Abstract
The interaction between antipolysaccharide (anti-PS) antibodies and their antigens was investigated by the use of isothermal titration calorimetry to determine the thermodynamic binding constant (K), the change in the enthalpy of binding (ΔH), and the binding density (N) to high-molecular-weight PSs. From these values, the change in the entropy of binding (ΔS) was calculated. The thermodynamic parameters of binding to high-molecular-weight capsular PSs are reported for two monoclonal antibodies (MAbs) with different specificities for meningococcal serogroup C PS, five MAbs specific for different pneumococcal serotypes, and the Fab fragments of two antipneumococcal MAbs. The K values were in the range of 106 to 107 M−1, and these values were 1 to 2 orders of magnitude greater than the previously reported K values derived from antibody-oligosaccharide interactions. The ΔH associated with binding was favorable for each MAb and Fab fragment. The ΔS associated with binding was also generally favorable for both the MAbs and the Fab fragments, with the exception of the anti-serotype 14 MAb and its Fab fragment. N provides information regarding how densely MAbs or Fabs can bind along PS chains and, as expressed in terms of monosaccharides, was very similar for the seven MAbs, with an average of 12 monosaccharides per bound MAb. The value of N for each Fab was smaller, with five or seven monosaccharides per bound Fab. These results suggest that steric interactions between antibody molecules are a major influence on the values of N of high-affinity MAbs to capsular PSs.
Much detailed information regarding the thermodynamic parameters of monoclonal antibody (MAb) binding to oligosaccharides is available (8, 9, 11, 15, 26, 29, 38, 39, 43). However, very little thermodynamic information regarding binding of MAbs or Fab fragments to intact polysaccharides (PSs) is available. Of particular interest is understanding the density of MAb or Fab binding along high-molecular-weight PS chains.
Isothermal titration microcalorimetry (ITC) can be used to investigate the thermodynamics of molecular interactions such as the binding of a MAb to its epitope (10). The thermodynamic binding constant (K), the change in the enthalpy of binding (ΔH), and the stoichiometry or density of binding (N) can all be determined directly from one ITC experiment. ITC directly measures the heat evolved (or consumed) in liquid samples upon the mixing of precise, known amounts of a ligand into a solution of a macromolecule. The heat released (or consumed) after each addition of ligand is directly proportional to the amount of binding that occurs. The data are analyzed by using fitting models to calculate ΔH, K, and N. ΔH is proportional to the magnitude of the inflection of the binding isotherm, K is derived from the slope at the midpoint of the binding isotherm, and N is derived from the midpoint of the rise or the inflection point of the binding isotherm. The change in free energy (ΔG) and the change in the entropy of binding (ΔS) are calculated values, since ΔG is derived from K, that is, ΔG = −RTlnK, where R is the universal gas constant, T is the temperature of the interaction, and ΔS is derived from ΔG by the equation ΔG = ΔH − TΔS.
We report here on the thermodynamic parameters of binding, K, ΔH, and ΔS, as well as N, for two MAbs with different specificities for Neisseria meningitidis serogroup C capsular PS (MnC PS) and five MAbs and two Fab fragments specific for Streptococcus pneumoniae serotype 4, 14, 6B, 9V, and 19F capsular PSs (Pn PSs).
MATERIALS AND METHODS
The MnC PS and the Pn PSs were obtained from Wyeth Vaccines Research. The average molecular masses of the PSs were 360 kDa for MnC PS, 500 kDa for the serotype 4 PS, 850 kDa for the serotype 14 PS, 890 kDa for the serotype 6B PS, 900 kDa for the serotype 9V PS, and 940 kDa for the serotype 19F PS. The O-acetyl content of the MnC PS was 2.24 μmol/mg, which is equivalent to approximately 0.7 O-acetyl groups per N-acetylneuraminic acid residue. The methods for the production and purification of anti-MnC PS MAbs Mn207-3 (immunoglobulin G1 [IgG1]), specific for O-acetylated epitopes of MnC PS, and Mn46-1 (IgG1), not specific for O-acetylated epitopes of MnC PS, were reported previously (16). Anti-Pn PS MAbs Pn31-1 (IgG1), Pn36-1 (IgG1), Pn45-1 (IgG1), and Pn42-1 (IgG2b), specific for serotype 4, 6B, 9V, and 14 capsular PSs, respectively, were produced in SJL mice immunized with a mixture of nine Pn PS-CRM197 conjugates containing serotype 1, 4, 5, 6B, 9V, 14, 18C, 19F, and 23F capsular PSs. MAb 63-1 (IgG2b), specific for the serotype 19F PS, was produced in Swiss Webster mice immunized with the same nine-valent mixture of Pn PS conjugates. Splenocytes were harvested from selected animals and were fused to hypoxanthine-aminopterin-thymidine-sensitive nonsecreting myeloma cells by a standard methodology. The resultant culture media were tested for their reactivities with the appropriate capsular PS by enzyme-linked immunosorbent assay and were subcloned at limiting dilution. All of the anti-Pn PS MAbs reported in this study mediate the opsonophagocytosis of pneumococcal cells of the appropriate serotypes.
