Abstract
Aims/Hypothesis
Islet transplantation is a potential cure for diabetes; however, rates of graft failure remain high. We sought to determine whether amyloid deposition is associated with reduced beta cell volume in islet grafts and the recurrence of hyperglycaemia following islet transplantation.
Methods
We transplanted streptozotocin-diabetic mice with 100 islets from human islet amyloid polypeptide transgenic mice that have the propensity to form islet amyloid (n=8–12) or from non-transgenic mice that do not develop amyloid (n=6–10) in sets of studies that lasted one or six weeks.
Results
Plasma glucose before and for one week after transplantation was similar in mice that received transgenic or non-transgenic islets, and at that time amyloid was detected in all transgenic grafts and, as expected, in none of the non-transgenic grafts. However, over six weeks following transplantation, plasma glucose increased in transgenic but remained stable in non-transgenic islet graft recipients (p<0.05). At six weeks, amyloid was present in 92% of the transgenic grafts and in none of the non-transgenic grafts. Beta cell volume was reduced by 30% (p<0.05), beta cell apoptosis was two-fold higher (p<0.05), while beta cell replication was reduced by 50% (p<0.001) in transgenic compared to non-transgenic grafts. In summary, amyloid deposition in islet grafts occurs prior to the recurrence of hyperglycaemia and its accumulation over time is associated with beta cell loss.
Conclusion/Interpretation
Islet amyloid formation may explain in part the non-immune loss of beta cells and recurrence of hyperglycaemia following clinical islet transplantation.
Keywords: islet amyloid, islet transplantation, hyperglycaemia, beta cell apoptosis, beta cell replication
INTRODUCTION
Islet transplantation is a potential treatment for diabetes [1]. Despite the initial promise of a high success rate of insulin independence with the Edmonton protocol of steroid-free immunosuppression [2], a recent follow-up study reported that only 10% of alloislet transplant recipients remained insulin independent five years after transplantation [3]. The decrease in islet graft function cannot be explained solely by immune mechanisms as a decrease in graft function has been demonstrated in autoislet transplant recipients [4]. Thus, non-immune factors are a critical component of long-term islet graft failure. Islet amyloid may be one of these non-immune factors.
Amyloid has been shown to form in human islets as early as two weeks following transplantation into nude mice [5, 6] and has recently been observed in a case in which a sample of transplanted islets was obtained from a human subject [7]. However, these studies using human islets did not determine whether amyloid deposition is associated with beta cell loss in transplanted islets and the recurrence of hyperglycaemia [8]; thus, the answer to this critical question remains unknown.
Islet amyloid is frequently observed in patients with type 2 diabetes but less frequently in non-diabetic individuals [9–11] and is associated with the loss of beta cells [12, 13]. The unique amyloidogenic constituent of islet amyloid is the peptide islet amyloid polypeptide (IAPP) or amylin [14, 15], which is a normal secretory product of the beta cell [16]. Human IAPP (hIAPP) can aggregate to form fibrils and eventually amyloid deposits. While hIAPP-derived amyloid fibrils have been demonstrated to reduce cell viability and induce beta cell death in vitro [17, 18], other studies have suggested hIAPP oligomers to be the mediators of cell cytotoxicity [19–21].
While it would be ideal to use human islets to address questions regarding non-immune factors in islet transplantation, their availability is limited. Further, their use to study the consequences of amyloid formation on islet transplantation by definition lacks control islets that do not have the propensity to form amyloid and thus would allow attribution of differences in outcomes to amyloid formation. On the other hand, while rodent islets are plentiful, they never form amyloid as rodent islet amyloid polypeptide is not amyloidogenic [22]. Thus, we and others have developed hIAPP transgenic mice to study islet amyloid [23–26]. With our mouse model, we have observed in vivo islet amyloid deposits that are morphologically identical to those in humans [27] and result in the loss of beta cells and impaired insulin secretion [28].
We hypothesized that amyloid deposition in transplanted islets contributes to islet graft failure and thus recurrence of hyperglycaemia. To address this hypothesis, we undertook this study in which we transplanted hIAPP transgenic or non-transgenic islets (as controls that lack the propensity to develop amyloid) into syngeneic streptozotocin-diabetic mice and followed them for one or six weeks in order to address the following specific questions: Does amyloid form in islet grafts following islet transplantation and, if so, is this associated with the recurrence of hyperglycaemia and a reduction in graft beta cell volume? Does amyloid form prior to the recurrence of hyperglycaemia? If beta cell volume is decreased with amyloid formation, is this associated with an increase in beta cell apoptosis and/or a decrease in beta cell replication?
