Abstract
The activation of caspases represents a crucial turning point during a batch or a fed-batch culture of mammalian cells. It not only affects the quantity but also the quality of the recombinant glycoprotein produced. In this study, the activation of various caspases, the release of intracellular sialidase and the changes in sialylation pattern of a recombinant product, erythropoietin (EPO), in the culture medium were analyzed in both batch and fed-batch cultures. In both setups, all caspase activities peaked at the culture time point at which decline of cell viability was most pronounced. In addition, the release of intracellular lactate dehydrogenase (LDH) was also tracked during these cultures. The increase in LDH activity in the medium coincided with the increase of intracellular caspase activities, the release of sialidase and the observed decline in cell viability, suggesting that the LDH activity in the medium can be used as an indirect indicator of apoptotic cell death in bioreactors. Isoelectric focusing (IEF) coupled with double blotting was employed to analyze the changes in sialylation pattern of the recombinant EPO. This assay resulted in a prompt resolution of secreted EPO isoforms in a time course format. IEF profile of batch culture showed relatively consistent product sialylation compared to fed-batch culture, which showed gradual band shifts towards the isoforms with fewer sialic acid as the culture progressed. These data provided a guideline for the optimal time point to terminate the culture and collect products in batch and fed-batch cultures.
Keywords: Apoptosis, Caspase activation, CHO cells, Erythropoietin, Isoelectric focusing (IEF), Lactate dehydrogenase (LDH), Sialidase
Introduction
Characterization of mammalian cell culture processes is essential for the economical production of therapeutic recombinant glycoproteins. The in vivo activity of many recombinant glycoproteins is highly dependent upon their glycan structures. Terminal sialic acid residues at the N-linked glycans influence a glycoprotein’s circulatory half-life significantly as it prevents the recognition by the asialo-glycoprotein receptor on the surface liver cells, which are chiefly responsible for systemic clearance of glycoproteins in the blood (Higuchi et al. 1992; Misaizu et al. 1995; Spivak and Hogans 1989; Takeuchi et al. 1989).
Desialylation of recombinant glycoproteins in cell culture media is largely attributed to the release of intracellular sialidase by dead cells (Gramer and Goochee 1993; Gramer et al. 1995). Cell death in bioreactors can be triggered by various factors. These factors include inordinate shear stress, nutrient depletion, waste accumulation, hypoxia and high osmolality (Al-Rubeai and Singh 1998; Chen et al. 2001; Ryu and Lee 1999). Under the influence of these factors, cell death in bioreactors can occur in two different forms, namely cell necrosis and apoptosis. Necrosis involves the physical rupturing of cells and is often caused by excessive mechanical agitation or aeration in bioreactors. Apoptosis, on the other hand, is a gene-regulated physiological response and is morphologically characterized by cell shrinkage, membrane blebbing, chromatin condensation and DNA fragmentation. To date, apoptosis is regarded as the principal form of cell death in cell culture (Goswami et al. 1999); though the exact pathway involved remains unclear. Breakdown of apoptotic bodies results in the release of intracellular contents, such as sialidase, into the medium which can be harmful to the recombinant product.
Apoptosis is characterized by the activation of a family of cysteine–aspartate proteases, known as caspases (Degterev et al. 2003; Hengartner 2000). In viable cells, caspases are expressed as inactive zymogens. Under the action of various apoptosis inducing signals, inactive caspase precursors are mechanistically activated. A unique hallmark of caspases is that they specifically cleave after an aspartic acid in the target protein. As different caspases prefer different amino acid sequences at the cleavage site, synthetic substrates have been generated for the analysis of various human caspases (Thornberry et al. 1997).
Apoptosis occurs through the mitochondrial pathway or the death receptor pathway (Jiang and Wang 2004; Ashkenazi and Dixit 1998). At the apex of these two apoptosis pathways are the initiator caspases, caspase-8 for the death receptor pathway and caspase-9 for the mitochondrial pathway. Active caspase-8 or caspase-9 in turn activates downstream effector caspases, such as caspase-3, caspase-6 and caspase-7, which lead to the execution of apoptosis.
Traditional methods employed in the analysis of protein glycosylation require large amount of purified product. In addition, it is a time-consuming process. As glycosylation microheterogeneity is known to occur during the course of cell culture (Floch et al. 2004; Harmon et al. 1996), alternative methods need to be developed for the routine monitoring of glycan structures of recombinant glycoproteins. Isoelectric focusing (IEF) has been successfully employed for the detection of recombinant human erythropoietin (EPO) isoforms in urine samples of athletes (Lasne and de Ceaurriz 2000; Lasne et al. 2002). Separation of EPO glycoforms is based on pI differences due to different sialylation profiles exhibited by recombinant and endogenous EPO. In contrast to traditional glycan analysis approaches, IEF requires a much smaller sample size and it eliminates the need for complicated protein purification and sample preparation. It does not compromise the resolution as IEF can clearly distinguish one isoform from another that differs by only one sialic acid residue.
