Abstract
Purpose:
To establish the protective efficacy against late complications of electron therapy using customized lead eye shields in cases with orbital and periorbital lesions.
Methods
Between 1982 and 2006, 16 patients with 22 orbital and periorbital lesions were treated by electron therapy. Customized lead eye shields were prepared and placed in the respective patients’ eyes during each fraction of electron therapy. The toxicity and local control rates were analyzed.
Results
The preparation period for the customized lead eye shields was 2 days. The shields could be used throughout the treatment period in all the patients. No evidence of radiation cataract was observed in 15 of the 16 patients. None of the patients developed corneal ulceration or evidence of lead poisoning.
Conclusion
Customized lead eye shields could be made relatively quickly, and electron therapy for orbital and periorbital lesions could be undertaken safely without any late complication.
Key Words: Lead eye shields, Electron therapy, Lymphoma, Neoplasm
Introduction
Electron therapy is often undertaken for the treatment of ocular and periocular lesions, such as lymphoma or carcinoma, either alone or in combination with surgery or chemotherapy [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15].
When electron therapy is undertaken for orbital and periorbital lesions, it has been suggested that the use of an eye shield, block or disk is beneficial for preventing late complications, such as radiation cataract, keratopathy, retinopathy and optic neuropathy [16], unless they would impair the local therapeutic effect [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Eye shields, blocks and disks are often made of lead [1,2,3,4,5,6,7,8,9,10,11,12,13,14], and sometimes tungsten [17]. These eye shields must fulfill 4 requirements to be useful medical devices: (1) they should be harmless to normal orbital tissue; (2) they should shield the cornea and lens sufficiently from radiation toxicity; (3) they should be suitable for use throughout the treatment period to achieve effective protection, and (4) they should not attenuate the local therapeutic effect by also partially shielding the planning target volume [6, 7]. To meet all the requirements of eye shields stated above, we undertook preparation of contact lens-shaped customized lead eye shields at our institution. The aim of this study was to analyze the efficacy of customized lead eye shields for the patients and their effectiveness in avoiding late complication.
Methods
Patient and Tumor Characteristics
A total of 16 patients seen between 1982 and 2006 with 22 orbital and periorbital lesions, including 9 malignant lymphoma patients, 6 carcinoma (up to 2 cm) patients and 1 metastatic patient, were included in the study (table 1). There were 8 male and 8 female patients, with a median age of 64 years (range 26–84 years). Fourteen lesions were unilateral, and 8 lesions were bilateral. Four patients had bilateral lesions and 2 patients had separate unilateral lesions; these 6 patients were treated in 2 separate sessions for the 2 lesions. The remaining 10 patients were treated in a single session. The characteristics of the tumors and the histological subtypes and stages of the malignant lymphoma are shown in table 2. The stage of the malignant lymphoma was determined based on the results of physical examination, laboratory blood examination, imaging and bone marrow biopsy. The tumor locations in the patients are shown in table 3; they were on the eyelid in 12 patients, conjunctiva in 3 patients and cutaneous around the orbit in 1 case.
Table 1.
Patient characteristics
| Gender, n (%) | |
| Male | 8 (50) |
| Female | 8 (50) |
| Age at diagnosis, years | |
| Median | 64 |
| Range | 26–84 |
| Laterality | |
| Unilateral lesions, n (%) | 14 (64) |
| Bilateral lesions, n (%) | 8 (36) |
| Median follow-up, months | 25 |
Table 2.
Tumor characteristics
| Factor | n (%) |
|---|---|
| Malignant lymphoma | 9 (56) |
| Mucosa-associated lymphoid tissue (IAE) | 3 |
| Mycosis fungoides (IVA) | 2 |
| B cell small lymphocytic lymphoma | 2 |
| IAE | 1 |
| IIIAE | 1 |
| Diffuse medium cell lymphoma (IAE) | 1 |
| Follicular lymphoma (grade 2; IVB) | 1 |
| Meibomean gland carcinoma | 2 (13) |
| Squamous cell carcinoma | 2 (13) |
| Basal cell carcinoma | 1 (6) |
| Poorly differentiated carcinoma | 1 (6) |
| Cutaneous metastasis of parotid gland carcinoma | 1 (6) |
Table 3.
