Abstract
Apolipoprotein F (Apo F) is a protein component of several lipoprotein classes including HDL. It is also known as lipid transfer inhibitor protein (LTIP) based on its ability to inhibit lipid transfer between lipoproteins ex vivo.
Objective
We sought to investigate the role of Apo F in HDL metabolism.
Methods and Results
Adeno-associated viruses (AAV) based on serotype 8, were used to overexpress either murine or human ApoF in mice. Overexpression of murine ApoF significantly reduced total cholesterol levels by 28% (p < 0.001), HDL by 27% (p < 0.001), and phospholipid levels by 19% (p < 0.001). Overexpression of human Apo F had similar effects. Human Apo F was nearly exclusively HDL-associated in mice. In agreement with this finding, greater than 90% of the Apo F in human plasma was found on HDL3, with only a small amount on LDL. Overexpression of mouse Apo F accelerated the plasma clearance of [3H]-cholesteryl ether labeled HDL. Plasma from mice overexpressing Apo F showed improved macrophage cholesterol efflux on a per HDL-C basis.
Conclusions
Apo F overexpression reduces HDL cholesterol levels in mice by increasing clearance of HDL-CE. Apo F may be an important determinant of HDL metabolism and reverse cholesterol transport.
Apolipoprotein F (Apo F) is a 29 kDa sialoglycoprotein found in the HDL and LDL fractions of human plasma1–4. It is also known as lipid transfer inhibitor protein (LTIP) for its ability to inhibit lipid transfer between lipoproteins ex vivo5. Apo F (LTIP) is an endogenous inhibitor of CETP-mediated cholesteryl ester (CE) and triglyceride (TG) transfer6, 7. In vitro, Apo F can selectively inhibit transfer events involving LDL8, and thereby indirectly increases the movement of CE from HDL to VLDL9, 10. Accordingly, Apo F has been postulated to play an important role in HDL metabolism and reverse cholesterol transport (RCT)11, 12.
Apo F originates in the liver as a 326 amino acid precursor, which consists of a small signal peptide followed by a large proprotein of 290 amino acids. The proprotein is cleaved to release the 162 amino acid C-terminus which makes up the mature secreted form of the protein3. Apo F is an unusual apolipoprotein in that it is heavily glycosylated with both O- and N-linked sugar groups. These glycosylations render the protein very acidic with an isoelectric point of 4.5, and result in a molecular mass about 40% greater than predicted.
Recent shotgun proteomics studies have identified Apo F as a component of the human HDL particle 13. This renewed our interest in Apo F as a candidate gene involved in HDL metabolism. Little is known about the effects or function of Apo F in vivo, and no overexpression model has been reported. In order to test the hypothesis that Apo F modulates HDL metabolism, we generated a recombinant adeno-associated virus serotype 8 (AAV8) vector to somatically overexpress Apo F in mouse liver. In this report, we show that stable overexpression of Apo F: 1) reduces HDL cholesterol levels, 2) accelerates plasma clearance of HDL-CE, and 3) results in HDL particles that have increased ability to accept cholesterol from the macrophage.
Methods
Plasmid Construction
An expression clone encoding mouse Apo F (Genbank BC010815) was obtained from the American Type Culture Collection. A human Apo F expression clone (Genbank NM_001638) was purchased from Origene. The ApoF cDNAs were subcloned into the pcDNA3.1(+)/V5-His TOPO vector (Invitrogen) to generate C-terminal tagged versions. Site-directed mutagenesis was preformed using the Quikchange II XL kit (Stratagene). Primer sequences used for cloning are listed in Supplemental Table I. All clones were verified by sequencing at the Department of Medical Genetics DNA Sequencing Facility (University of Pennsylvania, School of Medicine).
Virus Construction
Recombinant AAVs were engineered to overexpress Beta Galactosidase (Lac Z), mouse Apo F (mApoF), or human Apo F (hApoF). AAV8 was generated as previously described14 using a chimeric packaging construct in which the Rep gene from AAV2 has been fused with the Cap gene from AAV8.
