Abstract
Tuberculosis (TB) is a major cause of morbidity and mortality, especially in developing countries. Despite significant limitations, microscopy remains the cornerstone of the global TB control strategy. As the TB epidemic escalates, new diagnostic methods that are accurate and also economical and simple to manufacture and deploy are urgently needed. Although several promising antigens have been identified and evaluated in recent years, the reproducible production of high-quality recombinant mycobacterial proteins with minimal batch-to-batch variation is difficult, laborious, and expensive. To determine the feasibility of devising a synthetic peptide-based diagnostic test for TB, we have delineated the immunodominant epitopes of three candidate antigens, Ag85B, BfrB, and TrxC, that were previously identified to be immunogenic in TB patients. The results demonstrate that combinations of carefully selected synthetic peptides derived from highly immunogenic proteins can be the basis for devising an immunodiagnostic test for TB.
According to World Health Organization estimates, ∼9 × 106 new cases of tuberculosis (TB) and ∼2 × 106 TB-related deaths occur every year. The major proportion (>90%) of these cases and deaths occur in low-income developing countries where the diagnosis of TB is based primarily on the examination of sputum smears for acid-fast bacilli. Although microscopy is highly specific, it has some serious limitations, such as the fact that is has a low and variable sensitivity, is time-consuming, and is tedious. The need for multiple visits to provide specimens and obtain results before treatment can be initiated also leads to high rates of dropout of infectious patients (30). A rapid and economical diagnostic test that can provide an accurate means for the identification of sputum smear-positive TB cases is a major diagnostic priority of TB control programs in countries where TB is endemic.
Antibody detection-based diagnostic assays have been successfully devised for several infectious diseases (7, 15, 26, 30), but efforts to develop a serodiagnostic test for TB have yielded disappointing results for several decades and no currently available commercial immunodiagnostic tests for TB provide high sensitivity and specificity (24). However, interest in developing these assays for the diagnosis of TB continues due to their adaptability to simple, rapid formats, such as the dipstick and lateral-flow cassette formats, which are extremely useful in the field, where a sophisticated laboratory infrastructure and highly trained personnel are not available (8, 30).
Our earlier studies of the humoral immune responses elicited at different stages of active TB in humans demonstrated that only a small subset of the Mycobacterium tuberculosis culture filtrate proteins is recognized by antibodies in both patients with early TB and patients with advanced TB and in the presence of human immunodeficiency virus (HIV) coinfection (14, 20-23). Our earlier studies also identified M. tuberculosis malate synthase (Rv1837c) and MPT51 (Rv3803c) to be immunodominant antigens that are recognized by antibodies from ∼80% of the smear-positive HIV-negative (HIV−) and TB-positive (TB+) patients and HIV-positive (HIV+) and TB+ patients (2, 28). Although the performance of the two antigens has been validated with TB patients from different countries by several investigators (1, 2, 9, 10, 16, 18), additional antigens that can further enhance sensitivity are needed.
In recent years, antigen discovery has been accelerated by the development of high-throughput screening assays such as microarray screening that allows the rapid selection of target potential candidate antigens from large numbers of proteins (22, 25). In our quest for additional antigens that may be useful for devising an antibody detection-based diagnostic test for TB, we recently screened protein microarrays of fractionated native cytosolic and culture filtrate proteins of M. tuberculosis with sera from HIV− TB+ patients with early TB (noncavitary, sputum smear-negative TB) or advanced TB (sputum smear-positive cavitary TB), as well as HIV+ TB+ patients (22). This systematic exploration of biomarkers for inclusion in immunodiagnostic tests for TB identified 15 antigens, some of which have been investigated earlier (LAM, the 45-kDa Apa protein [Rv1860], Ag85A [Rv3804c], Ag85B [Rv1886c], malate synthase [Rv1837c], the 38-kDa PstS1 protein [Rv0934], HspX [Rv2031c], and MPT64 [Rv1980c]) and others that have not previously been investigated as B-cell antigens (the 19-kDa LpqH protein [Rv3763], BfrB [Rv3841], LppZ [Rv3006], SecE2 [Rv0379], SodC [Rv0432], and TrxC [Rv3914]).