Though IEF was previously employed in the analysis of EPO glycoforms in batch culture (Yoon et al. 2003, 2004), the published method required reverse phase HPLC purified EPO samples of up to 10 μg. In this paper, we proposed the use of isoelectric focusing, in conjunction with double blotting (Lasne 2001, 2003), as a simplified method to analyze the sialylation profile of secreted EPO produced by a recombinant CHO cell line in both batch and fed-batch cultures, without the need for any form of preliminary sample purification. Due to the sensitivity and specificity of double blotting, sample requirement was reduced by 50-fold to only 200 ng.
In addition to sialidase, many other cellular components could be released by the dead cells. One such component is lactate dehydrogenase (LDH). As LDH can be easily monitored we used LDH as an indicator of cell death during a batch and a fed-batch culture. When both the productivity and the quality (sialylation pattern) of the recombinant EPO in the medium were considered, our data provided a guideline as when to stop the bioreactor and collect the products.
Materials and methods
Cell line and culture conditions
CHO-K1 EPO HT # 1 was a single clone recombinant CHO cell line that stably expressed recombinant human erythropoietin with a six His tag attached to the C-terminal. It was engineered by transfecting a CHO-K1 (ATCC CRL-9618) cell line with a pcDNA 3.1(+) (Invitrogen) construct, containing human erythropoietin cDNA tagged with six histidine codons at the 3′ end. Stable transfectants were selected using G418 (Gibco) at 1 mg ml−1 and single clones were isolated and maintained in DMEM (Gibco) supplemented with 10% (v/v) FBS (Gibco).
CHO-K1 EPO HT # 1 was adapted to serum free suspension culture and maintained in HyQ PF-CHO (Hyclone), supplemented with 2 g l−1 sodium bicarbonate (Sigma) and 0.1% (v/v) Pluronic F-68 (Gibco). Glucose and glutamine concentrations were 17 and 4 mM, respectively. Suspension culture was agitated via an orbital shaker (Ika) set at 110 rpm and kept in a 37°C humidified incubator with 8% (v/v) CO2.
Bioreactor setup and set point controls
Two-liter doubled-walled, round-bottom glass vessels with heated water jackets (B. Braun Biotech) were employed in the bioreactor runs. Exponentially growing cells from a 2nd day seed culture were used to inoculate both the batch and the fed-batch bioreactors at a seeding density of 3.0 × 105 cells ml−1. The initial working volume of each bioreactor was 1.5 l. Process control set points were controlled using a digital control unit (DCU, B. Braun Biotech). Each bioreactor was configured with a standard 60 mm diameter, 45° pitch blade impeller and agitation rate was set at 110 rpm. Aeration was achieved through the headspace and silicone membrane tubing basket (B. Braun Biotech). Dissolved oxygen (DO) concentration was monitored using a polarographic electrode (Mettler Toledo) and maintained at 50% air saturation at 1 atm using an air/N2 mix or O2/air mix set at 1 slpm. Culture pH was controlled at 7.1, using intermittent CO2 gas sparging (maximum 0.2 slpm) or 7.5% (w/v) NaHCO3 solution. Culture temperature was controlled at 37°C via the integrated water jacket. HyQ PF-CHO was employed as the initial basal medium for both batch and fed-batch cultures.
Feeding strategy for the fed-batch operation
Two separate feed media were formulated for controlling glucose and glutamine concentrations. The glutamine feed medium was prepared from a proprietary blend comprising of 10 × salt-free, glucose-free, and glutamine-free DMEM/F12 (Hyclone), supplemented with 1 × HyQ PF-CHO. Glutamine concentration was 200 mM (Sigma). The glucose feed medium was a 1 M glucose solution (Sigma).
Predictive feed forward control was employed in the fed-batch setup. Discrete control of glutamine and glucose were achieved by feeding the projected amounts of glutamine and glucose that would be consumed by the cells over the forecast intervals. This was estimated by assuming that the specific growth rate, specific glucose and glutamine consumption rates during the forecast interval would be the same as that in the current sampling interval (Sauer et al. 2000). Glucose and glutamine concentration set points were 0.5 and 0.15 g l−1, respectively. Periodically, about 10 ml of culture were sampled from each reactor twice a day at 9 AM and 5 PM. Culture supernatants and cell pellets, after centrifugation at 2,000 rpm (Beckman), were aliquoted for various analyses and kept at − 20°C.
Cell density and cell viability determination
Viable cell density and cell viability of culture samples were measured using Cedex (Innovatis), based on trypan blue staining. Standard deviation obtained in each reading (based on 20 slide images) was used in the computation of cell count error.
EPO quantification
EPO concentration of supernatant samples was determined using an enzyme-linked immuno-sorbent assay (ELISA) kit (Roche). Appropriate serial dilutions were performed on samples prior to ELISA analysis. ELISA assay was duplicated for each sample to ensure reproducibility.
