Abstract
An Arabidopsis (Arabidopsis thaliana) double mutant impaired in starch biosynthesis and the triose phosphate/phosphate translocator (adg1-1/tpt-1) is characterized by a diminished utilization of photoassimilates and the concomitant consumption of reducing power and energy produced in the photosynthetic light reaction. In order to guarantee survival, the double mutant responds to this metabolic challenge with growth retardation, an 80% decline in photosynthetic electron transport, diminished chlorophyll contents, an enhanced reduction state of plastoquinone in the dark (up to 50%), a perturbation of the redox poise in leaves (increased NADPH/NADP ratios and decreased ascorbate/dehydroascorbate ratios), hyperstacking of grana thylakoids, and an increased number of plastoglobules. Enhanced oxygen consumption and applications of inhibitors of alternative mitochondrial and chloroplast oxidases (AOX and PTOX) suggest that chlororespiration as well as mitochondrial respiration are involved in the enhanced plastoquinone reduction state in the dark. Transcript amounts of PTOX and AOX were diminished and nucleus-encoded components related to plastidic NADH reductase (NDH1) were increased in adg1-1/tpt-1 compared with the wild type. Cytochrome b559, proposed to be involved in the reoxidation of photosystem II, was not regulated at the transcriptional level. The hyperstacking of grana thylakoids mimics adaptation to low light, and increased plastoglobule numbers suggest a response to enhanced oxidative stress. Altered chloroplast organization combined with perturbations in the redox poise suggests that adg1-1/tpt-1 could be a tool for the in vivo study of retrograde signaling mechanisms controlling the coordinated expression of nucleus- and plastome-encoded photosynthetic genes.
The triose phosphate/phosphate translocator (TPT) is the major metabolite transporter of the chloroplast inner envelope membrane (Flügge et al., 2003) and is believed to be the main exporter of photoassimilates in the light. However, a reduced activity of the TPT by antisense inhibition in potato (Solanum tuberosum; Riesmeier et al., 1993; Heineke et al., 1994) or tobacco (Nicotiana tabacum; Häusler et al., 1998, 2000a, 2000b) plants and, more recently, investigations of the Arabidopsis (Arabidopsis thaliana) tpt-1 mutant (Schneider et al., 2002) suggested that even an almost complete absence of the TPT in tpt-1 could be compensated by an increased turnover of transitory starch and consequently the export of maltose and Glc (Weise et al., 2004) in the light. The proposed increase in daytime starch mobilization was independently demonstrated by Walters et al. (2004) with the same mutant allele (ape2 [for acclimation of photosynthesis to the environment2] = tpt-1) under high light but not under low light. Interestingly, ape2 has been isolated in a forward genetics screen for mutants with an impaired acclimation to high light intensities (Walters et al., 2003).
In order to address constraints in primary metabolism in the tpt-1 mutant background (Schneider et al., 2002), the importance of starch as a temporary carbohydrate buffer was tested by genetic crosses of tpt-1 with adg1-1, a starch-free mutant that lacks the catalytic subunit of ADP-Glc pyrophosphorylase (Lin et al., 1988) or with sex1-1 (Caspar et al., 1991), a starch excess mutant deficient in glucan, water dikinase (Yu et al., 2001; Ritte et al., 2002), resulting in a low or abolished starch mobilization. The adg1-1/tpt-1 double mutant is severely retarded in growth and hence reduced in final size. It exhibits maximum photosynthetic electron transport rates of only 10% to 20% of the wild type or the single mutants (Schneider et al., 2002) and resembles phenotypically a transgenic potato line with a combined antisense repression of the TPT and AGPase (Hattenbach et al., 1997). As the transcript level of the TPT gene was diminished, but not completely absent in tpt-1, it is likely that the viability and fertility of adg1-1/tpt-1 is based on the low residual TPT activity. In tpt-1, a TPT transport activity of up to 10% of the wild type could be determined in 4-week-old plants (Schneider et al., 2002). Moreover, adg1-1/tpt-1 experiences the same light intensities as the single mutants or the wild type during growth. It is questionable, therefore, how the adg1-1/tpt-1 mutant can survive in conditions in which the Calvin cycle, as the major sink for ATP and NADPH, is hampered by the largely diminished capacity to export the fixed carbon in the form of triose phosphates combined with the lack of ability to direct the fixed carbon into the biosynthesis of starch.
Here, the consequences of diminished photoassimilate utilization on photosynthesis, the redox poise, and the chloroplast ultrastructure are elucidated. It is shown that adg1-1/tpt-1 exhibits an altered contribution of chlororespiration as well as mitochondrial respiration in the dark. Chlororespiration is a transfer of electrons from NAD(P)H or other reduced metabolic intermediates to molecular oxygen at the thylakoid membrane involving the plastoquinone (PQ) pool and components of the thylakoid membrane like the chloroplast alternative oxidase (PTOX) and the largely plastome-encoded NADH dehydrogenase complex (NDH1). Both components have been characterized at the molecular level in Arabidopsis and other species (for reviews, see Peltier and Cournac, 2002; Rumeau et al., 2007). Ultrastructural analyses of the double mutant compared with the single mutants revealed a hyperstacking of grana thylakoids and an increased number of plastoglobules. It is conceivable that the coordinated expression of nucleus- and plastome-encoded photosynthetic genes is impaired and adapts to the new requirements in adg1-1/tpt-1, which guarantees the survival of the plant and which could form a platform for the in vivo analysis of retrograde redox signals.
RESULTS
High Chlorophyll a Ground Fluorescence Revealed an up to 50% Reduction of QA in Dark-Kept adg1-1/tpt-1 Plants
The severe impairment of leaf primary metabolism in the adg1-1/tpt-1 double mutant resulted in an 80% diminished maximum photosynthetic electron transport rate compared with the single mutants and the wild type (Schneider et al., 2002). Measurements of modulated chlorophyll a (Chl a) fluorescence also indicate that the ratio of variable to maximum Chl a fluorescence yield (Fv/Fm) was significantly lower in adg1-1/tpt-1 (by about 45%) compared with the wild type or the single mutants (Table I). This decline in Fv/Fm was due to a drastically enhanced Fo (ground fluorescence) rather than a diminished Fm (Table I), as would be indicative for photoinhibition or the onset of senescence (Maxwell and Johnson, 2000). The increase in the Fo value observed in adg1-1/tpt-1 indicates a partial reduction of QA, the primary quinone electron acceptor of PSII, and hence also of PQ in the dark (Kruk and Karpinski, 2006). Oxidized QA is capable of quenching Fv almost completely (i.e. Fv is close to zero in the dark-adapted state), which is the case for the wild type and both single mutants. However, in adg1-1/tpt-1 plants, variable fluorescence in addition to Fo contributed significantly to the dark Chl a fluorescence yield, indicating a pronounced reduced state of QA. From determinations of Fo values in the presence or absence of the PSII inhibitor dichlorophenyl dimethyl urea, it was estimated that QA was reduced by up to 50% in dark-kept adg1-1/tpt-1 plants compared with the wild type (data not shown).
Table I.
Chl a fluorescence parameters of dark-adapted wild-type, tpt-1, adg1-1, and adg1-1/tpt-1 plants
The absolute values of the Chl a fluorescence parameters Fo and Fm represent voltage changes measured by the pulse amplitude modulation fluorometer. Care was taken that the leaf areas of wild-type and mutant plants used for Chl a fluorescence determinations were the same. The data represent means of five measurements. The numbers in parentheses indicate fold changes in the individual parameters relative to the wild type.
| Plant | Fo | Fm | Fv/Fm |
|---|---|---|---|
| mV | |||
| Wild type | 280 ± 30 (1.00) | 1,350 ± 90 (1.00) | 0.79 ± 0.01 (1.00) |
| tpt-1 | 250 ± 20 (0.89) | 1,220 ± 5 (0.91) | 0.80 ± 0.10 (1.01) |
| adg1-1 | 240 ± 20 (0.86) | 1,100 ± 10 (0.81) | 0.78 ± 0.02 (0.98) |
| adg1-1/tpt-1 | 680 ± 5 (2.41)a | 1,240 ± 190 (0.92) | 0.45 ± 0.09 (0.56)a |
Significantly different (P < 0.001) as calculated using the Welch test.
Specific excitation of PSI reaction centers by far red (FR) illumination resulted only in a minor quenching of Fo (5%) in adg1-1/tpt-1 plants (Fig. 1A), suggesting limitations on PSI electron acceptors (i.e. NADP) and/or a fraction of reduced QA not accessible to reoxidation by PSI. The slow recovery of Fo after switching off FR light most likely reflects a back transfer of electrons to the QA/PQ system. In contrast, quenching of Fo by FR illumination was absent in the wild type (Fig. 1B), indicating that QA was completely oxidized in the dark-adapted state. There was no further decline of Fo detected in adg1-1/tpt-1 when the duration of FR illumination was extended to up to 2 h (data not shown). Even long-term illumination (140 min) with an extremely high photon flux density (PFD) of FR light (1,300 μmol m−2 s−1) had little additional effect on Fo quenching (15%), and it also resulted in a 5% Fm quenching (data not shown). Again, these data suggest an electron acceptor limitation at PSI or a fraction of QA, which is completely reduced but not accessible to reoxidation by PSI. The increased reduction state of QA in dark-kept adg1-1/tpt-1 plants most likely reflects a high electron pressure on the thylakoid redox system combined with a limitation on the electron flow from a donor to O2 via PQ in a process known as chlororespiration, involving PTOX and NDH1 (Peltier and Cournac, 2002; Rumeau et al., 2007).
Figure 1.
Typical traces of modulated Chl a fluorescence determined with adg1-1/tpt-1 or wild-type plants after 20 min of dark adaptation (A and B) or in actinic light (C and D). The arrows indicate the time points when FR illumination or actinic light (AL) was turned on or off. The spikes in the kinetics during illumination indicate the applications of saturated light pulses. The dashed lines represent Fo and Fm determined in the dark-adapted state following the application of a saturated light pulse (duration, 1 s).
Photoinhibitory Chl a Quenching during Illumination Is Enhanced in adg1-1/tpt-1 Plants
Illumination with actinic light caused a dramatic decline in the yield of variable Chl a fluorescence (Ft − Fo) in adg1-1/tpt-1 far below Fo, particularly at PFDs above those the plants had experienced during growth (Fig. 1C; Supplemental Fig. S1, A–F). Such a drop in Fo was absent in the wild type (Fig. 1D) and both single mutants (data not shown). Moreover, following exposure of adg1-1/tpt-1 plants to high PFDs for extended time periods (30–60 min), Ft declined to levels comparable to Fo observed in dark-adapted wild-type or single mutant plants (Supplemental Fig. S1F). It is thus likely that Ft approached the true Fo emitted from the antenna pigments in adg1-1/tpt-1 in high light. Nonphotochemical Chl a quenching (NPQ), indicative of thermal energy dissipation, was enhanced by 20% to 60% in adg1-1/tpt-1 plants compared with the wild type (Schneider et al., 2002) following long-term illumination (30–60 min) at PFDs above 100 μmol m−2 s−1 (Supplemental Fig. S1G). Moreover, the reduction state of QA, expressed as 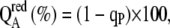 remained close to 60% in adg1-1/tpt-1 (i.e. little above the reduction state in the dark-adapted state) irrespective of the PFD applied, whereas it increased hyperbolically from zero in the dark-adapted state to 40% at the highest PFD applied in the wild type (Supplemental Fig. S1H). In those cases where Fo was quenched during illumination in adg1-1/tpt-1, it increased again asymptotically immediately after darkening (Fig. 1C; Supplemental Fig. S1, C–F) but failed to attain Fo of the dark-adapted state after illumination with high light (Supplemental Fig. S1, D–F), suggesting the occurrence of photoinhibition.
remained close to 60% in adg1-1/tpt-1 (i.e. little above the reduction state in the dark-adapted state) irrespective of the PFD applied, whereas it increased hyperbolically from zero in the dark-adapted state to 40% at the highest PFD applied in the wild type (Supplemental Fig. S1H). In those cases where Fo was quenched during illumination in adg1-1/tpt-1, it increased again asymptotically immediately after darkening (Fig. 1C; Supplemental Fig. S1, C–F) but failed to attain Fo of the dark-adapted state after illumination with high light (Supplemental Fig. S1, D–F), suggesting the occurrence of photoinhibition.
