Abstract
Purpose
The efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer (NSCLC) has been linked to activating mutations in the EGFR gene. So far these mutations have been extensively characterized in established cell lines. The aim of this study was to determine the effects of EGFR mutations on downstream signaling in human tumor specimens.
Methods
We have looked for mutations of the EGFR gene in specimens of 67 patients with NSCLC and correlated these with EGFR phosphorylation and the activity of its three main downstream signaling cascades Akt, MAPK and Stat3 by immunohistochemistry.
Results
We show that the phosphorylation of tyrosine residues 922 and 1173, but not 1068, are primarily affected by the activating EGFR mutations. Akt activity was significantly higher in patients with EGFR mutations but we found no difference in Stat3 or MAPK phosphorylation. Our results suggest that EGFR mutations not only increase receptor activity, but also alter responses of downstream signaling cascades in human NSCLCs and that these finding differ from results obtained in cell lines.
Electronic supplementary material
The online version of this article (doi:10.1007/s00432-008-0509-9) contains supplementary material, which is available to authorized users.
Keywords: EGFR, NSCLC, Akt, MAPK, Stat3
Introduction
Lung cancer remains to be the leading cause of cancer related deaths in North America and Europe despite advances in surgical and chemotherapeutic interventions (Jemal et al. 2007). The epidermal growth factor receptor (EGFR) is often overexpressed in non-small cell lung cancer (NSCLC) and considered to play a key role in carcinogenesis due to its effects on cell cycle progression, apoptosis, angiogenesis and metastasis (Stoscheck and King 1986; Sobol et al. 1987; Ciardiello and Tortora 2001). This has motivated the development of new NSCLC targeted chemotherapeutic drugs including the EGFR specific tyrosine kinase inhibitors (TKI) Gefitinib (Iressa®) and Erlotinib (Tarceva®). Unfortunately, however, only an unexpectedly small group of patients benefit from these TKIs (Fukuoka et al. 2003; Kris et al. 2003). It has been shown previously that activating mutations in the kinase domain of the EGFR strongly correlate with the clinical response to EGFR targeted tyrosine kinase inhibiting therapies (Lynch et al. 2004; Paez et al. 2004).
EGFR/ErbB-1, HER2/ErbB-2, HER3/ErbB-3 and HER4/ErbB-4 make up the EGFR superfamily. Specific ligands, including the epidermal growth factor (EGF) and transforming growth factor-α, have been discovered for all ErbB-receptors except for HER2. Upon ligand binding, the receptor dimerizes to form a homo or heterodimer with another member of the EGFR superfamily. Dimerization then initiates an autophosphorylation of specific tyrosine residues on the intracellular domain. The EGFR can be autophosphorylated on tyrosine 974, 992, 1045, 1068, 1086, 1148 and 1173. The receptor can also be activated by the Src kinase through phosphorylation of tyrosine 845 and 1101. Distinct downstream signaling cascades are initiated by the EGFR depending on its phosphorylation pattern. The most important ones are mitogen-activated protein kinases (MAPK), Akt (Protein Kinase B) and the signal transducer and activator of transcription protein 3 (Stat3) (Jorissen et al. 2003).
MAPK can be phosphorylated by the EGFR via protein kinase C (PKC). Phospholipase C-gamma (PLCγ) is able to bind directly with its SH2 domain to the EGFR when phosphorylated on Tyr-992 and Tyr-1173 (Chattopadhyay et al. 1999). Activated PLCγ catalyzes the hydrolysis of phosphatidyl-inositol (4,5) diphosphate, producing the second messengers 1,2-diacylglycerol (DAG) and inositol 1,3,5-triphosphate (IP3). IP3 triggers a Ca2+ influx, while DAG is a cofactor in the activation of PKC. MAPK signaling can also be activated by the P-Tyr-1068 EGFR. Phospho-tyrosine 1068 EGFR recruits the Grb2/Sos complex to the plasma membrane where Sos1 induces Ras to exchange its GDP for GTP (Batzer et al. 1994). Ras in turn can activate Raf-1, which through a series of kinases, leads to the phosphorylation and nuclear translocation of Erk1 and Erk2 (Johnson and Vaillancourt 1994).
EGFR induced Akt signaling is initiated through phosphatidylinositol-3-kinase class Ia (PI3-K Ia). It is activated by the SH2 domain of the p85 adaptor protein binding to the phosphotyrosine residue of the EGFR. PI3-K Ia produces PIP3, which is one of the best-characterized stimulators of serine/tyrosine kinase Akt. It binds to Akt and the complex is then translocated into the plasma membrane, where Akt is phosphorylated by phosphoinositide dependent kinases (Jorissen et al. 2003).
