Abstract
The relative contributions of the hydrophilic surfactant proteins (SP)-A and -D to early inflammatory responses associated with lung dysfunction after experimental allogeneic hematopoietic stem cell transplantation (HSCT) were investigated. We hypothesized that the absence of SP-A and SP-D would exaggerate allogeneic T cell-dependent inflammation and exacerbate lung injury. Wild-type, SP-D-deficient (SP-D−/−), and SP-A and -D double knockout (SP-A/D−/−) C57BL/6 mice were lethally conditioned with cyclophosphamide and total body irradiation and given allogeneic bone marrow plus donor spleen T cells, simulating clinical HSCT regimens. On day 7, after HSCT, permeability edema progressively increased in SP-D−/− and SP-A/D−/− mice. Allogeneic T cell-dependent inflammatory responses were also increased in SP-D−/− and SP-A/D−/− mice, but the altered mediators of inflammation were not identical. Compared with wild-type, bronchoalveolar lavage fluid (BALF) levels of nitrite plus nitrate, GM-CSF, and MCP-1, but not TNF-α and IFN-γ, were higher in SP-D-deficient mice before and after HSCT. In SP-A/D−/− mice, day 7 post-HSCT BALF levels of TNF-α and IFN-γ, in addition to nitrite plus nitrate and MCP-1, were higher compared with mice lacking SP-D alone. After HSCT, both SP-A and SP-D exhibited anti-inflammatory lung-protective functions that were not completely redundant in vivo.
Keywords: T cells, transplantation, nitric oxide, idiopathic pneumonia syndrome, hematopoietic stem cell transplantation
in addition to pulmonary host defense functions, recent evidence indicates that the hydrophilic surfactant proteins A and D (SP-A and SP-D) contribute to the hyporesponsive immunological state in the lung (9). SP-A and SP-D, synthesized and secreted by alveolar type II cells, exhibit direct effects on T cells, and may also regulate T cell responses indirectly by their effects on cells of the innate (i.e., macrophages) immune system (33).
SP-D may suppress T cell activation and immune responses by binding allergens or endotoxin (17, 27), limiting antigen presentation by lung dendritic cells (12), and inhibiting T cell proliferation via IL-2-dependent and IL-2-independent mechanisms (4). Although SP-A has also been shown to inhibit T cell proliferation (4), this in vitro effect of SP-A has been questioned after it was noted that some SP-A preparations contained immunosuppressive levels of TGF-β1 (25), which may be responsible, at least in part, for the SP-A-mediated inhibition of T cell proliferation.
SP-A and SP-D belong to the collectin family of proteins that share a collagen-like region and a calcium-dependent globular carbohydrate-recognition domain. Despite structural similarity, SP-A and SP-D exhibit distinct effects on lung immunity (26). The phenotypic differences between mice lacking SP-A or SP-D are clear evidence that the immune-regulatory functions of SP-A and SP-D are not completely redundant in vivo. Under specific pathogen-free environment, the lungs of unstressed SP-A−/− mice appear histologically normal. In contrast, SP-D-deficient mice exhibit three- to fourfold increase in the number of large macrophages that are foamy in appearance (24). In addition, the lack of SP-D leads to a state of persistent T cell activation (8) and altered nitric oxide (.NO) metabolism associated with enhanced detection of harmful nitrated proteins (2). To further investigate the functions of SP-A and SP-D in vivo, mice deficient in both SP-A and SP-D (SP-A/D−/−) have been developed (15). The lung pathology in SP-A/D−/− resembled that seen in SP-D−/− mice, although intense patchy lung inflammation was noted. Forty-eight hours after Pseudomonas aeruginosa respiratory infection, bacterial counts and levels of macrophage inflammatory peptide-2 (CXCL1) were higher in the lungs of SP-A/D−/− mice compared with wild-type and SP-A−/− mice (10). The relative contribution of SP-A and SP-D in a noninfectious model of T cell-dependent inflammation has not been investigated.
A mouse model of T cell-dependent lung injury after allogeneic hematopoietic stem cell transplantation (HSCT) has been established by Panoskaltsis-Mortari et al. (32). This model represents noninfectious early-onset acute lung injury after HSCT, known as idiopathic pneumonia syndrome (IPS). IPS is a significant cause of death after HSCT and accounts for the majority of complications involving the lung in the early posttransplant period (23). Our studies have shown that the lung dysfunction is caused by the influx of host monocytes and donor T cells into the lungs of lethally conditioned mice and is manifested by decreased dynamic compliance and increased permeability edema (11, 32). To investigate the role of endogenous SP-A in early inflammatory response and lung dysfunction after allogeneic HSCT, our laboratories previously performed HSCT experiments in SP-A-sufficient and SP-A-deficient (SP-A−/−) mice. On day 7 after allogeneic HSCT, mice lacking SP-A exhibited increased levels of TNF-α, IFN-γ, and nitrite plus nitrates, the stable byproducts of .NO, in bronchoalveolar lavage fluids (BALF). Enhanced expression of proinflammatory cytokines and .NO was associated with increased nitrated proteins in alveolar macrophages, increased markers of lung dysfunction, and accelerated early post-HSCT mortality (36). The role of SP-D in the presence or absence of SP-A in modifying the inflammatory response and lung dysfunction after allogeneic HSCT remains unknown.
