Abstract
Astrocytes constitute a major class of glial cells in the CNS, and play crucial roles in physiological functioning, performance and maintenance of the CNS, as well as promotion of neuronal migration and maturation. Astrocytes have also been directly and indirectly implicated in the pathophysiology of various trauma occurrences, development of neurodegenerative diseases and nerve regeneration. To further understand mechanisms by which astrocytes elicit these effects, the first critical step in the study of astrocytes is the preparation of purified astrocytes cultures. Here we describe a simple and convenient procedure for producing rat primary astrocyte cultures of high purity, viability and proliferation. For astrocyte culture, we have optimized the isolation procedures and cultivation conditions including coating substrates, enzyme digestion, seeding density and composition of the culture medium. Using immunofluorescent antibodies against GFAP and OX-42 in combination of Hoechst 33342 fluorescent staining, we found that the purity of the astrocyte cultures was >99%. Astrocytes had high viability as measured by 3-(4, 5-dimethyl-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay. In addition, flow cytometric analysis was used to measure and observe variations in the cell cycle after 1–2 passages and proliferation of astrocytes was detected with a high percentage of cells stand in S+G2/M phase. Therefore, the method described here is ideal for experiments, which require highly pure astrocyte cultures.
Keywords: Astrocyte, Cell culture, Spinal cord, Immunofluorescence, EGF , l-valine, Glutamate, Sorbitol
Introduction
Astrocytes are believed to play integral roles in the normal CNS functioning as well as CNS pathology (Travis 1994; Largo et al. 1996). Recent studies have demonstrated that astrocytes can act as regulators of cerebral blood flow, various trophic factors, glutamate metabolism, potassium buffering and neuronal-glial signaling (Walz 1987, 1989; Gallo et al. 1996; Allan 2005; Kim et al. 2003; Haberg et al. 2006). Astrocytes also associate with synapses integrate neuronal inputs and release transmitters that modulate synaptic sensitivity (Hansson and Ronnback 2003). Additionally, astrocytes participate in formation and rebuilding of synapses and play a prominent role in the protection and repair of nervous tissues after damage. It was found that neuronal activities could trigger ATP release from astrocytes, which modulate synaptic transmission. Astrocytes also exert transient modification of synaptic activity and sustain synchronized neuronal activities (Zhang et al. 2003). Finally, astrocytes may be a useful source of pluripotent cells. For example, astrocytes in injured adult rat spinal cord may acquire the potential of neural stem cells, and were found to be undergoing a process of de-differentiation (Lang et al. 2004). Many researchers have created in vitro astrocyte culture systems from the spinal cord due to relative ease and convenience. However, one significant obstacle in isolating astrocytes from spinal cord is minimizing contamination from sources within the microenvironment of spinal cord. Therefore, we have created a method to culture highly pure primary astrocytes which have the ability to de-differentiate.
To study the role of astrocyte physiological and pathophysiological properties, it is critical to establish highly pure, viable astrocyte culture systems. Much of what we know about astrocyte properties has come from studies using cultured embryonic or neonatal cells. Some researchers have shown that mature astrocytes differ from embryonic and neonatal cells in receptor expression, which may present confounding factors for experimenters (Goldman 1996; Mi and Ben 1999). Therefore, the use of astrocytes derived from mature animal CNS is necessary in some cases. But the developmental stage at which astrocytes are isolated is of prime importance. Unfortunately, astrocytes are difficult to isolate from mature mammalian spinal cord because of the abundance of connective tissue and the highly differentiated cells. Few cells are successfully isolated from spinal cord according to previous procedures. Moreover, contamination by fibroblasts, microglia, oligodendrocytes, endothelial cells, ependymal cells and neurons may cause a difficult problem in long-term astrocyte cultures. Therefore eliminating the contaminating cells is crucial for harvesting highly pure astrocytes in primary culture. Previous techniques have been applied including immunopanning, complement-killing, fluorescence-activated cells sorting, mechanical shaking and use of chemical inhibitors in culture. Each of them has intrinsic merits, but multiple strategies have faced time- and resource-consuming procedures and technical complexity. Some strategies lead to lower cell viability.