In order to separate individual components of NPQ (energy quenching [qE], state transition [qT], and photoinhibitory quenching [qI]), a Chl a fluorescence quench analysis was carried out according to Quick and Stitt (1989) after short-term illumination (4 min) at different PFDs. Furthermore, it was assumed that antenna fluorescence in adg1-1/tpt-1 was similar to the wild-type level and hence well below the apparent Fo. The results of the quench analysis (Supplemental Fig. S2) are summarized in Table II for an intermediate PFD (240 μmol m−2 s−1) and a high PFD (680 μmol m−2 s−1). Relaxation of Chl a fluorescence following preillumination at a low PFD (77 μmol m−2 s−1) yielded little information on individual NPQ types (Supplemental Fig. S2, A and B). In contrast to long-term illumination, the application of actinic light for 4 min did not result in an appreciable increase of NPQ in adg1-1/tpt-1 plants relative to the wild type at a PFD of 240 μmol m−2 s−1 (Table II). Moreover, qE was lower in adg1-1/tpt-1 compared with the wild type (Table II), suggesting a diminished proton gradient across the thylakoid membrane and the concomitant thermal energy dissipation via the xanthophyll cycle (Färber et al., 1997; Ruban and Horton, 1999; Avenson et al., 2004). The largest difference in NPQ between the wild type and adg1-1/tpt-1 was due to photoinhibitory quenching (i.e. the parameter qI was increased by factors of 1.6 and 2 after preillumination with intermediate and high PFDs, respectively). Photoinhibited plants recovered completely from short- or long-term illumination with a high PFD within 24 h (data not shown). There was also a trend for an increase in qT in adg1-1/tpt-1 at a high PFD (Table II), which reflects state transition (i.e. phosphorylation of LHCII and the concomitant transfer of excitation energy from PSII to PSI). Table II again shows that 1 − qP (i.e. the reduction state of QA) was increased significantly in adg1-1/tpt-1 plants compared with the wild type (Supplemental Fig. S1H; Schneider et al., 2002).
Table II.
Chl a fluorescence parameters qP, NPQ, qE, qT, and qI of adg1-1/tpt-1 compared with the wild type
Rosette leaves were illuminated for 4 min at the PFD indicated and darkened for a further 16 min while supplying saturating light pulses at increasing time intervals. The Chl a fluorescence parameters were determined graphically from the relaxation kinetics shown in Supplemental Fig. S2, assuming a similar antenna fluorescence in adg1-1/tpt-1 plants as in wild-type plants. The data represent means ± se of three to five measurements. Statistical analysis was performed according to the Welch test. Note that the individual NPQ types are correlated as follows: (1 − NPQ) = (1 − qE)·(1 − qT)·(1 − qI).
| Fluorescence Parameters | PFD
|
|||
|---|---|---|---|---|
| 240 μmol m−2 s−1
|
680 μmol m−2 s−1
|
|||
| Wild Type | adg1-1/tpt-1 | Wild Type | adg1-1/tpt-1 | |
| 1 − qP | 0.244 ± 0.049 | 0.597 ± 0.006a | 0.483 ± 0.029 | 0.634 ± 0.025a |
| NPQ (qN) | 0.623 ± 0.033 | 0.552 ± 0.046 | 0.774 ± 0.016 | 0.796 ± 0.022 |
| qE | 0.456 ± 0.045 | 0.227 ± 0.058a | 0.680 ± 0.029 | 0.589 ± 0.047 |
| qT | 0.118 ± 0.001 | 0.117 ± 0.010 | 0.112 ± 0.007 | 0.166 ± 0.033 |
| qI | 0.216 ± 0.010 | 0.342 ± 0.045a | 0.200 ± 0.025 | 0.401 ± 0.017b |
P < 0.05.
P < 0.02.
Oxygen Deprivation and Inhibitor Treatments Indicate Enhanced Chlororespiration and Mitorespiration in adg1-1/tpt-1 Plants
The impact of respiratory processes on dark Chl a fluorescence yield of adg1-1/tpt-1 and wild-type plants was analyzed in an O2-free atmosphere (i.e. by fumigation with N2 gas) or by the application of inhibitors known to affect components of chlororespiration, cyclic electron transport, or aspects of mitochondrial respiration (mitorespiration).
As shown in Figure 2A, Fo was further enhanced by fumigation with N2 in adg1-1/tpt-1 but not in wild-type plants (Fig. 2B) within 6 to 8 min. This surplus of Fo could be rapidly quenched to the initial level observed in air by FR illumination and could be restored to the increased level within 5 min after FR light was turned off. Replacing N2 by air resulted again in a rapid drop of Fo to the level observed at the start of the experiment. These fast responses of Fo toward O2 deprivation or FR illumination suggest that components directly involved in thylakoid electron transfer were affected. Fumigation with 2% O2 resulted in a less marked increase in Fo compared with the complete deprivation of O2 (data not shown). In contrast, as a control, fumigation with CO2-free air did not affect Fo in adg1-1/tpt-1 (data not shown). Neither O2 deprivation nor illumination with FR caused any substantial changes of Fo in the wild type (Fig. 2B). Moreover, in order to visualize small variations in Fo, an at least 4-fold amplification of the fluorescence signal was required (Fig. 2B, inset). The substantial changes in Fo, due to the removal of O2 and/or illumination with FR, clearly indicate that respiratory processes are indeed involved in the dark reduction of the PQ/QA system in adg1-1/tpt-1 plants.
Figure 2.
Effects of oxygen deprivation and FR illumination on traces of modulated Chl a fluorescence yield of dark-adapted leaves of adg1-1/tpt-1 (A) or wild-type (B) plants. Rosette leaves were fumigated in a Perspex chamber with either air or, in order to create an oxygen-free atmosphere, N2 gas. Where indicated, FR was either turned on or off. In order to detect small changes in the dark fluorescence yield (Fo) in leaves of wild-type plants in response to N2 or FR treatment, the signal had to be amplified 4-fold compared with adg1-1/tpt-1 (B, inset). Fm indicates the maximum Chl a fluorescence yield following the application of a saturated light pulse (duration, 1 s).
In an attempt to separate chlororespiration from mitochondrial respiration, octyl gallate (OG), an effective inhibitor of PTOX (Josse et al., 2003), or salicylhydroxamic acid (SHAM), an inhibitor of AOX (Peltier and Cournac, 2002), was applied in time-dependent (Fig. 3, A–C) or concentration-dependent (Fig. 3D) series. Furthermore, cyclic electron transfer from reduced ferredoxin to PQ at the cytochrome b/f complex, which might be involved in QA reduction in the light, can be inhibited by micromolar concentrations of antimycin A (Joët et al., 2001; Munekage et al., 2004; Rumeau et al., 2007). The application of 1 mm OG or 2.5 mm SHAM, either individually or in combination, to excised leaves caused a slow decline in the Fv/Fm due to an increase in Fo (data not shown) in adg1-1/tpt-1 but not in wild-type plants (Fig. 3A). Interestingly, the Fv/Fm recovered almost completely after FR illumination in OG-fed, but not in SHAM-fed, leaves of adg1-1/tpt-1 (Fig. 3B). A combined feeding of OG and SHAM even resulted in an almost complete reduction of QA in adg1-1/tpt-1 leaves (Fig. 3A), which could be partially reverted by FR illumination (Fig. 3B). In actinic light (duration, 1 min), the quantum efficiency of electron transport through PSII (ΦPSII) was almost completely inhibited when OG or SHAM was fed individually or in combination to adg1-1/tpt-1 leaves (Fig. 3C). However, ΦPSII also dropped in the untreated controls after 3.5 h in leaves of both adg1-1/tpt-1 and wild-type plants. In adg1-1/tpt-1, Fv/Fm declined in a dose-dependent manner upon inhibitor treatment (Fig. 3D). Moreover, high concentrations of SHAM, but not of OG, diminished Fv/Fm also in the tpt-1 and adg1-1 single mutants (Fig. 3D). Feeding of up to 5 μm antimycin A did not affect Fv/Fm in adg1-1/tpt-1, in the single mutants, or in the wild type. Only at high concentrations of antimycin A (50 μm) was a slight recovery of the Fv/Fm observed in adg1-1/tpt-1 plants (Fig. 3D). These data suggest a contribution of both chlororespiration and mitorespiration to the enhanced Fo in adg1-1/tpt-1. In particular, the fast recovery of Fv/Fm from OG treatment upon FR illumination (Fig. 2) points to the involvement of processes taking place directly at the thylakoid membrane, whereas the lack of Fv/Fm recovery from SHAM treatment suggests a different target or a more complex or unspecific mode of action of this inhibitor when applied in an in vivo system. Indeed, SHAM has been shown to stimulate soluble peroxidases involved in NADH oxidation (see “Discussion”). Furthermore, the absence of any effect of antimycin A on Fv/Fm in adg1-1/tpt-1 rules out any involvement of the components of cyclic electron transport in dark QA reduction.
Figure 3.
Impact of inhibitor treatment on modulated Chl a fluorescence parameters in leaves of the wild type, the adg1-1/tpt-1 double mutant, and the tpt-1 and adg1-1 single mutants. Fv/Fm of dark-adapted plants (A), ΦPSII after 1 min of illumination with actinic light at a PFD of 120 μmol m−2 s−1 (B), and Fv/Fm during FR illumination at 1 min after actinic light was turned off (C) were determined in a time course after inhibitor application. Excised leaves of the wild type or adg1-1/tpt-1 were plunged into solutions containing 1 mm OG and 2.5 mm SHAM either individually or in combination. Chl a fluorescence parameters were determined at 0 h (white bars), 3.5 h (light gray bars), and 6 h (dark gray bars) after inhibitor treatment. As a control, leaves were incubated in 1% methanol. The leaf samples were kept in low light (PFD of 5 μmol m−2 s−1) during incubation. In a second experiment (D), Fv/Fm was determined after treatment of leaves of the wild type, both single mutants, and the double mutant with increasing concentrations of OG, SHAM, or antimycin A for 3 h. All data represent means ± se of three replicates.
