Abstract
Background
Discontinuous pulmonary arteries (PAs) may develop in patients with single-ventricle heart disease from a variety of causes. We investigated factors associated with successful connection of nonconfluent PAs in patients with a cavopulmonary circulation.
Methods
We reviewed 49 patients who underwent connection of discontinuous PAs with or after a bidirectional Glenn (n = 29) or Fontan (n = 20) procedure at a median age of 7.9 years. PA continuity was established by direct anastomosis in 27, interposition graft in 19, and transcatheter recanalization in 3. Survival was 92% ± 4% at 1 year and 89% ± 5% at 5 years.
Results
Recurrent PA occlusion was documented in 7 patients, 5 within 10 days of PA connection. The only factor associated with shorter freedom from PA occlusion was sole supply of blood flow to 1 lung by systemic-to-PA collaterals before connection (66% ± 14% vs 95% ± 4% freedom from occlusion at 6 months, p = 0.03). Among the 45 early survivors, freedom from PA reintervention or occlusion was 83 ± 6% at 1 year and 55 ± 9% at 3 years.
Conclusions
Discontinuous PAs can be successfully connected in most patients with a cavopulmonary circulation, although nonconfluent PAs appear to increase the risk of poor outcome after Fontan. Recurrent PA occlusion was usually diagnosed in the early postoperative period. In patients with sole supply to 1 lung through collaterals, shunt placement before PA connection may optimize outcome. A low threshold for investigation of the reconnected PA is warranted.
In individuals with a Fontan connection, systemic venous return is transmitted directly to the pulmonary circulation, with no intervening subpulmonary ventricle to add kinetic energy to the system. Because many of the important adverse consequences of a Fontan circulation are related to elevated systemic venous pressure, low total resistance to pulmonary blood flow is critical to maintaining a healthy Fontan circulation.
In patients with functionally univentricular heart disease, obstruction or discontinuity of the pulmonary arteries (PAs) may develop for a variety of reasons, both intrinsic and iatrogenic. Such abnormalities may pose a risk to the subsequent development of the distal pulmonary vasculature and likely to the overall success of the Fontan circulation. Adequate PA size was one of the criteria originally specified by Fontan and Choussat [1] for selection of candidates to undergo a Fontan operation. A number of studies, including reports from our center, have found that PA size or abnormalities are important determinants of outcome in this population [2, 3, 4 and 5], although other reports have challenged the significance of PA size per se in affecting outcome after a Fontan procedure [6 and 7].
Despite these conflicting reports about PA size, most clinicians consider pulmonary vascular anatomy and function to be important factors in considering the risk of a Fontan procedure in any given patient. Accordingly, it is important to understand the progression, management, and implications of specific PA anomalies in patients who have or are anticipated to undergo a Fontan procedure.
An obstruction of the central PAs may develop in individuals with functionally univentricular heart disease due to a variety of causes, including PA coarctation at the site of ductal insertion and prior surgical procedures on the central PAs, such as a systemic-to-PA shunt, augmentation arterioplasty, or a classic Glenn procedure. Little is known about the effectiveness of treatment for anatomic abnormalities of the PAs in individuals with a functionally univentricular circulation [8]. In the setting of a cavopulmonary connection, PA flow has minimal pulsatility. The effects of this circulatory feature on pulmonary vascular function and remodeling are unknown. In the setting of significant PA obstruction or discontinuity, the absence of pulsatility may also be a factor in the growth and adaptation of the pulmonary vasculature to reperfusion occurring after a period of hypoperfusion or nonperfusion.
In this study we reviewed patients with a functionally univentricular circulation who underwent procedures to restore PA continuity simultaneous with or subsequent to a bidirectional Glenn or Fontan procedure. Our primary objective was to assess underlying anatomic and therapeutic factors associated with failure to recruit nonconfluent central PAs into a continuous cavopulmonary circulation.
Patients and Methods
The database of the Cardiovascular Program at Children's Hospital Boston was searched for patients with a functionally univentricular circulation who underwent connection of discontinuous central PAs at the time of or after a bidirectional superior cavopulmonary connection (bidirectional Glenn procedure) or total cavopulmonary connection (Fontan procedure). Patients were characterized according to age, underlying anatomic diagnosis, previous surgical procedures on the PAs, cause of PA discontinuity (congenital, secondary to closure of an arterial duct, or iatrogenic), specific cause of iatrogenic PA discontinuity if applicable, hypoplasia of atretic/nondominant PA, stenosis of distal PA branches, presence of pulmonary arteriovenous malformations, and known duration of PA discontinuity.
PA diameters were measured just distal to the origin of the upper lobe branch to avoid the potentially confounding effect of cavopulmonary anastomosis or shunt insertion on the measurement. To assess relative hypoplasia, the ratio of left and right PA diameters was calculated, with the larger of the 2 vessels as the denominator. The PA diameter or area indexed to body surface area was not analyzed because this measure may vary with patient size, and there was a wide range of patient age and size in this series.
