Abstract
A case-control study was performed in the Kruger National Park (KNP), South Africa, to find out whether impala (Aepyceros melampus) were more likely to harbor tetracycline-resistant Escherichia coli (TREC) in their feces when they drank from rivers that contained these bacteria than when they drank from rivers that were uncontaminated with TREC. The following five perennial rivers were selected: the Crocodile, the Letaba, the Olifants, the Sabie, and the Sand. Samples of river water (n = 33) and feces (n = 209), collected at 11 different sites, were cultured for E. coli. The resulting colonies were screened for tetracycline resistance by use of the Lederberg replica plating method (breakpoint, 4 mg/liter). A resistant and/or a susceptible isolate was then selected from each sample and subjected to the CLSI MIC broth microdilution test for tetracyclines. Among the 21 water specimens contaminated by E. coli, 19.05% (n = 4) were found to be resistant by the MIC method (breakpoint, ≥8 mg/liter). This led to the Crocodile, Olifants, and Letaba rivers being classified as TREC positive. Among the 209 impala feces sampled, 191 were positive for the presence of E. coli (91.38%). Within these (n = 191), 9.95% (n = 19) of the isolates were shown to be TREC by the MIC method. It was found that 1.11% (n = 1) of the E. coli isolates cultured from the feces of the control group (n = 90) were TREC, in comparison with 17.82% (n = 18) of those in feces from the exposed group (n = 101). The calculation of the odds ratio showed that impala drinking from TREC-contaminated rivers were 19.3 (2.63 to 141.69) times more likely to be infected with TREC than were unexposed impala. This is a significant finding, indicating that surface water could be a possible source of antimicrobial resistance in naïve animal populations and that impala could act as sentinels for antimicrobial resistance.
The number of antimicrobial-resistant (AMR) bacteria in the environment increases exponentially with the misuse of antimicrobials, as a result of increasing selective pressure on bacterial populations (29, 41, 46). Furthermore, AMR is increasing, and its spread between different bacterial strains in different habitats has been demonstrated (19, 25, 38, 43, 47, 51, 52). This has led the World Organization for Animal Health and the World Health Organization to identify AMR as a major threat to the well-being of humans and animals (10, 29, 54, 55). Programs for the surveillance of AMR have been initiated in many countries (1, 9, 17, 18, 21, 28, 31, 42, 48). Although there are several studies assessing AMR in Escherichia coli populations of animal origin, not much work has been done on the ecology of AMR (5, 37). The spread of AMR into environments where antibiotics are not used is a possibility that has not yet been well researched, although it has been postulated that water could disseminate AMR (58).
Tetracyclines are the most common class of antimicrobials sold for the treatment of livestock in South Africa, as they are essential for the treatment of common tick-borne diseases, such as anaplasmosis and heartwater (33). They are also used to treat a variety of erosive diseases in livestock. Furthermore, they are registered for oral use in these species and can be used without prescription (27). In humans, they are also used in malaria prophylaxis and therapy in areas such as the Kruger National Park (KNP), South Africa, where there is resistance to the chloroquines (4). It has been shown that tetracycline-resistant Escherichia coli (TREC) is highly prevalent in food animals in South Africa (32, 36). Recent studies have shown that the various tet genes, coding for tetracycline resistance, can be exchanged between bacteria, including enteric bacteria, in many ecosystems (6, 19). Since E. coli is present in the digestive tracts of both humans and animals and the potential for the dissemination of tetracycline resistance is thus likely to be high, it was chosen as the indicator organism for this study. It has been postulated that commensal bacterial populations in the intestinal tracts of unexposed animals should have low levels of AMR (37). Thus, TREC in unexposed wildlife can be used as a model to study the dissemination of AMR in the environment. The aim of this study was to investigate whether wild impala (Aepyceros melampus) in a natural environment and unexposed to antimicrobial treatment with tetracyclines but drinking from contaminated rivers were more likely to contain TREC in their feces than unexposed wild impala in a similar environment.
MATERIALS AND METHODS
Study area.
