Abstract
Background
The impact of recent guidelines for early detection and prevention of chronic kidney disease (CKD) on patient awareness of disease and factors that might be associated with awareness have not been well-described.
Methods
Awareness rates were assessed in 2992 adults (≥20 years) with CKD stages 1-4 from a nationally representative, cross-sectional survey (National Health and Nutrition Examination Survey 1999-2004). Awareness of CKD was defined by an answer of yes to: “Have you ever been told you have weak or failing kidneys?” Potential predictors of awareness included demographic, access to care, clinical, and lifestyle factors, which were assessed by standardized interviewer-administered questionnaires and physical exams. We examined independent associations of patient characteristics with awareness in those with CKD stage 3 (N=1314) over 6 years using multivariable logistic regression.
Results
Awareness improved over time in stage 3 only [4.7% (95% CI, 2.6-8.5%), 8.9% (7.1-11.2%), and 9.2% (6.1-13.8%) for 1999-2000, 2001-2002, and 2003-2004, respectively; P = 0.037, adjusted for age, sex, race]. Having proteinuria [OR=3.04 (1.62-5.70)], diabetes [OR=2.19 (1.03-4.64)], and hypertension [OR=2.92 (1.57-5.42)] and being male [OR=2.06 (1.15-3.69)] were all statistically significantly associated with greater awareness among persons with CKD stage 3 after adjustment. CKD awareness increased almost 2-fold for CKD stage 3 over recent years but remains quite low. Persons with risk factors for CKD (proteinuria, diabetes, hypertension, male sex) were more likely to be aware of their stage 3 disease.
Conclusion
Renewed and innovative efforts should be made to increase CKD awareness among patients and providers.
Introduction
Chronic kidney disease (CKD) is a growing problem in the U.S., with an estimated prevalence of 9-12% in 1999-2000 (1;2). However, the majority of persons with CKD, especially those in early stages, may be unaware of their disease. Although there have been recent efforts to increase awareness both among providers, by dissemination of guidelines for the definition and staging of severity of CKD (3), and more recently to the public (4), there is a lack of evidence about whether awareness of CKD has increased over time.
Better management of CKD can slow progression of CKD, prevent complications, and reduce cardiovascular-related outcomes (3;5;6). Early referral to a nephrologist has been shown to improve outcomes for those who progress to end-stage renal disease (7). Even if quality improvement initiatives are accepted and implemented by the medical community, patients must still seek timely treatment in order to be exposed to these initiatives. However, because CKD in its early stages is usually silent, without remarkable symptoms, patients may not be aware of their disease. Even persons who have been identified as having early-stage CKD by evidence of kidney damage or reduced kidney function through regular screening may not understand their diagnosis or recognize the importance of treatment.
Some patient characteristics may make screening more likely. For example, patients who are at high risk because of diabetes or hypertension may be screened more frequently. The likelihood of being aware could differ by age, sex, or race; for example, there is a greater prevalence of CKD among elderly patients (1;2), but older patients may also be less likely to be screened, or CKD may not be recognized in older patients, because of competing health issues. Whether the patient is insured or has a regular provider could also affect CKD awareness. Finally, a healthy lifestyle (as indicated by such factors as no smoking, low alcohol intake, and high physical activity) may make a person less likely to have CKD but also more attentive to presence of disease and practice of preventive behaviors, which modify risk.
Previous studies have reported on static CKD awareness estimates in the U.S. population in 1999-2000 (1;8). To our knowledge, none have examined the trends in CKD awareness over time, with more recent data, nor have the associations of awareness with characteristics of persons with CKD been extensively explored. We sought to examine the prevalence of disease awareness among adults with CKD and the factors that may be associated with CKD awareness in a long-running, national survey of U.S. citizens, the National Health and Nutrition Examination Survey (NHANES).
Methods
Study Design
The NHANES surveys are currently conducted every 2 years by the National Center for Health Statistics to examine disease prevalence and trends over time in different cross-sectional representative samples of non-institutionalized U.S. civilian residents. The survey consists of a standardized in-home interview and a physical examination and blood and urine collection at a mobile examination center (MEC). Participants gave informed consent. The protocol was approved by an institutional review board.