The Expression Levels of Alternative Oxidases and Components of the NDH1 Complex Are Differentially Affected in adg1-1/tpt-1 Compared with the Wild Type
As shown above, the increased reduction state of QA in darkened adg1-1/tpt-1 leaves could be further enhanced by O2 deprivation or by inhibitors of alternative plastidial or mitochondrial oxidases. Therefore, we analyzed whether components of mitorespiration or chlororespiration were affected at the transcriptional level. Arabidopsis contains five genes encoding AOX isoforms, from which AOX1a represents the major form in leaves (Clifton et al., 2006). In contrast to AOX, PTOX is a single-copy gene (Wu et al., 1999). The largely plastome-encoded NDH1 also contains three nucleus-encoded subunits, NDH-M, -N, and -O (Rumeau et al., 2005). In addition, for the correct assembly of NDH1, the nucleus-encoded protein CHLORORESPIRATION REDUCTION6 (CRR6) is required. The lack of this factor in the crr6 mutant results in the absence of NDH1 activity and a failure to transiently reduce the PQ pool after turning off actinic light (Munshi et al., 2006). Moreover, the plastome-encoded cytochrome b559 (PsbE) is most likely involved in the reoxidation of reduced PQ in the dark (Bondarava et al., 2003). Table III shows that both AOX1a and PTOX were down-regulated in adg1-1/tpt-1 compared with the wild type, most pronounced during the dark period. A general trend for a down-regulation of AOX1a in the dark also became apparent for the wild type, which is consistent with microarray data of light series (efp browser: http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi). In contrast, NDH-N showed little deregulation after 8 h in the light or after 12 and 16 h in the dark but was down-regulated after 2 h in the light in adg1-1/tpt-1 plants (Table III). Interestingly, the NDH1 assembly factor CRR6 was more than 2-fold up-regulated in adg1-1/tpt-1 plants after 8 h in the light and at the end of the regular 12-h dark period, suggesting an increased abundance of NDH1 in the double mutant. Cytochrome b559 lacked any trend of transcriptional regulation during the light/dark cycle or in adg1-1/tpt-1 relative to wild-type plants (Table III). It can be stated that a decline in AOX and PTOX expression combined with an increase in NDH1 would be consistent with an enhanced reduced state of QA in adg1-1/tpt-1 during the dark period.
Table III.
Relative expression profiles of genes involved in mitorespiration or chlororespiration as well as of a component of PSII determined by real-time RT-PCR
Relative expression levels were assessed by the ΔΔCt method using two biological and technical replicates for each gene. As references for nucleus- and plastome-encoded genes, ubiquitin-conjugated enzyme 21 (At5gG25760; Czechowski et al., 2005) and a ribosomal protein (RPL23.2; AtCg01300) were used. In all cases, sd values of the individual Ct levels were less than 10% of the mean values. The relative transcript levels are expressed as log2 ratios. In columns 1 and 2, the relative expression levels were referred to the time point 2 h in the light for the wild type (1) or adg1-1/tpt-1 (2); in column 3, expression levels in adg1-1/tpt-1 were compared with the wild type at each time point. Genes up- or down-regulated relative to the respective controls are indicated with roman or italic numbers, respectively. In addition, up- or down-regulated genes above or below a threshold of ±1 are indicated with boldface numbers.
| Light/Dark Series |
AOX1a
|
PTOX
|
NDH1 (NDH-N)
|
NDH1 (CRR6)
|
Cytochrome b559 (PsbE)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| 2 h of light | – | – | −0.51 | – | – | −0.37 | – | – | −1.54 | – | – | −0.32 | – | – | −0.01 |
| 8 h of light | −0.55 | −0.71 | −0.66 | −0.41 | −0.61 | −0.57 | −1.43 | −0.11 | 0.21 | −1.24 | 0.77 | 1.28 | 0.31 | 0.39 | 0.09 |
| 12 h of dark | −1.33 | −3.35 | −2.52 | −0.17 | −0.50 | −0.70 | −1.54 | −0.08 | 0.35 | −0.32 | 1.51 | 1.11 | −0.09 | 0.05 | 0.08 |
| 16 h of dark | −2.84 | −3.47 | −1.14 | 0.08 | −1.62 | −2.07 | −2.93 | −1.91 | −0.09 | 0.08 | 0.04 | −0.76 | −0.18 | 0.10 | 0.28 |
Oxygen Gas Exchange Is Impaired in the Light and Dark But Does Not Correlate with Photosynthetic Electron Transport Rates in adg1-1/tpt -1
The enhanced reduction state of QA in adg1-1/tpt-1 due to chlororespiration and mitorespiration in the dark was reflected by changes of O2 gas-exchange rates in the light and dark. On average, dark O2 consumption was increased by a factor of 4, from 0.5 μmol m−2 s−1 in the wild type to about 2 μmol m−2 s−1 in adg1-1/tpt-1 (Fig. 4, A and B; Supplemental Fig. S3B). In order to assess the impact of the light intensity on dark respiration, O2 gas-exchange rates were determined in alternating dark/light cycles at increasing PFDs (Fig. 4A). At a PFD similar to the growth light of the plants (80 μmol m−2 s−1), the wild type evolved O2 at a substantial rate, whereas adg1-1/tpt-1 barely compensated dark O2 consumption. At an intermediate PFD (390 μmol m−2 s−1), adg1-1/tpt-1 also produced O2, albeit at a rate of about 15% compared with the wild type. Moreover, the rate of O2 evolution was linearly related to time in the wild type but declined in adg1-1/tpt-1 after 3 min in the light. Likewise, O2 consumption in adg1-1/tpt-1 was enhanced immediately upon darkening and recovered to a steady state within 2 min, whereas O2 consumption in the wild type instantaneously dropped to rates observed in the dark-adapted state. These differences between the wild type and adg1-1/tpt-1 were most pronounced at a high PFD of 780 μmol m−2 s−1 (Fig. 4A). The different impact of the PFD on O2 gas-exchange rates between adg1-1/tpt-1 and the wild type is summarized in Supplemental Figure 4 (A and B). In contrast to the wild type, in adg1-1/tpt-1 rates of O2 evolution with increasing PFD (Supplemental Fig. S4A) were very much dependent on the rate of O2 consumption in the intermittent dark periods (Supplemental Fig. S4B).
Figure 4.
A, Time course of O2 gas exchange of wild-type and adg1-1/tpt-1 plants. Excised rosette leaves were kept in the dark for at least 30 min and then illuminated with PFD of 80 μmol m−2 s−1 (L1), 392 μmol m−2 s−1 (L2), or 780 μmol m−2 s−1 (L3) interrupted by dark periods (D) as indicated by arrows. B to D, Combined determinations of O2 gas exchange (B) and photosynthetic ETR (C) in response to increasing PFDs in wild-type and adg1-1/tpt-1 plants as well as light dependencies of the ratios of ETR and O2 evolution (ETR/O2 ratio) in the wild type (circles) and adg1-1/tpt-1 (squares; D). The data represent two independent sets of experiments. The leaf area inside the oxygen electrode chamber varied between 4 and 5 cm2. The chamber temperature was kept constant at 25°C.
Light saturation curves of net O2 evolution in high CO2 (Fig. 4B) as well as photosynthetic electron transport rates (ETRs; Fig. 4C) revealed light compensation points for O2 evolution at PFDs of below 8 and 35 μmol m−2 s−1 in the wild type and adg1-1/tpt-1, respectively. Surprisingly, the light-saturated rates of O2 evolution were decreased by only 50% (from 8 to about 4 μmol m−2 s−1), whereas ETR was decreased by 80% (from 50 to 10 μmol m−2 s−1) in adg1-1/tpt-1 compared with the wild type (Schneider et al., 2002). In low light (i.e. at a PFD of up to 100 μmol m−2 s−1), the ETR/O2 ratios were close to the theoretical value of 4 in the wild type and increased linearly with increasing PFDs to a value of about 6 in high light (i.e. a PFD of 400 μmol m−2 s−1), indicating higher portions of O2 consumption by mitorespiration and/or the Mehler reaction (Fig. 4D). In contrast, adg1-1/tpt-1 showed high ETR/O2 ratios in low light (indicating increased rates of O2 consumption relative to O2 evolution), whereas with increasing PFD, ETR/O2 ratios even fell far below those of the wild type, suggesting a high portion of O2 evolution uncoupled from ETR through PSII. It is hence conceivable that ETR in adg1-1/tpt-1 is underestimated. Interestingly, the ETR/O2 curves of both the wild type and adg1-1/tpt-1 intersected at about the PFD the plants experienced during growth (i.e. between 50 and 100 μmol m−2 s−1; Fig. 4D).
Altered Levels of Pyridine Nucleotides and Ascorbate Point at an Impaired Redox Poise in adg1-1/tpt-1 in Light and Dark Conditions
The lack of significant Fo quenching in adg1-1/tpt-1 by FR illumination suggested PSI electron acceptor (i.e. NADP) limitation (Fig. 1A). Therefore, we analyzed the levels of important redox components of the mesophyll (i.e. pyridine nucleotides [NADP, NADPH, NAD, and NADH] and ascorbate). In the light, the total contents of NADP and NADPH were significantly lowered by approximately 50% in adg1-1/tpt-1 compared with the wild type (Table IV). Moreover, the NADPH/NADP ratios in adg1-1/tpt-1 were increased substantially compared with the wild type by factors of 4.2 and 9.3 after 2 and 11.5 h in the light, respectively (Table IV). Interestingly, after a prolonged time in darkness (15 h), the NADPH/NADP ratios were still appreciably higher in the double mutant (0.64) compared with the wild type (0.15), suggesting a more reduced stroma in dark-kept adg1-1/tpt-1 plants. Moreover, the total contents of NADP(H) in the wild type declined to a similar level as in adg1-1/tpt-1 after a prolonged time in darkness.
Table IV.
Effects of light/dark treatment on contents and ratios of pyridine nucleotides as well as on Asc and DAsc in the wild type and the adg1-1/tpt-1 double mutant
The PFD at the plant level ranged between 80 and 100 μmol m−2 s−1. The data represent means ± se. Statistical analysis was conducted according to the Welch test.
| Sample | Treatment
|
|||||
|---|---|---|---|---|---|---|
| 2 h of Light
|
11.5 h of Light
|
15 h of Dark
|
||||
| Wild Type | adg1-1/tpt-1 | Wild Type | adg1-1/tpt-1 | Wild Type | adg1-1/tpt-1 | |
| Pyridine nucleotides (nmol g−1 fresh weight) | ||||||
| NADP | 17.04 ± 1.61 | 4.88 ± 0.51a | 15.99 ± 0.82 | 5.78 ± 0.80b | 4.59 ± 0.49 | 3.15 ± 0.53 |
| NADPH | 3.40 ± 1.13 | 4.08 ± 0.63 | 1.34 ± 0.25 | 4.52 ± 0.32b | 0.70 ± 0.53 | 2.03 ± 0.69 |
| NADPH/NADP ratio | 0.200 | 0.84 | 0.084 | 0.78 | 0.152 | 0.64 |
| NAD | 18.66 ± 1.73 | 11.81 ± 0.93c | 16.44 ± 3.61 | 12.79 ± 0.76 | 23.11 ± 4.06 | 17.92 ± 0.46 |
| NADH | 1.20 ± 0.59 | 0.77 ± 0.17 | 0.68 ± 0.31 | 1.00 ± 0.36 | 0.39 ± 0.28 | 0.38 ± 0.15 |
| NADH/NAD ratio | 0.0641 | 0.0654 | 0.0416 | 0.0786 | 0.0169 | 0.0211 |
| Asc and DAsc (μmol g−1 fresh weight) | ||||||
| Asc + DAsc | 2.15 ± 0.39 | 1.74 ± 0.24 | 5.17 ± 0.68 | 1.53 ± 0.64a | 2.63 ± 0.37 | 1.60 ± 0.30c |
| Asc | 1.80 ± 0.50 | 0.48 ± 0.15c | 2.47 ± 0.64 | 0.09 ± 0.10c | 1.97 ± 0.15 | 0.73 ± 0.22a |
| Asc/DAsc ratio | 5.14 | 0.38 | 0.91 | 0.06 | 2.08 | 0.84 |
P < 0.01.