Signal transducer and activator of transcription 3 may also be activated by the EGFR. Tyrosine-705 phosphorylation of Stat3 requires EGFR and Src kinase activity, but is independent of JAK2. Stat3 phosphorylation on Tyr-705 is required for its dimerization, nuclear translocation and DNA binding. The EGFR initiated Ras/Raf/MAPK pathway may also lead to the phosphorylation of Stat3 on Ser727 via Erk1/Erk2, important for maximal transcription activity (Alvarez et al. 2006).
To further understand the molecular and biological effects of EGFR mutations in NSCLC we investigated EGFR phosphorylation and its main downstream signaling pathways.
Materials and methods
Tumor samples
All tumor samples were collected from the archives of the Institute of Pathology, University of Bonn (Germany). The samples were formalin fixed and paraffin embedded as part of routine diagnostic procedures. All tumors were clinically and pathologically identified as being the primary and only neoplastic lesion. Non-distinct samples were discarded. Grading and staging was independently performed by at least two experienced pathologists, and in accordance with WHO guidelines (Brambilla et al. 2001). Patients’ data were obtained from clinical records provided by the Department of Surgery, University of Bonn, Germany.
DNA-isolation/PCR/sequencing
Paraffin embedded tissue sections (3–5 μm) were micro-dissected for tumor tissue. DNA was isolated using Applied Biosystems’ (Foster City, CA, USA) NucPrep® Kit according to the manufactures instructions. DNA concentrations and purity were tested with a spectrophotometer and agarose gel electrophoresis. PCR primers were purchased from Proligo (Boulder, CO, USA). Standard PCR protocols were used and all batches included both positive and negative controls. Agarose gel electrophoresis confirmed the PCR products, which were then purified using Applied Biosystems’ (Foster City, CA, USA) CentriSep™ Columns. Cycle sequencing was performed according to Applied Biosystems’ recommended protocols and analyzed on an Applied Biosystems (Foster City, CA, USA) Prism® 310 Genetic Analyzer.
Immunohistochemistry
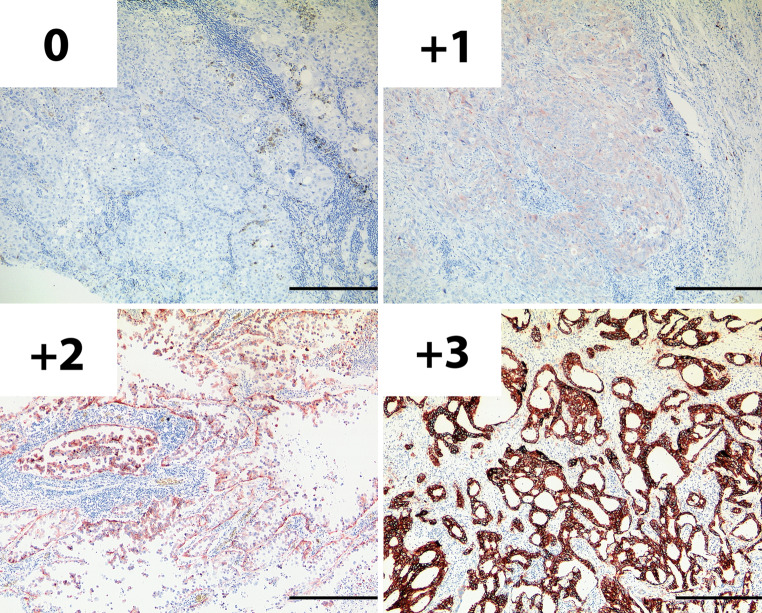
Sections (3–5 μm) were stained as previously described (Heukamp et al. 2006). All antibodies were purchased from New England Biolabs GmbH (Frankfurt am Main, Germany) and used according to the manufactures instructions [Phospho-EGFR Tyr-992 (#2235), Phospho-EGFR Tyr1068 (#2236), Phospho-EGFR Tyr1173 53A5 (#4407), Phospho-Akt Ser473 736E11 (#3787), Phospho-Stat3 Tyr705 (#9145), Phospho-p44/p42 MAPK Tyr202/Tyr204 (#4376)]. Staining intensity was individually evaluated by two pathologists, blind to the EGFR-mutation status. Conflicting results were discussed and re-evaluated by a panel of three pathologists including the two original pathologists and a third investigator from the Institute of Pathology, University Hospital Bonn. We employed a four-tier scoring system, similar to those used in other EGFR-immunohistochemistry (IHC) studies (Selvaggi et al. 2004; Parra et al. 2004; Haura et al. 2005; Hirsch et al. 2003; Atkins et al. 2004). For EGFR and phosphotyrosine-EGFR IHC only membrane staining was evaluated: 0 no or undefined background staining, 1+ weak and discontinuous, 2+ distinct and of moderate intensity, 3+ strong and complete (Fig. 1). The same scoring system was used for P-Akt, P-MAPK and P-Stat3 IHC, but in these cases only cytoplasmic and nuclear staining was assessed. Samples scoring 0 or +1 were considered as negative staining and samples assessed a +2 or +3 as positive staining. Of the 469 slides analyzed, 37 (8%) received conflicting grades by the two pathologists and in 4% of all cases the conflict was between +1 and +2 on the four-tier scoring system. All conflicts were resolved by the blinded panel.
