Abstract
Objective
Leukocytes are activated in women with preeclampsia, but the class of leukocytes that infiltrates the maternal vasculature and, therefore, most likely to cause vascular dysfunction is not known.
Methods
Subcutaneous fat biopsies were obtained at cesarean section or abdominal surgery from 7 normal nonpregnant women, 7 normal pregnant women, and 7 preeclamptic women. Tissues were immunohistochemically stained for CD14, a monocyte/macrophage antigen, CD99, a lymphocyte antigen, and CD66b, a neutrophil antigen.
Results
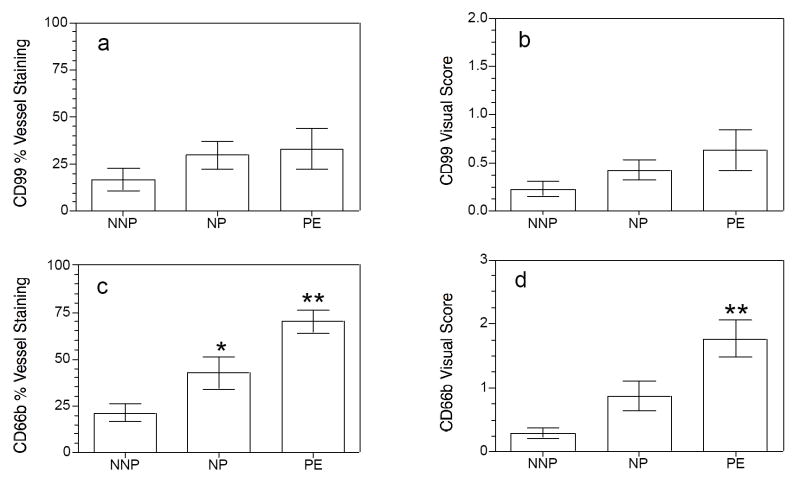
CD14 stained cells were found infiltrated into fat tissue but were not present in vessels for any of the groups. CD99 stained cells were present in approximately 20-30% of vessels with no difference among groups. CD66b stained cells were present in all groups with a significantly greater percentage of vessels stained for preeclamptic than normal pregnant or normal nonpregnant women (70±6 vs. 43±9 vs. 21±5%, respectively, P<0.01). CD66b cells were the most abundant cell type that infiltrated vessels of preeclamptic women.
Conclusions
1) There was significantly more neutrophils adhered to endothelium and infiltrated into the intimal space in the maternal systemic vasculature of preeclamptic women than in normal pregnant women or normal nonpregnant women; 2) There was no significant difference in lymphocyte infiltration among the patient groups and lymphocytes were present in much lower numbers than neutrophils; 3) Monocytes/macrophages were found in fat tissue but not in vessels. We speculate that neutrophils are the class of leukocytes that cause the majority of vascular cell dysfunction in preeclamptic women.
Keywords: Preeclampsia, pregnancy, neutrophils, lymphocytes, macrophages
INTRODUCTION
Maternal circulating leukocytes are activated in pregnancy and further activated in preeclampsia 1-5. The major leukocyte classes, neutrophils, lymphocytes and monocytes, are all activated. Leukocyte activation likely occurs as they circulate through the intervillous space and are exposed to oxidized lipids secreted by the placenta 6-10. As these activated leukocytes then reenter the maternal systemic circulation, they could be involved in causing vascular dysfunction associated with preeclampsia.
Macrophages are widely studied for their role as foam cells in atherosclerotic plaques, Lymphocytes are part of the adaptive immune system involved in the production of antibodies to combat disease. Neutrophils are usually thought of as part of the innate immune system and the first line of defense against infection at the site of a wound, but recently, we found that neutrophils infiltrate systemic vascular tissue in women with preeclampsia and that this is associated with vascular inflammation 11, 12. The other leukocyte classes might also infiltrate the maternal vasculature in preeclampsia and be involved in causing vascular dysfunction.
In the present study, we obtained subcutaneous fat biopsies from normal nonpregnant women, normal pregnant women and preeclamptic women to compare the extent of vascular infiltration of the major classes of leukocytes. We immunostained the tissues using antibodies directed against markers of monocytes/macrophages, lymphocytes and neutrophils. We hypothesized that neutrophils would be the major class of leukocyte infiltrating the vasculature because they are the most numerous class of leukocyte and their numbers increase during pregnancy.