The purpose of this study was to determine the effects of SP-D deficiency and simultaneous absence of SP-A and SP-D on allogeneic T cell-dependent inflammation and IPS injury. Expanding on our previous results using SP-A−/− mice, we hypothesized that SP-D−/− and SP-A/D−/− mice will exhibit a progressive increase in markers of lung inflammation and injury in the early post-HSCT period. Our results indicate that SP-A and SP-D have anti-inflammatory lung-protective functions by both overlapping and distinct mechanisms.
METHODS
Mice.
SP-D−/− and SP-A/D−/− mice were generated from embryonic stem cells targeted with replacement-type vectors as previously described (15). All strains were backcrossed 10 generations onto a C57/BL6 (B6) background. Wild-type B6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used as wild-type mice. Mice were housed in barrier containment and were weaned on day 21. The protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. For allogeneic HSCT, inbred B10.BR (H2K) donor mice (Jackson Laboratories) were 6–8 wk of age, and recipients were used at 8–10 wk of age. Sentinel mice were found to be negative for 15 known murine viruses, including CMV, K-virus, and pneumonia virus of mice.
Pre-HSCT conditioning.
Mice received intraperitoneal injections of cyclophosphamide (Cy) (Cytoxan; Bristol Myers Squibb, Seattle, WA) 120 mg·kg−1·d−1 on days −3 and −2 pre-HSCT. On the day before HSCT, all recipient mice were lethally total body irradiated (7.5 Gy) by X-ray at a dose rate of 0.41 Gy/min.
HSCT.
Our HSCT and IPS generation protocols have been described previously (36). Briefly, donor bone marrow was T cell depleted with anti-Thy 1.2 monoclonal antibody (clone 30-H-12, rat IgG2b; kindly provided by Dr. David Sachs, Massachusetts General Hospital, Cambridge, MA) plus complement (Neiffenegger, Woodland, CA). For each experiment, a total of five to ten recipient mice per treatment group were transplanted via caudal vein with 20 × 106 T cell-depleted bone marrow cells with 15 × 106 allogeneic spleen T cells as a source of IPS-causing T cells.
Bronchoalveolar lavage.
Mice were killed on day 7 after HSCT after an intraperitoneal injection of pentobarbital sodium, and the thoracic cavity was partially dissected. The trachea was cannulated with a 22-gauge angiocatheter, infused with 1 ml of ice-cold sterile PBS, and withdrawn. This was repeated once, and return fluids were combined. BALF was immediately centrifuged at 500 g for 10 min at 4°C to pellet cells. The initial 1.5 ml of BALF from each individual mouse was used for biochemical analysis. BALF cell pellets from each group of mice were combined, and the cell differential was determined in samples cytospun onto glass slides and stained with Wright-Giemsa.
BALF analysis.
Cell-free BALF TNF-α, IFN-γ, MCP-1 (CCL2), and granulocyte/macrophage colony-stimulating factor (GM-CSF) levels were determined by performing sandwich ELISA using murine-specific commercial kits (sensitivity 1.5–3 pg/ml; R&D Systems, Minneapolis, MN). In addition, Th2-type cytokines IL-4, IL-5, and IL-13 were assayed by Luminex method (Austin, TX) using murine-specific bead sets (R&D Systems). For the cytokine analyses, all specimens were assayed in duplicate and results averaged. Nitrite in BALF was measured according to the Griess method after the conversion of nitrate to nitrite with the reduced NADH-dependent enzyme nitrate reductase (Calbiochem, La Jolla, CA). BALF total protein was determined using the bicinchoninic acid (Sigma, St. Louis, MO) method with bovine serum albumin as the standard.
Macrophage/monocyte culture.
The BALF cell pellets from mice in each treatment group were combined, washed twice in cold PBS, and resuspended in RPMI 1640 medium (Celox Laboratories, St. Paul, MN) containing 5% FCS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Total cell number was determined with a hemacytometer. A total of 2 × 105 cells/well was added to flat-bottom 96-well microtiter plates (Costar, Cambridge, MA), and macrophages were allowed to adhere for 1 h at 37°C in 5% CO2 in air, followed by removal of unbound cells. More than 95% of adherent cells were macrophages. The cells were maintained in culture at 37°C for 48 h in 5% CO2 in air. At termination of cell culture, supernatants were aspirated from individual culture wells for measurement of TNF-α by ELISA, nitrite by the Griess method, and lactic dehydrogenase (LDH) by the colorimetric CytoTox 96 assay (Promega, Madison, WI). Cells were washed twice with PBS and lysed with lysis solution (10×, Triton X-100, Promega), and cellular LDH release was measured. Total (supernatant + cellular) LDH values were used to correct for possible differences in adherent cell number between groups. TNF-α and nitrite readings were adjusted accordingly using the non-HSCT group as an assigned reference value for 2 × 105 cells (the number of cells originally plated per well).
Immunohistochemistry.
In one to two mice per group per experiment, a mixture of 1 ml of optimal cutting temperature medium (Miles Laboratories, Elkhart, IN) and PBS (3:1) was infused into the trachea. The lung was snap-frozen in liquid nitrogen and stored at −80°C. Frozen sections were cut 4 μm thick, mounted onto glass slides, and fixed for 10 min in 3% paraformaldehyde at 4°C. Nonantigenic sites were blocked with 10% normal goat serum (Sigma) for 30 min at 23°C followed by incubation overnight at 4°C with rabbit polyclonal anti-nitrotyrosine antibody (NTAb; 1:50 dilution, Upstate Biotechnology, Lake Placid, NY). In control measurements, tissues were incubated with NTAb in the presence of 10 mM nitrotyrosine or with nonspecific rabbit IgG (20 μg/ml). All sections were incubated with a secondary antibody, goat anti-rabbit IgG conjugated with horseradish peroxidase (1:500 dilution), for 45 min at 23°C, followed by the addition of 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA) chromogenic substrate. The sections were counterstained with hematoxylin, dehydrated, overlaid with Permount (Sigma), and sealed with coverslips.