To avoid the above mentioned disadvantages, we devised here a simple and convenient method that yields astroglia-rich primary cultures from spinal cord of 15–20 days old Sprague Dawley (SD) rat. At this developmental stage, the majority of astrocytes of rat pinal cord have already differentiated as shown acquisition of GFAP and loss/down-regulation of vimentin and nestin (Ling 1976; Valentino et al. 1983; Warf et al. 1991; Mcdermott et al. 2005), which are markers we use to islolate and identify astrocyts. The method involves minimal successive procedures combining astrocyte biological and biochemical characteristics. Modified chemically defined medium (MCM) was used to stimulate astrocyte growth or proliferation and helped to eliminate contaminating cells. Glucose and d-valine were replaced with sorbitol and l-valine in growth media. Meningeal tissues were removed by stripping to reduce fibroblasts during the spinal cord isolation process. To reduce direct distress or irritation to dissociated cells, a low concentration mixture of three enzymes was used to digest tissues and cells were plated sequentially at high density. The astrocytes purified with this simple technique get to >99% purity. We hope this method will be a useful experimental tool to test function of astrocytes in vitro.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) with glucose and l-valine, or without glucose and with d-valine, D-Hank’s, HEPES, glucose, dispase, collagenase and fetal calf serum (FCS) were purchased from GIBCO (USA). Sorbitol, penicillin G, streptomycin, glutamine, trypsin, papain and ethylenediamine tetraacetic acid (EDTA), bovine serum albumin (BSA) and phosphate-buffered saline (PBS) were purchased from Sigma (USA). Fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse IgG was purchased from Molecular Probe (USA). Mouse anti-rabbit glial fibrillary acidic protein (GFAP) antiserum was from Santa Cruz (USA). A Hoechst 33342 staining Kit was purchased from Molecular Probes (USA); 3-(4, 5-dimehthyl-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) and poly-l-lysine were purchased from Sigma (USA). Cell culture dishes, plates and flasks were purchased from Nunco (Denmark).
Preparation and purification of astrocyte culture
Primary astrocyte cultures were made from 15–20 days old SD rats and all experimental procedures were performed in strict accordance with the recommendations of the European Economic Community (86/609/EEC) for care and use of laboratory animals.
Timed-pregnant SD rats were deeply anesthetized under cold standard operating procedure (Phifer and Terry 1986). The skin and muscle overlying the spinal column were quickly removed and mid-cervical to lumbar portions of the spinal cord were dissected and pooled on D-Hank’s balanced sodium salts without Ca2+ and Mg2+ on ice. The meninges and the capillaries were carefully and quickly peeled away with fine forceps (No. 5; Dumont & Files). The tissues were rinsed three times with D-Hank’s balanced sodium salts without Ca2+ and Mg2+ and were minced into small pieces with an iris scalpel.
The spinal cord tissue was incubated in 3 ml of D-Hank’s (2 mM l-glutamine and 25 mM HEPES) containing 0.125% trypsin, 0.05% collagenase and 0.03% dispase for 25 min at 37°C with gentle shaking at 5-min intervals. After the last shaking, the solution was allowed to sit for 5 min, then the supernatant was aspirated carefully. This procedure was repeated one more time. At the end of the total 50 min digestion, 2 ml DMEM containing 10% FCS was added to inactivate the activity of enzymes for 5 min, the tissue was then centrifuged for 5 min at 1,000 rpm and washed with 10 ml D-Hank’s balanced salt solution. The tissue pellet was incubated at 37°C for 20 min in 2 ml of D-Hank’s (2 mM l-glutamine and 25 mM HEPES) containing 30 U ml−1 activated papain. After the 20 min digestion, the 2 ml of D-Hank’s (2 mM l-glutamine and 25 mM HEPES) containing 30 U ml−1 activated papain was removed, and 3 ml DMEM containing 10% FCS was added. The dissociated single cell suspension was easily generated with five cycles of trituration through a fire-polished Pasteur pipette or a No. 23 hypodermic needle. The suspension was further passed through sterile nylon gauze (80 μm pore size) and centrifuged at 500 × g for 10 min at room temperature. The supernatant was removed and the pellet was resuspended in DMEM containing 20% FCS supplemented with penicillin/streptomycin (50 U and 50 µg/ml) (CM). The cell yield was 8 × 106 viable cells/rat. Cells were plated into 24-well plate containing 12-mm diameter coverslips or T-25 flasks coated with 25 μg ml−1 poly-l-lysine at a density of 1 × 105 cells/well and 3 × 106 cells/flask, respectively.
After 48–60 h in culture, the medium was changed to MCM. The components of MCM are DMEM without glucose and with D-Val (Gibco, Cat.No.1196-025-M), 25 mM sorbitol, 25 μg ml−1 EGF, 1 mM glutamate, 1 μM glucose oxidase. Cultures were fed twice a week by replacement of 1/3 volume of medium with fresh medium. Control cultures were fed with DMEM with 20% FCS and penicillin/streptomycin (50 U and 50 µg/ml). After the cultures were grown for an additional 15 days, the cells were detached from the culture flasks with 0.125% trypsin and 0.02% EDTA. Cells were plated on 12-mm diameter sterile coverslips-coating poly-l-lysine placed in 24-well plate or 48-well plate at density of 1 × 105 cells/well and 1 × 104 cells/well, respectively.
