Abstract
Although oxytocin (OT) and oxytocin receptor (OTR) are known for roles in parturition and milk let-down, they are not hypothalamus-restricted. OT is important in nurturing and opposition to stress. Transcripts encoding OT and OTR have been reported in adult human gut, and OT affects intestinal motility. We tested the hypotheses that OT is endogenous to the enteric nervous system (ENS) and that OTR signaling may participate in enteric neurophysiology. Reverse transcriptase polymerase chain reaction confirmed OT and OTR transcripts in adult mouse and rat gut and in precursors of enteric neurons immunoselected from fetal rats. Enteric OT and OTR expression continued through adulthood but was developmentally regulated, peaking at postnatal day 7. Coincidence of the immunoreactivities of OTR and the neural marker Hu was 100% in the P3 and 71% in the adult myenteric plexus, when submucosal neurons were also OTR-immunoreactive. Co-localization with NeuN established that intrinsic primary afferent neurons are OTR-expressing. Because OTR transcripts and protein were detected in the nodose ganglia, OT signaling might also affect extrinsic primary afferent neurons. Although OT immunoreactivity was found only in ~1% of myenteric neurons, extensive OT-immunoreactive varicosities surrounded many others. Villus enterocytes were OTR-immunoreactive through postnatal day 17; however, by postnatal day 19, immunoreactivity waned to become restricted to crypts and concentrated at crypt-villus junctions. Immunoelectron microscopy revealed plasmalemmal OTR at enterocyte adherens junctions. We suggest that OT and OTR signaling might be important in ENS development and function and might play roles in visceral sensory perception and neural modulation of epithelial biology.
Indexing terms: nodose, crypt villus junction, irritable bowel syndrome, maternal deprivation, autism spectrum disorder
Oxytocin (OT) is a nine-amino acid peptide that is best known for its ability to stimulate labor and milk ejection in humans (Sala et al., 1974), rats (Wakerley et al., 1973), cows (Graf, 1969, 1970), and other mammals. OT, however, clearly has additional functions. OT and its receptor (OTR) have been found in many brain regions (Fenelon et al., 1993; Yoshimura et al., 1996) as well as in a number of peripheral organs including the testis (Gerendai and Csernus, 1995), nonpregnant uterus and oviduct (Ayad et al., 1991), thymus (Geenen et al., 1987; Argiolas et al., 1990; Melis et al., 1993), pancreas (Amico et al., 1988; Jeng et al., 1996), heart (Cicutti et al., 1999; Gutkowska et al., 2000), kidney (Jasper et al., 1995; Kiss and Mikkelsen, 2005), and breast (Sapino et al., 1998; Cassoni et al., 2006). Much recent interest has focused on the behavioral effects of OT (Welch et al., 2005; Welch and Ruggiero, 2005). For example, OT release by nurturing stimuli has been proposed to mediate a psycho-physiological pattern of activities that are associated with relaxation and opposition to stress (Uvnas-Moberg and Petersson, 2005).
OT also plays a role in social motivation (Nelson and Panksepp, 1998) and affects areas of the brain that are responsible for social recognition (Ferguson et al., 2001). OT, furthermore, has been implicated in early environmental conditioning of stress adaptation patterns (Carter, 1998), and it selectively decreases the maternal separation response of rat pups, an effect that mimics social contact (Insel and Winslow, 1991). OT manipulations in early life, moreover, can change the response of an infant to its mother. For example, peripheral administration of oxytocin to maternal rats reinstates the ability of postnatal day 8–9 (P8–9) sucking offspring to orient correctly to nipples despite removal of cues by washing of the ventral surface of the mother or ligation of the nipples (Singh and Hofer, 1978). Significantly, OT antagonists inhibit maternal behavior (Pedersen et al., 1994; Boccia et al., 2007), suggesting that endogenous OT plays an essential role in this activity.
In addition to its effects on behavior, milk let-down, and parturition, OT acts on the bowel to modulate motility, secretion, blood flow, cell turnover, and release of neurotransmitters, paracrine factors, and hormones (Iseri et al., 2005). Transcripts encoding OT and those encoding OTR have been reported to be present in most segments of the adult human gut (Monstein et al., 2004). More recently, oxytocin immunoreactivity has been observed in myenteric and submucosal neurons of the human bowel (Ohlsson et al., 2006b). OTR protein, however, has not previously been identified in the gut despite the presence in the bowel of OTR-encoding transcripts.
The reported effects of OT on gastrointestinal motility appear to be species-dependent. For example, OT accelerates (Hashmonai et al., 1979; Petring, 1989), and OTR antagonists delay (Ohlsson et al., 2006a), gastric emptying in humans, but OT delays gastric emptying in rats (Wu et al., 2002, 2003). The effects of OT on the human stomach appear to be direct; however, the gastric effects of OT in rats are blocked by cholecystokinin antagonists and thus are probably mediated indirectly by cholecystokinin. OT acts as a visceral analgesic and may thus interfere with the transmission of visceral nociceptive signals to the CNS (Louvel et al., 1996; Spiller, 2002; Ohlsson et al., 2005). For this reason, intranasal OT treatment has been proposed to relieve the pain, discomfort, and depression associated with the irritable bowel syndrome (IBS).
The current study was undertaken to determine whether endogenous OT signaling could be a significant factor in the physiology of the bowel. To do so, we sought to verify whether OTR protein as well as transcript is expressed in the bowel, and if so, to locate its sites of expression. We also sought to verify the expression and localization of OT in the rodent gut and to relate the sites of endogenous OT to OTRs. Because, as noted above, OT is important in nurture (Numan, 1988; Welch and Ruggiero, 2005; Welch et al., 2006) and is expressed in milk (Takeda et al., 1986), we also investigated the developmental regulation of enteric OT and OTR. The rat provides a good model system to enable controlled studies to be carried out to understand how OTR signaling affects gut function from development to adulthood.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats from Hilltop Lab Animals (Scottdale, PA) were housed in AAALAC-approved facilities of the New York State Psychiatric Institute and Columbia University. Dams were individually caged with their litters and were provided food and water ad libitum. The Institutional Animal Care and Utilization Committees of Columbia University and the New York State Psychiatric Institute approved all experimental procedures. Timed-pregnant rats were euthanized by exposure to CO2. Fetuses from embryonic day 14–18 (E14–18) were rapidly removed from the gravid uterus and anesthetized by cooling for the collection of tissue. The entire bowel was dissected from fetuses. Portions of each gut region were fixed for immunocytochemistry or frozen with liquid nitrogen for analysis of RNA and protein. P0–adult rats were euthanized by intraperitoneal injection of ketamine and xylazine and, as with fetal bowel, sections of gut were frozen with liquid nitrogen for analysis of RNA and protein. For immunocytochemistry, pups were deeply anesthetized with ketamine/xylazine and perfused sequentially through the heart with 10–15 ml of heparinized 0.9% saline and 15–30 ml of a 4% solution of formaldehyde (freshly generated from paraformaldehyde) in sodium phosphate buffer (pH 7.4). Adults were perfused with 200 ml saline and 500 ml of 4% formaldehyde.
RT-PCR
Total RNA was isolated from segments of rat fetal gut with Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions and stored for further use at −80°C. Complementary DNA (cDNA) was prepared from 3 µg of total RNA by reverse transcription in a 30 µl reaction volume with 0.5 µg random hexamer primers, 0.5 mM dNTPs, 40 U RNAsin, and 400 U Maloney Murine Leukemia Virus reverse transcriptase (MMLV; Promega, Madison, WI). Pairs of oligonucleotide primers for amplification of cDNA encoding β-actin, OT, and OTR were designed from published rat cDNA sequences. Sequence analysis confirmed the identities of all polymerase chain reaction (PCR) products. For sequencing, the TA-cloning kit (Invitrogen) was employed to subclone PCR products into pGEM-T Easy vectors (Promega). The dideoxynucleotide-chain termination method was then used in the DNA Facility of Columbia University to sequence inserts in two individual clones. The sequences of the PCR products obtained from the gut with the indicated primers were identical to those of the appropriate regions of the GenBank sequences of the amplified cDNAs.
RT-PCR was used to quantify messenger RNA encoding OT and OTR in the intestine. The expression of OT and OTR was normalized to that of β-actin, a housekeeping gene that is not thought to be subject to regulation. The real-time reaction contained 5 µl of the cDNA along with the 5′ and 3′ primers for OT (forward: 5′-CGAGCG CTGAGCC-3′; reverse: 5′-TCTGGCAGAATCTG-3′) or OTR (forward: 5′-CGACCCTGAGTCTGCCTTCT-3′; reverse: 5′-GCCCTAAAGGTATCATCACAAAGC-3′) and SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). β-Actin was detected by using a dual-labeled fluorogenic probe (5′-FAM/3′-MGB probe, Applied Biosystems). mRNA levels were quantified with a GeneAmp 7500 sequence detection system (Applied Biosystems). Duplicate reactions of each standard or sample were incubated for 2 minutes at 50°C, denatured for 10 minutes at 95°C, and subjected to 40 cycles of annealing at 60°C for 20 seconds, extension at 60°C for 1 minute, and denaturation at 95°C for 15 seconds. A melting point analysis was finally carried out to validate amplification reactions detected with the SYBR Green I dye. Samples were incubated at 95°C for 60 seconds, at 67°C for 15 seconds, and then from 67°C to 95°C with a transition rate of 0.2°C/second. Data were analyzed with computer assistance employing the TaqMan 7500 software.
Immunoselection
Crest-derived cells were immunoselected from the fetal rat gut with antibodies to the common neurotrophin receptor p75NTR at E16 (Chalazonitis et al., 1998, 2004). The fetal bowel was dissociated with collagenase to provide a single-cell suspension. Antibodies to p75NTR and goat anti-rabbit secondary antibodies coupled to magnetic beads were sequentially applied to the cells (Miltenyi Biotec, Auburn, CA), and the suspension was passed through a column in a magnetic field (Miltenyi Biotec). Crest-derived cells express p75NTR and thus become decorated by beads and are retained, whereas non-crest-derived cells are not. The enriched population of crest-derived cells is finally eluted by withdrawal of the magnetic field.