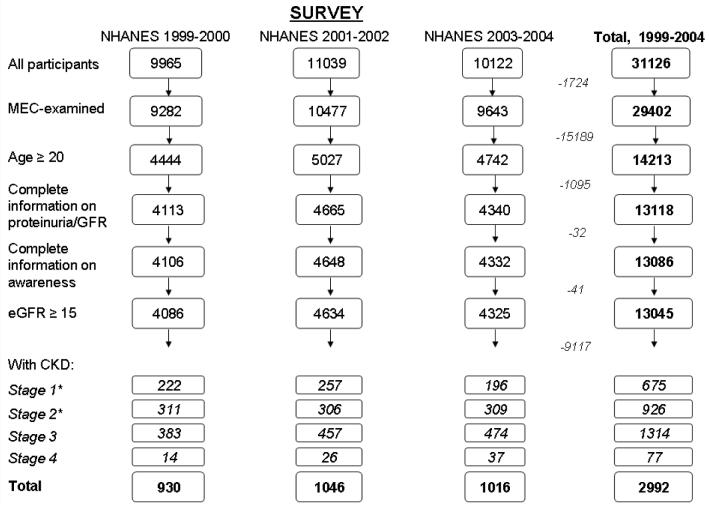
Here we examined data from the 1999-2000, 2001-2002, and 2003-2004 (9) NHANES surveys. Our study was limited to NHANES participants from 1999-2004 who: underwent the MEC exam; were at least 20 years old; had information on awareness, age, sex, race, creatinine [to calculate estimated GFR (eGFR)], and proteinuria (if eGFR>60 ml/min/m2); had an eGFR of at least 15 ml/min/1.73 m2; and had CKD stages 1-4 (n=930, 1046, and 1016 for the three surveys, respectively) (see Fig. 1).
Figure 1.
NHANES participants, 1999-2004, who met inclusion criteria for this study. *Stages 1 and 2 defined by single measurement of albuminuria only; persistent albuminuria data not available.
Measurements
Self-reported demographic characteristics (age, sex, race, educational level), access to care (insurance, routine healthcare site, time since last doctor visit), lifestyle factors (smoking, activity, alcohol use), and diagnoses (CKD, diabetes, hypertension) were obtained during the interview portions of the surveys. Blood pressure was measured during the MEC visit, and the average of all available measurements (up to four) was used. Height and weight, used in the calculation of body mass index (BMI), were also measured during this exam. Random spot urine samples were obtained, and urine albumin and creatinine were measured using frozen specimens. Urine albumin was measured using solid-phase fluorescence immunoassay; urine creatinine was measured using the modified Jaffe kinetic method in the same laboratory. Serum creatinine was measured by the modified kinetic method of Jaffe using different analyzers in different survey years.
Definitions
The outcome variable was awareness of CKD. Participants who answered “yes” to the question “Have you ever been told you have weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence)?” during the interview were defined as being aware of their CKD.
eGFR was calculated according to the modified MDRD formula for calibrated creatinine: eGFR=175 × [(calibrated serum creatinine in mg/dl)−1.154] × age−0.203 × (0.742 if female) × (1.210 if African-American) (10;11). Serum creatinine was calibrated for 1999-2000 participants using the formula: calibrated serum creatinine=1.013*(original serum creatinine in mg/dl) + 0.147. No correction was required for calibrated serum creatinine in participants in the 2001-2002 or 2003-2004 surveys (12). Proteinuria was considered to be present at urinary albumin-to-creatinine ratios of 17-250 mg/g (microalbuminuria) and >250 mg/g (macroalbuminuria) for males and 25-355 mg/g (microalbuminuria) and >355 mg/g (macroalbuminuria) for females (13). The independent variable of presence and stage of CKD in the participants was determined using eGFR and presence of proteinuria according to the Kidney Disease Outcomes Quality Initiative guidelines (3). Because urine protein measurements in NHANES are cross-sectional, we did not have data on persistent proteinuria, and the definitions of stages were therefore modified as: stage 1, eGFR > 90 ml/min per 1.73 m2 and presence of proteinuria; stage 2, eGFR 60-89 ml/min per 1.73 m2 and presence of proteinuria; stage 3, eGFR 30-59 ml/min per 1.73 m2; stage 4, 15-29 ml/min per 1.73 m2; and stage 5, <15 ml/min per 1.73 m2.
Other independent variables included sex, diabetes, and hypertension. Diabetes was defined by answer of “yes” to the question “have you ever been told by a doctor that you have diabetes or sugar diabetes?”; hypertension was defined by an answer of “yes” to “have you ever been told by a doctor that you have hypertension, also called high blood pressure?,” an average systolic blood pressure ≥140 mmHg, or average diastolic blood pressure ≥90 mmHg.
Statistical Methods
The proportion aware was calculated by CKD stage and survey year. Variance of proportions was estimated with Taylor series linearization. Exploration of factors associated with awareness was limited to CKD stage 3 due to small sample sizes for stage 4 and potential misclassification due to short-term variability in microalbuminuria in stages 1 and 2 (14). Proportion aware was calculated by patient characteristics and by year, and those characteristics that were shown or thought a priori to be associated with awareness, were examined in logistic models predicting awareness by year.
All analyses were performed using the svy commands in Stata v 9.2 to account study design weights, strata, and pseudostrata. Appropriate NHANES 2-year and 6-year MEC weights were used; 6-year weights were calculated as: wtmec6yr=2/3 × wtmec4yr (if survey year = 1999-2002) and wtmec6yr=1/3 × wtmec2yr (if survey year = 2003-2004) (15).