Immunofluorescence staining and cell purity assessment
Immunoflourscence (IF) staining was carried out to characterize the cell purity on different days, 2, 4, 6, 8, 10, 14 and 16 days. Cells were rinsed with PBS, fixed in 4% paraformaldehyde for 20 min at room temperature, then washed with Dulbecco’s phosphate-buffered saline (0.01M PBS, pH7.2) three times for 5 min. The cells were blocked in 10% normal donkey serum for 1 h at room temperature and incubated with 0.01% (v/v) Triton X-100 for 5 min. The cells were subsequently incubated at 4°C overnight with primary mouse anti-GFAP (1:1000 dilution) diluted with 1% (w/v) BSA dissolved in PBS (BSA/PBS). Controls were carried out under identical conditions except that primary antibodies were replaced with PBS, and in some cases with matching pre-immune sera. The cells were washed with PBS for three times and incubated with the corresponding secondary antibody. For detection of GFAP, fluorescein isothiocyanate-labeled donkey anti-mouse IgG (1:500 Molecular Probes) was used and incubated for 2 h. After the cells were rinsed three times in PBS for 5 min, Hoechst 33342 was added and incubated for 20 min. After washing with PBS, the coverslips were observed under fluorescent or confocal microscope at 180 × magnification. All cells show nuclear blue positive staining with Hoechst 33342 and only astrocytes show positive staining with GFAP. The total area of each observation field was 0.45 mm2 and 15 observation fields were chosen randomly. The mean ± SEM of 15 field counts from at least four different cultures were determined as the purity index—the proportion of GFAP-positive cells in total cells. To further confirm astrocyte purity, we also carried out OX-42 immunofluorescence staining to identify the microglia, the staining procedure and determination for proportion of OX-42 positive cells were the same as the procdure for astrocytes.
MTT assay
Cell proliferation and viability were determined using the MTT method (Mosmann 1983). Approximately 10,000 cells/well were seeded in 48-well plates (Nunco) and cultured with MCM. The initial number of viable cells at the time of culture, termed t = 0, was determined to correct for differences in starting cell number between experiments and to monitor changes in cell number over time. At the indicated times, 10 μl of 50 mg ml−1 MTT tetrazolium was added directly to the 300 μl culture media and the cells were allowed to incubate for 4 h. After the incubation, the medium from each well was gently removed by aspiration and 200 μl Dimethylsulfoxide (DMSO) was added to each well followed by incubation and shaking for 10 min to dissolve the insoluble formazan. The culture plate with DMSO solutions were measured in a microplate reader at 570 nm (reference wave length 630 nm, Dynatech MR4000, Japan) to determine the number of viable cells at 1, 2, 4, 6, 8 and 10 days after culture with MCM. All data presented in the present report were obtained from 5 independent experiments.
Analysis of cell cycle
When astrocytes in flasks reached 70–80% confluence, subculture was carried out in 35 mm plastic Petri culture dishes at a density of 5 × 105cells/dish. After culture with MCM for 3 days in vitro, a 0.05% trypsin and 0.02% EDTA solution was added without dislodging the cell sheet. Cells were observed through an inverted microscope for 3–6 min. If the cells began to be round or lift off the dishes, the trypsin/EDTA solution was removed immediately. If the cells do not become detached within seven minutes, incubate an additional 1–2 min. The trypsinization was inactivated by washing the bottom of the dishes with CM. Cells were mixed with the medium by gently pipeting up and down five times. The cell suspensions were centrifuged and washed with 8 ml Hanks thoroughly and an aliquot was removed for cell counting. Cultured astrocytes were subcultured for two passages, and the astrocytes from each passage were subcultured and harvested. The harvested cells were washed twice in ice-cold PBS by centrifugation, and fixed in 70% ethanol at 4°C overnight. Subsequently, the cells were incubated with 10 μl RNAse A (5 mg ml−1) at 37°C for 30 min and cellular DNA was stained with Propidium Iodide100 mg ml−1 for 30 min. Distribution of the cell cycle was determined by flow cytometry (ProfileII, Coulter, USA). The proliferation index was calculated using the following equation: Proliferation index (PI) (%) = (S + G2/M)/ (G0/G1 + S + G2/M) × 100%.