IgG MAbs were purified from ascitic fluid generated in SCID mice (Taconic, Germantown, NY) to >95% purity, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with protein A resin. Monovalent Fab fragments for Pn31-1 and Pn42-1 were generated by papain digestion of the IgG.
ITC.
ITC experiments were performed on a VP-ITC titration microcalorimeter (Microcal Inc., Northhampton, MA). The MAbs, Fabs, and capsular PSs were codialyzed against 1× phosphate-buffered saline (pH 7.4; Cellgro Mediatech, Herndon, VA) to standardize the buffer conditions and reduce the heat of mixing. The sample cell (1.4071 ml) was maintained at 25°C and filled with MAb solution at concentrations ranging from 3 to 7 μM or Fab solution at concentrations ranging from 1 to 9 μM. The injection syringe (nominal volume, 250 μl) was filled with the appropriate capsular PS solution. Typical concentrations of PS, expressed as the molarity of the repeat units, ranged from 100 to 1,000 μM for the experiments with MAbs and from 30 to 500 μM for the experiments with Fabs. During the titration experiments, the sample solution was stirred at 450 rpm with the paddle-shaped injection syringe. After the establishment of the baseline stability, 2 μl of solution was injected to remove possible air bubbles at the syringe opening. Subsequently, a succession of approximately 35 microinjections of constant volume (typically 7 to 10 μl) was made, with each injection separated by 3 min to allow the heat signal to return to the baseline. The isothermal titration curves were recorded and analyzed with ORIGIN (version 5.0) software (Microcal Inc.), provided with the VP-ITC instrument. A monovalent binding model was used to calculate the thermodynamic parameters.
Determination of MAb and Fab concentrations.
The exact concentration of purified MAb or Fab used in each microcalorimetry experiment was determined by measurement of the absorbance at 280 nm.
Determination of PS concentration.
The concentrations of PS used in each ITC experiment were determined by use of a combination of size-exclusion chromatography and multiangle laser light scattering. Three Synchropak SEC columns with nominal pore sizes of 100, 300, and 4,000 Å, respectively, were used in series. The column effluent was monitored with a variable-wavelength UV detector for protein detection, a Wyatt Technology MiniDawn multiangle laser light scattering detector, and a differential refractive index detector. The outputs from the last two detectors were combined to calculate absolute molecular weights without the use of standards or estimates. The refractive index detector response was calibrated using dextran.
RESULTS
Thermodynamic characterization of MAb binding to MnC PS.
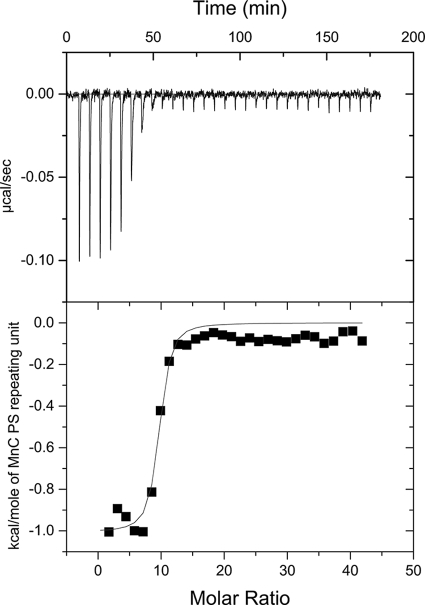
Two different anti-MnC PS MAbs, Mn207-3, specific for O-acetylated epitopes, and Mn46-1, not specific for O-acetylated epitopes, were utilized in the ITC experiments to investigate MAb binding to O-acetylated MnC PS. There was no evidence of precipitation in the reaction cell as a result of MAb cross-linking of PS. Large shifts in the baselines of the isotherms would occur when immunoprecipitates form in the reaction cell. The binding isotherms had stable baselines (Fig. 1), and the reaction mixtures were not turbid when they were removed at the end of the experiment, suggesting that significant immunoprecipitation did not occur under the conditions of these experiments. The thermodynamic parameters of binding were very similar for both MnC PS MAbs (Table 1). The values of K were in the micromolar−1 range, and both ΔH and ΔS were favorable for binding.
FIG. 1.
Isotherm (top panel) and nonlinear least-squares fit of the data (bottom panel) from a representative ITC experiment with MnC PS and MAb 46-1.
TABLE 1.