Results
Proportion of Persons with CKD Who Are Aware of Their Disease
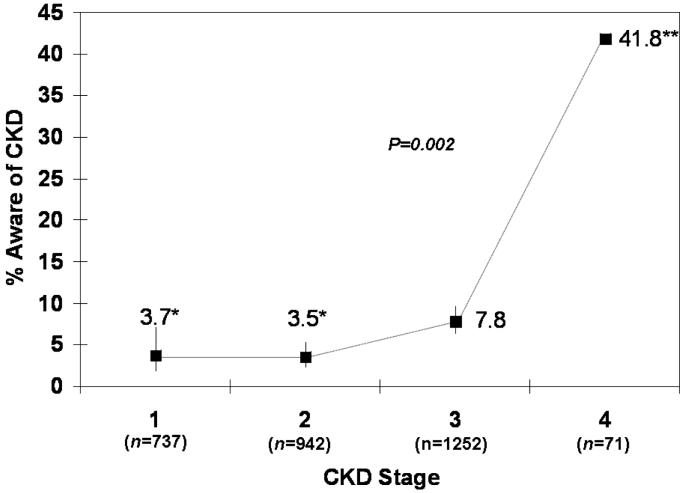
Overall, there were 2992 NHANES participants with CKD stages 1-4 who met the inclusion criteria (Fig. 1) from 1999 to 2004 (675 stage 1, 926 stage 2, 1314 stage 3, and 77 stage 4). Of these, only 6.0% reported being told that they had weak or failing kidneys (24 stage 1, 36 stage 2, 111 stage 3, and 34 stage 4 participants). The proportion aware differed substantially by CKD stage (Fig. 2), with awareness in stages 1 and 2 being less than half that in stage 3, and awareness in stage 4 being nearly 6 times greater than that in stage 3 (P=0.002). However, even at stage 4, fewer than half the subjects were aware that they had CKD.
Figure 2.
Proportion of subjects with CKD who were aware of their disease by CKD stage, NHANES 1999-2004. P for trend across stage adjusted for age, sex, and race. *Stages 1 and 2 defined by single measurement of albuminuria only; persistent albuminuria data not available. **No SE estimates due to small sample size. Bars, 95% CI.
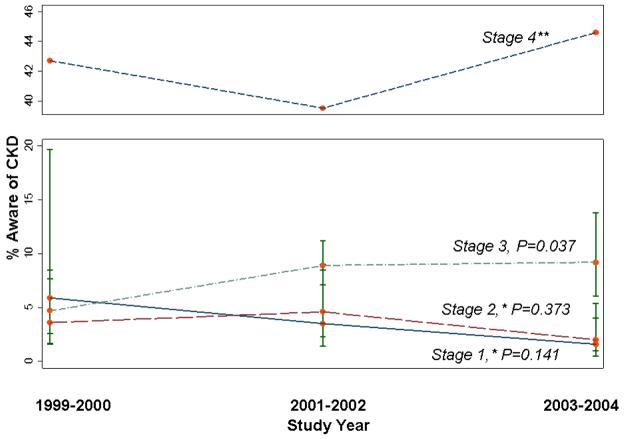
CKD awareness did not change over time for participants with CKD overall (stages 1-4: 1999-2000, 5.2%; 2001-2002, 6.7%; and 2003-2004, 6.0%; P=0.391). Awareness did increase for participants with CKD stage 3 (Fig. 3), with proportion aware being greater in 2001-2004 than in 1999-2000 (P=0.037). For stages 1 and 2, there were slight downward trends in awareness over time that were not statistically significant (P=0.141 and 0.373, respectively); awareness in CKD stage 4 did not change significantly over time. Awareness for CKD stage 3 was comparable to awareness for CKD stages 1 and 2 in 1999-2000, but in 2001-2004 awareness was higher for CKD stage 3 (Fig. 3).
Figure 3.
Proportion of subjects with CKD stages 1-4 who were aware of their disease by survey year, NHANES 1999-2004. P for trend across years adjusted for age, sex, and race. *Stages 1 and 2 defined by single measurement of albuminuria only; persistent albuminuria data not available. **No SE estimates due to small sample size. Bars, 95% CI.
Factors Associated with CKD Awareness in CKD Stage 3
A total of 1314 participants had CKD stage 3 from 1999 to 2004; of these, 7.8% were aware of their CKD (Table 1). Participants with CKD stage 3 who were non-Hispanic black or Mexican-American had higher rates of awareness than participants of non-Hispanic white or other races. A greater proportion of males were aware of their CKD than females. CKD awareness was far greater, but still less than one-fifth, among participants who had proteinuria, diabetes, hypertension, and obesity than those who did not. Those with less education and those with a routine site for healthcare were also more likely to be aware of their CKD, but these associations were not statistically significant.
Table 1.