Cell Culture
HEK293 cells were grown in Dulbecco’s modification of Eagle’s media (DMEM) containing 10% fetal bovine serum and 1% antibiotic / antimycotic. Cells were seeded into 12 well plates and transiently transfected with 1 µg of plasmid DNA with Lipofectamine (Invitrogen). Twenty four hours later, the media were changed to serum free DMEM. The cells were incubated overnight and harvested the following day.
Western Blotting
Immunoblotting was performed as described previously 15. Antibodies and dilutions are described in the supplemental methods- please see www.ahajournals.org.
Mice
Male wild type C57BL/6 mice were obtained from Jackson Labs. Mice were fed a standard chow diet (LabDiet number 5053) ad libitum. AAVs were delivered by intraperitoneal injection. In all cases, fasting plasma was obtained by retro-orbital bleeding while the mice were under isofluorane anaesthesia. Animal experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Lipid and Lipoprotein Analyses
Total cholesterol, HDL cholesterol, phospholipids, triglycerides, and alanine aminotransferase (ALT) levels were measured with the use of diagnostic reagents from Sigma. Pooled plasma from each group (160 µl), was separated by FPLC gel filtration. Total cholesterol in each fraction was measured using the Total Cholesterol E kit from Wako.
HDL Kinetic study
Human HDL3 was isolated from pooled human plasma and labeled with [3H]-cholesteryl hexadecyl ether (cholesteryl-1,2-3H, Perkin Elmer) as described previously 16. The fractional catabolic rate (FCR) was determined from the area under the plasma decay curves fitted to a bicompartmental model using the WinSAAM software assuming a plasma volume equivalent to 3.5% of body weight.
Reverse Cholesterol Transport Assay
In vivo RCT assay was performed as previously described using acetylated LDL loaded J774 macrophages labeled with [3H]-Cholesterol17.
Cholesterol Efflux Assays in Bone Marrow-derived Macrophages
Efflux assays were performed as previously described 18. Briefly, cells were loaded with 2 µCi / ml of [3H]-Cholesterol and 25 µg / ml of acetylated LDL for 24 hours. The cells were pre-incubated for 18 hours with an LXR agonist (GW3965, 10 µM) to upregulate ABCA1 and ABCG1 expression. Where indicated, cells were treated with 20 µM probucol for 2 hours before starting. Efflux to pooled plasma (final 2.5%) was performed for two hours at 37°C. Free cholesterol (FC) efflux was calculated as the percentage of the counts in the media divided by the sum of the counts in the media and the FC counts in the cells. ABCA1-dependent efflux was defined as the total FC efflux minus the FC efflux for cells pretreated with probucol (specific ABCA1 inhibitor). Efflux was normalized to HDL-C levels by dividing by the plasma HDL cholesterol concentration (mg / dl).
Statistical Analysis
All values are shown as the mean +/− standard deviation. A two-tailed student’s t-test was used to test for statistical significance. Detailed methods are available as supplemental information online- please see www.ahajournals.org.
Results
Proteolytic processing and N-linked glycosylation of Apo F
In order to investigate the effects of Apo F on lipoprotein metabolism, we first examined the protein in vitro. To study the synthesis and processing of Apo F, we made C-terminally tagged versions of mouse and human Apo F (Figure 1A) and transiently transfected them into HEK293 cells. Human Apo F produced two sets of bands in the media: a doublet around 33 kDa (normally 29 kDa without the tag), as well as a higher band at 55 kDa (Figure 1, lane 2). In contrast, mouse Apo F produced a single band in the media around 35 kDa (Figure 1, lane 9). In order to delineate the differences between the proprotein and mature Apo F, we mutated arginine 164 at the predicted proprotein cleavage site to alanine (R165 in mApo F). This substitution completely inhibited processing to the mature form (Figure 1, lanes 7 and 10), revealing that the upper band in the media represents the Apo F proprotein.
Figure 1.