Values of K, ΔH, and ΔS for MAbs and Fabsa
| Antibody designation | Specificity | Ig isotype or Fab | K (μM−1) | ΔH (cal·mol−1) | ΔS (cal·mol−1·K−1) | TΔS (cal·mol−1)b |
|---|---|---|---|---|---|---|
| Mn207-3 | O-acetylated MnC | IgG1 | 0.59, 0.68 | −1,110, −1,144 | 22.7, 22.8 | 6,759, 6,807 |
| Mn46-1 | Non-O-acetylated MnC | IgG1 | 3.5 ± 3 | −954 ± 126 | 26.2 ± 2.1 | 7,798 ± 638 |
| Pn31-1 | Pn4 | IgG1 | 34 ± 7 | −7,000 ± 1,000 | 10 ± 4 | 3,085 ± 1,100 |
| Pn31-1 | Pn4 | Fab | 10 ± 2 | −8,700 ± 780 | 2.8 ± 2.8 | 832 ± 852 |
| Pn36-1 | Pn6B | IgG1 | 3.8 ± 0.3 | −4,800 ± 200 | 13.9 ± 0.9 | 4,131 ± 248 |
| Pn45-1 | Pn9V | IgG1 | 3.6 ± 0.9 | −2,030 ± 40 | 23.2 ± 0.7 | 6,902 ± 203 |
| Pn42-1 | Pn14 | IgG2b | 12 ± 2 | −13,000 ± 1,000 | −12 ± 4 | −3,519 ± 1,137 |
| Pn42-1 | Pn14 | Fab | 2.1 ± 0.3 | −12,200 ± 2,400 | −12 ± 8 | −3,539 ± 2,357 |
| Pn63-1 | Pn19F | IgG2b | 4.3 ± 0.5 | −8,100 ± 900 | 3 ± 3 | 529 ± 826 |
For Mn207-3, values from two independent experiments are reported. For all other MAbs and Fabs, the values reported are the averages of three experiments ± standard deviations.
T = 298.15 K.
Thermodynamic characterization of antibody binding to Pn PS.
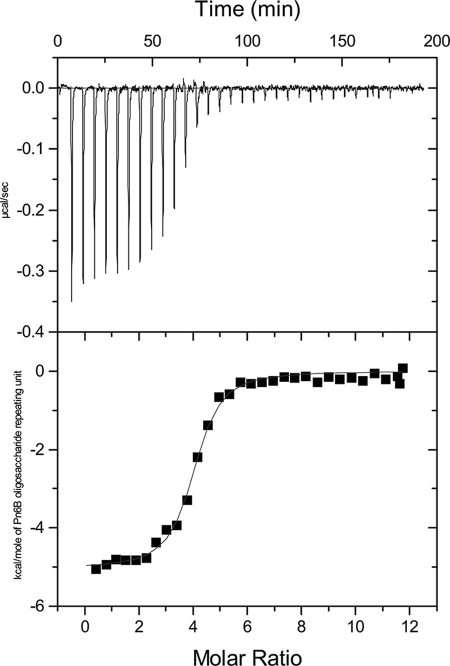
ITC was used to investigate the binding of five MAbs specific for different Pn PSs: Pn31-1, specific for serotype 4 PS; Pn36-1, specific for serotype 6B PS; Pn45-1, specific for serotype 9V PS; Pn42-1, specific for serotype 14 PS; and Pn63-1, specific for serotype 19F PS. Again, there was no evidence of immunoprecipitation at the concentrations of MAb and PS used for these experiments. A typical binding isotherm for a Pn PS is shown in Fig. 2. As with the interaction between MnC PS-specific MAbs and the MnC PS, the values of K for the anti-Pn PS interactions were all in the micromolar−1 range (Table 1). All of the Pn PS interactions were driven by a large, favorable ΔH. With the exception of serotype 14, ΔS was also favorable for binding.
FIG. 2.
Isotherm (top panel) and nonlinear least-squares fit of data (bottom panel) from a representative ITC experiment with Pn6B PS and MAb Pn36-1.
Thermodynamic characterization of Fab binding to Pn PSs.
Fab fragments were produced for Pn31-1, specific for serotype 4 PS, and Pn42-1, specific for serotype 14 PS; and the binding of the Fab fragments to the respective PS was investigated by ITC. The value of K for Fab Pn31-1 to serotype 4 PS was in the micromolar−1 range, but it was 3.4-fold less than that for the corresponding MAb (Table 1). Similarly, the value of K for Fab Pn42-1 to serotype 14 PS was 5.7-fold less than that for the corresponding MAb (Table 1). Similar to the Pn42-1 IgG, the binding of the Pn42-1 Fab fragment to the PS was driven entirely by a large, favorable ΔH, with an unfavorable ΔS upon binding.
Values of N for MAb binding to PSs.
Since the molar concentration of oligosaccharide repeating units was known, the value of N in terms of repeat units was determined by nonlinear least-squares regression analysis of the calorimetric data. N is one of the regression parameters and derives from the inflection point, or midpoint, of the rise of the isotherm. Table 2 summarizes the values of N for the two anti-MnC PSs and the five anti-Pn PS MAbs.
TABLE 2.
Values of N for MAbs and Fabs
| Antibody designation | Specificity | Isotype or Fab | No. of residues/repeat | Na | No. of monosaccharides/ bound MAb or Fab |
|---|---|---|---|---|---|
| Mn207-3 | O-acetylated MnC | IgG1 | 1 | 9.3, 12 | 10.7 |
| Mn46-1 | Non-O-acetylated MnC | IgG1 | 1 | 10.8 ± 1.6 | 10.8 |
| Pn31-1 | Pn4 | IgG1 | 4 | 2.9 ± 0.5 | 11.6 |
| Pn31-1 | Pn4 | Fab | 4 | 1.24 ± 0.09 | 5.0 |
| Pn36-1 | Pn6B | IgG1 | 3 + ribitol phosphate | 4.0 ± 0.3 | 12.0 |
| Pn45-1 | Pn9V | IgG1 | 5 | 3.58 ± 0.06 | 17.9 |
| Pn42-1 | Pn14 | IgG2b | 4 | 3.4 ± 0.3 | 13.6 |
| Pn42-1 | Pn14 | Fab | 4 | 1.8 ± 0.4 | 7.2 |
| Pn63-1 | Pn19F | IgG2b | 3 + phosphate | 3.1 ± 0.3 | 9.3 |
For Mn207-3, values from two independent experiments are reported. For all other MAbs, the values reported are the average of three experiments ± standard deviations.
