Abstract
Cyclic AMP (cAMP) is an important signal transduction second messenger that is commonly used as a functional mirror on the actions of G protein-coupled receptors that can activate or inhibit adenylate cyclases. A radioimmunoassay for cAMP with femtomole sensitivity was first reported by Steiner more than 30 years ago, and there have been several subsequent modifications that have improved this assay in various ways. Here we describe additional improvement to existing methods that markedly improve speed and reduce cost without sacrificing sensitivity, and is also adaptable to analysis of cGMP. The primary antibody is coupled directly to magnetic beads that are then separated from unbound marker using filtration on microplates. This eliminates the need for a secondary antibody, and markedly increases throughput. In addition, we report a simple, reproducible, and inexpensive method to make the radiomarker used for this assay. Although still requiring the use of radioactivity, the resulting method retains a high degree of accuracy and precision, and is suitable for low-cost high-throughput screening. Use of aspects of this method can also improve throughput in other radioimmunoassays.
Introduction
Cyclic AMP (3′,5′-cyclic adenosine monophosphate; cAMP) is a key second messenger involved in numerous intracellular signaling pathways (Antoni, 2000; McPhee et al., 2005). Production of cAMP is controlled by the membrane-bound family of adenylate cyclases (ACs) that convert adenosine triphosphate to cAMP. The activity of most of the ACs is regulated by heterotrimeric GTP-binding proteins (e.g., Gαs/olf, Gαi/o) that directly interact with the intracellular region of GPCRs and can both increase or decrease enzyme activity (Hanoune and Defer, 2001). In addition, phosphodiesterases can catalyze the degradation of cAMP (Weishaar, 1986).
The measurement of adenylate cyclase activity can be accomplished using radiometric assays that follow the incorporation of a radioactive precursor into cAMP (Salomon, 1979; Schulz and Blum, 1985). More commonly, however, a variety of methods that quantify cAMP have been used both for assessment of adenylate cyclase activity, as well as for measuring tissue content of cAMP or breakdown of this second messenger. A major advance for the field was the development by Steiner et al. (1972) of a radioimmunoassay (RIA) for cAMP that offered a high degree of sensitivity and specificity that was soon improved by Harper and Brooker (Harper and Brooker, 1975). Attempts at automating this assay actually led to a commercial instrument (Brooker et al., 1976), but this proved unwieldy.
More recently, other methods for quantifying cAMP have used different radiometric or reporter gene strategies (Williams, 2004). Recently developed radiometric assays such as Flashplate technology (NEN/Perkin Elmer) and scintillation proximity assays (SPA, Amersham Biosciences) are based on the competition of [125I]-labeled cAMP and analyte cAMP, resulting in the production of light when the labeled compound is in close proximity to a solid scintillant surface. These assays are convenient and reproducible, but are often more expensive than traditional radiometric methods and generally speaking less sensitive. Reporter-gene assays utilize cell lines expressing reporter enzymes such as luciferase, green fluorescent protein (GFP), and β-lactamase. Levels of intracellular cAMP are detected via the expression level of a reporter gene that is modulated by transcription factor binding to upstream cAMP response elements (CRE). Reporter-gene assay are generally less expensive than the radiometric assays discussed above, however, they are often plagued by high false-positive hit rates. Several novel, non-radiometric methods to quantify cAMP also have recently become available. These assays involve the use of luminescent proximity (ALPHAScreen®) (Ullman et al., 1994), enzyme complementation technology (DiscoveRx, HitHunter™ EFC), or electrochemiluminescence (Meso Scale Discovery) to detect receptor-mediated changes in intracellular cAMP. Each method is readily compatible with automated high throughput screening (HTS), and often demonstrates a high level of sensitivity, but requires a high degree of instrumentation to maximize throughput putting it beyond the reach of most academic labs.
For this reason, the RIA (or to a lesser extent, protein binding assays using PKA-enriched tissue) remains the most widely used technique. There has been a recent report detailing an improved procedure for this RIA (Post et al., 2000). Indeed, there are commercial kits available (e.g., Amersham Biosciences) that utilize secondary antibody bound to magnetizable polymer beads, and are separated by magnetic separation or centrifugation. Using the dopamine D1 receptor as a model system, we now describe improvements to this procedure that decrease the number of experimental steps, the assay time, and the assay cost, without sacrificing accuracy or precision. In addition, we describe a rapid method for the routine production of the [125I]-labeled cAMP derivative that is used as the radiomarker in this RIA.