Tumor sites
| Site | n (%) |
|---|---|
| Eyelid | 12 (75) |
| Conjunctiva | 3 (19) |
| Cutaneous around orbit | 1 (6) |
Preparation of Customized Lead Eye Shields
Customized lead eye shields were prepared by maxillofacial prosthodontists prior to the electron therapy [18]. Under local ophthalmic anesthesia, the prosthodontists applied impression material (Ophthalmic Moldite Powder, Danker Laboratories, Sarasota, Fla., USA) onto the surface of the patient's globe (fig. 1a). Then we prepared a working plaster cast (New plastone, GC Corp., Tokyo, Japan) of the impression. The base of the eye shield for space of lead was apolymerized with 1-mm thick resin (Acron, GC Corp.) and subsequently 1-mm thick lead was coated with 1-mm thick resin. A handle made of the resin was attached at the marking of the lower eyelid margin. Finally, the surface of the lead eye shield was polished well (fig. 1b, c). Experienced maxillofacial prosthodontists took 2 days to prepare a customized lead eye shield.
Fig. 1.
The preparation of the lead eye shield. a Impression of the eye surface. b Lead eye shield (frontal view). c Lead eye shield (side view).
Effect of the Lead Eye Shield
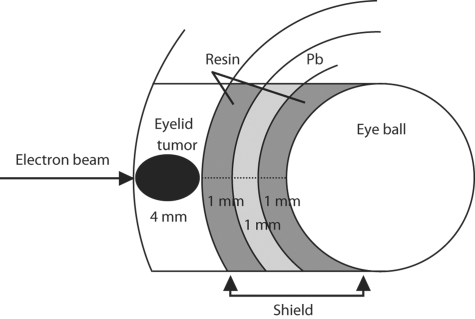
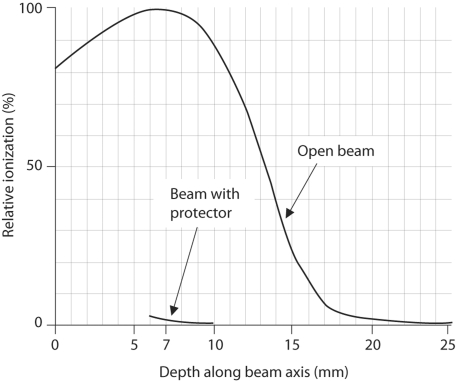
Figure 2 is a schematic drawing of the eye shield. The distance between the surface of the tumor and the globe would be 7 mm in a hypothetical case with an eyelid tumor 4 mm thick, with the application of the lead eye shield. When en face 4MeV electron therapy was administered, the relative ionization of the surface of the globe surface under the lead eye shield on the beam central axis was experimentally confirmed as 3.3%(fig. 3) [19]. At our institution, the applied dose of electron irradiation is specified for a relative dose of 80%. Therefore, the exposed dose of the surface of the globe under the lead eye shield was estimated to be 4% of the total applied dose.
Fig. 2.
Schema of shielding eye.
Fig. 3.
Central axis dose curve of 4MeV electron. Relative ionization of the surface of the globe was 3.3% when the depth along the beam axis was 7 mm.
Due to backscatter from the lead eye shield, a dose of 1.0 Gy at dmax is increased to 1.2 Gy under experimental measurement. We took this phenomenon into consideration when determining the applied dose, and the toxicities at the under surface of the eyelid were within permissible levels.
Statistic Analysis and Follow-Up
The cumulative local control rates of the tumors were estimated using the Kaplan-Meier method. The median follow-up period was 25 months (range 1–290 months), calculated from the initial date of the electron therapy. Both the primary disease and ophthalmologic follow-up were performed.
Results
Treatment
The customized lead eye shields were placed in the patients’ eyes during each fraction of electron therapy. The lead eye shields were thoroughly irrigated with normal saline before daily insertion into patients’ eyes. Protective administration of antibiotic ophthalmic solution was continued during the treatment period. The shields could be used throughout the treatment period in all the patients, and showed no deterioration. All the cases were treated by 4MeV electron therapy using anterior fields (median port size 16 cm2). The median fraction dose was 2.5 Gy (range 2–4 Gy). Gross tumor volume was determined by macroscopic, pathologic findings or clinical imaging. Adequate margins (2–3 cm) for clinical target volume and planning target volume were added to gross tumor volume. For lymphomas, the median total dose was 30 Gy (range 24 Gy/8 fr to 40 Gy/16–20 fr); 4 patients were treated by chemoradiation, 4 were treated by radiation alone and 1 was treated by postoperative irradiation. For carcinomas, the median dose was 35 Gy (range 27.5 Gy/11 fr to 50.4 Gy/18 fr). Among the 6 patients with primary carcinoma, 1 patient was treated by electron therapy alone (50.4 Gy/18 fr) and the remaining 5 had undergone surgery prior to the electron therapy (range 36 Gy/9 fr to 50 Gy/20 fr). Of these 5, 4 were suspected to have residual tumor and 1 patient had local recurrence. The patient with cutaneous metastasis was treated by electron therapy alone (range 27.5 Gy/11 fr to 30 Gy/12 fr). Figure 4 shows the therapeutic process and the therapeutic outcome in a patient with malignant lymphoma who was treated by 4MeV electron therapy (35 Gy/14 fr/25 days) with a customized lead eye shield.