Establishment of PA Continuity
PA continuity was established either surgically or by transcatheter recanalization of the occluded PA segment. In patients treated surgically, the reconstruction was performed with a tubular interposition graft or by direct connection of the nonconfluent PA segments when possible, with or without augmentation using a patch. In a subset of patients who underwent PA reconnection at the time of a bidirectional Glenn procedure, a systemic-to-PA shunt was placed before or at the same procedure as PA reconstruction.
Data Analysis
The primary outcome was recurrent PA occlusion, which was assessed as a time-related function, freedom from diagnosis of recurrent PA occlusion, using Kaplan-Meier analysis and Cox regression. Additional outcomes included survival, takedown of the Glenn or Fontan connection, freedom from reintervention on the PAs, and status of the circulation at the time of most recent follow-up. Independent variables assessed for association with these outcomes include the demographic, diagnostic, and procedural variables described in the previous sections. The Children's Hospital Committee for Clinical Investigation approved the study protocol without a requirement for individual patient consent.
Results
Patients
From 1985 to 2006, 49 patients underwent connection of discontinuous PAs at the time of or after a bidirectional Glenn or Fontan procedure. The median age when the PAs were connected was 7.9 years (range, 5 months to 37 years). As summarized in Table 1, all patients had undergone prior surgical intervention on the PAs, and most had PA discontinuity at the site of prior surgical procedures (Fig 1 and Fig 2).
Table 1.
Diagnostic and Historical Data
| Variable | Patients, No. (%) |
|---|---|
| Primary diagnosis | |
| Tricuspid atresia | 13 (27) |
| Single ventricle/double-inlet left ventricle | 9 (18) |
| Heterotaxy-associated univentricular heart disease | 9 (18) |
| Pulmonary atresia, intact ventricular septum | 7 (14) |
| Hypoplastic left-heart syndrome | 6 (12) |
| Other | 5 (10) |
| Prior interventions on the PAs | |
| Any | 49 (100) |
| Systemic-to-PA shunt | 41 (84) |
| Blalock-Taussig or central shunt | 40 (82) |
| Waterston or Potts shunt | 8 (16) |
| Multiple systemic-to-PA shunts | 19 (39) |
| Classic Glenn shunt | 19 (39) |
| Surgical pulmonary arterioplasty | 12 (24) |
| PA banding | 7 (14) |
| Connection of discontinuous PAsa | 3 (6) |
| Proximate cause of discontinuous PAs | |
| Iatrogenicb | 40 (82) |
| Classic Glenn shunt | 19 (39) |
| Related to systemic-to-PA shunt(s)c | 11 (22) |
| Related to BDG and pulmonary arterioplasty | 10 (20) |
| Associated with closure of arterial duct | 7 (14) |
| Congenital nonconfluence | 2 (4) |
BDG = bidirectional Glenn; PA = pulmonary artery.
Before superior cavopulmonary connection.
Related to or at the site of prior surgical procedure.
In 5 of these 11 patients, PA discontinuity was related to a prior Waterston or Potts shunt.
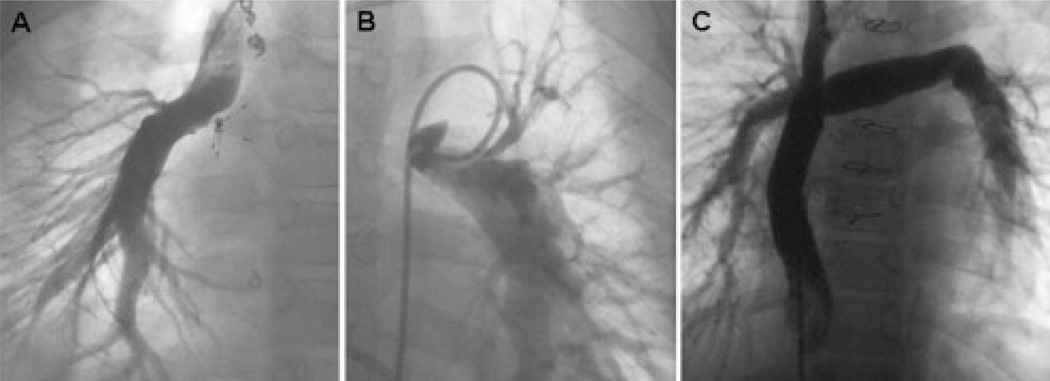
Figure 1.
(A) Discontinuous pulmonary arteries (PAs) after a classic Glenn procedure, with a (B) systemic-to-PA shunt providing flow to the left PA. The umbrella occlusion device at the junction of the superior vena cava and right PA in Panel A was placed to close a residual superior vena cava–right atrial communication. (C) A tube graft was used successfully to reconstruct the central PAs at the time of Fontan completion.