N is the number of repeat units, on average, per bound MAb at saturation and is not necessarily the number of repeat units filling a MAb binding site. For example, Mn46-1 IgG bound, on average, approximately once every 11 repeat units of MnC PS and Pn31-1 IgG bound approximately once every three repeat units of the serotype 4 PS. The size of the oligosaccharide repeat units for the PSs studied ranged from one to five monosaccharides (Table 2). When N, in terms of the repeat units, was multiplied by the number of monosaccharides in a repeat unit, the number of monosaccharides between bound MAbs varied from 9.3 to 17.9 (Table 2) but ranged from 10.7 to 13.6 for five of the seven MAbs studied. The general similarity of the values of N (in terms of monosaccharides) for seven MAbs with distinct PS specificities suggests that steric interactions between IgG molecules may have a large influence on the density of MAb binding to a PS at saturation.
Density of Fab binding to Pn PSs.
If the N of MAb binding to a PS is influenced by steric interactions between bound IgG, a denser binding of Fab fragments would be predicted. This was, in fact, observed for the two Fab fragments described in this report (Table 2). The values of N in terms of monosaccharides decreased from 11.6 for the Pn31-1 IgG to 5.0 for the Fab fragment and from 13.6 for Pn42-1 IgG to 7.2 for the Fab fragment.
DISCUSSION
Immune complexes can be formed when bivalent MAbs interact with multivalent antigens, such as PSs. Such immune complexes can lead to insoluble precipitates that would interfere with detailed analyses of antibody-antigen binding in solution. Most previously published reports of thermodynamic investigations of anti-PS antibodies and their antigens have avoided this issue by using small oligosaccharides that present a single or a limited number of epitopes and/or monovalent Fab fragments to avoid the formation of large insoluble immune complexes (8, 9, 11, 15, 26, 29, 38, 39, 43). The affinities (K values) of the interactions reported to date have been in the range of 104 to 105 M−1. Thus, relatively high concentrations of MAbs or Fabs and oligosaccharides were needed in order to produce a measurable ΔH. If bivalent antibodies (e.g., MAbs) and multivalent antigens (e.g., PSs) are used at high concentrations, insoluble immune complexes may be formed and microcalorimetric experiments cannot be performed. However, if dilute solutions can be used, the small immune complexes can remain soluble and microcalorimetric data can be collected.
The work reported here is the first to describe the thermodynamic parameters of binding for several intact IgG MAbs to high-molecular-weight PSs and the N of anti-PS IgG to high-molecular-weight PSs. Microcalorimetric measurements were carried out with relatively dilute solutions of MAbs (3 to 7 μM) and PSs (100 to 1,000 μM, in terms of repeat units), since the K values for the IgG-PS interactions described in this study were 1 to 2 orders of magnitude greater (106 to 107 M−1) than those reported previously (8, 9, 11, 15, 26, 29, 38, 39, 43). The increased affinities measured for these antibodies may result from the favorable conformations assumed by the higher-molecular-weight PS employed in this study. However, it is also possible that the higher affinities measured in this study are the result of the use of highly immunogenic glycoconjugates to generate these MAbs. The increased affinity allowed the use of dilute solutions of reactants and the avoidance of problems associated with precipitation. The relatively small differences in the K values of the Fab against intact IgG, on the order of threefold for IgG1 and sixfold for IgG2b, suggest that the binding of the IgG is primarily monovalent and support the use of a monovalent binding model for calculation of the thermodynamic parameters. As was previously reported for other antibody-carbohydrate interactions (8, 9, 11, 15, 26, 29, 38, 39, 43), the binding of all seven MAbs and two Fabs to their respective PSs was driven enthalpically. The ΔH values for the two Fabs were similar to those for the MAbs: −7,000 cal·mol−1 and −8,700 cal·mol−1 for the MAb and the Fab of Pn31-1 (specific for serotype 4 PS), respectively, and −13,000 cal·mol−1 and −12,200 cal·mol−1 for the MAb and the Fab of Pn42-1 (specific for serotype 14 PS), respectively. For six of the seven MAbs, the ΔS values for binding to the PSs were favorable under the experimental conditions. The generally favorable ΔS values observed in this study suggest that significant cross-linking of the PSs did not occur and further support the use of a monovalent binding model.
