Abstract
Controlling cell proliferation during cell culturing is an effective way to improve the production yield in mammalian cell culture. We examined the effect of temperature shifts (TS) under pH control conditions in Chinese hamster ovary cells. When we shifted the culture temperature from 37 °C to 31 °C before a stationary phase at pH 6.8 (TS/pH 6.8), cell viability remained high, and the final human monoclonal antibody (hMab) concentration increased to 2.3 times that in the culture remaining at 37 °C. However, there were no significant effects on the cell viability or production yield with the same TS at pH 7.0 (TS/pH 7.0). The average specific hMab productivity and mRNA level of TS/pH 7.0 were the same as that of TS/pH 6.8. The control of cell growth by the TS or the addition of rapamycin was effective in the maintenance of cell viability, but there was no significant increase of the average specific hMab productivity in the culture where cell proliferation was controlled with rapamycin. The hMab mRNA concentration decreased to 55%–65% at a 37 °C culture with the addition of actinomycin D. In contrast, actinomycin D did not affect the mRNA level in the TS culture. This result suggested that the increase in the mRNA level in the TS condition was caused by an increase in mRNA stability. In this study, we show that TS can produce two unrelated effects: a prolongation of cell longevity and an improvement in mRNA stability.
Keywords: CHO cell, Temperature shift, pH condition, Fed-batch culture, Actinomycin D
Introduction
Recent studies suggest that controlling the proliferation of cells and maintaining high cell viability during cell culturing can lead to enhanced productivity and higher product yields, thereby improving process performance. Strategies for controlling cell growth include the expression or addition of cell cycle inhibitors like p27 (Kaufmann et al. 2001), p21cip1 (Watanabe et al. 2002), and rapamycin (Balcarcel and Stephanopoulos 2001); or exposure to hyperosmotic pressure (Ryu et al. 2000) or low culture temperature. Low temperature cultivation is a simple and very effective method of controlling cell proliferation (Reuveny et al. 1993). It has been known for many years that the growth rate of mammalian cells decreases if the cultivation temperature is lower than 37 °C. Mammalian cells show a remarkably high viability and a decreased proliferation rate at temperatures between 27 °C and 32 °C (Kaufmann et al. 1999; Giard et al. 1982). To maximize the beneficial effect of lowering the culture temperature, in order to control cell proliferation, the temperature was reduced after cell growth reached a suitable cell density under the condition of 37 °C (Hendrick et al. 2001; Schatz et al. 2003). Although many researchers have attempted to increase product yields by low temperature cultivation, their results have varied (Sureshkumar and Mutharasan 1991; Bloemkolk et al. 1992; Weidemann et al. 1994), mainly because of differences in specific productivity as a result of controlling cell growth in low temperature cultivation conditions. The relationship between prolonging cell longevity and increasing specific productivity is unclear. To aid in clarifying this phenomena, we investigate the relationship between a temperature shift (TS) and other culture conditions in Chinese hamster ovary (CHO) cells. Some culture conditions, such a low pH, sometimes gives rise to negative effects on cell growth and production yield because it halts cell growth and changes cell metabolism (Varley and Birch 1999; Sauer et al. 2000). Here, we show that the optimization of the pH condition in the TS culture is important for maintaining high viability and increasing specific human monoclonal antibody (hMab) productivity. To clarify the relationship between prolonging cell longevity and increasing specific hMab productivity, we treated our cell line with rapamycin in order to control the cell cycle progression without shifting the temperature. Rapamycin prevents the progression of cell division from the G1 to the S phase of the cell cycle. To determine whether the mechanism of mRNA changes is caused by an increase in the rate of synthesis of the mRNA or by a decrease in its degradation rate, we studied mRNA stability using actinomycin D as an inhibitor of RNA synthesis in a TS culture.