Several immunodiagnostic tests based on synthetic peptides derived from carefully selected highly antigenic proteins have been devised for viral, bacterial, and parasitic diseases in recent years (3, 6, 17). In the current studies, we have screened libraries of overlapping peptides of immunogenic proteins identified from our microarray studies to investigate the feasibility of using selected peptides rather than recombinant proteins to devise a diagnostic test for TB. We selected antigen 85B (Ag85B) and two newly identified antigens, BfrB and TrxC, for the current investigations. Both Ag85B and the 38-kDa protein have provided similar sensitivities with different cohorts of smear-positive HIV− TB+ patients (5; K. R. Steingart, N. Dendukuri, M. Henry, I. Schiller, P. Nahid, P. C. Hopewell, A. Ramsay, M. Pai, and S. Laal, submitted for publication). However, when they were tested with the same patients, although the performance characteristics of the two antigens were similar for smear-positive TB+ patients, the former antigen provided higher sensitivities with smear-negative HIV− TB+ patients and HIV+ TB+ patients (22). The choice of the new antigens BfrB and TrxC was also based on their performance with all three types of patients, as well as on their low molecular weights, which reduces the repertoire of peptides that needs to be tested. All three proteins have been dissected to identify their immunodominant regions. The results demonstrate that the screening of libraries of overlapping linear peptides with sera from patients and healthy subjects can successfully identify subsets of immunogenic peptides (epitopes) that can be the basis of a sensitive and specific diagnostic test for TB.
MATERIALS AND METHODS
Study populations.
Serum specimens obtained from 96 subjects who provided informed consent were included in these studies. Of these, 49 subjects were female and 47 were male, and their ages ranged from 16 to 62 years. Sera were obtained from three groups of subjects, as described below.
(i) PPD− healthy subjects (n = 13).
Eleven of the 13 purified protein derivative (PPD)-negative (PPD−) subjects were immigrants from countries where TB is endemic (India or China) who were likely to be vaccinated with Mycobacterium bovis BCG, and 2 were born in the United States. All these subjects were working in the Manhattan Veterans Affairs Medical Center (VAMC).
(ii) PPD+ healthy subject (n = 23).
Fifteen of the 23 PPD-positive (PPD+) individuals were immigrants from countries where TB is endemic (India, China, or Cameroon); 8 of them were bled within 2 to 12 weeks of their arrival in the United States. Most of these individuals were also vaccinated with BCG. Seven PPD+ subjects were from the United States, Europe, or Japan; none of them had been vaccinated with BCG. All these subjects were also employed at VAMC.
(iii) TB+ patients (n = 60).
Patients who were diagnosed with TB on the basis of the fact that their sputum smears were positive for acid-fast bacilli were recruited from the Lala Ram Sarup Institute of Tuberculosis and Respiratory Diseases, New Delhi, India. Since the PPD skin test fails to discriminate between latent and active infection while microscopy definitively identifies active TB, these patients were not tested for their PPD reactivities. Also, the seroprevalence of HIV among TB patients from the New Delhi area has been reported to be ∼1.0% (11, 29); and so in view of the low seroprevalence, a lack of a significant risk for HIV infection in the patients’ histories, as well as confidentiality concerns, the HIV status of the TB patients was not tested and these patients were considered HIV−.
Mapping of immunogenic regions of the proteins.
Thirty-two overlapping peptides (peptides A1 to A32; 20 amino acids in length with an overlap of 10 amino acids) covering the entire Ag85B protein sequence were synthesized commercially by use of the PEPscreen format (Sigma Genosys, The Woodlands, TX). Similar peptide libraries were also synthesized for BfrB (peptides B1 to B18) and TrxC (peptides T1 to T11). Each peptide was labeled with a biotin residue at the N terminus. The peptides were dissolved in dimethyl sulfoxide. Specific antipeptide antibodies were detected in sera by a modified enzyme-linked immunosorbent assay (ELISA) in which the peptides were captured in streptavidin-coated wells (Streptawell plates; Roche) via the biotin residue to ensure equivalent binding. Briefly, 50 μl of each peptide (2.5 μg/ml) diluted in blocking buffer (7.5% fetal bovine serum [HyClone, Logan, UT] and 2.5% bovine serum albumin in phosphate-buffered saline [PBS]) was added to the ELISA plates and the plates were incubated for 1 h at 37°C. Subsequently, 50 μl of diluted sera (1:20 in 0.1× blocking buffer) from patients and healthy subjects was added and the plates were incubated for 60 min at 37°C. After four washes with PBS-Tween 20 (0.05% Tween 20 in PBS), a mixture of alkaline phosphatase-conjugated protein A (1:2,000) and anti-human immunoglobulin A (IgA; 1:1,000) was added to each well and the plates were incubated for 1 h at 37°C. The simultaneous detection of both IgG and IgA antibodies has previously been demonstrated to enhance the sensitivity of detection without reducing the specificity (4; Steingart et al., submitted). The wells were washed six times with PBST, and the bound enzyme-conjugated antibodies were detected with p-nitrophenylphosphate substrate (1 mg/ml p-nitrophenylphosphate in 10% diethanolamine buffer containing 0.5 mM MgCl2, pH 9.8). The plates were read at 405 nm. The cutoff was determined by calculating the mean optical density (OD) obtained with sera from PPD+ and PPD− individuals plus 3 standard deviations (SDs).