MATERIALS AND METHODS
Study Subjects
Subcutaneous fat biopsies were collected from patients at MCV Hospitals, Virginia Commonwealth University Medical Center. Subcutaneous fat biopsies were used because subcutaneous fat is a highly vascularized tissue representative of the systemic vasculature. Fat biopsies (approximately 1 cm × 1 cm × 1 cm) were collected at the time of cesarean section from normal pregnant patients (NP, n = 7) and preeclamptic patients (PE, n = 7) or at the time of abdominal surgery from normal nonpregnant patients (NNP, n = 7). Preeclampsia was defined as sustained blood pressure of ≥ 140/90 mmHg with readings at least 6 hours apart and proteinuria (300mg/24 hr or ≥1+ urine dipstick). Patient demographic data are presented in Table 1. For the preeclamptic patients, 6 had severe preeclampsia, 6 delivered preterm and 1 had IUGR. Fat biopsies were placed immediately in 10% neutral buffered formalin. Cesarean sections for normal pregnant women were performed because of previous cesarean section or secondary to latent herpes simplex virus or fetal malposition. Surgeries for normal nonpregnant women were performed for removal of fibroids or for tissue biopsies. Exclusion criteria were chorioamnionitis, maternal infection, active STDs, diabetes, cardiovascular disease and smoking. All patients were matched for body mass index (BMI) and were not in labor. Informed consent was obtained prior to surgery. The Office of Research Subjects Protection of Virginia Commonwealth University approved this study.
TABLE 1.
Patient Demographic Information
| NNP (n = 7) | NP (n = 7) | PE (n = 7) | |
|---|---|---|---|
| Age, years | 37.9±2.8*** | 22.6±0.7 | 24.6±1.7 |
| Pre-pregnancy BMI | 25.8±0.9 | 29.7±2.6 | 29.0±3.1 |
| Systolic BP, mmHg | 125±6 | 114±5 | 173±5*** |
| Diastolic BP, mmHg | 77±6 | 67±3 | 106±3*** |
| Proteinuria (mg/24 hrs) | NA□ | ND□ | 670±176 |
| Dipstick | NA | ND | 3.0 (2-4) |
| Parity | NA | 1.0 (1.0-3.0) | 0.0 (0.0-3.0) |
| Gestational Age, weeks | NA | 39.5±0.3 | 32.9±1.1*** |
| Infant Birth Weight, gms | NA | 3256±142 | 1804±297** |
P<0.01
P<0.001 as compared to other groups
NA – not applicable, ND – not determined
Data are presented as mean ± SE, except Dipstick and Parity are presented as median and range.
Immunohistochemistry
Tissue was paraffin embedded and cut in 10 μm sections. Vessels were stained for CD 14, a monocyte/macrophage antigen, CD 99, a lymphocyte antigen, and CD66b, a granulocyte antigen. CD66b is upregulated and secreted upon granulocyte activation. CD66b staining primarily represents neutrophils because they comprise 96% of the granulocyte population. On the day of staining, tissue sections were incubated in 3% H2O2 in methanol for thirty minutes to quench endogenous tissue peroxidase. Antigen retrieval was performed using a low-set pressure cooker incubation in 10 mM citrate buffer for 5 minutes. CD14 slides were additionally incubated for ten minutes in a 0.125% trypsin solution to enhance antigen retrieval. Tissues were stained with the following antibodies: 1) mouse monoclonal IgG anti-human CD14 (titer 1:50, Zymed/Invitrogen, South San Francisco, CA), 2) mouse IgG anti-human CD99 (titer 1:400, Serotec, Oxford, UK), 3) mouse monoclonal IgM anti-human CD66b (1:50, BD BioSciences, San Diego, CA). Negative controls for CD14 and CD99 were stained with a mouse monoclonal isotype IgG antibody (pre-diluted, Zymed/Invitrogen) and negative controls for CD66b were stained with a mouse IgM monoclonal isotype control (pre-diluted, BD BioSciences, San Diego, CA). Monocytes, lymphocytes and neutrophils isolated from whole blood and separated by Histopaque 1077/1119 density centrifugation served as positive controls for CD14, CD99 and CD66b. Immunohistochemical staining was performed at room temperature using a Zymed SuperPicture Kit (Zymed Laboratories, San Francisco, CA), according to the manufacture’s instructions. Slides were processed by hand and tissue was counterstained with Hematoxylin. DAB reagent resulted in brown staining of antigens.