N-linked glycosylation and cleavage of Apo F. A. Expression constructs for human and mouse Apo F. B. N-linked glycosylation and propeptide cleavage mutants in HEK293 cells (Amino acid substitutions given above each lane). Arrows indicate the mature peptide (Mature), proprotein (Pro), and maturely glycosylated proprotein (Pro *).
Apo F is glycosylated with both O-linked and N-linked sugar groups12, increasing its size from the predicted 17.5 kDa to the observed molecular mass of 29 kDa3. Human Apo F contains three predicted N-linked glycosylation sites not found in the mouse protein (Figure 1A, Supplemental Figure I). Two of these sites are in the proprotein (N118 and N139), and one lies in the mature peptide (N267). Site directed mutagenesis was used to mutate these asparagine residues to alanine. The proprotein in the media was glycosylated at both N118 and N139 (Figure 1B, lanes 3–5), as mutation of either or both of these residues reduced the molecular mass of the upper band. The mature form of Apo F appears to be variably glycosylated at N267, as mutation of this residue abolished the upper band of the doublet in the media (Figure 1B, lane 6). Similar results were seen in the cell lysates (Figure 1B, bottom panel). Apo F in the cell lysates was found to be both PNGase F and EndoH-sensitive, consistent with N-glycosylated proteins that have not yet been processed by Mannosidase II in the medial Golgi. Apo F in the media was PNGase F-sensitive but Endo H-resistant, indicating that the secreted forms of Apo F are maturely glycosylated (Supplemental Figure II).
Generation of a polyclonal antibody to human Apo F and detection of Apo F in human HDL
An Adeno-Associated Virus (AAV) encoding human Apo F was used to generate a rabbit polyclonal antibody. The antibody did not recognize mouse Apo F in plasma (Supplemental Figure IIIA, lane 1- control) even when overexpressed (Supplemental Figure IIIA, lane 3- mApoF). Two sets of bands were visible in plasma from mice overexpressing human Apo F, including a doublet at 29 kDa and an additional band around 45 kDa (Supplemental Figure IIIA, lane 4- hApoF). The upper band is most likely uncleaved Apo F proprotein (Figure 1B). We used this antibody to examine Apo F in human lipoproteins. Surprisingly, greater than 90% of the Apo F was found on HDL3, with only a small amount on LDL (Supplemental Figure IIIB). Apo F was not detected on VLDL or HDL2. The Apo F proprotein could not be detected in human plasma (Supplemental Figure IV).
AAV8-mediated overexpression of Apo F in vivo
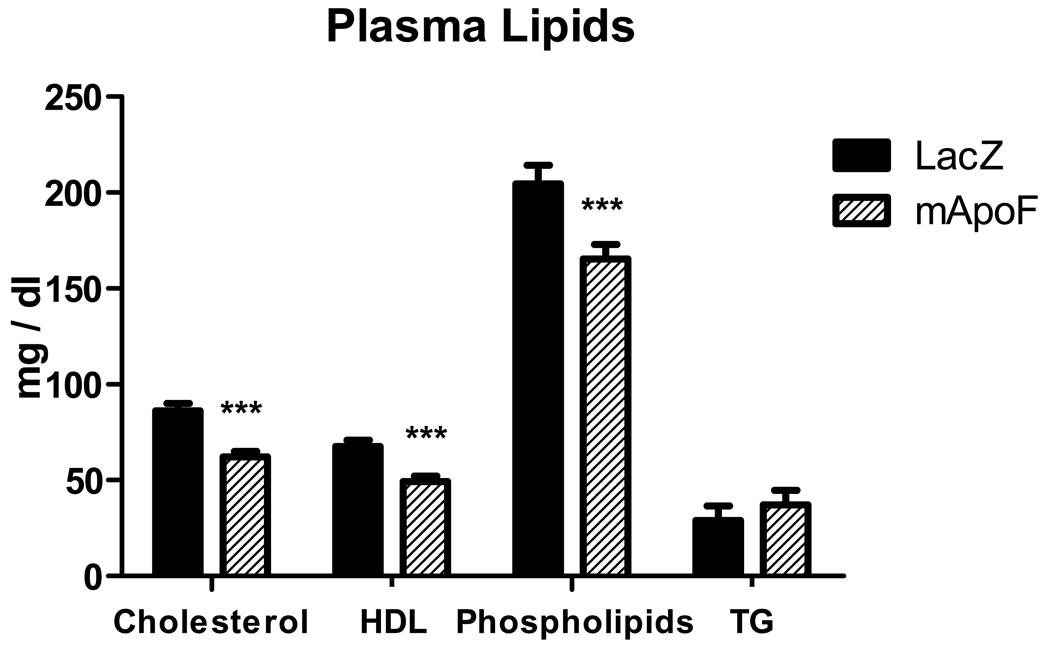