All peptides were screened for their reactivities with the serum samples once, and the peptides from Ag85B and BfrB that were recognized by antibodies in sera from at least 50% of the 60 TB patients were tested for their reactivities with the same serum panel on two additional separate occasions for validation. When the peptide library for TrxC was screened, only one peptide was recognized by antibodies in sera from >50% of the TB patients; for this protein, peptides that were recognized by antibodies in sera from at least 40% of the 60 TB patients were retested for validation. For all peptides, sera showing positive reactivities two of three or three of three times were considered positive.
Statistical analysis.
Comparison of the reactivities of peptides with sera from PPD− and PPD+ healthy controls as well as sera from PPD+ and PPD− healthy controls and TB patients was performed by calculating the P value by the nonparametric Mann-Whitney test with Prism (version 5) software (GraphPad Software, Inc. San Diego, CA). A P value of <0.05 was considered statistically significant.
An algorithm for defining the best diagnostic subset of the peptides was derived. The algorithm aims to determine the smallest best-performing subset of a set of N peptides for possible use as a diagnostic test for TB. The test result for a serum sample based on a candidate subset of peptides is deemed positive if any of the peptides in the candidate set are deemed to be reactive with the serum sample. The input to this algorithm consists of two matrices of zeros and ones, in which the ones indicate reactivity and zeros indicate a lack of reactivity between a serum sample and one of the peptides. The N columns of each matrix correspond to peptides, and the several rows correspond to the samples of sera from different subjects. One matrix is obtained from the sera from TB patients, and the other matrix is obtained from the sera from the controls. The algorithm attempts to examine every one of the 2N − 1 subsets of the N peptides. Sensitivity is approximated as the percentage of serum samples from TB patients for which at least one peptide of the candidate subset is deemed reactive. Specificity is approximated as the percentage of the control sera with which none of the peptides in the candidate subset was deemed reactive. Candidate subsets of size K whose specificity exceeds a specified threshold (97% in our computations) are sorted by sensitivity. A maximally sensitive candidate (there may be more than one) is selected. Starting with K equal to 1, this process is repeated for increasing subset sizes K until no further improvement is seen in the highest sensitivity obtained.
RESULTS
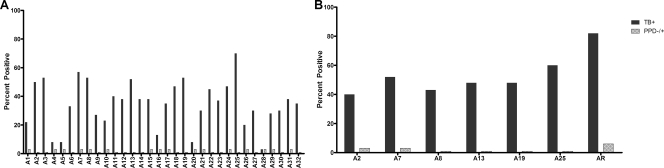
Ag85B has previously been considered to be a potential candidate for inclusion in a serodiagnostic test for TB by other investigators, and studies with several cohorts from different geographical settings have shown that anti-Ag85B antibodies are present in ∼60% of smear-positive TB patients (5, 13, 19, 27). When the reactivities of the overlapping peptides of Ag85B were tested with sera from PPD− and PPD+ subjects, there was no significant difference in the OD values between the two groups of subjects with any of the 32 peptides (P = 0.08 to 0.96). The mean OD of all 36 PPD− and PPD+ healthy subjects plus 3 SDs was taken as the cutoff to determine positive responses in the TB patients. As expected, there was a wide variation (8 to 68%) in the recognition of individual peptides with the sera from TB patients (Fig. 1A). Evaluation of the reactivity of individual patient serum sample with the different peptides showed that sera that contained antibodies to the poorly immunogenic peptides generally also recognized the highly immunogenic peptides, and retesting of the former peptides was unlikely to make any significant contribution to increased sensitivity. In view of the large numbers of peptides and the serum volumes required, an arbitrary cutoff of >50% was used as the criterion for the selection of immunogenic peptides for further studies. Seven peptides of Ag85B (peptides A2, A3, A7, A8, A13, A19, and A25) met the criteria and were retested for validation of their reactivities. Six of the seven peptides (all peptides that met the criteria except peptide A3) continued to provide similar reactivities (within 10% of that determined by the screening assay) (Table 1 and Fig. 1B). Eighty-two percent (49/60) of the TB patients had antibodies to at least one of the six peptides, while 6% (2/36) of the healthy PPD− and PPD+ subjects were positive.