CKD awareness in NHANES participants 1999-2004 (stage 3 only), by patient characteristics
| Characteristic | Proportion (%) of stage 3 CKD participants who were ever told that they had weak or failing kidneys (95% CI) |
||||
|---|---|---|---|---|---|
| 1999-2000 | 2001-2002 | 2003-2004 | All years, 1999-2004 |
||
| N | 383 | 457 | 474 | 1314 | |
| All patients | 4.7 (2.6-8.5) | 8.9 (7.1-11.2) | 9.2 (6.1-13.8) | 7.8 (6.3-9.6) | |
| Demographics | |||||
| Age | |||||
| <65 | 3.9 (1.1-12.7)* | 8.9 (4.9-15.5) | 15.1(7.1-29.3)* | 9.2 (6.1-13.8) | |
| ≥65 | 5.2 (2.5-10.3) | 9.0 (6.1-13.0) | 6.8 (4.0-11.3) | 7.1 (5.4-9.3) | |
| P | 0.685 | 0.975 | 0.095 | 0.325 | |
| Race | |||||
| Non-Hispanic White | 4.3 (1.9-9.5)* | 7.5 (4.9-11.3) | 9.0 (5.4-14.5) | 7.1 (5.3-9.5) | |
| Non-Hispanic Black | 11.2 (5.0-23.4)* | 24.3 (11.8-43.5)* | 22.7 (10.3-42.8)* | 19.3 (12.9-27.9) | |
| Mexican-American | 14.3 (5.8-31.0)* | 11.0 (2.6-36.8)* | 9.0 (2.3-29.6)* | 11.6 (6.1-21.2)* | |
| P | 0.299 | 0.061 | 0.081 | 0.040 | |
| Sex | |||||
| Female | 1.7 (0.7-4.4)* | 6.6 (3.9-10.9) | 7.4 (5.0-11.0) | 5.4 (4.0-7.4) | |
| Male | 9.9 (4.5-20.3)* | 12.4 (7.5-19.8) | 12.1 (5.9-23.3) | 11.6 (8.2-16.2) | |
| P | 0.003 | 0.150 | 0.190 | 0.004 | |
| Education | |||||
| <High school | 2.9 (1.8-4.6) | 14.6 (10.5-20.1) | 13.5 (5.9-28.2)* | 10.3 (7.2-14.4) | |
| High school/GED+ | 5.7 (2.6-12.4) | 6.6 (4.0-10.6) | 7.8 (4.7-12.6) | 6.8 (5.0-9.2) | |
| P | 0.169 | 0.021 | 0.261 | 0.108 | |
| Access to Care: | |||||
| Health insurance | |||||
| Yes | 4.8 (2.6-8.8)* | 8.9 (7.2-10.8) | 9.5 (6.3-14.0) | 7.9 (6.5-9.7) | |
| No | 4.3 (0.8-19.6)* | 13.0 (2.3-48.7)* | 0** | 6.6 (1.9-20.4)* | |
| P | 0.876 | 0.612 | 0.556 | 0.756 | |
|
Routine site for healthcare |
|||||
| Yes | 4.9 (2.6-8.8)* | 9.2 (7.2-11.8) | 9.4 (6.1-14.3) | 8.1 (6.5-10.0) | |
| No | 2.0 (0.2-16.9)* | 0.7 (0.0-6.4)* | 4.0 (0.4-29.7)* | 2.3 (0.6-9.0)* | |
| P | 0.395 | 0.006 | 0.418 | 0.058 | |
| Clinical: | |||||
| Proteinuria | |||||
| None | 1.5 (0.4-5.3)* | 5.0 (2.8-9.0)* | 6.0 (3.2-11.1) | 4.5 (3.0-6.7) | |
| Microalbuminuria | 6.4 (2.1-17.7)* | 14.3 (9.5-21.1) | 12.2 (6.0-23.3) | 11.0 (7.6-15.5) | |
| Macroalbuminuria | 27.7 (11.6-52.7)* | 32.0 (17.2-51.5) | 47.8 (20.7-76.2) | 33.0 (22.4-45.6) | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |
| Diabetes | |||||
| No | 3.7 (1.7-7.9)* | 7.1 (5.4-9.3)* | 6.4 (3.8-10.5) | 5.9 (4.6-7.5) | |
| Yes | 12.2 (3.5-34.4)* | 20.3 (8.1-42.4) | 19.1 (9.5-34.6) | 17.8 (11.1-27.4) | |
| P | 0.083 | 0.049 | 0.016 | <0.001 | |
| Hypertension | |||||
| No | 3.6 (1.2-10.0)* | 1.5 (0.4-5.3)* | 5.3 (1.7-15.4)* | 3.4 (1.8-6.4)* | |
| Yes | 5.8 (3.1-10.6)* | 14.2 (11.2-17.9) | 11.3 (8.0-15.7) | 10.9 (9.1-13.0) | |
| P | 0.361 | <0.001 | 0.148 | <0.001 | |
| Lifestyle: | |||||
| Obesity (BMI ≥30 kg/m2) | |||||
| No | 3.7 (1.5-8.8)* | 11.3 (7.7-16.1) | 7.4 (4.1-13.2) | 5.7 (3.9-8.2) | |
| Yes | 6.6 (2.8-14.9)* | 5.7 (2.8-10.9)* | 12.5 (7.3-20.6) | 10.4 (7.6-13.9) | |
| P | 0.317 | 0.085 | 0.166 | 0.012 | |
|
Any moderate physical activity in past 30 days |
|||||
| No | 5.3 (2.6-10.5)* | 11.5 (8.1-16.0) | 10.2 (5.4-18.7) | 9.1 (6.8-12.0) | |
| Yes | 3.7 (1.0-12.6)* | 5.8 (2.3-13.5)* | 8.1 (4.3-14.5) | 6.1 (3.8-9.5) | |
| P | 0.600 | 0.199 | 0.590 | 0.162 | |
| Current smoking status | |||||
| No | 3.5 (1.1-10.0)* | 13.1** | 25.1** | 8.7** | |
| Yes | 9.6 (3.1-26.2)* | 10.2** | 7.6** | 14.0** | |
| P | 0.183 | — | — | — | |
Proportions adjusted for sampling weights for each survey year.