To produce antibodies to p75NTR, the immunizing antigen used was a peptide with the sequence SGECCKACNLGEGVAQPCGANQTVCEPCLDSVTFSDVVSATEPCKPCT ECLGLQSMSAPCVEADDAV - CRCSYGYYQDEETGRCEACSVCGVGS GLFFSC - QDKQNTVCEECPEGTYSDE (Huber and Chao, 1995), which corresponds to amino acids 43–161 of the protein (Lee et al., 1992). The antibody recognizes rat p75NTR. The sequence of amino acids 41–158 of rat p75NTR is identical to that of mouse p75NTR. When the antibodies to p75NTR are applied to frozen sections of bowel with targeted mutations of the gene encoding p75NTR, no immunostaining is observed (Chalazonitis et al., 2008).
Characterization of antibodies used for OTR immunoreactivity
Two antibodies were used to investigate OTR expression in the gut. The first was a generous gift from Gloria Hoffman, PhD (University of Maryland). This polyclonal antibody was raised against a peptide with the sequence WQNLRLKTAAAA (Adan et al., 1995). The second anti-OTR was purchased from Sigma-Aldrich (St. Louis, MO) but raised by MBL International (Woburn MA). The sequence of the immunizing peptide, CAEGNRTAGPPRRNEALARVE (amino acids 21–42), was determined in the protein core at Columbia University by analyzing the control peptide furnished by MBL International. Neither antibody is suitable for Western blot analysis (Sigma technical assistance; Adan et al., 1995). Specificity of the antibodies was therefore validated by four other methods: 1) immunostaining of central nervous system (CNS) tissue demonstrated sites of immunoreactivity in regions found by others to contain OTR immunoreactivity and cells that express transcripts encoding OTR (Adan et al., 1995) (data not illustrated); 2) no immunostaining was observed when the primary antibodies were omitted; 3) no immunostaining was observed when primary antibodies were preadsorbed with the immunizing peptides (LifeSpan Biosciences, Seattle, WA); and 4) no immunostaining was observed when primary antibodies were applied to fixed, frozen bowel removed from transgenic mice that lack OTR (Takayanagi et al., 2005) (knockout mice were donated by Jeffrey Mogil at McGill University, Montreal, Canada; data not illustrated).
Immunocytochemistry
Light microscopy
Small pieces of fixed duodenum, jejunum, ileum, and colon were removed and postfixed for 2 hours in individual glass vials containing 4% formaldehyde (from paraformaldehyde) in 0.1 M phosphate-buffered saline (PBS), pH 7.4. Tissue blocks were then washed, cryoprotected overnight at 4° C in a solution of 30% sucrose in 0.1 M PBS, and frozen with dry ice. Sections were cut with a sliding microtome (30 µm). All incubations were carried out with free-floating sections in separate test wells on a rotator table. Endogenous peroxidase activity was inhibited with H2O2 for 15 minutes, and nonspecific binding sites were blocked by preincubating sections for 30 minutes with 1% bovine serum albumin (BSA) in TBS. Sections were incubated overnight at 4°C with primary rabbit polyclonal antibodies to the OTR (diluted 1:2,000; Sigma) in TBS containing 0.1% BSA, to which 0.25% Triton X-100 was added to facilitate antibody penetration. Sections were then washed with TBS (10 minutes × 3), treated sequentially with avidin (15 minutes) and biotin (15 minutes) to prevent nonspecific staining, and incubated for 1 hour with biotinylated goat anti-rabbit secondary antibodies (1:200; Vector, Burlingame, CA).
Sites of antibody binding were finally visualized by using the ABC method (Vector) with horseradish peroxidase (HRP) and 3,3′-diaminobenzadine (DAB) according to the manufacturer’s directions. Alternatively, laminar preparations were examined as whole mounts. These were permeabilized with a solution containing 1% Triton X-100, 4% horse serum, and 1% BSA. Preparations were exposed overnight in a humidified chamber at 4°C to rabbit primary antibodies to OT (diluted 1:100; Serotec, Bicester, UK) or OTR (diluted 1:5,000; Sigma or donated by Dr. Hoffman; see above). The antibodies to OT were raised against the immunogen oxytocin-CYIQNCPLG-NH2. Their specificity was verified by absorption by the immunogen; no immunostaining was observed when preadsorbed antibodies were applied to whole mounts of laminar preparations of bowel. Sites of OT or OTR antibody binding were located with biotinylated donkey anti-rabbit secondary antibodies (diluted 1:200; Jackson ImmunoResearch, West Grove, PA) and Alexa 488 or 594 (diluted 1:100). Primary antibodies were either omitted or exposed to adsorbed sera as controls. In addition, as a further control, immunostaining was carried out on tissues from mice lacking OTR (donated by Jeffrey Mogil, McGill University, Montreal, Canada) (Takayanagi et al., 2005).
When preparations were doubly immunostained with human antibodies to Hu to identify neurons (diluted 1:1,000; ANNA-1; contributed by Dr. Miles Epstein, University of Wisconsin, Madison, WI) (Fairman et al., 1995) or murine antibodies to NeuN (diluted 1:800; Chemicon; Temecula, CA) to identify putative primary afferent neurons (Poole et al., 2006; Van Nassauw et al., 2005), species-specific secondary antibodies labeled with contrasting fluorophores were employed (Alexa 488 or 594; diluted 1:400). NeuN antibodies are monoclonals that were raised against brain cell nuclei (Mullen et al., 1992). Immunoreactivity appears in neuronal nuclei shortly after cells withdraw from the cell cycle and is seen in most but not in all neurons of rats, humans, and other vertebrates; for example, NeuN immunoreactivity is lacking in Purkinje cells, olfactory bulb mitral cells, retinal photoreceptor cells, and sympathetic chain ganglia (Mullen et al., 1992; Wolf et al., 1996). In subsets of neurons, including intrinsic primary afferent neurons (IPANs) of the guinea pig ENS, NeuN immunoreactivity is not restricted to nuclei, but can also be seen in cell bodies and dendrites (Mullen et al., 1992; Wolf et al., 1996; Van Nassauw et al., 2005; Poole et al., 2006). The antigen recognized by antibodies to NeuN has not been completely characterized, but it is known to exist in multiple phosphorylated isoforms of 46 and 48 kDa (Mullen et al., 1992; Lind et al., 2005).
The ANNA-1 antibody to Hu was derived from the serum of a human patient with a small cell carcinoma of the lung and a severe peripheral neuropathy (Fairman et al., 1995). The antibodies react with proteins of 35–42 kDa, which are members of a family of RNA binding proteins that are related to the neuronally restricted Elav RNA binding proteins of Drosophila. The neuronal specificity of the ANNA-1 antibody to Hu for the ENS has been well established in a number of studies (Fairman et al., 1995; Young et al., 2005; D’Autreaux et al., 2007), and antibodies to Hu have been shown to be useful to detect the total population of enteric neurons (Phillips et al., 2004).
To locate OT immunoreactivity in the gut, the dissected longitudinal muscle with adherent myenteric plexus was incubated with colchicine (2.5 × 10−5 M) overnight at 37°C in 10 ml of DMEM/F12 (Mediatech, Herndon, VA) without glutamine. The medium was continually gassed with a mixture of 95% O2 and 5% CO2. The tissue was pinned to balsa wood supports, with a cutout to facilitate oxygenation from both sides of the preparation. Following incubation, the preparations were fixed and processed for immunocytochemistry as described above.
Electron microscopy
Tissue was fixed for 3 hours at room temperature in a mixture of 4% formaldehyde (from paraformaldehyde) and 0.1% glutaraldehyde in 0.1% phosphate buffer. After rinsing overnight in PBS, the specimens were embedded in 8% gelatin at 37°C. The gelatin-embedded tissue was then postfixed with 4% formaldehyde (from paraformaldehyde) for 3 days and again rinsed with PBS. Sections were cut at ~40 µm with a Vibratome. The sections were quenched with 0.5% NaBH4 in 0.1 M phosphate buffer for 30 minutes and again washed with PBS. Sections were blocked with 10% normal goat serum, and primary antibodies to OTR (Hoffman; diluted 1:5,000) were applied overnight at 4°C. The specificity of the antibodies was established at the light microscopic level (see above). Sites of immunoreactivity were visualized by using biotinylated goat anti-rabbit secondary antibodies and a preformed avidin-biotin complex (Vectastain Eite ABC kit, Vector) according to the manufacturer’s directions. Peroxidase activity was detected with DAB and peroxide generated from glucose and glucose oxidase (Shu et al., 1988). Sections were finally embedded in epoxy resin (Epon 812) for thin sectioning. Sections were examined with a JEOL 1200 transmission electron microscope with or without light counterstaining with lead citrate and uranyl acetate. Specimens were always compared with sections processed without exposure to primary antibodies. Images were obtained on photographic negatives, which were digitized, converted to positive images, and arranged in figures by using Adobe (San Jose, CA) Photoshop Cs3 software.
Retrograde tracing
The retrograde tracer Fluoro-Gold (FG; 10 mg/kg; Fluorochrome, Englewood, CO) was injected intraperitoneally to label neurons that project to intra-abdominal viscera. This technique has previously been validated (Powley et al., 1987; Berthoud and Powley, 1992, 1993). After its intraperitoneal injection, FG does not spread systemically and has been shown specifically to label neurons of the dorsal vagal complex (Krowicki et al., 1997) and to demonstrate the adequacy of subdiaphragmatic vagotomies (Powley et al., 1987). Intra-abdominal injections of FG also label virtually all neurons of the bowel wall (Berthoud and Powley, 1993). FG is itself fluorescent; however, permanent preparations and greater sensitivity can be obtained when FG-labeled tissue is immunostained with antibodies to FG. In the current experiments, rabbit antibodies to FG were employed (Fluorochrome; diluted 1:100). Sites of antibody binding were visualized with donkey anti-rabbit secondary antibodies coupled to Alexa 488; diluted 1:400; Invitrogen).