FIG. 1.
Epitope mapping of Ag85B. (A) Overlapping peptides of Ag85B were screened for their reactivities with sera from TB patients and healthy PPD+ and PPD− subjects. Peptides that were recognized by none of the healthy control sera are depicted as minimal bars (1% reactivity). (B) Validation of reactivities of peptides identified to be recognized by >50% of the serum samples from TB patients during initial screening. AR, additive reactivity obtained with the six validated peptides.
TABLE 1.
Amino acid sequences of immunogenic peptides
| Peptide name | Amino acid sequence |
|---|---|
| A2 | WGRRLMIGTAAAVVLPGLVG |
| A7 | DIKVQFQSGGNNSPAVYLLD |
| A8 | NNSPAVYLLDGLRAQDDYNG |
| A13 | DWYSPACGKAGCQTYKWETF |
| A19 | QQFIYAGSLSALLDPSQGMG |
| A25 | VANNTRLWVYCGNGTPNELG |
| B7 | MLVQHLLDRDLRVEIPGVDT |
| B13 | GEQFMQWFLQEQIEEVALMA |
| T4 | DFWATWCGPCKMVAPVLEEI |
| T5 | KMVAPVLEEIATERATDLTV |
| T11 | GAKGKAALLRELSDVVPNLN |
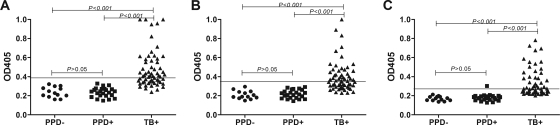
By itself, peptide A25 provided the highest sensitivity, being recognized by sera from 36/60 (60%) of the TB patients (Fig. 1B and 2A). In the different ELISAs with A25, the ODs for the healthy PPD− subjects ranged from 0.14 to 0.44, and those for the PPD+ subjects ranged from 0.13 to 0.38. There was no statistical difference in the OD values between these subjects (P = 0.46 to 0.78). In each experiment, the cutoff for the identification of antibody-positive TB patients was calculated as the mean OD plus 3 SDs for the entire healthy control group (PPD− and PPD+). The cutoff ODs ranged from 0.31 to 0.49 in the three assays. The OD values for the antibody-positive TB patients ranged from above the cutoff to as high as 2.26. These values were significantly different (P < 0.001) from those for the non-TB subjects (Fig. 2A).
FIG. 2.
Reactivities of individual serum samples from PPD− healthy subjects (•), PPD+ healthy subjects (▪), and TB patients (▴) with peptides A25 (A), B13 (B), and T11 (C). OD values obtained from one representative experiment are shown. OD values above 1.0 are depicted as 1.0. There were no statistically significant differences between the OD values obtained with sera from PPD+ or PPD− individuals with any of the peptides. The OD values for the sera from TB patients were statistically different from those for the PPD− and the PPD+ healthy subjects. OD405, OD at 405 nm.
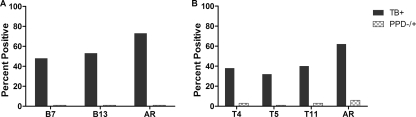
The reactivities of sera from PPD− and PPD+ subjects with the overlapping peptides of BfrB were also similar (P = 0.17 to 0.92 for all peptides except B14, for which P = 0.04). As was observed with the Ag85B peptides, individual BfrB peptides differed in their reactivities, being recognized by antibodies from 8 to 58% of the TB patients (data not shown). Five peptides were recognized by antibodies in sera from ≥50% of the TB patients during the initial screen and were retested. The reactivities with peptides B7 and B13 were similar (within 10% of the value obtained by the screening assay), while the reactivities with peptides B8, B9, and B11 were lower. Sixty percent (36/60) of the TB patients had antibodies directed against at least one of the two peptides (peptides B7 and B13) (Table 1 and Fig. 3A).
FIG. 3.
Validation of reactivities of BfrB peptides (A) and TrxC peptides (B) which were identified to be recognized by the sera from TB patients during the initial screening. AR, additive reactivity obtained with the validated peptides.
