Abstract
The folding mechanism of two closely related proteins in the intracellular lipid binding protein family, human bile acid binding protein (hBABP) and rat bile acid binding protein (rBABP) were examined. These proteins are 77% identical (93% similar) in sequence Both of these single domain proteins fit well to a two-state model for unfolding by fluorescence and circular dichroism at equilibrium. Three phases were observed during the unfolding of rBABP by fluorescence but only one phase was observed during the unfolding of hBABP, suggesting that at least two kinetic intermediates accumulate during the unfolding of rBABP that are not observed during the unfolding of hBABP. Fluorine NMR was used to examine the equilibrium unfolding behavior of the W49 side chain in 6-fluorotryptophan labeled rBABP and hBABP. The structure of rBABP appears to be more dynamic than that of hBABP in the vicinity of W49 in the absence of denaturant, and urea has a greater effect on this dynamic behavior for rBABP than for hBABP. As such, the folding behavior of highly sequence related proteins in this family can be quite different. These differences imply that moderately sized proteins with high sequence and structural similarity can still populate quite different structures during folding.
Keywords: protein denaturation, kinetics, fluorine NMR, β-sheet, folding initiation, molten globule
INTRODUCTION
One major question in protein folding is how dissimilar sequences can achieve similar final structures. Most small proteins (<100 residues) with high structural homology appear to conserve the nature of the transition state and the folding pathway even when sequence homologies are low.1 However, the folding of larger proteins with similar structures is considerably more complex, and alternative folding paths with different numbers and types of intermediates can be observed.1 One family of proteins with this complex folding behavior is the intracellular lipid binding proteins.
These mostly β-sheet proteins (127 –134 residues) typically have quite dissimilar sequences (pairwise sequence identities ranging from 8 to 40%), despite having very similar structures.2,3 The folding mechanism of a number of proteins in this family has been examined, and considerable heterogeneity in the number and types of intermediates has been observed.4–7 The overall rates of folding of these proteins vary by over 2 orders of magnitude despite having nearly identical structures and contact orders. A recent study showed that single amino acid substitutions in the hydrophobic core of these proteins had predictable effects on the number and types of intermediates observed,8 suggesting a role for the structure and stability of a folding initiation site in later folding events.
These studies examined proteins with different ligand specificities, tissue distributions, and genes, all of which contribute to the high degree of sequence variation among these proteins.2,3 The folding of functionally identical proteins in this family from the same tissue in different species has not been examined. Since single amino acid changes were sufficient to change the apparent folding mechanism of two proteins in the family,8 it was of interest to examine the same gene product from different species, in order to determine if any difference in folding mechanism could be observed for much more similar sequences. This study compares the folding of the rat and human versions of bile acid binding protein (rBABP and hBABP, respectively, also known as ileal lipid binding protein or gastrotropin). These two proteins have 77% sequence identity and 93% sequence similarity. The structure of rBABP is not known, but is highly likely to be nearly identical to that of hBABP9 (Figure 1), especially considering that the structural homology of proteins in this family is as great as that for the same protein determined by NMR and X-ray crystallography.2,4 Despite their sequence and structural homology, significant differences in the folding behavior of these proteins was observed.
Figure 1.
Cartoon structure of hBABP (pdb code 1o1u).
MATERIALS AND METHODS
Protein Source and Purification
The expression vectors for rBABP and hBABP have been described.10 Unlabeled proteins were expressed in E. coli strain MG1655 in terrific broth. 6FTrp-rBABP and 6FTrp-hBABP were expressed in E. coli strain KS463, a tryptophan auxotroph obtained from the Yale E. coli genetic stock center. The media and methods used to produce labeled proteins were identical to those used for labeling intestinal fatty acid binding protein with 6FTrp.11
The proteins were extracted from E. coli using the freeze thaw method12 and purified from the soluble fraction as previously described.4 Protein purity was demonstrated by the presence of a single band on a SDS polyacrylamide gel. The extinction coefficients were calculated at 280 nm: 0.80 mg−1cm−1 for hBABP and 6FTrp-hBABP and 0.90 mg−1cm−1 for rBABP and 6FTrp-rBABP.13
Reagents
Urea stock solutions (approximately 10 M, ultrapure, Amresco) were prepared and stored at −20 °C as previously described.14 On the day of an experiment, a working solution of about 9 M urea was prepared by adding the buffer components to a freshly thawed urea stock solution. All experiments were performed at 25 °C in 25 mM NaPO4, 75 mM NaCl, and 0.1 mM EDTA at pH 8. Actual denaturant concentrations were determined by refractive index measurements with a Milton Roy Abbe-3 refractometer at 25 °C.15 All buffers were filtered through 0.2 µm Whatman nylon membranes before use. All chemicals were reagent grade.