Materials and methods
Cell culture
The parental cell line was a protein-free medium adapted CHO DHFR− cell line derived from CHO DG44. The hMab production cell line was constructed by introducing a vector DNA containing a human CMV promoter provided by Biogen-IDEC Inc. (Reff 1997) into the parent cell, selecting transfectants and then amplifying the gene copy number. EX-CELL 302 (SAFC BIOSCIENCES) supplemented with 4 mM glutamine was used in this study for the initial medium. To provide nutrients, 10× modified DMEM/F12 medium supplemented with 100 g L−1 glucose and 100 mM glutamine solution were used as feeding mediums. The culture vessels used were 2-L scale stirred bioreactors or shake flasks (working volume: 40 mL). The initial temperature of the culture was maintained at 37 °C, which was lowered to 31 °C by the temperature control units after several days of cultivation. The pH of the culture was kept not lower than 7.0 or 6.8 by the addition of 0.5 M NaOH. To maintain cell growth, feeding media were added to the vessels to prevent glucose or glutamine depletion.
Analysis
The viable cell density (VCD) and viability were determined using the cell count in the trypan blue dye exclusion method. The antibody titer was quantified with HPLC. The average specific hMab productivity was determined by the slope of the hMab concentration versus the integral of the viable cell density (IVCD) (Fox et al. 2004).
Isolation of total RNA
To obtain total RNA, a culture sample containing 1 × 107 cells was harvested from each culture vessel. After centrifugation for 5 min at 300 × g and washing with PBS, the total RNA was isolated using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol. The RNA concentration and purity were determined photometrically at 260/280 nm.
Northern Blot analysis
Twenty μg of RNA were subjected to 1% formaldehyde agarose-gel electropheresis in a MOPS running buffer (20 mM MOPS, 5 mM Na-acetate, 1 mM EDTA). The gels were blotted onto positively charged nylon membranes (MILLIPORE) by capillary transfer using 20× SSC (3 M NaCl, 0.3 M Na3-citrate). After blotting, the membranes were rinsed and baked dry in an oven for 2 h at 80 °C. As a probe, DIG-labeled heavy chain (HC) and light chain (LC) homologous region fragments using a DIG Labeling Kit (Roche) and DIG-labeled β-Actin RNA probe (Roche) were used. The membrane was prehybridized at 55 °C for 30 min in Easy Hyb (Roche), and the denatured probe was added and allowed to hybridize at 55 °C for 16 h. The membrane was then washed for 2 × 5 min in 2× SSC/0.1% SDS and for 2 × 15 min in 0.1× SSC/0.1% SDS at 65 °C. To detect the RNA-RNA hybrid, the membrane was preincubated for 30 min in blocking solution and then incubated for 30 min with anti-DIG-AP conjugate (diluted 1:10000). A 2 × 15 min washing step was followed by equilibration in a detection buffer for 5 min. Detection was performed with the chemiluminescence substrate CSPD (Roche).
Quantitative RT-PCR (Q-PCR) analysis
For the RT-PCR reaction, the TaqMan One-step RT-PCR Master Mix Reagents Kit (Applied Biosystems) containing 900 nM forward primer, 900 nM reverse primer and a 250 nM TaqMan probe was used at 50 μL tube−1. Amplification and detection were performed using the ABI PRISM 7900HT Sequence Detector System (Applied Biosystems) with the following profile: 1 cycle at 48 °C, 1 cycle at 95 °C for 10 min, and 40 cycles each at 95 °C for 15 s and 60 °C for 1 min. Samples were deemed positive in any given cycle when the value of the emitted fluorescence was greater than the threshold value calculated by the instrument’s software (Sequence Detector System 2.0).
mRNA stability analysis
In experiments to determine hMab mRNA stability, the cells were maintained for 24 h at a culture temperature of 37 °C or were reduced to 31 °C after 3 days cultivation. These cells were chased for 0, 12 or 24 h in the presence of 5.0 μg mL−1 of actinomycin D using shake flasks. The pH of the culture was kept not lower than 6.8 by the addition of 0.5 M NaOH. At the end of each chase period, the culture samples containing 1 × 107 cells were washed with PBS, total RNAs were collected, and hMab transcripts were quantified by Q-PCR as described above.