The specificity of labeling with antibodies to FG was validated by verifying that the same cells and subcellular structures were doubly labeled by FG native fluorescence and FG immunoreactivity (Supplemental Fig. 1). The OTR immunoreactivity of nodose neurons was studied in preparations in which the neurons were simultaneously labeled by retrograde transport of FG. For this purpose goat primary antibodies to OTR were employed (diluted 1:100; Santa Cruz Biotechnology, Santa Cruz, CA) and visualized with donkey anti-goat secondary antibodies coupled to Alexa 594 (diluted 1:400; Invitrogen). The antibodies were raised against the sequence YLKGRRLGETSASKKSNSSSFVLSHRSSSQRSCSQPSTA (amino acids 350–389 of the human OTR).
Photomicrograph production and processing
Photomicrographs were obtained with a Q Imaging digital camera attached to a Leica DMRXA2 microscope and an Apple computer with Openlab or Volocity 4.0 software (Improvision, Lexington, MA). Additional photomicrographs were obtained by using a Photometrics CoolSnap CF camera attached to a Nikon Eclipse E600 microscope and a PC computer with IPLab software (version 3.7, Scanalytics, BD Biosciences, Rockville, MD). Electron micrography was performed by using procedures described previously. An image editing software program Adobe Photoshop 10.0 was used to merge images and/or to adjust brightness and contrast.
RESULTS
Transcripts encoding the OTR are found in the gut and the nodose ganglion
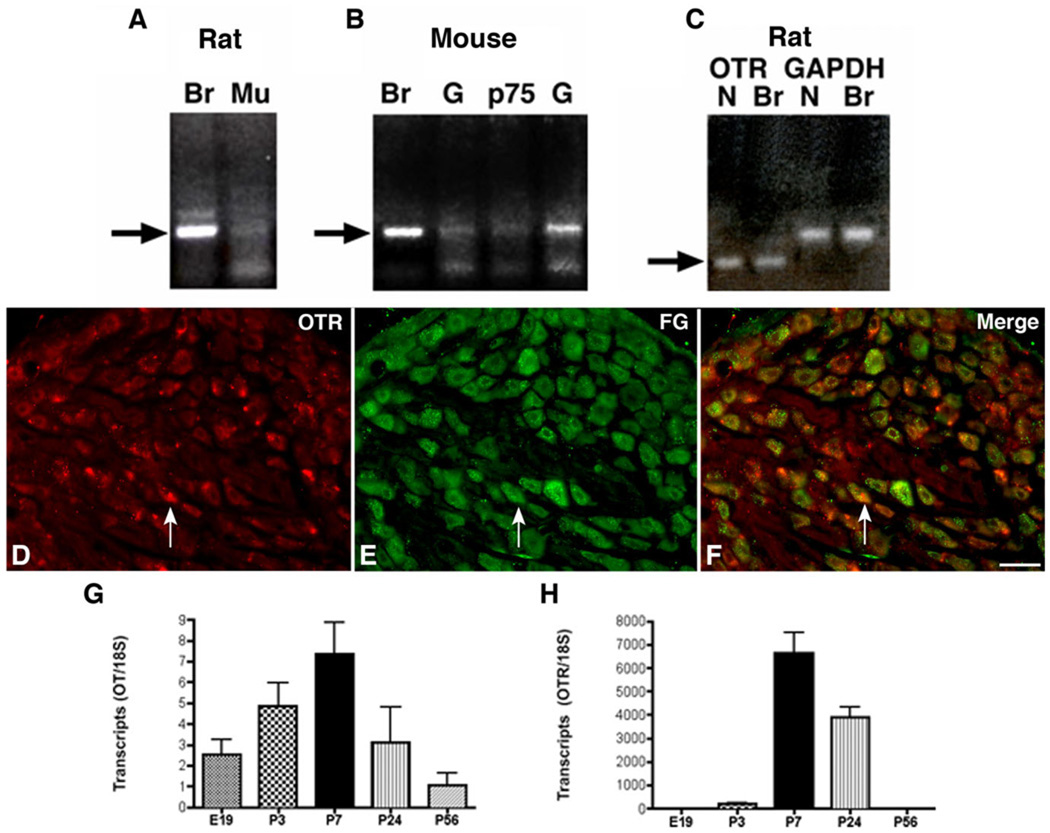
RT-PCR was used to determine whether expression of the OTR could be detected in rodent bowel. Rat and mouse gut were investigated. Rat and mouse brain were also studied as positive controls. Transcripts encoding the OTR were detected in both rat (Fig. 1A) and mouse gut (Fig. 1B). OTR transcripts were detectable in whole mouse gut (Fig. 1B), in isolated samples of rat intestinal mucosa (Fig. 1A), and in isolated neural crest-derived cells immunoselected from embryonic day 16 (E16) mouse gut with antibodies to p75NTR (Fig. 1B). These observations confirm that the OTR and its transcript are expressed in the bowel and that enteric OTR-expressing cells are found both within the mature intestinal mucosa and the population of precursor cells that gives rise to the ENS. Because of the postulated ability of OT to relieve gastrointestinal discomfort, we tested the idea that the OTR is expressed by the extrinsic sensory innervation of the gut, as well as by intrinsic elements of the ENS and bowel wall. RT-PCR revealed the presence of transcripts encoding the OTR in the rat nodose ganglion (Fig. 1C). Therefore, the sensory neurons that innervate the bowel may express OTR. To determine whether protein as well as transcripts is expressed, OTR immunoreactivity was demonstrated in sections of nodose ganglia.
Figure 1.
Transcripts encoding OTR are expressed in the rodent gut and nodose ganglion. A: Reverse transcriptase polymerase chain reaction (RT-PCR) showing OTR transcripts in rat brain and in isolated samples of rat intestinal mucosa. Br, brain; Mu, mucosa. B: OTR transcripts were detectable in whole mouse gut and in isolated neural crest-derived cells immunoselected from the E16 mouse gut with antibodies to the p75 neurotrophin receptor (p75). G, gut. C: Messenger RNA (mRNA) encoding OTR found by RT-PCR in the nodose ganglion. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used as an internal RT-PCR control. N, nodose. D–F: OTR immunoreactivity and retrograde labeling 1 week after intra-abdominal administration of Fluoro-Gold (FG) were simultaneously demonstrated in rat nodose ganglia. D: OTR immunoreactivity. E: FG immunolabeling. F: Merged image. The arrow indicates an example of a neuron doubly labeled with antibodies to OTR and FG. G: Transcripts encoding OT are developmentally regulated in the rat large intestine. Real-time PCR was employed to quantify transcripts encoding OT as a function of age from E19 to P56. Expression peaks at P7. H: Transcripts encoding OTR are developmentally regulated in the rat large intestine. Real-time PCR was employed to quantify transcripts encoding OTR as a function of age from E19 to P56. Expression peaks at P7 where it is coincident with that of OT. A magenta-green copy of this image is available as Supplementary Figure 2. Scale bar = 50 µm in F (applies to D–F).
The retrograde tracer FG was also injected into the peritoneal cavity to label the subset of nodose neurons that project intra-abdominally. Antibodies to OTR immunostained a large proportion of nodose neurons (Fig. 1D). As anticipated, 1 week after the injection of FG, many nodose neurons were labeled by retrograde transport (Fig. 1E). Substantial numbers of these neurons were doubly labeled by antibodies to OTR (Fig. 1F). These data support the idea that vagal sensory neurons innervating the intra-abdominal viscera express OTR.
The expression of transcripts encoding both OT and OTR were developmentally regulated. Transcripts encoding OT (Fig. 1G) and those encoding OTR (Fig. 1H) were each detected in the developing rat small and large intestine as early as E18 and peaked at P7 before declining to reach the adult level, which was sustained through P56. These data are compatible with the idea that OT is synthesized locally within the bowel and utilized for signaling via the OTR, which is expressed by enteric effectors.
Myenteric neurons express OTR immunoreactivity
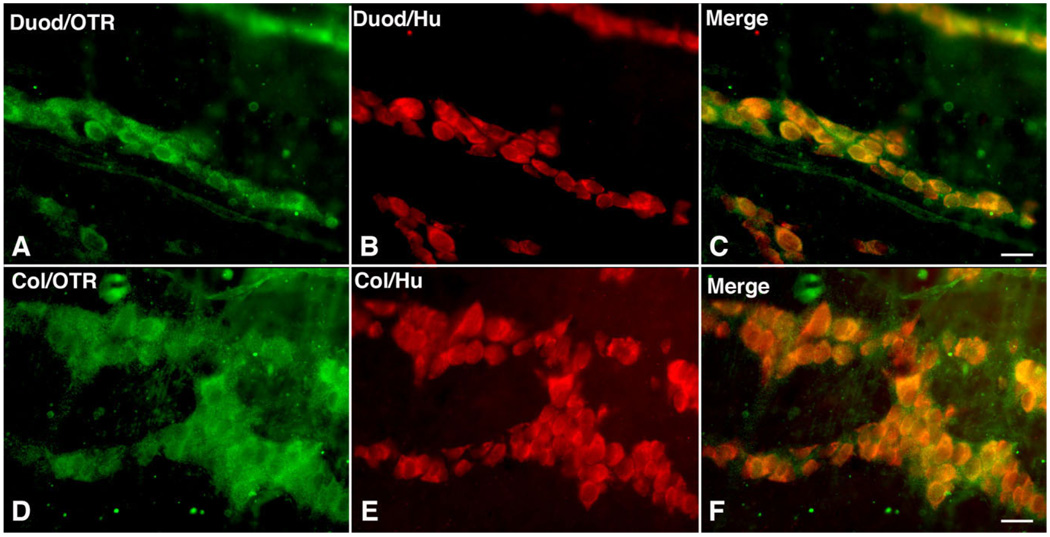
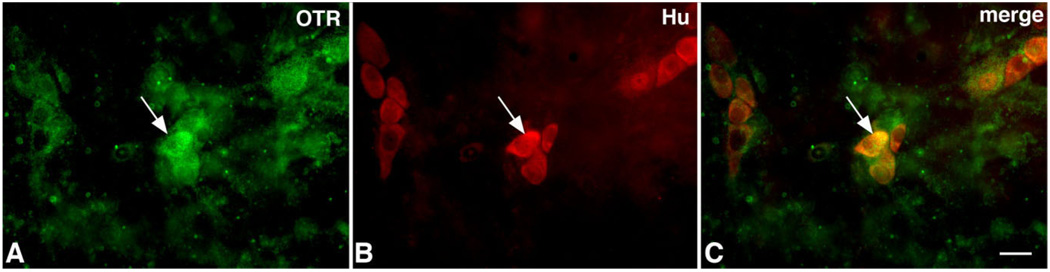
Immunocytochemistry was used to locate cells in the bowel that express OTR. Laminar preparations of the bowel wall were employed to permit OTR-immunoreactive cells to be quantitatively evaluated in whole mounts containing the myenteric or submucosal plexuses. Because the precursors of enteric neurons were found to express the receptor and both OT and the OTR are developmentally regulated (Fig. 1B,G,H), the gut was examined during early postnatal development as well as at maturity. The pan-neuronal marker Hu was used, both to determine whether cells that express OTR immunoreactivity are neurons, and to ascertain the total number of neurons in order to be able to estimate the proportion that are OTR-immunoreactive. In the P3 duodenum (Fig. 2A–C) and colon (Fig. 2D–F), a complete coincidence was found between OTR and Hu immunoreactivity. In the layer of the myenteric plexus, therefore, only neurons are OTR-immunoreactive and all neurons express the receptor.