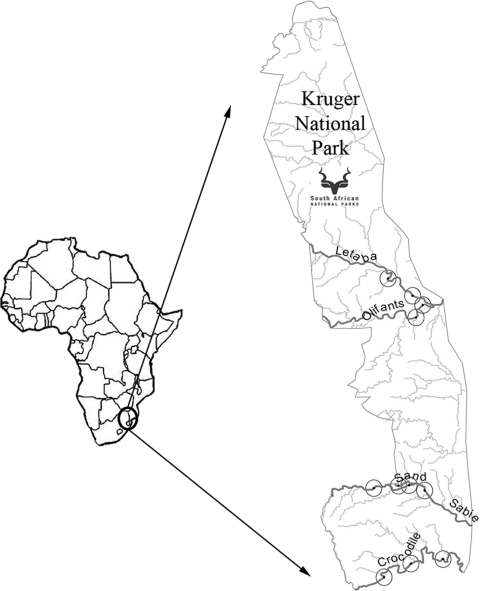
The KNP in South Africa was selected as the study area for this research. This conservancy area covers a surface area of 18,989 km2. It has free-ranging animals that have never been treated with antimicrobials or had contact with feces from domesticated animals or humans. Practically speaking, these animals could be considered a naïve population when it comes to antimicrobial drug-induced AMR. Five rivers in the area, with different levels of pollution, were chosen for the study. These were the Crocodile, the Letaba, the Olifants, the Sabie, and the Sand rivers (Fig. 1). The first characteristic considered for the choice of the rivers was their location, as all of them had to be situated where there were large numbers of impala. Secondly, perennial rivers were considered so it would be possible to take samples in different periods of the year and so some would have been polluted by animal and human waste before entering the KNP.
FIG. 1.
Study area, sampled rivers, and sampling points.
Study design.
In terms of the case-control study design (44), rivers were divided into those considered polluted and those considered unpolluted on the basis of a retrospective study of data on the chemical composition of water. Once water samples were cultured, the presumption was found to be correct, and the Crocodile, Letaba, and Olifants rivers were classified as TREC positive, while the Sabie and Sand rivers were classified as TREC negative. Impala drinking from these two groups of rivers were similarly classified as exposed and unexposed to TREC. A total of 11 points along these rivers were sampled on three separate occasions, including 3 points on the Crocodile, 2 on the Letaba, 2 on the Olifants, 3 on the Sabie, and 1 on the Sand (Fig. 1). Special attention was given to selecting points that had a minimum distance between them of 10 km or a physical barrier, thus avoiding mixing between the highly territorial impala herds. The river sampling points were selected on the basis of a study of retrospective data on impala distribution obtained from the KNP and on observational data. On the same day that water was collected, impala herds were selected within a 5-km radius of the river samples, and after tracking them to confirm their drinking points, freshly voided fecal pellets (approximately 10 g) were collected.
Sample size.
The estimation of the minimum sample size (nmin) of feces needed for the case and the control groups was found by applying the equation of Cobb (12, 44), as follows: nmin = {[(r + 1)/r][(p̄)(1 − p̄)(Zβ + Zα/2)2/(p1 − p2)2]}, where n is an estimation of sample size in the case group, r is the ratio of controls to cases, p̄ is the average prevalence of exposed [(p1 + p2)/2)] cases, p1 is the estimated prevalence of cases, p2 is the estimated prevalence of controls, Zα/2 is the normal standard deviation for type II error, and Zβ is the normal standard deviation for type I error. The estimated prevalence of cases was derived from the following formula: p1 = (OR p2)/[p2(OR − 1) + 1], where OR is the expected odds ratio and p2 is the estimated prevalence of controls.
When the expected maximum prevalence of TREC in the control group was estimated to be 5% and the minimum OR was 5, the expected case prevalence was calculated as 0.21. In addition, when equal numbers of cases and controls (r = 1) are collected, and assuming a power of 80% with a significance level of 0.05 (Zα/2 = 0.84 and Zβ = 1.96), the minimum sample size for cases and controls is as follows: nmin = {[(1 + 1)/1][(0.13)(1 − 0.13)(0.84 + 1.96)2/(0.21 to 0.05)2]} = 89. Taking into consideration that most of the parameters in the equation were assumed and that the larger the sample size the closer is the possibility of a true estimation, about 105 samples were collected per group.
Sample collection.
Each water point was sampled three different times during the year (March, May, and July), using geographical positioning system coordinates recorded with an e-Trex instrument (Garmin, Johannesburg, South Africa) and transferred to Mapsource (Garmin) software. Samples of flowing river water (500 ml) were collected into sterile glass bottles (Lasec, Johannesburg, South Africa). A five-step sampling technique was used, as described by the Department of Water Affairs and Forestry (14). Within a distance of 5 km from each water sampling point, about 10 g of fresh fecal pellets was collected from the top of fecal middens, using a sterile latex glove. Five fecal samples were collected per water sampling point from all the rivers, with the exception of the Sabie River, where 10 samples were collected. The samples were stored in sterile plastic sample bottles (PlastPro, Johannesburg, South Africa) in a cooler bag at ±4°C until their arrival at the laboratory.