Extracellular sialidase activity analysis
Extracellular sialidase activity was determined by fluorescence emission according to a modified method (Gramer and Goochee 1993), using 4-methylumbelliferyl-α-d-N-acetylneuraminic acid (4MU-NANA, Sigma) as substrate. Eleven μl of 4-MU-NANA stock solution (3.3 mM 4-MU-NANA in 0.9 M KH2PO4 pH 7.2) was added to 89 μl of supernatant sample and the reaction mixture was incubated at 37°C. The fluorescence generated was measured at intervals of 2 min for 2 h using a microplate reader (Tecan GENios) at an excitation wavelength of 365 nm and emission wavelength of 450 nm. Extracellular sialidase activity of each sample was calculated based on the rate of increase in fluorescence. Samples were run in duplicate to ensure reproducibility.
Lactate dehydrogenase (LDH) activity analysis
LDH activity in the culture media was analyzed using a Cytotoxic Detection Kit (Roche) according to the supplied manufacturer’s protocol. Medium samples from batch culture were used as undiluted while samples from fed-batch cultures were diluted 10 × with PBS. Samples were run in duplicate to ensure reproducibility.
Intracellular caspase activities assay
Harvested CHO cell pellets were lysed with chilled lysis buffer (5 mM DTT, 10 mM HEPES pH 7.5, 2 mM EDTA, 0.1% CHAPS) on ice for 10 min and cell lysates were collected via centrifugation at 13,000 rpm for 5 min (Eppendorf). Protein concentration of these cell lysates was assayed using Coomassie Plus (Pierce) against known BSA protein standards (Pierce) by determining absorbance at 595 nm. Samples containing equal loading of total protein were incubated with their respective assay buffers and para-nitroaniline (pNA) labeled caspase substrates in excess at 37°C in 96 well plates. Release of free pNA chromophores from cleaved substrates by caspases were monitored by tracking changes in absorbance at wavelength of 405 nm, using a microplate reader (Tecan) over a period of 2.5 h in 15 min intervals. Caspase activity of each sample was subsequently calculated from the rate of increase in free pNA. Caspase substrates used were DEVD-pNA (for caspase-3), IETD-pNA (for caspase-8), LEHD-pNA (for caspase-9), VDVAD-pNA (for caspase-2), WEHD-pNA (for caspase-1, −4 and −5) and VEID-pNA (for caspase-6). Buffers and substrates were used as instructed in Chemicon’s colorimetric assay kits (APT163, APT167, APT169, APT173, APT165 and APT171) for the respective caspases.
Western blotting assay for caspase-3 activation
Harvested CHO cell pellets were lysed and protein concentration of these cell lysates was assayed using methods described in the previous section. Cell lysates containing 50 μg of protein were loaded for each sample, resolved via 12% polyacrylamide SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membrane (Bio-rad). Rabbit polyclonal antibody which recognizes the full length human caspase-3 and the large fragment of caspase-3 resulting from cleavage (Cell Signaling) was used as primary antibody, while horseradish-peroxidase (HRP) conjugated goat anti-rabbit IgG (H + L) antibody (Jackson ImmunoResearch) was employed as secondary antibody. Detection was carried out using ECL detection reagent (Amersham Biosciences) and exposed using chemiluminescent detection film (Roche).
Isoelectric focusing (IEF)
Supernatant samples were concentrated via Microcon® (Millipore) or diluted to 1000 IU EPO/L with dilution buffer (1% BSA, 50 mM Tris–HCl pH 7.4) prior to isoelectric focusing in a 1.3 mm thick, 5% T, 3% C polyacrylamide gel (Bio-rad) containing 7 M urea (Fluka), 1.2% (w/v) 2–4, 1.2% (w/v) 4–6, 1.2% (w/v) 6–8, 0.4 % (w/v) 2–11 ampholytes (Serva) and 5% (w/v) sucrose (Sigma). After prefocusing at 250 V and 10°C for 30 min (using 0.5 M NaOH as catholyte and 0.5 M H3PO4 as anolyte), 20 μl of diluted/concentrated samples were applied onto rectangular pieces of filter paper, placed 0.5 cm from the cathode end of the gel. Electrophoresis was conducted on the Multiphor II Electrophoresis system (Amersham-Pharmacia) at 1 W per cm of gel length and stopped at 3,600 Vh. EPO isoforms were specifically revealed by a double-blotting method using monoclonal anti-human EPO AE7A5 (R&D Systems) as primary antibody and biotinylated goat anti-mouse IgG (H + L) (Pierce) as secondary antibody, according to previously described methods (Lasne 2001, 2003). Chemiluminescent images of these IEF blots were detected with a charge coupled device (CCD) camera (Fuji), using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce).
Luminescence intensity analysis and sialylation quality evaluation
Luminescence intensity spectrum corresponding to the isoelectric profile of each sample was obtained using “AIDA 1D-Evaluation” software from Fuji. Quantitative sialylation quality of each sample was evaluated based on both the distribution of luminescence intensity and the degree of sialylation associated with the respective isoforms.