Equilibrium Studies
Unfolding transitions as a function of denaturant concentration were monitored by circular dichroism (CD) and fluorescence. A Jasco J-710 spectropolarimeter was employed to follow changes in secondary structure in the far-UV portion of the CD spectrum.8 The dependence of the signal change on urea concentration was monitored at 216, 218, and 222 nm. No significant differences in fit parameters were observed for any wavelength. Fluorescence studies were carried out with a PTI QuantaMaster luminescence spectrometer.8 Samples ranging in denaturant concentrations from 0 to 8.5 M urea were prepared using a Hamilton Microlab titrator at protein concentrations of 0.1 – 0.2 mgs/ml.16 The data were corrected for the background signal of the buffer and urea solutions. For comparison of denaturation curves, the data for rBABP, 6FTrp-rBABP and 6FTrp-hBABP were normalized from 0 to 1, where 0 and 1 were assigned to the lowest and highest observed intensity, respectively. For hBABP, the data were also normalized to 0 to 1, but 0 was assigned to the highest observed intensity and 1 was assigned to the lowest observed intensity, so that all of the transitions would be in the same direction. The dependence of the signal change on urea concentration was monitored at 333, 340, 355, and 370 nm for all proteins. No significant differences in fit parameters were observed at any wavelength. Nonlinear least-squares fit to the equilibrium data were generated using KaleidaGraph (Synergy Software) as previously described.5,17 No significant differences in the fitting parameters were observed at different protein concentrations. All fits in Table 1 were to a minimum of two independent data sets.
Table 1.
Summary of fits to a two state model for unfolding.
| Protein (Method) | ΔGH2O (kcal mol−1) |
mG (kcal mol−1 M−1) |
Midpoint (M) |
|---|---|---|---|
| rBABP (Fluor – 340 nm) | 4.84 ± 0.36 | −1.61 ± 0.12 | 3.01 ± 0.03 |
| rBABP (CD – 218 nm) | 4.51 ± 0.29 | −1.51 ± 0.16 | 2.99 ± 0.14 |
| 6FTrp-rBABP (Fluor – 340 nm) | 3.81 ± 0.19 | −1.34 ± 0.06 | 2.83 ± 0.03 |
| 6FTrp-rBABP (CD – 218 nm) | 3.61 ± 0.45 | −1.26 ± 0.14 | 2.87 ± 0.14 |
| hBABP (Fluor – 370 nm) | 6.48 ± 0.20 | −1.81 ± 0.05 | 3.58 ± 0.01 |
| hBABP (CD – 218 nm) | 5.93 ± 0.58 | −1.61 ± 0.16 | 3.68 ± 0.07 |
| 6FTrp-hBABP (Fluor – 340 nm) | 5.76 ± 0.19 | −1.55 ± 0.05 | 3.73 ± 0.03 |
| 6FTrp-rBABP (CD – 218 nm) | 6.10 ± 0.39 | −1.64 ± 0.10 | 3.72 ± 0.03 |
| 6FTrp-hBABP in 10% D2O (Fluor – 340 nm) | 5.99 ± 0.52 | −1.55 ± 0.13 | 3.86 ± 0.03 |
| 6FTrp-hBABP in 10% D2O (NMR) | 6.86 ± 0.35 | −1.78 ± 0.20 | 3.85 ± 0.04 |
Stopped-Flow Fluorescence Kinetic Studies
The kinetics of unfolding and refolding of all of the proteins were monitored using an Applied Photophysics DX-17MV Stopped flow Spectrophotometer. Excitation was at 290 nm (2 nm bandpass) using a 0.2 cm path length cell at 25 °C. Emission intensity was monitored above 305 nm at 90° through a WG305 filter (Oriel). Two drive syringes (2.5 ml and 0.5 ml) were used to mix 5 parts of denaturant or buffer solution with one part of protein solution in buffer with or without denaturant (0.26 mg/ml, final concentration). Five to seven kinetic traces were averaged for each denaturant concentration. The dead time of this instrument is 5 – 10 msec, depending on the final denaturant concentration.14 Data collected during the dead time were excluded from the analysis.