Isolation of E. coli.
Once the water samples arrived at the laboratory, they were prefiltered using a paper filter (1336/2; Munktell, Krugersdorp, South Africa) and then filtered through a 47-mm-wide sterile cellulose nitrate filter with a 45 ± 2-μm pore diameter (Sartorius, Krugersdorp, South Africa). Each membrane was placed in 10 ml of peptone water (Oxoid, Basingstoke, United Kingdom) and incubated overnight at 37°C. The next day, seven serial 10-fold dilutions were made of the broth culture, and 0.1-ml aliquots of the last three dilutions (10−7, 10−6, and 10−5) were spread plated onto MacConkey agar plates (Oxoid). Each fecal sample was mixed thoroughly, and 1 g was put into a sterile bottle with 10 ml of physiological saline solution (BR 53; Oxoid). Six serial 10-fold dilutions were made using physiological saline, and 0.1-ml aliquots of the last three dilutions (10−7, 10−6, and 10−5) were spread plated onto MacConkey agar (CM0507-CM7b; Oxoid) and incubated overnight at 37°C.
First screen for tetracycline resistance, using the replica plating method.
One MacConkey plate for each sample that showed the growth of a good number (30 to 50) of separate colonies with the morphology of E. coli was copied, following the replica plating method of Lederberg (23, 30). In brief, a sterile velvet-covered disk the size of the plate was pressed onto the chosen MacConkey plate, removed, and printed onto an unused MacConkey agar plate, to which 4 μg/ml of doxycycline hydrochloride powder (p13177; Medpet, Johannesburg, South Africa) was added. After incubation, six lactose-fermenting colonies, three showing tetracycline resistance at 4 μg/ml and three not showing resistance, were selected from each MacConkey plate.
Identification of E. coli.
Selected colonies were streaked onto 5% horse blood-enriched Columbia agar (CM0331 Columbia agar base; Oxoid) and incubated at 37°C overnight for identification purposes. Resistant (at 4 μg/ml) and sensitive pure cultures were identified as E. coli when they were gram-negative short rods that were oxidase negative, catalase positive, spot indole positive, motile, and citrate negative. Once E. coli had been isolated and identified from each sample, it was stored in 2.2-ml Nunc cryotubes containing 1 ml brain heart infusion broth (Oxoid) and 1 ml 10% glycerol (Merck Pty., Ltd.) in an ultracool freezer (Forma Scientific) at −86°C.
Tetracycline resistance susceptibility determination.
The MIC of tetracycline was determined on all the stored E. coli isolates by using the guidelines of the Clinical Laboratory Standards Institute and freshly grown overnight cultures (11). The accuracy of the method was checked by testing the control strain E. coli ATCC 25922 and by performing the test in triplicate. The MIC was read as the lowest concentration inhibiting visible growth. The test was repeated when the MIC for the quality control strain disagreed by more than one dilution from the expected results, when the MIC results showed a disagreement of more than one dilution, or when the two results were around the cutoff value.
Data analysis.
Data were entered and analyzed using Microsoft Excel (2003) software. Quantitative data were analyzed for significant corelations. The strength of association was calculated between TREC-positive and -negative water sources and fecal samples by using the OR. The significance of the OR was then estimated using the Mantel-Haenszel χ2 test, with a degree of freedom of 1 and a P value of 0.0001. Maps were elaborated using ArcGIS (ESRI).
RESULTS
The initial classification of rivers into polluted and unpolluted categories based on their upstream land use (56, 57) was confirmed by the MIC results. While TREC isolates comprised 0% of the E. coli strains from water samples collected from the Sabie and Sand rivers, TREC isolates made up 25% of the E. coli strains from water samples collected from the Crocodile, Olifants, and Letaba rivers. In particular, the maximum number of TREC isolates was detected from the Letaba River, where 50% of the E. coli strains isolated were tetracycline resistant, followed by 20% from the Olifants River and 14.28% from the Crocodile River.