Peptide B13 provided the highest sensitivity, being recognized by sera from 53% of the TB patients (Fig. 2B and Fig. 3A). As was observed with peptide A25, the results of the three ELISAs with B13 were highly reproducible. In the different ELISAs with B13, the ODs for the healthy PPD− subjects ranged from 0.15 to 0.35 and those for the PPD+ subjects ranged from 0.13 to 0.34. There was no statistical difference in the OD values between these subjects (P = 0.30 to 0.51). The cutoff OD values ranged from 0.35 to 0.39 in the three assays. The OD values for the antibody-positive TB patients ranged from above the cutoff to as high as 1.28. These values were significantly different (P < 0.001) from those for the non-TB subjects.
When the peptide library of TrxC was tested, as was observed with the vast majority of peptides from Ag85B and BfrB, there was no significant difference between the two groups of healthy controls in the reactivities of their sera with any of the 11 peptides (P = 0.16 to 0.99). Individual peptides were recognized by serum antibodies from 17 to 68% of the TB patients (data not shown), but only one peptide (peptide T2) was recognized by sera from ≥50% of the patients. For this antigen, peptides recognized by sera from ≥40% of the patients were selected for use in the validation. Of the five TrxC peptides (peptides T2, T4, T5, T10, and T11) that were retested, only peptides T4, T5, and T11 continued to provide reproducible reactivities (Table 1). Together, the three peptides provided 62% sensitivity (Fig. 3B).
Peptide T11 was the best-recognized peptide in the TrxC library, being reproducibly recognized by sera from 40% of the TB patients (Fig. 2C and 3B). In the different ELISAs with T11, the ODs for the healthy PPD− subjects ranged from 0.14 to 0.303 and those for the PPD+ subjects ranged from 0.138 to 0.457. There was no statistically significant difference in the OD values between these subjects (P = 0.34 to 0.72). The cutoff OD values ranged from 0.35 to 0.39 in the three assays. The OD values for the antibody-positive TB patients ranged from above the cutoff to as high as 1.37. These values were significantly different (P < 0.001) from those for the non-TB subjects.
When the additive reactivities of three peptides providing the highest sensitivities (peptides A25, B13, and T11) from each of the three antigenic proteins were computed, antibodies could be detected in sera from 43/60 (72%) of the TB patients. The additive reactivity with all 11 peptides that were validated by three assays was also determined; 87% (52 of 60) of the TB patients had antibodies to at least 1 of the 11 peptides. Four of the 36 healthy subjects showed OD values that were above the cutoff, with one subject each showing OD values above the cutoff with peptides A2, A7, T4, and T11. When the data for all 11 peptides were analyzed by use of the algorithm for the identification of the subsets that can provide high sensitivity, several subsets of peptides that included four to eight peptides that provided sensitivities ranging from 76 to 82% and >97% specificity were identified (Table 2). The minimal subset of peptides that provided 82% sensitivity at a specificity of 97% was a subset comprising six peptides (peptides A7, A8, A13, A25, B7, and T5).
TABLE 2.
Peptide subsets providing high sensitivity
| Peptide combinationa | Sensitivity (%) |
|---|---|
| A7, A8, A13, A25 | 76 |
| A7, A13, A19, A25 | 76 |
| A2, A8, A13, A19, A26 | 76 |
| A7, A8, A13, A19, A25 | 78 |
| A7, A13, A25, B7, T5 | 80 |
| A7, A8, A13, A25, B7 | 80 |
| A7, A13, A25, B7, B13, T5 | 80 |
| A7, A8, A13, A19, B13, T5 | 80 |
| A7, A13, A19, A25, B7, T5 | 80 |
| A8, A13, A25, B13, T4, T5 | 80 |
| A7, A8, A13, A25, B7, B13 | 80 |
| A7, A8, A13, A25, B13, T5 | 80 |
| A7, A8, A13, A25, B7, T5 | 82 |
| A7, A8, A13, A19, A25, B7, T5 | 82 |
| A7, A8, A13, A25, B7, B13, T5 | 82 |
| A7, A8, A13, A19, A25, B13, T5 | 82 |
| A7, A8, A13, A19, A25, B7, B13, T5 | 82 |
All subsets showed >97% specificity.