Western blots.
Equal volumes (20 μl) of BALF were solubilized in 0.1 M Tris·HCl buffer (that contained 50 μM DTT, 0.01% bromophenol blue, 1% SDS, and 10% glycerol) and boiled for 5 min. The proteins were resolved by 10% SDS polyacrylamide gels, transferred to nitrocellulose paper, and probed with polyclonal anti-rabbit SP-A or SP-D antibodies (1:10,000 dilution) followed by alkaline phosphatase-conjugated goat anti-rabbit IgG (1:7,500 dilution) as the secondary antibody. Bound antibody was detected using nitro blue tetrazolium and a 5-bromo-4-chloro-3-indolyl-1-phosphate kit (Sigma).
Pulmonary function analysis.
Pulmonary mechanics in pentobarbital-anesthetized ventilated mice were measured following the method described by Diamond and O'Donnell (7) with slight modifications. In brief, after careful dissection of the neck, a short metal cannula was inserted into the trachea and secured with 3.0 silk. A polyethylene catheter was inserted orally into the lower third of the esophagus to estimate pleural pressure. The animal was then placed into a plethysmograph (Buxco Electronics, Sharon, CT) and connected to a mouse ventilator (Harvard Apparatus, March-Hugstetten, Germany) set at a respiratory rate of 150 breaths/min and a tidal volume of 200 μl. Respiratory flow signal was measured through a flow transducer (Sen Sym SCXL004, Buxco Electronics) connected to the plethysmograph. Lung volume was obtained by electric integration of the flow signal. Intraesophageal and airway pressure were measured with a pressure transducer (Validyne DP45, Buxco Electronics) directly connected to their respective ports. These data were fed into a computer through a preamplifier (MaxII, Buxco Electronics), and the data were analyzed with the Biosystem XA software (Buxco Electronics). When the signal was stable, delivered tidal volume was varied from 350 to 100 μl in 50-μl decrements, and for each delivered volume, the effective tidal volume, transpulmonary pressure, and dynamic compliance (Cdyn) were measured. Body temperature was maintained at 37°C throughout the experiment.
RESULTS
Decreased dynamic lung compliance during SP-D deficiency.
In the first set of experiments, we determined the effects of endogenous SP-D on markers of lung injury and inflammation after allogeneic HSCT. To assess lung mechanics during SP-D deficiency, lung Cdyn was measured in anesthetized and ventilated mice on day 7 after allogeneic HSCT. At a delivered tidal volume of 200 μl, Cdyn was significantly decreased in both groups of mice post-HSCT, and a significantly greater decrease was seen in SP-D−/− mice compared with SP-D+/+ (Fig. 1). These results are consistent with increased lung dysfunction during SP-D deficiency after allogeneic HSCT.
Fig. 1.
Exacerbated lung dysfunction in SP-D−/− mice on day 7 after allogeneic hematopoietic stem cell transplantation (HSCT). B6 SP-D+/+ and SP-D−/− were conditioned with cytoxan and total body irradiation and given bone marrow from B10.BR mice and inflammation-inducing donor spleen T cells. Mice were placed in a single-chamber plethysmograph and ventilated with delivered tidal volume of 100–350 μl. Effective tidal volume was measured and transpulmonary pressure was calculated using airway and intraesophageal pressures. Shown is lung compliance at a delivered tidal volume of 200 μl. Values are means ± SE for n = 3 different mice in each experimental group. *P < 0.05 compared with non-HSCT SP-D+/+ mice. +P < 0.05 between SP-D+/+ and SP-D−/− mice after HSCT. Closed bars, SP-D+/+; open bars, SP-D−/−.
Increased BALF cellularity during SP-D deficiency.
BALF return volumes collected on day 7 after transplantation were similar in all groups (>90% of instilled volume). BALF from non-HSCT SP-D−/− mice contained 2–3× higher number of inflammatory cells compared with B6 mice (15.7 ± 1.3 × 104/ml vs. 4.5 ± 0.4 × 104/ml, respectively; n = 8; P < 0.05), most likely macrophages as has been previously reported (39). Macrophages from SP-D−/− were large in size and foamy in appearance as observed on cytospun BALF samples. Exposure of SP-D+/+ and SP-D−/− mice to irradiation and allogeneic T cells increased the number of inflammatory cells contained in BALF collected on day 7 after HSCT (8.1 ± 0.6 × 104/ml vs. 25.9 ± 4.1 × 104/ml, respectively; n = 8; P < 0.05) and shifted the cellular differential toward more lymphocytes. On day 7 after allogeneic HSCT, BALF cell differential was not significantly modified in SP-D−/− vs. SP-D+/+ mice (30–40% T cells, 50–60% alveolar macrophages and monocytes, and 1–10% neutrophils and other cell types). The large foamy appearance of alveolar macrophages in SP-D−/− mice persisted after allogeneic HSCT (Fig. 2).
Fig. 2.