P < 0.001.
P < 0.05.
For NAD(H), there were only minor differences in total contents and ratios between the wild type and adg1-1/tpt-1 (Table IV). The major portion of NAD(H) was found in the oxidized state. However, in adg1-1/tpt-1, the NADH/NAD ratios increased relative to the wild type after 11.5 h in the light and also after 15 h in the dark. Interestingly, in adg1-1/tpt-1, the decline in the total NADP(H) contents from about 10.3 nmol g−1 fresh weight at the end of the light period to about 5.2 nmol g−1 fresh weight after a prolonged time in darkness was mirrored by an increase in total NAD(H) contents, from 13.8 to 18.3 nmol g−1 fresh weight (Table IV), suggesting an efficient interconversion of both pyridine nucleotide pools. Moreover, in adg1-1/tpt-1, the summed contents of all pyridine nucleotides remained relatively constant in the light and dark (between 21.5 and 24.1 nmol g−1 fresh weight) but showed larger variations between 40.3 nmol g−1 fresh weight after 2 h in the light and 28.8 nmol g−1 fresh weight after 15 h in the dark in the wild type.
As it is likely that the redox poise of the mesophyll is impaired in the presence of respiratory inhibitors, the effects of OG and SHAM on pyridine nucleotide levels in adg1-1/tpt-1, both single mutants, and the wild type were analyzed after 1.5 h of incubation (Supplemental Table S1). Compared with intact plants (Table IV), excised leaves of the wild type and adg1-1/tpt-1 showed only minor changes in pyridine nucleotide levels in the controls. In addition, the levels of the individual pyridine nucleotides in tpt-1 and adg1-1 were not significantly different from those in the wild type (Supplemental Table S1). Interestingly, OG caused a steep decline in NADP contents in the wild type and the whole set of mutant plants, whereas NADPH levels were not significantly altered compared with the controls, which resulted in a general steep increase in NADPH/NADP ratios (Supplemental Table S1). NAD levels were slightly decreased in adg1-1/tpt-1, the wild type, and adg1-1 but not in tpt-1, whereas NADH contents, in the presence of OG, were increased in adg1-1 and adg1-1/tpt-1 but decreased in tpt-1. These changes in NAD and NADH contents caused a low NADH/NAD ratio in tpt-1 but elevated NADH/NAD ratios in the wild type, adg1-1, and, most pronounced, in adg1-1/tpt-1 compared with the controls (Supplemental Table S1). The presence of SHAM resulted in a dramatic decline in NADH contents below the detection limit in the wild type and both single mutants but led to an increase in NADH contents in adg1-1/tpt-1. Likewise, contents of both NADP and NADPH declined in the wild type and both single mutants but remained almost unaffected in adg1-1/tpt-1. The differential effects of OG and SHAM on pyridine nucleotide levels point at different targets and/or modes of actions of the individual inhibitors. Moreover, the substantial increase in NADPH/NADP and NADH/NAD ratios after OG application in adg1-1/tpt-1 compared with the wild type suggests a block of electron transfer from reduced pyridine nucleotides to O2 via NDH1, PTOX, and PQ, which in turn leads to an enhanced reduction state of QA.
Chloroplasts contain between 20% and 40% of the total ascorbate (Asc) in the mesophyll (Foyer et al., 1983), which acts as a scavenger of reactive oxygen species (ROS; Noctor and Foyer, 1998) and, together with the glutathione system, plays an important role in the Mehler peroxidase reaction (Asada, 1999). Asc is probably also directly involved in energy dissipation via the xanthophyll cycle in the thylakoid lumen (Müller-Moulé et al., 2002). In the wild type, the total contents of Asc and dehydroascorbate (DAsc) increased during the light period; they exhibited highest contents after 11.5 h in the light and lowest contents after 15 h in the dark or after 2 h in the light (Table IV). The major portion of the Asc/DAsc system in the wild type consisted of Asc (75% to 84%) in both the light and the dark. In contrast, the total contents of Asc + DAsc were lower in adg1-1/tpt-1 compared with the wild type and lacked large changes during the light/dark cycle (Table IV). Moreover, the Asc/DAsc ratio decreased from 5.14 in the wild type to 0.38 in adg1-1/tpt-1 after 2 h in the light and further declined to about 0.06 after 11.5 h in the light. Even after 15 h in the dark, Asc/DAsc ratios remained low in adg1-1/tpt-1 (0.84 compared with 2.08 in the wild type). The large decline in Asc/DAsc ratios in adg1-1/tpt-1 compared with the wild type suggests an enhanced requirement for the elimination of ROS in the double mutant, in particular after several hours in the light.
Grana Thylakoid Hyperstacking, Increased Abundance of Plastoglobules, and Altered Composition of Photosynthetic Pigments and Lipids in adg1-1/tpt-1
The consequences of an enhanced reduction state in the stroma caused by a strongly impaired primary metabolism on the chloroplast ultrastructure, contents of photosynthetic pigments, and the lipid composition were further investigated. Ultrastructural analyses (Fig. 5A) revealed a pronounced hyperstacking of grana thyalakoids and more abundant plastoglobules in adg1-1/tpt-1 compared with the single mutants or the wild type. As expected, starch granules were absent in the starch-free lines. In tpt-1, both the number and size of starch granules per chloroplast showed a trend of a slight increase compared with the wild type (Table V). More settled differences in the chloroplast ultrastructure between the individual plant lines emerged from a statistical analysis of up to 20 electromicrographs per line (Table V). A comparison of chloroplast cross-section areas between the plant lines revealed significantly larger chloroplasts in tpt-1 (factor 1.2), whereas the chloroplast size was significantly diminished by 17% in adg1-1 and by 26% in adg1-1/tpt-1 compared with the wild type. These changes in cross-section area were reflected in alterations of the maximum width rather than the maximum length of the chloroplasts (Table V). As the most obvious feature, a hyperstacking of grana thylakoids in adg1-1/tpt-1 emerged (Fig. 5A), which was reflected in a 1.8-fold increase in the overall cross-section area of grana thylakoids per chloroplast in adg1-1/tpt-1 compared with the wild type (Table V). Moreover, an increase in grana thylakoid size was also significant in tpt-1 (1.2-fold) and adg1-1 (1.3-fold) compared with the wild type. These statistical differences were supported by distributions of stacked grana size classes between the lines (Fig. 5B). In the wild type, the maximum grana cross-section area ranged between 0.02 and 0.04 μm2, with about 65% of the total grana thylakoid area. In contrast, there was a proportional increase toward larger size classes (0.04–0.06 μm2) in tpt-1 and more pronounced in adg1-1. In adg1-1/tpt-1, a much broader distribution of stacked grana size classes compared with the wild type or the single mutants was observed. Although the maximum grana cross-section area ranged between 0.02 and 0.08 μm2 in adg1-1/tpt-1, there were also size classes between 0.14 and 0.24 μm2, which were found in neither the wild type nor the single mutants. The hyperstacking of grana thylakoids in the mutants (particularly in adg1-1/tpt-1) relative to the wild type strongly suggests a higher abundance of PSII relative to PSI and is reflected in an altered composition of photosynthetic pigments.
Figure 5.
Chloroplast ultrastructure analyses of wild-type, tpt-1, adg1-1, and adg1-1/tpt-1 plants. A, The images taken by an transmission electron microscope represent typical examples of chloroplasts from the individual lines either as overviews or as close-ups of the same chloroplasts. Bars = 1 μm and 0.5 μm for the overviews and close-ups, respectively. The cross-section areas of grana thylakoids (containing more than two stacks) were determined for eight to 20 chloroplast images per line and grouped into size classes. B, The relative grana size class distribution was compared between the lines. C, The number of plastoglobules was counted in 20 chloroplast cross sections per line and grouped into plastoglobule number classes per chloroplast, and the relative distribution of plastoglobule numbers per chloroplast was compared between the individual lines.
Table V.
Analyses of the chloroplast ultrastructure, pigment, and lipid composition in wild-type, tpt-1, adg1-1, and adg1-1/tpt-1 plants
Areas of chloroplast cross sections as well as maximum lengths and widths of 20 chloroplast images were determined from electromicrographs. The ratio of cut grana area to chloroplast area was determined for eight to 20 chloroplasts of each line. n.d., Not detectable. Plants harvested for pigment analyses were grown in growth cabinets under controlled environmental conditions in a light/dark cycle of 12 h/12 h. Numbers in parentheses represent percentage values relative to the wild type. If not stated otherwise, the data represent means ± se of three independent measurements. A statistical analysis of the data sets between the mutant lines and the wild type was conducted according to the Welch test.