Results
Growth profiles of batch and fed-batch cultures
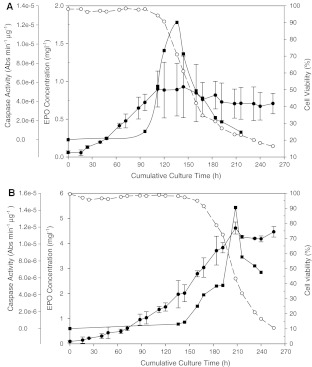
Viable cell density profiles of both batch and fed-batch cultures of CHO-K1 EPO HT #1 exhibited a trend as shown in Fig. 1A, B, with characteristic logarithmic, stationary and death phases. A maximum viable cell density of 5.07 × 106 cell ml−1 was observed at a culture time of 96 h for batch culture. Maximum viable cell density of fed-batch culture reached 8.56 × 106 cell ml−1 at a culture time of 136 h. Cumulative integral viable cell density showed an approximate 2-fold increase in fed-batch culture over batch culture. Decline in culture viabilities was observed at 120 and 168 h for batch and fed-batch cultures, respectively.
Fig. 1.
Cell viability and caspase activation during batch and fed-batch cultures. (A) Viable cell density, cell viability and intracellular caspase-2, −3, −5, −6, −8, −9 like activity profiles of batch culture. (•) Viable cell density; (○) cell viability; (
 ) caspase-2 like activity; (■) caspase-3 like activity; (
) caspase-2 like activity; (■) caspase-3 like activity; (
 ) caspase-5 like activity; (▴) caspase-6 like activity; ( × ) caspase-8 like activity; (▵) caspase-9 like activity. (B) Viable cell density, cell viability and intracellular caspase-2, −3, −5, −6, −8, −9 like activity profiles of fed-batch culture. (C) Western blot analysis of caspase-3 activation during the course of a batch culture. (D) Western blot analysis of caspase-3 activation during the course of a fed-batch culture
) caspase-5 like activity; (▴) caspase-6 like activity; ( × ) caspase-8 like activity; (▵) caspase-9 like activity. (B) Viable cell density, cell viability and intracellular caspase-2, −3, −5, −6, −8, −9 like activity profiles of fed-batch culture. (C) Western blot analysis of caspase-3 activation during the course of a batch culture. (D) Western blot analysis of caspase-3 activation during the course of a fed-batch culture
Intracellular caspase activity profiles of batch and fed-batch cultures
Intracellular caspase activity profiles during the entire course of batch and fed-batch cultures, in a time course format, were previously unreported. The intracellular caspase activities at various time points, in both batch and fed-batch cultures, were analyzed using Chemicon’s caspase assay kits, containing six different substrates designed for various human caspases. As shown in Fig. 1A, intracellular caspase-2, caspase-3, caspase-6, caspase-8 and caspase-9 like activity peaks were observed at a culture time point of 136 h and cell viability of 71% in batch culture. In fed-batch culture, caspase-2, caspase-3, caspase-6, caspase-8 and caspase-9 like activity peaks were located at a culture time point of 208 h and corresponding cell viability of 43% (Fig. 1B).
Caspase-3 Western blots of batch and fed-batch cultures
Activation of inactive zymogene of caspases is often used as a benchmark to signify the occurrence of apoptotic death. Among the 14 identified caspases, cleavage of effector caspase-3 is often employed by investigators to verify the activation of the caspase signaling cascade (Li et al. 2001; Tse and Rabbitts 2000). Western blot was performed to track the cleavage of caspase-3 in a time course format for batch and fed-batch cultures. As shown in Fig. 1C, D, inactive caspase-3 zymogene (35 kDa) was cleaved into smaller fragments (30 and 19 kDa) during the later stages of culture in both batch and fed-batch setups, which further accentuated that apoptosis was indeed activated in batch and fed-batch cultures. The 19 kDa fragment is the large subunit of active capsase-3. In batch culture, cleavage of capase-3 was first observed at 112 h of culture, which corresponded to a culture viability of 94.2%. Similarly, in fed-batch culture, cleavage of capase-3 was first observed at 136 h of culture, which corresponded to a culture viability of 98.1%. Maximum amounts of cleaved caspase-3 subunits were detected at 136 and 208 h in batch and fed-batch cultures, respectively, which concurred with the occurrence of the caspase-3 activity peaks in the caspase activity assay. This antibody was known to recognize human, mouse and rat caspase-3, as shown here, it also recognize Chinese hamster caspase-3. Since the antibody only recognizes the full length and the large fragment (p20 subunit) of caspase-3, the small subunit of caspase-3 was not detected.
