Abstract
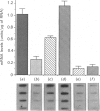
Keloids are benign cutaneous tumours characterized by excess deposition of collagen, specifically type I collagen. We report here that collagen biosynthesis, as measured by hydroxyproline synthesis, was markedly inhibited by 65-80% by the combination of endothelial cell growth factor (ECGF) supplement and heparin in keloid fibroblast cultures. Fibroblast cultures that were incubated with ECGF alone also demonstrated a measurable decrease of approx. 50% in collagen synthesis compared with control cultures. The inhibition of collagen synthesis was related to the down-regulation of collagen gene expression. Quantitative measurements of mRNA-cDNA hybrids revealed that the gene expression of collagen type I was decreased by more than 80% by heparin and ECGF. Markedly diminished levels of mRNA encoding collagen type I were also observed in cultures incubated with ECGF alone. The results show that ECGF and heparin elicit a negative regulatory effect on collagen production, and that this inhibition is due largely to the down-regulation of the pro-alpha 1(I) of type I collagen gene. Furthermore, ECGF has a potent suppressive effect, and heparin provides an additive effect to this inhibitory phenomenon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abergel R. P., Pizzurro D., Meeker C. A., Lask G., Matsuoka L. Y., Minor R. R., Chu M. L., Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985 May;84(5):384–390. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- Ala-Kokko L., Rintala A., Savolainen E. R. Collagen gene expression in keloids: analysis of collagen metabolism and type I, III, IV, and V procollagen mRNAs in keloid tissue and keloid fibroblast cultures. J Invest Dermatol. 1987 Sep;89(3):238–244. doi: 10.1111/1523-1747.ep12471056. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Kolbe M., Weil D., Chu M. L. Human cellular fibronectin: comparison of the carboxyl-terminal portion with rat identifies primary structural domains separated by hypervariable regions. Biochemistry. 1985 May 21;24(11):2698–2704. doi: 10.1021/bi00332a016. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Kambe A. M., Dobson D. E., Spiegelman B. M. Heparin potentiation of 3T3-adipocyte stimulated angiogenesis: mechanisms of action on endothelial cells. J Cell Physiol. 1986 May;127(2):323–329. doi: 10.1002/jcp.1041270221. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wright T. C., Karnovsky M. J. Regulation of vascular smooth muscle cell growth by heparin and heparan sulfates. Semin Thromb Hemost. 1987 Oct;13(4):489–503. doi: 10.1055/s-2007-1003525. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Damon D. H., Lobb R. R., D'Amore P. A., Wagner J. A. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J Cell Physiol. 1989 Feb;138(2):221–226. doi: 10.1002/jcp.1041380202. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. P., Jung W. K., Costa D. E., Rajaratnam J. B. Effects of heparin on vascularization of artificial skin grafts in rats. Exp Mol Pathol. 1988 Apr;48(2):244–251. doi: 10.1016/0014-4800(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Rosenberg R., Haering W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. II. In vitro studies. Circ Res. 1980 Oct;47(4):578–583. doi: 10.1161/01.res.47.4.578. [DOI] [PubMed] [Google Scholar]
- Joseph-Silverstein J., Rifkin D. B. Endothelial cell growth factors and the vessel wall. Semin Thromb Hemost. 1987 Oct;13(4):504–513. doi: 10.1055/s-2007-1003526. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- LeBaron R. G., Hök A., Esko J. D., Gay S., Hök M. Binding of heparan sulfate to type V collagen. A mechanism of cell-substrate adhesion. J Biol Chem. 1989 May 15;264(14):7950–7956. [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. N., Thomas K. A., Di Salvo J., Levine E. M. Stabilization by heparin of acidic fibroblast growth factor mitogenicity for human endothelial cells in vitro. J Cell Physiol. 1989 Sep;140(3):439–448. doi: 10.1002/jcp.1041400306. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Pollack S. V., Pinnell S. R. Keloids: a review. J Am Acad Dermatol. 1981 Apr;4(4):461–470. doi: 10.1016/s0190-9622(81)70048-3. [DOI] [PubMed] [Google Scholar]
- Oikarinen H., Oikarinen A. I., Tan E. M., Abergel R. P., Meeker C. A., Chu M. L., Prockop D. J., Uitto J. Modulation of procollagen gene expression by retinoids. Inhibition of collagen production by retinoic acid accompanied by reduced type I procollagen messenger ribonucleic acid levels in human skin fibroblast cultures. J Clin Invest. 1985 May;75(5):1545–1553. doi: 10.1172/JCI111859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen J., Hsiao L. L., Jaakkola S., Sollberg S., Aumailley M., Timpl R., Chu M. L., Uitto J. Activation of collagen gene expression in keloids: co-localization of type I and VI collagen and transforming growth factor-beta 1 mRNA. J Invest Dermatol. 1991 Aug;97(2):240–248. doi: 10.1111/1523-1747.ep12480289. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. B., Trupin J. S., Myers J. C., Broquist A. H., Smith J. C., Myles M. E., Russell J. D. Differential glucocorticoid regulation of collagen mRNAs in human dermal fibroblasts. Keloid-derived and fetal fibroblasts are refractory to down-regulation. J Biol Chem. 1989 Aug 15;264(23):13730–13735. [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Dodge G. R., Sorger T., Kovalszky I., Unger G. A., Yang L., Levine E. M., Iozzo R. V. Modulation of extracellular matrix gene expression by heparin and endothelial cell growth factor in human smooth muscle cells. Lab Invest. 1991 Apr;64(4):474–482. [PubMed] [Google Scholar]
- Tan E. M., Levine E., Sorger T., Unger G. A., Hacobian N., Planck B., Iozzo R. V. Heparin and endothelial cell growth factor modulate collagen and proteoglycan production in human smooth muscle cells. Biochem Biophys Res Commun. 1989 Aug 30;163(1):84–92. doi: 10.1016/0006-291x(89)92102-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C., Koliakos G. G., Charonis A. S., Vogel A. M., Reger L. A., Furcht L. T. Heparin type IV collagen interactions: equilibrium binding and inhibition of type IV collagen self-assembly. J Biol Chem. 1988 Dec 15;263(35):19112–19118. [PubMed] [Google Scholar]
- Uitto J., Perejda A. J., Abergel R. P., Chu M. L., Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5935–5939. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Perejda A. J., Abergel R. P., Chu M. L., Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5935–5939. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Santa Cruz D. J., Eisen A. Z. Connective tissue nevi of the skin. Clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980 Nov;3(5):441–461. [PubMed] [Google Scholar]