Statistical analysis
All data are presented as mean ± SD. The statistical significance was determined using one-way ANOVA with SPSS 11.5 software (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
After 48 h in culture, many of the cells dissociated from the spinal cord had adhered to the culture plates and exhibited various morphologies. Some of cells were flat and polygonal while others were round, bipolar and irregular. A few cells extended thin and long processes. At this stage the cell types could not be determined because many of them had not matured to their final morphology. It is likely that polygonal cells with processes or irregular cells were astrocytes, the flat polygonal cells were contaminating cells such as fibroblasts, and the phase-bright cells with small soma and short processes were oligodendrocytes. When cells were grown for 2–3 days in CM, a switch to MCM was performed. After cultured in MCM for two days (Fig. 1a), the cells proliferated as control cells (Fig. 1b) did, and the morphology and cellular types in cultures were similar in both culture media. We found that if the primary cultures were maintained further in MCM for five days in vitro, the astrocyte-like cells rapidly multiplied to form a confluent monolayer in some fields (data not shown). The majority of the cells were astrocyte-like cells, and the flat fibroblasts and oligodendrocyte-like cells were rare. Furthermore, the astrocyte-like cells fed with MCM were morphologically homogeneous compared with astrocytes in CM. CM cultures were rapidly contaminated by fibroblasts.
Fig. 1.
Phase-contrast micrographs of primarily dissociated cells from spinal cord under different conditions. (a) Two days in CM followed by a switch to MCM for additional two days. (b) CM for four days, astrocyte-like cells with polygonal irregular shape could be seen in field (arrow). (c) Cells in CM over 12 days, contaminating cells such as fibroblasts (arrowheads) and oligodendrocytes (arrow) are marked. (d) Highly enriched population of astrocyte after MCM over 12 days. Bar = 200 μm
Inspection of living cultures through a phase-contrast microscope on 12–15 days after switching to MCM revealed a change in cell type and different cell morphologies seen in CM-fed compared with MCM-fed cells. Flat fibroblasts with irregular shape and oligodendrocytes could be seen in each field, and some fields were devoid of astrocytes. With longer culture period beyond 12–14 days, non-astrocytic cells became more abundant, and eventually overgrew the entire culture (Fig. 1c). However, when cultured in MCM over 12 days, astrocytes became the more abundant cell type, and their processes extended to form a complicated net. Contaminating cells in these cultures were rare (Fig. 1d).
Immunofluorescence staining and cell purity determination
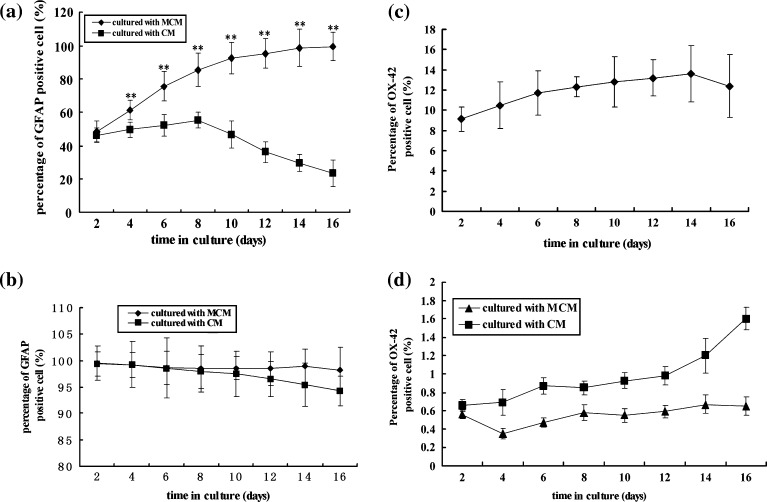
Astrocytes purity can be examined by using astrocytic marker such as GFAP immunofluorescence and Hoechst 33342 double staining. GFAP IF-staining was present throughout the cell cytoplasm and was relatively homogeneous. Intense labeling for Hoechst 33342 was prominent only in the nucleus. Inspection of astrocyte purity from different culture periods revealed that cultures fed with MCM resulted in enriched GFAP–positive cells compared to control cultures. On the fifth day after switched to MCM, astrocyte purity was still low (48.5%), but was higher than that in controls (cultured in CM) (46.3%) (Figs. 2a and 3a). Prolonged cultures in MCM rapidly increased astrocyte purity. After 12–15 days the percentage of GFAP-positive cells reached over 98%. In controls, after 4 days in vitro, the percentage of GFAP-positive cells was only 49.5%, and with prolonged culture (Over 12 days), the proportion decreased to 29.5% (Figs. 2a, 3b). To further confirm MCM selectivity for astrocyte culture, the astrocytes were passaged with MCM. After being cultured for 2, 4, 6, 8, 10, 12, 14, 16 days, the astrocytes achieved higher purity determined to be over 99% (Figs. 2b and 3c). While being sub-cultured with CM, the purity of astrocytes gradually decreased (Figs. 2b and 3d), however, it remained over 95%. These data show that astrocytes primarily cultured with MCM have high purity, and MCM can selectively promote astrocyte growth whereas cells fed with CM have decreased astrocytic purity. As for microglia, the proportion of OX-42 positive cells ranged between 9.12 and 12.4% when fed with CM without a switch to MCM (Fig. 2c). Whereas cells from MCM further sub-cultured and fed with MCM and CM, respectively, the corresponding proportions were below 1 and 1.4% (Figs. 2d and 3e, f).