Figure 2.
Neurons are OTR-immunoreactive in the developing (P3) myenteric plexus. Whole mount preparations of longitudinal muscle with adherent myenteric plexus. A–C: Duodenum. D–F: Colon. A: Duodenum; OTR immunoreactivity. B: Duodenum; Hu immunoreactivity identifying neurons. C: Duodenum; merged image. Note that OTR and Hu immunoreactivities are coincident. D: Colon; OTR immunoreactivity. E: Colon; Hu immunoreactivity identifying neurons. F: Colon; merged image. Note that OTR and Hu immunoreactivities are coincident. A magenta-green copy of this image is available as Supplementary Figure 3. Scale bar = 20 µm in C (applies to A–C) and F (applies to D–F).
Myenteric intrinsic primary afferent neurons express OTR immunoreactivity
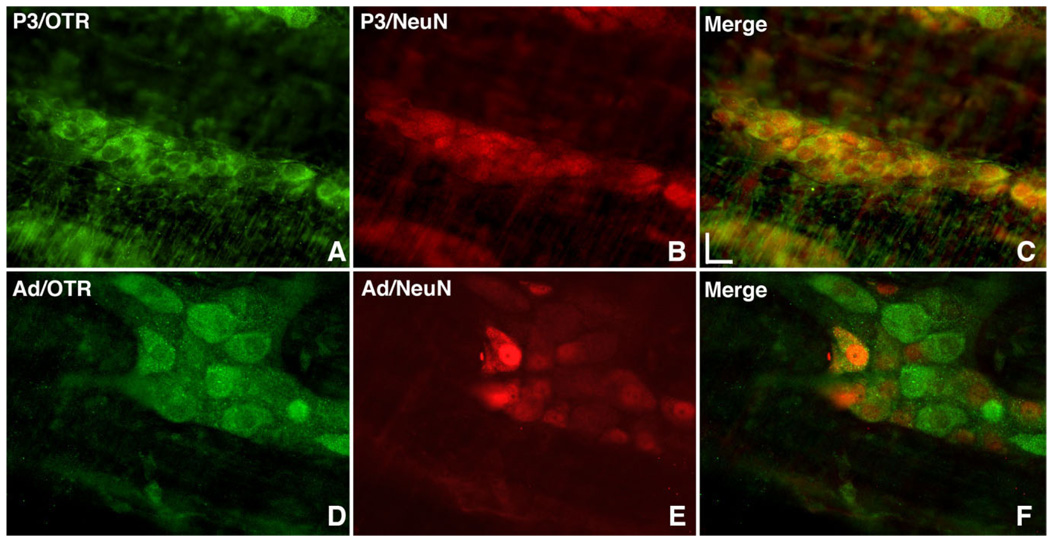
The distribution of OTR immunoreactivity in the perinatal ENS at P3 was compared with that of the mature adult bowel (Fig. 3). At P3, virtually all neurons of the small and large intestines were again found to be OTR-immunoreactive (Fig. 3A–C). The OTR was not expressed quite as widely in the adult (Fig. 3D–F) as in the perinatal gut (compare Fig. 3A with Fig. 3D); computer analysis of overlap revealed that OTR immunoreactivity was coincident with that of Hu in 71.4 ± 4.6% of enteric neurons. To help determine the type of myenteric neuron that expressed OTR, the immunoreactivity of the IPAN marker NeuN (Brookes, 2001; Chiocchetti et al., 2003; Hind et al., 2005) was located simultaneously with that of OTR. Although at least some NeuN immunoreactivity can be observed exclusively in the nuclei of many other types of enteric neuron, NeuN is found both in nuclei and in the cytoplasm of guinea pig IPANs (Van Nassauw et al., 2005), which has been taken as a defining characteristic of the IPAN class of cell. In rat at P3, the nuclei of almost all enteric neurons were NeuN-immunoreactive (Fig. 3B); thus, OTR-expressing neurons exhibited NeuN-fluorescent nuclei (Fig. 3C).
Figure 3.
OTR immunoreactivity is developmentally regulated and in adult myenteric plexus is expressed in IPANs. Whole mount preparations of longitudinal muscle with adherent myenteric plexus. A–C: P3 rat colon. D–F: Adult rat colon. A: OTR immunoreactivity. B: NeuN immunoreactivity; uniform immunostaining of developing neurons precludes using NeuN to identify IPANs in the developing gut. C: Merged image. D: OTR immunoreactivity. E: NeuN immunoreactivity is present in both the cytoplasm and nucleus of IPANs. F: Merged image. IPANs are included in the OTR-immunoreactive subset of myenteric neurons. A magenta-green copy of this image is available as Supplementary Figure 4. Scale bar = 20 µm in C (applies to A–F).
In the adult myenteric plexus, NeuN immunoreactivity was still seen, although less strongly than at P3, in the nuclei of many, but not all myenteric neurons (Fig. 3E); however, in the subset of cells that fit the definition of IPANs in the guinea pig ENS (Van Nassauw et al., 2005), which exhibited both cytoplasmic and nuclear NeuN immunoreactivity, OTR immunoreactivity in the adult rat was very prominent (Fig. 3F). At P3, NeuN was not a good type-specific neural marker because the distinction between cytoplasmic and nuclear NeuN immunoreactivity was less clear than in the adult myenteric plexus.
Submucosal neurons express developmentally regulated OTR immunoreactivity
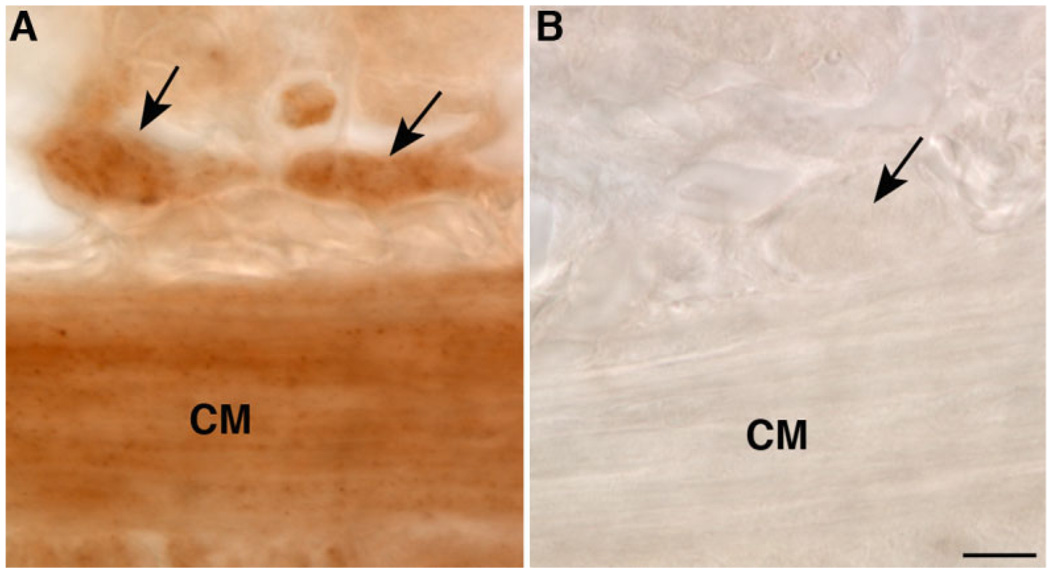
Because the submucosal layer of the perinatal gut could not be dissected and examined as a laminar preparation, the submucosal plexus in developing rats was examined in sections cut through the bowel. OTR immunoreactivity was first observed in the submucosal plexus at postnatal day 19 (Fig. 4). At this age, OTR immunoreactivity was also seen in the muscularis externa (Fig. 4) and in the endothelia of venules (not illustrated). In adult rats, OTR receptor immunoreactivity was strong (Fig. 5A) and coincident with the immunoreactivity of Hu (Fig. 5B,C) in a subset of neurons. Additional neurons showed weak OTR immunoreactivity, also coincident with Hu (Fig. 5A–C). These observations suggest that OTR expression is widespread in the ENS, is present in both submucosal and myenteric plexuses, and is developmentally regulated; in mature animals the OTR-expressing subset of enteric neurons includes IPANs.
Figure 4.
Submucosal neurons are OTR-immunoreactive. Cryostat sections; immunoreactivity visualized with HRP. A: Submucosal ganglia. B: Control (primary antibodies omitted). The arrows point to submucosal ganglia. Immunostaining obscures cell boundaries. CM, circular muscle. Scale bar = 30 µm B (applies to A,B).
Figure 5.
Coincident immunoreactivities of Hu and OTR confirm that OTR-immunoreactive cells in submucosal ganglia are neurons. A: Duodenum; OTR immunoreactivity. B: Duodenum; Hu immunoreactivity identifying neurons. C: Duodenum; merged image: note that OTR and Hu immunoreactivities are coincident. A magenta-green copy of this image is available as Supplementary Figure 5. Scale bar = 20 µm in C (applies to A–C).