The nonlinear least square fit of the fluorescence kinetic data to monophasic, monophasic plus steady state, biphasic, and triphasic models was accomplished with the program supplied by Applied Photophysics. The goodness of the fit to the various models was determined using published criteria.18,19
19F-NMR Studies
NMR data were collected on a Bruker AMX-500 spectrometer using a 5 mm Bruker hydrogen/fluorine dual channel probe at 470.54 MHz. The 19F spin lattice relaxation times were determined using the inversion recovery method and were <0.5 sec for all protein resonances. The pulse interval and pulse width were 2.5 sec and 15.2 µsec, respectively. The sweep widths ranged from 9000 to 12,000 Hz, and the acquisition time was 0.7 sec. The spectral offset and sweep width depended on the labeled protein and standard used, but always extended at least 500 Hz beyond the frequencies observed for the native resonances. Spectra were collected with decoupling of the aromatic ring protons during data collection. At least 256 transients were collected for each sample. 3-fluorotyrosine was used as an external chemical shift and concentration standard, because hBABP and rBABP bind the previously used external standard 4-fluorophenylalanine with milliMolar affinity. Proteins were dialyzed against 25 mM PO4, 75 mM NaCl, 0.1 mM EDTA at pH 8 at concentrations greater than 10 mg/ml. Five mg aliquots of each protein were placed in 2 ml eppendorf tubes and sufficient buffer was added to bring the final volume to 0.6 ml. The proteins were lyophilized, and stored at −20 °C until use. The lyophilized samples were dissolved in the appropriate volumes of water and 8 M urea containing 10% D2O for a final volume of 0.6 ml at each concentration of urea.
Spectra were collected at 25 °C and processed with the NUTS software program (Acorn NMR, Livermore, CA) using 5 Hz of line broadening. Line widths were determined with the NUTS software. Simulations were performed with WINDNMR20 (http://www.chem.wisc.edu/areas/reich/plt/windnmr.htm) to calculate populations of the native and unfolded states as previously described.21
RESULTS AND DISCUSSION
Spectral comparison
The fluorescence spectra of native hBABP and rBABP were quite different (Figure 2), despite only one amino acid substitution (L90 in hBABP is V90 in rBABP) whose sidechain is within 6 Å of the W49 side chain in the hBABP structure. The native state fluorescence intensity for rBABP was approximately three times that of hBABP, and the wavelength of maximal fluorescence was red shifted 6 nm for rBABP compared to hBABP, suggesting greater solvent exposure for this tryptophan in rBABP than in hBABP. Similar differences in fluorescence intensity and maximal wavelength of fluorescence were observed for the native state of the respective 6FTrp-labeled proteins (Figure 2). The weak fluorescence of native hBABP compared to unfolded hBABP required different data processing procedures than the other proteins, as described in the methods. The fluorescence properties (wavelength of maximal intensity and overall intensity) of the unfolded states of the 6FTrp-labeled and unlabeled proteins were identical to each other, as expected for the complete exposure of the tryptophan side chain to solvent in the extended unfolded state. The spectrum of unfolded 6FTrp-rBABP is shown as an example in Figure 2. The CD spectra of the native and unfolded states of all of the labeled and unlabeled proteins were identical (data not shown), suggesting that the secondary structures of these proteins were identical.
Figure 2.
Fluorescence spectra of native hBABP (○), rBABP (×), 6FTrp-hBABP (□), and 6FTrp-rBABP (◊) in buffer, and unfolded 6FTrp-rBABP (●) in buffer with 8.0 M urea.
Equilibrium unfolding
The equilibrium unfolding of rBABP has been previously reported.4,8 Like rBABP, the equilibrium unfolding of hBABP fits well to a two state model for the unfolding transition (Figure 3), with no significant accumulation of intermediates detected at equilibrium by both methods. Similar midpoints and stability for unfolding were observed by CD and fluorescence for each protein (Table 1). Regardless of the method used, all of the transitions by fluorescence and CD were completely reversible and independent of protein concentration. The stability of hBABP was greater than that of rBABP, with a midpoint for unfolding with urea more than 0.5 M urea higher than that of rBABP. Similar differences in stability have been observed for single site mutations of proteins in this family,8,21 and there are 29 amino acid changes (19 conservative) between these two sequences. As such, it is not possible to relate the stability differences to any specific amino acid change.