Estimates have low precision (SEs ≥ 30% of estimate).
No SEs due to single sampling units or zero sample sizes.
Within individual survey years, the associations of awareness with patient characteristics were generally similar to those seen in the overall study period (Table 1), although statistical significance was often lost in these small subgroups. For example, male sex was associated with increased awareness for all years, but the association was only statistically significant for 1999-2000. The association of CKD awareness with proteinuria in these stage 3 participants was statistically significant for all three survey years, with macroalbuminuria being far more predictive than microalbuminuria. Diabetes and hypertension were statistically significantly associated with CKD awareness in 2001-2002 and 2003-2004 only.
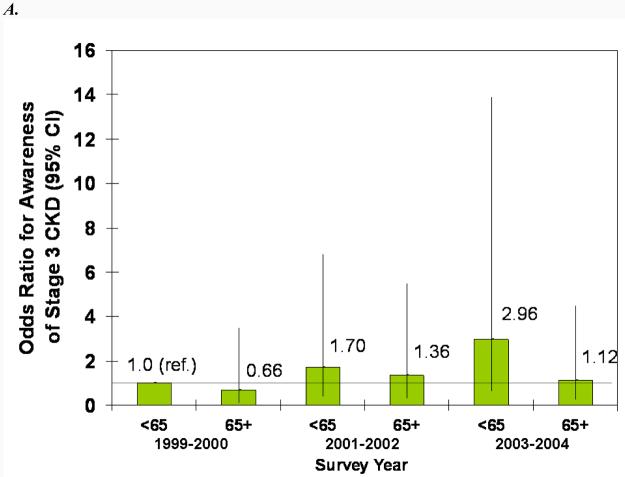
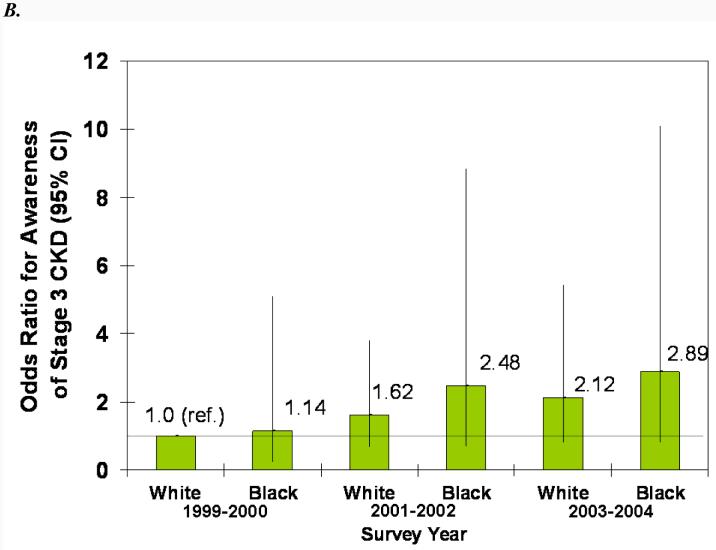
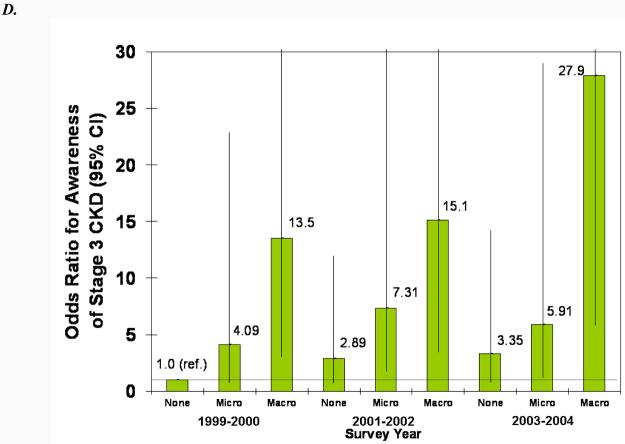
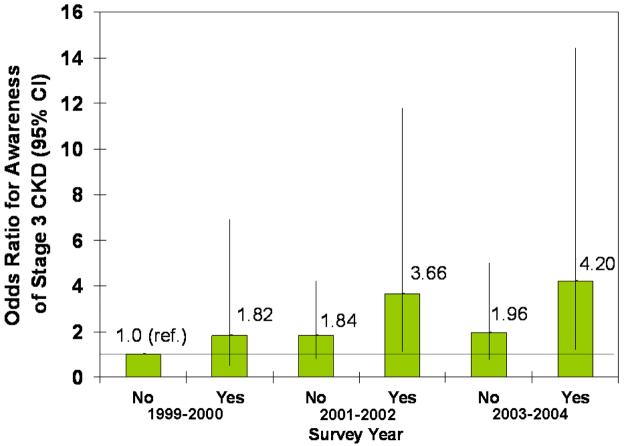
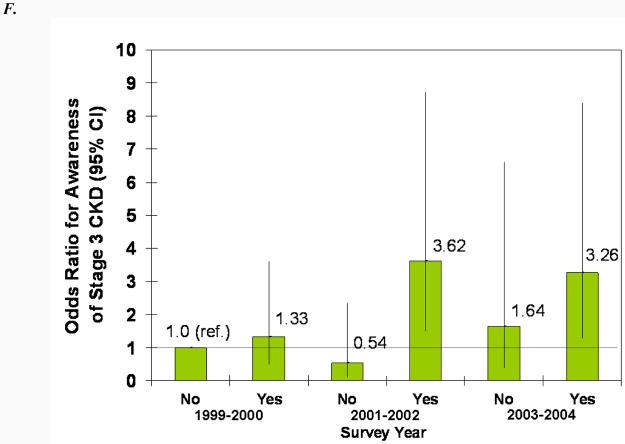
In adjusted models, male sex, proteinuria, diabetes, and hypertension were all statistically significantly associated with greater odds of CKD awareness (Fig. 4). Older age was associated with less awareness overall, and black race was associated with more awareness, but the associations were not statistically significant. The increase in awareness over time can be seen in both the younger and older and white and black subgroups (Fig. 4, A and B). Similarly, awareness increased in males and females from 1999-2002, but males remained more likely to be aware in all three survey years (Fig. 4C). Regardless of proteinuria subgroup (Fig. 4D), awareness increased after 1999-2001, with macroalbuminuria being a greater predictor than microalbuminuria in all three survey years. The same trend was seen for diabetes (Fig. 4E), with awareness increasing over time and diabetics remaining more likely to be aware. Hypertensive participants were more likely to be aware, especially in the last two survey years, but it is unclear whether awareness increased in non-hypertensive participants (Fig. 4F).