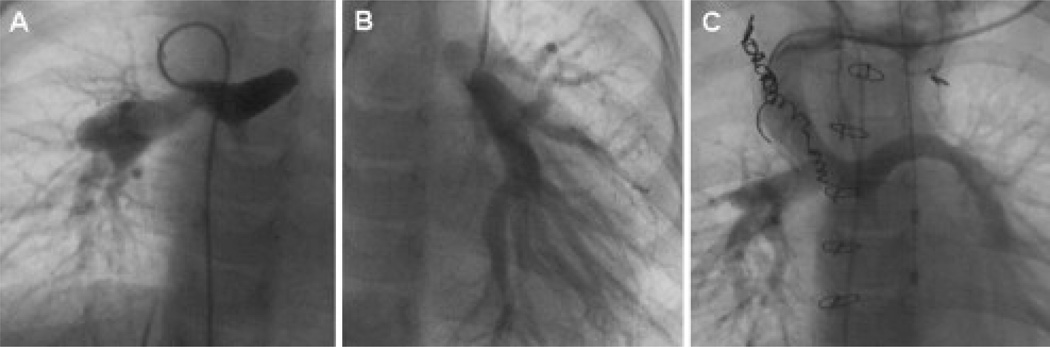
Figure 2.
Discontinuous pulmonary arteries (PAs) due to ductal closure, with consequent left PA atresia. (A) The initial palliation in this patient with a right-sided systemic-to-PA shunt, which is seen supplying the right PA and filling the central PA and main PA remnant. (B) After diagnosis of discontinuous PAs, an additional systemic-to-PA shunt was placed to supply with left lung, (C) before the reconnection of the left and right PAs at the time of bidirectional superior cavopulmonary anastomosis.
Pulmonary blood flow to the 2 lungs arose, by definition, from different sources with many different arrangements, as summarized in Table 2. Angiographic stenosis of lobar or segmental PA branches was present in 7 patients. In 7 patients with a superior cavopulmonary anastomosis, pulmonary arteriovenous malformations were present in the lung supplied by the cavopulmonary anastomosis. The median ratio of diameters of the discontinuous branch PAs was 0.75 (range, 0.2 to 0.99). In 19 patients blood flow to 1 lung was supplied solely by systemic-to-PA collaterals. In 7 of these patients, including 4 with nonconfluent PAs after a bidirectional Glenn procedure and PA augmentation, a shunt was placed after the diagnosis of discontinuous PAs but before PA reconstruction (range, 5 months to 3 years prior) in an effort to restore growth and function to the hypoperfused PA (Fig 3).
Table 2.
Sources of Pulmonary Blood Flow Before Connection of Discontinuous Pulmonary Arteries
| Patients, No. |
PA Occlusion |
Early Death or Fontan/Glenn Takedown |
|
|---|---|---|---|
| Unilateral superior cavopulmonary anastomosis | 30 | 5 | 5 |
| Contralateral lung | |||
| Systemic–to-PA shunt (1 or more) | 12a | 1 | 0 |
| Antegrade from ventricle | 9 | 1 | 3 |
| Systemic–to-PA collaterals | 7 | 3 | 1 |
| Contralateral superior cavopulmonary anastomosis | 1 | 0 | 1 |
| Classic atriopulmonary Fontan connection | 1 | 0 | 0 |
| No superior cavopulmonary anastomosis | 14 | 2 | 2 |
| Separate systemic–to-PA shunts to each lung | 9b | 1 | 1 |
| Unilateral systemic–to-PA shunt, systemic–to-PA collaterals | 5 | 1 | 1 |
PA = pulmonary artery.
At the time PA discontinuity was diagnosed, 7 (3a and 4b, respectively) of these patients had sole supply to 1 lung by systemic-to-PA collaterals, but a systemic-to-PA shunt was placed to the nonconfluent PA before establishment of PA continuity.
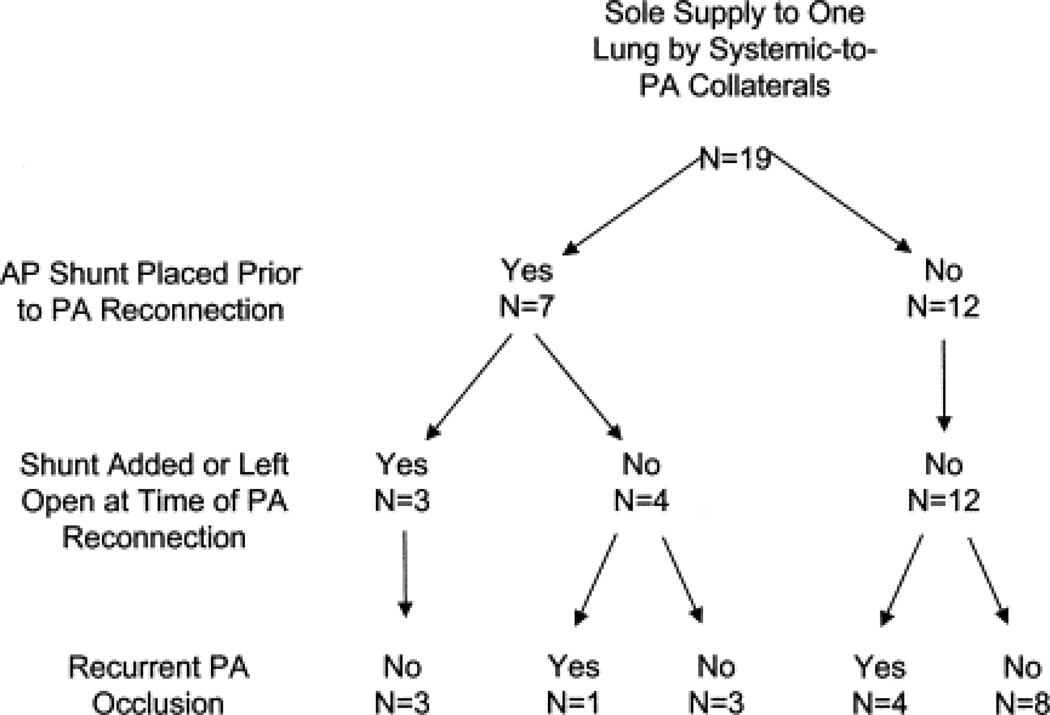
Figure 3.