Figure 3.
Dependence of fluorescence (370 nm,□) and CD (218 nm, ○) intensity on urea concentration for hBABP.
Refolding kinetic studies
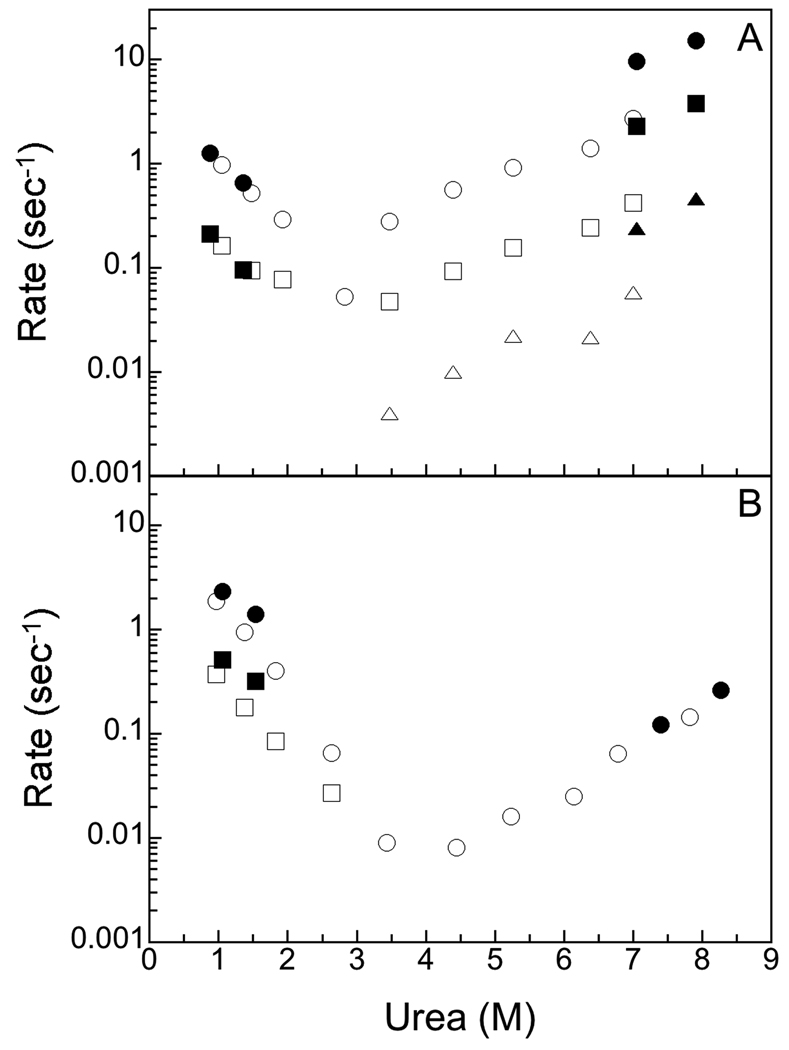
The kinetics of refolding of hBABP and rBABP were very similar (Figure 4). The folding of rBABP has been reported previously.4,8 Two phases were observed for both proteins during refolding, and the entire expected amplitude change was observed for each final concentration of denaturant (no burst phases). The faster of the two folding phases accounted for the entirety of the folding transition observed by CD, and the majority of the fluorescence change. A second slower refolding phase of smaller amplitude was observed by fluorescence that resulted in native state fluorescence. Overall, the data fits a sequential folding pathway (U => I1 => N). The intermediate state (I1) appears to have the spectral characteristics of a molten globule, with native like amounts of secondary structure as monitored by CD, but lacking at least some of the specific tertiary interactions typical of the native state.22 A kinetic intermediate with similar spectral properties has been observed for several other proteins in this protein family.5–7 The overall rates of folding of hBABP and rBABP were very similar, suggesting that similar transition state(s) and intermediate structure(s) are present during the refolding process for these closely related proteins.
Figure 4.