Extracellular sialidase activity profiles of batch and fed-batch cultures
The release of sialidase from dead cells into culture medium and the concomitant desialylation of recombinant product is a major problem caused by apoptosis during cell culture processes. The sialidase activities of batch and fed-batch culture media at various time points are shown in Fig. 2A, B. The sialidase activities of both batch and fed-batch cultures, during the initial 120 h of culture were found to be consistently low, which implied that sialidase release was not a secretory event. Peak sialidase activities of both batch and fed-batch cultures, occurred at a sample time point subsequent of the caspase-3 activity peak. Coupled with the fact that the initial increase in sialidase activities for both batch and fed-batch cultures (see arrows in Fig. 2A, B) were accompanied by a marked increase in caspase activities; these observations suggest that extracellular sialidase activities, in both batch and fed-batch cultures, were due to intracellular sialidase release, which in turn was an event downstream of apoptosis. The sialidase activity in the fed-batch culture medium was much higher than that in the batch culture medium could be due to the higher cell density in the fed-batch culture.
Fig. 2.
Release of cellular sialidase and LDH in batch and fed-batch cultures. (A) Cell viability, extracellular sialidase activity and LDH activity profiles of the same batch culture shown in Fig. 1A. (○) Cell viability; (▴) LDH activity; (■) sialidase activity. (B) Cell viability, extracellular sialidase activity and LDH activity profiles of the same fed-batch culture shown in Fig. 1B
LDH activity profiles of batch and fed-batch cultures
The release of LDH has been used as a generic indicator of cell death (Wagner et al. 1992). LDH activity in the culture medium of both batch and fed-batch cultures were tested and the results are shown in Fig. 2A, B. The initial increase in LDH activities in both batch and fed-batch medium coincided with the increase in sialidase activity in the media and the increase in caspase activities. The onset of cell death occurred at the same time point for both methods (indicated by arrows in Fig. 2A, B). Intuitively, LDH release could also indicate that significant amount of other intra-cellular enzymes, such as proteases and glycosidases, were also indiscriminately released into the medium during the death phase of culture.
Volumetric EPO yield in batch and fed-batch cultures
In batch culture, a peak EPO concentration of 0.9 mg l−1 was obtained at a culture time point of 112 h, which was 32 h before the observed caspase-3 peak (Fig. 3A). The reason for this premature halt in EPO production could be due to nutrient depletion, which was inevitable in batch culture. In fed-batch culture, a peak EPO concentration of 4.61 mg l−1 was established at a culture time point of 208 h. This was the same time point at which caspase activities peaked (Fig. 3B). In fed-batch culture, volumetric EPO yield continued to escalate even at rather low cell viabilities (70→ 45%) as shown in Fig. 3B. This could be due to the availability of nutrients in fed-batch culture, which provided an impetus for the remaining viable cells to actively secrete EPO. Once these cells are committed towards apoptosis (signified by the observed caspase-3 peak), these cells would cease to produce the recombinant product.
Fig. 3.
Productivity of EPO in batch and fed-batch cultures. (A) Volumetric EPO yield, intracellular caspase-3 like activity and cell viability profiles of the same batch culture shown in Fig. 1A. (•) EPO concentration; (■) caspase-3 like activity; (○) cell viability. (B) Volumetric EPO yield, intracellular caspase-3 like activity and cell viability profiles of the same fed-batch culture shown in Fig. 1B. (•) EPO concentration; (■) caspase-3 like activity; (○) cell viability
IEF profiles of EPO in batch and fed-batch cultures
The sialylation quality of secreted EPO at various culture time points in both batch and fed-batch cultures was analyzed by IEF. Sialylation quality was evaluated based on the sialylation pattern of secreted EPO resolved through IEF. As shown in Fig. 4A, a highly consistent EPO sialylation profile was observed throughout the entire batch culture. The most sialylated EPO product, the band I, which was the nearest to the anode, was not detected after a culture period of 184 h. In the fed-batch culture, gradual band shifts could be observed from Fig. 4B as the culture progressed. The most sialylated EPO product, the band I, was not detected after 144 h, while bands, II and III, which were progressively less sialylated, were not detected in the medium after 192 and 216 h, respectively.
Fig. 4.
Changes in sialylation pattern of EPO in batch and fed-batch cultures analyzed with IEF. (A) IEF profile of secreted EPO in the batch culture. (B) IEF profile of secreted EPO in the fed-batch culture. (C) Band intensity spectrum of a batch culture sample obtained using “AIDA 1D-Evaluation” software from Fuji
In batch culture, sialidase activity peaked at a culture time point of 184 h, concurring with the loss of the most sialylated band I in the IEF profile (Figs. 2A, 4A). The peak sialidase activity of fed-batch culture occurred at a culture time of 216 h and corresponded with the loss of band III in the IEF profile (Figs. 2B, 4B). Loss of bands I, II and III in fed-batch culture and loss of band I in batch culture occurred at normalized sialidase activities of 0.29, 1.12, 2.03 and 0.64 FU min−1 μl−1 of media, respectively. Significant loss of hypersialylated species in fed-batch culture during the death phase, as compared to batch culture, could be attributed to a larger total cell density, which directly correlated to larger amount of sialidase released, leading to more extensive desialylation.