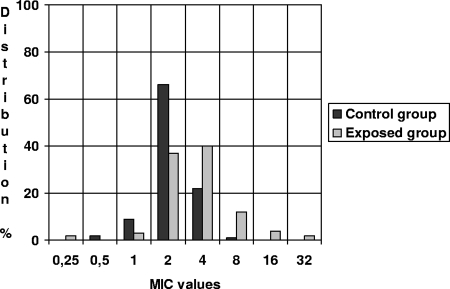
It was found that 91.38% of the overall fecal samples cultivated contained E. coli. The different distributions of the TREC MICs among the feces for the river groups are displayed in Fig. 2. The percentage of fecal TREC isolates per river is reported in Table 1. Among the fecal TREC isolates, 42.1%, 36.68%, and 15.72% of the total resistant samples were collected from impala in areas close to the Crocodile, Letaba, and Olifants rivers, respectively. Only 5% of the TREC samples originated from the control group of impala, who were drinking from the Sand River. Furthermore, the highest MICs originated from the exposed group (Table 2). For the control group, none of the MICs for tetracycline resistance exceeded 8 mg/liter, while in the exposed group 11.88%, 3.96%, and 1.98% of the samples had MICs of 8, 16, and 32 mg/liter, respectively. There were thus clear differences between the two groups. This was further supported by the fact that the calculated OR was 19.3 (2.63 to 41.69). This result clearly shows that feces of impala drinking from TREC-positive rivers were more likely to contain TREC than were feces of impala drinking from TREC-negative rivers. The χ2 test was run to find out whether the value was significant, and the result was 14.76, confirming that the association (OR) between exposed and unexposed groups and the exposure factor (TREC) was highly significant (P < 0.0001).
FIG. 2.
Bar chart showing distributions of fecal TREC MICs in the control and exposed groups.
TABLE 1.
Prevalence of TREC isolates in fecal samples per river according to MIC results (breakpoint, ≥8 mg/liter)
| River | No. of samples | No. of E. coli-positive samples | % TREC-positive samples |
|---|---|---|---|
| Sand | 15 | 14 | 7.14 |
| Sabie | 89 | 76 | 0 |
| Crocodile | 45 | 42 | 19.05 |
| Olifants | 30 | 29 | 10.34 |
| Letaba | 30 | 30 | 23.33 |
| Total | 209 | 191 | 9.95 |
TABLE 2.
Distribution of tetracycline MICs for E. coli strains isolated from impala feces (n = 191) obtained from different river zones
| River | % of samples with MIC (mg/liter)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |
| Sand | 28.57 | 64.29 | 7.14 | |||||
| Sabie | 2.63 | 10.53 | 72.37 | 14.47 | ||||
| Crocodile | 23.81 | 57.14 | 9.52 | 7.14 | 2.38 | |||
| Olifants | 6.9 | 3.45 | 51.72 | 27.59 | 10.34 | |||
| Letaba | 6.67 | 40 | 30 | 16.67 | 3.33 | 3.33 | ||
DISCUSSION
TREC and river pollution.
The results confirmed that impala drinking from contaminated rivers have a statistically higher level of TREC among the commensal flora in their intestines than do unexposed impala. The TREC-contaminated rivers (Crocodile, Letaba, and Olifants rivers) were found to receive water from processed urban and industrial sewage as well as runoff from agriculture, forestry, and farming activities (56, 57). In contrast, the Sabie-Sand catchment areas and river banks are characterized by conservation areas and small villages (56, 57). It is generally accepted that both human and veterinary medicines include a relatively large proportion of tetracycline, administered both parenterally and orally, due to tropical diseases in South Africa (27), and it is postulated that this would result in both AMR elements and tetracycline residues appearing in sewage. The potential exchange of AMR elements during waste treatment has been recognized (15, 16, 24, 40, 49). In fact, Reinthaler et al. (34) observed that AMR was more common in effluents from sewage treatment plants than in those before treatment, indicating that there is a possibility of the exchange of resistance genes in sewage treatment plants (34).
Models used for evaluation of AMR.
The model used in this research was a cheap, simple, yet successful system that could possibly be extended to include testing for other classes of antimicrobials and bacteria. The replica plating test (23) was used as a screening test, and the more accurate MIC (11) was used to classify the isolates. A cutoff of 8 mg/liter was selected for the MIC test because according to the CLSI, the MIC breakpoints of E. coli for tetracycline are ≤4 mg/liter for sensitive, 8 mg/liter for intermediately resistant, and ≥16 mg/liter for highly resistant bacteria (11). Since the bacteria isolated did not come from an environment with a high selective pressure and the results were not important for the determination of therapeutic success, as in the case of clinical environments, the intermediate and highly resistant strains were considered together as indicators of resistance. The 4-mg/liter breakpoint, in contrast, was chosen for screening with the replica plating test to eliminate sensitive bacteria without accidentally eliminating borderline colonies that were resistant at a level of 8 mg/liter when tested later using MIC testing. A recently published study seems to have experimented with a new method that can give the same, if not more accurate, results in less time (50). It is suggested that future studies could be done to compare the two methods for the detection of TREC.