DISCUSSION
The studies described here demonstrate that the three antigens (antigens Ag85B, BfrB, and TrxC) that were identified to be targets of the antibody responses in TB patients in the microarray analysis of cytosolic and culture filtrate proteins of M. tuberculosis are indeed highly immunogenic in these patients. The screening of overlapping peptides of Ag85B demonstrated that, as expected, the protein has both immunogenic regions (epitopes) and nonimmunogenic regions and that the use of peptides encompassing the immunogenic regions enables the detection of anti-Ag85B antibodies in sera from TB patients. Although the reactivities of the same sera with the Ag85B protein were not tested, it is interesting that previous studies, including ours, have demonstrated the presence of anti-Ag85B antibodies in ∼60% of the smear-positive TB patients (5, 19, 22, 27) and that the use of a single immunodominant peptide (peptide A25) was sufficient to obtain a sensitivity similar to that described previously and a high specificity (97%). Interestingly, our earlier studies have demonstrated that patients who are at the same stage of TB have antibodies to a homogeneous repertoire of antigens (20, 22). The current studies show that the repertoire of epitopes that is recognized by individual patients is also rather homogeneous, and the use of only six immunodominant peptides of Ag85B was sufficient to identify 82% of the Indian TB patients. Whether the same epitopes will be recognized by patients with different genetic backgrounds and whether antibodies to these immunodominant epitopes of Ag85B (which has homologues in some other mycobacteria) exist in patients with other mycobacterial infections remains to be tested, but these results demonstrate that the use of dominant epitopes from highly immunogenic antigens could be the basis of a simple diagnostic test for TB. Previous studies with native culture filtrate-derived BfrB and TrxC indicated that ∼50% of the smear-positive TB patients have antibodies directed against these antigens (22). The systematic stepwise screening and validation enabled the identification of immunodominant epitopes of BfrB and TrxC, and as was observed for Ag85B, the use of a small number of these peptides was sufficient to obtain a sensitivity similar to or greater than that achieved with the parent protein.
Several studies have demonstrated that the detection of antibodies directed against multiple antigens provides an improvement in sensitivity over that achieved by the use of a single antigen (2, 10, 28). However, neither the additive reactivity of the three most immunogenic peptides nor the subset of peptides from the three antigens that was defined by the algorithm provided better sensitivity than the immunodominant peptides of Ag85B alone (∼80%). These results suggest that although BfrB and TrxC are immunogenic in TB patients, patients who have antibodies to these antigens also have antibodies to Ag85B and the use of additional epitopes from these antigens provides no increase in sensitivity. Several other immunogenic proteins identified in our previous studies are currently being mapped, since additional epitopes will be required to further enhance the sensitivity.
Although for a majority of the peptides the results of the initial screen and the subsequent validation assays were similar (within 10%), for some peptides (e.g., peptides B8 and T2), the reactivities with the same sera were significantly lower than those when they were retested for validation. In the case of peptide T2, the peptide gradually precipitated out of solution, but for the other peptides, we were unable to determine the cause for the decreasing reactivity. Thus, all synthetic peptides are not equally stable and the reproducibility of results needs to be carefully examined. Interestingly, none of the peptides selected for validation provided a dramatic increase in sensitivity on retesting, and it is unlikely that peptides that provided a low sensitivity in the first round of selection contained dominant epitopes. The OD values obtained with some peptides may not be sufficient to allow clear discrimination in a rapid test, and further optimization by constructing chimeric multiepitope peptides (10) or peptides attached to multiplexed microbeads (12) may be required to devise a commercial assay.
Although these studies provide the proof of principle that selected peptides can replace the parent proteins in immunodiagnostic tests for TB and a peptide-based diagnostic test is feasible, much work remains to be done. At the minimum, a rapid test that can replace microscopy must have a sensitivity of >90% for smear-positive patients and a specificity of >95%; the optimal diagnostic test would have sensitivities of >95% for smear-positive TB patients and ∼65% for smear-negative TB patients and a specificity of >95% (30). Thus, additional immunogenic peptides need to be identified. Moreover, the current studies included only 60 TB patients, all of whom were from the same geographical region; whether the same peptides (epitopes) will be recognized by antibodies in sera from TB patients from other geographical settings and genetic backgrounds and in sera from larger cohorts of patients remains to be determined. Obtaining sera from culture-confirmed smear-negative patients from countries where TB is endemic is difficult, since cultures are rarely performed in these settings; but 30 to 40% of the TB patients presenting to health care facilities are sputum smear negative, and the performance of these peptides in smear-negative patients with culture-confirmed TB must be evaluated. Since TB is a major opportunistic disease in HIV+ patients, it is also important to determine if the same epitopes will also be recognized in HIV+ TB+ patients. Lastly, the specificities obtained with these peptides and peptide subsets need to be evaluated with sera from larger numbers and types of non-TB subjects from additional settings where TB is endemic.