Wright-Giemsa stain of bronchoalveolar lavage fluid (BALF) cell pellets from nontransplanted (non-HSCT) SP-D+/+ and SP-D−/− mice, and on day 7 after allogeneic HSCT. Non-HSCT SP-D−/− mice contained increased number of inflammatory cells and the presence of large foamy macrophages. After HSCT, there was further enhancement of the number of inflammatory cells and foamy appearance of alveolar macrophages in lungs of SP-D−/− mice.
Increased levels of chemoattractants MCP-1 and GM-CSF, but not proinflammatory cytokines TNF-α and IFN-γ, during SP-D deficiency.
Despite the increased number of inflammatory cells in the lungs of non-HSCT SP-D−/− mice, levels of the proinflammatory cytokines TNF-α and IFN-γ were low to non-detectable and no different from SP-D+/+ non-HSCT mice (Fig. 3, A and B). In contrast to SP-D+/+ mice, levels of the chemoattractants MCP-1 and GM-CSF were elevated in BALF of non-transplanted (non-HSCT) SP-D−/− mice (Fig. 3, C and D). Both inflammatory mediators and chemokines were significantly increased in BALF collected on day 7 after allogeneic HSCT. Whereas BALF MCP-1 and GM-CSF were higher in SP-D−/− vs. SP-D+/+ mice (Fig. 3, C and D), levels of TNF-α and IFN-γ in SP-D+/+ and SP-D−/− mice were not different (Fig. 3, A and B). These data indicate that SP-D has a role in regulation of the aforementioned chemoattractants, but not the proinflammatory cytokines TNF-α and IFN-γ, after allogeneic HSCT. The Th2-type cytokines IL-4, IL-5, and IL-13 were below detection limits in all experimental groups in both wild-type and gene-targeted mice.
Fig. 3.
Absence of SP-D enhances MCP-1 and granulocyte/macrophage colony-stimulating factor (GM-CSF) BALF levels before and after allogeneic HSCT. Cytoxan/total body irradiation (Cy/TBI)-recipient SP-D+/+ and SP-D−/− B6 were infused with inflammation-inducing donor T cells. BALF was collected on day 7 after HSCT. TNF-α (A), IFN-γ (B), MCP-1 (C), and GM-CSF (D) were measured by sandwich ELISA. Values are means ± SE for n = 8–12 mice/group from 3 experiments. *P < 0.05 compared with non-HSCT SP-D+/+ mice. +P < 0.05 between SP-D+/+ and SP-D−/− mice after HSCT. Closed bars, SP-D+/+; open bars, SP-D−/−.
Increased .NO metabolites during SP-D deficiency.
Before transplantation, BALF from non-HSCT SP-D−/− vs. wild-type mice contained increased levels of the stable .NO byproducts, nitrite plus nitrate (Fig. 4). Exposure to allogeneity and conditioning regimens increased day 7 after HSCT BALF levels of nitrite plus nitrate in both wild-type and gene-targeted mice, but these .NO metabolites remained significantly higher in SP-D−/− compared with similarly treated wild-type mice (Fig. 4). To determine the contribution of alveolar macrophages and lung-infiltrating monocytes to the production of .NO and TNF-α, 2 × 105 cells were allowed to adhere to the bottom of 96-well plates, and cell-free supernatant was collected after 48 h of culture to measure nitrite and TNF-α levels. We have shown that macrophages obtained on day 7 after allogeneic HSCT continue to spontaneously generate .NO and TNF-α for at least 48 h in culture, and this production of mediators reflects the in vivo inflammatory environment from which the cells were extracted (37). Macrophages/monocytes obtained from non-HSCT wild-type and SP-D−/− failed to produce .NO and TNF-α. After allogeneic HSCT, macrophages from SP-D−/− vs. SP-D+/+ mice produced more .NO (65.2 ± 8.9 vs. 34.5 ± 8.8 μM/2 × 105 cells, respectively; n = 8; P < 0.05). In contrast, TNF-α levels in supernatant of cultured macrophages/monocytes collected on day 7 after allogeneic HSCT from wild-type and SP-D−/− mice were similar (1.70 ± 0.33 vs. 1.55 ± 0.26 ng/ml/2 × 105 cells, respectively; n = 8; P > 0.05). Nitrative stress was assessed by detection of antigenic sites related to nitrotyrosine. Compared with lung sections from non-HSCT mice, sections obtained from HSCT wild-type mice given allogeneic T cells exhibited increased nitrotyrosine immunostaining that was mainly observed in alveolar epithelium and inflammatory cells. Nitrotyrosine staining was at least as intense in SP-D−/− compared with SP-D+/+ mice following allogeneic HSCT and was mainly observed in the alveolar epithelium and large foamy alveolar macrophages (Fig. 5).
Fig. 4.
Absence of SP-D enhances BALF·NO levels before and after allogeneic HSCT. Cy/TBI-recipient SP-D+/+ and SP-D−/− B6 were infused with inflammation-inducing donor T cells. BALF was collected on day 7 after HSCT. Nitrate was reduced to nitrite before measurement by the Griess reaction. Values are means ± SE for n = 8–12 mice/group from 3 experiments. *P < 0.05 compared with nontransplanted (non-HSCT) SP-D+/+ mice. +P < 0.05 between SP-D+/+ and SP-D−/− mice after HSCT. Closed bars, SP-D+/+; open bars, SP-D−/−.
Fig. 5.