Fig. 1.
An example of the four-tier scoring system used for evaluating the staining intensity in EGFR and phosphotyrosine-EGFR IHC, here demonstrated with an antibody targeting the EGFR independent of phosphorylation. 0 no or undefined background staining, 1+ weak and discontinuous membrane staining, 2+ distinct membrane staining of moderate intensity, 3+ strong and complete plasma membrane staining. Samples with 1+ or 2+ staining intensity were considered positive. The black bar represents 400 μm
Statistics
Statistical analysis was performed using Statistica 6.0© by StatSoft Inc. (Tulsa, OK, USA). Groups were compared using 2 × 2 tables and tested for significance with Fishers exact P (two-tailed) test. The level of significance was set to P ≤ 0.05.
Results
All samples were selected from the archives of the Institute of Pathology, University of Bonn, Germany (Table 1). The collection included 67 non-small cell lung tumors from 24 female and 43 male patients between the ages 43–87. All cancer patients underwent surgery been 1995 and 2003. None of the patients received chemo- or radiation therapy before surgery. NSCLC was the primary and only malignancy. The 67 NSCLC were further classified in 52 adenocarcinomas, of which 12 were bronchioloavelolar, and 15 squamous cell carcinomas.
Table 1.
Characteristics of the patient collective with regard to EGFR mutation status
| EGFR-WT | EGFR-M | |
|---|---|---|
| Sex | ||
| Male | 41 | 2 |
| Female | 14 | 10 |
| Histology | ||
| Adenocarcinoma | 31 | 9 |
| BAC | 9 (23%) | 3 (25%) |
| Squamous-cell carcinoma | 15 | 0 |
| Smoking status | ||
| Never | 15.2% (n = 5) | 22.2% (n = 2) |
| Former | 30.3% (n = 10) | 22.2% (n = 2) |
| Smoker | 54.5% (n = 18) | 55.6% (n = 5) |
| Mean age at surgery | 65.1 | 59.9 |
BAC bronchioloalveolar carcinoma
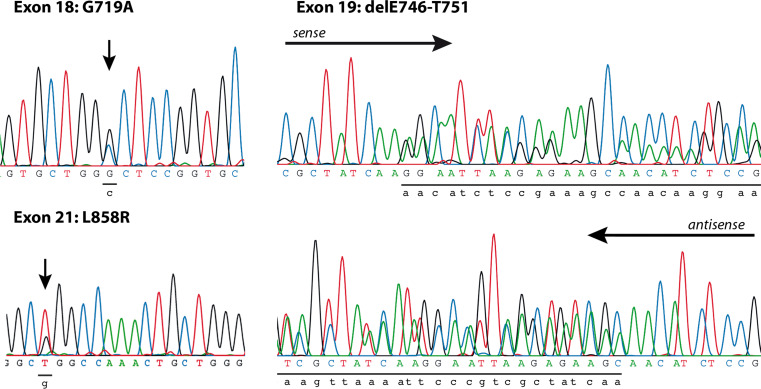
DNA was extracted from paraffin embedded tumor samples, dissected from normal adjacent tissue. Exons 18, 19 and 21 of the EGFR gene, which are known to be sites for activating mutations, were sequenced. In a total of twelve samples (18%) mutations were detected, as shown in Table 2. Two cases had point mutations resulting in amino acid substitutions in exon 18, six had deletions of 12–18 bases in exon 19 and four point mutations lead to substitutions in exon 21 (Fig. 2). Although women accounted for only approximately 30% of the collective, ten out of the 12 EGFR mutations (EGFR-M) were from women. All were adenocarcinomas and 22% of these patients had never smoked.
Table 2.