Flow diagram demonstrates outcome of pulmonary artery (PA) reconstruction among patients with sole supply to 1 lung by systemic-to-PA collaterals at the time of diagnosis of PA discontinuity.
Establishment of PA Continuity
The median duration from the diagnosis of discontinuous PAs to establishment of continuity was 1.9 years (range, 1 day to 29 years), and among patients in whom PA discontinuity was not intentional (as part of a classic Glenn procedure) was 26 days (range, 1 day to 25 years). PA continuity was established surgically in 46 patients and by transcatheter recanalization and stenting in 3 (Table 3). In patients managed with an interposition graft, graft diameters were 6 to 19 mm and were typically several millimeters larger than the distal PA to which they were sewn.
Table 3.
Timing and Method of Procedures to Establish Pulmonary Artery Continuity
| Variable | Patients, No. |
PA Occlusion |
Early Death or Fontan/Glenn Takedown |
|---|---|---|---|
| Timing of connection | |||
| During BDG | 21 | 2 | 1 |
| After BDG but before Fontan completion | 6 | 0 | 0 |
| During Fontan procedure | 17 | 3 | 6 |
| After Fontan procedure | 1 | 1 | 0 |
| After a failed Fontan was taken down | 2 | 1 | 0 |
| During conversion from atriopulmonary Fontan to lateral tunnel | 2 | 0 | 0 |
| Method of connection | |||
| Surgical | 46 | 3 | |
| Direct connection | 27 | 0 | |
| With patch augmentation of the connection | 10 | 0 | |
| Glutaraldehyde-fixed autologous pericardium | 4 | 0 | |
| Expanded PTFE | 3 | 0 | |
| Autologous PA tissue | 2 | 0 | |
| Bovine pericardium | 1 | 0 | |
| Tubular interposition graft | 19 | 4 | |
| Ring-supported tube of expanded PTFE | 18 | 4 | |
| Autologous superior vena cava graft | 1 | 0 | |
| Transcatheter recanalization and stenting | 3 | 0 |
BDG = bidirectional Glenn; PA = pulmonary artery; PTFE = polytetrafluoroethylene.
The circulation at the conclusion of the procedure to establish PA continuity was a bidirectional Glenn in 29 patients and a Fontan in 20; the specific timing of the procedures is summarized in Table 3. In 10 of the 29 patients with a Glenn connection at the time of PA reconstruction, a systemic-to-PA shunt was placed (n = 7) or an existing shunt was left patent (n = 3) to maintain pulsatile flow to the previously atretic PA.
Survival and Acute Surgical Failure
Four of the 20 patients who underwent connection of discontinuous PAs at the time of or after a Fontan operation died, 3 after takedown of the Fontan connection, and 2 others had the Fontan taken down to an intermediate palliative circulation within the first 60 days postoperatively. In 4 of these 6 patients, occlusion of (n = 3) or high vascular resistance in (n = 1) the reconnected PA was clearly a contributing factor in the outcome. No early deaths occurred after PA connection in the setting of a bidirectional Glenn, but in 1 patient the bidirectional Glenn was taken down to a central shunt the same day. The 45 early survivors were followed up for a median of 7 years (range, 0.7 to 21 years). Two patients died during follow-up: one 15 years after a Fontan procedure at which discontinuous PAs were connected and one after heart transplantation 6 years after connection of discontinuous PAs. Survival was 92% ± 4% at 1 year and 89% ± 5% at 5 years. Two patients underwent heart transplantation for ventricular failure 1.5 and 3.6 years after connection of discontinuous PAs.