AAVs based on serotype 8 were constructed to overexpress human or mouse Apo F under the control of the liver-specific thyroxine binding globulin promoter19. Wild type male C57BL/6 mice were injected with 1 × 1012 genome copies (GC) of AAV encoding either Lac Z or hApoF and followed for four weeks. Human Apo F overexpression reduced HDL-C by 15% (p < 0.05). Phospholipids paralleled the decrease in HDL with a 19% reduction (p < 0.05). Triglyceride levels were not affected. In addition, plasma alanine aminotransferase (ALT) levels were not substantially elevated (mean < 150 IU / L), confirming that the lipid effects are not due to hepatotoxicity (data not shown). Pooled plasma from mice infected with AAV-Lac Z or AAV-hApoF was separated by FPLC. Human Apo F overexpression reduced cholesterol in all fractions. The HDL-C peak was noticeably decreased, but not shifted in either direction, indicating that particle size was not affected (Figure 2). Apo F was found to be strictly HDL-associated in these mice. Interestingly, human Apo F appeared to preferentially associate with the larger mouse HDL fractions. The same strategy was used to overexpress mouse Apo F in mice. Mouse Apo F overexpression gave effects similar to those observed for human Apo F. Total cholesterol levels were reduced by 28% (p < 0.001) at four weeks. Accordingly, HDL-C decreased by 27% (p < 0.001), and phospholipids fell by 19% (p < 0.001) relative to control animals (Figure 3). As with human Apo F, plasma triglycerides and ALT levels were not affected. Apo F was overexpressed 25-fold relative to the endogenous Apo F message in the liver as determined by quantitative RT-PCR (data not shown). Mouse Apo F reduced cholesterol across all lipoprotein fractions (Supplemental Figure V).
Figure 2.
AAV overexpression of human ApoF reduces HDL cholesterol. Total cholesterol was measured in FPLC fractions of pooled plasma from mice overexpressing either Beta-Galactosidase (LacZ) or human ApoF (hApoF). Apo A-I, Apo E, and human Apo F were detected by Western blotting.
Figure 3.
AAV overexpression of mouse Apo F reduces HDL cholesterol levels. Total plasma cholesterol, HDL cholesterol, phospholipids, triglycerides in mice overexpressing LacZ or mApoF (*** p< 0.001).
Effects of Apo F overexpression on HDL turnover
We hypothesized that Apo F might decrease HDL cholesterol levels by increasing cholesteryl ester removal from the plasma. A kinetic study was performed to examine the plasma clearance of human HDL3-labeled cholesteryl ether. Mice were injected with AAV-LacZ or AAV-mApoF three weeks before the kinetic study. We chose to overexpress mouse Apo F in this and subsequent experiments, since it is plausible that human Apo F may not as efficiently interact with mouse SR-BI or other receptors in the liver. At two weeks, total cholesterol in the plasma was reduced by 19% (p < 0.05), and HDL-C decreased by 20% (p < 0.05) (Supplemental Figure VI, A). Plasma clearance of HDL-CE was significantly faster in the mice overexpressing mouse Apo F. This was reflected in a 24% increase in the fractional catabolic rate− 0.220 +/− 0.028 HDL3 pools / hr for mApoF versus 0.178 +/− 0.012 HDL3 pools / hr for LacZ (p<0.05) (Supplemental Figure VI, B).