Dependence of the rates of folding and unfolding on urea concentration by fluorescence (open symbols) and CD (closed symbols) for hBABP. The gray lines are the folding and unfolding rates for rBABP fit to an exponential dependence on denaturant concentration.
Unfolding kinetic studies
Considerable differences were observed during the unfolding of these proteins (Figure 4). The first study of the unfolding of rBABP detected two phases during unfolding.4 Instrument improvements showed the presence of an additional small amplitude slow phase that was not detected in the original study.8 Thus, three phases were observed during the unfolding of rBABP by fluorescence, with the entire secondary structure change as monitored by CD occurring at the same rate as the second unfolding rate observed by fluorescence.8 Assuming a single pathway for unfolding, (N => I1 => I2 => U), the first intermediate has the same molten globule like properties of the intermediate observed during refolding. Previous double jump experiments have shown that the I1 intermediate is on path during refolding.4 In contrast, I2 lacks any significant secondary structure by CD criteria, but its fluorescence properties are not identical to that of the extended unfolded state.8 This intermediate may correspond to a folding initiation site, where a limited number of residues may be involved in potentially native-like contacts prior to the formation of stable secondary structure.8 Both types of kinetic intermediates have been observed during unfolding for other members of the iLBP family.4–8 Although it is possible that the multiple phases observed during unfolding could be due to multiple native states, each of which unfolds at a different rate, this explanation is unlikely, because the second phase has no detectable secondary structure component by CD. Thus, the slower unfolding native state would have to have little if any secondary structure. Further, natural abundance 1H13C HSQC experiments showed that the methyl resonances of the protein are not broadened by exchange, and the number of resonances observed correlated well with the expected number of methyl groups for both proteins (data not shown), arguing against multiple conformations of the native state for rBABP.
Unlike rBABP, only one rate was observed during the unfolding of hBABP (Figure 4). This phase accounted for the entire expected amplitude of the unfolding transition by both fluorescence and CD. This transition is similar to that of the middle rate during the unfolding of rBABP, since all of the secondary and most of the tertiary structure breaks down during the middle phase for rBABP. The observed unfolding rate for the single unfolding transition of hBABP is much slower than that of the middle unfolding transition of rBABP. Thus, slower unfolding rather than faster refolding appears to be responsible for the increased equilibrium stability of hBABP compared to rBABP. The rates of unfolding have been correlated with the thermal stabilities of thermophilic, mesophilic and psychrotrophic 3-Isopropylmalate dehydrogenases.23
It is not clear why I1 and I2 are not observed during the unfolding of hBABP. The most likely explanation is that both kinetic intermediates are not stable enough relative to the native and extended unfolded states to accumulate to detectable concentrations during unfolding, and thus cannot be observed. Both of these proteins fit well to a two state model for the unfolding process at equilibrium, implying that all of the observed kinetic intermediates are inherently less stable than either the native or extended unfolded states. If these kinetic intermediates are even less stable for hBABP, they might not be detected even though they are present. The increased concentrations of denaturant required to unfold this protein might also make the intermediates more difficult to detect.
6FTrp labeled rBABP
Previous studies of the folding of intestinal fatty acid binding protein (IFABP) by NMR have shown the presence of an equilibrium intermediate that was not detectable by either CD or fluorescence.11,21 These experiments involved the replacement of the aromatic amino acids of the of the protein with fluorine labeled aromatic amino acids.21 The single tryptophan (W49) of rBABP and hBABP was replaced with 6-fluorotryptophan (6FTrp) to determine if a similar intermediate could be detected for these proteins. The dependence of the fluorine spectra of 6FTrp-rBABP on urea is shown in Figure 5. As expected for a protein with one tryptophan, one resonance was observed for the native and unfolded states. Unlike the previously published results for 6FTrp-IFABP,11,21 4FPhe-IFABP,21 3FTyr-IFABP,21 and 6FTrp-hBABP (shown below), the native resonance for 6FTrp-rBABP was very broad (80 – 120 Hz), and the peak width increased as the concentration of urea increased. The transition from the native to the unfolded state by NMR occurred over the same concentration range as that observed by fluorescence for the labeled protein (Figure 6A), but the behavior of the native state resonance precluded the accurate determination of the populations of the native and unfolded states. The simplest explanation for this behavior is that the tryptophan sidechain has multiple conformations in the native state that are in conformational exchange on a timescale that causes chemical exchange line broadening. The addition of urea either increases the rate of exchange between the conformers, and/or increases the number of conformers present, causing increased line broadening at higher concentrations of denaturant. As described above, the natural abundance 1H13C HSQC experiments argue against multiple conformations for all of the sidechains of rBABP, but do not preclude mobility of individual sidechains. It has been speculated that increased dynamic movement might be necessary to bind the large steroid ligands bound by this protein.24 Addition of ligand appears to sharpen this resonance at 0M urea, suggesting that ligand binding fixes the position of the tryptophan side chain (data not shown).