| Variable | Wild Type (n = 20) | tpt-1 (n = 20) | adg1-1 (n = 20) | adg1-1/tpt-1 (n = 20) |
|---|---|---|---|---|
| Chloroplast dimensions and composition | ||||
| Chloroplast area (μm2) | 11.93 ± 0.44 | 14.29 ± 0.67a | 9.90 ± 0.47a | 8.82 ± 0.55b |
| Chloroplast length (μm) | 6.24 ± 0.21 | 5.86 ± 0.16 | 6.26 ± 0.17 | 5.77 ± 0.18 |
| Chloroplast width (μm) | 2.40 ± 0.07 | 2.93 ± 0.10b | 1.90 ± 0.05b | 1.95 ± 0.10b |
| Width/length ratio | 0.39 ± 0.02 | 0.51 ± 0.02b | 0.31 ± 0.01a | 0.34 ± 0.02 |
| Grana thylakoid area per chloroplast area (%) | 14.57 ± 1.16 (n = 8) | 17.43 ± 0.67c (n = 20) | 18.62 ± 1.06c (n = 8) | 26.37 ± 2.02b (n = 8) |
| Average no. of plastoglobules per chloroplast | 5.95 ± 0.64 | 7.40 ± 0.64 | 6.05 ± 0.28 | 12.90 ± 1.47b |
| Average no. of starch grains per chloroplast | 1.55 ± 0.23 | 1.70 ± 0.26 | n.d. | n.d. |
| Starch grain area per chloroplast (μm2) | 0.48 ± 0.08 | 0.56 ± 0.10 | n.d. | n.d. |
| Area per starch grain (μm2) | 0.33 ± 0.06 | 0.35 ± 0.05 | n.d. | n.d. |
| Pigment contents and ratios | ||||
| Total chlorophyll (μg g−1 fresh weight) | 1,461 ± 15 (100) | 1,570 ± 92 (107) | 1,167 ± 26 (78)b | 1,048 ± 12 (72)b |
| Chl a/b | 2.68 ± 0.09 (100) | 2.71 ± 0.06 (101) | 2.10 ± 0.26 (78) | 1.69 ± 0.06 (63)b |
| Carotenoids (μg g−1 fresh weight) | 47.0 ± 4.8 (100) | 50.1 ± 3.2 (107) | 37.4 ± 1.7 (79) | 39.0 ± 4.9 (83) |
| Chlorophyll/carotenoid ratio | 31.1 ± 1.2 (100) | 31.3 ± 0.6 (101) | 31.2 ± 0.2 (101) | 26.9 ± 0.2 (84) |
| Total lipids (mg g−1 fresh weight) | 5.1 ± 0.5 | 4.7 ± 0.6 | 4.8 ± 0.2 | 4.4 ± 0.3 |
| Polar lipids (mol %) | ||||
| MGDG | 49.6 ± 2.6 | 48.3 ± 2.3 | 45.9 ± 5.2 | 39.9 ± 1.6b |
| Phosphatidylglycerol | 8.4 ± 1.4 | 7.3 ± 1.2 | 7.8 ± 1.4 | 10.1 ± 2.2 |
| Digalactosyl diacylglycerol | 16.9 ± 1.1 | 18.5 ± 0.9 | 17.2 ± 0.6 | 16.4 ± 0.9 |
| Phosphatidylinositol | 1.5 ± 0.9 | 1.1 ± 0.6 | 1.6 ± 0.1 | 2.3 ± 0.3c |
| Sulfolipid | 1.2 ± 0.4 | 1.4 ± 0.6 | 1.6 ± 0.5 | 1.9 ± 0.1b |
| Phosphatidylethanolamine | 8.7 ± 0.9 | 10.1 ± 2.0 | 10.5 ± 2.0 | 10.8 ± 0.5b |
| PC | 13.8 ± 1.5 | 13.3 ± 1.3 | 15.4 ± 3.1 | 18.7 ± 3.8 |
P < 0.01.
P < 0.001.
P < 0.05.
Total chlorophyll contents were significantly diminished in adg1-1/tpt-1 by about 30% and were also lower in adg1-1 (20%; Table V). In contrast, total chlorophyll contents in tpt-1 showed a trend of a slight increase. The Chl a/b ratio, which is an indicator for the stoichiometry of the photosystems (in particular of their light-harvesting complexes [LHCs]), was significantly lower in adg1-1/tpt-1 (Chl a/b = 1.69) and also in adg1-1 (Chl a/b = 2.1) compared with the wild type (Chl a/b = 2.63) and tpt-1. A decrease in the Chl a/b ratio suggests an increase in the abundance of LHCs of PSII relative to PSI. Contents of carotenoids exhibited less pronounced differences between the individual lines (Table V). Carotenoid contents in tpt-1 appeared to be slightly elevated and hence correlated with the increase in total chlorophyll contents in this mutant. Despite a 30% decrease in chlorophyll contents in adg1-1/tpt-1, carotenoid contents remained relatively high compared with the wild type and the single mutants (Table V), resulting in a pronounced decline in the chlorophyll/carotenoid ratio in adg1-1/tpt-1 compared with the wild type and the single mutants. The latter finding suggests that the capacity to trap singlet oxygen by carotenoids in the antenna per molecule of chlorophyll might be increased.
The altered chloroplast ultrastructure in adg1-1/tpt-1 was not directly correlated with membrane lipid contents and composition. However, the total leaf lipid content was strongly decreased in adg1-1/tpt-1 compared with the wild type or the single mutants (Table V), leaving the fatty acid composition of lipids unaltered between the lines (data not shown). Lipid quantification revealed that the amounts of the two galactolipids, monogalactosyl diacylglycerol (MGDG) and digalactosyl diacylglycerol, and of the phospholipids were comparable in the wild type and in both single mutants, whereas the MGDG content was strongly reduced in adg1-1/tpt-1, with a concomitant increase in phosphatidylcholine (PC; Table V). Both galactolipids form the major constituents of the thylakoid membrane (Ort, 1986). The reduction in the amount of the plastidic galactolipid MGDG and the increase in PC (which is mostly extraplastidic), together with the reduction in total lipids, would suggest that the portion of thylakoids was decreased in adg1-1/tpt-1. This observation, however, is in contrast with the ultrastructural analysis of adg1-1/tpt-1, which indicated grana hyperstacking as one of the most prominent features. Hence, the drop in MGDG contents observed in adg1-1/tpt-1 most likely reflects the reduction in chloroplast size or number rather than a decreased abundance of thylakoids. There was also an increase in sulfolipids, phosphatidylethanolamine, and phosphatidylinositol in adg1-1/tpt-1, with a similar trend in adg1-1 (Table V).
A second obvious ultrastructural feature was the 4.4-fold increase in the average number of plastoglobules in adg1-1/tpt-1 compared with the wild type (Table V). Plastoglobules have been shown to form subcompartments of the stroma thylakoids and to contain components and enzymatic activities also found in the thylakoids (Austin et al., 2006). As the plastoglobule number distribution in Figure 5C shows, the majority of chloroplasts from the wild type and both single mutants contained between three and eight plastoglobules, whereas the plastoglobule number distribution in chloroplasts of adg1-1/tpt-1 revealed a minimum of six and a maximum of 23 plastoglobules per chloroplast.
DISCUSSION
In this report, compensatory and adaptive changes in plant performance caused by diminished photoassimilate utilization have been characterized in the Arabidopsis double mutant adg1-1/tpt-1 (Schneider et al., 2002). The metabolism of adg1-1/tpt-1 is strongly impaired in such a way that the known paths to export photoassimilates in the light (i.e. TP) and dark (i.e. maltose and Glc) are either restricted (knockdown of the TPT) or blocked (lack of starch, due to a defect in AGPase; Fig. 6, A and B). Therefore, one can raise the question of how the plants are actually capable of surviving. One obvious way to adapt to such a limitation in primary metabolism would be to decrease biomass production. Hence, the retarded growth of adg1-1/tpt-1 (Schneider et al., 2002) could be entirely due to the incomplete restriction in TP export by the TPT in the light (i.e. a residual activity of up to 10%) and would suggest that a complete lack of the TPT in a starch-free background ultimately leads to lethality of the plants. This assumption is currently being tested by crosses of adg1-1 with a complete knockout mutant of the TPT. Apart from the reduced size and a decline in total chlorophyll contents by at most 30% compared with the wild type, adg1-1/tpt-1 lacks any further visible phenotype. This is at least surprising, however, as the consumption of energy and reducing power during photosynthesis must be kept extremely low due to the lack of significant sinks. Moreover, adg1-1/tpt-1 has to cope with the same light intensities as the wild type or the single mutants during growth, which is expected to be perceived as a severe light stress by the plant.
Figure 6.
Schematic overview of the response of Arabidopsis to a block of daily carbon export combined with a lack of starch in adg1-1/tpt-1 plants (B, D, F, and G) compared with wild-type plants (A, C, E, and G). The impairment of primary metabolism, as indicated in A and B, results in smaller chloroplasts, grana hyperstacking, and an increased number of plastoglobules (C and D). The enhanced reduction state of QA in the dark combined with increased NADPH/NADP ratios (G) in the light and dark point at an increased flux through chlororespiration in adg1-1/tpt-1 (E and F; modified from Rumeau et al., 2007). The gray arrows indicate the path of electrons in the light, starting from water splitting to NADPH generation, whereas the black arrows mark the probable path of electrons from NAD(P)H or reduced metabolites to molecular oxygen via NDH, PQ, and PTOX. Fd, Ferredoxin; FNR, ferredoxin NADPH reductase; PC, plastocyanin. The enhanced requirement to detoxify ROS is reflected in a large decrease in the Asc/DAsc ratio in adg1-1/tpt-1 compared with the wild type (G). Note that the thickness of the arrows in the metabolic diagrams reflects the relative velocities through individual pathways.
The response of adg1-1/tpt-1 to this environmental challenge can be summarized as follows (see also Fig. 6): (1) NADPH/NADP ratios were increased and Asc/DAsc ratios were diminished in both the light and the dark (Fig. 6G), which was accompanied by (2) an increased reduction state of QA in dark-adapted plants and the failure to reoxidize QA via the excitation of PSI by FR illumination. Deprivation of O2 or the application of inhibitors of the alternative oxidases PTOX or AOX point at an involvement of (3) chlororespiration and mitorespiration in the increased reduction state of QA in the dark (Fig. 6, E and F). This observation was supported by (4) enhanced dark O2 consumption in adg1-1/tpt-1 and (5) a 2-fold up-regulation of the NDH1 assembly factor CRR6. Application of high light (i.e. at PFDs above the PFD the plants experienced during growth) led to (6) an enhanced photoinhibition in adg1-1/tpt-1. Most prominently, (7) the ultrastructure of the chloroplast exhibited hyperstacking of grana thylakoids in adg1-1/tpt-1 (Fig. 6, C and D), indicating an altered stoichiometry of the photosystems, which was supported by a decline in Chl a/b ratios. Such changes in photosystem stoichiometry suggest alterations in retrograde signaling that coordinate the expression of plastome- and nucleus-encoded photosynthesis genes (Rodermel, 2001; Pesaresi et al., 2007). Finally, (8) the significant increase in the number of plastoglobules in adg1-1/tpt-1 (Fig. 6D) can be interpreted in the broadest sense as a stress response (Austin et al., 2006).
Enhanced Chlororespiration and Mitorespiration in adg1-1/tpt-1
The adg1-1/tpt-1 double mutant exhibits a significantly increased reduction state of QA in the dark (Table I; Figs. 1 and 2; Supplemental Fig. S1), which is most likely linked to chlororespiration (Bennoun, 1982; Nixon, 2000; Peltier and Cournac, 2002; Rumeau et al., 2007). The basic molecular mechanisms of chlororespiration have been partially resolved (for reviews, see Peltier and Cournac, 2002; Rumeau et al., 2007). For example, PTOX knocked out in the Arabidopsis immutans mutant (Wu et al., 1999; Aluru and Rodermel, 2004) is involved in carotenoid biosynthesis at the step of phytoene desaturase. A possible further function of PTOX is the protection of PQ from overreduction, in particular during dark-light transients as well as in the regulation of cyclic electron transport (Casano et al., 2000; Joët et al., 2002; Rumeau et al., 2007). As a second major component of chlororespiration as well as cyclic electron transport, the largely plastome-encoded NDH1 complex has been identified on the basis of sequence homologies to the mitochondrial complex 1 (Peltier and Cournac, 2002; Rumeau et al., 2007). The absence of NDH1 in the Arabidopsis crr2 mutant results in a lack of NPQ reduction by stromal reductants after turning off actinic light (Hashimoto et al., 2003). A similar effect was observed recently for the pifi (for postillumination chlorophyll fluorescence increase) mutant, which is defective in a nucleus-encoded stromal protein but contains an intact NDH1 complex (Wang and Portis, 2007). The PIFI protein represents most likely a stromal component of chlororespiration. A further enhanced reduction state of QA in adg1-1/tpt-1 after O2 deprivation in the dark or after application of the PTOX inhibitor OG supports the contribution of chlororespiration in dark QA reduction (Fig. 3). Moreover, OG application resulted in substantially increased NADPH/NADP and NADH/NAD ratios in adg1-1/tpt-1 compared with the wild type, thus supporting the idea that a block in electron transfer from reduced pyridine nucleotides to O2 via NDH1, PTOX, and PQ leads to an enhanced reduction state of QA. Interestingly, following OG treatment, NADPH/NADP but not NADH/NAD ratios were also increased in tpt-1 and less pronounced in adg1-1, without any major effect on the QA reduction state, an observation that requires further detailed investigation. At the transcriptional level, PTOX as well as AOX1a were down-regulated, and a component associated with NDH1 function (i.e. CRR6) was up-regulated in adg1-1/tpt-1 compared with the wild type, which might lead in turn to an imbalance between QA reduction and reoxidation and hence to an increased reduction state of QA. Moreover, it was recently shown that PTOX and AOX1a are coregulated under a variety of conditions (Rosso et al., 2006).