Results
Effect of the pH condition in temperature shift cultivation
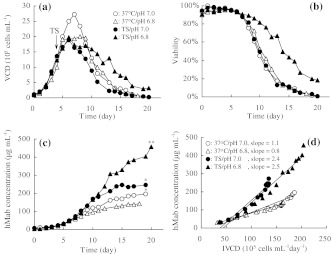
It is necessary to clarify the relationship between the TS and culture parameters such as pH because the effect of TS is influenced by the culture conditions. The pH condition sometime gives rise to negative effect on cell growth and production yield. We examined the effect of TS and the pH condition using 2-L bioreactors. The initial temperature of the culture was maintained at 37 °C. After 4 days of cultivation, it was lowered to 31 °C. The pH of the culture was kept not lower than 7.0 or 6.8 by the automatic addition of 0.5 M NaOH. Figure 1 shows the effect of TS at a pH of 6.8 (TS/pH 6.8) and a pH of 7.0 (TS/pH 7.0). In the culture remaining at 37 °C, the maximum viable cell density (Fig. 1a) was reduced by the pH of 6.8 (37 °C/pH 6.8), but the pH level did not have an effect on cell viability (Fig. 1b), the final hMab concentration (Fig. 1c) or the average specific hMab productivity determined by the slope of the hMab concentration versus IVCD (Fig. 1d) compared to the culture at pH 7.0 (37 °C/pH 7.0). Independent of the pH, the cell growth rate decreased when the temperature was lowered to 31 °C (Fig. 1a), where the maximum viable cell density was 30% lower than that of the culture at 37 °C/pH 7.0. In the TS/pH 6.8 condition, the cell viability after 11 days of cultivation was twice as high as that in the culture under the other conditions (Fig. 1b). The cell viability of TS/pH 6.8 remained high during culturing. The final antibody concentration was 460 mg L−1, which is 2.3 times as high as 200 mg L−1 for the 37 °C/pH 7.0 culture (Fig. 1c). The average specific hMab productivity of TS/pH 6.8 increased to 2.3 times that of the culture at 37 °C/pH 7.0 (Fig. 1d). However, there was no significant effect on keeping the cell viability or increasing production yield under the TS/pH 7.0 condition, despite control of the cell growth. Interestingly, the average specific hMab productivity of TS/pH 7.0 was 2.4 μg 105 cell−1 day−1 (Fig. 1d). It was the same as that of TS/pH 6.8 and increased to 2.2 times that of the culture at 37 °C/pH 7.0. This result indicates that the specific hMab productivity did not correlate with prolonging cell longevity.
Fig. 1.
The profiles of temperature shift cultures in pH 7.0 or 6.8 conditions using 2-L bioreactors. (a) VCD, (b) Viability, (c) hMab concentration, (d) hMab concentration as a function of IVCD. The average specific hMab productivity was determined by the slope of the hMab concentration versus IVCD. Results are mean of multiple experiments. (* n = 3, ** n = 4)
Northern Blot and Q-PCR analysis to determine the antibody mRNA level of temperature shift cultivation
Northern blot analysis was performed to determine whether antibody upregulation upon exposure of the cells to 31 °C occurred at the mRNA level (Fig. 2). These cells were collected just before shifting the temperature (day 4) and 4 days after TS cultivation (day 8) from the same culture described in Fig. 1. Figure 2 shows the mRNA levels after 4 days cultivation at 37 °C/pH 7.0 (Lane 1) and 37 °C/pH 6.8 (Lane 2) conditions and after 8 days cultivation at 37 °C/pH 7.0 (Lane 3), TS/pH 7.0 (Lane 4) and TS/pH 6.8 (Lane 5). These results indicate that higher antibody expression level resulted from the increasing of mRNA level. The mRNA levels of antibody in these TS cultures clearly increased compared to the culture at 37 °C during the same culture period regardless of the pH levels. To quantify the result of Northern blot analysis, we also determined the mRNA level of the same samples described above by Q-PCR. The mRNA of the HC and LC were normalized to β-actin. The percentage under the bands were the relative mRNA values against 37 °C/pH 7.0 on day 8 quantified by Q-PCR. These relative values for the HC and LC mRNAs under the TS culture increased to 1.9–2.9 times that of the 37 °C culture during the same culture period regardless of the pH levels. This result is clearly reflected in Fig. 1d.