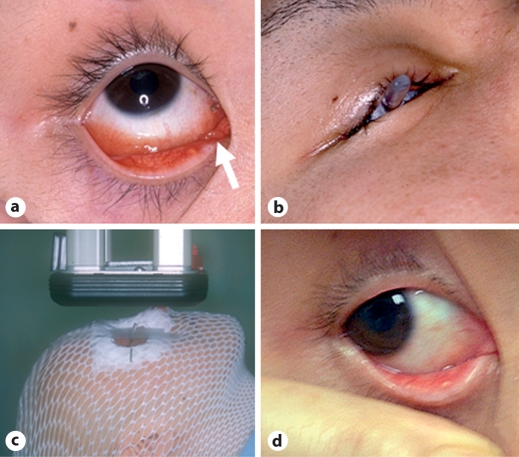
Fig. 4.
The 4MeV electron therapy process (35 Gy/14 fr/25 days) in a 26-year-old male with MALT lymphoma. a Lymphoma of the right inner canthus (white arrow). b Application of the lead eye shield during the electron therapy. c Electron therapy in progress. d The tumor vanished by 2.5 years after the electron therapy.
Local Control
The overall cumulative local control rate in the 22 tumors was 78.8% at 5 years. None of the cases of malignant lymphoma developed local recurrence during the follow-up period (median 29 months, range 4–106 months). One male patient with poorly differentiated adenocarcinoma and 1 male patient with carcinoma of the meibomian glands developed local recurrence.
Toxicity of Radiation Therapy
Normal tissue complications were assessed based on National Cancer Institute's common toxicity criteria (NCI-CTC) and Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) criteria. Acute toxicities were evaluated by NCI-CTC criteria, and late toxicities by RTOG/EORTC criteria. The acute toxicities were as follows: conjunctival adverse reactions in 5 patients (dacryorrhea in 4, eye discharge in 4, congestion in 1 and hemorrhage in 1), dermatitis in 5, local skin pigmentation in 3, subcutaneous edema in 2 and ophthalmalgia in 1 patient. All the acute toxicities were NCI-CTC grade 1. Late toxicities, including blepharoptosis (grade 2), pruritus of the eyelid (grade 1) and ophthalmalgia (grade 1) appeared in 1 patient. One 78-year-old female patient who was treated for bilateral mycosis fungoides of the eyelids at different times developed progressive simultaneous bilateral cataract (Emery-Little classification grade 3) after 27 and 12 months, respectively, as calculated from the date of initiation of the electron therapy. None of the patients showed any evidence of radiation keratopathy, retinopathy, optic neuropathy, or lead poisoning.
One patient was diagnosed as having mild superficial punctate keratopathy 1 month after the electron therapy, which could be attributed to irritation by the lead eye shield. The patient received instillation therapy of sodium chondroitin sulfate and a nonsteroidal anti-inflammatory agent, and was cured by a month and a half after the treatment.
Discussion
Radiation therapy for orbital and periorbital lesions without the use of an eye shield can cause various late adverse effects, including radiation cataract, keratopathy, retinopathy and optic neuropathy, all of which are associated with reduced visual acuity [16]. The most frequently encountered adverse effect in cases of orbital and periorbital lesions treated by electron therapy is radiation cataract. In this study, vision could be successfully maintained in most of the patients with the use of customized lead eye shields during the electron therapy. One patient developed bilateral cataract 27 and 12 months after the electron therapies, respectively. Although radiation cataract cannot be entirely ruled out, we regarded it highly probable that the cataract in this patient represented senile cataract, at least on 1 side, for the following reasons: although the treatments for the disease on each side were administered at different points in time, the cataract progressed simultaneously on both sides, and typically, radiation cataract occurs between 2 and 3 years after radiotherapy [20, 21].
In cases with lesions of the bulbar conjunctiva, excessive shielding of the eye sometimes compromises the local therapeutic effect [6, 7]. The use of customized lead eye shields allowed avoidance of shielding lesions in the bulbar conjunctiva in our 3 patients, because local recurrence did not develop in any of these cases with lesions of the bulbar conjunctiva. In this study, all of the local recurrences occurred in eyelid carcinomas and their development was not related to compromise of local control by excessive shielding. We are certain of the benefits of using an eye shield, unless the lesion extends to the corneoscleral limbus, especially for young patients.