MATERIALS AND METHODS
Animals
Islet donors were 8–10 week old hemizygous transgenic mice expressing hIAPP in their pancreatic islet beta cells [25] and non-transgenic littermates (F1 C57BL/6 x DBA/2J). Mice for breeding were obtained from a commercial source (Jackson, Bar Harbor, ME) and all breeding was done locally. All islet recipients were syngeneic non-transgenic male mice (F1 C57BL/6 x DBA/2J), rendered diabetic at 7–9 weeks of age by a single intra-peritoneal injection of streptozotocin (Sigma, St. Louis, MO, USA; 200 mg/kg body weight), freshly dissolved in citrate buffer (pH 4.5). Mice were fed a moderate fat diet containing 9% fat by weight (PicoLab # 5058, Brentwood, MO, USA) that we have previously shown to be permissive for islet amyloid formation [27]. Mice had free access to food and water. The study was approved by the Institutional Animal Care and Use Committee at the VA Puget Sound Health Care System.
Islet Isolation and Transplantation
Islets for transplantation were isolated from 8 to 10 week old hIAPP transgenic and non-transgenic mice as described previously [29]. Briefly, following anaesthesia with pentobarbital (100 mg/kg i.p.), mice were killed by cervical dislocation. Collagenase P (0.5 mg/ml in RPMI) was injected into the pancreas via the common bile duct. The pancreas was then removed and incubated in a water bath for 15 minutes at 37°C. Pancreas tissue was disrupted by manual shaking for one minute. Islets were purified by Histopaque-1077 density centrifugation and washed twice in RPMI medium before being handpicked. These islets were cultured in RPMI medium containing 11.1 mmol/l glucose at 37°C, 5% CO2 for 90 minutes, after which they were placed into PE50 tubing using a Hamilton syringe (Fisher Scientific, Pittsburgh, PA, USA) for centrifugation to form a pellet for transplantation.
Mice with plasma glucose levels ≥22.2 mmol/l five days after streptozotocin treatment were used as islet transplant recipients. Mice were anaesthetised with sodium pentobarbital (100 mg/kg i.p.), the left kidney was then exposed by a small lumbar incision. The tip of the PE50 tubing was placed into a hole made in the kidney capsule, the islets were injected under the capsule and the hole sealed by cauterization. The peritoneum and muscle were sutured in layers; the skin incision was closed with staples and the animals made their post-operative recovery under a warm lamp. 100 hIAPP transgenic or non-transgenic islets were thus transplanted under the left kidney capsule through PE50 tubing and islet loss was <5% in all mice (by manual counting).
Study Design – One Week and Six Week Studies
Mice were followed for one or six weeks following transplantation. Plasma glucose and body weight were measured eight days (the day of streptozotocin injection) before, two days before and the day of islet transplantation in both studies. Following transplantation, plasma glucose and body weight were measured every two to three days until the mice were killed. Plasma glucose was determined using a glucose oxidase method on non-fasting blood samples obtained from the lateral saphenous vein between 12:00 pm and 5:00 pm.
In the one-week study, mice were anesthetized one week after transplantation and the graft-bearing kidney removed. These mice were monitored for another week before they were killed. For the six-week study, mice were anesthetized on day 42, the graft-bearing kidney was removed and the mice killed immediately.