OTR immunoreactivity is developmentally regulated in the mucosal epithelium
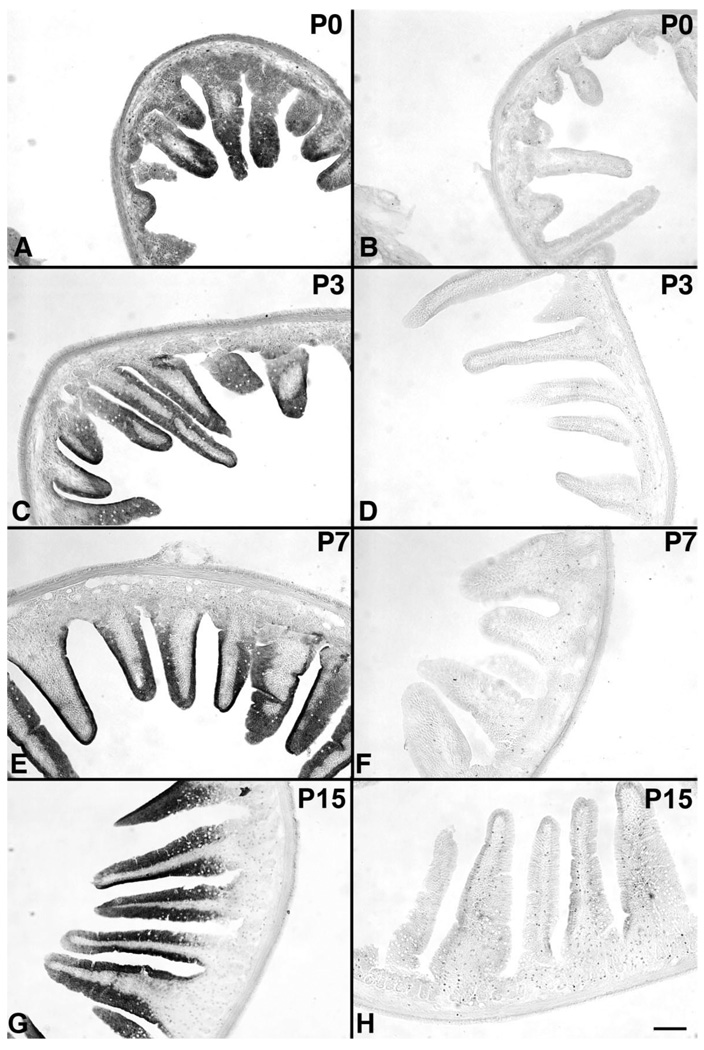
OTR immunoreactivity in the bowel was not limited to the ENS, but was also observed in the mucosal epithelium. In perinatal rats, examined between P0 and P15, OTR immunoreactivity was found in the developing epithelium and was prominent in enterocytes lining growing villi (Figs. 6A,C,E,G, 7A,C). OTR immunoreactivity was a specific function of the binding of primary antibodies, and thus no OTR immunostaining was observed either when the primary antibodies were omitted (Figs. 6B,D,F, 7B) or when sections were exposed to antibodies that were adsorbed with the immunizing peptide (Fig. 6H). After P15, as the crypt-villus distinction became more apparent, OTR immunoreactivity decreased in the villi, and OTR-immunoreactive cells formed prominent clusters at the crypt-villus junction (Fig. 7D–G). Within these cells, OTR immunoreactivity was intense at apices, below the brush border, and in intracellular vesicles (Fig. 7F,F inset, G inset). The apical cytoplasm of crypt epithelial cells also displayed modest OTR immunoreactivity (Fig. 7E). Once acquired, the clustering of OTR-immunoreactive epithelial cells at crypt-villus junctions was stably maintained through adulthood. The pattern of apical OTR immunoreactivity (Fig. 7F inset, G inset) suggested that the OTR might be associated with junctional complexes and was thus investigated further.
Figure 6.
OTR immunoreactivity is present in villus enterocytes in early postnatal bowel. Sections exposed to primary antibodies at each age (A,C,E,G) are displayed in the left column. The corresponding control sections, which were not exposed to primary antibodies (B,D,F) or were exposed to antibodies that were preadsorbed with the immunizing peptide (H), are displayed in the right column. Pup ages: A,B (P0); C,D (P3); E,F (P7); G,H (P15). Scale bar = 100 µm in H (applies to A–H).
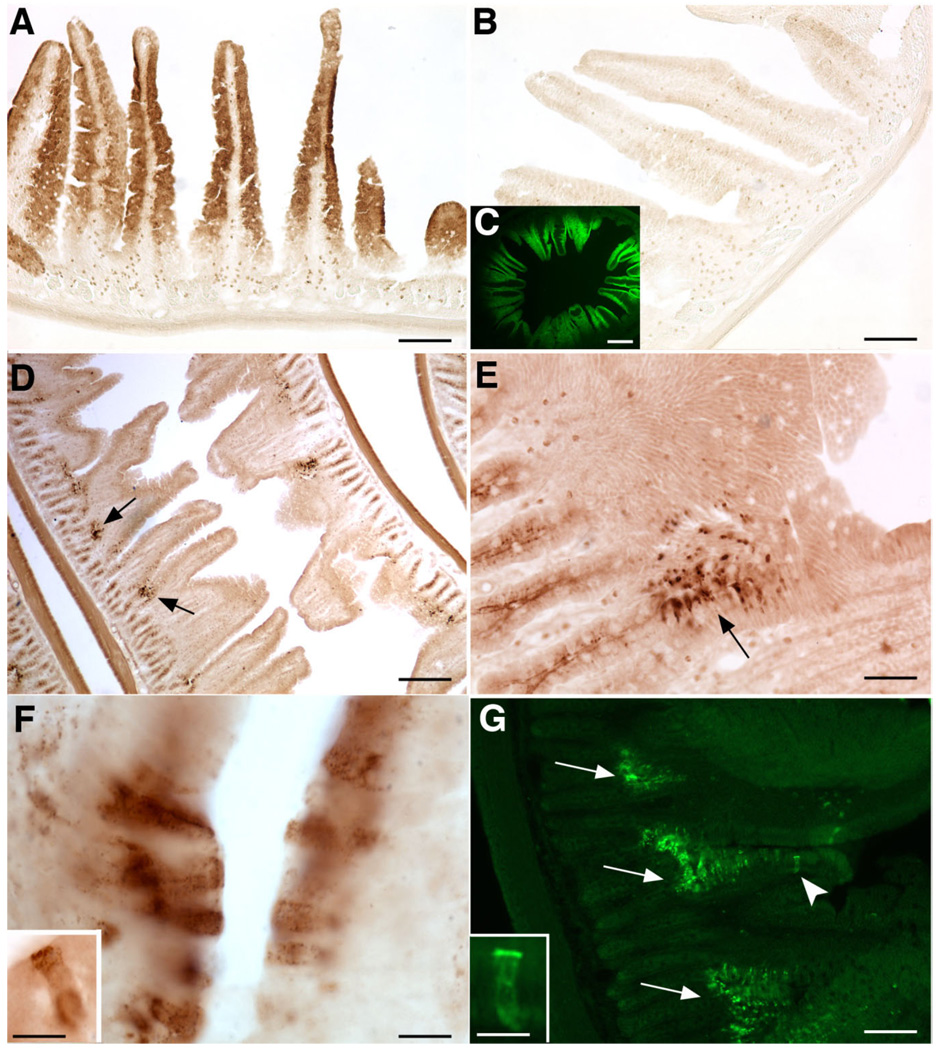
Figure 7.
OTR immunoreactivity in the mucosal epithelium is developmentally regulated in the rat small intestine. A: At P0 enterocytes on the walls of villi are OTR-immunoreactive. B: If antibodies to OTR are omitted (control), the villus epithelium is not stained. C: Because of the OTR immunoreactivity of enterocytes, villi display OTR immunofluorescence. D–G: At P15 OTR immunoreactivity is modest in crypts and concentrated in enterocytes at crypt-villus junctions. Little or no OTR immunoreactivity remains in enterocytes of the walls of villi. (Compare D with A). E: Higher magnification of a portion of the field shown in D. F, F inset: The cells at the crypt-villus interface display intense OTR immunoreactivity, which is prominent in a band in the apical cytoplasm, just below the microvillus border. G: A high concentration of OTR-immunofluorescent cells is located at the crypt-villus junction (arrows). Crypt epithelial cells also display apical OTR immunoreactivity (arrowhead), although it is much less intense than that of cells at crypt-villus junctions. G inset: The apical OTR-immunoreactive band is well seen in a fluorescent image. Scale bar = 100 µm in A,B,E,G; 400 µm in C,D; 10 µm in F; 10 µm in insets to F,G.