Recurrent PA Occlusion
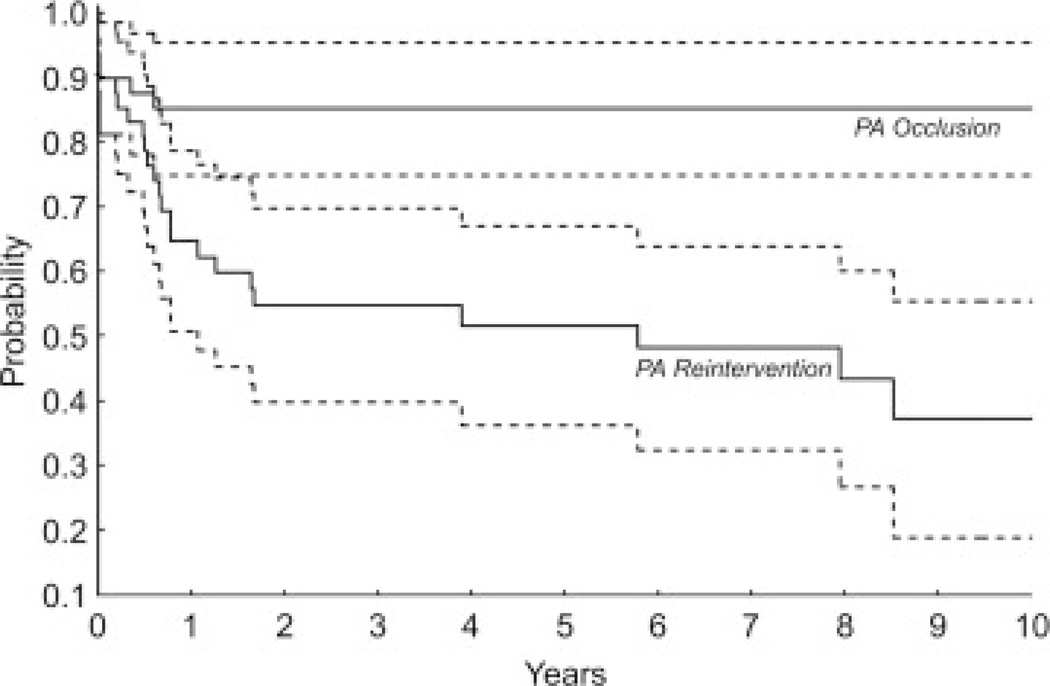
Recurrent PA occlusion was documented in 7 patients, 5 within the first 10 days after the procedure to establish PA connection and all within 8 months. Among early survivors, freedom from diagnosis of PA occlusion was 87% ± 5% at 6 months and 85% ± 5% at 1 year (Fig 4). In 4 of the 7 patients who presented with PA occlusion, blood flow to 1 lung was supplied solely by systemic-to-PA collaterals before PA connection (Fig 4). By multivariable Cox regression analysis, the only factor associated with shorter freedom from PA occlusion was sole supply of blood flow to 1 lung before connection by systemic-to-PA collaterals (66% ± 14% vs 95% ± 4% freedom from occlusion at 6 months, p = 0.03).
Figure 4.
Kaplan-Meier curves depict freedom from PA occlusion (top, solid line) and freedom from either PA occlusion or reintervention (bottom, solid line). The dotted lines show the 95% confidence intervals.
In 6 of the 7 patients diagnosed with PA occlusion, attempts were made to restore flow to the occluded PA again by revision, thrombectomy, or stenting of the occluded PA segment (n = 4), or placement of a systemic-to-PA shunt to the PA distal to the occlusion (n = 3), or both. Reocclusion was documented in 3 of the 4 patients in whom PA revision was performed, at which time there was no source of pulmonary blood flow to the affected lung other than systemic-to-PA collaterals; the PA remained patent in the patient whose reconnection was achieved by transcatheter recanalization and stenting.
Reintervention on the Reconnected PAs
Twenty-three patients underwent a total of 50 reinterventions on the PAs related to the site of connection, none of whom were among the early deaths. Among the 45 early survivors, freedom from PA reintervention or occlusion after connection of discontinuous PAs was 83% ± 6% at 1 year and 55% ± 9% at 3 years (Fig 4). By multivariable Cox regression analysis, factors associated with a shorter freedom from PA occlusion or reintervention after PA connection included sole supply of 1 lung with systemic-to-PA collaterals (β = 1.5, p = 0.009), lobar/segmental branch PA stenosis (β = 2.4, p < 0.001), and younger age at PA connection (β = 0.29, p = 0.11). The first PA reintervention and subsequent reinterventions are listed in Table 4; only one of these reinterventions was performed at the time of a subsequent Fontan operation. Freedom from placement of a PA stent was 86% ± 5% at 1 year, 75% ± 6% at 3 years, and 69% ± 7% at 5 years.
Table 4.