Characterization of islet graft morphology: amyloid severity, beta cell volume, beta cell apoptosis and beta cell replication rates
After nephrectomy, the islet graft was fixed in phosphate buffered paraformaldehyde (4% wt/vol, pH 7.4) and embedded in paraffin. The entire islet graft was cut into 5 μm sections. Sections were examined for islet amyloid following thioflavin S staining (0.5% wt/vol in water), and for beta cells using insulin immunostaining with an anti-insulin antibody (1:2000; Sigma, St. Louis, MO, USA) followed by Cy-3-conjugated secondary antisera (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Graft sections were co-stained with terminal deoxynucleotidyl transferase biotin-mediated dUTP nick end-labeling (TUNEL; In Situ Cell Death Detection kit, Roche Applied Sciences, Indianapolis, IN, USA ) and insulin or the nuclear proliferative marker Ki-67 (1:50; DAKO, Carpinteria, CA, USA) and insulin to detect apoptotic or replicating beta cells, respectively. All sections were counterstained with Hoechst 33258 (2 μg/ml; Sigma, St. Louis MO, USA) to identify cell nuclei. Islet graft area, insulin positive area and amyloid area were determined using a computer-based quantitative system [30]. Amyloid severity was calculated as Σ amyloid area (μm2)/Σ islet graft area (μm2) × 100 (%). Beta cell volume (μm3) was determined as the product of beta cell area and the interval between selected sections (Σ beta cell area [μm2] × 50 [μm]). Apoptotic beta cells were identified by manual counting of insulin positive cells with TUNEL positive nuclei while replicating beta cells were similarly identified by insulin positive cells having Ki-67 positive nuclei. The total number of islet graft cells was determined using the same approach we have previously used to calculate total islet cell number [31]. At least 1,200 cells were counted per graft. We have previously shown that the proportion of apoptotic and replicating beta cells calculated per total number of beta cells are highly correlated with the respective proportions expressed per total number of islet cells in pancreas sections [31]. Therefore, beta cell apoptotic and replication rates are reported as a percentage of total islet graft cell number. All histological assessments were performed in a blinded manner.
Characterization of islets before transplantation and in control pancreata
A piece of pancreas from each mouse used as an islet donor was excised prior to islet isolation, fixed and analyzed to ensure amyloid was not present prior to transplantation. Amyloid was not detected in pancreata from any of the hIAPP transgenic or non-transgenic donor mice. Similarly, no amyloid was detected in pancreata from 14–16 week old, non-transplanted, non-diabetic, hIAPP transgenic mice age-matched for transplant recipients at the end of the study. Finally, the volume of beta cells in samples of 100 isolated islets (transgenic: n=5, non-transgenic: n=5) from 8–10 week old mice that were age-matched with donors was not different between the two groups (data not shown).
Data Analysis and Statistical Methods
Data are expressed as mean ± SEM. Two group comparisons were performed using Mann-Whitney test. Correlation analysis was performed with Pearson’s correlation coefficient. The presence and severity of amyloid deposition between the two groups were compared using Fisher’s exact probability test. Plasma glucose over time was evaluated by repeated measure analysis of variance (ANOVA). Comparison of graft failure as defined by recurrence of hyperglycaemia was performed by Kaplan-Meier analysis. A p<0.05 was considered significant.
RESULTS
Plasma glucose pre and post islet transplantation
In animals that were followed for one week following transplantation of 100 islets, non-fasting plasma glucose before streptozotocin injection was not different between mice that received hIAPP transgenic islets (11.3±0.6 mmol/l) and in those that received non-transgenic islets (12.5±0.7 mmol/l). Similarly plasma glucose before islet transplantation (transgenic islet recipients: 31.8±1.9, non-transgenic islet recipients: 34.7±1.3 mmol/l) did not differ between groups (Figure 1a). Transplantation was associated with a similar decrease in non-fasting plasma glucose levels in both groups of mice by day 2 and it remained similar in both groups one week post-transplantation (Figure 1a). At that time, nephrectomy of the graft-bearing kidney resulted in the recurrence of diabetes as measured one week post nephrectomy in all islet graft recipients (transgenic: 25.4±2.2, non-transgenic: 28.7±3.6 mmol/l). Plasma glucose did not differ between the two groups at any time point.
Figure 1.
Plasma glucose levels (panels a and b) and body weight (panels c and d) before and after streptozotocin-induced diabetes and after islet transplantation (TX) in mice that received 100 non-transgenic islets or 100 hIAPP transgenic islets and were followed for one week (panels a and c; non-transgenic: open circles, n=6; transgenic: solid circles, n=8) or for six weeks following islet transplantation (panels b and d; non-transgenic: open squares, n=10; transgenic: solid squares, n=12). Plasma glucose was similar in both studies for the first week after transplantation (panels a and b) and then increased gradually over six weeks following islet transplantation in mice that received transgenic islets (panel b). Body weight was similar between mice receiving transgenic or non-transgenic islets in both studies (panels c and d). * p<0.05 for mice transplanted with transgenic versus non-transgenic islets.