Data Analysis
Vessels between 10 μm and 200 μm were analyzed. An average of 26 vessels were analyzed for each patient. Lumen width was measured for each vessel. Vessel staining for CD14, CD99 and CD66b was graded using a visual score ranging from zero to four (absent to intense staining) by an investigator blinded to the patient group. Objectivity of the visual score was verified by optical density measurements of staining using image analysis software (IP Lab, Scanalytics, Inc., Fairfax, VA). Tissues were also evaluated for percent of vessels with staining.
Statistical Analysis
Visual score data across patient groups for CD99 and CD66b were analyzed by Kruskal-Wallis test with Dunn’s post hoc test to determine differences between patient groups. For optical density measurements, one-way ANOVA was used with Newman-Keuls post-hoc test. Spearman r regression analysis was performed to correlate visual scores with optical density measurements. Percent of vessels with staining were analyzed by one-way ANOVA with Newman-Keuls post-hoc test. When data were analyzed within each patient group for CD antigens, Mann-Whitney or unpaired t-test were used because there was no vessel staining for CD14. Bar graph data are reported as means ± SE. A statistical software application was used (Prism, GraphPad Software, Inc., San Diego, CA).
RESULTS
CD14 stained cells were found infiltrated into fat tissue but were not present in vessels for any of the groups. CD99 stained cells were present in approximately 20-30% of vessels with no difference among groups (33 ± 11% for PE vs. 30 ± 7% for NP vs. 17 ± 6% for NNP, P>0.05, Fig. 1). There was no difference for visual score of staining intensity for CD99 among groups (0.6 ± 0.2 for PE vs. 0.4 ± 0.1 for NP vs. 0.2 ± 0.1 for NNP, P>0.05). CD66b stained cells were present in all groups with a significantly greater percentage of vessels stained for preeclamptic women than normal pregnant or normal nonpregnant women (70 ± 6% vs. 43 ± 9% vs. 21 ± 5%, respectively, P<0.01, Fig. 1). Visual score of staining intensity for CD66b was also significantly higher for preeclamptic women than for normal pregnant or normal nonpregnant women (1.8 ± 0.3 for PE vs. 0.9 ± 0.2 for NP vs. 0.3 ± 0.1 for NNP, P<0.01). The percentage of vessels with staining for CD66b was significantly higher than CD99 only for preeclamptic women (70 ± 6% vs. 33 ± 11%, P<0.05). The percentage of vessels with CD66b staining was not significantly higher than CD99 staining for normal pregnant or normal nonpregnant women (43 ± 9% vs. 30 ± 7% for NP and 21 ± 5% vs. 17 ± 6% for NNP, P>0.05). The number of leukocytes per stained vessel was calculated. The number of CD66b stained cells per vessel was significantly greater than CD99 stained cells per vessel only for the preeclamptic group (3.5 ± 0.9 vs. 1.1 ± 0.3 cells/vessel, respectively, P<0.05). There were no significant differences between the number of CD66b or CD99 stained cells per vessel for the normal pregnant group (1.8 ± 0.6 vs. 1.1 ± 0.3 cells/vessel, respectively, P>0.05) or the normal nonpregnant group (0.7 ± 0.2 vs. 0.6 ± 0.2 cells/vessel, respectively, P>0.05).
Figure 1.