Pulmonary Artery Reinterventions After Connection of Nonconfluent Pulmonary Arteries
| Type of Reintervention | Reintervention, No. | |
|---|---|---|
| First | Subsequent | |
| Total patients | 23 | 14 |
| Total procedures | 25 | 25 |
| Procedure | ||
| Transcatheter reinterventions | ||
| PA occlusion recanalization and stenting | 1 | 1 |
| PA stent placementa | 7 | 5 |
| PA angioplasty or stent redilation | 9 | 13 |
| Stenting/redilation of systemic–to-PA shunt | 1 | 3 |
| Surgical reinterventions | ||
| Fontan takedown and PA thrombectomyb | 2 | … |
| Systemic–to-PA shunt placement | 4 | 1 |
| Conversion of BDG to classic Glenn | 1 | … |
| PA interposition graft replaced | … | 1 |
| Augmentation pulmonary arterioplasty | … | 1 |
BDG = bidirectional Glenn; PA = pulmonary artery.
Does not include patients who underwent recanalization and stenting as the primary reconnection procedure.
In 3 patients who underwent takedown of the Fontan (n = 2) or BDG (n = 1) without a clear problem related to the PA connection, the takedown procedure was not counted as a PA reintervention.
Follow-Up Circulatory Status
At a median follow-up of 7 years (range, 0.5 to 21 years) in the 43 surviving patients, 29 had a Fontan circulation with continuous PAs and flow to both lungs, 12 had some form of intermediate palliative circulation, 1 had received a transplant, and 1 was found to have recurrent left PA occlusion after establishment of continuity and had a functionally single-lung Fontan, with no flow from the Fontan connection to the left PA. Pulmonary blood flow in the 12 patients with an intermediate palliative circulation was supplied through a variety of sources. Seven patients had a bidirectional superior cavopulmonary connection; 3 of these 7 had no other source of pulmonary blood flow, and 4 had a systemic-to-PA shunt. In 3 of the 4 patients with a bidirectional Glenn and a systemic-to-PA shunt, there was central or peripheral branch PA stenosis, or both, resulting in effectively unilateral flow of the Glenn. The other 5 patients had a classic Glenn connection, with flow to the contralateral lung only through systemic-to-PA collaterals in 3, antegrade from the ventricle in 1, and through a systemic-to-PA shunt in 1 patient, who also had multiple peripheral branch PA stenoses.
Overall, 32 patients were alive with continuous branch PAs that were successfully included in a Fontan (n = 29) or a bidirectional Glenn circulation (n = 3) in which there was no significant PA hypoplasia or obstruction. The only factor associated with failure to survive with a continuous central pulmonary circulation was the occurrence of postconnection PA occlusion (odds ratio, 0.68; 95% confidence interval, 0.49 to 0.96; p = 0.007).
Comment
In most patients who underwent reconnection of discontinuous central PAs at the time of or after a superior or total cavopulmonary connection, the PA reconstruction remained patent. In a subset of patients, however, recurrent PA occlusion occurred, acutely in most cases. Patients with systemic-to-PA collaterals as the sole source of pre-reconstruction blood flow to 1 lung were at increased risk of occlusion. Repeated attempts to connect the occluded PA were performed in 4 of these 7 patients, but succeeded in only 1. No technical or procedural factors were associated with an increased risk of PA occlusion. Even when the PA connection remained patent, reintervention was common, with 55% ± 9% freedom from PA reintervention or occlusion 3 years after connection of discontinuous PAs.
Although there is debate about the importance of PA size in predicting outcome after a Fontan procedure [2,3, 4, 5, 6 and 7], our findings and those of others reinforce the notion that the integrity of the pulmonary circulation is critically important in optimizing the chance of a successful univentricular palliation. In this series, 30% of patients undergoing connection of discontinuous PAs at the time of or after a Fontan connection experienced acute Fontan failure (death or Fontan takedown). At the most recent follow-up, only 32 of 49 patients were alive with a successful Fontan circulation or an intact bidirectional Glenn in which there were no major abnormalities in the pulmonary circulation.
In a recent study, we found that 8 of 49 patients who underwent acute or subacute takedown of a failing Fontan circulation to an intermediate palliative circulation had discontinuous PAs at the time of the Fontan procedure [9]. In 7 of these 8 patients, PA continuity was restored at the time of the Fontan operation (2 of these 7 patients are not included in the present series because the Fontan and PA reconstruction procedures were performed elsewhere; only the Fontan takedown was done at our institution). Of these 7 patients, 4 were short-term survivors after Fontan takedown, but the PAs in all 4 had become discontinuous again at the time of takedown.
This finding is consistent with previous series in which reconstruction of discontinuous PAs at the time of Fontan operation often failed to result in a patent connection [6 and 8]. Although patients may survive with a Fontan connection to a single lung, the longer-term health of an acutely successful single-lung Fontan circulation is unknown [6, 8, 10 and 11].
The optimal management of patients with palliated functionally univentricular heart disease and substantially unbalanced pulmonary blood flow is not clear. This series included patients undergoing either bidirectional Glenn or Fontan procedures, with discontinuous PAs due to a variety of causes. The heterogeneity introduced by these factors complicates our assessment of optimal management for each subgroup.