A band intensity spectrum corresponding to an early batch culture sample is shown in Fig. 4C. Peaks to the right correspond to the more sialylated EPO isoforms, while percentages displayed on top of peaks represent the relative distribution of each EPO isoform in the resolved culture sample. A quantitative analysis of these band intensity spectrums is shown in Fig. 5. These data represent the relative total sialic acid on the EPO molecule at different time points. In contrast to batch culture, sialylation quality of EPO in fed-batch culture deteriorated even more during the late logarithmic to stationary phase of culture. It should be pointed out that the derived numerical index shown in Fig. 5 is not an absolute quantification of EPO sialic acid content but a relative comparison between product sialylation of EPO at various time points of both batch and fed-batch cultures.
Fig. 5.
Sialylation quality profile of EPO in batch and fed-batch cultures and its association with cell viability. (▵) Sialylation quality score of the batch culture; (
 ) sialylation quality score of the fed-batch culture; (▴) cell viability of the batch culture; (■) cell viability of the fed-batch culture. The numbers shown here is not an absolute quantification of the sialic acid content of EPO but a relative comparison of sialylation between EPO products at various time points of both batch and fed-batch cultures
) sialylation quality score of the fed-batch culture; (▴) cell viability of the batch culture; (■) cell viability of the fed-batch culture. The numbers shown here is not an absolute quantification of the sialic acid content of EPO but a relative comparison of sialylation between EPO products at various time points of both batch and fed-batch cultures
Discussion
Apoptosis is broadly regarded as the principal mechanism responsible for death in cell cultures (Goswami et al. 1999; Vives et al. 2003). Our results further confirmed this proposition. In both batch and fed-batch cultures, all caspase activities peaked at the same time point at which cell viability declined most abruptly. In spite of the fact that caspase-8 and caspase-9 are initiator caspases, both enzymes peaked at the same time point as effector caspase-3 and caspase-6. This could be attributed to the large interval between consecutive samplings (8 or 16 h), which limited the sensitivity of this assay (since enzymatic reactions involving initiator and effector caspase activation are likely to be rather spontaneous). When analyzed with these synthetic short peptides as substrates, peak activity of caspase-3 was the highest, followed by caspase-2, caspase-6, caspase-9 and caspase-8.
Caspase-1, −4 and −5 activities were absent in both batch and fed-batch cultures since the substrate (WEHD-pNA) for these three caspases was not cleaved. This observation was not surprising. Caspase-1 was reported to be involved specifically in inflammation rather than apoptosis (Zeuner et al. 1999), and thus unlikely to be activated under batch and fed-batch culture conditions. Caspase-4 and −5 were not found in the mouse genome (Reed et al. 2003) and have not been reported in rats either. It is very likely that caspase-4 and −5 would be absent in CHO cells, since Chinese hamsters are closely related to rats and mice.
The apoptotic pathways involved in batch and fed-batch cultures could be different, since the fundamental aim of fed-batch culture is to overcome nutrient limitation in batch culture. However, results from the caspase activity assay seem to imply that both caspase-8 and caspase-9 were activated in batch and fed-batch cultures, suggesting that both the mitochondrial and death receptor pathways might be involved. However, before any conclusion can be reached, there is a need to demonstrate the actual activation of the respective caspases in batch or fed-batch cultures, using alternative methods such as immuno-blotting. Although a particular substrate may be cleaved most efficiently by a specific caspase, many other caspases can also contribute to the cleavage of that substrate, possibly with a lower efficiency. For example, the cleavage of “caspase-8 substrate” shown in Fig. 1A, B should be treated as a combined effect of several caspases, i.e. caspase-8 itself and possibly several other caspases. According to Thornberry et al. (1997), these caspase substrates were derived combinatorially, based on specificity for recombinant human caspases; but even then, these sequences were demonstrated not to be highly specific in their caspase recognition abilities (Talanian et al. 1997).
Intracellular sialidase release has been reported to be the chief determinant for the deterioration in sialylation profiles of recombinant cell cultures during the death phase (Gramer and Goochee 1993; Gramer et al. 1995). The loss of the three most sialylated EPO bands and the appearance of several less sialylated bands during the death phase of fed-batch culture (Fig. 4B) closely correlated with the increase in extracellular sialidase activity, suggesting sialic acid residues on the EPO molecules could possibly be liberated by sialidase action. However, extent of sialylation of EPO in fed-batch culture was observed to be inferior to that of batch culture (Fig. 5), even during the late logarithmic phase of culture. This observation was inferred from the disappearance of the most sialylated band I, in fed-batch culture after only 144 h. Since sialidase activity detected in the medium during this time frame was found to be low (∼0.29 FU min−1 μl−1), this phenomenon is unlikely to be caused by sialidase release.