Experimental procedures and Results
Materials and reagents
Dihydrexidine was synthesized according to procedures previously published (Brewster et al., 1990). Acetic anhydride, dopamine, IBMX, pargyline, propranolol, SKF38393, and triethyleneamine, and 2′-O-monosuccinyladenosine 3′:5′ monophosphate tyrosyl methyl ester (ScAMP-TME) were purchased from Sigma-Aldrich (St. Louis MO). HEPES was obtained from Research Organics, Inc. (Cleveland OH). Dulbecco’s modified eagle’s media (DMEM), penicillin/streptomycin, and fetal bovine serum (FBS) were purchased from Gibco/Invitrogen. UniFilter-96 GF/B RIA filter plates, Microscint™ 20, and Na125I were purchased from Perkin-Elmer (Waltham, MA, USA). Donkey anti-goat antibody was purchased from Jackson ImmunoResearch (West Grove, PA, USA). Amine terminated BioMag® beads were purchased from Polysciences, Inc. (Warrington, PA, USA), and pre-conjugated Biomagnetic Particles (BMP) to donkey anti-goat secondary was obtained from Rockland, Inc (Gilbertsville, PA, USA).
Sample Generation and storage
cAMP is a relatively heat and acid stable compound that does not require special storage. The following procedure illustrates a common way that samples are generated from a GPCR-based cellular system, but the assay that follows can be used for almost any matrix.
Cell culture
Human epithelial kidney (HEK-hD1) cells transiently transfected with human D1 dopamine receptor using pcDNA3.1 vector (Invitrogen) cells were maintained using Dulbecco’s Modified Eagles’ Medium with 50 U/mL of penicillin, 50 μg/mL of streptomycin (Gibco), and supplemented with 10% fetal bovine serum at 37°C, 5% CO2. Saturation binding experiments with the D1-selective antagonist [3H]SCH23390 using membrane homogenates provided a Bmax of approximately 4.5 pmol/mg protein.
Cell membrane adenylate cyclase assay
Assay buffer was prepared containing 100 mM HEPES, 4 mM MgCl2, 2 mM EDTA, 100 mM NaCl 10 μM pargyline, 500 μM IBMX, 0.1% ascorbic acid, pH 7.4. Drug dilutions were prepared at a range of 10-4 to 10-10 M with three replicates per drug treatment. Diluted drugs, ATP (2 mM), GTP (5 μM), phosphocreatine (20 mM), creatine phosphokinase (185 U/tube) and propranolol (100 μM to block endogenous β1-adrenergic endogenous receptors) were added in a total volume of 100 μL in each well of a 48-well plate. The reaction was initiated by addition of HEK-hD1 cell membranes. Plates then were vortexed briefly, and incubated at 30°C for 15 min. The reaction was terminated with 500 μL 0.1 M HCl, and stored at 4°C. Prior to transferring samples for the RIA, plates are centrifuged for 5 min at 2,500 g using a RC-3B centrifuge from Sorvall Instruments (H2000B rotor) to pellet cellular debris. Plates will keep indefinitely at 4°C following the assay.
cAMP Radioimmunoassay
Iodination reaction
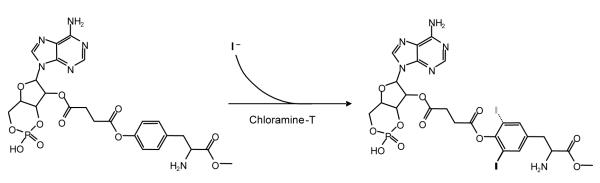
The radiomarker 2′-O-[4-monosuccinyladenosine 3′:5′-cyclic monophosphate-3-[125I]iodotyrosyl methyl ester (hereafter termed ([125I]cAMP-ScTME).was first reported by Steiner et al. (Steiner et al., 1972) can be purchased commercially. For laboratories that will run a reasonable number of such assays, it is technically simple and inexpensive to synthesize this in the laboratory as outlined below. The overall reaction scheme as outlined by Steiner and coworkers (Steiner et al., 1972) is shown in Figure 1.