Fig. 2.
Northern blot and Q-PCR analysis of HC and LC mRNA levels in various culture conditions. RNAs were sampled 4 days after inoculation at pH 7.0 (Lane 1) or 6.8 (Lane 2) conditions and 8 days after inoculation at 37 °C/pH 7.0 (Lane 3), TS/pH 7.0 (Lane 4) and TS/pH 6.8 (Lane 5). The percentage under the bands were the relative mRNA values against 37 °C/pH 7.0 on day 8 quantified by Q-PCR. The mRNA value of the HC and LC was normalized to β-actin
Relationship between prolonging cell longevity and increasing specific hMab productivity
To clarify the relationship between prolonging cell longevity and increasing specific hMab productivity, we used rapamycin to control the cell cycle progression without shifting the temperature and compared the effect of the rapamycin addition to that of shifting the temperature. Shake flasks (working volume: 40 mL) were used as culture vessels. The initial temperature of the culture was maintained at 37 °C. Before a stationary growth phase, either the culture temperature was lowered to 31 °C or 100 ng mL−1 rapamycin was added to the culture. The pH of the culture was kept not lower than 6.8 by the addition of 0.5 M NaOH. The culture profiles for the two conditions are shown in Table 1. In the cultures under the cell growth controlling conditions, the cell growth rate decreased, and the day to achieve the maximum VCD delayed 2 days that of the 37 °C culture. But the cell viabilities at 11 days of cultivation were 2.4 and 2.9 times as high as that of the culture that remained at 37 °C. The cell viabilities of the cell growth controlling conditions remained high during culturing. The final hMab concentrations in the TS culture and rapamycin-added cultures increased to 1.6 and 1.3 times, respectively, that of the 37 °C culture. The rapamycin treatment condition was effective in prolonging the cell viability and increasing hMab yields, but there was a difference of the average specific hMab productivity between these two cell growth controlling conditions. The average specific hMab productivity after the TS was 2.3 μg 105 cell−1 day−1, which is clearly higher than 1.7 μg 105 cell−1 day−1 for the culture at 37 °C. In contrast, the average specific hMab productivity of culture with the cell growth controlled by rapamycin was only 1.6 μg 105 cell−1 day−1, a result which shows no significant difference compared to the 37 °C culture. This result suggests that the control of cell growth is effective for cell longevity but does not correlate with increase of specific hMab productivity. We also confirmed these results by Northern blot analysis of the HC and LC expressions under the addition of rapamycin (Fig. 3). There were no differences between the HC and LC mRNA levels either without (Lane 1) or with (Lane 2) the addition of rapamycin.
Table 1.
The culture profiles of samples where cell growth was controlled either by temperature shifts or rapamycin
| Culture condition | 37 °C | Temperature shifta | Rapamycin additionb |
|---|---|---|---|
| Maximum VCD (105 cells mL−1) | 19 | 18 | 18 |
| Day to achieve maximum VCD (day) | 6 | 8 | 8 |
| Viability at day 11 (%) | 29 | 70 | 85 |
| Final hMab concentration (μg mL−1) | 216 | 352 | 279 |
| IVCD (105 cells mL−1 day−1) | 122 | 147 | 158 |
| Average specific hMab productivityb (μg 105 cells−1 day−1) | 1.7 | 2.3 | 1.6 |
a Before the stationary growth phase, the cell proliferation was controlled by a temperature shift down to 31 °C or by a rapamycin addition
b The average specific hMab productivity was calculated by the slope of the hMab concentration versus IVCD. Results are mean of 3 experiments
Fig. 3.