Fig. 2.
(a) Effect of MCM on percentage of GFAP positive cells primarily cultured from rat spinal cord for various durations. In each group, the value represents mean ± SD obtained from three replicates in six wells. ** P < 0.01 for MCM vs. CM at the same time point. (b) Effect of MCM on percentage of GFAP positive cells after subcultured with MCM for various durations. In each group, the value represents mean ± SD obtained from three replicates in six wells. **Compared with CM, P < 0.01 namely, fed with MCM vs with CM at same time point. (c) Percentage of OX-42 (a marker of microglia) positive cells in CM for different time. (d) Effect of MCM on percentage of OX-42 positive cells after sub-cultured with MCM for different time. Cells were cultured in MCM for 15 days, then sub-cultured with MCM or CM for different time. In each group, the value represents mean ± SD obtained from repeated test for three times in six wells
Fig. 3.
Photomicrographs of dual staining for GFAP immunofluorescence (green) and OX-42 (green) with Hoechst 33342 (blue). (a) Fed with CM for four days. Cytoplasm (green, arrow) and non-astrocytes (blue, arrowhead). (b) CM cultures at 12 days. (c), (d): MCM or CM over 12 days, respectively, after having been precultured in MCM for 12 days. Bar = 200 μm. (e), (f) Fed with CM or MCM over 12 days. The positive double staining for OX-42 and Hoechst 33342 were microglial cells (arrow). Non-microglial cells only labeled in nucleus (arrowheads). Bar = 250 μm
Cell proliferation and viability
To further confirm that the MCM could induce stably and highly proliferative astrocytes in culture, the purified astrocytes were continually sub-cultured in MCM for 16 days. On day 1, 2, 4, 6, 8 and 10 days, cell proliferation or viability was assessed by MTT assay. As shown in Fig. 4, there was a dramatic increase in cell viability and proliferation compared to those in controls. Over five different time points, the OD (Optical density) value of cells fed with MCM increased from 0.257 to 0.927, as for CM culture, the OD value of cells increased from 0.263 to 0.473, showing only two-fold increase. In Fig. 5, the color-dense formazan showed that the activity of cellular mitochondrial dehydrogenases was higher in MCM culture than in CM culture, which revealed that the MCM could promote astrocyte proliferation or viability.
Fig. 4.
Effect of MCM on proliferation and viability of astrocytes subcultured with MCM for different culture durations. ** P < 0.01 μm, * P < 0.05 Fed with MCM vs. with CM at same time point
Fig. 5.
Photomicrographs of formation of cell color-dense formazan from MTT assay. (a) Fed with CM for 10 days. (b) Fed with MCM for 10 days. Bar = 200 μm
Changes of cell cycle
To determine if cells fed with MCM have strong division capacity, cells were sub-cultured for two passages with MCM for three days, and then analyzed with flow cytometry. Shown in Table 1 are the changes in the percentage of cells in each cycle phase (G0/G1, S, G2/M). We found that there was a 20.1% (Passage I) and 17.9% (Passage II) increase in S and G2/M phases, and a corresponding quantitative decrease in the percent in G0/G1 phases. In control groups, there was little cell transition from G0/G1 to S and G2/M phase, and a large percentage of cells were in G0/G1 phase arrest. These data indicated that MCM significantly facilitated transition of astrocyte from stationary phase (G0/G1) to DNA synthesis (S phase) and mitosis (G2/M phase), which leads to proliferation of astrocytes. These data along with the data from MTT assay consistently suggest that MCM increased viable cell numbers by promoting astrocytes in S and G2/M cell cycle arrest.
Table 1.