The application of SHAM, an inhibitor of mitochondrial AOX, also led to a further increase in dark QA reduction in adg1-1/tpt-1, suggesting that cyanide-insensitive mitorespiration might also be involved in the disturbed redox poise in adg1-1/tpt-1 (Fig. 3). However, there are problems of principle with the application of inhibitors in intact systems. In particular for SHAM, Diethelm et al. (1990) could demonstrate that, besides its inhibitory effects on AOX, SHAM is capable of stimulating soluble peroxidases involved in the oxidation of NADH in the presence of O2. In green tissues, SHAM inhibits CO2 assimilation completely in high light. NADH oxidation might indeed explain the strong decrease in NADH levels after SHAM application in the wild type and both single mutants (Supplemental Table S1). Hence, an analysis of the involvement of AOX or other mitochondrial redox systems in increased respiration rates would require more reliable methods, such as isotope-labeling experiments. The involvement of mitorespiration, nevertheless, would require a more complex communication between the chloroplasts and the mitochondria via metabolites such as malate, oxaloacetate, or 2-oxoglutarate (Krömer et al., 1988; Heineke et al., 1991; Raghavendra and Padmasree, 2003).
Taking the severe increase in the dark respiration rate in adg1-1/tpt-1 relative to wild-type plants into account (Fig. 4), the question of the carbon source for respiration arises. As the residual activity of the TPT appears be insufficient for the accumulation of substantial carbohydrate reserves in the form of soluble sugars within the mesophyll of adg1-1/tpt-1, it is conceivable that the remaining photosynthetic carbon flux is diverted, for instance, in the direction of fatty acid biosynthesis in the light. This would also consume reducing power and energy formed in the light reaction of photosynthesis. Fatty acids might then be subjected to β-oxidation in the subsequent dark period and hence would contribute to the increased reduction state of pyridine nucleotide pools. After prolonged time in darkness, metabolism in adg1-1/tpt-1 might well be halted and the redox systems would remain in a stationary, highly reduced state, an assumption that needs to be addressed in the background of crosses between adg1-1 and a complete knockout mutant of the TPT.
Changes in Chl a fluorescence properties similar to those in adg1-1/tpt-1 could also be brought about by long-term exposure of tobacco plants to CO2-free air (Durchan et al., 2001). The stressed plants exhibited an increase in Fo, which, in contrast to adg1-1/tpt-1, could be reversibly decreased by FR illumination. Furthermore, in Beta vulgaris, iron deficiency also caused a dark reduction of the PSII acceptor side (Belkhodja et al.,1998), which was accompanied by a similar perturbation of pyridine nucleotide pools as observed for adg1-1/tpt-1. Moreover, a single amino acid exchange in the β-subunit of cytochrome b559 (the nucleus-encoded psbF gene of PSII) in transgenic tobacco plants resulted in Chl a fluorescence characteristics similar to those of adg1-1/tpt-1 (Bondarava et al., 2003). Cytochrome b559 is probably involved in the reoxidation of reduced PQ in the dark. In contrast to adg1-1/tpt-1, grana thylakoids in the tobacco mutant were underdeveloped, indicating that the hyperstacking of grana thylakoids is not a prerequisite for the abnormal fluorescence characteristics observed in adg1-1/tpt-1. Moreover, cytochrome b559 transcript levels remained unaffected in adg1-1/tpt-1.
Increased Photoinhibitory Chl a Quenching in adg1-1/tpt-1
Plants have developed strategies to counteract light stress, such as by increasing NPQ (Maxwell and Johnson, 2000). NPQ was increased in adg1-1/tpt-1 compared with the wild type after long-term illumination with high PFDs (Supplemental Fig. S1G; Schneider et al., 2002). However, the major portion of NPQ, qE, was lowered significantly in adg1-1/tpt-1 compared with the wild type after short-term illumination (Table II), suggesting a less steep pH gradient across the thylakoid membrane and, concomitantly, a decline in thermal dissipation via xanthophylls associated with the antenna complexes of PSII (Müller et al., 2001). In contrast, photoinhibition (qI) was significantly increased in adg1-1/tpt-1 compared with the wild type at both intermediate and high PFDs. The phenomenon of photoinhibition has been studied extensively (Powles, 1984; Horton and Hague, 1988; Long et al., 1994), and several models have emerged on its mechanism, such as photodamage and repair of the D1 protein of PSII, which occurs depending on the reduction state of QA (Melis, 1999), as well as reverse proton pumping by thylakoid ATPase under cold stress (Müller et al., 2001). For an effective repair of the D1 protein, it has to be posttranslationally modified by phosphorylation (Baena-Gonzáles et al., 1999). Moreover, a separate mechanism has been described for the photoinhibition of PSI, involving the chloroplast NDH1 complex (Teicher et al., 2000). In adg1-1/tpt-1, increased photoinhibition was observed even after short-term illumination with high light. The mechanisms leading to photoinhibition in adg1-1/tpt-1, however, have not been further addressed in this report. The third NPQ component, qT, is attributed to state transition, involving the phosphorylation of LHCII, the subsequent uncoupling of LHCII from PSII, and excitation transfer to PSI. In adg1-1/tpt-1, qT was slightly enhanced compared with the wild type after illumination with a high PFD (Table II). However, state transition has not been considered to play a major role in the protection of the photosystems (Müller et al., 2001). Recently, the kinase responsible for the phosphorylation of LHCII was identified in Arabidopsis (STN7; Bellafiore et al., 2005) as well as a homolog involved in the phosphorylation of PSII core proteins (STN8; Bonardi et al., 2005). STN8 appears to be involved in D1 phosphorylation but seems not to be directly involved in D1 repair. Interestingly, besides its role in state transition, STN7 appears also to be essential for long-term acclimation to changing light conditions (Bonardi et al., 2005) and seems to be linked to signal pathways leading to the acclimation of photosynthesis to environmental cues. Notably, the ape2 (= tpt-1) mutant was found in a screen aimed at the identification of mutants affected in such acclimation processes (Walters et al., 2003), suggesting that, apart from the obvious constraints in primary metabolism, signaling mechanisms controlling the expression of nucleus- and plastid-derived photosynthesis genes are also likely to be affected. Interestingly, tpt-1 exhibits an expression profile of deregulated genes (encoding mainly plastid-localized proteins) similar to that of the genome-uncoupled mutant gun5 (Biehl et al., 2005). There is, however, no obvious link between the defect in tetrapyrrole biosynthesis (gun5) and the impaired export of photoassimilates in the light (tpt-1). It is likely that common regulatory mechanism control gene expression in both mutants, an observation that deserves further investigation.
Oxidative Stress, Hyperstacking of Grana Thylakoids, and Increased Plastoglobule Number in adg1-1/tpt-1
Chloroplasts are the main source for ROS (i.e. singlet oxygen, superoxide anions, hydrogen peroxide, and hydroxyl radicals; Mittler et al., 2004). One source for superoxide anion radicals is the Mehler reaction (pseudocyclic electron transport), a process in which electrons are transferred from ferredoxin to molecular oxygen rather than to NADP. Superoxide radicals are subsequently detoxified by superoxide dismutase(s) and the Asc/glutathione system involving ascorbate peroxidases (Asada, 1999). The reduction of hydrogen peroxide to water eventually consumes NADPH generated by the photosynthetic light reaction. Interestingly, Asc levels were significantly lower in adg1-1/tpt-1 relative to the wild type in both the light and the dark, suggesting an increased requirement to detoxify this ROS (Table IV). A source for singlet oxygen is the excited triplet state of chlorophyll molecules in the photosystems. Recent data provide evidence that singlet oxygen appears to be the only ROS involved in photooxidative damage, such as lipid peroxidation (Triantaphylides et al., 2008). In the antenna complexes, carotenoids efficiently quench chlorophyll triplet states and thus prevent singlet oxygen generation inside the antenna (Frank and Cogdell, 1996). However, triplet states of PSII reaction centers appear to be unprotected from singlet oxygen formation by carotenoids. Recent data indicate that triplet states of P680 are scavenged by tocopherol (Krieger-Liszkay and Trebst, 2006). Strikingly, despite a 30% reduction in total chlorophyll contents, carotenoid contents were less affected in adg1-1/tpt-1, suggesting a higher ratio of carotenoid to chlorophyll molecules in the antenna and hence a more efficient quenching of chlorophyll triplet states compared with the wild type and the single mutant. Moreover, the increased abundance of plastoglobules in adg1-1/tpt-1 and their proposed function in tocopherol biosynthesis (Austin et al., 2006) might form a link to the protection of PSII by tocopherol. Besides their destructive potential, ROS produced in the chloroplast can modulate a number of cellular responses (reviewed by Baier and Dietz, 2005), and they are likely to be involved in redox signaling. The growth of unicellular algae (Escoubas et al., 1995) and higher plants (Pfannschmidt et al., 1999) at light qualities that selectively excite PSII or PSI suggested that changes in the redox state of PQ might act as the primary chloroplast redox signal. It is likely that ROS and ascorbate peroxidases are also involved in this process (Surpin et al., 2002). The impact of the light quality (PSI or PSII light) and hence of retrograde plastid redox signals on nuclear gene expression has been studied extensively in Arabidopsis wild-type and mutant plants affected in photomorphogenesis (Fey et al., 2005). It is likely that the hyperstacking of grana thylakoids, as observed in adg1-1/tpt-1, is linked to an altered redox poise in the stroma and the QA/PQ system. Moreover, hyperstacking of grana observed in chloroplasts of adg1-1/tpt-1 resembles that of plants grown in low light (Meier and Lichtenthaler, 1981). Thus, adg1-1/tpt-1 shows a low-light phenotype of grana stacking even in normal growth light, an aspect that deserves further investigation. The stacked grana regions contain only PSII and LHCII, whereas the cytochrome b/f complex and PSI as well as ATPase are associated with stroma thylakoids and the fringes of the grana thylakoids (Andersson and Anderson, 1980; Barber, 1982; Olive and Vallon, 1991). Moreover, components of chlororespiration appear to be associated with stroma thylakoids as well (Rumeau et al., 2007). The massive increase in stacked grana regions hence suggests more abundant PSII and LHCII relative to PSI in adg1-1/tpt-1 compared with the wild type (Fig. 5A). This notion was supported by a significant decline in the Chl a/b ratio in adg1-1/tpt-1, indicating a higher abundance of LHCII. Interestingly, both single mutants also showed a trend for increased grana stacking, indicating that the underlying mechanisms that trigger modifications in thylakoid organization in adg1-1/tpt-1 were already activated in tpt-1 and adg1-1. Surprisingly, the contents of total membrane lipids and of MGDG (a major constituent of the thylakoid membrane) declined in adg1-1/tpt-1 relative to the wild type by 14% and 19%, respectively, whereas PC was increased by 1.4-fold (Table V). These results resemble membrane lipid changes observed in plants suffering from chlorotic stress. For example, nitrogen deprivation in Arabidopsis causes reductions in chlorophyll content and in the amount of thylakoid membranes, accompanied by a strong decrease in MGDG content (Gaude et al., 2007). However, in adg1-1/tpt-1, the drop in lipid content correlates with decreases in both chloroplast size (Table V) and total chlorophyll contents (Table V) rather than with a diminished thylakoid membrane abundance.