Immunoperoxidase staining of lung sections taken from non-HSCT (unmanipulated) wild-type B6 mice, and on day 7 after allogeneic HSCT from B6 and SP-D−/− mice. Sections were incubated with nitrotyrosine antibody (NTAb) or with NTAb in the presence of 10 mM nitrotyrosine (NTblock). Increased staining of epithelium and inflammatory cells was observed after allogeneic HSCT. Shown is a representative experiment, which was repeated once (original magnification, ×100; resolution power, ×40 objective lens). NTAb binding was specific because it was completely blocked in the presence of excess antigen (NTblock).
SP-A levels during SP-D deficiency.
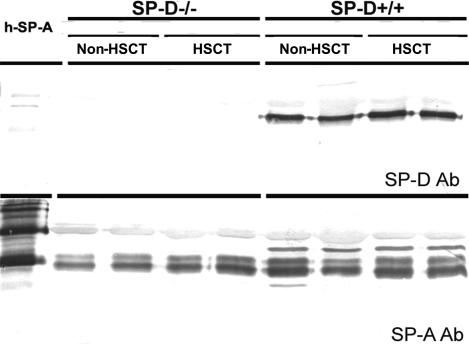
Because the functions of SP-A and SP-D may overlap and SP-A may compensate for SP-D deficiency, we evaluated SP-A protein in BALF of SP-D+/+ and SP-D−/− mice using Western blotting. Although semiquantitative, Western blots exhibited decreased SP-A protein during SP-D deficiency before and on day 7 after allogeneic HSCT (n = 4). A representative Western blot is shown in Fig. 6. These findings are consistent with previously reported data that SP-A protein content in large- and small-aggregate fractions of BALF from SP-D−/− mice is ∼50% of levels in BALF from SP-D+/+ mice (1).
Fig. 6.
Western blots of equal BALF volume (20 μl) obtained from nontransplanted (non-HSCT) and on day 7 after allogeneic HSCT from SP-D+/+ and SP-D−/− B6 mice. BALF SP-D was detected using anti-rabbit SP-D antibody. SP-D was undetectable in SP-D−/− mice. BALF SP-A was detected using anti-rabbit SP-A antibody. SP-A protein levels were decreased in SP-D−/− mice compared with SP-D+/+ mice. Shown is a representative Western blot that was repeated twice.
To investigate the role of SP-A during SP-D deficiency, and confirm our previous results of the anti-inflammatory functions of SP-A following allogeneic HSCT, we performed additional HSCT experiments and compared allogeneic T cell-dependent inflammatory responses in wild-type, SP-D−/−, and SP-A/D−/− mice. All transplanted mice progressively lost weight after allogeneic HSCT. On day 7 after allogeneic HSCT, weight loss in wild-type vs. SP-D−/− vs. SP-A/D−/− mice was not different (mean weight loss 21.2, 20.5, and 21.9% in wild-type, SP-D−/−, and SP-A/D−/− mice, respectively; P > 0.05, n = 12 in each group). Compared with B6 mice, non-HSCT unmanipulated (nonirradiated and nontransplanted) SP-D−/− and SP-A/D−/− mice each exhibited comparably increased BALF total protein levels, which was higher than non-HSCT wild-type mice (Fig. 7A). However, on day 7 after allogeneic HSCT, BALF total protein levels were progressively increased in BALF of B6, SP-D−/−, and SP-A/D−/− mice (P < 0.05 in SP-D−/− vs. B6; SP-A/D−/− vs. SP-D−/− mice) (Fig. 7A). These data indicate that the simultaneous absence of SP-A and SP-D results in significantly increased permeability edema compared with absence of SP-D alone and implicate a role for SP-A in maintaining the integrity of the alveolar epithelial barrier after allogeneic HSCT. Similarly, nitrite plus nitrate levels were higher in control SP-A/D−/− compared with SP-D−/− mice, indicative of a role for SP-A in regulation of .NO levels during SP-D deficiency (Fig. 7B). On day 7 after allogeneic HSCT, .NO levels in BALF were significantly increased compared with pretransplant levels. This increase in .NO was most pronounced in mice lacking both SP-A and SP-D (Fig. 7B).
Fig. 7.
The simultaneous absence of SP-A and SP-D increases permeability edema and BALF .NO levels. BALF collected from nontransplanted (non-HSCT) SP-A/D−/− mice contained higher levels of nitrite plus nitrate compared with SP-D−/− or wild-type mice. On day 7 after allogeneic HSCT, BALF recovered from SP-A/D−/− mice contained higher levels of total protein (A) and nitrite plus nitrate levels (B) compared with SP-D−/− or wild-type mice. Protein levels were determined using the bicinchoninic acid method with bovine serum albumin as the standard. Nitrate was reduced to nitrite before measurement by the Griess reaction. Values are means ± SE for n = 8–12 mice/group from 3 experiments. *P < 0.05 compared with nontransplanted (non-HSCT) SP-D+/+ mice. +P < 0.05 between SP-D+/+ and SP-D−/− mice after HSCT. ^P < 0.05 comparing SP-A/D−/− and SP-D−/− mice. Closed bars, SP-D+/+; hatched bars, SP-D−/−; open bars, SP-A/D−/−.
Increased levels of TNF-α, IFN-γ, and MCP-1 during the simultaneous absence of SP-A and SP-D.