Figure 1.
Reaction scheme for synthesis of 2′-O-[4-monosuccinyladenosine 3′:5′-cyclic monophosphate-3-iodotyrosyl methyl ester. Conditions described in Methods (molar excess of precursor) favor the formation of the monoiodinated product (see Figure 2).
The following reagents and buffers are required:
0.5 M phosphate buffer, pH 7.6. We usually make this by titrating 15 mL of 0.5 M K2HPO4 with ca. 1.5 mL of NaH2PO4 to pH 7.6.
0.05 M phosphate buffer (pH 7.6). This is prepared by adding 10 mL of the 0.5 M phosphate to 90 mL H2O.
Carrier-free Na125I. We usually use 2 or 5 mCi. If more than 5 mCi is used, the amount of precursor should be increased proportionally,
Precursor ScAMP-TME [2′-O-monosuccinyladenosine 3′:5′ monophosphate tyrosyl methyl ester; Sigma M2257]. From the 1 mg commercial size, we make 1-1.5 mL of a stock solution containing 0.1 mg/mL of distilled water. Aliquots (50 μL) are added to microfuge tubes, labeled, and frozen at -20 C. A single aliquot is used for each radioiodination. The frozen precursor appears stable for several years.
Chloramine-T: (20 mg/10 mL 0.05 M PO4).
Sodium metabisulfite: (24 mg/10 mL 0.05 M PO4).
The reaction procedure is as follows. Briefly, 80 μL of the 0.5 M phosphate buffer pH 7.6 is added directly to the container in which carrier-free Na125I (Perkin Elmer) arrives. We usually iodinate with 5 mCi, but this can be varied. Then, the whole content of one of the thawed aliquots of ScAMP-TME (5 μg/50 μL H2O) is added, the cap screwed back on, and the vial mixed on a vortexer for 15 sec. Following this, Chloramine T (100 μL of 2 mg/mL solution) is added, and timing begun as the mixture is vortexed. After ∼45 sec, the reaction is terminated by addition of sodium metabisulfite (200 μL of 2.4 mg/mL solution). [Safety note: Unreacted 125I is potentially volatile, and a potential health hazard. The use of concentrated (0.5 M) phosphate buffer insures that the reaction solution does not become acidic, a condition favoring the liberation of molecular iodine. In addition, this reaction is done in a chemical hood.]
Purification of iodinated product
It is necessary to separate the monoiodinated cAMP-ScTME from free iodine, diiodinated cAMP-ScTME and other minor by-products. Although this can be done using batch chromatography with reverse phase Sep-Pak cartridges (Oehlenschlager et al., 1990), we have dedicated an archaic isochratic HPLC system and fraction collector for this purpose. The total reaction volume (∼500 μL) is injected using a Rheodyne 7125 Injector (500 μL loop). The isocratic separation (0.8 mL/min) uses a C18 reverse phase column (Inertsil ODS 2-5 μm, Metachem Technologies). The column effluent is collected by a fraction collector (0.5 min samples). As noted above, unreacted 125I is a potential health hazard, and for the separation, 100 μL of 0.1 M sodium hydroxide is added to the first ten tubes on the fraction collector to insure that all unreacted iodine remains in the form of soluble sodium iodide rather than molecular iodine.
The radioactivity is estimated using a hand-held radioactivity detector (or one can count 1 μL aliquots), and the tubes with the highest radioactivity (usually 3-4 tubes) are pooled together, diluted with 1.5 volumes of methanol, and then divided into two or more aliquots for storage at -20°C. Under these conditions, the marker is usable for a minimum of four months, although there is a significant loss of material due to decay.