Figure 4.
Association of CKD awareness over time with age (A), race (B), sex (C), proteinuria (D), diabetes (E), and hypertension (F) in NHANES participants 1999-2004 (stage 3 only). Odds ratios are adjusted for other patient characteristics shown plus education and health insurance. *, no estimates, failure predicted perfectly.
After adjustment for other factors, having a routine site for health care (adjusted OR for 1999-2004=2.56, 95% CI, 0.63-10.3), having a high school education or greater (adjusted OR for 1999-2004=0.70, 95% CI, 0.35-1.38), being uninsured (adjusted OR for 1999-2004=0.92, 95% CI, 0.25-3.36), and being obese (adjusted OR for 1999-2004=1.45, 95% CI, 0.83-2.54) were not associated CKD awareness.
Discussion
We found that disease awareness among U.S. adults with CKD, as defined by KDOQI staging (3), was generally quite low. Even at CKD stage 4, fewer than half of the persons with CKD were aware of their disease. There were increases in CKD awareness after 2000, consistent across subgroups but seen only in those with CKD stage 3, which are arguably impressive for this short period; however, awareness among these persons was still fewer than 1 in 10. Substantial recent efforts to increase awareness among nephrologists (dissemination of KDOQI staging in 2002 (3)) and general physicians and persons in the general public (formation of the National Kidney Disease Education Program by the National Institutes of Health in 2001 (16) and the initiation of a free screening program by the National Kidney Foundation, the Kidney Early Education Program, piloted in 1997-1999 and continuing today (17)) have not produced high levels of awareness among patients since their implementation several years ago. However, changes in guidelines (3) coupled with recent increased reporting of eGFR (16) may be at least partially responsible for differential increase in awareness for CKD stage 3 versus stages 1 and 2. CKD stages 1 and 2 are identified through the presence of proteinuria, which may not be as commonly, or consistently, detected.