In contrast, the ΔS values for antibody-oligosaccharide interactions reported in the literature have generally been unfavorable (8, 9, 11, 15, 26, 29, 38, 39, 43). The binding of small, conformationally mobile oligosaccharides to large IgG molecules may freeze one conformation (44), resulting in unfavorable ΔS values. However, the experiments described here utilized large PSs that may be less conformationally mobile. If the conformation recognized by a MAb is favored in the large PS, binding of the MAb would not decrease the conformational mobility or freedom of the PS and would not contribute unfavorably to ΔS. The one exception observed was MAb Pn42-1 and its corresponding Fab fragment, which had unfavorable ΔS values for binding to serotype14 PS. Pn42-1 (MAb and Fab) had the largest, most favorable ΔH (approximately −13,000 cal·mol−1), which was offset by an unfavorable ΔS (approximately −12 cal·mol−1·K−1). Such enthalpy-entropy compensation has been reported for many antibody-carbohydrate interactions (8, 12). The serotype 14 PS is an uncharged polymer and is highly conformationally mobile (20). Despite the conformational flexibility of the serotype 14 PS, a conformational epitope consisting of at least four repeating units has been proposed for the serotype 14 PS (21, 45). The unfavorable ΔS of MAb (or Fab) binding to the serotype 14 PS may therefore be the result of the freezing or locking of this mobile PS into one conformation in which a specific conformational epitope is available for binding.
Thermodynamic parameters have also been reported for antibody interactions with proteins and haptens. For a series of MAbs to hen egg lysozyme, ΔH values ranged from −9,400 cal·mol−1 to −22,600 cal·mol−1 (4, 5, 17, 36). A series of MAbs directed against the hapten, 4-hydroxy-3-nitrophenylacetyl caproic acid, showed ΔH values ranging from −11,200 cal·mol−1 to −18,300 cal·mol−1 (34, 41). Rabbit polyclonal antibodies directed against the hapten 2,4-dinitrophenyl-lysine showed a similar range of ΔH values, from −13,900 cal·mol−1 to −22,800 cal·mol−1 (2, 3, 14). The binding of both the antilysozyme and the antihapten MAbs was driven by large negative ΔH values, with unfavorable or small contributions from ΔS. The larger decreases in ΔH exhibited by the antilysozyme and antihapten MAbs therefore reflect the higher affinities of these antibodies than those of anti-PS antibodies.
The saturation density of MAb binding to high-molecular-weight PSs was also evaluated in this study. The data in Table 2 demonstrate that the MAbs evaluated can bind at approximately every 9 to 18 monosaccharide residues at saturation, with five of the seven MAbs binding at approximately every 11 to 14 monosaccharide residues at saturation. The relative consistency of the N values of the anti-Pn PS and the anti-MnC PS IgG MAbs at saturation suggests that N may derive from steric interactions between the bound IgG rather than from the density of presentation of the specific epitope along the PS. The similar N values for the two anti-MnC PS MAbs may be explained by the O-acetyl content of the MnC PS, 2.24 μmol/mg, or approximately 0.7 O-acetyl groups/sialic acid residue in the PS, which may have enabled both antibodies to bind at the maximum density permitted by steric interactions between bound IgG molecules. Consistent with this model, the Fabs of MAbs Pn31-1 and Pn42-1 were able to bind at every 5.0 and 7.2 monosaccharide residues at saturation, respectively, or at roughly double the density of the binding of intact Pn31-1 and Pn42-1 IgG antibodies. These Fab binding densities are also fairly consistent with those found in earlier studies, which showed that two repeat units, or 8 to 10 monosaccharides, is the minimum binding unit for MAbs and polyclonal antibodies to group B streptococcal type III PS and serotype 14 PS (21, 46); 6 to 10 monosaccharides is the minimum binding unit for group B meningococcal PS (24); and isomaltohexaose, which consists of 6 monosaccharides, is the optimal inhibitor of antidextran antibodies (19). In another study, the data describing the binding of a Fab fragment to a 41-mer oligosaccharide of α-(2-8)-linked sialic acid best fit a two-epitope model, or 15 to 20 monosaccharides per binding site (11).
Modeling studies carried out with the IgA antigalactan MAb J539 suggested that the binding site was filled by a tetrasaccharide (13) or a pentasaccharide (40). However, the lower size limit for oligosaccharides needed to demonstrate detectable binding to antibodies is not clearly defined, and very low affinity interactions of smaller oligosaccharides and monosaccharides with antibodies can be detected by using sufficiently sensitive methodologies. Microcalorimetric methods have been used to demonstrate the low-affinity binding of di-, tri-, and tetrasaccharide haptens to the antidextran IgM antibody MOPC-104E (47); methylglycosides and di- and trisaccharides to IgA antidextran and antigalactan antibodies (27); and a Salmonella serogroup B trisaccharide epitope to an IgG MAb (38, 39). The binding of two IgA antigalactans to tri- and tetrasaccharides (18) and the binding of methylgalactosides and di- and trisaccharides to an IgM anti-Shigella dysenteria type 1 O antigen (28) were also quantified by fluorescence spectroscopy.