Northern blot of HC and LC mRNA levels in the culture with or without rapamycin. After 4 days of cultivation, 100 ng mL−1 rapamycin was added to the culture. RNAs were sampled 7 days after inoculation. Lane 1 is the culture without rapamycin, Lane 2 is the culture with rapamycin
mRNA stability analysis in the TS culture
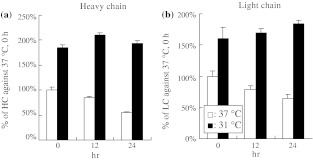
To determine whether the mechanism of the mRNA changes is caused by an increase in its rate of synthesis or by a decrease in its degradation rate, we studied mRNA stability using actinomycin D in the TS culture. After 3 days cultivation at 37 °C, the cells were maintained at 37 °C or reduced to 31 °C for 24 h. These cells were then chased for 0, 12 or 24 h in the presence of 5.0 μg mL−1 of actinomycin D using shake flasks. The pH of the culture was kept not lower than 6.8 by the addition of 0.5 M NaOH. At the end of each chase period, the cells were collected and hMab transcripts were quantified by Q-PCR. In the culture remaining at 37 °C, the mRNA of the HC decreased after 24 h incubation with actinomycin D to 55% at 0 h (Fig. 4a), while that of the LC decreased to 65% at 0 h (Fig. 4b). In contrast, actinomycin D did not affect the mRNA level in the TS culture after 24 h incubation. The result suggests that the increase of the mRNA level in the TS condition was caused by an increase in mRNA stability.
Fig. 4.
A TS affects the stability of hMab mRNA. The cells maintained 24 h at 37 °C or at 31 °C after 3 days cultivation at 37 °C were chased for 0, 12 or 24 h in the presence of 5.0 μg mL−1 of actinomycin D. At the end of each chase period, the cells were counted and hMab transcripts were quantified by Q-PCR. (a) Percentage of HC against 37 °C at 0 h, (b) Percentage of LC against 37 °C at 0 h. Error bars indicate SD (n = 3)
Discussion
A temperature shift cultivation is an effective way to improve the production yield in a mammalian cell culture. Some groups have said that the low temperature effects on specific productivity appear to be cell-line dependent (Ducommun et al. 2002; Yoon et al. 2003). We examined the combination of shifting the temperature and shifting the timing using shake flasks to determine the most effective condition for production yield in our cell line. The shift in temperature and in timing significantly influenced the cell proliferation rate and antibody productivity (data not shown). The most effective condition for our cell line was a culture temperature shift from 37 °C to 31 °C before a stationary growth phase (3–4 days after inoculation). The suppressive effect on cell growth by a TS was minimized, but the cell viability remained high. The final hMab concentration increased to 3 times that of the culture remaining at 37 °C. The pH of the culture was kept not lower than 6.8 by the addition of 0.5 M NaOH. We selected this condition for our cell line and attempted to optimize other culture conditions for scaling up.
To establish a process for commercial production, it is very important to clarify the relationships among culture conditions. We examined the effect of a TS on the pH condition using 2-L bioreactors (Fig. 1). In the TS/pH 6.8 condition, cell viability was maintained at a high level, and the final hMab concentration increased to 2.3 times that of 37 °C culture. Interestingly, there was no effect on cell viability or production yield in 37 °C/pH 6.8 or TS/pH 7.0, which suggests that there is a positive effect of the interaction between the TS and pH conditions. On the other hand, the average specific hMab productivity of TS/pH 7.0 was the same as that of TS/pH 6.8 and increased up to 2.2 times that of the culture at 37 °C. This result indicates that the TS condition affects both the longevity of cell viability and specific hMab productivity but that the pH in the TS condition influences only the longevity of cell viability. It suggests that there is an unknown effect that increases the specific hMab productivity in the TS condition.
Northern blot and Q-PCR analyses were performed to determine whether antibody upregulation upon the exposure of the cells to 31 °C occurred at the mRNA level (Fig. 2). The mRNA levels of the antibody in the TS cultures increased to 1.9–2.9 times that in the culture remaining at 37 °C in the same culture period regardless of these pH levels. This result also indicates that the only effect of the pH is on maintaining cell viability and that the pH does not affect the mRNA level.