Sakamoto and colleagues [8] recently reported a series of 20 patients with functionally single-ventricle heart disease and unilateral PA hypoplasia or pulmonary venous obstruction in whom “intrapulmonary-artery septation” was performed at the time of superior cavopulmonary connection. They isolated the affected (ie, hypoplastic or obstructed) lung from the cavopulmonary connection with an intra-PA septation patch, and a systemic-to-PA shunt was placed to supply the affected lung, usually in conjunction with PA or pulmonary venous augmentation [8].
This strategy provides higher pressure, pulsatile flow to the underdeveloped PA, presumably optimizing the opportunity for pulsatile flow–mediated growth while protecting the normal PA from the potentially adverse effects of such flow. At the same time, it provides a stable, low-velocity/pressure source of flow to the good lung and decreases the volume load on the functionally single ventricle. Many of the patients in the series of Sakamoto and colleagues [8] had pulmonary venous obstruction rather than PA disease, and few had discontinuous PAs, but their strategy appears to be a logical balanced approach that is conceptually similar to our practice of revascularizing the hypoperfused discontinuous PA with a shunt before or simultaneous with a bidirectional Glenn procedure.
Hemodynamic factors such as volume and pulsatility of blood flow are thought to play an important role in stimulating PA growth. Studies in animal models of unilateral PA ligation have demonstrated altered expression of important modulators of pulmonary vascular resistance, including components of the nitric oxide and endothelin networks [12, 13 and 14]. In patients with a passive pulmonary circulation (ie, a superior or total cavopulmonary connection), regional differences in pulmonary vascular resistance, as may result from pulmonary hypoperfusion or injury, may facilitate persistent low flow to the affected region of lung, and in combination with abnormalities in the coagulation system [15 and 16] and the presence of freshly operated on tissue or synthetic patch/graft material, may predispose to thrombosis or occlusion of a reconnected PA, particularly in the early postoperative period.
Although the biology and physiology of the pulmonary circulation in the setting of a cavopulmonary connection are not well characterized, a few studies have shown abnormalities of the endothelin and nitric oxide systems in these patients [17 and 18], but the relationship between these changes and pulmonary vascular remodeling after PA reconnection or other PA intervention is unknown.
Reconnection of discontinuous PAs is performed in patients with a variety of conditions, most often complex tetralogy of Fallot [19, 20 and 21]. In most cases the reconnected PAs remain patent. The population of patients studied in this report differs from other groups of patients undergoing PA reconnection insofar as flow in the cavopulmonary pathway is nonpulsatile and low pressure, which may have implications for PA remodeling in response to hypoperfusion/nonperfusion and reperfusion, as discussed previously. This consideration is difficult to assess in a human population, but forms a theoretic basis for providing an additional or obligate source of pulmonary blood flow to the hypoperfused lung before restoration of PA continuity in patients with nonconfluent PAs and a functional univentricular circulation. Staged reconstruction in patients with no controlled source of or very small volumes of flow to the affected lung may increase the likelihood of successful recruitment. The time course of pulmonary vascular remodeling and growth in these circumstances is not known, and accordingly, patients managed with this approach should be monitored closely to maximize the benefits of shunting without incurring the hemodynamic consequences related to high combined pulmonary blood flow or pulmonary vascular disease in the shunted lung.
This study has several limitations. The retrospective design and relatively extended period during which patients underwent PA reconnection, along with the small size and heterogeneity of the cohort, complicate the assessment of our data. Also, this series includes only patients with PA discontinuity, and our findings may not be applicable to patients with confluent but severely stenotic PAs, which is probably a more common situation.
In conclusion, discontinuous PAs can be successfully connected in most patients with a cavopulmonary circulation, although patients with nonconfluent PAs appear to be at a relatively high risk for a poor outcome after a Fontan operation. When recurrent PA occlusion occurred, it was almost always diagnosed in the early postoperative period. Particularly in patients with no central source of flow to the affected lung (ie, systemic-to-PA collaterals as the sole source of flow), placement of a shunt before establishing PA continuity may provide a hemodynamic stimulus for PA growth, facilitate transcatheter rehabilitation of distal PA stenoses, and optimize the ultimate success of PA reconnection. A low threshold for investigation of occlusion or stenosis of the reconnected PA is warranted and may increase the chances of preserving a robust pulmonary circulation, which is critically important in this population of patients.