BALF collected on day 7 after allogeneic HSCT from mice lacking both SP-A and SP-D contained significantly higher levels of TNF-α, IFN-γ, and MCP-1 compared with wild-type mice and with mice deficient in SP-D alone (Fig. 8, A–C). In contrast, BALF GM-CSF levels after allogeneic HSCT in SP-A/D−/− were not different compared with SP-D−/− mice (Fig. 8D). During SP-D deficiency, these data support a role for SP-A in modulating allogeneic HSCT-induced upregulation of TNF-α, IFN-γ, and MCP-1, but not GM-CSF. Similar to SP-D−/− mice, the Th2-type cytokines IL-4, IL-5, and IL-13 were below detection limits in BALF from both non-HSCT and transplanted SP-A/D−/− mice.
Fig. 8.
BALF levels of TNF-α, IFN-γ, and MCP-1 are increased in SP-A/D−/− vs. SP-D−/− and wild-type mice after allogeneic HSCT. Cy/TBI-recipient SP-D+/+, SP-D−/−, and SP-A/D−/− B6 mice were infused with inflammation-inducing donor T cells. BALF was collected on day 7 after HSCT. TNF-α (A), IFN-γ (B), MCP-1 (C), and GM-CSF (D) were measured by sandwich ELISA. Values are means ± SE for n = 8–12 mice/group from 3 experiments. *P < 0.05 compared with nontransplanted (non-HSCT) SP-D+/+ mice. +P < 0.05 between SP-D+/+ and SP-D−/− mice after HSCT. ^P < 0.05 comparing SP-A/D−/− and SP-D−/− mice. Closed bars, SP-D+/+; hatched bars, SP-D−/−; open bars, SP-A/D−/−.
DISCUSSION
The major findings of this study are that the hydrophilic surfactant proteins modify the early inflammatory response after allogeneic HSCT and severity of IPS injury. On day 7 after allogeneic HSCT, SP-D−/− and SP-A/D−/− mice exhibited enhanced allogeneic T cell-dependent inflammation and increased permeability edema. Specifically, BALF from mice lacking SP-D contained increased levels of .NO, GM-CSF, and MCP-1. GM-CSF is implicated in the pathogenesis of macrophage cell changes observed in SP-D−/− and SP-A/D−/− mice (14). BALF from transplanted mice lacking both SP-A and SP-D contained, in addition, higher levels of TNF-α and IFN-γ. These results are consistent with differential anti-inflammatory protective effects of SP-A and SP-D after allogeneic HSCT.
There are several possible explanations for exacerbated IPS injury in mice lacking SP-D. In our murine IPS model, injury has been shown to correlate with the early induction of TNF-α following infusion of allogeneic T cells in conditioned mice (34). However, despite evidence of cellular activation in lungs of nontransplanted control SP-D−/−, BALF TNF-α levels were below detection limits, and cultured alveolar macrophages collected from non-HSCT SP-D−/− mice did not generate TNF-α. Furthermore, SP-D+/+ and SP-D−/− generated similar levels of TNF-α and IFN-γ after allogeneic HSCT. We concluded that increased lung dysfunction in SP-D−/− vs. wild-type mice is not caused by enhanced production of the proinflammatory cytokines TNF-α and IFN-γ after allogeneic HSCT. Instead, BALF from SP-D−/− vs. wild-type mice before and after HSCT contained increased levels of nitrite and nitrates, the stable byproducts of .NO metabolism. High levels of .NO and nitrative stress may have contributed to severe lung dysfunction during SP-D deficiency. Evidence indicates that increased .NO expression and generation of nitrative stress have been implicated in infectious and noninfectious lung injury models, including our IPS model (11), bleomycin-induced pulmonary damage (5), and Pneumocystis carinii respiratory infection (2). Importantly, a recent study identified iNOS as a primary mediator of the inflammatory response that is observed in SP-D−/− mice (3). Selective inhibition of iNOS in 10-wk-old SP-D−/− mice attenuated BALF inflammatory cell count, macrophage size, and cytokine/chemokine expression (3). Other potential protective mechanisms of SP-D may include its ability to enhance the elimination of apoptotic and necrotic cells (6), to suppress oxidative stress, and to decrease the production of matrix metalloproteinase (38).
BALF protein levels were higher in SP-A/D−/− vs. SP-D−/−, indicating increased permeability edema during the simultaneous absence of SP-A and SP-D. This increased BALF protein in SP-A/D−/− mice was associated with enhanced levels of .NO, TNF-α, IFN-γ, and MCP-1. These results are consistent with our previous findings that mice lacking SP-A exhibit increased lung levels of TNF-α, IFN-γ, .NO, and nitrative stress (36). We concluded that increased TNF-α and IFN-γ levels after allogeneic HSCT in SP-A/D−/− mice are mainly caused by the absence of SP-A. The progressive increase in .NO levels in wild-type vs. SP-D−/− vs. SP-A/D−/− mice after allogeneic HSCT is probably the result of the absence of both surfactant proteins. Simultaneous overproduction of the proinflammatory cytokines and .NO may clarify, at least in part, the reason why SP-A/D−/− mice exhibited the most severe lung dysfunction, manifested by exaggerated permeability edema.