Apo F overexpression and reverse cholesterol transport
Since Apo F overexpression accelerated the plasma clearance of HDL-CE, we wondered whether Apo F would promote RCT. To address this possibility, we performed an in vivo RCT assay. Mice overexpressing Lac Z or mApoF were injected with [3H]-cholesterol labeled J774 macrophages and followed for 48 hours. Apo F overexpression reduced HDL-C levels in these mice by 21% relative to control animals (p < 0.001), consistent with previous observations (data not shown). Surprisingly, despite a markedly reduced HDL peak in the FPLC profile, the 3H counts in HDL were almost identical to control animals at 48 hours after macrophage injection (Figure 4A). This suggests that despite lower HDL cholesterol levels, Apo F-containing HDL is still able to accept nearly normal levels of macrophage-derived cholesterol. Plasma counts of [3H]-cholesterol were not significantly different throughout the experiment (Figure 4B). This might be expected as plasma counts are a result of both macrophage cholesterol efflux, and clearance of HDL cholesterol by the liver. Interestingly, liver counts were significantly increased with Apo F overexpression (34%, p < 0.05) (Figure 4C), consistent with the HDL kinetic study. Bile and Fecal 3H counts were not significantly different between groups (Figure 4D and E), and notably were not decreased with Apo F despite the reduction in HDL cholesterol.
Figure 4.
The effects of Apo F on RCT. A. Lipoprotein profile 48 hours after macrophage injection- total cholesterol (left), [3H]-cholesterol (right). B. [3H]-cholesterol in plasma following macrophage injection. C. Final 3H counts in bile. D. Final 3H counts in liver. E. Total fecal 3H counts (* p < 0.05).
Apo F and macrophage cholesterol efflux
To characterize the functional activity of HDL from control and Apo F overexpressing mice, we examined the capacity of plasma to promote cholesterol efflux from bone marrow derived macrophages. Despite a roughly 20% decrease in HDL cholesterol and phospholipids, plasma from mice overexpressing mouse Apo F demonstrated only 5% lower capacity to promote efflux from cells (p < 0.05) (Figure 5A). When free cholesterol efflux was normalized to HDL cholesterol concentration, plasma from Apo F animals showed 19% higher efflux (p < 0.001). This higher per unit efflux capacity was due primarily to changes in ATP-binding cassette transporter A1 (ABCA1)-independent efflux, as determined by pretreatment with probucol (22% increase, p < 0.001) (Figure 5B). ABCA1-dependent efflux trended towards an increase per HDL-C, but this did not reach statistical significance. Additional results from experiments addressing the role of Apo F in cholesterol efflux as well as CETP inhibition are included in supplemental information available online at www.ahajournals.org.
Figure 5.
Plasma from mice overexpressing Apo F has improved efflux capacity per HDL-C. A. Free cholesterol efflux to plasma from mice overexpressing Lac Z or mApoF B. Efflux capacity to plasma normalized to HDL-C concentrations. Significant differences indicated with * p < 0.05 and *** p < 0.001.
Discussion
We used AAVs based on serotype 8 to overexpress Apo F in mouse liver. Human and mouse Apo F overexpression both reduced HDL cholesterol levels, providing the first concrete in vivo evidence that Apo F is involved in HDL metabolism. Overexpression of mouse Apo F improved the clearance of HDL cholesterol ester from the plasma, and resulted in HDL particles that are more efficient acceptors of macrophage-derived cholesterol. In addition, we have gained important insights into the biology of Apo F as it relates to HDL cholesterol and RCT.