Acknowledgements
This work was supported by the National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Choussat, Fontan F, Besse P, Vallot F, Chauve A, Bricaud H. Selection criteria for Fontan's procedure. In: Anderson RH, Shinebourne EA, editors. Paediatric cardiology 1977. Edinburgh: Churchill Livingstone; 1978. pp. 559–566. [Google Scholar]
- 2.Gentles TL, Mayer JE, Jr, Gauvreau K, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 3.Fontan F, Fernandez G, Costa F, et al. The size of the pulmonary arteries and the results of the Fontan operation. J Thorac Cardiovasc Surg. 1989;98:711–719. [PubMed] [Google Scholar]
- 4.Knott-Craig CJ, Julsrud PR, Schaff HV, Puga FJ, Danielson GK. Pulmonary artery size and clinical outcome after the modified Fontan operation. Ann Thorac Surg. 1993;55:646–651. doi: 10.1016/0003-4975(93)90268-m. [DOI] [PubMed] [Google Scholar]
- 5.Senzaki H, Isoda T, Ishizawa A, Hishi T. Reconsideration of criteria for the Fontan operation: Influence of pulmonary artery size on postoperative hemodynamics of the Fontan operation. Circulation. 1994;89:266–271. doi: 10.1161/01.cir.89.1.266. [DOI] [PubMed] [Google Scholar]
- 6.Zachary CH, Jacobs ML, Apostolopoulou S, Fogel MA. One-lung Fontan operation: hemodynamics and surgical outcome. Ann Thorac Surg. 1998;65:171–175. doi: 10.1016/s0003-4975(97)01015-1. [DOI] [PubMed] [Google Scholar]
- 7.Bridges ND, Farrell PE, Jr, Pigott JD, 3rd, Norwood WI, Chin AJ. Pulmonary artery index: A nonpredictor of operative survival in patients undergoing modified Fontan repair. Circulation. 1989;80:I216–I221. [PubMed] [Google Scholar]
- 8.Sakamoto K, Ikai A, Fujimoto Y, Ota N. Novel surgical approach ‘intrapulmonary-artery septation' for Fontan candidates with unilateral PA hypoplasia or pulmonary venous obstruction. Interact Cardiovasc Thorac Surg. 2007;6:150–154. doi: 10.1510/icvts.2005.124925. [DOI] [PubMed] [Google Scholar]
- 9.Almond CS, Mayer JE, Thiagarajan RR, Blume ED, del Nido PJ, McElhinney DB. Outcome after Fontan failure and takedown to an intermediate palliative circulation. Ann Thorac Surg. 2007;84:880–887. doi: 10.1016/j.athoracsur.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 10.Tchervenkov CI, Chedrawy EG, Korkola SJ. Fontan operation for patients with severe distal pulmonary artery stenosis, atresia, or a single lung. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:68–75. doi: 10.1053/pcsu.2002.31505. [DOI] [PubMed] [Google Scholar]
- 11.Schmauss D, Kaczmarek I, Sachweh J, et al. Successful single lung Fontan operation in 2 children: case reports. Heart Surg Forum. 2007;10:E331–E333. doi: 10.1532/HSF98.20071032. [DOI] [PubMed] [Google Scholar]
- 12.Shi W, Cernacek P, Hu F, Michel RP. Endothelin reactivity and receptor profile of pulmonary vessels in postobstructive pulmonary vasculopathy. Am J Physiol. 1997;273:H2558–H2564. doi: 10.1152/ajpheart.1997.273.6.H2558. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Yung GL, Marsh JJ, et al. Pulmonary vascular remodeling distal to pulmonary artery ligation is accompanied by upregulation of endothelin receptors and nitric oxide synthase. Exp Lung Res. 2000;26:287–301. doi: 10.1080/019021400404555. [DOI] [PubMed] [Google Scholar]
- 14.Fadel E, Michel RP, Eddahibi S, et al. Regression of postobstructive vasculopathy after revascularization of chronically obstructed pulmonary artery. J Thorac Cardiovasc Surg. 2004;127:1009–1017. doi: 10.1016/j.jtcvs.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 15.Odegard KC, McGowan FX, Jr, Zurakowski D, et al. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg. 2003;125:1260–1267. doi: 10.1016/s0022-5223(02)73605-2. [DOI] [PubMed] [Google Scholar]
- 16.Odegard KC, McGowan FX, Jr, DiNardo JA, et al. Coagulation abnormalities in patients with single-ventricle physiology precede the Fontan procedure. J Thorac Cardiovasc Surg. 2002;123:459–465. doi: 10.1067/mtc.2002.120010. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu T, Imai Y, Kurosawa H, et al. Effects of dilutional and modified ultrafiltration in plasma endothelin-1 and pulmonary vascular resistance after the Fontan procedure. Ann Thorac Surg. 2002;73:861–865. [PubMed] [Google Scholar]
- 18.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–3208. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 19.Murphy DN, Winlaw DS, Cooper SG, Nunn GR. Successful early surgical recruitment of the congenitally disconnected pulmonary artery. Ann Thorac Surg. 2004;77:29–35. doi: 10.1016/s0003-4975(03)01504-2. [DOI] [PubMed] [Google Scholar]
- 20.Stamm, Friehs I, Zurakowski D, et al. Outcome after reconstruction of discontinuous pulmonary arteries. J Thorac Cardiovasc Surg. 2002;123:246–257. doi: 10.1067/mtc.2002.119700. [DOI] [PubMed] [Google Scholar]
- 21.Agnoletti G, Boudjemline Y, Bonnet D, Sidi D, Vouhé P. Surgical reconstruction of occluded pulmonary arteries in patients with congenital heart disease: effects on pulmonary artery growth. Circulation. 2004;109:2314–2318. doi: 10.1161/01.CIR.0000129273.50975.F4. [DOI] [PubMed] [Google Scholar]