Leukocyte infiltration into vessels of subcutaneous fat in normal nonpregnant (NNP), normal pregnant (NP) and preeclamptic (PE) women. Lymphocytes indicated by CD99 staining infiltrated approximately 20 – 30% of subcutaneous fat vessels with no significant differences among groups (panel a). Visual score of vessel infiltration was also not different among groups for CD99 (panel b). In contrast, neutrophil infiltration indicated by CD66b staining showed significant differences among groups. Preeclamptic women had a significantly higher percentage of vessels stained for CD66b than normal pregnant women which had a significantly higher percentage than normal nonpregnant women (panel c). The visual score for CD66b vessel staining was significantly higher for preeclamptic women than for normal pregnant or normal nonpregnant women. Monocyte/macrophages indicated by CD14 staining were observed in the fat tissue, but not in the vessels. Data represent mean ± SE. * P < 0.05, ** P < 0.01
Representative examples of the immunohistochemistry results are shown in Figure 2. CD14 stained macrophages were present in adipocytes (panels b, c), but were not observed in vessels. CD99 staining of lymphocytes was present in vessels and in adipocytes (arrows, panels d-f), but there were few CD99 stained cells per vessel and there was no difference in the amount of staining among the groups (panel d, NNP; panel e, NP; panel f, PE). In contrast to CD14 and CD99 staining, CD66b staining of neutrophils was significantly different among the groups (panels g-i). Panel g shows a vessel from a NNP patient with no stained cells. Panel h shows a NP patient with a few brown stained cells in the lumen of two vessels. Most often when there was staining in NP patients, the CD66b stained cells were in the lumen rather than adhered onto endothelium and infiltrated into the vessel. In contrast, vessels of PE patients showed neutrophils fattened onto the endothelium and infiltrated into the intimal space (panel i).
Figure 2.
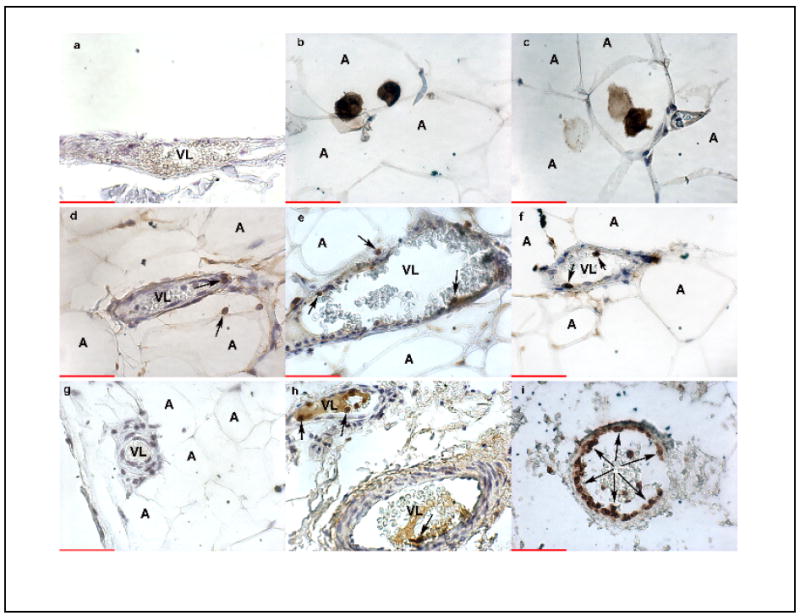
Representative examples of leukocyte infiltration into vessels of subcutaneous fat in NNP, NP and PE women. Panel a, IgG negative control in a PE patient; panel b, CD 14 staining of macrophages in adipocytes of a NP patient; panel c, CD 14 staining in a PE patient; panel d, CD99 staining of lymphocytes in NNP patient; CD99 staining in a NP patient; CD99 staining in a PE patient; panel g, CD66b staining of neutrophils in a NNP patient; panel h, CD66b staining in a NP patient; panel i, CD66b staining in a PE patient. CD14 staining of monocytes/macrophages was not present in vessels, but was present in the adipocytes. CD99 staining of lymphocytes was present in vessels and in adipocytes (arrows, panels d-f), but there were few CD99 stained cells per vessel and there was no difference in the amount of staining among the groups. Only CD66b staining of neutrophils showed a significant difference with PE vessels showing significantly more staining than NP or NNP vessels. Panel i shows the lumen of a PE vessel almost completely lined with neutrophils adhered to endothelium (arrows). In contrast, when neutrophils were present in NP vessels, there were few neutrophils and they were usually present in the lumen and not adhered to endothelium (arrows, panel h). Panel g shows no CD66b staining in a NNP vessel. Brown color indicates specific staining of antigens. VL, vessel lumen; A, adipocyte. All images are 400X. Scale bar indicates 50 microns.