Many investigators have demonstrated that rapamycin produces cell cycle arrest, preventing the progression of cells division from the G1 to the S phase in the cell cycle (Hung et al. 1996). The control of cell growth by the addition of rapamycin was effective in prolonging cell viability for CRL 1606 hybridoma (Balcarcel and Stephanopoulos 2001). To clarify the relationship between prolonging cell longevity and increasing specific hMab productivity, we selected rapamycin to control the cell cycle progression without shifting the temperature and compared the effect of the rapamycin addition with that of the temperature shift. Controlling cell proliferation by a TS or rapamycin were both effective in the maintenance of cell viability (Table 1), but there was no significant increase in the average specific hMab productivity in the culture with rapamycin. We also confirmed these results by Northern blot analysis of the HC and LC expressions under the addition of rapamycin (Fig. 3). There were no differences between the HC and LC mRNA levels either with or without rapamycin. These data suggest that only controlling cell proliferation does not induce an increase in specific hMab productivity. In other words, there remains an unknown factor that influences an increase in specific hMab productivity.
Actinomycin D is an inhibitor of RNA synthesis by binding to DNA at dG residues. To determine whether the mechanism of mRNA changes is caused by an increase in its synthesis rate or a decrease in its degradation rate, we studied mRNA stability using actinomycin D in a TS culture. The reagent decreased the mRNA level in the culture remaining at 37 °C, but this decrease did not occur in 24 h in the TS condition (Fig. 4). The increase of the mRNA level in the TS culture may be caused by the residual effect of the shifting temperature. This result indicates that the increase in the mRNA level in the TS condition is caused by an increase in mRNA stability. Only the TS/pH 6.8 condition may induce the expression of CIRP (cold inducible RNA-binding protein) working as an RNA chaperone (Nishiyama et al. 1997). Actinomycin D induces apoptosis caused by the inhibition of RNA synthesis, and the cell viability of the 37 °C culture decreased to 40%. Despite the same treatment, the cell viability did not decrease in the TS condition. Actinomycin D retains the activity at 31 °C according to the report of the actinomycin D treatment at ambient temperature (Paul and Brune 2002). The result also indicates that the mRNA level was maintained in the TS culture.
In this study, we show that a TS can produce two independent effects: the prolongation of cell longevity caused by controlling cell growth and the improvement in mRNA stability. The prolongation of cell longevity is affected by the pH condition, but this has no influence on mRNA stability. Only the exposure to a low pH and low temperature induces longevity and an improved mRNA stability. We also suggest that an unknown effect exists that increases specific hMab productivity in the TS condition. To improve the production yield, it is important that we get a better understanding that will allow control of these effects in a TS culture. It is very interesting to note that a slight pH difference induces an antiapoptotic condition, although the explanation for this is not clear at this time. We believe that this study is useful for not only improving the process performance but also for being able to scale up bioreactors and increase the robustness of the process.
Glossary
- CHO cell
Chinese hamster ovary cell
- HC
Heavy chain
- hMab
Human monoclonal antibody
- IVCD
Integral of viable cell density
- LC
Light chain
- TS
Temperature shift
- TS/pH 6.8
Temperature shift cultivation, pH 6.8
- TS/pH 7.0
Temperature shift cultivation, pH 7.0
- VCD
Viable cell density
- 37 °C/pH 6.8
Culture at 37 °C, pH 6.8
- 37 °C/pH 7.0
Culture at 37 °C, pH 7.0
References
- Balcarcel RR, Stephanopoulos G. Rapamycin reduces hybridoma cell death and enhances monoclonal antibody production. Biotechnol Bioeng. 2001;76:1–10. doi: 10.1002/bit.1020. [DOI] [PubMed] [Google Scholar]
- Bloemkolk J, Gray MR, Merchant F, Mosmann TR. Effect of temperature on hybridoma cell cycle and Mab production. Biotechnol Bioeng. 1992;40:427–431. doi: 10.1002/bit.260400312. [DOI] [PubMed] [Google Scholar]