Wildlife and conservancy areas as models for environmental research.
Wildlife populations, such as impala, within large nature reserves are useful for the study of the dissemination of AMR in nature, as they have never been treated with antimicrobials, have no direct contact or access to the feces of domesticated animals and humans, and often live in areas with low population densities. They can thus be used to study the role that environmental pollution, in particular polluted water, plays in the dissemination of tetracycline resistance. Impala proved to be a good model of a näive population, as they are widely distributed in different ecosystems, have a known social structure, and most of all, are territorial (39). Thus, an association with TREC in rivers used as a drinking water source proved feasible. It was critical in the design of the study to minimize any source of tetracycline resistance for the impala other than water. There was no possibility that the impala population studied had ever had contact with any treated animals. The KNP is fenced all around, no pets are allowed, the public are not permitted to walk around unsupervised, and staff are encouraged to practice good hygiene, such as fecal burial. Migratory birds that may have come into contact with resistant bacteria elsewhere could feasibly pose a very small risk of transfer of AMR (45). The other risk factor is the presence of natural resistance of E. coli to tetracycline, which will always be present at a low level in a bacterial population, even if the bacterium has never been exposed to antimicrobials. Many microbes produce antibiotics (bacteriocins) that give them a competitive advantage, but the levels of bacteriocins and consequent AMR are low in natural compartments (20). Even this small amount of natural resistance would obviously contribute a background AMR value for both groups (case and control), but it did not negatively affect the validity of this study. The presence of bacteria producing tetracycline in soil is also known, but the level has always been recorded as very low or under detection limits when synthetic antibiotics are not used (20, 35). Furthermore, most E. coli isolates originate from feces and only survive well under moist conditions (7, 8, 53). The dry conditions of most of the soil in the KNP would not be conducive to the survival of this bacterium. Thus, we can assume that water was the main source of AMR for impala in this study. Davison postulated the presence of tetracycline resistance in water, both in bacterial flora and as naked DNA (13). By selecting only E. coli for testing, naked DNA or other bacterium-related AMR transmission could have been missed during the current study. Kümmerer also suggested that resistance would be more common in commensal bacteria (including E. coli) from animals treated with antibiotics (20). It is thus felt that E. coli was a good indicator of the status of tetracycline resistance, both in animals and in water.
Water and its role in the transmission of AMR.
The presence of AMR in aquatic sources can be considered an area of concern for human public health. This study showed a relationship for AMR in enteric flora of untreated impala drinking from a water source polluted by AMR bacteria (that is, TREC), and the same could be true for humans. Even groundwater from wells or boreholes used for drinking could be a source for the spread and transfer of AMR strains of bacteria. Since previous studies reported that drinking water systems can fail to distribute bacterium-free water (3, 22, 26), it is possible that some of these bacteria could carry AMR into household tap water. It should be noted that bacteria that are resistant to multiple antibiotics have previously been isolated from chlorinated drinking water (2, 43). Moreover, even commercially available bottled water can represent a risk. In a recent study, it was found that 80% of heterotrophic bacteria isolated from mineral water at different stages of the bottling process were resistant to one or more antibiotics (26).
Conclusions.
It was concluded from this research that unexposed wild herbivores, such as impala, are good sentinels for the environmental dissemination of AMR through surface water. The results of this study showed that impala drinking from TREC-contaminated rivers were 19.3 (confidence interval, 2.63 to 41.69) times more likely to be infected with TREC than were unexposed impala. This is a highly significant finding (P < 0.0001), leading to the opinion that surface waters are an important source of TREC. It is thus possible that the same would be true for humans and other animal species that have access to AMR bacterium-polluted waters, emphasizing the importance of a broader definition of water quality for public health. Once the risks associated with the dissemination of AMR in water have been studied fully, it may be necessary for AMR tests to be added to the currently used tests for water quality assessment.
Acknowledgments
This work was partly supported through grants from the Ministero degli Affari Esteri, Direzione Generale per la Promozione e Cooperazione Culturale, Rome, Italy, between the Facoltà di Medicina Veterinaria, Università degli Studi di Perugia, Italy, and the Faculty of Veterinary Medicine, University of Pretoria, South Africa. The Research Committee of the Faculty of Veterinary Science, University of Pretoria, is also thanked for funding.
