Coggan C, Dawe M, Sheridan N. Risk factors associated with the transition from acute to chronic occupational back pain. Spine. 2002;27:92–98. doi: 10.1097/00007632-200201010-00022. [DOI] [PubMed] [Google Scholar]
- Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24:75–79. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol. 2004;468:24–56. doi: 10.1002/cne.10873. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Wang X, Harden RN, Paice JA, Apkarian AV. Brain dynamics for perception of tactile allodynia (touch-induced pain) in postherpetic neuralgia. Pain. 2008;138:641–656. doi: 10.1016/j.pain.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Godbolt AK, Waldman AD, MacManus DG, Schott JM, Frost C, Cipolotti L, Fox NC, Rossor MN. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology. 2006;66:718–722. doi: 10.1212/01.wnl.0000201237.05869.df. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Aalto S, Rinne JO, Scheinin H, Taiminen T, Nagren K, Eskola O, Jaaskelainen SK. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003a;106:43–48. doi: 10.1016/s0304-3959(03)00275-6. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, Luutonen S, Nagren K, Jaaskelainen S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003b;101:149–154. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- Hagelberg N, Jaaskelainen SK, Martikainen IK, Mansikka H, Forssell H, Scheinin H, Hietala J, Pertovaara A. Striatal dopamine D2 receptors in modulation of pain in humans: a review. Eur J Pharmacol. 2004;500:187–192. doi: 10.1016/j.ejphar.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hagen KB, Jamtvedt G, Hilde G, Winnem MF. The updated cochrane review of bed rest for low back pain and sciatica. Spine. 2005;30:542–546. doi: 10.1097/01.brs.0000154625.02586.95. [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Harris IA, Young JM, Rae H, Jalaludin BB, Solomon MJ. Factors associated with back pain after physical injury: a survey of consecutive major trauma patients. Spine. 2007a;32:1561–1565. doi: 10.1097/BRS.0b013e318067dce8. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007b;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- Hartvigsen J, Christensen K, Frederiksen H, Petersen HC. Genetic and environmental contributions to back pain in old age: a study of 2,108 danish twins aged 70 and older. Spine. 2004;29:897–901. doi: 10.1097/00007632-200404150-00015. [DOI] [PubMed] [Google Scholar]
- Heneweer H, Aufdemkampe G, van Tulder MW, Kiers H, Stappaerts KH, Vanhees L. Psychosocial variables in patients with (sub)acute low back pain: an inception cohort in primary care physical therapy in The Netherlands. Spine. 2007;32:586–592. doi: 10.1097/01.brs.0000256447.72623.56. [DOI] [PubMed] [Google Scholar]
- Heymans MW, de Vet HC, Knol DL, Bongers PM, Koes BW, van Mechelen W. Workers’ beliefs and expectations affect return to work over 12 months. J Occup Rehabil. 2006;16:685–695. doi: 10.1007/s10926-006-9058-8. [DOI] [PubMed] [Google Scholar]
- Hughes RN. Responsiveness to brightness change in male and female rats following treatment with the partial agonist of the N-methyl-D-aspartate receptor, D-cycloserine. Behav Brain Res. 2004;152:199–207. doi: 10.1016/j.bbr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Jellema P, van der Windt DA, van der Horst HE, Blankenstein AH, Bouter LM, Stalman WA. Why is a treatment aimed at psychosocial factors not effective in patients with (sub)acute low back pain? Pain. 2005;118:350–359. doi: 10.1016/j.pain.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Inui K, Tamura Y. Electrophysiological studies on human pain perception. Clin Neurophysiol. 2005;116:743–763. doi: 10.1016/j.clinph.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Keeley P, Creed F, Tomenson B, Todd C, Borglin G, Dickens C. Psychosocial predictors of health-related quality of life and health service utilisation in people with chronic low back pain. Pain. 2008;135:142–150. doi: 10.1016/j.pain.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131:3–4. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Lima D, Almeida A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Prog Neurobiol. 2002;66:81–108. doi: 10.1016/s0301-0082(01)00025-9. [DOI] [PubMed] [Google Scholar]