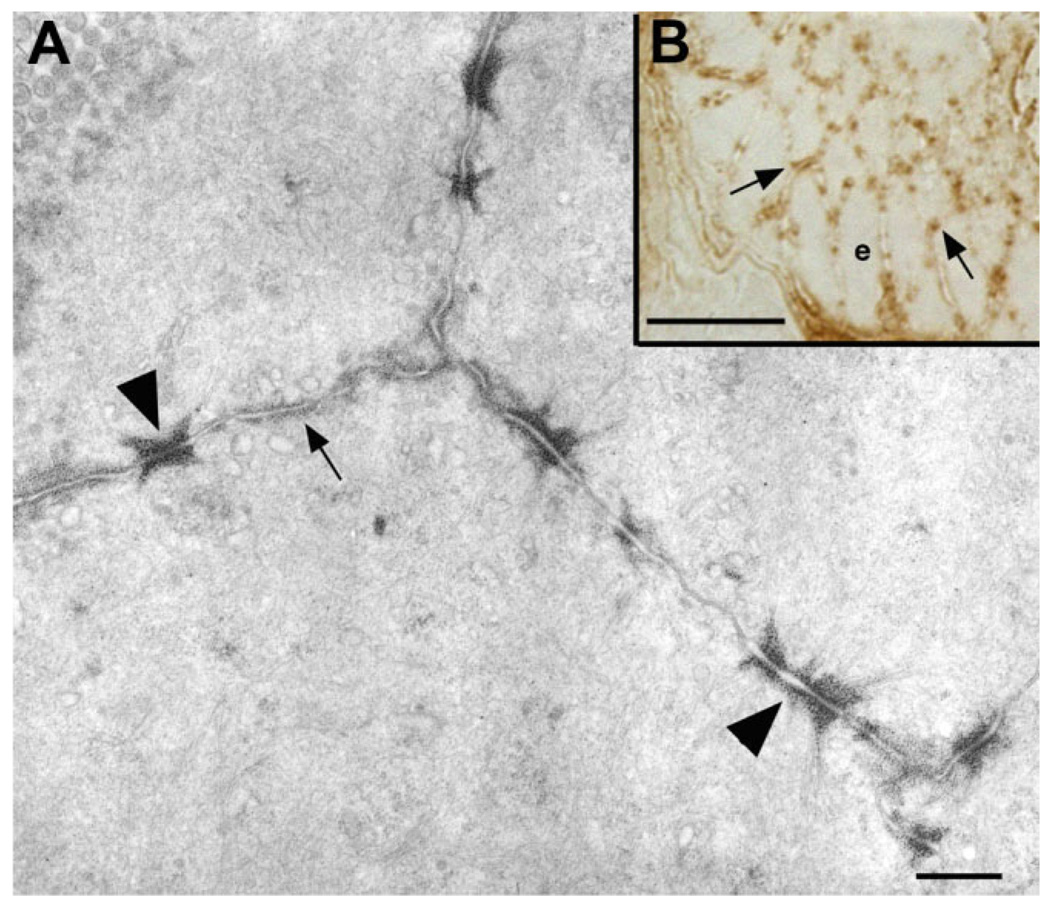
Thin sections were cut through the immunostained epithelium, which were then examined by electron (Fig. 8A) or light microscopy (Fig. 8B). Although little OTR immunostaining was observed in or on enterocyte microvilli (Fig. 8A), intense OTR immunoreactivity was found in association with junctional complexes and, to a more moderate extent, the adjoining lateral plasma membrane (Fig. 8A,B). The immunoreactivity appeared to be associated with the dense material found at the sites of insertion of actin filaments into zonulae adherens. These filaments, however, were not themselves OTR-immunoreactive.
Figure 8.
OTR immunoreactivity is concentrated at apical adherens junctions in enterocytes at the crypt-villus junction in rat duodenum. A: Electron micrograph (EM) of a thin section cut tangentially through the apical OTR-immunoreactive band of cells at the crypt-villus junction. OTR immunoreactivity, demonstrated by pre-embedding staining, is visualized by the osmiophilic DAB reaction product. Although OTR immunoreactivity is present in most of the plasma membrane ringing each enterocyte (arrows), it is especially prominent in accumulation in the cytosolic dense material associated with junctions identified by the insertions of thin filaments as adherens junctions (arrowheads). Little or no immunoreactivity is seen in the plasma membrane of microvilli. B: Light micrograph of a semithin section cut tangentially through the apical cytoplasm of OTR-immunoreactive cells at a crypt-villus junction. Punctate OTR immunoreactivity fills the intercellular space, corresponding to the adherens junctions seen in the matching EM section. Scale bar = 500 nm in A; 10 µm in B.
Terminal varicosities in the ENS are OT-immunoreactive
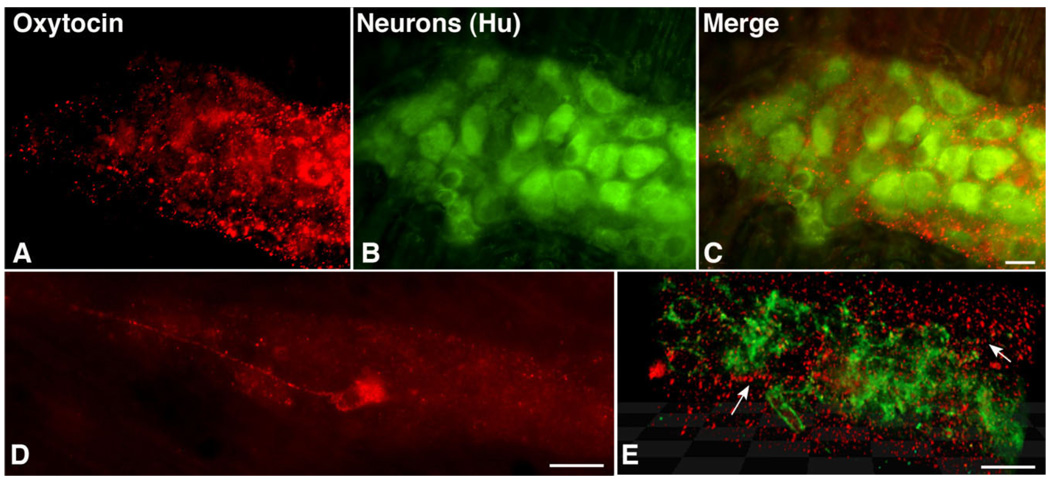
The widespread expression of OTR in the ENS and its location on the cell bodies and proximal processes of neurons suggest that OT can influence these cells. Pituitary-derived OT could reach the ENS by way of the circulation. However, there is a blood-myenteric plexus barrier (Gershon and Bursztajn, 1978), which, like the blood-brain barrier, could restrict access of circulating OT to enteric neurons. We thus tested the possibility that OT-immunoreactive neurons are intrinsic to the ENS and that OT is present in axon terminals. OT immunoreactivity was highly punctate in nature and intense in axonal varicosities in the neuropil of myenteric ganglia (Fig. 9A,C,E). The OT-immunoreactive varicosities often surrounded myenteric neurons, suggesting that some enteric neurons receive an oxytocinergic innervation (Fig. 9C,E). OT-immunoreactive neuronal perikarya were also observed. However, these cells were not seen unless the bowel had been pretreated with colchicine to inhibit axonal transport and promote accumulation of peptide in cell bodies. Within cell bodies, OT immunoreactivity was highly granular in appearance, suggesting, as does the ability of colchicine to induce OT to accumulate in cell bodies, that OT is stored intracellularly in vesicles. The OT-immunoreactive neurons (Fig. 9D) were relatively uncommon (<1% of enteric neurons) and were not seen in most ganglia. Despite the paucity of OT-immunoreactive neurons, OT-immunoreactive varicosities were widespread throughout the myenteric plexus (Fig. 9C,E).
Figure 9.
OT-immunoreactive cells are rare but OT-immunoreactive varicosities are widespread throughout the myenteric plexus. Colchicine treated to maximize peptide accumulation in neuronal perikarya. A: OT immunoreactivity is punctate and limited to ganglia. B: Neurons are identified by immunostaining with antibodies to Hu. C: Merged image. Hu-immunoreactive nerve cells are outlined by OT-immunoreactive varicosities in the ganglionic neuropil. D: OT immunoreactivity in a myenteric neuron. E: Deconvoluted projection of a Z stack of images taken at 0.5-µm intervals through a myenteric ganglion in which Hu immunofluorescence is green and OT immunofluorescence is red. OT-immunoreactive varicosities are abundant in the ganglionic neuropil and some varicose neurites can be tracked (arrows). A magenta-green copy of this image is available as Supplementary Figure 6. Scale bar = 25 µm in C (applies to A–C); 30 µm in D,E.
DISCUSSION
We tested the ideas that OT is produced locally in the bowel and that its signaling via enteric OTRs is a potential contributor to gastrointestinal physiology. Transcripts encoding OT and those encoding the OTR were, in fact, expressed in the gut. Interestingly, expression of both were developmentally regulated and each peaked, coincidentally, at postnatal day 7. Nevertheless, although this pattern of developmental regulation is consistent with a role for OT/OTR signaling in the maturation of the bowel, the observation that OT and OTR expression continues in later life implies that the function of enteric OT/OTR signaling is unlikely to be restricted to development. Varicose OT-immunoreactive nerve fibers were found within the ENS. OTR-immunoreactive protein was also observed in the majority of enteric neurons, but was found, as well, in cells of the mucosal epithelium and on submucosal venules. When freshly fixed tissue was examined, OT immunoreactivity was seen only in the varicosities of fibers within enteric ganglia; however, when tissue was incubated with colchicine overnight, OT immunoreactivity accumulated in nerve cell bodies, which then reached the threshold of sensitivity required for their immunocytochemical detection. OT-immunoreactive neuronal perikarya were relatively rare (<1% of Hu-immunoreactive total neurons) and not seen in all ganglia. Although OT-immunoreactive neuronal perikarya were not observed in the submucosal plexus, because they are rare even in the myenteric plexus, it is not possible to conclude that there are no such cells in any submucosal ganglia.
These observations suggest that OT is probably synthesized by a small subset of myenteric neurons, which branch extensively to innervate the large proportion of enteric neurons that are OTR-expressing. These OTR-expressing target neurons are found in both the myenteric and submucosal plexuses, and because OTR transcripts and protein were also found in the nodose ganglia, enteric terminals of extrinsic sensory nerves would also have to be considered potential targets of enteric OT. It is possible that oxytocinergic neurons innervate submucosal venules and mucosal epithelial cells, as do IPANs and enteric secretomotor neurons. No direct evidence, however, of a vascular or mucosal innervation by oxytocinergic neurites was found. Alternatively, OT, which is present in breast milk (Takeda et al., 1986), could be ingested during suckling and, at least prior to weaning, provide OT to stimulate epithelial OTR.