For animals followed for six weeks, the pattern of plasma glucose levels during the first week was similar to those in the one-week study. Non-fasting plasma glucose prior to streptozotocin (transgenic: 12.7±0.4; non-transgenic: 12.9±0.4 mmol/l), prior to transplantation (transgenic: 27.8±0.8, non-transgenic: 28.5±1.0 mmol/l) and two days after transplantation (transgenic: 11.6±0.9, non-transgenic: 12.1±1.3 mmol/l) did not differ between the two groups (Figure 1b). After that, plasma glucose in both groups rose slightly and similarly until 12 days after transplantation (transgenic: 14.6±1.0, non-transgenic: 14.1±1.0 mmol/l). However, thereafter plasma glucose in the group of mice transplanted with transgenic islets continued to rise over time reaching 16.2±1.7 mmol/l six weeks after transplantation. In contrast, in the mice that received non-transgenic islets, plasma glucose decreased slightly and remained stable, being 11.9±0.6 mmol/l at six weeks post transplantation. These differences in plasma glucose profiles over time meant that this measure was significantly greater in mice transplanted with transgenic islets from 29 to 40 days after transplantation (p<0.05).
Body weight pre and post islet transplantation
In the one-week study, prior to the injection of streptozotocin, body weight was similar in mice that subsequently received hIAPP transgenic islets (34.1± 1.4 g) and in those that received non-transgenic islets (32.1± 1.2 g) (Figure 1c). Similarly, for animals that were followed for six weeks, prior to the induction of diabetes, body weight was not different between groups of mice that either received hIAPP transgenic islets (33.9±0.9 g) or, non-transgenic islets (33.5±0.8 g) (Figure 1d). In the one-week study, body weight decreased rapidly in both groups after streptozotocin injection and continued to do so up until the end of the first week after islet transplantation (Figure 1c). In the mice followed for six weeks, body weight decreased similarly after streptozotocin injection and for one week after transplantation (transgenic islet recipients: 29±0.7, non-transgenic islet recipients: 29±0.7 g). Thereafter, body weight increased gradually in both groups until the mice were killed six weeks following transplantation (transgenic islet recipients: 30.1±0.7, non-transgenic islet recipients: 30.9±0.7 g). Body weight did not differ between groups at any time (Figure 1d).
Histological assessment of amyloid formation, beta cell volume and rates of beta cell apoptosis and beta cell replication in islet grafts
One-week post transplantation, amyloid was present in all transgenic grafts with a severity of 0.2±0.06% while, as expected, no amyloid was detected in any of the non-transgenic grafts (Figures 2a, 2c and 3a). Beta cell volume one week post-transplantation did not differ significantly between groups (transgenic: 3.7± 0.8, non-transgenic: 5.9±1.4×107μm3, p=0.20) (Figure 3c). One week post-transplantation, beta cell apoptosis rates were also not different in transgenic and non-transgenic islet grafts (transgenic: 0.20±0.03%, non-transgenic: 0.12±0.04%, p=0.10) (Figure 3e). At this same time point, beta cell replication rates also did not differ in transgenic and non-transgenic islet grafts (transgenic: 0.7±0.11%, non-transgenic: 0.9±0.11%, p=0.22) (Figure 3g).
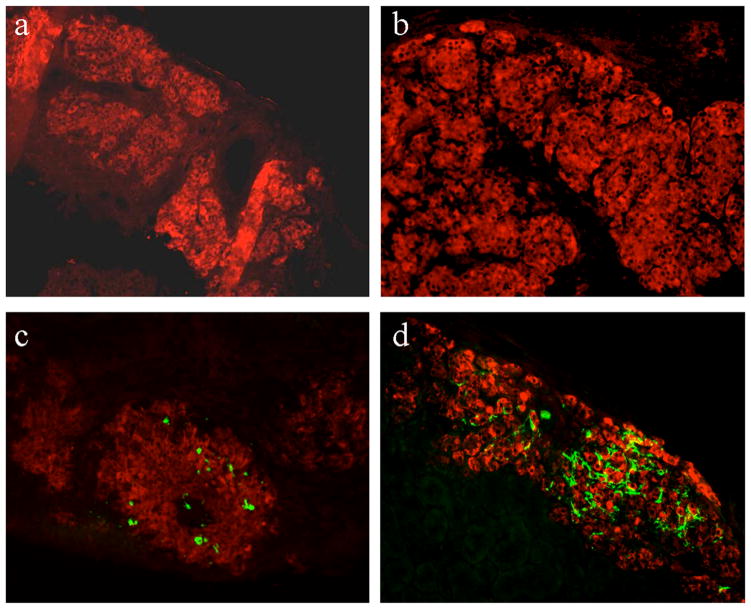
Figure 2.