The underlying mechanisms leading to a constitutive alteration in chloroplast ultrastructure, which, in the case of adg1-1/tpt-1, appears to be independent from the quality and intensity of the growth light, deserve further investigation. Likewise, the discrepancy between ETR and O2 evolution observed in adg1-1/tpt-1 (Fig. 4C) with increasing light intensities might be linked to changes in the chloroplast ultrastructure (i.e. a heterogeneity of Chl a fluorescence emission within stacked grana regions) and will be addressed in future studies. It is conceivable that central regions of the grana stacks contain completely reduced QA in the light and dark, whereas the fringe regions of the grana stacks adjacent to the stroma thylakoids perform wild-type-like photosynthesis. Such heterogeneity in Chl a fluorescence emission combined with photoinhibitory NPQ would be consistent with an underestimation of ETR in the double mutant.
CONCLUDING REMARKS
The data presented in this report show that a double mutant defective in starch biosynthesis combined with drastically reduced TPT activity shows a biochemical phenotype that at least partially resembles plants that were grown for several days in CO2-free air or that were iron limited. The lack of significant sinks for reducing power and energy generated by the light reaction of photosynthesis is hence perceived as severe (light/oxidative) stress by the plants, and adaptive strategies are installed to minimize damage and to retain substantial chlorophyll contents and basic photosynthetic rates. Adaptive strategies are reflected most prominently in the altered thylakoid organization and the higher abundance of plastoglobules. This double mutant, or transgenic approaches aimed at a transient knockdown of starch biosynthesis in a tpt mutant background or, vice versa, an inhibition of TPT expression in a starch-free background, could form a platform for the investigation of retrograde signaling mechanisms. Moreover, certain aspects linked to the organization of components of the photosynthetic electron transport chain, or the way the double mutant handles ROS or the disturbance of redox poise in general, deserve more thorough future investigations, in particular with the help of a complete knockout of the TPT in a starch-free background (if viable). Moreover, compensational changes in metabolic fluxes (e.g. by the induction of other known plastidial metabolite transporters and/or alternative routes of carbon utilization) could be more thoroughly addressed in a starch-free mutant that completely lacks TPT activity.
MATERIALS AND METHODS
Plant Growth
Unless stated otherwise, Arabidopsis (Arabidopsis thaliana) plants were initially germinated and grown on soil for 5 to 6 weeks in a temperature-controlled Percival growth cabinet at day/night temperatures of 22°C/18°C and a 12-h-light/12-h-dark-cycle. The average PFD at the plant level was 80 to 100 μmol m−2 s−1.
Photosynthesis Measurements
Modulated Chl a fluorescence emission from the upper surface of the leaf was measured with a pulse amplitude modulation fluorometer (PAM-2000 or PAM-2100; Walz; Schreiber et al., 1986). The ΦPSII and photosynthetic ETR were calculated according to Genty et al. (1989). Inhibitors (SHAM, OG, and antimycin A) were supplied to excised leaves, which were plunged into the solution for 30 s and than further incubated with their petioles in the solution for up to 6 h. FR light was either supplied by the integrated source of the pulse amplitude modulation fluorometer at a PFD of 50 μmol m−2 s−1 (maximum at λ = 735 nm) or by a Schott lamp combined with a Schott filter (RG9). The latter allowed a maximum PDF of 1,300 μmol m−2 s−1 (at a λ between 700 and 800 nm) at the highest intensity of the Schott lamp. Under these conditions, the maximum PFD of photosynthetic active radiation detectable was 6.5 μmol m−2 s−1 (at a λ between 690 and 700 nm). For the determination of absolute Chl a fluorescence levels, care was taken that the illuminated leaf areas were of similar size. NPQ relaxation kinetics were determined according to Quick and Stitt (1989) with an automated run implemented in the PAM 2100 software.
Oxygen gas exchange was determined with a leaf disc electrode (Hansatech) in a temperature-controlled measuring electrode disc chamber. Individual leaves were cut, and the area was determined by the imaging and weighing method. The effective chamber volume in the presence of the leaf sandwich varied between 4.8 and 5 mL. Light was supplied by a light-emitting diode source (Hansatech) with a maximum PDF of 780 μmol m−2 s−1 at the leaf level. The oxygen electrode was equipped with an inlet for a PAM 2100 fiber optic, which was located at a distance of 1 cm above the surface of the leaves. The Fv/Fm determined under these conditions with the individual plant lines were identical to those determined under standard conditions.
Determination of Pyridine Nucleotides, Ascorbate, Photosynthetic Pigments, and Lipids
Pyridine nucleotides in leaf extracts were determined by the enzymatic cycling method (Lowry et al., 1961b) essentially as described by Matsumora and Miyachi (1980), taking into account different stabilities of oxidized and reduced forms of pyridine nucleotides in alkaline or acidic solutions (Lowry et al., 1961a).
Asc and DAsc were determined colorimetrically essentially as described by Henninger (1981) and the application note by Roche (product no. 0409677).
Photosynthetic pigments were extracted with 100% acetone. Chlorophyll contents as well as Chl a/b ratios were assayed in 80% acetone and calculated as described by Graan and Ort (1984). Total carotenoids were determined in the same extracts at a wavelength of 480 nm, and the contents were calculated using the following equation: car = E480 + 0.144·E663 − 0.638·E645.
Lipids were isolated from leaves and separated by thin-layer chromatography according to Dörmann et al. (1995). Lipids were isolated from thin-layer chromatography plates and quantified by gas chromatography as fatty acid methyl esters using pentadecanoic acid (15:0) as an internal standard (Browse et al., 1986).
Real-Time Reverse Transcription-PCR
Total RNA was extracted with guanidinium thiocyanate-phenol-chloroform modified after Chomczynski and Sacchi (1987). The RNA preparations were treated with DNA-free DNase (Ambion; http://www.ambion.com) to remove contaminations of genomic DNA. For a two-step reverse transcription (RT)-PCR analysis, total RNA (5 μg per reaction) was reverse transcribed into cDNAs using Reverse Transcriptase BioScript (Bioline; http://www.bioline.com) according to the manufacturer's instructions. Subsequently, the cDNAs obtained formed the templates in real-time PCR experiments with gene-specific primers (for primer sequences, see Supplemental Table S2) in a 7300 Real-Time PCR System (Applied Biosystems; www.appliedbiosystems.com) using the SYBR Green I Master Kit (Roche Diagnostics) according to the manufacturer's instructions. The Ct, defined as the PCR cycle at which a statistically significant increase of reporter fluorescence is detected, was used as a measure for the starting copy number of the target genes. Relative quantification of expression levels was performed using the comparative Ct method (Ramakers et al., 2003). The relative value for the expression level of each gene was calculated by the equation Y = 2−ΔΔCt, where ΔCt is the difference between control and target products [ΔCt = Ct(GENE) − Ct(ACT) and ΔΔCt = ΔCt(mutant) − ΔCt(wt)]. Thus, the calculated relative expression values are normalized to the expression levels in the wild type (wild type = 1, i.e. a log2 ratio of 0). The efficiency of each primer pair was tested using wild-type cDNA as a standard template, and the RT-PCR data were normalized according to the relative efficiency of each primer pair. Two different technical replicates and two independent sets of plants were used for the analyses.
Electron Microscopy
Leaf tissue pieces (about 1 mm2) were fixed with 2.5% glutaraldehyde in 50 mm phosphate buffer, pH 7.4, for 4 to 6 h at 4°C. After three rinses in distilled water, the samples were postfixed in 1% osmium tetroxide in distilled water for 1 h at 4°C, dehydrated in a graduated ethanol series, including a step with 1% uranyl acetate (in 50% ethanol, 2 h, 4°C), embedded in Epon resin, and polymerized at 65°C for approximately 48 h. Ultrathin sections (60–70 nm) were cut with a diamond knife (Diatome) on a Leica Ultracut UCT ultramicrotome (Leica Microsystems) and mounted on pioloform-coated copper grids. The sections were stained with lead citrate and uranyl acetate (Reynolds, 1963) and viewed with a Carl Zeiss EM 902A transmission electron microscope at 80 kV. Micrographs were taken using EMS electron microscopy film (Maco).
Statistical Analysis
Significance analysis was determined using the Welch test, which allows for unequal variances (Lorenz, 1984).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Traces of modulated Chl a fluorescence of an adg1-1/tpt-1 leaf.
Supplemental Figure S2. Relaxation kinetics of Chl a fluorescence in adg1-1/tpt-1 and wild-type plants.
Supplemental Figure S3. The impact of the PFD on O2 evolution and respiratory O2 consumption.
Supplemental Table S1. Effect of inhibitor treatment on pyridine nucleotide levels and ratios in the wild type, the adg1-1 and tpt-1 single mutants, and the adg1-1/tpt-1 double mutant.
Supplemental Table S2. Primer pairs used in real-time RT-PCR experiments.
Supplementary Material
Acknowledgments
We thank Dr. Uwe Rascher (Forschungszentrum Jülich) for the determination of the transmission PFD spectra of FR filters.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to J.S.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Rainer E. Häusler (rainer.haeusler@uni-koeln.de).
The online version of this article contains Web-only data.