DISCUSSION
Although all classes of leukocytes are activated in the maternal circulation in preeclampsia, only neutrophils were found to significantly infiltrate the systemic vascular. Seventy % of vessels in preeclamptic women showed staining for neutrophils as compared to only 33% of vessels for lymphocytes, and for those vessels with leukocytes present, there were three times more neutrophils per vessel than lymphocytes. Lymphocytes were present in the vessels of all groups, but preeclamptic women did not show an increase as compared to normal pregnant or normal nonpregnant women. Monocytes were found infiltrated into fat tissue, but they were not present in vessels.
These findings indicate that neutrophils are the class of leukocyte most likely to cause vascular dysfunction in preeclamptic women. We recently reported that the systemic vascular of preeclamptic women was in an inflamed state and that this inflammation was associated with extensive endothelial adhesion and infiltration of neutrophils 11, 12. We observed many markers of inflammation present in the vasculature of preeclamptic women. Endothelial cells and vascular smooth muscle cells showed activation of nuclear factor-κB (NF-κB) and increased expression of gene products regulated by NF-κB, such as cyclooxygenase-2 (COX-2), intercellular adhesion molecule-1, and interleukin-8. Neutrophils that were present in the vessel lumen and neutrophils that were flatten and adhered onto the endothelium also showed activation of NF-κB and increased expression of COX-2.
Neutrophils release toxic substances, such as reactive oxygen species (ROS), tumor necrosis factor-alpha (TNFα), thromboxane, myeloperoxidase, and matrix metalloproteinase-8 (MMP8), all of which have the ability to cause vascular dysfunction. For example, ROS and myeloperoxidase could cause oxidative stress which has been observed in the systemic vasculature of preeclamptic women by the presence of nitrotyrosine staining which is a marker for the pro-oxidant, peroxynitrite 13. Peroxynitrite is formed when superoxide interacts with nitric oxide, so neutrophil release of superoxide could be a source of superoxide for the formation of peroxynitrite present in the vasculature of preeclamptic women. TNFα is a well recognized inflammatory cytokine. Thromboxane is a potent vasoconstrictor and so could contribute to hypertension. Myeloperoxidase inactivates nitric oxide 14, which is a potent vasodilator, so neutrophil release of myeloperoxidase could also contribute to hypertension by diminishing vascular levels of nitric oxide. MMP8 is a collagenase and its release by neutrophils onto the vasculature could cause a loss of cellular integrity resulting in edema.
Although macrophages are widely studied in cardiovascular disease, neutrophils are the most likely class of leukocyte to be involved in vascular inflammation because they are part of the innate immune system. They not only cause inflammation, but they also respond to inflammation. Neutrophils bind to inflamed endothelium and extravasate into the tissues where they release their toxic compounds 15. Usually this is at the site of a wound where the neutrophil products kill bacteria, but increasing evidence implicates neutrophil infiltration in systemic inflammatory disorders, such as rheumatoid arthritis 16.
A number of investigators have described an increase in soluble endothelial adhesion molecules in maternal blood of preeclamptic women 17-19, and increased expression of ICAM-1 is present in the vasculature of preeclamptic women 11. Increased expression of endothelial adhesion molecules, such as ICAM-1, would play a key role in neutrophilendothelial adhesion and subsequent neutrophil infiltration. Once infiltrated into the intimal space, the life-span of the neutrophil would increase because exposure to vascular smooth muscle colony-stimulating factors decreases apoptosis 20. The number of neutrophils increases 2.5-fold by 30 weeks of gestation in normal pregnancy 21 and the number increases further in preeclampsia 22. Neutrophil numbers likely increase in pregnancy as a result of increased levels of colony-stimulating factors 23, 24.
In preeclampsia, neutrophil activation likely occurs as they circulate through the intervillous space and are exposed to oxidized lipids secreted by the placenta 6-10. Oxidized lipids are potent activators of neutrophils and cause their expression of COX-2 which regulates their release of thromboxane, TNFα and superoxide 25, 26. Neutrophils obtained from preeclamptic women express significantly more COX-2 than neutrophils obtained from normal pregnant women or normal nonpregnant women 27.
In conclusion, there was significantly more neutrophil endothelial adhesion and infiltration into the intimal space in the systemic vasculature in preeclamptic women than in normal pregnant women or normal nonpregnant women. There was no significant difference in lymphocyte infiltration among the patient groups and lymphocytes were present in much lower numbers than neutrophils in the vasculature of preeclamptic women. Monocytes/macrophages were found in fat tissue but not in vessels. This study indicates that neutrophils are the class of leukocyte most likely to cause vascular cell dysfunction in preeclamptic women.