The distribution of OTR immunoreactivity was, like OTR transcripts, developmentally regulated. In the immature bowel, virtually all myenteric neurons were OTR-immunoreactive. In the adult gut, however, the proportion of neurons with demonstrable OTR immunoreactivity was reduced to 71.4 ± 4.6%. The OTR-immunoreactive subset of neurons included all of those that displayed both cytoplasmic and nuclear NeuN immunoreactivity. Although these cells meet the definition of NeuN-labeled IPANs that has been established for the guinea pig intestine (Van Nassauw et al., 2005), this definition has not yet been validated for the rat ENS. The developmentally regulated distribution of OTR is compatible with the possibility that OT/OTR signaling plays both an early role in development and/or maturation of the ENS and a later role in modulation of enteric neurotransmission, perhaps by affecting the sensitivity and/or irritability of OTR-expressing IPANs. The temporal and spatial pattern of developmental regulation of epithelial OTR was particularly striking. Although OTR immunoreactivity extended to the tips of villi in the developing gut, by P15, OTR immunoreactivity was restricted to crypt cells and was most highly concentrated in a set of cells at the crypt-villus junctions where the immature precursors of the crypts develop into functioning villus absorptive cells. Although this distribution is compatible with the possibility that OT/OTR signaling affects or regulates epithelial maturation, crypt cells are secretory. OT/OTR signaling might thus affect secretion and/or maturation.
Effects exerted on the gut by OT could be direct or indirect, mediated through the OT-induced release of another molecule. Direct actions of OT on enteric neurons, mediated by the stimulation of their G protein-coupled OTR, are probably linked to increases in cyclic AMP and activation of protein kinase A. This type of signaling can exert either acute effects on enteric neurons or long-term actions such as neuronal survival or maturation of the intestinal epithelium. Because OT has been demonstrated to release other neurotransmitters/neuromodulators, such as vasoactive intestinal peptide (VIP) and acetylcholine (ACh) from the gut (Bitar et al., 1980), these or other similar molecules could also be responsible for the ability of OT to provoke short- or long-term changes in enteric behavior. VIP, for example, is a potent neuroprotective agent (Arciszewski and Ekblad, 2005; Arciszewski et al., 2008).
The source of the OT that stimulates enteric OTR might be hypothalamic and/or enteric. Hypothalamic neurons, projecting to the neurohypophysis, release OT into the systemic circulation. In the same way that systemic delivery of OT can induce milk let-down and uterine contraction at parturition, it might also be sufficient to activate enteric OTR. Nurturing stimuli, such as touch and warmth, which are known to affect OT release from hypothalamic neurons (Uvnas-Moberg and Petersson, 2005), might thus be able to affect intestinal motility or sensation by acting on OTR in the ENS. Particularly in view of the developmental regulation of enteric OTR, it is plausible that this action of OT could contribute to the ability of the OT-releasing behaviors of holding, breast feeding, carrying, and rocking to relieve the apparent discomfort of infantile colic (Howard et al., 2006). Alternatively or additionally, the source of OT might be the intrinsic oxytocinergic innervation of the ENS. As yet, nothing is known about what type of stimulation or input activates enteric oxytocinergic neurons. One might postulate that these cells, like their hypothalamic counterparts, respond and act to oppose the effects of stress on the bowel. Indeed OT has been found to oppose acetic acid-induced inflammation of the rat intestine (Iseri et al., 2005), which is consistent with a local action to counteract stress. Synchronous entrainment of OT-mediated responses of the brain and gut might thus be important in ENS development and maintenance.
Separation of offspring from mothers early in postnatal life has been shown to lead to long-lasting changes in bowel function. These changes include visceral hypersensitivity (van den Wijngaard et al., 2005; Welting et al., 2005; Eutamene et al., 2007), increased intestinal permeability (Soderholm et al., 2002; Gareau et al., 2006, 2007), and enhanced intestinal motility. Similarly, long-lasting effects on the bowel occur in response to local irritation of the developing gut itself (Al-Chaer et al., 2000). Corticotrophin-releasing factor (CRF) and/or the CRF-related ligands urocortin 1–3, all of which act on the CRF receptors CRF1 and CRF2, have been implicated in the mediation of the gut’s long-term response to stress (Martinez et al., 2004; Tache et al., 2005; Million et al., 2006). Separation of offspring from mothers and irritation of the developing bowel are both manipulations that would deprive the subjected infants of OT release and thus OTR stimulation. Whether the source of OT is maternal milk, the pup’s hypothalamus, or enteric oxytocinergic neurons, OT/OTR stimulation might be able to oppose at least some of the detrimental and long-lasting effects of CRF and urocortins on the behavior of the bowel.
It is tempting to speculate that visceral hypersensitivity, which is an important component of irritable bowel syndrome (IBS) (Drossman, 1999; Tillisch and Mayer, 2005), arises, in part, because of an endogenous maladaptation that involves enteric OT/OTR signaling. This dysfunction might arise from an environmental perturbation, such as child abuse, which has been linked to the acquisition of long-term visceral hypersensitivity (Talley et al., 1994, 1998; Ringel et al., 2008). Child abuse might well be a stimulus that mimics in humans effects elicited in rats by maternal deprivation. Both may deprive the ENS of the nurturing effects of OT/OTR signaling. Gastrointestinal discomfort, analogous to the visceral hypersensitivity seen in IBS, is significantly more common in children with autistic spectrum disorders (ASDs) than in typically developing children or in children with other developmental disabilities (Valicenti-McDermott et al., 2006). Because of the impairment in social interactions that is the hallmark of ASD, children with an ASD will be unable to receive the nurture offered by their parents. Nurture that is not detected by a child would be expected to lead to the same outcome as nurture not given (Welch and Ruggiero, 2005; Welch et al., 2006) and reduce OT/OTR within the bowel. Low levels of serum OT have been reported in a subset of children with ASD, although the contribution of enteric sources of OT to serum levels is unknown (Modahl et al., 1998). Regardless of whether a defect in OT/OTR signaling is causally related to ASD-associated gastrointestinal discomfort, extrinsic OT might be able to provide relief from it. There is, for example, evidence that OT is helpful in the treatment of ASD (Hollander et al., 2003, 2007).
We conclude that OT/OTR signaling could play an important role both in normal enteric physiology and possibly in the pathophysiology of functional gastrointestinal disorders. Because OT and OTR transcripts and protein are both found within the ENS, OT/OTR signaling does not have to depend on hypothalamic secretion of OT. Because many enteric neurons express OTR, however, OT in the systemic circulation is likely to exert enteric effects. Therefore, hypothalamic and enteric sources of OT may, in appropriate circumstances, act in synchrony. The developmental regulation of OT, which is coincident with that of OTR, strongly suggests that OT/OTR signaling plays a role in development of the ENS and the enteric epithelium. However, the continuing expression of both OT and OTR suggests that the role of this signaling is not restricted to the immature bowel. Because nurturing stimuli are known to provoke OT and to act through OT/OTR signaling, we propose that OT/OTR signaling mediates the effects of these stimuli on the gut. This demonstration of the OT/OTR signaling apparatus within the ENS provides a good reason to test this hypothesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Valerie Boone for immunocytochemistry, Wanda Setlik for electron microscopy, Zhi Shan Li for deconvolution, Christine Chang for excellent technical assistance and Sue Ann Power for close editing. We also thank Dr. Gloria Hoffman at the University of Maryland for providing the oxytocin receptor antibodies, as well as Dr. Jeffrey Mogil at McGill University, Montreal, Canada for providing oxytocin receptor knockout mice.
LITERATURE CITED
- Adan RA, van Leeuwen FW, Sonnemans MA, Hoffman G, Verbalis JG, Burbach JP. The rat oxytocin receptor. cDNA cloning and immunocytochemical localization in brain, pituitary, mammary gland and uterus. Adv Exp Med Biol. 1995;395:345–346. [PubMed] [Google Scholar]
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Amico JA, Finn FM, Haldar J. Oxytocin and vasopressin are present in human and rat pancreas. Am J Med Sci. 1988;296:303–307. doi: 10.1097/00000441-198811000-00003. [DOI] [PubMed] [Google Scholar]
- Arciszewski MB, Ekblad E. Effects of vasoactive intestinal peptide and galanin on survival of cultured porcine myenteric neurons. Regul Pept. 2005;125:185–192. doi: 10.1016/j.regpep.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Arciszewski MB, Sand E, Ekblad E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul Pept. 2008;146:218–223. doi: 10.1016/j.regpep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Stancampiano R, Mauri A, Gessa GL. Hypothalamic modulation of immunoreactive oxytocin in the rat thymus. Peptides. 1990;11:539–543. doi: 10.1016/0196-9781(90)90056-b. [DOI] [PubMed] [Google Scholar]
- Ayad VJ, Guldenaar SE, Wathes DC. Characterization and localization of oxytocin receptors in the uterus and oviduct of the non-pregnant ewe using an iodinated receptor antagonist. J Endocrinol. 1991;128:187–195. doi: 10.1677/joe.0.1280187. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- Bitar KN, Said SI, Weir GC, Saffouri B, Makhlouf GM. Neural release of vasoactive intestinal peptide from the gut. Gastroenterology. 1980;79:1288–1294. [PubMed] [Google Scholar]
- Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Marrocco T, Sapino A, Allia E, Bussolati G. Oxytocin synthesis within the normal and neoplastic breast: first evidence of a local peptide source. Int J Oncol. 2006;28:1263–1268. [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Vinson EN, MacLennan AJ, Gershon MD. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: interactions with neurotrophin-3. Dev Biol. 1998;198:343–365. [PubMed] [Google Scholar]
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes WA, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti R, Poole DP, Kimura H, Aimi Y, Robbins HL, Castelucci P, Furness JB. Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons. Cell Tissue Res. 2003;311:11–22. doi: 10.1007/s00441-002-0652-6. [DOI] [PubMed] [Google Scholar]