Representative sections of transplanted islet grafts under the kidney capsule stained for amyloid with thioflavin S (green) and insulin (red) (original magnification ×20). As expected, no amyloid was detected in non-transgenic islet grafts at one week (a) and six weeks (b) after transplantation. Amyloid deposits are present in transgenic islet grafts at one week (c) and six weeks (d) following transplantation.
Figure 3.
Severity of amyloid deposition, beta cell volume, rate of beta cell apoptosis and rate of beta cell replication in non-transgenic and transgenic islet grafts at one week (a, c, e and g; non-transgenic: open bars, transgenic: solid bars) and six weeks (b, d, f and h; non-transgenic: open bars, transgenic: solid bars) post islet transplantation. Amyloid deposits were found in all eight transgenic grafts one week after islet transplantation (panel a) and were associated with lower beta cell volume (panel c), increased rate of beta cell apoptosis (panel e) and decreased rate of beta cell replication (panel g), although these did not reach statistical significance. Amyloid deposits were present in 11 of 12 transgenic islet grafts six weeks after islet transplantation (panel b) and were associated with a 30% reduction in beta cell volume (panel d), a two-fold higher rate of beta cell apoptosis (panel f) and a 50% decrease in beta cell replication (panel h), all of which were statistically significant. As expected, no amyloid was detected in any of the non-transgenic islet grafts at one week or six weeks post islet transplantation (panels a and b), as mouse IAPP is not amyloidogenic. * p<0.05; ** p<0.001; *** p<0.0001; for transgenic versus non-transgenic islet grafts.
Six weeks after transplantation, amyloid deposition was detected in 11 of 12 transgenic islet grafts with a severity of 0.33±0.12% in the transgenic grafts and again, as expected, in none of the non-transgenic grafts (Figures 2b, 2d and 3b; p<0.0001). Beta cell volume was reduced by 30% in transgenic compared to non-transgenic grafts (transgenic: 6.9±0.7×107, non-transgenic: 9.9±1.0×107 μm3; p<0.05; Figure 3d). Increasing amyloid severity was associated with decreasing beta cell volume in transgenic grafts (r=−0.65, p<0.05) and with increasing plasma glucose levels at 40 days after transplantation (r=0.75, p<0.005). In islet grafts harvested six weeks following transplantation, beta cell apoptosis was two-fold higher in transgenic than in non-transgenic grafts (transgenic: 0.07±0.01%, non-transgenic: 0.03±0.01%, p=0.02; Figure 3f), while beta cell replication was reduced by 50% in transgenic grafts compared to non-transgenic grafts (transgenic: 0.51±0.08%, non-transgenic: 1.00±0.09%, p<0.001; Figure 3h). Figure 4 illustrates TUNEL- and Ki-67 positive beta cells in non-transgenic and transgenic islet grafts at six weeks following islet transplantation.
Figure 4.
Staining of apoptotic and replicating beta cells with TUNEL (a, c) and Ki-67 (b, d) in non-transgenic (a, b) and transgenic (c, d) islet grafts six weeks post transplantation (original magnification ×40). Arrows indicate apoptotic and replicating beta cells. Nuclear staining is in blue and insulin staining is in red (a, c) and green (b, d).
Effect of amyloid formation on beta cell measures in hIAPP transgenic islet grafts of mice with and without recurrence of hyperglycaemia
Recurrence of hyperglycaemia, as a measure of graft failure, was defined as plasma glucose >13.9 mmol/l on three consecutive occasions during the six-week follow up period. The first time point at which this outcome was reached was at three weeks and by six weeks, 50% of the mice that received transgenic islets had a recurrence of hyperglycaemia (Figure 5). In contrast, none of the ten mice that received non-transgenic islets had a recurrence of hyperglycaemia (p=0.01 versus transgenic).
Figure 5.
Long-term recurrence of hyperglycaemia as defined by plasma glucose levels >13.9 mmol/l occurred in 50% of mice that received transgenic (solid squares, n=12) but in none of the mice that received non-transgenic islets (open squares, n=10) over the six weeks following transplantation. * p=0.01 for mice transplanted with transgenic versus non-transgenic islets.
To assess whether there were differences in transgenic grafts that were and were not associated with graft failure, we compared graft morphology in grafts from mice with (n=6) and without recurrence of hyperglycaemia (n=6). Amyloid deposits were detected in all six transgenic grafts with and in five out of six transgenic grafts without recurrence of hyperglycaemia (Table 1). While amyloid severity and the rate of beta cell apoptosis were no different in the two groups, the rate of beta cell replication was lower (p<0.01) and beta cell volume tended to be lower (p=0.06) in the grafts from the mice with recurrence of hyperglycaemia. Body weights did not differ between these two sub-groups at any time point.