Acknowledgments
Supported by a grant to SWW from the National Institutes of Health (HL069851)
Footnotes
Presented at the 53rd Annual Meeting of the Society for Gynecologic Investigation, March 22-25, 2006, Toronto, CANADA
This is an electronic version of an article published in Hypertension in Pregnancy, 27: 396-405, 2008. Hypertension in Pregnancy is available online at informaworld™, http://www.informaworld.com/smpp/content~content=a905424225~db=all?jumptype=ale rt&alerttype=author,email
References
- 1.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 2.Greer IA, Haddad NG, Dawes J, Johnstone FD, Calder AA. Neutrophil activation in pregnancy-induced hypertension. Br J Obstet Gynaecol. 1989;96:978–982. doi: 10.1111/j.1471-0528.1989.tb03358.x. [DOI] [PubMed] [Google Scholar]
- 3.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 4.Tsukimori K, Maeda H, Ishida K, Nagata H, Koyanagi T, Nakano H. The superoxide generation of neutrophils in normal and preeclamptic pregnancies. Obstet Gynecol. 1993;81:536–540. [PubMed] [Google Scholar]
- 5.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 6.Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregn. 1994;13:1–32. [Google Scholar]
- 7.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SW, Vaughan JE, Wang Y, Roberts LJ., II Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–96. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 9.Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. Am J Obstet Gynecol. 1993;169:1462–6. doi: 10.1016/0002-9378(93)90419-j. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SW, Wang Y, Jesse R. Placental production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. Hypertens Pregn. 1996;15:101–111. [Google Scholar]
- 11.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–7. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 12.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48.e1–48.e8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33:83–9. doi: 10.1161/01.hyp.33.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–4. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 15.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. New York: W.H. Freeman; 2000. [Google Scholar]
- 16.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 17.Krauss T, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol. 1997;177:443–449. doi: 10.1016/s0002-9378(97)70213-8. [DOI] [PubMed] [Google Scholar]
- 18.Austgulen R, Lien E, Vince G, Redman CW. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 1997;71:53–8. doi: 10.1016/s0301-2115(96)02647-4. [DOI] [PubMed] [Google Scholar]
- 19.Abe E, Matsubara K, Ochi H, Ito M, Oka K, Kameda K. Elevated levels of adhesion molecules derived from leukocytes and endothelial cells in patients with pregnancy-induced hypertension. Hypertens Pregnancy. 2003;22:31–43. doi: 10.1081/PRG-120016793. [DOI] [PubMed] [Google Scholar]
- 20.Stanford SJ, Pepper JR, Burke-Gaffney A, Mitchell JA. Cytokine-activated human vascular smooth muscle delays apoptosis of neutrophils: relevance of interactions between cyclo-oxygenase-2 and colony-stimulating factors. FASEB J. 2001;15:1813–5. doi: 10.1096/fj.00-0670fje. [DOI] [PubMed] [Google Scholar]
- 21.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, et al. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77:1032–7. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- 22.Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45:229–31. doi: 10.1159/000009973. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara K, Ochi H, Kitagawa H, Yamanaka K, Kusanagi Y, Ito M. Concentrations of serum granulocyte-colony-stimulating factor in normal pregnancy and preeclampsia. Hypertens Pregnancy. 1999;18:95–106. doi: 10.3109/10641959909009614. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Ohkura T, Inaba N. Elevation of serum macrophage colony-stimulating factor before the clinical manifestations of preeclampsia. Am J Obstet Gynecol. 2003;189:1356–60. doi: 10.1067/s0002-9378(03)00674-4. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan JE, Walsh SW. Neutrophils from pregnant women produce thromboxane and tumor necrosis factor-alpha in response to linoleic acid and oxidative stress. Am J Obstet Gynecol. 2005;193:830–5. doi: 10.1016/j.ajog.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan JE, Walsh SW, Ford GD. Thromboxane mediates neutrophil superoxide production in pregnancy. Am J Obstet Gynecol. 2006;195:1415–20. doi: 10.1016/j.ajog.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 27.Bachawaty T, Washington S, Walsh SW. Neutrophils of preeclamptic women express cyclooxygenase-2. J Soc Gynecol Invest. 2006;13(Suppl):227A. [Google Scholar]