- Lombard MC, Nashold BS, Jr, be-Fessard D, Salman N, Sakr C. Deafferentation hypersensitivity in the rat after dorsal rhizotomy: a possible animal model of chronic pain. Pain. 1979;6:163–174. doi: 10.1016/0304-3959(79)90123-4. [DOI] [PubMed] [Google Scholar]
- MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51:160–167. doi: 10.1002/art.20236. [DOI] [PubMed] [Google Scholar]
- Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manek NJ, MacGregor AJ. Epidemiology of back disorders: prevalence, risk factors, and prognosis. Curr Opin Rheumatol. 2005;17:134–140. doi: 10.1097/01.bor.0000154215.08986.06. [DOI] [PubMed] [Google Scholar]
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- Melzack R, Casey K. Sensory, motivational, and central contol determinants of pain. In: Kenshalo DR, editor. The skin senses. Charles C. Thomas; Springfield: 1968. pp. 423–443. [Google Scholar]
- Merskey H, Bogduk N. Classification of chronic pain 1994 [Google Scholar]
- Meyer T, Cooper J, Raspe H. Disabling low back pain and depressive symptoms in the community-dwelling elderly: a prospective study. Spine. 2007;32:2380–2386. doi: 10.1097/BRS.0b013e3181557955. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2007;132:108–123. doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monconduit L, Villanueva L. The lateral ventromedial thalamic nucleus spreads nociceptive signals from the whole body surface to layer I of the frontal cortex. Eur J Neurosci. 2005;21:3395–3402. doi: 10.1111/j.1460-9568.2005.04160.x. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Nathan PW. The gate-control theory of pain. A critical review. Brain. 1976;99:123–158. doi: 10.1093/brain/99.1.123. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. Clinico-anatomical correlation in anterolateral cordotomy. In: Bonica JJ, editor. Advances in pain research and therapy. Raven press; New York: 1979. pp. 921–926. [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Okuda T, Baba I, Fujimoto Y, Tanaka N, Sumida T, Manabe H, Hayashi Y, Ochi M. The pathology of ligamentum flavum in degenerative lumbar disease. Spine. 2004;29:1689–1697. doi: 10.1097/01.brs.0000132510.25378.8c. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann N Y Acad Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- Pallay RM, Seger W, Adler JL, Ettlinger RE, Quaidoo EA, Lipetz R, O’Brien K, Mucciola L, Skalky CS, Petruschke RA, Bohidar NR, Geba GP. Etoricoxib reduced pain and disability and improved quality of life in patients with chronic low back pain: a 3 month, randomized, controlled trial. Scand J Rheumatol. 2004;33:257–266. doi: 10.1080/03009740410005728. [DOI] [PubMed] [Google Scholar]
- Pattany PM, Yezierski RP, Widerstrom-Noga EG, Bowen BC, Martinez-Arizala A, Garcia BR, Quencer RM. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am J Neuroradiol. 2002;23:901–905. [PMC free article] [PubMed] [Google Scholar]
- Praemer A, Furnes S, Rice DP. Musculoskeletal conditions in the United States. AAOS; Rosemont: 1992. pp. 1–99. [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Pye SR, Reid DM, Smith R, Adams JE, Nelson K, Silman AJ, O’Neill TW. Radiographic features of lumbar disc degeneration and self-reported back pain. J Rheumatol. 2004;31:753–758. [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MC, Williams CS, Gill TM. The relationship between psychological factors and disabling musculoskeletal pain in community-dwelling older persons. J Am Geriatr Soc. 2003;51:1092–1098. doi: 10.1046/j.1532-5415.2003.51357.x. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Saade NE, Baliki M, El Khoury C, Hawwa N, Atweh SF, Apkarian AV, Jabbur SJ. The role of the dorsal columns in neuropathic behavior: evidence for plasticity and non-specificity. Neuroscience. 2002;115:403–413. doi: 10.1016/s0306-4522(02)00417-7. [DOI] [PubMed] [Google Scholar]
- Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Penad O, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- Schnitzer TJ, Ferraro A, Hunsche E, Kong SX. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage. 2004;28:72–95. doi: 10.1016/j.jpainsymman.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Schultz IZ, Crook J, Meloche GR, Berkowitz J, Milner R, Zuberbier OA, Meloche W. Psychosocial factors predictive of occupational low back disability: towards development of a return-to-work model. Pain. 2004;107:77–85. doi: 10.1016/j.pain.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Bountra C, Tracey I. Pharmacological FMRI in the development of new analgesic compounds. NMR Biomed. 2006;19:702–711. doi: 10.1002/nbm.1076. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007;97:3651–3659. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- Senapati AK, Lagraize SC, Huntington PJ, Wilson HD, Fuchs PN, Peng YB. Electrical stimulation of the anterior cingulate cortex reduces responses of rat dorsal horn neurons to mechanical stimuli. J Neurophysiol. 2005;94:845–851. doi: 10.1152/jn.00040.2005. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez J, Corbetta M, Buckner R, Miezin FM, Raichle ME, Petersen S. Common blood flow changes across visual task: II decreases in cerebral cortex. J Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci U S A. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren P, Christrup LL, Petersen MA, Hojsted J. Neuropsychological assessment of chronic non-malignant pain patients treated in a multidisciplinary pain centre. Eur J Pain. 2005;9:453–462. doi: 10.1016/j.ejpain.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care. 2008;31:980–981. doi: 10.2337/dc07-2088. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine. 2003;28:2540–2545. doi: 10.1097/01.BRS.0000092372.73527.BA. [DOI] [PubMed] [Google Scholar]
- Stromme JT, Myhrer T. Impaired visual memory in rats reared in isolation is reversed by D-cycloserine in the adult rat. Eur J Pharmacol. 2002;437:73–77. doi: 10.1016/s0014-2999(02)01282-7. [DOI] [PubMed] [Google Scholar]
- van den Bosch MA, Hollingworth W, Kinmonth AL, Dixon AK. Evidence against the use of lumbar spine radiography for low back pain. Clin Radiol. 2004;59:69–76. doi: 10.1016/j.crad.2003.08.012. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Scholten RJ, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000;25:2501–2513. doi: 10.1097/00007632-200010010-00013. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Wand BM, O’Connell NE. Chronic non specific low back pain - Subgrouping or a single mechanism? BMC Musculoskelet Disord. 2008:9–11. doi: 10.1186/1471-2474-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Sweet WH. Pain and the neurosurgeon. CC Thomas; New York: 1969. [Google Scholar]
- Willburger RE, Ehiosun UK, Kuhnen C, Kramer J, Schmid G. Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine. 2004;29:1655–1661. doi: 10.1097/01.brs.0000133645.94159.64. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood PB, Patterson JC, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: A randomized controlled clinical trial. Pain. 2008;136:271–280. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Plasticity and pain: role of the dorsal hon. In: McMahon SB, Koltzenburg M, editors. Textbook of pain. Churchill-Livingstone; New York: 2006. pp. 91–105. [Google Scholar]
- Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25:11107–11116. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H, Malick A, Chamberlin NL, Burstein R. Cardiovascular and neuronal responses to head stimulation reflect central sensitization and cutaneous allodynia in a rat model of migraine. J Neurophysiol. 1999;81:479–493. doi: 10.1152/jn.1999.81.2.479. [DOI] [PubMed] [Google Scholar]
- Yezierski RP. Spinal cord injury: a model of central neuropathic pain. Neurosignals. 2005;14:182–193. doi: 10.1159/000087657. [DOI] [PubMed] [Google Scholar]
- Young CC, Greengerg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: A model including cognitive, affective, and trauma factors. Pain. 2008;134:69–79. doi: 10.1016/j.pain.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Zhao ZQ. Anterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleus. Eur J Neurosci. 2005;22:1141–1148. doi: 10.1111/j.1460-9568.2005.04302.x. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]