Characteristics of patients with mutant EGFR NSCLC
| Number | Age at surgery | Sex | Tumor type | Grading | pT | pN | pM | Mutation type | Smoking status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | Adenocarcinoma | G2 | 2 | 1 | Exon 18—E709G, G719C | Former | |
| 2 | 62 | F | Adenocarcinoma | G2 | 2 | 0 | Exon 18—G719A | Smoker | |
| 3 | 55 | F | Adenocarcinoma | G2 | 1 | 2 | Exon 19—delE746-A750 | Smoker | |
| 4 | 67 | F | BAC | G2 | 2 | 0 | Exon 19—delE746-T751 | Never | |
| 5 | 50 | F | Adenocarcinoma | G3 | 1 | 0 | Exon 19—delE746-S752 | Smoker | |
| 6 | 57 | F | BAC | G2 | 2 | 2 | 1 | Exon 19—delE746-T751 | |
| 7 | 69 | F | Adenocarcinoma | G2 | 2 | Exon 19—delE746-T751 | |||
| 8 | 53 | F | Adenocarcinoma | G3 | 2 | 1 | 1 | Exon 19—delE746-A750 | Never |
| 9 | 50 | F | BAC | G2 | 1 | 0 | Exon 21—L858R | Former | |
| 10 | 61 | M | Adenocarcinoma | G1-G2 | 2 | 1 | Exon 21—L858R | ||
| 11 | 48 | M | Adenocarcinoma | G2-G3 | 1 | 1 | Exon 21—L858R | Smoker | |
| 12 | 71 | F | Adenocarcinoma | G3 | 1 | 1 | Exon 21—G857R | Smoker |
BAC bronchioloalveolar carcinoma
Fig. 2.
Examples of mutations found in the kinase domain of the EGFR. We found E709G, G719C and G719A substitutions in Exon 18. The deletions in Exon 19 ranged from delE746-A750 to delE746-S752. Both the L858R and G857R substitution were found in Exon 21
The expression status of the EGFR was determined using semi-quantitative IHC. Forty-nine samples (73%) expressed the EGFR. There was no difference in frequency between tumors without EGFR mutations (EGFR-WT) and EGFR-M tumors (75 vs. 73%). This result indicates that the mutant status of the EGFR did not influence its expression level. We therefore analyzed the activation status of the EGFR by determining the phosphorylation of Tyr-992, Tyr-1068 and Tyr-1173 using specific antibodies. In the entire collection, EGFR was activated and phosphorylated in 33 samples (49%). All three tyrosines showed similar phosphorylation frequencies (P-Tyr-992, 28%; P-Tyr-1068, 24%; and P-Tyr-1173 21%).
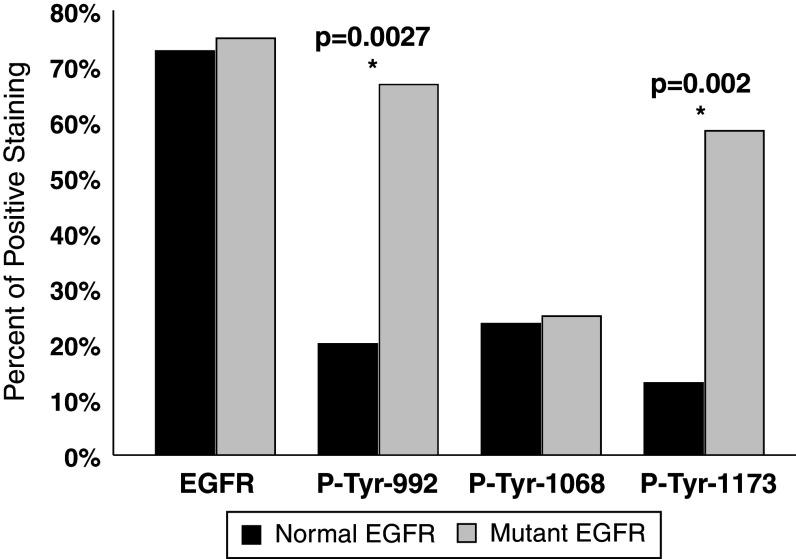
In contrast, when analyzed according to the mutation status, there was a striking difference in phosphorylation between EGFR-WT and EGFR-M (Fig. 3). Of the mutated tumors 83% showed phosphorylation in at least one of the tyrosines, while only 42% of normal tumors were phosphorylated (P = 0.0115). We found the most striking difference with Tyr-992 and Tyr-1173: for Tyr-992, 67% of the EGFR-M tumors were phosphorylated, in contrast to 20% of EGFR-WT samples (P = 0.0027). Tyr-1173 was phosphorylated in 58% of EGFR-M tumors but only in 13% of EGFR-WT samples (P = 0.002). Thus, mutations in the EGFR seem to coincide with enhanced receptor activation.
Fig. 3.