Published estimates of the lengths of extended PS chains range from approximately 0.43 nm to 0.56 nm per monosaccharide residue (1, 19, 25). Therefore, the spacing of the binding of the Fabs along the PS calculated from these studies, about 3 nm per bound Fab at saturation, is relatively consistent with published estimates of the size of an antibody binding site (19) and with estimates of the dimensions of an Fab fragment (30, 31, 35, 42), suggesting that anti-PS antibodies of moderately high affinities are capable of binding as densely along the PS chain as permitted by steric interactions between the IgG molecules and may therefore recognize structural or linear epitopes rather than conformational epitopes. Recognition of immunodominant and functionally important conformational epitopes by polyclonal antibodies and MAbs has been proposed for the group B streptococcal type III PS (7, 46), the serotype 14 PS (20, 21, 45), and the Neisseria meningitidis serogroup B PS (6, 11, 23, 24). In contrast, there is evidence to suggest that conformational epitopes may not be present or may not play an important role in generating functional immune responses for serotype 3, 6A, 18C, 19F, or 23F PS (22) or pneumococcal serogroup 9 capsular PS (32, 33).
IgG binding at near saturation levels may not be required for anti-capsular PS antibodies to fix C1q and initiate the classical complement cascade, as C1q can span approximately 35 nm between bound IgG (37). Nevertheless, high-affinity MAbs will bind more densely than lower-affinity MAbs at the same concentration and thus may be more effective at initiating complement fixation and show greater activity in opsonic and bactericidal models of protection.
Acknowledgments
We thank Maya Koster and Olivier Marcq for valuable discussions and technical assistance.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Adams, E. L., P. A. Kroon, G. Williamson, and V. J. Morris. 2003. Characterization of the heterogeneous arabinoxylans by direct imaging of individual molecules by atomic force microscopy. Carbohydr. Res. 338771-780. [DOI] [PubMed] [Google Scholar]
- 2.Barisas, B. G., S. J. Singer, and J. M. Sturtevant. 1972. Thermodynamics of the binding of 2,4-dinitrophenyl and 2,4,6-trinitrophenyl haptens to the homologous and heterologous rabbit antibodies. Biochemistry 112741-2744. [DOI] [PubMed] [Google Scholar]
- 3.Barisas, B. G., J. M. Sturtevant, and S. J. Singer. 1971. Thermodynamics of the binding of haptens to rabbit anit-2,4-dinitrophenyl antibodies. Biochemistry 102816-2821. [DOI] [PubMed] [Google Scholar]
- 4.Bhat, T. N., G. A. Bentley, G. Boulot, M. I. Greene, D. Tello, W. Dall'Acqua, H. Souchon, F. P. Schwarz, R. A. Mariuzza, and R. J. Poljak. 1994. Bound water molecules and conformational stabilization help mediate an antigen-antibody association. Proc. Natl. Acad. Sci. USA 911089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braden, B. C., A. Cauerhff, W. Dall'Acqua, B. A. Fields, F. A. Goldbaum, E. L. Malchiodi, R. A. Mariuzza, R. J. Poljak, F. P. Schwarz, X. Ysern, et al. 1995. Structure and thermodynamics of antigen recognition by antibodies. Ann. N. Y. Acad. Sci. 764315-327. [DOI] [PubMed] [Google Scholar]
- 6.Brisson, J. R., H. Baumann, A. Imberty, S. Perez, and H. J. Jennings. 1992. Helical epitope of the group B meningococcal alpha(2-8)-linked sialic acid polysaccharide. Biochemistry 314996-5004. [DOI] [PubMed] [Google Scholar]
- 7.Brisson, J. R., S. Uhrinova, R. J. Woods, M. van der Zwan, H. C. Jarrell, L. C. Paoletti, D. L. Kasper, and H. J. Jennings. 1997. NMR and molecular dynamics studies of the conformational epitope of the type III group B Streptococcus capsular polysaccharide and derivatives. Biochemistry 363278-3292. [DOI] [PubMed] [Google Scholar]
- 8.Brummell, D. A., V. P. Sharma, N. N. Anand, D. Bilous, G. Dubuc, J. Michniewicz, C. R. MacKenzie, J. Sadowska, B. W. Sigurskjold, B. Sinnott, et al. 1993. Probing the combining site of an anti-carbohydrate antibody by saturation-mutagenesis: role of the heavy-chain CDR3 residues. Biochemistry 321180-1187. [DOI] [PubMed] [Google Scholar]
- 9.Bundle, D. R., E. Eichler, M. A. Gidney, M. Meldal, A. Ragauskas, B. W. Sigurskjold, B. Sinnott, D. C. Watson, M. Yaguchi, and N. M. Young. 1994. Molecular recognition of a Salmonella trisaccharide epitope by monoclonal antibody Se155-4. Biochemistry 335172-5182. [DOI] [PubMed] [Google Scholar]
- 10.Bundle, D. R., and B. W. Sigurskjold. 1994. Determination of accurate thermodynamics of binding by titration microcalorimetry. Methods Enzymol. 247288-305. [DOI] [PubMed] [Google Scholar]