Preparation of primary antibody conjugation to amine-terminated beads
The primary α-3′-5′-cyclic monophosphate antibody was conjugated to BioMag® Amine-terminated beads (50 mg/mL) as directed by the provided protocol (Polysciences, Inc). Lyophilized antibody was reconstituted in distilled water to a final concentration of 0.5 mg/mL, and dialyzed in coupling buffer (0.01 M pyridine in distilled water, pH 6.0), changing the buffer three-times over a 9 hr period. The beads then were prepared by washing with coupling buffer, and magnetically separating three times. Glutaraldehyde solution (5% glutaraldehyde in coupling buffer) was mixed with the BioMag® beads, and reacted for three hours with rotation. The beads were washed four times with coupling buffer, and antibody was added to the beads with rotation for 16-24 hrs. Glycine quenching solution (1 M glycine, pH 8.0) was combined with beads and rotated for 30 min. Primary α-cAMP-beads were mixed a volume of 20 mL of storage buffer (0.01 M Tris, 0.1% NaN3, 0.1% w/v BSA, 0.15 M NaCl, 1 mM EDTA, pH 7.4), and stored at 4°C. The antibody-bead conjugate was used for up to three months with no appreciable sign of degradation. Fidelity of the conjugate was assessed by determining the ratio of binding between two sets of tubes, one containing radiolabeled cAMP bound to primary antibody and the other containing only radiolabeled cAMP. A ratio of 0.2-0.3 was found to be ideal while less than 0.2 led to inconsistent replicates.
Radioimmunoassay
cAMP standards (2 nM-500 nM) and sample aliquots (5 μL) were transferred from the 48-well microplate in which the cAMP formation was performed to 96-well Skatron plates containing Macrowell tube strips. Sodium acetate buffer (50 mM, pH 6.75) was added to the sample wells to bring the total volume up to 50 μL. Samples that contain cAMP outside of the range of the standard curve can be diluted with additional sodium acetate. An acetylating mixture of TEA/AA (2:1 ratio) was added (5 μL) to the wells and vortexed. Acetylation increases assay sensitivity presumably by creating a structure that more closely resembles the original hapten. 125I-cAMP was then added within 30 minutes of acetylation. Optimal ranges for radioactivity were determined to be between 280 cpm/μL to 320 cpm/μL for iodinated 125I-cAMP-scTME. An aliquot (20 μL) of conjugated-primary antibody then was added to bind labeled and unlabeled cAMP (in 50 mM sodium acetate, 0.1% BSA, pH 4.75). Plates were incubated overnight at 4°C. Radioimmunoassay reactions were terminated by filtration with UniFilter-96 plates (Perkin-Elmer) with dH2O. Plates were washed three times and then dried at 50°C for 1 hour. Microscint™ 20 fluid (50 μL) was added to the wells and counted on a TopCount NXT (Perkin-Elmer) for 2 min or 2σ = 5%.
Data Analysis
Standard data were fit to a one-site binding competition model using Prism 4 (GraphPad Inc,, San Diego CA USA). Sample data were fit by interpolation using standard data to obtain fmol cAMP values. A sigmoidal regression model was used to fit the data to obtain EC50 and maximal efficacy values over the complete dose range (10-4-10-10 M).
Discussion
It should be underscored that the radiosynthesis of the marker does not require a UV detector or radioactivity detector to perform this separation, as a lab radioactivity monitor can easily distinguish the tubes that contain the desired material. Moreover, although we use a dedicated HPLC system for this work, the separation could be optimized for a SepPak, although the disadvantage is that it is difficult to verify the separation. This would not save significant time, but does not require a dedicated “hot” HPLC.
Elimination of secondary antibody allows direct detection
All prior procedures have used secondary antibodies to separate free and antibody-bound 125I-cAMP-ScTME after the incubation of the analytical samples with the primary antibody. Techniques have included ammonium sulfate precipitation (Steiner et al., 1972), charcoal-albumin (Harper and Brooker, 1975), and more recently, polyethylene glycol-assisted secondary separation of bound and unbound 125I-cAMP (Amersham Biosciences) in which samples are pelleted by centrifugation, excess fluid in each tube decanted or aspirated, and bound radioactivity quantified. Subsequent modifications of this method have used secondary antibody conjugated to magnetic beads for detection of cAMP. All of these procedures are relatively laborious and we therefore examined whether both cost and time savings might result from elimination of the use of secondary antibody. We hypothesized that the primary antibody could be conjugated directly to Biomag® amine-terminated beads (see Materials and Methods), and then used in a one-step assay. We therefore used the beads prepared as described above.