- Cicutti NJ, Smyth CE, Rosaeg OP, Wilkinson M. Oxytocin receptor binding in rat and human heart. Can J Cardiol. 1999;15:1267–1273. [PubMed] [Google Scholar]
- D’Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development. 2007;134:2237–2249. doi: 10.1242/dev.003814. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Review article: an integrated approach to the irritable bowel syndrome. Aliment Pharmacol Ther. 1999;13 suppl 2:3–14. doi: 10.1046/j.1365-2036.1999.0130s2003.x. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthesy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- Fairman CL, Clagett-Dame M, Lennon VA, Epstein ML. Appearance of neurons in the developing chick gut. Dev Dyn. 1995;204:192–201. doi: 10.1002/aja.1002040210. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Poulain DA, Theodosis DT. Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neuroscience. 1993;53:77–89. doi: 10.1016/0306-4522(93)90286-o. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–88. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198–G203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- Geenen V, Legros JJ, Franchimont P, Defresne MP, Boniver J, Ivell R, Richter D. The thymus as a neuroendocrine organ. Synthesis of vasopressin and oxytocin in human thymic epithelium. Ann N Y Acad Sci. 1987;496:56–66. doi: 10.1111/j.1749-6632.1987.tb35746.x. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Csernus V. Effect of intratesticular administration of oxytocin on testicular steroidogenesis in immature rats. Andrologia. 1995;27:291–297. doi: 10.1111/j.1439-0272.1995.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol. 1978;180:467–488. doi: 10.1002/cne.901800305. [DOI] [PubMed] [Google Scholar]
- Graf GC. Ejection of milk in relation to levels of oxytocin injected intramuscularly. J Dairy Sci. 1969;52:1003–1007. doi: 10.3168/jds.S0022-0302(69)86684-1. [DOI] [PubMed] [Google Scholar]
- Graf GC. Ejection of milk in relation to oxytocin injected intravenously. J Dairy Sci. 1970;53:1283–1285. doi: 10.3168/jds.S0022-0302(70)86381-0. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, Mukaddam-Daher S, McCann SM. Oxytocin is a cardiovascular hormone. Braz J Med Biol Res. 2000;33:625–633. doi: 10.1590/s0100-879x2000000600003. [DOI] [PubMed] [Google Scholar]
- Hashmonai M, Torem S, Argov S, Barzilai A, Schramek A. Prolonged post-vagotomy gastric atony treated by oxytocin. Br J Surg. 1979;66:550–551. doi: 10.1002/bjs.1800660809. [DOI] [PubMed] [Google Scholar]
- Hind A, Migliori M, Thacker M, Staikopoulos V, Nurgali K, Chiocchetti R, Furness JB. Primary afferent neurons intrinsic to the guinea-pig intestine, like primary afferent neurons of spinal and cranial sensory ganglia, bind the lectin, IB4. Cell Tissue Res. 2005;321:151–157. doi: 10.1007/s00441-005-1129-1. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Howard CR, Lanphear N, Lanphear BP, Eberly S, Lawrence RA. Parental responses to infant crying and colic: the effect on breastfeeding duration. Breastfeed Med. 2006;1:146–155. doi: 10.1089/bfm.2006.1.146. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin gene occur by different mechanisms. Dev Biol. 1995;167:237–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT. Central administration of oxytocin modulates the infant rat’s response to social isolation. Eur J Pharmacol. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Jasper JR, Harrell CM, O’Brien JA, Pettibone DJ. Characterization of the human oxytocin receptor stably expressed in 293 human embryonic kidney cells. Life Sci. 1995;57:2253–2261. doi: 10.1016/0024-3205(95)02218-8. [DOI] [PubMed] [Google Scholar]
- Jeng YJ, Lolait SJ, Strakova Z, Chen C, Copland JA, Mellman D, Hellmich MR, Soloff MS. Molecular cloning and functional characterization of the oxytocin receptor from a rat pancreatic cell line (RINm5F) Neuropeptides. 1996;30:557–565. doi: 10.1016/s0143-4179(96)90039-6. [DOI] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin—anatomy and functional assignments: a minireview. Endocr Regul. 2005;39:97–105. [PubMed] [Google Scholar]
- Krowicki ZK, Sharkey KA, Serron SC, Nathan NA, Hornby PJ. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K. Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res. 2005;79:295–302. doi: 10.1002/jnr.20354. [DOI] [PubMed] [Google Scholar]
- Louvel D, Delvaux M, Felez A, Fioramonti J, Bueno L, Lazorthes Y, Frexinos J. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996;39:741–747. doi: 10.1136/gut.39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Wang L, Million M, Rivier J, Tache Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1744. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Melis MR, Stancampiano R, Argiolas A. Oxytocin- and vasopressin-like immunoreactivity in the rat thymus: characterization and possible involvement in the immune response. Regul Pept. 1993;45:269–272. doi: 10.1016/0167-0115(93)90218-w. [DOI] [PubMed] [Google Scholar]
- Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, McRoberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Tache Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept. 2004;119:39–44. doi: 10.1016/j.regpep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Truedsson M, Bengtsson M, Torstenson R, Sjolund K, Bjornsson ES, Simren M. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol Motil. 2005;17:697–704. doi: 10.1111/j.1365-2982.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Bjorgell O, Ekberg O, Darwiche G. The oxytocin/vasopressin receptor antagonist atosiban delays the gastric emptying of a semisolid meal compared to saline in human. BMC Gastroenterol. 2006a;6:11. doi: 10.1186/1471-230X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson B, Truedsson M, Djerf P, Sundler F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul Pept. 2006b;135:7–11. doi: 10.1016/j.regpep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Petring OU. The effect of oxytocin on basal and pethidine-induced delayed gastric emptying. Br J Clin Pharmacol. 1989;28:329–332. doi: 10.1111/j.1365-2125.1989.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Hargrave SL, Rhodes BS, Zopf DA, Powley TL. Quantification of neurons in the myenteric plexus: an evaluation of putative pan-neuronal markers. J Neurosci Methods. 2004;133:99–107. doi: 10.1016/j.jneumeth.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res. 2006;325:413–422. doi: 10.1007/s00441-006-0181-9. [DOI] [PubMed] [Google Scholar]
- Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253:R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Sala NL, Luther EC, Arballo JC, Cordero Funes JC. Oxytocin reproducing reflex milk ejection in lactating women. J Appl Physiol. 1974;36:154–158. doi: 10.1152/jappl.1974.36.2.154. [DOI] [PubMed] [Google Scholar]
- Sapino A, Cassoni P, Stella A, Bussolati G. Oxytocin receptor within the breast: biological function and distribution. Anticancer Res. 1998;18:2181–2186. [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Hofer MA. Oxytocin reinstates maternal olfactory cues for nipple orientation and attachment in rat pups. Physiol Behav. 1978;20:385–389. doi: 10.1016/0031-9384(78)90317-7. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1257–G1263. doi: 10.1152/ajpgi.00314.2002. [DOI] [PubMed] [Google Scholar]
- Spiller R. Pharmacotherapy: non-serotonergic mechanisms. Gut. 2002;51 suppl 1:i87–i90. doi: 10.1136/gut.51.suppl_1.i87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend Med. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Kuwabara Y, Mizuno M. Concentrations and origin of oxytocin in breast milk. Endocrinol Jpn. 1986;33:821–826. doi: 10.1507/endocrj1954.33.821. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA. Pain perception in irritable bowel syndrome. CNS Spectr. 2005;10:877–882. doi: 10.1017/s1092852900019830. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K, Petersson M. [Oxytocin, a mediator of anti-stress, well-being, social interaction, growth and healing] Z Psychosom Med Psychother. 2005;51:57–80. doi: 10.13109/zptm.2005.51.1.57. [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott M, McVicar K, Rapin I, Wershil BK, Cohen H, Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J Dev Behav Pediatr. 2006;27(2 suppl):S128–S136. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R, Welting O, de Jonge W, Boeckxstaens G. Parental care, mast cells and visceral hypersensitivity. J Pediatr Gastroenterol Nutr. 2005;41 suppl 1:S12–S13. doi: 10.1097/01.scs.0000180288.36118.1f. [DOI] [PubMed] [Google Scholar]
- Van Nassauw L, Wu M, De Jonge F, Adriaensen D, Timmermans JP. Cytoplasmic, but not nuclear, expression of the neuronal nuclei (NeuN) antibody is an exclusive feature of Dogiel type II neurons in the guinea-pig gastrointestinal tract. Histochem Cell Biol. 2005;124:369–377. doi: 10.1007/s00418-005-0019-7. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Dyball RE, Lincoln DW. Milk ejection in the rat: the result of a selective release of oxytocin. J Endocrinol. 1973;57:557–558. doi: 10.1677/joe.0.0570557. [DOI] [PubMed] [Google Scholar]
- Welch MG, Ruggiero DA. Predicted role of secretin and oxytocin in the treatment of behavioral and developmental disorders: implications for autism. In: Dhossche D, editor. GABA in autism and related disorders Vol. 71, International Review of Neurobiology. New York: Elsevier/Academic Press; 2005. pp. 273–315. [DOI] [PubMed] [Google Scholar]
- Welch MG, Welch-Horan TB, Anwar M, Anwar N, Ludwig RJ, Ruggiero DA. Brain effects of chronic IBD in areas abnormal in autism and treatment by single neuropeptides secretin and oxytocin. J Mol Neurosci. 2005;25:259–274. doi: 10.1385/JMN:25:3:259. [DOI] [PubMed] [Google Scholar]
- Welch MG, Northrup RS, Welch-Horan TB, Ludwig RJ, Austin CL, Jacobson JS. Outcomes of prolonged parent-child embrace therapy among 102 children with behavioral disorders. Complement Ther Clin Pract. 2006;12:3–12. doi: 10.1016/j.ctcp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Welting O, Van Den Wijngaard RM, De Jonge WJ, Holman R, Boeckxstaens GE. Assessment of visceral sensitivity using radio telemetry in a rat model of maternal separation. Neurogastroenterol Motil. 2005;17:838–845. doi: 10.1111/j.1365-2982.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Wu CL, Hung CR, Chang FY, Pau KY, Wang JL, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflugers Arch. 2002;445:187–193. doi: 10.1007/s00424-002-0925-7. [DOI] [PubMed] [Google Scholar]
- Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Pharmacological effects of oxytocin on gastric emptying and intestinal transit of a non-nutritive liquid meal in female rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:406–413. doi: 10.1007/s00210-003-0690-y. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kimura T, Watanabe D, Kiyama H. Differential expression of oxytocin receptor mRNA in the developing rat brain. Neurosci Res. 1996;24:291–304. doi: 10.1016/0168-0102(95)01003-3. [DOI] [PubMed] [Google Scholar]
- Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res. 2005;320:1–9. doi: 10.1007/s00441-004-1057-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.