Secreted Apo F is known to arise from cleavage of a much larger precursor 3. We created Apo F variants that could not be cleaved to generate the mature peptide. Surprisingly, the proprotein was still secreted into the media. The presence of maturely glycosylated proprotein in the media suggests that Apo F is processed to the mature form at the cell surface or after secretion. Human Apo F contains the sequence RVGRS at the cleavage site, whereas the mouse sequence is RAKRS. This RAKR sequence in mouse is a consensus furin recognition sequence of the format R-X-R/K-R. It will be interesting to determine whether Apo F is a bona fide target of furin or if another proprotein convertase is responsible for its processing. Interestingly, the Apo F proprotein could be detected in plasma and on isolated HDL when overexpressed in mice (Supplemental Figure III and IV). Although we did not detect the Apo F proprotein in human plasma, our data suggests that it can be secreted. Cleavage of the secreted proprotein may represent a regulatory mechanism for converting a potentially inactive version of Apo F to its active form.
Apo F is known to be heavily glycosylated with both O- and N-linked sugar moieties. Glycosylation is a requirement for full LTIP activity 12. Based upon sequence, it has long been thought that asparagine 267 in the mature peptide is N-glycosylated 3. We have shown that the human Apo F protein is in fact variably N-glycosylated at N267, giving rise to the doublet seen at 29 kDa in plasma. In addition, we have shown that the Apo F proprotein is glycosylated at N118 and N139. These glycosylations are Endo H sensitive in cell lysates, but resistant in the media (Supplemental Figure II). The presence of maturely glycosylated Endo H-resistant proprotein in the media supports the idea that Apo F is processed after secretion. It is curious that the human protein has evolved these three N-linked glycosylation sites, including one in the mature peptide. Despite 60% total amino acid identity, mouse and human Apo F have a great deal of sequence divergence near the C-terminus (Supplemental Figure I). It is tempting to speculate that these differences may have evolved for the purpose of fine tuning CETP activity in humans.
In our experiments, human Apo F was strictly HDL-associated in wild type mice. A small amount could be found on LDL in mouse strains lacking the LDL receptor (Supplemental Figure VII and VIII). In agreement with this, Apo F in human plasma was found predominantly in the HDL3 fraction, with a very small amount on LDL (Supplemental Figure III,B). The presence Apo F on human HDL is consistent with very early reports 1–3, 7. Apo F was originally purified and cloned using human HDL as the starting material 3. This conflicts with reports that Apo F is predominantly LDL-associated 5, 8. A recent study has reconciled this discrepancy, confirming that 75% of the Apo F protein is present on HDL, and only about 25% on LDL 4. LTIP activity was found only in the LDL fractions in this report- while the HDL associated Apo F existed as part of a 470 kDa inactive complex with a density in the HDL3 range. These findings are in general agreement with our observations. It is noteworthy that we found Apo F on HDL3 and not HDL2 (Supplemental Figure III,B). This is surprising, since human Apo F preferred to associate with the larger HDL particles in the mouse profile (Figure 2B) 4. As has been suggested4, it is possible that the protein content of Apo F-containing particles places their density in the HDL3 range despite a larger size.
Overexpression of mouse and human Apo F both significantly reduced HDL cholesterol levels. This suggests that despite their differences, mouse and human Apo F share some overlapping functional properties. Apo F overexpression had no effect on SR-BI and ABCA1 mRNA or protein levels (Supplemental Figure IX and X), ruling out obvious effects at the level of the liver that would impact HDL production or selective uptake. Since neither mouse nor human Apo F overexpression significantly altered the size of the HDL particles (by FPLC), it is unlikely that the effects are due to altered LCAT activity (Figure 2 and Supplemental Figure V). Furthermore, the ratio of cholesteryl ester to free cholesterol did not change with Apo F overexpression (data not shown), nor was the lipid composition of the HDL from these mice affected (Supplemental Table II).