The ability to adapt serodiagnostic tests to rapid formats that can provide point-of-care diagnosis in settings where a sophisticated laboratory infrastructure and highly trained personnel are not available makes them especially attractive for use in the resource-limited settings where TB is endemic. It is estimated that ∼10 to 20% of the patients investigated for TB are finally diagnosed to be afflicted with this disease; thus, ∼45 × 106 to 90 × 106 tests for TB need to be performed globally every year. An ideal TB diagnostic test not only must provide a high sensitivity and a high specificity but must also be amenable to mass production (30). Although recombinant proteins are easier to obtain than their native counterparts and have been used for the detection of specific antibodies in several diagnostic tests, they are prone to degradation and misfolding, making the reproducible production of high-quality recombinant proteins with minimal batch-to-batch variation difficult, laborious, and expensive. In contrast, the large-scale synthesis of peptides allows the easier production of homogeneous antigenic moieties, most peptides are relatively stable, and the use of peptides eliminates the inclusion of cross-reactive regions of the protein antigens, thus increasing the specificity over that which can be achieved with full-length proteins (6, 17). Thus, carefully selected synthetic peptides derived from highly immunogenic proteins can provide uniform, well-defined antigens for use in an immunodiagnostic test for TB. The current studied provides the proof of concept that peptides (epitopes) derived from carefully selected immunogenic antigens can be used to devise a serodiagnostic test for TB.
Acknowledgments
This work was supported by the NIH/FIC AIDS and TB international training and research program (grant Tw001409), a grant from NIAID, NIH (grant AI056257), and a VAMC merit review award.
We thank Krishna K. Singh for his review and comments during the preparation of the manuscript.
Footnotes
Published ahead of print on 12 November 2008.
REFERENCES
- 1.Abebe, F., C. Holm-Hansen, H. G. Wiker, and G. Bjune. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66176-191. [DOI] [PubMed] [Google Scholar]
- 2.Achkar, J. M., Y. Dong, R. S. Holzman, J. Belisle, I. S. Kourbeti, T. Sherpa, R. Condos, W. N. Rom, and S. Laal. 2006. Mycobacterium tuberculosis malate mynthase- and MPT51-based serodiagnostic assay as an adjunct to rapid identification of pulmonary tuberculosis. Clin. Vaccine Immunol. 131291-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcaro, M. C., E. Peroni, P. Rovero, and A. M. Papini. 2003. Synthetic peptides in the diagnosis of HIV infection. Curr. Protein Pept. Sci. 4285-290. [DOI] [PubMed] [Google Scholar]
- 4.Bethunaickan, R., A. R. Baulard, C. Locht, and A. Raja. 2007. Antibody response in pulmonary tuberculosis against recombinant 27kDa (MPT51, Rv3803c) protein of Mycobacterium tuberculosis. Scand. J. Infect. Dis. 39867-874. [DOI] [PubMed] [Google Scholar]
- 5.Daniels, T. M. 1996. Immunodiagnosis of tuberculosis, p. 223-231. In W. R. Rom and S. Garay (ed.), Tuberculosis. Little, Brown & Company, Inc., Boston, MA.
- 6.Gomara, M. J., and I. Haro. 2007. Synthetic peptides for the immunodiagnosis of human diseases. Curr. Med. Chem. 14531-546. [DOI] [PubMed] [Google Scholar]
- 7.Granberg, C., A. Mansikka, O. P. Lehtonen, H. Kujari, R. Gronfors, H. Nurmi, I. Raiha, M. R. Stahlberg, and R. Leino. 1993. Diagnosis of Helicobacter pylori infection by using Pyloriset EIA-G and EIA-A for detection of serum immunoglobulin G (IgG) and IgA antibodies. J. Clin. Microbiol. 311450-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamasur, B., J. Bruchfeld, M. Haile, A. Pawlowski, B. Bjorvatn, G. Kallenius, and S. B. Svenson. 2001. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J. Microbiol. Methods 4541-52. [DOI] [PubMed] [Google Scholar]
- 9.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 382354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain, S. K., J. K. Aggarwal, S. Rajpal, and U. Baveja. 2000. Prevalence of HIV infection among tuberculosis patients in Delhi—a sentinel surveillance study. Indian J. Tuberc. 4721-26. [Google Scholar]
- 12.Khan, I. H., R. Ravindran, J. Yee, M. Ziman, D. M. Lewinsohn, M. L. Gennaro, J. L. Flynn, C. W. Goulding, K. DeRiemer, N. W. Lerche, and P. A. Luciw. 2008. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin. Vaccine Immunol. 15433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laal, S. 1996. Humoral responses to Mycobacterium tuberculosis, p. 335. In W. N. Rom and S. M. Garay (ed.), Tuberculosis. Little, Brown & Company, Inc., Boston, MA.