To expedite the radioimmunoassay (RIA), we attempted to eliminate the use of secondary antibody by conjugating anti-succinyl-cAMP antiserum to Biomag® amine-terminated beads (see Materials and Methods). Following the conjugation of antiserum to Biomag® beads, we compared the ability of cAMP antiserum to bind cAMP standards. After incubation, the free radiomarker and that bound to the primary antibody-conjugated BioMag® beads were separated using a 96-well harvester and UniFilter-96 GF/B plates (1 μm pore size, PerkinElmer), thus enabling detection of bound radioactivity using a high throughput plate counter (Perkin-Elmer TopCount NXT). Samples (10 μL) were transferred to Macrowell tube strips (using a 12-channel electronic pipette) and necessary reagents were added as described in the Experimental Procedures section. Following overnight incubation with 30 μL of primary antibody (1:40 dilution), samples were harvested using Filtermate Harvester (Packard) and plates were dried for ∼1 h. Scintillation fluid (50 μL) was added to each well, and plates were counted on a TopCount NXT. Cross-well variation was corrected for following the manufacturer’s protocol. Not only does this result in useful standard curves, but application to a well-characterized system (the dopamine D1 receptor) results in EC50 values consistent with earlier literature. Our results demonstrate that cAMP antiserum conjugated to beads can be used to separate bound and free 125I-cAMP with the method of separation utilized in this study.
Optimization of cAMP antiserum conditions
To determine optimal conditions for cAMP antiserum binding, we assessed the ability of the antibody to bind cAMP under variable assay conditions. cAMP standards were incubated with antiserum volumes of 50 μL and 10 μL (1:40 dilution in 50 mM sodium acetate, pH 6.75) for 2 h at room temperature, and overnight at 4°C. All assay conditions yielded viable standard curves (Figure 3). As anticipated, the total amount of cAMP bound was greater for samples incubated with a 50 μL volume of cAMP antiserum than samples incubated with 10 μL. Incubation overnight resulted in increased levels of binding for both dilutions compared to the samples incubated for 2 h at room temperature. These results indicate that antiserum conditions (i.e. dilution, volume, and time of incubation) can be altered according to individual preference and assay requirements. For future experiments we chose to use an overnight incubation using 30 μL (per well) of cAMP antiserum at a 1:40 dilution.
Figure 3.
cAMP standard curves generated under varying assay conditions. Standards were incubated for 2 hrs. at room temperature with 50 μL (A) and 10 μL (B) primary antibody and overnight at 4 C [50 μL (C), 10 μL (D)]. Each assay condition yielded a viable standard curve, indicating that the conditions can be tailored according to the user’s needs.
Assay precision and accuracy
To assess the feasibility of our new cAMP method, we performed an RIA using assay conditions as described by Amersham and our new method. The adenylate cyclase portion of the assay was conducted as described in the Experimental Procedures section. Samples were drawn from the same adenylate cyclase plate and cAMP concentrations were measured using both RIA methods. We determined that the efficacy (Figure 4) and potency of dopamine and SKF38393 was the same for the old method and our new method (Figure 4). In light of the fact that some assays are limited by their ability to distinguish full and partial agonists (Williams, 2004), it is noteworthy that our method easily detects compounds with partial agonist activity (e.g., SKF38393).
Figure 4.
Measurement of D1 dopamine receptor-mediated cAMP accumulation utilizing [Left panel] secondary antibody-PEG assisted RIA method, and [right panel] our new RIA method (primary antibody conjugated to beads. cAMP production was measured using HEK293 cell membranes transiently expressing human D1 dopamine receptors. Data are expressed as % maximal cAMP stimulation caused by dopamine. The curves shown represent mean ± SEM for quadruplicate determinations of cAMP accumulation from four separate experiments.