Apo F overexpression did not substantially change Apo AI or Apo E levels in wild type mice as assessed by western blotting (Supplemental Figure XI). Apo F was overexpressed in human Apo AI transgenic mice to more precisely measure Apo AI levels. Human Apo F overexpression lowered HDL levels by 25% relative to mice receiving the control virus (Lac Z). This drop in HDL cholesterol was accompanied by a 19% drop in Apo AI levels relative to baseline measurements (Supplemental Figure XII, A). The reduction in Apo AI was observed in both the HDL and non-lipoprotein fractions in these mice (Supplemental Figure XII, B). It is conceivable that the tight association of Apo F with the HDL particle displaces Apo AI, and thereby promotes catabolism of the particle. This seems less likely as the HDL particle size was not altered (by FPLC), and free Apo AI levels (in the non-lipoprotein fraction) were also reduced to a similar extent in these mice (Supplemental Figure XIII).
Overexpression of Apo F accelerated the plasma clearance of HDL cholesteryl ether, as reflected by a 24% increase in the fractional catabolic rate. This finding is in agreement with the increased liver counts observed in the RCT study. Since SR-BI and LDL receptor protein levels were not affected (Supplemental Figure X), the increased liver uptake is probably due to the presence of Apo F on HDL. Apo F may improve the HDL particle’s interaction with SR-BI, or possibly even serve as a weak ligand for another liver-expressed receptor. Apo F could modify the particle in such a way that uptake of CE is improved. Alternatively, Apo F is known to form particles in the HDL density range either along with Apo A-I, Apo A-I and Apo-II, or with apolipoprotein F as the sole apolipoprotein 1. Perhaps these Apo F-only particles can also play a unique role in shuttling CE to the liver.
Plasma from mice overexpressing Apo F was a better acceptor for macrophage derived cholesterol on a per HDL-C basis. This increased efflux capacity was due primarily to changes in ABCA1 independent efflux, as would be expected from the presence of Apo F on the larger HDL fractions. In loaded J774 macrophages, HDL isolated from mice overexpressing human Apo F was a better acceptor in cholesterol efflux assays (Supplemental Figure XIV and XV). The non-lipoprotein fraction, containing free human Apo AI as well as the free human Apo F protein, showed significantly reduced efflux relative to that from control mice. Taken together, these results indicate that Apo F overexpression produces HDL particles that are more efficient acceptors of cholesterol from the macrophage.
Apo F overexpression consistently lowered HDL cholesterol levels in mice. Surprisingly, this did not adversely affect the rate of RCT, as mice overexpressing Apo F had similar counts of macrophage-derived cholesterol in the feces. This paradox can be explained by two factors: 1) an increased per unit capacity of HDL to accept cholesterol in cases of Apo F overexpression, and 2) accelerated clearance of HDL-CE from the plasma. Increased flux of macrophage-derived cholesterol through the plasma compartment was able to maintain RCT at levels seen in control mice, despite lower steady state levels of HDL.
We have demonstrated that Apo F overexpression reduces HDL cholesterol levels in mice- the first in vivo data implicating Apo F in HDL metabolism. Furthermore, our results indicate that Apo F has favorable effects on the reverse cholesterol transport pathway which are independent of its predicted effects on CETP function. Apo F overexpression increased clearance of CE from the plasma, and resulted in HDL particles that were better acceptors for macrophage-derived cholesterol. Based on the data, we believe that high levels of Apo F would be atheroprotective. It will be important to determine whether Apo F levels or genetic variants have a relationship to coronary risk in humans.
Supplementary Material
Acknowledgments
A) Sources of Funding: This work was supported by NIH grants 5-P01-HL-059407-09 and 5R01-HL 055323-11. B) Acknowledgements- The rabbit polyclonal antibody used to detect the LDL receptor was a gracious gift of Dr. Gene C. Ness. C) Disclosure – The authors have no conflicts of interest to disclose.