- 11.Evans, S. V., B. W. Sigurskjold, H. J. Jennings, J. R. Brisson, R. To, W. C. Tse, E. Altman, M. Frosch, C. Weisgerber, H. D. Kratzin, et al. 1995. Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 Å resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific for alpha-(2→8)-polysialic acid. Biochemistry 346737-6744. [DOI] [PubMed] [Google Scholar]
- 12.Gabius, H. J. 1998. The how and why of protein-carbohydrate interaction: a primer to the theoretical concept and a guide to application in drug design. Pharm. Res. 1523-30. [DOI] [PubMed] [Google Scholar]
- 13.Glaudemans, C. P., P. Kovac, and K. Rasmussen. 1984. Mapping of subsites in the combining area of monoclonal anti-galactan immunoglobulin A J539. Biochemistry 236732-6736. [DOI] [PubMed] [Google Scholar]
- 14.Halsey, J. F., and R. L. Biltonen. 1975. The thermodynamics of hapten and antigen binding by rabbit anti-dinitrophenyl antibody. Biochemistry 14800-804. [DOI] [PubMed] [Google Scholar]
- 15.Harris, S. L., L. Craig, J. S. Mehroke, M. Rashed, M. B. Zwick, K. Kenar, E. J. Toone, N. Greenspan, F. I. Auzanneau, J. R. Marino-Albernas, B. M. Pinto, and J. K. Scott. 1997. Exploring the basis of peptide-carbohydrate cross-reactivity: evidence for discrimination by peptides between closely related anti-carbohydrate antibodies. Proc. Natl. Acad. Sci. USA 942454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, S. L., H. Tsao, L. Ashton, D. Goldblatt, and P. Fernsten. 2007. Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 14397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbits, K. A., D. S. Gill, and R. C. Willson. 1994. Isothermal titration calorimetric study of the association of hen egg lysozyme and the anti-lysozyme antibody HyHEL-5. Biochemistry 333584-3590. [DOI] [PubMed] [Google Scholar]
- 18.Jolley, M. E., S. Rudikoff, M. Potter, and C. P. Glaudemans. 1973. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry 123039-3044. [DOI] [PubMed] [Google Scholar]
- 19.Kabat, E. A. 1966. The nature of an antigenic determinant. J. Immunol. 971-11. [PubMed] [Google Scholar]
- 20.Kadirvelraj, R., J. Gonzalez-Outeirino, B. L. Foley, M. L. Beckham, H. J. Jennings, S. Foote, M. G. Ford, and R. J. Woods. 2006. Understanding the bacterial polysaccharide antigenicity of Streptococcus agalactiae versus Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 1038149-8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1998. Streptococcus pneumoniae type 14 polysaccharide-conjugate vaccines: length stabilization of opsonophagocytic conformational polysaccharide epitopes. Infect. Immun. 662441-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laferriere, C. A., R. K. Sood, J. M. de Muys, F. Michon, and H. J. Jennings. 1997. The synthesis of Streptococcus pneumoniae polysaccharide-tetanus toxoid conjugates and the effect of chain length on immunogenicity. Vaccine 15179-186. [DOI] [PubMed] [Google Scholar]
- 23.Lifely, M. R., C. Moreno, and J. C. Lindon. 1987. An integrated molecular and immunological approach towards a meningococcal group B vaccine. Vaccine 511-26. [DOI] [PubMed] [Google Scholar]
- 24.Michon, F., J. R. Brisson, and H. J. Jennings. 1987. Conformational differences between linear alpha (2-8)-linked homosialooligosaccharides and the epitope of the group B meningococcal polysaccharide. Biochemistry 268399-8405. [DOI] [PubMed] [Google Scholar]
- 25.Moorhouse, R., W. T. Winter, S. Arnott, and M. E. Bayer. 1977. Conformation and molecular organization in fibers of the capsular polysaccharide from Escherichia coli M41 mutant. J. Mol. Biol. 109373-391. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Loennies, S., L. Brade, C. R. MacKenzie, F. E. Di Padova, and H. Brade. 2003. Identification of a cross-reactive epitope widely present in lipopolysaccharide from enterobacteria and recognized by the cross-protective monoclonal antibody WN1 222-5. J. Biol. Chem. 27825618-25627. [DOI] [PubMed] [Google Scholar]
- 27.Nashed, E. M., and C. P. Glaudemans. 1996. Observations on the binding of four anti-carbohydrate monoclonal antibodies to their homologous ligands. J. Biol. Chem. 2718209-8214. [DOI] [PubMed] [Google Scholar]
- 28.Pavliak, V., E. M. Nashed, V. Pozsgay, P. Kovac, A. Karpas, C. Chu, R. Schneerson, J. B. Robbins, and C. P. Glaudemans. 1993. Binding of the O-antigen of Shigella dysenteriae type 1 and 26 related synthetic fragments to a monoclonal IgM antibody. J. Biol. Chem. 26825797-25802. [PubMed] [Google Scholar]