References
- Aluru MR, Rodermel SR (2004) Control of chloroplast redox by the IMMUTANS terminal oxidase. Physiol Plant 120 4–11 [DOI] [PubMed] [Google Scholar]
- Andersson B, Anderson JM (1980) Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta 593 427–440 [DOI] [PubMed] [Google Scholar]
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50 601–639 [DOI] [PubMed] [Google Scholar]
- Austin JR 2nd, Frost E, Vidi PA, Kessler F, Staehelina LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenson TJ, Cruz JA, Kramer DM (2004) Modulation of energy-dependent quenching of excitons in antennae of higher plants. Proc Natl Acad Sci USA 101 5530–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Gonzáles E, Barbato R, Aro EM (1999) Role of phosphorylation in the repair cycle and oligomeric structure of photosystem II. Planta 208 196–204 [Google Scholar]
- Baier M, Dietz KJ (2005) Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. J Exp Bot 56 1449–1462 [DOI] [PubMed] [Google Scholar]
- Barber J (1982) Influence of surface charges on thylakoid structure and function. Annu Rev Plant Physiol 33 261–295 [Google Scholar]
- Belkhodja R, Morales F, Quilez R, Lopez-Millan AF, Abadia A, Abadia J (1998) Iron deficiency causes changes in chlorophyll fluorescence due to the reduction in the dark of the photosystem II acceptor side. Photosynth Res 56 265–276 [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433 892–895 [DOI] [PubMed] [Google Scholar]
- Bennoun P (1982) Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA 79 4352–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biehl A, Richly E, Noutsos C, Samalini F, Leister D (2005) Analysis of 101 nuclear transcriptomes reveals 23 distinct regulons and their relationships to metabolism, chromosomal gene distribution and co-ordination of nuclear and plastid gene expression. Gene 344 33–41 [DOI] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437 1179–1182 [DOI] [PubMed] [Google Scholar]
- Bondarava N, De Pascalis L, Al-Babili S, Goussias C, Golecki JR, Beyer P, Bock R, Krieger-Liszkay A (2003) Evidence that cytochrome b559 mediates the oxidation of reduced plastoquinone in the dark. J Biol Chem 278 13554–13560 [DOI] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152 141–145 [DOI] [PubMed] [Google Scholar]
- Casano LM, Zapata JM, Martin M, Sabater B (2000) Chlororespiration and poising of cyclic electron transport: plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J Biol Chem 275 942–948 [DOI] [PubMed] [Google Scholar]
- Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C (1991) Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol 95 1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi S (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162 156–159 [DOI] [PubMed] [Google Scholar]
- Clifton R, Millar AH, Whelan J (2006) Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochim Biophys Acta 1757 730–741 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis thaliana. Plant Physiol 139 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethelm R, Miller MG, Shibles R, Stewart CR (1990) Effect of salicylhydroxamic acid on respiration, photosynthesis, and peroxidase activity in various plant tissues. Plant Cell Physiol 31 179–185 [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durchan M, Vacha F, Krieger-Liszkay A (2001) Effects of severe CO2 starvation on the photosynthetic electron transport chain in tobacco plants. Photosynth Res 68 203–213 [DOI] [PubMed] [Google Scholar]
- Escoubas J-M, Lomas M, La Roche J, Falkowski PG (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinol pool. Proc Natl Acad Sci USA 92 10237–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber A, Young AJ, Ruban AV, Horton P, Jahns P (1997) Dynamics of xanthophyll-cycle activity in different antenna subcomplexes in the photosynthetic membranes of higher plants (the relationship between zeaxanthin conversion and nonphotochemical fluorescence quenching). Plant Physiol 115 1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey V, Wagner R, Bräutigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmüller R, Pfannschmidt T (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280 5318–5328 [DOI] [PubMed] [Google Scholar]
- Flügge UI, Häusler RE, Ludewig F, Fischer K (2003) Functional genomics of phosphate antiport systems of plastids. Physiol Plant 118 475–482 [Google Scholar]
- Foyer C, Rowell J, Walker D (1983) Measurement of ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 257 239–244 [DOI] [PubMed] [Google Scholar]
- Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63 257–264 [DOI] [PubMed] [Google Scholar]
- Gaude N, Bréhélin C, Tischendorf G, Kessler F, Dörmann P (2007) Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J 49 729–739 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- Graan T, Ort DR (1984) Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem 259 14003–14010 [PubMed] [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36 541–549 [DOI] [PubMed] [Google Scholar]
- Hattenbach A, Müller-Röber B, Nast B, Heineke D (1997) Antisense-repression of both ADP-glucose pyrophosphorylase and triose phosphate translocator modifies carbohydrate partitioning in potato leaves. Plant Physiol 115 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Flügge UI (2000. a) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum). II. Assessment of control coefficients of the triose phosphate/phosphate translocator. Planta 210 383–390 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Nicolai P, Fischer K, Fischer KL, Flügge UI (2000. b) Control of carbon partitioning and photosynthesis by the triose phosphate/phosphate translocator in transgenic tobacco plants (Nicotiana tabacum). I. Comparative physiological analysis of tobacco with antisense repression and overexpression of the triose phosphate/phosphate translocator. Planta 210 371–382 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Schlieben NH, Schulz B, Flügge UI (1998) Compensation of decreased triosephosphate/phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta 204 366–376 [DOI] [PubMed] [Google Scholar]
- Heineke D, Kruse A, Flügge UI, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW (1994) Effect of antisense repression of the chloroplast triosephosphate translocator on photosynthetic metabolism in transgenic potato plants. Planta 193 174–180 [Google Scholar]
- Heineke D, Riens B, Grosse H, Hoferichter P, Flügge UI, Heldt HW (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol 95 1131–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger G (1981) Enzymatische Bestimmung von L-Ascorbinsäure in Lebensmitteln, Pharmazeutika und biologischen Flüssigkeiten. Alimenta 20 12–14 [Google Scholar]
- Horton P, Hague A (1988) Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts. IV. Resolution of non-photochemical quenching. Biochim Biophys Acta 932 107–115 [Google Scholar]
- Joët T, Cournac L, Horvath EM, Medgyesy P, Peltier G (2001) Increased sensitivity of photosynthesis to antimycin A induced by inactivation of chloroplast ndhB gene: evidence for the participation of NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol 125 1919–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around photosystem I in C(3) plants: in vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128 760–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse EM, Alcaraz JP, Laboure AM, Kuntz M (2003) In vitro characterization of a plastid terminal oxidase (PTOX). Eur J Biochem 270 3787–3794 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Trebst A (2006) Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction center. J Exp Bot 57 1677–1684 [DOI] [PubMed] [Google Scholar]
- Krömer S, Stitt M, Heldt HW (1988) Mitochondrial oxidative phosphorylation participating in photosynthetic metabolism of a leaf cell. FEBS Lett 226 352–356 [Google Scholar]
- Kruk J, Karpinski S (2006) An HPLC-based method of estimation of the total redox state of plastoquinone in chloroplasts, the size of the photochemically active plastoquinone-pool and its redox state in thylakoids of Arabidopsis. Biochim Biophys Acta 1757 1669–1675 [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J (1988) Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) lacking ADPglucose pyrophosphorylase activity. Plant Physiol 86 1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45 633–662 [Google Scholar]
- Lorenz RJ (1984) Grundbegriffe der Biometrie. In RJ Lorenz, J Vollmar, eds, Biometrie. Gustav Fischer Verlag, Stuttgart, Germany, pp 174–175
- Lowry OH, Passonneau JV, Rock MK (1961. a) The stability of pyridine nucleotides. J Biol Chem 236 2756–2759 [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Schulz DW, Rock MK (1961. b) The measurement of pyridine nucleotides by enzymatic cycling. J Biol Chem 236 2746–2752 [PubMed] [Google Scholar]
- Matsumora H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69 465–470 [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51 659–668 [DOI] [PubMed] [Google Scholar]
- Meier D, Lichtenthaler HK (1981) Ultrastructure of chloroplasts in radish seedlings grown at high- and low-light conditions in the presence of the herbicide bentazon. Protoplasma 107 195–207 [Google Scholar]
- Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4 130–135 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9 490–498 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa KI, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429 579–582 [DOI] [PubMed] [Google Scholar]
- Munshi MK, Kobayashi Y, Shikanai T (2006) Chlororespiratory reduction 6 is a novel factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Physiol 141 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon PJ (2000) Chlororespiration. Philos Trans R Soc Lond B Biol Sci 355 1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49 249–279 [DOI] [PubMed] [Google Scholar]
- Olive J, Vallon O (1991) Structural organization of the thylakoid membrane: freeze-fracture and immuno-cytochemical analysis. J Electron Microsc Tech 18 360–374 [DOI] [PubMed] [Google Scholar]
- Ort DR (1986) Energy transduction in oxygenic photosynthesis: an overview of structure and mechanism. In LA Staehlin, CJ Arntzen, eds, Encyclopedia of Plant Physiology, Vol 19. Springer Verlag, Berlin, pp 179–186
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53 523–550 [DOI] [PubMed] [Google Scholar]
- Pesaresi P, Schneider A, Kleine T, Leister D (2007) Interorganellar communication. Curr Opin Plant Biol 10 600–606 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397 625–628 [Google Scholar]
- Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35 15–44 [Google Scholar]
- Quick WP, Stitt M (1989) An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochim Biophys Acta 977 287–296 [Google Scholar]
- Raghavendra AS, Padmasree K (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8 546–553 [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 13 62–66 [DOI] [PubMed] [Google Scholar]
- Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Flügge UI, Schulz B, Heineke D, Heldt HW, Willmitzer L, Frommer WB (1993) Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA 90 6160–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Lloyd JR, Eckermann N, Rottmann A, Kossmann J, Steup M (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci USA 99 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodermel S (2001) Pathways of plastid-to-nucleus signalling. Trends Plant Sci 6 471–478 [DOI] [PubMed] [Google Scholar]
- Rosso D, Ivanov AG, Fu A, Geisler-Lee J, Hendrickson L, Geisler M, Stewart G, Krol M, Hurry V, Rodermel SR, Maxwell DP, Hüner NP (2006) IMMUTANS does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady-state photosynthesis. Plant Physiol 142 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Horton P (1999) The xanthophyll cycle modulates the kinetics of nonphotochemical energy dissipation in isolated light-harvesting complexes, intact chloroplasts, and leaves of spinach. Plant Physiol 119 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D, Bécuwe-Linka N, Beyly A, Louwagie M, Garin J, Peltier G (2005) New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 17 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30 1041–1051 [DOI] [PubMed] [Google Scholar]
- Schneider A, Häusler RE, Kolukisaoglu Ü, Kunze R, Van der Graaff E, Schwacke R, Catoni E, Desimone M, Flügge UI (2002) An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J 32 685–699 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10 51–62 [DOI] [PubMed] [Google Scholar]
- Surpin M, Larkin R, Chory J (2002) Signal transduction between the chloroplast and the nucleus. Plant Cell 14 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher HB, Møller BL, Scheller HV (2000) Photoinhibition of photosystem I in field-grown barley (Hordeum vulgare L.): induction, recovery and acclimation. Photosynth Res 64 53–61 [DOI] [PubMed] [Google Scholar]
- Triantaphylides C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ (2008) Singlet oxygen is the major reactive oxygen species involved in photo-oxidative damage to plants. Plant Physiol 148 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Ibrahim DG, Horton P, Kruger NJ (2004) A mutant of Arabidopsis lacking the triose-phosphate/phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiol 135 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Shepard F, Rogers JJM, Rolfe SA, Horton P (2003) Identifications of mutants of Arabidopsis thaliana defective in acclimation of photosynthesis to the light environment. Plant Physiol 131 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Portis AR Jr (2007) A novel nucleus-encoded chloroplast protein, PIFI, is involved in NAD(P)H dehydrogenase complex-mediated chlororespiratory electron transport in Arabidopsis. Plant Physiol 144 1742–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise SE, Weber APM, Sharkey TD (2004) Maltose is the major form of carbon exported from the chloroplast at night. Planta 218 474–482 [DOI] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The IMMUTANS variegation locus of Arabidopsis decodes a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Kofler H, Häusler RE, Hille D, Flügge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not the chloroplast hexose transporter. Plant Cell 13 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