After a careful analysis of the data shown in Figs. 2B, 5, we realized that the sialylation of EPO in the fed-batch culture started to drop even when the sialidase activity in the medium was still relatively low. A possible explanation for this observation is that the sialylation of recombinant EPO was affected by other factors. Presence of ammonium in culture medium has been reported to reduce both the extent of sialylation and antennarity of glycan structures (Yang and Butler 2000a, 2000b, 2002). In these experiments, the ammonium concentration was rather high in the fed-batch culture during the late logarithmic to stationary phase as compared to that of the batch culture (data not shown). Influx of less sialylated EPO variants secreted into the medium during this time frame is likely to adulterate and/or inundate hypersialylated EPO produced during the early logarithmic phase when ammonium concentration was low. This explanation would also justify the relatively “basic” sialylation profile that was detected by IEF during late logarithmic to stationary phase of culture, in addition to the action of the sialidase in the medium.
Lactate dehydrogenase (LDH) assay is a cell death assay based on measurement of LDH activity in the culture medium. LDH is a cytosolic enzyme but is rapidly released into the medium upon loss of membrane integrity. This assay was originally used to measure cell death via necrosis (Koh and Choi 1987). Lately it has been successfully used to measure apoptotic cell death as well (Lobner 2000; Koh et al. 1995; Koh and Cotman 1992). We clearly demonstrated that in both batch and fed-batch cultures the increase of LDH in the medium coincided with the activation of all the caspases inside the cell. Therefore, the release of LDH into the medium is very likely due to apoptotic death, rather than necrotic death. Thus, the increase of LDH activity in the culture medium can be used as an indicator for apoptotic cell death in the bioreactors.
In conclusion, we have systematically analyzed the activation of various caspases and the release of sialidase and LDH into the medium during batch and fed-batch cultures. All the caspase activities peaked at the time points when the viability dropped most dramatically. At the same time, sialidase and LDH, possibly other cellular contents as well, were released into the medium. As a result, the recombinant product, EPO in this case, gradually lost its sialic acid. IEF can be employed to monitor this change throughout the entire course of the culture. The key parameters, such as product yield and extent of product sialylation, can be used to optimize the harvest time point. Since LDH in the medium can be easily monitored it can serve as an indirect marker for apoptotic cell death in bioreactors.
Footnotes
Kok Hwee Chuan and Sing Fee Lim contributed equally to this work.
References
- Al-Rubeai M, Singh RP. Apoptosis in cell culture. Curr Opin Biotechnol. 1998;9:152–156. doi: 10.1016/S0958-1669(98)80108-0. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Chen KQ, Liu Q, Xie LZ, Sharp PA, Wang DIC. Engineering of a mammalian cell line for reduction of lactate formation and high monoclonal antibody production. Biotechnol Bioeng. 2001;72:55–61. doi: 10.1002/1097-0290(20010105)72:1<55::AID-BIT8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Floch FL, Tessier B, Chenuet S, Guillaume J-M, Cans P, Marc A, Goergen J-L. HPCE monitoring of the N-glycosylation pattern and sialylation of murine erythropoietin produced by CHO cells in batch processes. Biotechnol Prog. 2004;20:864–871. doi: 10.1021/bp0343211. [DOI] [PubMed] [Google Scholar]
- Goswami J, Sinskey AJ, Steller H, Stephanopoulos GN, Wang DIC. Apoptosis in batch cultures of Chinese Hamster Ovary cells. Biotechnol Bioeng. 1999;62:632–640. doi: 10.1002/(SICI)1097-0290(19990320)62:6<632::AID-BIT2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gramer MJ, Goochee CF. Glycosidase activities in Chinese Hamster Ovary cell lysate and cell culture supernatant. Biotechnol Prog. 1993;9:366–373. doi: 10.1021/bp00022a003. [DOI] [PubMed] [Google Scholar]
- Gramer MJ, Goochee CF, Chock VY, Brousseau DT, Sliwkowski MB. Removal of sialic acid from a glycoprotein in CHO cell culture supernatant by action of an extracellular CHO cell sialidase. Biotechnology (N Y) 1995;13:692–698. doi: 10.1038/nbt0795-692. [DOI] [PubMed] [Google Scholar]
- Harmon BJ, Gu XJ, Wang DIC. Rapid monitoring of site-specific glycosylation microheterogeneity of recombinant human interferon-γ. Anal Chem. 1996;68:1465–1473. doi: 10.1021/ac951229d. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Oh-eda M, Kuboniwa H, Tomonoh K, Shimonaka Y, Ochi N. Role of sugar chains in the expression of the biological activity of human erythropoietin. J Biol Chem. 1992;267:7703–7709. [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Meth. 1987;20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Koh JY, Cotman CW. Programmed cell death:its possible contribution to neurotoxicity mediated by calcium channel antagonists. Brain Res. 1992;587:233–240. doi: 10.1016/0006-8993(92)91002-V. [DOI] [PubMed] [Google Scholar]