Percent of NSCLC tumors staining positive in EGFR, P-Tyr-992, P-Tyr-1068 and P-Tyr-1173 immunohistochemistry with regard to their mutation status. There was no difference between EGFR-WT and EGFR-M in EGFR and P-Tyr-1068 IHC, but a drastic difference in P-Tyr-992 and P-Tyr-1173 staining
In order to determine which downstream signaling cascades were stimulated by the activated EGFR, we analyzed the phosphorylation status of Stat3, Akt and MAPK by semi-quantitative IHC (Fig. 4). P-Akt was positive in only 18 patients (27%), but of these eight where from EGFR-M samples (67%), while only 18% of EGFR-WT samples showed P-Akt immunostaining (P = 0.0017). In our collective, there was no significant difference in Stat3, which was phosphorylated in 41 tumors (83% EGFR-M; 56% EGFR-WT), nor in P-MAPK which was detected in 16 samples (33% EGFR-M, 22% EGFR-WT).
Fig. 4.
Percent of tumors with positive IHC staining in Stat3, Akt and MAPK; the three main downstream signaling cascades of the EGFR. Both P-Tyr-705-Stat3 and phospho-p44/p42 MAPK showed no difference between NSCLC tumors with mutations in the EGFR and those without. Staining of phospho-Akt, however, showed a significant difference between EGFR mutant and wild type tumors
Since all EGFR-M samples were adenocarcinomas and the EGFR-WT group included all 15 squamous cell tumors, we tested whether the observed results were due to this heterogeneous distribution. When all squamous cell tumors were excluded from the EGFR-WT group the observed patterns remained the same; Tyr-992 and Tyr-1173 of the EGFR and Akt were significantly more often phosphorylated in EGFR-M tumors (P = 0.0175, 0.0013 and 0.0035 respectively). Therefore, the different phosphorylation patterns observed are due to the EGFR mutation status and not a result of the histological subtype.
To test whether particular mutations correlate with specific EGFR phosphorylation patterns or activated signaling cascades, we spilt the EGFR-M group into exon 18, 19 and 21 subgroups and compared each to the EGFR-WT collective. There was a significant difference in Tyr-992 (exon 21 subgroup, P = 0.003), Tyr-1068 (exon 19 subgroup, P = 0.0082) and Akt phosphorylation (exon 18 and 19 subgroups, P = 0.0414 and 0.0212 respectively). A specific analyse is not possible due to the small group size but we found no significant differences between the EGFR mutation subgroups.
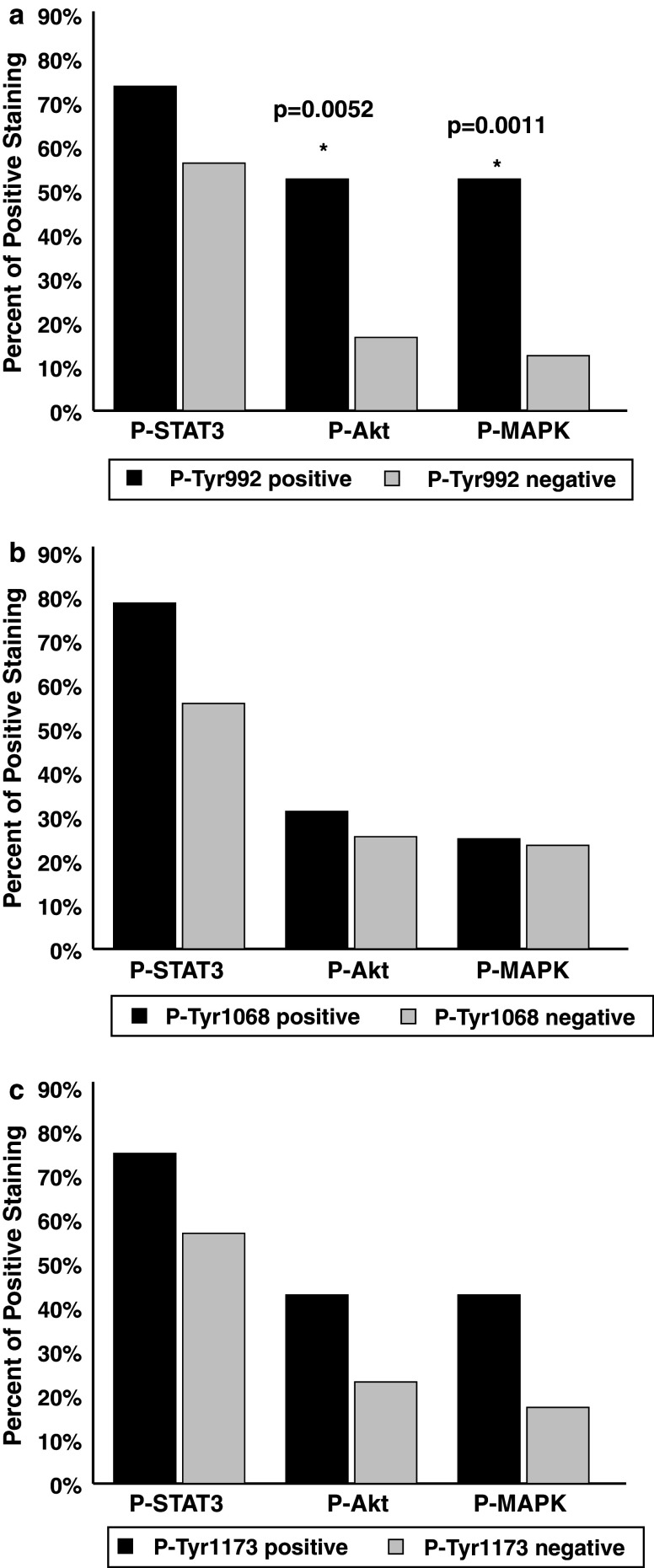
We finally investigated whether phosphorylation of specific EGFR tyrosine residues correlated with activation of distinct downstream signaling cascades (Fig. 5). Tyr-1068 phosphorylation showed no effect on the three signaling cascades investigated (Fig. 5b). MAPK and Akt reveal a slight trend towards increased activity when the EGFR was phosphorylated on Tyr-1173 (Fig. 5c). Phosphorylation of Tyr-992 however, significantly correlated with Akt and MAPK phosphorylation (P = 0.0052 and 0.0011 respectively) (see Fig. 5a).
Fig. 5.
Effect of EGFR specific tyrosine residue phosphorylation on downstream signaling cascades. a Phosphorylation of Tyr-992 correlates to Akt and MAPK activation. b Stat3, Akt and MAPK are not affected by Phospho-Tyr-1068. c Tyr-1173 does not directly correspond to Akt, MAPK or Stat3 phosphorylation
Discussion