Figure 5.
Spectra of 6FTrp-rBABP at the listed concentrations of urea. The chemical shift scale is from 3-fluoro-tyrosine.
Figure 6.
6A. Dependence of fluorescence intensity on urea concentration for 6FTrp-rBABP (□). 6B. Dependence of fluorescence intensity (□) and NMR population (●) on urea concentration for 6FTrp-hBABP. The line through the data is that of a fit to a two state model for stability for each data set. The gray lines in each figure are the fit to the unlabeled protein.
Unlike the other proteins in this family that have been previously labeled with 6FTrp,11,21 4FPhe,21 or 3FTyr,21 6FTrp-rBABP was significantly destabilized by the substitution (Figure 6A, Table 1). The midpoint of denaturation, the extrapolated change in free energy at 0 M denaturant, and the dependence of the free energy on denaturant concentration were all significantly decreased for the labeled protein. Mono-fluorinated amino acids are somewhat more hydrophobic than their unlabeled counterparts,25,26 and usually stabilize proteins slightly due to this increased hydrophobicity. W49 is completely buried in a hydrophobic pocket, but there must be an unexpected unfavorable interaction between the fluorine atom on the labeled sidechain and the rest of the protein that causes this destabilization. The destabilization may also be one of the causes of the line broadening observed for this resonance.
6FTrp labeled hBABP
Unlike 6FTrp-rBABP, the replacement of the tryptophan of hILBP with 6FTrp led to a protein that was slightly more stable than the unlabeled protein (Figure 6B, Table 1), as compared by the midpoint of the transition. The midpoint of denaturation appears to be a better estimate of stability than ΔGH2O given the apparent effects of fluorinated amino acids on the folding transition (see below). IFABP shows similar behavior when labeled with fluoro-aromatic amino acids.21 The equilibrium unfolding of 6FTrp-hBABP as monitored by fluorine NMR is shown in Figure 7. The native resonance of 6FTrp-hBABP has a relatively narrow peak width, 40 Hz, similar to that observed for the two tryptophan resonances in the native state for IFABP,21 instead of the broad resonance observed for 6FTrp-rBABP. There is a linear dependence of the resonance frequency for the native state on denaturant concentration, similar to that previously observed for some resonances in the 4-fluorophenylalanine labeled IFABP spectra.21 The fit of the changes in population of the native state to a two state model for unfolding are shown in Figure 6B. The parameters for this fit are similar to those for the transition observed by fluorescence for the labeled protein (Table 1). The slight increase in stability and midpoint for the NMR transition appears to be caused by the presence of 10% D2O in the NMR samples. Fluorescence unfolding transitions performed in 10% D2O also showed a slight increase in stability compared to experiments in 100% H2O (Table 1). The decrease of the dependence of ΔGH2O on urea concentration (mG) is consistently lower for fluoro-labeled proteins than for the unlabeled proteins (Table 1), as observed for fluoro-labeled IFABP.21 The simplest explanation for this phenomena is that the somewhat more hydrophobic fluorinated amino acids stabilize intermediates during the unfolding process, even if the intermediates cannot be detected by NMR.
Figure 7.
Spectra of 6FTrp-hBABP at the listed concentrations of urea. The chemical shift scale is from 3-fluoro-tyrosine.