In contrast to our findings, other investigators have shown that SP-A stimulates inflammatory responses and .NO expression (16, 22). A recent report indicates that the anti-inflammatory effects of SP-A are mainly caused by transforming growth factor-β present in SP-A preparations (25). Our findings of progressively increased levels of TNF-α, IFN-γ, and .NO in SP-A/D−/− compared with SP-D−/− mice after allogeneic HSCT, however, confirm that endogenous SP-A has a protective anti-inflammatory role and limits .NO generation in this model of lung injury induced by allogeneic donor T cells and the conditioning regimen. Clearly, the function of SP-A and SP-D depends on the environment present inside the lung. A seminal manuscript by Gardai and colleagues (9) demonstrated that SP-A and SP-D act in a dual manner, to enhance or suppress inflammatory mediator production depending on cellular binding orientation and the presence of pathogens (30). The importance of the injury model is also evident in most studies showing that SP-A−/− and SP-D−/− mice exhibit enhanced susceptibility to immune-mediated lung dysfunction (5, 13, 19, 35) but not during exposure to hyperoxic injury (18, 21). The most likely reason SP-D−/− mice are resistant to the damaging effects of hyperoxia is the presence of high levels of surfactant phospholipids in lungs of SP-D-deficient mice (20). Surfactant phospholipids have long been known to limit oxygen-induced lung damage (28).
The number and size of BALF cells increased during SP-D deficiency, possibly as a result of increased MCP-1 and GM-CSF, respectively (31). However, BALF cell differential was not modified, and the Th1-type (TNF-α and IFN-γ) in the absence of Th2-type cytokines (IL-4, IL-5, and IL-13) persisted during SP-D deficiency following allogeneic HSCT. Our results indicate that the absence of SP-D in recipient mice resulted in higher .NO and TNF-α levels in both BALF and in supernatant of cultured alveolar macrophages/monocytes. These data showing SP-D deficiency had similar effects on mediator production in both BALF and by isolated macrophages/monocytes are consistent with the notion that SP-D influenced BALF .NO and TNF-α generation after allogeneic HSCT, at least in part, by modulation of macrophages/monocytes. Before HSCT, however, BALF nitrite plus nitrate levels were higher in SP-D−/− vs. wild-type mice, yet macrophages from nontransplanted SP-D−/− mice failed to generate .NO. These results indicate that lack of SP-D may have altered .NO generation by effects on other cell type(s) in the lung. Alternatively, contamination of BALF by nitrite-containing serum in non-HSCT SP-D−/− mice may explain the increased BALF nitrite plus nitrate levels observed during SP-D deficiency.
A relevant recent clinical study revealed, using multivariate analysis, an association between pretransplant low-serum SP-D levels and development of IPS following allogeneic HSCT (29). The authors speculate that constitutively low-serum SP-D may reflect low alveolar levels, and, therefore, predispose recipients of HSCT to intense inflammation associated with allogeneity that can culminate in IPS injury.
One manifestation of lung injury in our murine IPS model is decreased lung dynamic compliance (32). On day 7 after allogeneic HSCT, lung compliance was significantly decreased in SP-D-deficient mice compared with B6 wild-type mice. In contrast, SP-D−/− mice on NIH Swiss and CD1 background have been shown to exhibit emphysema and increased lung compliance (39). SP-D−/− mice on B6 background, however, do not develop emphysema (1). Similarly, we did not observe histological evidence of emphysema in lungs collected from SP-D−/− and SP-A/D−/− mice before or after allogeneic HSCT.
In summary, the present study demonstrates that deficiency of SP-D and SP-A exacerbates the generation of early inflammatory responses associated with the development of IPS. The surfactant proteins exhibit overlapping, but not identical, anti-inflammatory protective roles. These data add to the accumulating evidence supporting the clinical use of SP-A and/or SP-D for the treatment of inflammatory and immune-mediated lung diseases.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R0-1-HL-67334 and HL-55209.
Acknowledgments
We gratefully thank Kathy Leigh-Godfrey for assistance in preparation of the manuscript. Present address for I. Y. Haddad: Banner Children's Hospital, 1400 S. Dobson Road, Mesa, AZ 85202.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Atochina EN, Beers MF, Hawgood S, Poulain F, Davis C, Fusaro T, Gow AJ. Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am J Respir Cell Mol Biol 30: 271–279, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Atochina EN, Gow AJ, Beck JM, Haczku A, Inch A, Kadire H, Tomer Y, Davis C, Preston AM, Poulain F, Hawgood S, Beers MF. Delayed clearance of pneumocystis carinii infection, increased inflammation, and altered nitric oxide metabolism in lungs of surfactant protein-D knockout mice. J Infect Dis 189: 1528–1539, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Atochina-Vasserman EN, Beers MF, Kadire H, Tomer Y, Inch A, Scott P, Guo CJ, Gow AJ. Selective inhibition of inducible NO synthase activity in vivo reverses inflammatory abnormalities in surfactant protein D-deficient mice. J Immunol 179: 8090–8097, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borron PJ, Mostaghel EA, Doyle C, Walsh ES, Heyzer-Williams MG, Wright JR. Pulmonary surfactant proteins A and D directly suppress CD3+/CD4+ cell function: evidence for two shared mechanisms. J Immunol 169: 5844–5850, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Casey J, Kaplan J, Atochina-Vasserman EN, Gow AJ, Kadire H, Tomer Y, Fisher JH, Hawgood S, Savani RC, Beers MF. Alveolar surfactant protein D content modulates bleomycin-induced lung injury. Am J Respir Crit Care Med 172: 869–877, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 169: 2892–2899, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Diamond L, O'Donnell M. Pulmonary mechanics in normal rats. J Appl Physiol 43: 942–948, 1977. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol 27: 24–33, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115: 13–23, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 34: 704–710, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad IY, Panoskaltsis-Mortari A, Ingbar DH, Yang S, Milla CE, Blazar BR. High levels of peroxynitrite are generated in the lungs of irradiated mice given cyclophosphamide and allogeneic T cells. A potential mechanism of injury after marrow transplantation. Am J Respir Cell Mol Biol 20: 1125–1135, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol 36: 94–102, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Umstead TM, Ponnuru P, Guo X, Hawgood S, Phelps DS, Floros J. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol 220: 72–82, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawgood S, Akiyama J, Brown C, Allen L, Li G, Poulain FR. GM-CSF mediates alveolar macrophage proliferation and type II cell hypertrophy in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 280: L1148–L1156, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, Edmondson J, Levitt S, Carlson E, Gillespie AM, Villar A, Epstein CJ, Poulain FR. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol 283: L1002–L1010, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Hickman-Davis J, Gibbs-Erwin J, Lindsey JR, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc Natl Acad Sci USA 96: 4953–4958, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikegami M, Carter K, Bishop K, Yadav A, Masterjohn E, Brondyk W, Scheule RK, Whitsett JA. Intratracheal recombinant surfactant protein D prevents endotoxin shock in the newborn preterm lamb. Am J Respir Crit Care Med 173: 1342–1347, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikegami M, Jobe AH, Whitsett J, Korfhagen T. Tolerance of SP-A-deficient mice to hyperoxia or exercise. J Appl Physiol 89: 644–648, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Ikegami M, Scoville EA, Grant S, Korfhagen T, Brondyk W, Scheule RK, Whitsett JA. Surfactant protein-D and surfactant inhibit endotoxin-induced pulmonary inflammation. Chest 132: 1447–1454, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ikegami M, Whitsett JA, Jobe A, Ross G, Fisher J, Korfhagen T. Surfactant metabolism in SP-D gene-targeted mice. Am J Physiol Lung Cell Mol Physiol 279: L468–L476, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Jain D, Atochina-Vasserman E, Kadire H, Tomer Y, Inch A, Scott P, Savani RC, Gow AJ, Beers MF. SP-D-deficient mice are resistant to hyperoxia. Am J Physiol Lung Cell Mol Physiol 292: L861–L871, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Janic B, Umstead TM, Phelps DS, Floros J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am J Physiol Lung Cell Mol Physiol 288: L317–L325, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation 63: 1079–1086, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlman MT, Jobe AH, Ikegami M, Whitsett JA, Fisher JH. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 273: 28438–28443, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Kunzmann S, Wright JR, Steinhilber W, Kramer BW, Blaser K, Speer CP, Schmidt-Weber C. TGF-beta1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T lymphocytes. Am J Physiol Lung Cell Mol Physiol 291: L747–L756, 2006. [DOI] [PubMed] [Google Scholar]
- 26.LeVine AM, Whitsett JA, Gwozdz JA, Richardson TR, Fisher JH, Burhans MS, Korfhagen TR. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J Immunol 165: 3934–3940, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Liu CF, Chen YL, Shieh CC, Yu CK, Reid KB, Wang JY. Therapeutic effect of surfactant protein D in allergic inflammation of mite-sensitized mice. Clin Exp Allergy 35: 515–521, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Loewen GM, Holm BA, Milanowski L, Wild LM, Notter RH, Matalon S. Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol 66: 1087–1092, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Nakane T, Nakamae H, Kamoi H, Koh H, Takeoka Y, Sakamoto E, Kanashima H, Nakamae M, Ohta K, Terada Y, Koh KR, Yamane T, Hino M. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 42: 43–49, 2008. [DOI] [PubMed] [Google Scholar]
- 30.O'Reilly P, Hickman-Davis JM, McArdle P, Young KR, Matalon S. The role of nitric oxide in lung innate immunity: modulation by surfactant protein-A. Mol Cell Biochem 234–235: 39–48, 2002. [PubMed] [Google Scholar]
- 31.Ochs M, Knudsen L, Allen L, Stumbaugh A, Levitt S, Nyengaard JR, Hawgood S. GM-CSF mediates alveolar epithelial type II cell changes, but not emphysema-like pathology, in SP-D-deficient mice. Am J Physiol Lung Cell Mol Physiol 287: L1333–L1341, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM, Wangensteen OD, Bitterman PB, Ingbar DH, Vallera DA, Blazar BR. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J Clin Invest 100: 1015–1027, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4: 252–257, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shukla M, Yang S, Milla C, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Absence of host tumor necrosis factor receptor 1 attenuates manifestations of idiopathic pneumonia syndrome. Am J Physiol Lung Cell Mol Physiol 288: L942–L949, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Wang JY, Reid KB. The immunoregulatory roles of lung surfactant collectins SP-A and SP-D in allergen-induced airway inflammation. Immunobiology 212: 417–425, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Milla C, Panoskaltsis-Mortari A, Hawgood S, Blazar BR, Haddad IY. Surfactant protein A decreases lung injury and mortality after murine marrow transplantation. Am J Respir Cell Mol Biol 27: 297–305, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Milla C, Panoskaltsis-Mortari A, Ingbar DH, Blazar BR, Haddad IY. Human surfactant protein A suppresses T cell-dependent inflammation and attenuates the manifestations of idiopathic pneumonia syndrome in mice. Am J Respir Cell Mol Biol 24: 527–536, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol 166: 7514–7519, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Ikegami M, Dey CR, Korfhagen TR, Whitsett JA. Reversibility of pulmonary abnormalities by conditional replacement of surfactant protein D (SP-D) in vivo. J Biol Chem 277: 38709–38713, 2002. [DOI] [PubMed] [Google Scholar]