- 14.Laal, S., K. M. Samanich, G. M. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Belisle. 1997. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin. Diagn. Lab. Imunnol. 449-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matter, L., and D. Germann. 1995. Detection of human immunodeficiency virus (HIV) type 1 antibodies by new automated microparticle enzyme immunoassay for HIV types 1 and 2. J. Clin. Microbiol. 332338-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee, S., N. Daifalla, Y. Zhang, J. Douglass, L. Brooks, T. Vedvick, R. Houghton, S. G. Reed, and A. Campos-Neto. 2004. Potential serological use of a recombinant protein that is a replica of a Mycobacterium tuberculosis protein found in the urine of infected mice. Clin. Diagn. Lab. Immunol. 11280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noya, O., M. E. Patarroyo, F. Guzman, and B. Alarcon de Noya. 2003. Immunodiagnosis of parasitic diseases with synthetic peptides. Curr. Protein Pept. Sci. 4299-308. [DOI] [PubMed] [Google Scholar]
- 18.Ramalingam, B., K. R. Uma Devi, and A. Raja. 2003. Isotype-specific anti-38 and 27 kDa (mpt 51) response in pulmonary tuberculosis with human immunodeficiency virus coinfection. Scand. J. Infect. Dis. 35234-239. [DOI] [PubMed] [Google Scholar]
- 19.Sada, E., L. E. Ferguson, and T. M. Daniel. 1990. An ELISA for the serodiagnosis of tuberculosis using a 30,000-Da native antigen of Mycobacterium tuberculosis. J. Infect. Dis. 162928-931. [DOI] [PubMed] [Google Scholar]
- 20.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 694600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samanich, K. M., J. T. Belisle, M. G. Sonnenberg, M. A. Keen, S. Zolla-Pazner, and S. Laal. 1998. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J. Infect. Dis. 1781534-1538. [DOI] [PubMed] [Google Scholar]
- 22.Sartain, M. J., R. A. Slayden, K. K. Singh, S. Laal, and J. T. Belisle. 2006. Disease state differentiation and identification of tuberculosis biomarkers via native antigen profiling. Mol. Cell. Proteomics 52102-2113. [DOI] [PubMed] [Google Scholar]
- 23.Singh, K. K., Y. Dong, J. T. Belisle, J. Harder, V. K. Arora, and S. Laal. 2005. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin. Diagn. Lab. Immunol. 12354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLOS Med. 41-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundaresh, S., A. Randall, B. Unal, J. M. Petersen, J. T. Belisle, M. G. Hartley, M. Duffield, R. W. Titball, D. H. Davies, P. L. Felgner, and P. Baldi. 2007. From protein microarrays to diagnostic antigen discovery: a study of the pathogen Francisella tularensis. Bioinformatics 23508-518. [DOI] [PubMed] [Google Scholar]
- 26.Taye, A., H. Chen, K. Duncan, Z. Zhang, L. Hendrix, J. Gonzalez, and W. Ching. 2005. Production of recombinant protein Pap31 and its application for the diagnosis of Bartonella bacilliformis infection. Ann. N. Y. Acad. Sci. 1063280-285. [DOI] [PubMed] [Google Scholar]
- 27.Van Vooren, J. P., A. Drowart, J. De Bruyn, P. Launois, J. Milan, E. Delaporte, M. Develoux, J. C. Yernault, and K. Huygen. 1992. Humoral responses against the 85A and 85B antigens of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. J. Clin. Microbiol. 301608-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanchu, A., Y. Dong, S. Sethi, V. P. Myneedu, A. Nadas, Z. Liu, J. Belisle, and S. Laal. 2008. Biomarkers for clinical and incipient tuberculosis: performance in a TB-endemic country. PLoS One 3e2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. 1997. Antituberculosis drug resistance in the world, 1997. World Health Organization, Geneva, Switzerland.
- 30.World Health Organization. 2006. Diagnostics for tuberculosis: global demand and market potential. World Health Organization, Geneva, Switzerland.