The flow cytometry analysis of cell cycle changes of astrocytes fed with chemically conditioned medium (MCM)(% Mean ± SD, n = 3)
| Groups | Percentage of cell cycle distribution | |||
|---|---|---|---|---|
| G0/G1 | S | G2/M | S + G2/M | |
| Control | 90.27 ± 0.76 | 6.22 ± 0.64 | 3.51 ± 0.54 | 9.73 ± 0.86 |
| Passage I | 70.14 ± 1.98 | 16.54 ± 0.16a | 13.32 ± 1.20a | 29.86 ± 2.03a |
| Passage II | 72.36 ± 1.35 | 15.43 ± 1.30a | 12.21 ± 0.54a | 27.64 ± 1.35sa |
aCompared with control group (fed with MCM vs. CM), P < 0.01. All the parameters of mean ± SD were obtained by triplicate measures of a given group
Discussion
The isolation and purification, or enrichment of astroglia from central nervous system tissues, require the integrated application of several strategies. Many approaches can be used to separate and purify a particular cell type, however, each method has its own limitations such as tedious procedure, complicated manipulation, high expenses for example. In the present study, we avoid these limitations by taking advantage of the distinct metabolic competences and properties of various cell populations in the CNS. We developed a comparatively easy and efficient method to successfully produce a highly enriched (>99%) population of astrocytes with strong proliferation from rat spinal cord.
Previous strategies for obtaining highly pure astrocytes have been focused on the use of immunopanning, complement-killing and fluorescence-activated cells sorting. These strategies can be time- and resource-consuming with low yield and viability of astrocytic cells. In rat spinal cord there are various types of cells: neurons, astrocytes, oligodendrocytes, fibroblasts, microglia and endothelial cells. Primary astrocyte cultures cannot avoid the contamination from other cell types, and these contaminating cells readily grow to be confluent. In our method for isolating and purifying astrocytes from spinal cord, we have reduced contaminating cells by carefully peeling away minute capillaries and meninges from spinal cord trunk using fine forceps. Because the fibroblast and endothelial cells are two major cellular components of meningeal coverings and accompanying minute capillaries, stripping away as much as possible, effectively reduced and even removed fibroblasts and endothelial cells in cultures. In addition, to carefully harvest astrocytes with high viability, we chose four enzymes to dissociate tissue. These enzymes are trypsin, collagenase, dispase and papain. Trypsinization is acute and it can cause direct distress or irritation to dissociated cells (Frangakis and Kimelberg 1984). Therefore, we applied trypsin at low concentration (0.125%). A mixture of the three enzymes, trypsin, collagenase, dispase, was used to facilitate dissociation and prevent a decrease in cell viability. In addition, papain, a mild and soothing enzyme, was applied to aid in keratose digestion.
A critical part of our method involved selecting the optimal medium for astrocytes cultures. The appropriate medium leads to astrocyte growth and proliferation, and inhibits or reduces the population of contaminating cells. The critical modifications in the modified culture medium (MCM) are the substitution of d-valine for l-valine and replacement of glucose by sorbitol. Some studies revealed that fibroblasts lack d-valine oxidase, and therefore fibroblasts are unable to convert d-valine into l-valine. Without l-valine, fibroblast cannot survive (Estin and Vernadakis 1986). Astrocytes possess appropriate enzymes for oxidizing fatty acids (i.e., glycogen phosphorylase, aldose reductase, and sorbitol dehydrogenase), so they do not require glucose or ketone bodies for respiration. Therefore, it is possible to selectively destroy oligodendrocytes, neurons, and microglia, which require glucose for respiration, by growing cells in a glucose-free medium containing both sorbitol and serum (Wiesinger et al. 1991). Previous studies have shown that the enzymes of the sorbitol pathway, aldose reductase and sorbitol dehydrogenase, and the sorbitol uptake system are present in rat astroglia-rich cultures, but could not be detected in oligodendrocyte- and neuron-rich cell cultures (Stahl et al. 1989; Wiesinger et al. 1990). Accordingly, the selectively cultured astrocyte can be grown in a glucose-free medium containing 25 μM sorbitol. Non-astrocytes can be effectively exterminated from cultures by using this modified chemically defined medium in that the capacity to utilize glucose as energy and l-valine as an essential amino acid for cellular anabolism, and the maintenance of proper nitrogen balance were deprived. In our study, 1 μM glucose oxidase was added to the medium for enzymatic degradation of glucose from FCS, which decreases the persistence of contaminating cells. In our culture system, we found that astrocyte purity increased to over 98% with prolonged culture periods, especially for 12 days in vitro which shows that this appropriate medium can effectively promote astrocytes growth and dramatically prevent non-astrocytic contamination. To further confirm our results, we also carried out staining microglial cells with OX-42, and monitoring its changes in percentage. It was found that the population was still below 1%, which shows that this appropriate medium can effectively promote astrocytes growth and dramatically prevent non-astrocytes persistence.