Bacteriology laboratory facilities were kindly made available by the Department of Veterinary Tropical Diseases, University of Pretoria, and the Veterinary Wildlife Section of Kruger National Park, South Africa. We thank Bruce Gummow for sharing his epidemiological knowledge, J. Grayling for her laboratory support, and the Kruger National Park staff for their collaboration.
Footnotes
Published ahead of print on 31 October 2008.
REFERENCES
- 1.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, J. L., D. S. Shigeno, J. J. Calomiris, and R. J. Seidler. 1981. Antibiotic-resistant bacteria in drinking water. Appl. Environ. Microbiol. 42:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry, D., C. Xi, and L. Raskin. 2006. Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol. 17:297-302. [DOI] [PubMed] [Google Scholar]
- 4.Bloland, P. B. 2001. Drug resistance in malaria. Department of Communicable Disease Surveillance and Response, WHO, Geneva, Switzerland. www.who.int/entity/drugresistance/publications/WHO_CDS_CSR_DRS_2001_4/en/. Accessed June 2008.
- 5.Boerlin, P., R. Travis, C. L. Gyles, R. Reid-Smith, N. J. H. Lim, V. Nicholson, S. A. McEwen, R. Friendship, and M. Archambault. 2005. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl. Environ. Microbiol. 71:6753-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan, A., N. Shapir, and M. J. Sadowsky. 2004. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Appl. Environ. Microbiol. 70:2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byappanahalli, M. N., and R. S. Fujioka. 1998. Evidence that tropical soil environment can support the growth of Escherichia coli. Water Sci. Technol. 38:171-174. [Google Scholar]
- 8.Byappanahalli, M. N., R. L. Whitman, D. A. Shively, W. T. E. Ting, C. C. Tseng, and M. B. Nevers. 2006. Seasonal persistence and population characteristics of Escherichia coli and enterococci in deep backshore sand of two freshwater beaches. J. Water Health 4:313-320. [DOI] [PubMed] [Google Scholar]
- 9.Caprioli, A., L. Busani, J. L. Martel, and R. Helmuth. 2000. Monitoring of antibiotic resistance in bacteria of animal origin: epidemiological and microbiological methodologies. Int. J. Antimicrob. Agents 14:295-301. [DOI] [PubMed] [Google Scholar]
- 10.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Cobb, K. 2004. Basic statistical principles for the clinical research scientist. www.stanford.edu/∼kcobb/precept.ppt. Accessed July 2006.
- 13.Davison, J. 1999. Genetic exchange between bacteria in the environment. Plasmid 42:73-91. [DOI] [PubMed] [Google Scholar]
- 14.Department of Water Affairs and Forestry. 2001. Quality of domestic water supplies—sampling guide. Water Research Commission, Pretoria, South Africa.
- 15.Díaz-Cruz, M. S., M. J. López de Alda, and D. Barceló. 2003. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. TrAC Trends Anal. Chem. 22:340-351. [Google Scholar]
- 16.Duriez, P., and E. Topp. 2007. Temporal dynamics and impact of manure storage on antibiotic resistance patterns and population structure of Escherichia coli isolates from a commercial swine farm. Appl. Environ. Microbiol. 73:5486-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin, A., J. Acar, F. Anthony, R. Gupta, T. Nicholls, Y. Tamura, S. Thompson, E. J. Threlfall, D. Vose, M. van Vuuren, D. G. White, H. C. Wegener, and M. L. Costarrica. 2001. Antimicrobial resistance: harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal-derived food. Rev. Sci. Tech. 20:859-870. [DOI] [PubMed] [Google Scholar]
- 18.Kaszanyitzky, É. J., M. Tenk, Á. Ghidán, G. Y. Fehérvári, and M. Papp. 2007. Antimicrobial susceptibility of enterococci strains isolated from slaughter animals on the data of Hungarian resistance monitoring system from 2001 to 2004. Int. J. Food Microbiol. 115:119-123. [DOI] [PubMed] [Google Scholar]
- 19.Kruse, H., and H. Sorum. 1994. Transfer of multiple drug resistance plasmids between bacteria of diverse origins in natural microenvironments. Appl. Environ. Microbiol. 60:4015-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kummerer, K. 2004. Resistance in the environment. J. Antimicrob. Chemother. 54:311-320. [DOI] [PubMed] [Google Scholar]
- 21.Lanz, R., P. Kuhnert, and P. Boerlin. 2003. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 91:73-84. [DOI] [PubMed] [Google Scholar]
- 22.LeChevallier, M. W., N. J. Welch, and D. B. Smith. 1996. Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lederberg, J., and E. M. Lederberg. 1952. Replica plating and indirect selection of bacterial mutants. J. Bacteriol. 63:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemunier, M., C. Francou, S. Rousseaux, S. Houot, P. Dantigny, P. Piveteau, and J. Guzzo. 2005. Long-term survival of pathogenic and sanitation indicator bacteria in experimental biowaste composts. Appl. Environ. Microbiol. 71:5779-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillehaug, A., B. Bergsjo, J. Schau, T. Bruheim, T. Vikoren, and K. Handeland. 2005. Campylobacter spp., Salmonella spp., verocytotoxic Escherichia coli, and antibiotic resistance in indicator organisms in wild cervids. Acta Vet. Scand. 46:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messi, P., E. Guerrieri, and M. Bondi. 2005. Antibiotic resistance and antibacterial activity in heterotrophic bacteria of mineral water origin. Sci. Total Environ. 346:213-219. [DOI] [PubMed] [Google Scholar]
- 27.MIMS. 2007. Index of veterinary speciality, vol. 45, p. 4. MIMS, Johannesburg, South Africa. [Google Scholar]
- 27a.NCCLS/CLSI. 2002. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standards. M31-A2. NCCLS/CLSI, Wayne, PA.