Neither 6FTrp-hBABP nor 6FTrp-rBABP show any sign of the chemical exchange intermediate observed for 6FTrp-IFABP.11,21 These studies showed evidence for a state in intermediate chemical exchange with the unfolded state for W82 in IFABP, but not W6 in the same protein. The absence of similar chemical exchange behavior does not prove that the equilibrium intermediate is not present in either hBABP or rBABP, but if the intermediate exists, either W49 does not participate in it, or it is populated at such a low level that it has no effect on the unfolded spectra. Preliminary studies of 4-fluoro-phenylalanine labeled hBABP suggest that at least three of the phenylalanine residues in hBABP participate in some structure that is in fast exchange with the extended unfolded state, but further experiments are necessary to identify the specific residues involved.
Kinetics of folding of 6FTrp-labeled proteins
One concern with the use of fluoro-tryptophan labeled proteins to examine protein folding is that the label would perturb the folding mechanism of the protein, because the properties of the fluorine labeled side chain are not identical to the unlabeled side chain.25,26 Figure 8 compares the dependence of the rates of folding and unfolding 6FTrp-rBABP and 6FTrp-hBABP on denaturant concentration. Overall, the mechanism of folding was not affected by the mutations, with the same number of phases observed for each transition. However, reflecting the changes in stability caused by the fluorine substitution, the rates of unfolding of 6FTrp-rBABP were increased, causing a decrease in the stability of the native state. The rates of folding of 6FTrp-hBABP were increased, reflecting the increased stability of the native state of 6FTrp-hBABP.
Figure 8.
Dependence of the rates of folding and unfolding on urea concentration for WT-rBABP (open symbols) and 6Ftrp-rBABP (closed symbols). 3B. Dependence of the rates of folding and unfolding on urea concentration for WT-hBABP (open symbols) and 6FTrp-hBABP (closed symbols).
In summary, significant differences were observed in the folding behavior of hBABP compared to rBABP, despite a sequence similarity of 93%. Kinetic data alone does not prove that the folding mechanisms are actually different from each other. The observed differences may be attributed to decreased stability for the same intermediate structures for the hBABP sequence, making the intermediates impossible to observe by these techniques. Regardless, it is clear that these single domain proteins (126–133 amino acids) show a rich divergence of spectral and structural intermediates during folding and unfolding, even when the sequence homology is high.
ACKNOWLEDGEMENTS
This NMR instrumentation and research in this project is funded, in part, under a grant from the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. Additional instrumentation support came from NIH 1 S10 RR021172.
Grant Sponsor: NIH; Grant number RO1 GM-57906
REFERENCES
- 1.Gunasekaran K, Eyles SJ, Hagler AT, Gierasch LM. Keeping it in the family: folding studies of related proteins. Curr Opin Struct Biol. 2001;11(1):83–93. doi: 10.1016/s0959-440x(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 2.Banaszak L, Winter N, Xu Z, Bernlohr DA, Cowan S, Jones TA. Lipid-binding proteins: A family of fatty acid and retinoid transport proteins. Adv Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- 3.Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: Molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11(5):175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 4.Dalessio PM, Ropson IJ. β-sheet proteins with nearly identical structures have different folding intermediates. Biochemistry. 2000;39(5):860–871. doi: 10.1021/bi991937j. [DOI] [PubMed] [Google Scholar]
- 5.Burns LL, Dalessio PM, Ropson IJ. Folding mechanism of three structurally similar β-sheet proteins. Proteins. 1998;33(1):107–118. doi: 10.1002/(sici)1097-0134(19981001)33:1<107::aid-prot10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Burns LL, Ropson IJ. Folding of intracellular retinol and retinoic acid binding proteins. Proteins. 2001;43(3):292–302. doi: 10.1002/prot.1040. [DOI] [PubMed] [Google Scholar]