EGF is a known mitogen for astroglial cells (Simpson et al. 1982) that initiates the mitogen for astroglial cells through EGF signal transduction pathways. Previous works have shown that EGF can stimulate the proliferation of primary astrocyte cultures obtained from rat cerebral hemispheres (Avola et al. 1988; 1993), and it strongly affects the morphology of astrocytes and induces upregulation of the glutamine synthase and S-100 (Avola et al. 1988; 1993). In the present study, the addition of EGF to this modified chemically defined medium significantly improved the proliferative capability of cultured astrocytes. We found that after 2, 4, 6 and 10 days in vitro, the medium containing 25 mM sorbitol supplemented with EGF resulted in astrocytes with high proliferation and viability. As measure with the MTT assay, the OD value of cells fed with MCM increased from 0.257 to 0.927, whereas in control cultures (fed with CM) the OD value of cells only increased from 0.263 to 0.473. Proliferation index (PI) for changes of cell cycle also exhibited a higher proportion of S and G2/M cell cycle arrest compared to controls. The results above are in agreement with findings from in vitro studies that EGF can stimulate proliferation of astroglial cells (Simpson et al. 1982).
Some previous studies revealed that astrocytes can express functional mGluR3 and mGluR5 (Miller et al. 1996; Liao and Chen 2001; Aronica et al. 2003). Activation of group I and II mGluRs have also been shown to regulate glial cell proliferation (Ciccarelli et al. 1997; Aronica et al. 2003) and glial glutamate transporter protein is also expressed in rat and human astrocytes in cultures (Aronica et al. 2001). These glutamate-stimulated cellular events are mediated through the mGluR activation. Excess glutamate causes excitoxic cell death. However, glutamate at certain concentrations can stimulate astrocyte proliferation. Therefore we supplemented 1 mM glutamate into medium. The addition of 1 mM glutamate to the culture medium resulted in an increase in proliferation in the astrocyte cultures. These results indicated that the chemically modified defined medium could enhance astrocyte proliferation by combined stimulus EGF together with glutamate.
In summary, we combined mechanical and enzymatic tissue dissociation together with sorbitol substituting for glucose and replacement of l-valine by d-valine in the culture medium to successfully grow homogenous astrocytes cultures. The supplement EGF and glutamate further enhanced astrocyte proliferation. The advantages of this method are that (1) the procedure to isolate astrocytes is fast and simple, (2) the cells are highly viable, (3) highly pure astrocytic population is achieved and (4) the cultures have strong proliferative capability. This approach proved feasible and useful for getting highly-rich astrocyte cultures, and is readily achieved with less time- and resource-consumption. This method may aid research in which pure astrocyte cultures are necessary.
Acknowledgements
This work was supported by the National Natural science foundation of China (30230450), Basic Research Program of China (2002CB515301).
References
- Allan S. The neurovascular unit and the key role of astrocytes in the regulation of cerebral blood flow. Cerebrovasc Dis. 2005;21:137–138. doi: 10.1159/000090447. [DOI] [PubMed] [Google Scholar]
- Aronica E, Catania MV, Geurts J, Yankaya J, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neurosci. 2001;105:509–520. doi: 10.1016/S0306-4522(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leonstra Troost D. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:1–13. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Avola R, Condorelli DF, Surrentino S, Turpeenoja L, Costa A, Giuffrida Stella AM. Effect of epidermal growth factor and insulin on DNA, RNA, and cytoskeletal protein labeing in parmary rat astrocyglial cell cultures. J Neurosci Res. 1988;19:230–238. doi: 10.1002/jnr.490190208. [DOI] [PubMed] [Google Scholar]
- Avola R, Reale S, Costa A, Insirello L, Spina-Purrello V, Giuffrida-Stella AM. Effects of bFGF and IGF-I on polyadenylated RNA and non-histone chromosomal protein labeling in cultured astrocytes. J Neurochem. 1993;61:200–210. doi: 10.1111/j.1471-4159.1993.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Ciccarelli R, Sureda FX, Casabona G, Di Iorio P, Caruso A, Spinella F. Opposite influence of the metabotropic glutamate receptor subtypes mGlu3 and -5 on astrocyte proliferation in culture. Glia. 1997;21:390–398. doi: 10.1002/(SICI)1098-1136(199712)21:4<390::AID-GLIA6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Estin C, Vernadakis A. Primary glial cells and brain fibroblasts: interactions in culture. Brain Res Bull. 1986;16:723–731. doi: 10.1016/0361-9230(86)90144-9. [DOI] [PubMed] [Google Scholar]