- 29.Pitner, J. B., W. F. Beyer, T. M. Venetta, C. Nycz, M. J. Mitchell, S. L. Harris, J. R. Marino-Albernas, F. I. Auzanneau, F. Forooghian, and B. M. Pinto. 2000. Bivalency and epitope specificity of a high-affinity IgG3 monoclonal antibody to the Streptococcus group A carbohydrate antigen. Molecular modeling of a Fv fragment. Carbohydr. Res. 32417-29. [DOI] [PubMed] [Google Scholar]
- 30.Poljak, R. J., L. M. Amzel, H. P. Avey, L. N. Becka, and A. Nisonoff. 1972. Structure of Fab′ new at 6 Å resolution. Nat. New Biol. 235137-140. [DOI] [PubMed] [Google Scholar]
- 31.Poljak, R. J., L. M. Amzel, H. P. Avey, B. L. Chen, R. P. Phizackerley, and F. Saul. 1973. Three-dimensional structure of the Fab′ fragment of a human immunoglobulin at 2.8-Å resolution. Proc. Natl. Acad. Sci. USA 703305-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutherford, T. J., C. Jones, D. B. Davies, and A. C. Elliott. 1994. Molecular recognition of antigenic polysaccharides: a conformational comparison of capsules from Streptococcus pneumoniae serogroup 9. Carbohydr. Res. 26597-111. [DOI] [PubMed] [Google Scholar]
- 33.Rutherford, T. J., C. Jones, D. B. Davies, and A. C. Elliott. 1994. NMR assignment and conformational analysis of the antigenic capsular polysaccharide from Streptococcus pneumoniae type 9N in aqueous solution. Carbohydr. Res. 26579-96. [DOI] [PubMed] [Google Scholar]
- 34.Sagawa, T., M. Oda, M. Ishimura, K. Furukawa, and T. Azuma. 2003. Thermodynamic and kinetic aspects of antibody evolution during the immune response to hapten. Mol. Immunol. 39801-808. [DOI] [PubMed] [Google Scholar]
- 35.Sarma, V. R., E. W. Silverton, D. R. Davies, and W. D. Terry. 1971. The three-dimensional structure at 6 Å resolution of a human γG1 immunoglobulin molecule. J. Biol. Chem. 2463753-3759. [PubMed] [Google Scholar]
- 36.Schwarz, F. P., D. Tello, F. A. Goldbaum, R. A. Mariuzza, and R. J. Poljak. 1995. Thermodynamics of antigen-antibody binding using specific anti-lysozyme antibodies. Eur. J. Biochem. 228388-394. [PubMed] [Google Scholar]
- 37.Shelton, E., K. Yonemasu, and R. M. Stroud. 1972. Ultrastructure of the human complement component, C1q. Proc. Natl. Acad. Sci. USA 6965-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigurskjold, B. W., E. Altman, and D. R. Bundle. 1991. Sensitive titration microcalorimetric study of the binding of Salmonella O-antigenic oligosaccharides by a monoclonal antibody. Eur. J. Biochem. 197239-246. [DOI] [PubMed] [Google Scholar]
- 39.Sigurskjold, B. W., and D. R. Bundle. 1992. Thermodynamics of oligosaccharide binding to a monoclonal antibody specific for a Salmonella O-antigen point to hydrophobic interactions in the binding site. J. Biol. Chem. 2678371-8376. [PubMed] [Google Scholar]
- 40.Suh, S. W., T. N. Bhat, M. A. Navia, G. H. Cohen, D. N. Rao, S. Rudikoff, and D. R. Davies. 1986. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-Å resolution. Proteins 174-80. [DOI] [PubMed] [Google Scholar]
- 41.Torigoe, H., T. Nakayama, M. Imazato, I. Shimada, Y. Arata, and A. Sarai. 1995. The affinity maturation of anti-4-hydroxy-3-nitrophenylacetyl mouse monoclonal antibody. A calorimetric study of the antigen-antibody interaction. J. Biol. Chem. 27022218-22222. [DOI] [PubMed] [Google Scholar]
- 42.Valentine, R. C., and N. M. Green. 1967. Electron microscopy of an antibody-hapten complex. J. Mol. Biol. 27615-617. [DOI] [PubMed] [Google Scholar]
- 43.Vyas, N. K., M. N. Vyas, M. C. Chervenak, M. A. Johnson, B. M. Pinto, D. R. Bundle, and F. A. Quiocho. 2002. Molecular recognition of oligosaccharide epitopes by a monoclonal Fab specific for Shigella flexneri Y lipopolysaccharide: X-ray structures and thermodynamics. Biochemistry 4113575-13586. [DOI] [PubMed] [Google Scholar]
- 44.Weimar, T., S. L. Harris, J. B. Pitner, K. Bock, and B. M. Pinto. 1995. Transferred nuclear Overhauser enhancement experiments show that the monoclonal antibody strep 9 selects a local minimum conformation of a Streptococcus group A trisaccharide-hapten. Biochemistry 3413672-13681. [DOI] [PubMed] [Google Scholar]
- 45.Wessels, M. R., and D. L. Kasper. 1989. Antibody recognition of the type 14 pneumococcal capsule. Evidence for a conformational epitope in a neutral polysaccharide. J. Exp. Med. 1692121-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wessels, M. R., A. Munoz, and D. L. Kasper. 1987. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc. Natl. Acad. Sci. USA 849170-9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zidovetzki, R., Y. Blatt, G. Schepers, and I. Pecht. 1988. Thermodynamics of oligosaccharides binding to a dextran-specific monoclonal IgM. Mol. Immunol. 25379-383. [DOI] [PubMed] [Google Scholar]