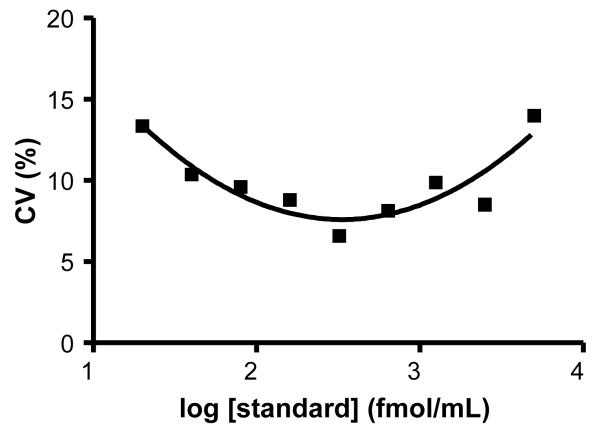
To assess the between-assay reproducibility for our method we pooled the standard deviation of duplicate samples for twenty assays (Figure 5). The Coefficient of Variation (CV) ranged from 7-13%, with the CV being 10% or less over a dynamic range of more than two orders of magnitude. This is an acceptable figure for an assay based on protein binding that uses radioactivity as its endpoint. It should be noted that a significant portion of the experimental variance is due to counting error (Mailman and Boyer, 1997; Motulsky, 2007), a factor that can be decreased by longer counting times if desired. It is known that this assay employs very good precision, and these data also show that it has good accuracy, both of which could be improved by longer counting time at the tradeoff of throughput.
Figure 5.
Precision profile demonstrates the Coefficient of Variation as a function of the concentration of cAMP standards.
Cost issues and alternative technology
In this study we have demonstrated an improved method of cAMP detection that allows for the quick, accurate measurement of femtomole levels of cAMP. A flowchart of this method is shown in Figure 6. We have eliminated the need for secondary antibody and time-consuming separation techniques. By altering the mode of detection and assay format, we have increased throughput and excluded laborious steps inherent to the previous method. Although our research focus is on whole-cell and membrane assays of Gαs/OLF, Gαi/o and Gαq/11 coupled GPCRs, the method is applicable to any measurement of cAMP and can be easily adapted for cGMP.
Figure 6.
Schematic flowchart of the described method.
The method summarized in Figure 6 significantly reduces the costs required to perform the assay. Modification of the assay format and method of detection has yielded a substantial reduction of the time, labor, and costs, as well as a decrease in reagents used for the previous method. At the time of submission of this manuscript, [125I]cAMP-ScTME cost $1,517 for 50 μCi (Perkin Elmer; NEX130050). Reagents suitable for dozens of radioiodinations cost less than $200, and 5 mCi of Na125I can be purchased from Perkin Elmer for $155 yielding a total cost of finished product for a single iodination of < $100/mCi, several-hundred-fold less than the commercial cost.
For the overall assay system we estimate our cost to be ca. $0.50/sample, several-fold less expensive than competing commercial systems. For example, one commercial ELISA assay costs $310 for a single 96 well assay plate, and also has a sensitivity of at least an order of magnitude less than the method we describe. Protein binding assays have also been used in the assay of cAMP for decades ((Brown et al., 1972; Ekins and Brown, 1972). Such protein binding assays are fast and suitable for high throughput, but are of much lower sensitivity, and also require preparation of the cAMP binding protein preparation and the use of a long-lived relatively expensive radioactive marker (i.e., 3H-cAMP). Finally, it should be obvious that this method could be easily adapted to the radioimmunoassay of cGMP. Indeed, the general approach used can also improve the throughput of any radioimmunoassay.
Figure 2.
Chromatogram of radioiodination. [Bottom tracing] shows injection of cAMP-Sc-TME precursor alone using conditions as described in Methods using 254 nm UV detection. The solvent front emerges at ∼ 2 min, and the precursor elutes at ∼ 6min. The signal in the solvent front and a detectable shoulder on the major peak is consistent with the 95% purity estimated by the supplier. [Top tracing] Actual results from a radioiodination. The monoiodinated product that is immunologically recognized elutes at ∼28 min, and is the fraction to be collected and used for the RIA. This fraction contains from 60-70% of the radioactivity in a typical reaction. The fraction eluting at ∼40 min also contains significant radioactivity (10-20%), and is presumably the diiodinated form. These two peaks account for ∼80% of the total radioactivity injected, with the remainder of the radioactivity largely eluting in the solvent front (representing unreacted iodine or highly polar reaction by-products).