- 28.Nel, H., M. van Vuuren, and G. E. Swan. 2004. Towards the establishment and standardization of a veterinary antimicrobial resistance surveillance and monitoring programme in South Africa. Onderstepoort J. Vet. Res. 71:239-246. [DOI] [PubMed] [Google Scholar]
- 29.Okeke, I. N., K. P. Klugman, Z. A. Bhutta, A. G. Duse, P. Jenkins, T. F. O'Brien, A. Pablos-Mendez, and R. Laxminarayan. 2005. Antimicrobial resistance in developing countries. II. Strategies for containment. Lancet Infect. Dis. 5:568-580. [DOI] [PubMed] [Google Scholar]
- 30.Osterblad, M., T. Leistevuo, and P. Huovinen. 1995. Screening for antimicrobial resistance in fecal samples by the replica plating method. J. Clin. Microbiol. 33:3146-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oteo, J., F. Baquero, A. Vindel, and J. Campos. 2004. Antibiotic resistance in 3113 blood isolates of Staphylococcus aureus in 40 Spanish hospitals participating in the European Antimicrobial Resistance Surveillance System (2000-2002). J. Antimicrob. Chemother. 53:1033-1038. [DOI] [PubMed] [Google Scholar]
- 32.Picard, J., and E. Sinthumule. 2002. VIRBAC antimicrobial database report, 2002. Department of Veterinary Tropical Disease, University of Pretoria, Pretoria, South Africa.
- 33.Potgieter, F. T., and W. H. Stoltsz. 2004. Bovine anaplasmosis, p. 594-616. In J. A. W. Coetzer and R. C. Tustin (ed.), Infectious diseases of livestock, 2nd ed., vol. 1. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 34.Reinthaler, F. F., J. Posch, G. Feierl, G. Wüst, D. Haas, G. Ruckenbauer, F. Mascher, and E. Marth. 2003. Antibiotic resistance of E. coli in sewage and sludge. Water Res. (Oxford) 37:1685-1690. [DOI] [PubMed] [Google Scholar]
- 35.Rysz, M., and P. J. J. Alvarez. 2004. Amplification and attenuation of tetracycline resistance in soil bacteria: aquifer column experiments. Water Res. (Oxford) 38:3705-3712. [DOI] [PubMed] [Google Scholar]
- 36.SANVAD. 2007. South African National Surveillance and Monitoring Programme for Resistance to Antimicrobial Drugs report 978-1-86854-673-2. University of Pretoria, Pretoria, South Africa.
- 37.Sayah, R. S., J. B. Kaneene, Y. Johnson, and R. A. Miller. 2005. Patterns of antimicrobial resistance observed in Escherichia coli isolates obtained from domestic- and wild-animal fecal samples, human septage, and surface water. Appl. Environ. Microbiol. 71:1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sengelov, G., B. Halling-Sorensen, and F. M. Aarestrup. 2003. Susceptibility of Escherichia coli and Enterococcus faecium isolated from pigs and broiler chickens to tetracycline degradation products and distribution of tetracycline resistance determinants in E. coli from food animals. Vet. Microbiol. 95:91-101. [DOI] [PubMed] [Google Scholar]
- 39.Skinner, J. D., and C. T. Chimimba. 2005. The mammals of the southern African subregion, 3rd ed. Cambridge University, Cambridge, United Kingdom.