TABLE 1.
Metabolic and graft characteristics of islet transplant recipients – 6 week study
| n | Day 40 Body Weight (grams) | Day 40 Plasma Glucose (mmol/l) | Amyloid Severity (%) | Beta cell Volume (× 107μm3) | TUNEL Positive beta cells (%) | Ki-67 Positive beta cells (%) | |
|---|---|---|---|---|---|---|---|
| Transgenic islet graft recipients with recurrence of hyperglycaemia | 6 | 30.0±1.0 | 20.0±2.6*,† | 0.49 ±0.22 | 5.6±0.9† | 0.06±0.02a | 0.32±0.08*,† |
| Transgenic islet graft recipients without recurrence of hyperglycaemia | 6 | 30.3±1.0 | 12.4±0.5 | 0.17±0.06 | 8.1±0.9 | 0.08±0.02† | 0.70±0.07† |
| Non-transgenic islet graft recipients | 10 | 30.9±0.6 | 11.9±0.6 | 0 | 9.9±1.0 | 0.03±0.01b | 1.00±0.09 |
Data are mean±SEM.
p<0.05 vs transgenic without recurrence of hyperglycaemia
p<0.05 vs non-transgenic
n=3
n=9
To determine whether amyloid formation affected beta cell viability under euglycemic conditions, we compared the metabolic and histological graft characteristics in the six mice that received transgenic islets and did not develop hyperglycaemia six weeks following transplantation and those from the ten mice that received non-transgenic islets. Plasma glucose levels and body weight did not differ significantly between the two groups at any time following islet transplantation. While beta cell volume did not differ between the transgenic and non-transgenic groups, the rate of beta cell apoptosis was higher (p=0.04) and the rate of beta cell replication lower (p=0.03) in the transgenic grafts (Table 1).
DISCUSSION
We have shown that amyloid deposition occurs as early as one week after transplantation of hIAPP transgenic islets and after six weeks, is associated with reduced beta cell volume and recurrence of hyperglycaemia. This deleterious effect of amyloid is associated with an increase in beta cell apoptosis and a decrease in beta cell replication.
Transplantation of both hIAPP transgenic and non-transgenic islets initially restored euglycaemia and plasma glucose concentrations were similar in both groups for the first week. At that time, we found amyloid deposits in all hIAPP transgenic islet grafts. Thus, amyloid deposition is an early event following islet transplantation and it occurs independent of hyperglycaemia. One week following transplantation, beta cell volume, beta cell apoptosis and beta cell replication were not different between hIAPP transgenic and non-transgenic islet grafts. However, the direction of these changes was similar to the transgenic and non-transgenic groups six weeks after islet transplantation and is in keeping with a deleterious effect of amyloid on the graft.
Six weeks after transplantation, beta cell volume was significantly reduced in the transgenic grafts and was inversely correlated with amyloid severity, in keeping with amyloid deposition following islet transplantation being associated with beta cell loss. The mechanism by which amyloid may be producing this loss of beta cells appears to be related to an increase in beta cell apoptosis, which was increased two-fold, coupled with a 50% reduction in beta cell replication. These findings are in keeping with in vitro studies that have demonstrated that amyloid fibrils and oligomers induce cell death by apoptosis and necrosis [17, 19] and that replicating beta cells have increased susceptibility to hIAPP induced apoptosis [32]. Our findings strongly suggest therefore that there is a dual negative effect of hIAPP-associated amyloid formation on beta cell turnover components, which may contribute to the loss of beta cell volume and the recurrence of hyperglycaemia that occurs following islet transplantation in humans.
Additional support for islet amyloid formation being responsible for beta cell loss and the recurrence of hyperglycaemia comes from the morphological analyses of islet grafts from mice that did and did not develop a recurrence of hyperglycaemia. Six weeks following islet transplantation, half of the mice that received transgenic islets had graft failure defined as a recurrence of hyperglycaemia. Amyloid was detected in hIAPP transgenic islet grafts, regardless of the glucose level, consistent with the one-week data. However, in mice that redeveloped hyperglycaemia, amyloid formation was associated with a tendency towards decreased beta cell volume and with decreased beta cell replication compared to mice that received transgenic islets and did not develop a recurrence of hyperglycaemia. Furthermore, hIAPP transgenic grafts from mice that did not redevelop hyperglycaemia exhibited an increased rate of apoptosis and a decreased rate of replication compared to non-transgenic islet grafts, despite the fact that the plasma glucose levels were not different in these two groups. Thus, the cytotoxic effect of amyloid on the beta cell was already evident in hIAPP transgenic islet grafts when the glucose level was still in the euglycemic range. We anticipate that with additional time, the effect of these changes would have resulted in the loss of more beta cells and the recurrence of hyperglycaemia in all mice transplanted with transgenic islets.