Small molecule tyrosine kinase inhibitors that bind to the kinase domain of the EGFR and inhibit its autophosphorylation have been developed for the therapy of lung cancer. The potency of these drugs has been linked to activating mutations in the EGFR. Molecular and biological effects of these mutations have been extensively characterized in cell cultures. We have now studied EGFR activation and signaling in tumor samples of patients with NSCLC. Our results strongly suggest important differences in the EGFR phosphorylation pattern between tissue cultures and tumor samples that are relevant to downstream signaling cascades.
EGF binding to its receptor triggers receptor dimerization and subsequent phosphorylation of intracellular tyrosine residues. Here, we analyzed the phosphorylation status of three tyrosine residues in the EGFR that have been shown to activate important downstream signaling pathways. Tyr-992 has mainly been associated with the activation of the MAPK and Akt pathways (Rotin et al. 1992; Vega et al. 1992; Sturla et al. 2005; Cao et al. 2005; Bishnupuri et al. 2006). MAPK signaling is stimulated via PLCγ/PKC, while Akt activation involves Src/PI3-K Ia/PIP3. Phosphorylation of Tyr-1068 activates Stat3 or MAPK. Stat3 activation may involve indirect mechanisms including, Grb2, Erk1/2, Src, and JAK. Tyr-1173 is a direct docking site for the SH2 domain of PLCγ, in turn initiating MAPK.
All tyrosine residues investigated here were evenly phosphorylated in 20–30% of EGFR-WT samples. Cell cultures studies demonstrated that the phosphorylation rate is significantly higher when the EGFR gene is mutated. Our tumor samples also showed an increased receptor phosphorylation, but the pattern was different. While mammary epithelial cell lines with a mutant EGFR displayed phosphorylation of Tyr-992 and Tyr-1068, but not of Tyr-1173 (Sordella et al. 2004), we detected an increased phosphorylation of Tyr-992 and Tyr-1173, but not of Tyr-1068 in human NSCLC samples. Thus, our results suggest that activating mutations in the EGFR gene primarily affect phosphorylation of Tyr-992 and Tyr-1173 in NSCLC.
Consequently, downstream signaling cascades induced by Tyr-992 and Tyr-1173 phosphorylation should be specifically up-regulated in mutant samples. We therefore studied the main targets of EGFR activity Akt, Stat3 and MAPK. While all have a somewhat increased activity in EGFR mutant tumors, we found the most prominent effect with Akt. Thus, Akt activation, most likely through P-Tyr-992 of mutant EGFR samples seems to be the most consistent finding in cell cultures and in primary tumors. Akt is known for its critical role in cell survival and apoptosis. It is overexpressed in many tumors including lung (Brognard et al. 2001), pancreatic (Cheng et al. 2001), thyroid (Vasko et al. 2004) and ovarian (Yuan et al. 2000) carcinomas. These findings suggest a predictive value of P-Akt for the TKI response in lung cancer patients. However, a recent study did not support this hypothesis by showing that EGFR gene copy number may be a predictor for the clinical benefit of TKIs, but not P-Akt (Hirsch et al. 2006). Thus, further investigations need to address whether EGFR mutations do not directly stimulate Akt activity or TKIs do not inhibit Akt phosphorylation by the mutant EGFR.