- 40.Smalla, K., H. Heuer, A. Götz, D. Niemeyer, E. Krögerrecklenfort, and E. Tietze. 2000. Exogenous isolation of antibiotic resistance plasmids from piggery manure slurries reveals a high prevalence and diversity of IncQ-like plasmids. Appl. Environ. Microbiol. 66:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinfield, H., P. Gerber, T. Wassenaar, V. Caste, M. Rosales, and C. De Haan. 2006. Livestock's new shadow: environmental issues and options. FAO, Rome, Italy.
- 42.SWARM. 2006. Swedish veterinary antimicrobial resistance monitoring 2006. The National Veterinary Institute (SVA), Uppsala, Sweden.
- 43.Swartz, M. 2002. Human diseases caused by foodborne pathogens of animal origin. Clin. Infect. Dis. 34:S111-S122. [DOI] [PubMed] [Google Scholar]
- 44.Thrusfield, M. V. 2005. Veterinary epidemiology. Blackwell Science Ltd., Oxford, United Kingdom.
- 45.Tsiodras, S., T. Kelesidis, I. Kelesidis, U. Bauchinger, and M. E. Falagas. 2008. Human infections associated with wild birds. J. Infect. 56:83-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turnidge, J. 2004. Antibiotic use in animals—prejudices, perceptions and realities. J. Antimicrob. Chemother. 53:26-27. [DOI] [PubMed] [Google Scholar]
- 47.van den Bogaard, A. E. 1997. Antimicrobial resistance—relation to human and animal exposure to antibiotics. J. Antimicrob. Chemother. 40:453-454. [DOI] [PubMed] [Google Scholar]
- 48.van den Braak, N., A. van Belkum, M. van Keulen, J. Vliegenthart, H. A. Verbrugh, and H. P. Endtz. 1998. Molecular characterization of vancomycin-resistant enterococci from hospitalized patients and poultry products in The Netherlands. J. Clin. Microbiol. 36:1927-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watkinson, A. J., G. B. Micalizzi, G. M. Graham, J. B. Bates, and S. D. Costanzo. 2007. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol. 73:5667-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkinson, A. J., G. R. Micalizzi, J. R. Bates, and S. D. Costanzo. 2007. Novel method for rapid assessment of antibiotic resistance in Escherichia coli isolates from environmental waters by use of a modified chromogenic agar. Appl. Environ. Microbiol. 73:2224-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weston, D. B. 1996. Environmental consideration in the use of antibacterial drugs in aquaculture, p. 140-165. In D. J. Baird, M. C. M. Beveridge, and L. A. Kelly (ed.), Aquaculture and water resource management. Blackwell Science, Oxford, United Kingdom.
- 52.White, D. G., J. Acar, F. Anthony, A. Franklin, R. Gupta, T. Nicholls, Y. Tamura, S. Thompson, E. J. Threlfall, D. Vose, M. van Vuuren, H. C. Wegener, and M. L. Costarrica. 2001. Antimicrobial resistance: standardisation and harmonisation of laboratory methodologies for the detection and quantification of antimicrobial resistance. Rev. Sci. Tech. 20:849-858. [DOI] [PubMed] [Google Scholar]
- 53.Whitman, R. L., M. B. Nevers, and M. N. Byappanahalli. 2006. Examination of the watershed-wide distribution of Escherichia coli along southern Lake Michigan: an integrated approach. Appl. Environ. Microbiol. 72:7301-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO. 2001. Global strategies for the containment of antimicrobial resistance. WHO, Geneva, Switzerland. http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_DRS_2001.2a.pdf. Accessed June 2008.
- 55.WHO, FAO, and OIE. 2006. Assessing the risk on public health of the use of antimicrobials in aquaculture, p. 1-10. In Antimicrobial use in aquaculture and antimicrobial resistance. Report of a joint FAO/OIE/WHO expert consultation on antimicrobial use in aquaculture and antimicrobial resistance, Seoul, Republic of Korea, 13 to 16 June 2006.
- 56.WRC. 2001. State of the river report 2001: Crocodile, Sabie-Sand and Olifants. Water Research Commission, Pretoria, South Africa.
- 57.WRC. 2001. State of the rivers report 2001: Letaba and Luvuvhu river systems. WRC report TT 165/01. Water Research Commission, Pretoria, South Africa.
- 58.Young, H.-K. 1993. Antimicrobial resistance spread in aquatic environments. J. Antimicrob. Chemother. 31:627-635. [DOI] [PubMed] [Google Scholar]