- Frangakis MV, Kimelberg HK. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochem Res. 1984;9:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- Gallo F, Morale MC, Farinella Z, Avola R, Marchetti B. Growth factors released from astroglial cells in primary culture participate in the cross talk between luteinizing hormone-releasing hormone (LHRH) neurons and astrocytes. Effects on LHRH neuronal proliferation and secretion. Ann N Y Acad Sci. 1996;784:513–516. doi: 10.1111/j.1749-6632.1996.tb16272.x. [DOI] [PubMed] [Google Scholar]
- Goldman JE. Developmental origins of astrocytes. In: Jessen KR, Richardson WD, editors. Glial cell development. Oxford: BIOS Scientific; 1996. p. 31. [Google Scholar]
- Haberg A, Qu H, Sonnewald U. Glutamate and GABA metabolism in transient and permanent middle cerebral artery occlusion in rat: importance of astrocytes for neuronal survival. Neurochem Int. 2006;48:531–540. doi: 10.1016/j.neuint.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Hansoson E, Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Kim JA, Tran ND, Wang SJ, Fisher MJ. Astrocyte regulation of human brain capillary endothelial fibrinolysis. Thromb Res. 2003;112:159–165. doi: 10.1016/j.thromres.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Lang B, Liu HL, Liu R, Feng GD, Jiao XY, Ju G. Astrocytes in injured adult rat spinal cord may acquire the potential of neural stem cells. Neurosci. 2004;128:775–783. doi: 10.1016/j.neuroscience.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Somjen GG, Martin Rio R, Herreras O. The effect of depressing glial function in rat brain in situ on ion homeostasis, synaptic transmission, and neuron survival. J Neurosci. 1996;16:1219–1296. doi: 10.1523/JNEUROSCI.16-03-01219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SL, Chen CJ. Tyrosine kinase signaling involved in glutamate-induced astrocyte proliferation. Neuroreport. 2001;12:3519–3522. doi: 10.1097/00001756-200111160-00029. [DOI] [PubMed] [Google Scholar]
- Ling EA. Study in the changes of the proportions and numbers of the various glial cell types in the spinal cord of neonatal and young adult rats. Acta Anat. 1976;96:188–195. doi: 10.1159/000144672. [DOI] [PubMed] [Google Scholar]
- Mcdermott KW, Barry DS, Mcmahon SS. Role of radial glia in cytogenesis patterning and boundary formation in the developing spinal cord. J Anat. 2005;207:241–250. doi: 10.1111/j.1469-7580.2005.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi HY, Ben AB. Purification and Characterization of Astrocyte Precursor Cells in the Developing Rat Optic Nerve. J Neurosci. 1999;19:1049–1061. doi: 10.1523/JNEUROSCI.19-03-01049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Sehati N, Romano C, Cotman CM. Exposure of astrocytes to thrombin reduces levels of the metabotropic glutamate receptor mGluR5. J Neurochem. 1996;67:1435–1447. doi: 10.1046/j.1471-4159.1996.67041435.x. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxic assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physio Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- Simpson DL, Morrison R, Velliis JD, Herschman HR. Epidermal growth factor binding and mitogenic activity on purified population of cells from central nervous system. J Neurosci Res. 1982;8:453–462. doi: 10.1002/jnr.490080233. [DOI] [PubMed] [Google Scholar]
- Stahl B, Wiesinger H, Hamprecht B. Characteristics of sorbitol uptake in rat glial primary cultures. J Neurochem. 1989;53:665–671. doi: 10.1111/j.1471-4159.1989.tb11755.x. [DOI] [PubMed] [Google Scholar]
- Travis J. Glia: the brain’s other cells. Science. 1994;266:970–972. doi: 10.1126/science.7973679. [DOI] [PubMed] [Google Scholar]
- Valentino KL, Jones EG, Kane SA. Expression of GFAP immunoreactivity during development of long fiber tracts in the rat CNS. Brain Res. 1983;285:317–336. doi: 10.1016/0165-3806(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of glial cells in the regulation of the brain ion microenvironment. Prog Neurobiol. 1989;33:309–333. doi: 10.1016/0301-0082(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Walz W. Swelling and potassium uptake in cultured astrocytes. Can J Physiol Pharmacol. 1987;65:1051–1057. doi: 10.1139/y87-166. [DOI] [PubMed] [Google Scholar]
- Warf BC, Fork-Seaning J, Miller RH. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J Neurosci. 1991;11:2477–2488. doi: 10.1523/JNEUROSCI.11-08-02477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger H, Schuricht B, Hamprecht B. Replacement of glucose by sorbitol in growth medium causes selection of astroglial cells from heterogeneous primary cultures derived from newborn mouse brain. Brain Res. 1991;550:69–76. doi: 10.1016/0006-8993(91)90406-L. [DOI] [PubMed] [Google Scholar]
- Wiesinger H, Thiess U, Hamprecht B. Sorbitol pathway activity and utilization of polyols in astroglia-rich primary cultures. Glia. 1990;3:277–282. doi: 10.1002/glia.440030407. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Chang-quan YE, Ge WP, Wu CP, Poo MM, Duan SM. ATP Released by astrocytes mediates glutamtergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/S0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]