CKD awareness rates, at <10% for CKD stage 3 and <50% for CKD stage 4, are still unacceptably low. The discrepancy between CKD awareness and awareness of other chronic diseases is quite large. Patients with hypertension and diabetes had awareness rates of 74% and 70%, respectively, in the same population during the same period (18;19). Both the National High Blood Pressure Education Program of the National Heart Lung and Blood Institute, founded in 1972 (20), and the National Diabetes Education Program (through the combined efforts of the National Institutes of Health, Centers for Disease Control, and more than 200 private organizations), founded in 1997 (21), ran aggressive public awareness campaigns for many years. Similar long-term, broad-scale efforts in CKD might increase awareness dramatically in the United States, especially if they target both practitioners, who could identify and treat affected individuals (7;22), and high-risk individuals, who could present to practitioners based upon their knowledge of CKD. Given that CKD not only can result in progression to end-stage renal disease and dependence on dialysis and transplantation but is also an independent risk factor for cardiovascular disease and mortality (23;24), the importance of increasing CKD awareness should not be underestimated.
Awareness was greater among some subgroups of patients. Black participants with CKD were more likely to be aware of their disease than whites. This race differential in patient awareness may reflect patient and physician perception of black race as a risk factor for CKD (25) or greater family history among these patients (26), which may result in more testing among these patients. Increased physician awareness of black race as a risk factor and greater communication of this risk to these patients may also have contributed to the greater CKD awareness seen in black patients. Although older patients are at increased risk for reduced kidney function and CKD, they were less likely to be aware of their disease. Whether this reflects less testing or acceptance of reduced kidney function on the part of the practitioners as a normal part of aging in this population is unknown. Also, although there is no evidence that males are at higher risk of developing CKD than females (27), males were far more likely to be aware of their CKD. Higher awareness among men may also be due to their higher serum creatinine, which physicians, especially those who are less aware of CKD and who use creatinine alone rather than age- and sex-adjusted eGFR, may recognize more readily as an abnormality. Physicians may also perceive men to be at higher risk than women and thus screen for CKD more often in these patients; or men's symptoms of CKD may be more pronounced or less likely to be attributed to other causes than women's symptoms.
Several clinical conditions made CKD awareness more likely as well. Those with hypertension and diabetes were far more likely to be aware of their disease. This greater likelihood may be because physicians recognize that these patients are at much greater risk for CKD. Patient awareness of their risk may also encourage patients to ask for CKD screening. Additionally, these patients are more likely to be seen frequently and thus be subjected to urine and blood testing as part of their regular care, making it more likely for CKD to be detected.
We expected that better access to care might lead to higher rates of disease awareness. However, we found no association between having health insurance and CKD awareness. This suggests that, even when patients have access to care, physician communication of risk and/or patient uptake of the information presented may be inadequate. Having a routine site for healthcare was marginally associated with greater CKD awareness, after adjustment for other factors. This may be due to those at greatest risk, including those with diabetes and hypertension, being more likely to have a routine site for healthcare because of the condition that puts them at risk for CKD. Given that having a routine site was associated with awareness after adjustment for these conditions, but that merely having health insurance was not, it is also possible that having an established relationship with a health care provider is more important to generating awareness than general access to care. Further efforts to enhance the patient-provider relationship, in the context of discussion of CKD, may improve awareness. Finally, we found that lifestyle factors, such as obesity, physical activity, smoking, and alcohol use were not associated with CKD awareness.
The urgency of making patients aware of CKD could be questioned on grounds that many patients die before they progress to a more severe stage of CKD and many are already being treated for diabetes and/or hypertension (28;29). However, there are still compelling reasons why patients would benefit from awareness of their CKD. First, they could be made aware of medication exposures that could influence progression, including over-the-counter non-steroidal anti-inflammatory agents and contrast agents using in imaging tests. Additionally, there is evidence that appropriate early treatment (with medications and hypertension control) could slow progression of CKD (29). Also, patients should be aware that current and future medications could require dose adjustment in the setting of CKD. Many conditions, such as heart disease and cognitive decline, may increase with severity of CKD, and awareness of disease may motivate patients to adopt preventive strategies for these conditions. Awareness might also make patients more vigilant with adherence to dietary recommendations for comorbid hypertension and diabetes, including lower salt, sugar, and fat intake, as well as other lifestyle changes.
There are several limitations to this study that deserve mention. First, the questionnaire item assessing awareness asked participants if they had ever been told they had weak or failing kidneys. Patients may not be told their kidneys are weak or failing, especially in early-stage CKD; rather they may be told they have decreased kidney function or protein in the urine. Thus, there is the possibility of misinterpretation of the questionnaire item by the participants; 1% of respondents without kidney disease answered “yes” to this question, indicating a small amount of misclassification of participant awareness and/or of early-stage disease. Second, proteinuria was a single measurement with no follow-up in NHANES, and CKD in its early stages is defined as persistent proteinuria. This lack of a confirmatory urine protein sample may have led to misclassification of participants with CKD stages 1 and 2. In fact, only 63% of subjects with albuminuria at first visit in NHANES III had albuminuria at a second visit (14). Misclassification of disease may have also occurred due to GFR estimation. Additionally, we do not know the duration of reduced kidney function and/or proteinuria, nor do we know the duration of symptoms, if any, that may have led the participant to seek medical treatment. The small number of participants with CKD, especially in single surveys, was another limitation. Provider factors, especially provider knowledge of CKD, quality of patient-provider communication, and specialist referral, may play a significant role in CKD awareness but could not be assessed using these data.