References
- 1.Koren E, McConathy WJ, Alaupovic P. Isolation and characterization of simple and complex lipoproteins containing apolipoprotein F from human plasma. Biochemistry. 1982;21:5347–5351. doi: 10.1021/bi00264a035. [DOI] [PubMed] [Google Scholar]
- 2.Olofsson SO, McConathy WJ, Alaupovic P. Isolation and partial characterization of a new acidic apolipoprotein (apolipoprotein F) from high density lipoproteins of human plasma. Biochemistry. 1978;17:1032–1036. doi: 10.1021/bi00599a014. [DOI] [PubMed] [Google Scholar]
- 3.Day JR, Albers JJ, Gilbert TL, Whitmore TE, McConathy WJ, Wolfbauer G. Purification and molecular cloning of human apolipoprotein F. Biochem Biophys Res Commun. 1994;203:1146–1151. doi: 10.1006/bbrc.1994.2302. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Greene DJ, Kinter M, Morton RE. Control of cholesteryl ester transfer protein activity by sequestration of lipid transfer inhibitor protein in an inactive complex. J Lipid Res. 2008 doi: 10.1194/jlr.M800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Driscoll DM, Morton RE. Molecular cloning and expression of lipid transfer inhibitor protein reveals its identity with apolipoprotein F. J Biol Chem. 1999;274:1814–1820. doi: 10.1074/jbc.274.3.1814. [DOI] [PubMed] [Google Scholar]
- 6.Son YS, Zilversmit DB. Purification and characterization of human plasma proteins that inhibit lipid transfer activities. Biochim Biophys Acta. 1984;795:473–480. doi: 10.1016/0005-2760(84)90175-9. [DOI] [PubMed] [Google Scholar]
- 7.Nishide T, Tollefson JH, Albers JJ. Inhibition of lipid transfer by a unique high density lipoprotein subclass containing an inhibitor protein. J Lipid Res. 1989;30:149–158. [PubMed] [Google Scholar]
- 8.Morton RE, Greene DJ. Regulation of lipid transfer between lipoproteins by an endogenous plasma protein: selective inhibition among lipoprotein classes. J Lipid Res. 1994;35:836–847. [PubMed] [Google Scholar]
- 9.Serdyuk AP, Morton RE. Lipid transfer inhibitor protein activity deficiency in normolipidemic uremic patients on continuous ambulatory peritoneal dialysis. Arterioscler Thromb Vasc Biol. 1997;17:1716–1724. doi: 10.1161/01.atv.17.9.1716. [DOI] [PubMed] [Google Scholar]
- 10.Serdyuk AP, Morton RE. Lipid transfer inhibitor protein defines the participation of lipoproteins in lipid transfer reactions: CETP has no preference for cholesteryl esters in HDL versus LDL. Arterioscler Thromb Vasc Biol. 1999;19:718–726. doi: 10.1161/01.atv.19.3.718. [DOI] [PubMed] [Google Scholar]
- 11.Morton RE, Nunes V, Izem L, Quintao E. Markedly elevated lipid transfer inhibitor protein in hypercholesterolemic subjects is mitigated by plasma triglyceride levels. Arterioscler Thromb Vasc Biol. 2001;21:1642–1649. doi: 10.1161/hq1001.096722. [DOI] [PubMed] [Google Scholar]
- 12.Morton RE, Gnizak HM, Greene DJ, Cho KH, Paromov VM. Lipid transfer inhibitor protein (apolipoprotein F) concentration in normolipidemic and hyperlipidemic subjects. J Lipid Res. 2008;49:127–135. doi: 10.1194/jlr.M700258-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc Diabetol. 2007;6:15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagor WR, Heller R, de Groh ED, Ness GC. Functional analysis of the hepatic HMG-CoA reductase promoter by in vivo electroporation. Exp Biol Med (Maywood) 2007;232:353–361. [PubMed] [Google Scholar]
- 16.Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J Biol Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
- 17.Tanigawa H, Billheimer JT, Tohyama J, Zhang Y, Rothblat G, Rader DJ. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation. 2007;116:1267–1273. doi: 10.1161/CIRCULATIONAHA.107.704254. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitajima K, Marchadier DH, Miller GC, Gao GP, Wilson JM, Rader DJ. Complete prevention of atherosclerosis in apoE-deficient mice by hepatic human apoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler Thromb Vasc Biol. 2006;26:1852–1857. doi: 10.1161/01.ATV.0000231520.26490.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.