- 7.Clark PL, Weston BF, Gierasch LM. Intrinsic tryptophans of CRABP I as probes of structure and folding. Protein Sci. 1996;5(6):1108–1117. doi: 10.1002/pro.5560050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalessio PM, Boyer JA, McGettigan JL, Ropson IJ. Swapping core residues in homologous proteins swaps folding mechanism. Biochemistry. 2005;44(8):3082–3090. doi: 10.1021/bi048125u. [DOI] [PubMed] [Google Scholar]
- 9.Kurz M, Brachvogel V, Matter H, Stengelin S, Thuring H, Kramer W. Insights into the bile acid transportation system: The human ileal lipid-binding protein-cholyltaurine complex and its comparison with homologous structures. Proteins. 2003;50(2):312–328. doi: 10.1002/prot.10289. [DOI] [PubMed] [Google Scholar]
- 10.Tochtrop GP, Richter K, Tang C, Toner JJ, Covey DF, Cistola DP. Energetics by NMR: Site specific binding in a positively cooperative system. Proc Natl Acad Sci USA. 2002;99(4):1847–1852. doi: 10.1073/pnas.012379199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ropson IJ, Frieden C. Dynamic NMR spectral analysis and protein folding: Identification of a highly populated folding intermediate of rat intestinal fatty acid binding protein by 19F NMR. Proc Natl Acad Sci USA. 1992;89(15):7222–7226. doi: 10.1073/pnas.89.15.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson BH, Hecht MH. Recombinant proteins can be isolated from E. coli cells by repeated cycles of freezing and thawing. Biotechnology. 1994;12(13):1357–1360. doi: 10.1038/nbt1294-1357. [DOI] [PubMed] [Google Scholar]
- 13.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4(11):2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ropson IJ, Dalessio PM. Fluorescence spectral changes during the folding of intestinal fatty acid binding protein. Biochemistry. 1997;36(28):8594–8601. doi: 10.1021/bi962983b. [DOI] [PubMed] [Google Scholar]
- 15.Pace CN. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- 16.Gualfetti PJ, Bilsel O, Matthews CR. The progressive development of structure and stability during the equilibrium unfolding of the R-subunit of tryptophan synthase from Escherichia coli. Protein Sci. 1999;8(8):1623–1635. doi: 10.1110/ps.8.8.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoro MM, Bolen DW. Unfolding free energy changes determined by the linear extrapolation method: Unfolding of phenylmethanesulfonyl R-chymotrypsin using different denaturants. Biochemistry. 1988;27(21):8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- 18.Mannervik B. Regression analysis, experimental error, and statistical criteria in the design and analysis of experiments for discrimination between rival kinetic models. Methods Enzymol. 1982;87:370–390. doi: 10.1016/s0076-6879(82)87023-7. [DOI] [PubMed] [Google Scholar]
- 19.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: A practical and nonmathematical review. FASEB J. 1987;1(5):365–374. [PubMed] [Google Scholar]
- 20.Reich HJ. WinDNMR - Dynamic NMR Spectra for Windows. J Chem Educ Software. 1996;3D2:38. [Google Scholar]
- 21.Ropson IJ, Boyer JA, Dalessio PM. A residual structure in unfolded intestinal fatty acid binding protein consists of residues that are neighbors in the native state. Biochemistry. 2006;45(8):2608–2617. doi: 10.1021/bi052091o. [DOI] [PubMed] [Google Scholar]
- 22.Ptitsyn OB. Molten globule and protein folding. Adv. Prot. Chem. 1995;47:83–229. doi: 10.1016/s0065-3233(08)60546-x. [DOI] [PubMed] [Google Scholar]
- 23.Graczer E, Varga A, Hajdu I, Melnik B, Szilagyi A, Semisotnov G, Zavodszky P, Vas M. Rates of unfolding, rather than refolding, determine thermal stabilities of thermophilic, mesophilic and psychrotrophic 3-Isopropylmalate dehydrogenases. Biochemistry. 2007;46(41):11536–11549. doi: 10.1021/bi700754q. [DOI] [PubMed] [Google Scholar]
- 24.Lucke C, Zhang F, Ruterjans H, Hamilton JA, Sacchettini JC. Flexibility is a likely determinant of binding specificity in the case of ileal lipid binding protein. Structure. 1996;4(7):785–800. doi: 10.1016/s0969-2126(96)00086-x. [DOI] [PubMed] [Google Scholar]
- 25.Xu ZJ, Love ML, Ma LYY, Blum M, Bronskill PM, Bernstein J, Grey AA, Hofmann T, Camerman N, Wong JTF. Tryptophanyl-tRNA synthetase from Bacillus subtilis. J Biol Chem. 1989;264:4304–4311. [PubMed] [Google Scholar]
- 26.Lee KH, Lee HY, Slutsky MM, Anderson JT, Marsh ENG. Fluorous effect in proteins: De novo design and characterization of a four-alpha-helix bundle protein containing hexafluoroleucine. Biochemistry. 2004;43(51):16277–16284. doi: 10.1021/bi049086p. [DOI] [PubMed] [Google Scholar]