Despite substantial recent efforts, both nationally and locally, to increase awareness of CKD in the community, the majority of those with CKD, as defined by decreased renal function and/or evidence of kidney damage, do not recall having been told by a physician that they have CKD. Renewed and greater efforts and resources may need to be directed toward dissemination activities by the government (e.g., Centers for Disease Control and National Kidney Disease Education Program) and private organizations (e.g., National Kidney Foundation, American Society of Nephrology, Renal Physicians Association, American College of Physicians) to increase awareness of CKD among practitioners and in the general community. Not only those with risk factors (diabetes, hypertension) but also those who are less likely to be aware, including older, female, and white patients, without diabetes or hypertension, and those without routine access to healthcare should be targeted more aggressively. Future studies of disease awareness among those with CKD should focus on intervention by examining patient, provider, and societal (e.g., public relations campaigns) factors that lead to better CKD awareness.
Acknowledgements
We thank the participants and staff of the NHANES survey. This project was supported under a cooperative agreement from the Centers for Disease Control and Prevention through the Association of American Medical Colleges, grant number U36/CCU319276, AAMC ID number MM-0997-07/07. Report contents are solely the responsibility of the authors and do not necessarily represent the official views of the AAMC or CDC. Dr. Powe is partially supported by grant K24DK02643 from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Footnotes
Meeting Presentation: This study was presented in part at the 2007 American Society of Nephrology annual meeting in San Francisco, California, on November 2, 2007.
Financial Disclosure: None of the authors have any conflicts of interest to report.
References
- 1.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol. 2005;16(1):180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation . Kidney Disease Outcomes Quality Initiative (K/DOQI) National Kidney Foundation; 2008. Available at: http://www.kidney.org/professionals/doqi. [Google Scholar]
- 4.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. Chronic kidney disease: common, harmful and treatable--World Kidney Day 2007. Am J Nephrol. 2007;27(1):108–112. doi: 10.1159/000099801. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42(5):1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 7.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137(6):479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nickolas TL, Frisch GD, Opotowsky AR, Arons R, Radhakrishnan J. Awareness of kidney disease in the US population: findings from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2000. Am J Kidney Dis. 2004;44(2):185–197. doi: 10.1053/j.ajkd.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey Data, 1999-2000, 2001-2002, and 2003-2004. U.S.Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2007. [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek J, et al. Expressing the MDRD study equation for estimating GFR with IDMS traceable(gold standard) serum creatinine values. J Am Soc Nephrol. 2005;16:69A. [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13(4):1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 14.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics Analytic and reporting guidelines: the National Health and Nutrition Examination Survey (NHANES) Available at: http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/analytical_guidelines.htm. Updated September 2006.
- 16.National Kidney Disease Education Program National Institutes of Health. Available at: http://nkdep.nih.gov/. November 16, 2007.
- 17.National Kidney Foundation Kidney Early Evaluation Program. National Kidney Foundation. 2008 Available at: http://www.kidney.org/news/keep/index.cfm.
- 18.Ostchega Y, Dillon CF, Hughes JP, Carroll M, Yoon S. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the national health and nutrition examination survey 1988 to 2004. J Am Geriatr Soc. 2007;55(7):1056–1065. doi: 10.1111/j.1532-5415.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 19.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 20.National Heart Lung and Blood Institute National High Blood Pressure Education Program. 2007 Available at: http://www.nhlbi.nih.gov/about/nhbpep/ [PubMed]
- 21.National Institute of Diabetes & Digestive & Kidney Diseases National Diabetes Education Program. 2007 Available at: http://ndep.nih.gov/
- 22.Lea JP, McClellan WM, Melcher C, Gladstone E, Hostetter T. CKD risk factors reported by primary care physicians: do guidelines make a difference? Am J Kidney Dis. 2006;47:72–77. doi: 10.1053/j.ajkd.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32(5):853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 24.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation The facts about chronic kidney disease. 2008 Available at: http://www.kidney.org/kidneyDisease/ckd/index.cfm#facts.
- 26.McClellan W, Speckman R, McClure L, Howard V, Campbell RC, Cushman M, et al. Prevalence and characteristics of a family history of end-stage renal disease among adults in the United States population: Reasons for Geographic and Racial Differences in Stroke (REGARDS) renal cohort study. J Am Soc Nephrol. 2007;18(4):1344–1352. doi: 10.1681/ASN.2006090952. [DOI] [PubMed] [Google Scholar]
- 27.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 29.Appel LJ, Wright JT, II, Greene T, Kusek JW, et al. the AASK Collaborative Research Group The long-term effects of renin-angiotensin system blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African-Americans. Arch Intern Med. doi: 10.1001/archinte.168.8.832. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]