It has been shown in tissue culture experiments that mutant EGFR tumor cell lines show higher basal levels of Tyr-705 phosphorylated Stat3 than corresponding cell lines without EGFR mutations. The basal level was further increased by EGF application. This increase was not affected by JAK2 and Src inhibitors and thus likely involved a yet unidentified kinase. Importantly, the TKI Gefitinib was not able to decrease basal P-Stat3 levels. These findings indicate that constitutive Tyr-705 phosphorylation is independent of EGFR kinase activity (Alvarez et al. 2006). In our collective over 60% of tumors stained positive in P-Tyr-705-Stat3 IHC. But, in contrast to previous studies in cell cultures, there was no significant difference between EGFR-WT and EGFR-M tumor samples. Stat3 Tyr-705 phosphorylation was also independent of the EGFR phosphorylation state. These results provide important evidence that Stat3 phosphorylation is not a result of EGFR kinase activity in NSCLC. Therefore, Stat3 activity may contribute to the carcinogenic potential of NSCLCs but independently of EGFR mutations.
In agreement with previous studies, we found that approximately 25% of all samples showed activated MAPK independently of mutations in the EGFR (Cappuzzo et al. 2004). However, the activity of MAPK was strongly correlated with Tyr-992 phosphorylation of the EGFR. Interestingly, MAPK phosphorylation was not elevated in mutant EGFR samples even though they show a significant increase in P-Tyr-992. Thus, MAPK activation via P-Tyr-992 is only important in EGFR-WT NSCLC. These findings suggest that EGFR mutations not only increase EGFR kinase activity, but also differentially alter interactions with downstream signaling cascades.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 6 Percent of tumors staining positive in EGFR, P-Tyr-992, P-Tyr-1068, P-Tyr-1173, P-Stat3, P-Akt and P-MAPK immunohistochemistry with regard to specific EGFR mutations. The EGFR subgroups were compared to EGFR-WT samples and the P-values are illustrated. The EGFR-M group and the EGFR-M subgroups display the same pattern in receptor phosphorylation and activity of downstream signaling cascades. (EPS 438 kb)
Acknowledgments
We thank Ellen Paggen for technical help with DNA sequencing and Christiane Esch for support with the immunohistochemistry. L.C.H. was supported by the BONFOR research council.
Abbreviations
- EGFR
Epidermal growth factor receptor
- NSCLC
Non-small cell lung cancer
- Akt
Protein kinase B
- MAPK
Mitogen activated protein kinase
- Stat
Signal transducer and activator of transcription protein
- TKI
Tyrosine kinase inhibitors
- PKC
Protein kinase C
- PLCγ
Phospholipase C-gamma
- DAG
1,2-Diacylglycerol
- IP3
Inositol 1,3,5-triphosphate
- Grb
Growth factor receptor-bound protein
- Sos
Son of sevenless
- SH2
Src homology 2
- GDP/GTP
Guanosine di-/tri-phosphate
- Erk
Extracellular signal-regulated kinases
- PI3-K Ia
Phosphatidylinositol-3-kinase class Ia
- WHO
World Health Organization
- IHC
Immunohistochemistry
References
- Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA (2006) Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res 66:3162–3168. doi:10.1158/0008-5472.CAN-05-3757 [DOI] [PubMed] [Google Scholar]
- Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Storkel S (2004) Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem 52:893–901. doi:10.1369/jhc.3A6195.2004 [DOI] [PubMed] [Google Scholar]
- Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J (1994) Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol 14:5192–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK (2006) Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology 130:137–149. doi:10.1053/j.gastro.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y (2001) The new World Health Organization classification of lung tumours. Eur Respir J 18:1059–1068. doi:10.1183/09031936.01.00275301 [DOI] [PubMed] [Google Scholar]
- Brognard J, Clark AS, Ni Y, Dennis PA (2001) Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61:3986–3997 [PubMed] [Google Scholar]
- Cao Z, Liu L, Van Winkle DM (2005) Met5-enkephalin-induced cardioprotection occurs via transactivation of EGFR and activation of PI3K. Am J Physiol Heart Circ Physiol 288:H1955–H1964. doi:10.1152/ajpheart.00256.2004 [DOI] [PubMed] [Google Scholar]
- Cappuzzo F, Magrini E, Ceresoli GL, Bartolini S, Rossi E, Ludovini V, Gregorc V, Ligorio C, Cancellieri A, Damiani S, Spreafico A, Paties CT et al (2004) Akt phosphorylation and gefitinib efficacy in patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 96:1133–1141 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Vecchi M, Ji Q, Mernaugh R, Carpenter G (1999) The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem 274:26091–26097. doi:10.1074/jbc.274.37.26091 [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR (2001) Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA 93:3636–3641. doi:10.1073/pnas.93.8.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G (2001) A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 7:2958–2970 [PubMed] [Google Scholar]