- Koh JY, Gwag BJ, Lobner D, Choi DW. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- Lasne F. Double-blotting: a solution to the problem of non-specific binding of secondary antibodies in immunoblotting procedures. J Immunol Meth. 2001;253:125–131. doi: 10.1016/S0022-1759(01)00355-6. [DOI] [PubMed] [Google Scholar]
- Lasne F. Double-blotting: a solution to the problem of nonspecific binding of secondary antibodies in immunoblotting procedures. J Immunol Meth. 2003;276:223–226. doi: 10.1016/S0022-1759(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Lasne F, Ceaurriz J. Recombinant erythropoietin in urine. Nature. 2000;405:635. doi: 10.1038/35015164. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, Crepin N, Ceaurriz J. Detection of isoelectric profiles of erythropoietin in urine:differentiation of natural and administered recombinant hormones. Anal Biochem. 2002;311:119–126. doi: 10.1016/S0003-2697(02)00407-4. [DOI] [PubMed] [Google Scholar]
- Li M, Shillinglaw W, Henzel WJ, Beg AA. The RelA (p65) subunit of NF-kB is essential for inhibiting double-stranded RNA-induced cytotoxicity. J Biol Chem. 2001;276:1185–1194. doi: 10.1074/jbc.M006647200. [DOI] [PubMed] [Google Scholar]
- Lobner D. Comparison of the LDH and MTT assays for quantifying cell death:validity for neuronal apoptosis? J Neurosci Meth. 2000;96:147–152. doi: 10.1016/S0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- Misaizu T, Matsuki S, Strickland TW, Takeuchi M, Kobata A, Takasaki S. Role of antennary structure of N-linked sugar chains in renal handling of recombinant human erythropoietin. Blood. 1995;86:4097–4104. [PubMed] [Google Scholar]
- Reed JC, Doctor K, Rojas A, Zapata JM, Stehlik C, Fiorentino L, Damiano J, Roth W, Matsuzawa S-I, Newman R et al (2003) Comparative analysis of apoptosis and inflammation genes of mice and humans. Genome Res 13:1376–1388 [DOI] [PMC free article] [PubMed]
- Ryu JS, Lee GM. Application of hypoosmolar medium to fed-batch culture of hybridoma cells for improvement of culture longevity. Biotechnol Bioeng. 1999;62:120–123. doi: 10.1002/(SICI)1097-0290(19990105)62:1<120::AID-BIT14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu LM. A high-yielding generic fed-batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng. 2000;67:585–597. doi: 10.1002/(SICI)1097-0290(20000305)67:5<585::AID-BIT9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Spivak JL, Hogans BB. The in vivo metabolism of recombinant human erythropoietin in the rat. Blood. 1989;73:90–99. [PubMed] [Google Scholar]
- Takeuchi M, Inoue N, Strickland TW, Kubota M, Wada M, Shimizu R, Hoshi S, Kozutsumi H, Takasaki S, Kobata A. Relationship between sugar chain structure and biological activity of recombinant human erythropoietin produced in Chinese Hamster Ovary cells. Proc Natl Acad Sci USA. 1989;86:7819–7822. doi: 10.1073/pnas.86.20.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP et al (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem 272:17907–17911 [DOI] [PubMed]
- Tse E, Rabbitts TH. Intracellular antibody-caspase-mediated cell killing: an approach for application in cancer therapy. Proc Natl Acad Sci USA. 2000;97:12266–12271. doi: 10.1073/pnas.97.22.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives J, Juanola S, Cairo JJ, Godia F. Metabolic engineering of apoptosis in cultured animal cells:implications for the biotechnology industry. Metab Eng. 2003;5:124–132. doi: 10.1016/S1096-7176(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Wagner A, Marc A, Engasser JM, Einsele A. The use of lactate dehydrogenase (LDH) release kinetics for the evaluation of death and growth of mammalian cells in perfusion reactors. Biotechnol Bioeng. 1992;39:320–326. doi: 10.1002/bit.260390310. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Effects of ammonia on CHO cell growth, erythropoietin production, and glycosylation. Biotechnol Bioeng. 2000a;68:370–380. doi: 10.1002/(SICI)1097-0290(20000520)68:4<370::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Effects of ammonia on the glycosylation of human recombinant erythropoietin in culture. Biotechnol Prog. 2000b;16:751–759. doi: 10.1021/bp000090b. [DOI] [PubMed] [Google Scholar]
- Yang M, Butler M. Effects of ammonia and glucosamine on the heterogeneity of erythropoietin glycoforms. Biotechnol Prog. 2002;18:129–138. doi: 10.1021/bp0101334. [DOI] [PubMed] [Google Scholar]
- Yoon SK, Song JY, Lee GM. Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Bioeng. 2003;82:289–298. doi: 10.1002/bit.10566. [DOI] [PubMed] [Google Scholar]
- Yoon SK, Choi LS, Song JY, Lee GM. Effect of culture pH on erythropoietin production by Chinese Hamster Ovary cells grown in suspension at 32.5 and 37.0 degree Celsius. Biotechnol Bioeng. 2004;89:345–356. doi: 10.1002/bit.20353. [DOI] [PubMed] [Google Scholar]
- Zeuner A, Eramo A, Peschie C, De Maria R. Caspase activation without death. Cell Death Differ. 1999;6:1075–1080. doi: 10.1038/sj.cdd.4400596. [DOI] [PubMed] [Google Scholar]