- Ducommun P, Ruffieux P, Kadouri A, Stockar U, Marison I. Monitoring of temperature effects on animal cell metabolism in a packed bed process. Biotechnol Bioeng. 2002;77:838–842. doi: 10.1002/bit.10185. [DOI] [PubMed] [Google Scholar]
- Fox SR, Patel UA, Yap MGS, Wang DC. Maximizing interferon-γ production by chinese hamster ovary cells through temperature shift optimization: Experimental and modeling. Biotechnol Bioeng. 2004;85:177–184. doi: 10.1002/bit.10861. [DOI] [PubMed] [Google Scholar]
- Giard DJ, Fleischaker RJ, Fabricant M. Effect of temperature on the production of human fibroblast interferon. Proc Soc Exp Biol Med. 1982;170:155–159. doi: 10.3181/00379727-170-41411. [DOI] [PubMed] [Google Scholar]
- Hendrick H, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J. Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology. 2001;36:71–83. doi: 10.1023/A:1014088919546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DT, Jamison TF, Schreiber SL. Understanding and controlling the cell cycle with natural products. Chem Biol. 1996;3:623–639. doi: 10.1016/S1074-5521(96)90129-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Mazur X, Fussenegger M, Bailey JE. Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng. 1999;63:573–582. doi: 10.1002/(SICI)1097-0290(19990605)63:5<573::AID-BIT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Mazur X, Marone R, Bailey JE. Comparative analysis of two controlled proliferation strategies regarding product quality, influence on tetracycline-regulated gene expression, and productivity. Biotechnol Bioeng. 2001;72:592–602. doi: 10.1002/1097-0290(20010320)72:6<592::AID-BIT1024>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899–908. doi: 10.1083/jcb.137.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Brune K. Stabilization of gene expression profiles in blood after phlebotomy. Clin Chem. 2002;48:2251–2253. [PubMed] [Google Scholar]
- Ryu JS, Kim TK, Chung JY, Lee GM. Osmoprotective effect of glycine betaine on foreign protein production in hyperosmotic recombinant chinese hamster ovary cell cultures differs among cell lines. Biotechnol Bioeng. 2000;70:167–175. doi: 10.1002/1097-0290(20001020)70:2<167::AID-BIT6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Reff ME (1997) Impaired dominant selectable marker sequence and intronic insertion strategies for enhancement of expression of gene product and expression vector systems comprising same. US Patent 5.648.267
- Reuveny S, Kim YJ, Kemp CW, Shiloach J. Effect of temperature and oxygen on cell growth and recombinant protein production in insect cell cultures. Applied Microbiol Biotechnol. 1993;38:619–623. doi: 10.1007/BF00182800. [DOI] [PubMed] [Google Scholar]
- Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L. A high-yielding, generic-fed batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67:585–597. doi: 10.1002/(SICI)1097-0290(20000305)67:5<585::AID-BIT9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Schatz SM, Kerschbaumer RJ, Gerstenbauer G, Kral M, Dorner F, Scheiflinger F. Higher expression of Fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng. 2003;84:433–438. doi: 10.1002/bit.10793. [DOI] [PubMed] [Google Scholar]
- Sureshkumar GK, Mutharasan R. The influence of temperature on a mouse–mouse hybridoma growth and monoclonal antibody production. Biotechnol Bioeng. 1991;37:292–295. doi: 10.1002/bit.260370313. [DOI] [PubMed] [Google Scholar]
- Varley J, Birch J. Reactor design for large scale suspension animal cell culture. Cytotechnology. 1999;29:177–205. doi: 10.1023/A:1008008021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Shuttleworth J, Al-Rubeai M. Regulation of cell cycle and productivity in NS0 cells by the over-expression of p21CIP1. Biotechnol Bioeng. 2002;77:1–7. doi: 10.1002/bit.10112. [DOI] [PubMed] [Google Scholar]
- Weidemann R, Ludwig A, Kretzmer G. Low temperature cultivation – A step towards process optimisation. Cytotechnology. 1994;15:111–116. doi: 10.1007/BF00762385. [DOI] [PubMed] [Google Scholar]
- Yoon SK, Song JY, Lee GM. Effect of low temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in chinese hamster ovary cells. Biotechnol Bioeng. 2003;82:289–298. doi: 10.1002/bit.10566. [DOI] [PubMed] [Google Scholar]