Acknowledgements
This work was supported by research grants MH082441, MH073910, and MH040537, and training grants GM007040 and ES007126.
Non-standard abbreviations
- AC
Adenylate cyclase
- cAMP
cyclic AMP adenosine 3′,5′-cyclic monophosphate
- DA
Dopamine
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- RIA
Radioimmunoassay
- SCH23390
7-chloro-8-hydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- SKF38393
7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- cAMP-ScTME
2′-O-[4-monosuccinyladenosine 3′:5′-cyclic monophosphate-3-iodotyrosyl methyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antoni FA. Molecular diversity of cyclic AMP signalling. Front Neuroendocrinol. 2000;21:103–132. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB. trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: a highly potent selective dopamine D1 full agonist. J.Med.Chem. 1990;33:1756–1764. doi: 10.1021/jm00168a034. [DOI] [PubMed] [Google Scholar]
- Brooker G, Terasaki WL, Price MG. Gammaflow: a completely automated radioimmunoassay system. Science. 1976;194:270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Brown BL, Ekins RP, Albano JD. Saturation assay for cyclic AMP using endogenous binding protein. Adv.Cyclic.Nucleotide.Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Ekins RP, Brown BL. Cyclic nucleotides and thyroid hormones: radioimmunoassay or protein-binding assay? Biochem.J. 1972;126:1P. doi: 10.1042/bj1260001pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu.Rev.Pharmacol.Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2′0 acetylation by acetic anhydride in aqueous solution. J.Cyclic.Nucleotide.Res. 1975;1:207–218. [PubMed] [Google Scholar]
- Mailman RB, Boyer J. Theory and practice of receptor characterization and drug analysis. 1997 http://www.pdg.cnb.uam.es/cursos/Barcelona2002/pages/Farmac/Comput_Lab/Radioligandos/Mailman_Boyer/index.htm.
- McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, Cooreman A, MacKenzie SJ. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem.Soc.Trans. 2005;33:1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ. Error in counting radioactivity. 2007 http://www.graphpad.com/articles/AZIntroStats_files/frame.htm#slide0271.htm.
- Oehlenschlager WF, Kubalak SW, Currie MG. A rapid and economical method of preparing radioiodinated cyclic nucleotide derivatives for use in radioimmunoassays. J.Immunoassay. 1990;11:109–118. doi: 10.1080/01971529008053262. [DOI] [PubMed] [Google Scholar]
- Post SR, Ostrom RS, Insel PA. Biochemical methods for detection and measurement of cyclic AMP and adenylyl cyclase activity. Methods Mol Biol. 2000;126:363–374. doi: 10.1385/1-59259-684-3:363. [DOI] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv.Cyclic.Nucleotide.Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Schulz R, Blum V. RIA determination and immunofluorescence localization of cyclic nucleotides in rainbow trout (Salmo gairdneri) testes. Gen.Comp Endocrinol. 1985;57:301–308. doi: 10.1016/0016-6480(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Steiner AL, Wehmann RE, Parker CW, Kipnis DM. Radioimmunoassay for the measurement of cyclic nucleotides. Adv.Cyclic.Nucleotide.Res. 1972;2:51–61. [PubMed] [Google Scholar]
- Ullman EF, Kirakossian H, Singh S, Wu ZP, Irvin BR, Pease JS, Switchenko AC, Irvine JD, Dafforn A, Skold CN. Luminescent oxygen channeling immunoassay: measurement of particle binding kinetics by chemiluminescence. Proc.Natl.Acad.Sci.U.S.A. 1994;91:5426–5430. doi: 10.1073/pnas.91.12.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar RE. Multiple molecular forms of phosphodiesterase: an overview. J Cyclic.Nucleotide.Protein Phosphor.Res. 1986;11:463–472. [PubMed] [Google Scholar]
- Williams C. cAMP detection methods in HTS: selecting the best from the rest. Nat.Rev.Drug Discov. 2004;3:125–135. doi: 10.1038/nrd1306. [DOI] [PubMed] [Google Scholar]