While the rate of beta cell apoptosis differed at six weeks by genotype, it also differed over time within both transgenic and non-transgenic islet grafts. Beta cell apoptosis was three-fold higher in the transgenic grafts, while it was four-fold higher in non-transgenic grafts at one week compared to six weeks. The finding of increased beta cell apoptosis in the first week post transplantation is not unexpected since considerable dynamic changes in islet cell turnover and graft tissue remodelling occur in the early post-transplant period [33]. This may explain why beta cell volume was low in both non-transgenic and transgenic islet grafts at one week compared to six weeks. Once transplanted islets survive this initial critical period, stressors that lead to long-term graft failure include both immune and non-immune factors.
We minimized the role of immunological factors in our study by using syngeneic donors and recipients and observed no immune infiltration in islet grafts at one or six weeks following transplantation (data not shown). Our experimental design using non-transgenic islets that do not have the propensity to, and did not, develop amyloid provided controls for other non-immune causes for islet transplantation failure such as ongoing hypoxia [34, 35] and altered metabolic milieu [36, 37] which are independent of amyloid formation. Further, we did not find evidence of light microscopy visible amyloid in donor pancreata at the time of islet isolation or in pancreata of non-diabetic, non-transplanted, hIAPP transgenic mice age matched to islet transplant recipients six weeks post-transplantation. Therefore, amyloid formation over six weeks in transplanted islets most likely explains the reduction in beta cell volume and subsequent increase in plasma glucose levels in hIAPP transgenic islet recipients.
It is possible that insulin release from the endogenous pancreas could have been responsible for differences in glycaemia in the different groups of mice. However, nephrectomy of the graft-bearing kidney at one week resulted in a return of plasma glucoses to pre-transplant values in both transgenic and non-transgenic islet recipients. While we did not perform nephrectomy on mice at six weeks, we have observed a similar recurrence of overt diabetes following nephrectomy 12 weeks after islet transplantation in both genotypes (unpublished observation). This is in keeping with the glycemic control post islet transplantation being due to a functioning islet graft rather than regeneration of endogenous beta cells in the pancreas of the streptozotocin-diabetic mice under the euglycemic conditions produced by islet transplantation.
In human islet transplantation, islets are transplanted intraportally, rather than under the renal capsule as in this study. Westermark et al [6] demonstrated that amyloid deposition occurs in human islets transplanted into the liver and spleen of nude mice; suggesting that it is not likely related to the site of transplantation. Of interest, widespread amyloid has recently been observed in a sample obtained from transplanted islets in a diabetic human [7]. Therefore, the deleterious effects of amyloid deposition on islet graft outcome in the present study are likely relevant to intraportally transplanted islets in human recipients.
In summary, we have observed that amyloid forms in transplanted islets as early as one week post transplantation and that this occurs prior to the recurrence of hyperglycaemia. Six weeks post transplantation amyloid formation in islet grafts is associated with beta cell volume loss and the redevelopment of hyperglycaemia. This effect is likely mediated by an amyloid associated increase in beta cell apoptosis and suppression of beta cell replication that would be required to prevent net beta cell loss. Thus, islet amyloid formation appears likely to be an important factor contributing to beta cell loss and the recurrence of hyperglycaemia in clinical islet transplantation and inhibition of amyloid formation may be a strategy that could improve the long-term survival of transplanted human islets.
Acknowledgments
We thank Shani Wilbur, Melissah Watts, Rahat Bhatti, Robin Vogel, Ruth Hollingworth and Rebekah Koltz for excellent technical support. Gordon Weir and Susan Bonner-Weir from Joslin Diabetes Center, Boston, are thanked for their valuable advice during the development of the study. This work was supported by the Department of Veterans Affairs and NIH grant DK-17047. K.K. was supported by the Manpei Suzuki International Diabetes Foundation.