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T et al (2003) Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 21:2237–2246. doi:10.1200/JCO.2003.10.038 [DOI] [PubMed] [Google Scholar]
- Haura EB, Zheng Z, Song L, Cantor A, Bepler G (2005) Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res 11:8288–8294. doi:10.1158/1078-0432.CCR-05-0827 [DOI] [PubMed] [Google Scholar]
- Heukamp LC, Fischer HP, Schirmacher P, Chen X, Breuhahn K, Nicolay C, Buttner R, Gutgemann I (2006) Podocalyxin-like protein 1 expression in primary hepatic tumours and tumour-like lesions. Histopathology 49:242–247. doi:10.1111/j.1365-2559.2006.02489.x [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21:3798–3807. doi:10.1200/JCO.2003.11.069 [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA Jr, Franklin WA, Dziadziuszko R, Thatcher N, Chang A, Parikh P, Pereira JR, Ciuleanu T, von Pawel J, Watkins C et al (2006) Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24:5034–5042. doi:10.1200/JCO.2006.06.3958 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66 [DOI] [PubMed] [Google Scholar]
- Johnson GL, Vaillancourt RR (1994) Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol 6:230–238. doi:10.1016/0955-0674(94)90141-4 [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW (2003) Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 284:31–53. doi:10.1016/S0014-4827(02)00098-8 [DOI] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D et al (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290:2149–2158. doi:10.1001/jama.290.16.2149 [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139. doi:10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500. doi:10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- Parra HS, Cavina R, Latteri F, Zucali PA, Campagnoli E, Morenghi E, Grimaldi GC, Roncalli M, Santoro A (2004) Analysis of epidermal growth factor receptor expression as a predictive factor for response to gefitinib (‘Iressa’, ZD1839) in non-small-cell lung cancer. Br J Cancer 91:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Margolis B, Mohammadi M, Daly RJ, Daum G, Li N, Fischer EH, Burgess WH, Ullrich A, Schlessinger J (1992) SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. Embo J 11:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaggi G, Novello S, Torri V, Leonardo E, De Giuli P, Borasio P, Mossetti C, Ardissone F, Lausi P, Scagliotti GV (2004) Epidermal growth factor receptor overexpression correlates with a poor prognosis in completely resected non-small-cell lung cancer. Ann Oncol 15:28–32. doi:10.1093/annonc/mdh011 [DOI] [PubMed] [Google Scholar]
- Sobol RE, Astarita RW, Hofeditz C, Masui H, Fairshter R, Royston I, Mendelsohn J (1987) Epidermal growth factor receptor expression in human lung carcinomas defined by a monoclonal antibody. J Natl Cancer Inst 79:403–407 [PubMed] [Google Scholar]
- Sordella R, Bell DW, Haber DA, Settleman J (2004) Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305:1163–1167. doi:10.1126/science.1101637 [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, King LE Jr (1986) Role of epidermal growth factor in carcinogenesis. Cancer Res 46:1030–1037 [PubMed] [Google Scholar]
- Sturla LM, Amorino G, Alexander MS, Mikkelsen RB, Valerie K, Schmidt-Ullrichr RK (2005) Requirement of Tyr-992 and Tyr-1173 in phosphorylation of the epidermal growth factor receptor by ionizing radiation and modulation by SHP2. J Biol Chem 280:14597–14604. doi:10.1074/jbc.M413287200 [DOI] [PubMed] [Google Scholar]
- Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, Burman KD, De Micco C et al (2004) Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet 41:161–170. doi:10.1136/jmg.2003.015339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega QC, Cochet C, Filhol O, Chang CP, Rhee SG, Gill GN (1992) A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol 12:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV, Cheng JQ (2000) Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene 19:2324–2330. doi:10.1038/sj.onc.1203598 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 6 Percent of tumors staining positive in EGFR, P-Tyr-992, P-Tyr-1068, P-Tyr-1173, P-Stat3, P-Akt and P-MAPK immunohistochemistry with regard to specific EGFR mutations. The EGFR subgroups were compared to EGFR-WT samples and the P-values are illustrated. The EGFR-M group and the EGFR-M subgroups display the same pattern in receptor phosphorylation and activity of downstream signaling cascades. (EPS 438 kb)