Abbreviations
- hIAPP
human islet amyloid polypeptide
- IAPP
islet amyloid polypeptide
- ANOVA
analysis of variance
References
- 1.Robertson RP. Successful islet transplantation for patients with diabetes--fact or fantasy? N Engl J Med. 2000;343:289–290. doi: 10.1056/NEJM200007273430409. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.Clayton HA, Davies JE, Pollard CA, White SA, Musto PP, Dennison AR. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the leicester general hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Eizirik DL, Pipeleers DG, Hellerstrom C, Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38:543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 6.Westermark GT, Westermark P, Nordin A, Tornelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108:193–203. doi: 10.3109/2000-1967-113. [DOI] [PubMed] [Google Scholar]
- 7.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 8.Westermark P, Andersson A, Westermark GT. Is aggregated IAPP a cause of beta-cell failure in transplanted human pancreatic islets? Curr Diab Rep. 2005;5:184–188. doi: 10.1007/s11892-005-0007-2. [DOI] [PubMed] [Google Scholar]
- 9.Bell ET. Hyalinization of the islets of Langerhans in nondiabetic individuals. The American journal of pathology. 1959;35:801–805. [PMC free article] [PubMed] [Google Scholar]
- 10.Clark A, Saad MF, Nezzer T, et al. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990;33:285–289. doi: 10.1007/BF00403322. [DOI] [PubMed] [Google Scholar]
- 11.Zhao HL, Lai FM, Tong PC, et al. Prevalence and clinicopathological characteristics of islet amyloid in chinese patients with type 2 diabetes. Diabetes. 2003;52:2759–2766. doi: 10.2337/diabetes.52.11.2759. [DOI] [PubMed] [Google Scholar]
- 12.Westermark P. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77:91–94. doi: 10.1517/03009734000000014. [DOI] [PubMed] [Google Scholar]
- 13.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 14.Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn SE, D’Alessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39:634–638. doi: 10.2337/diab.39.5.634. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 18.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. The Journal of biological chemistry. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 19.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 20.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. The FEBS journal. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 21.Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- 22.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox N, Schrementi J, Nishi M, et al. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM) FEBS Lett. 1993;323:40–44. doi: 10.1016/0014-5793(93)81444-5. [DOI] [PubMed] [Google Scholar]
- 24.Hoppener JW, Verbeek JS, de Koning EJ, et al. Chronic overproduction of islet amyloid polypeptide/amylin in transgenic mice: lysosomal localization of human islet amyloid polypeptide and lack of marked hyperglycaemia or hyperinsulinaemia. Diabetologia. 1993;36:1258–1265. doi: 10.1007/BF00400803. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessio DA, Verchere CB, Kahn SE, et al. Pancreatic expression and secretion of human islet amyloid polypeptide in a transgenic mouse. Diabetes. 1994;43:1457–1461. doi: 10.2337/diab.43.12.1457. [DOI] [PubMed] [Google Scholar]
- 26.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 27.Verchere CB, D’Alessio DA, Palmiter RD, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hull RL, Andrikopoulos S, Verchere CB, et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 29.Zraika S, Hull RL, Udayasankar J, et al. Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes. 2007;56:304–310. doi: 10.2337/db06-0430. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Hull RL, Vidal J, Cnop M, Kahn SE. Islet amyloid develops diffusely throughout the pancreas before becoming severe and replacing endocrine cells. Diabetes. 2001;50:2514–2520. doi: 10.2337/diabetes.50.11.2514. [DOI] [PubMed] [Google Scholar]
- 31.Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- 32.Ritzel RA, Butler PC. Replication increases beta-cell vulnerability to human islet amyloid polypeptide-induced apoptosis. Diabetes. 2003;52:1701–1708. doi: 10.2337/diabetes.52.7.1701. [DOI] [PubMed] [Google Scholar]
- 33.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 34.Carlsson PO, Mattsson G. Oxygen tension and blood flow in relation to revascularization in transplanted adult and fetal rat pancreatic islets. Cell Transplant. 2002;11:813–820. [PubMed] [Google Scholar]
- 35.Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87:5418–5423. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
- 36.Korsgren O, Jansson L, Andersson A. Effects of hyperglycemia on function of isolated mouse pancreatic islets transplanted under kidney capsule. Diabetes. 1989;38:510–515. doi: 10.2337/diab.38.4.510. [DOI] [PubMed] [Google Scholar]
- 37.Montana E, Bonner-Weir S, Weir GC. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]