For the preparation of the lead eye shields, Asbell et al. [1] used lead sheets with a thickness of 1/16 inch (0.159 cm), Jereb et al. [2] used blocks 1.4-cm thick, and Suh et al. [7] and Fitzpatrick et al. [8] used sheets 2-mm thick. We used 1 mm of lead with a covering of 1 mm of resin on both sides of the shielding. The dose to the surface of the globe under an eye shield was estimated to be 4% of the total applied dose. As this result shows, use of a 1-mm thick lead sheet may be sufficient for the preparation of a lead shield to prevent late toxicity. In consideration of continuous wear under a swelling eyelid during the treatment period, a thinner contact lens is preferable.
Regarding the material used in the shields, tungsten has been proven to be superior to lead for reducing backscatter, due to its higher density and lower atomic number [17]. Although we believe that backscatter from the lead eye shields does not cause significant complications from the observation results, trying to prepare customized tungsten eye shields might be a subject of future investigation.
Marriam et al. [20] warned that secondary radiation might cause punctate keratitis if the surface of the shield was rough. A smooth surface of the eye shield is also essential for guarding the cornea. We experienced 1 case of mild superficial punctate keratopathy which could be attributed to irritation by the shield. At our institution, resin is used as the covering material, because it is of stable quality and a smooth surface can be easily obtained. We regard our covering material as safe, because the heat-polymerizing acrylic resin used as covering for the lead eye shield was the same material as that which is used for soft contact lenses and artificial dentures.
Although Weaver et al. [17] expressed concern about deficit or deterioration of the coating, which could cause lead absorption, this problem has not been encountered during any treatment at our institution.
In conclusion, with the use of customized lead eye shields, electron therapy for orbital and periorbital lesions can be undertaken safely, with excellent local therapeutic results and protection against late complications.
Acknowledgements
The authors thank Dr. Masao Hoshina (Professor, School of Radiological Technology, Gunma Prefectural College of Health Sciences, Maebashi, Japan), Mr. Yukishige Kyuma (Radiological Center, Tokyo Medical and Dental University Hospital, Tokyo, Japan) and Dr. Masahiko Nakamura (MD, Department of Ophthalmology, Tohoku University, Sendai, Japan) for their helpful advice.
References
- 1.Asbell SO, Siu J, Lightfoot DA, Brady LW. Individualized eye shields for use in electron beam therapy as well as low-energy photon irradiation. Int J Radiat Oncol Biol Phys. 1980;6:519–521. doi: 10.1016/0360-3016(80)90070-x. [DOI] [PubMed] [Google Scholar]
- 2.Jereb B, Lee H, Jakobiec FA, Kutcher J. Radiation therapy of conjunctival and orbital lymphoid tumors. Int J Radiat Oncol Biol Phys. 1984;10:1013–1019. doi: 10.1016/0360-3016(84)90172-x. [DOI] [PubMed] [Google Scholar]
- 3.Smitt MC, Donaldson SS. Radiotherapy is successful treatment for orbital lymphoma. Int J Radiat Oncol Biol Phys. 1993;26:59–66. doi: 10.1016/0360-3016(93)90173-s. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Paulino AC, Buatti JM, Mayr NA, Wen BC. Curative radiotherapy for primary orbital lymphoma. Int J Radiat Oncol Biol Phys. 2002;54:818–823. doi: 10.1016/s0360-3016(02)02966-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee SW, Suh CO, Kim GE, Yang WI, Lee SY, Hahn JS, Park JO. Role of radiotherapy for primary orbital lymphoma. Am J Clin Oncol. 2002;25:261–265. doi: 10.1097/00000421-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Zhou P, Ng AK, Silver B, Li S, Hua L, Mauch PM. Radiation therapy for orbital lymphoma. Int J Radiat Oncol Biol Phys. 2005;63:866–871. doi: 10.1016/j.ijrobp.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Suh CO, Shim SJ, Lee SW, Yand WI, Lee SY, Hahn JS. Orbital marginal zone B-cell lymphoma of MALT: radiotherapy results and clinical behavior. Int J Radiat Oncol Biol Phys. 2006;65:228–233. doi: 10.1016/j.ijrobp.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick PJ, Thompson GA, Easterbrook WM, Gallie BL, Payne DG. Basal and squamous cell carcinoma of the eyelids and their treatment by radiotherapy. Int J Radiat Oncol Biol Phys. 1984;10:449–454. doi: 10.1016/0360-3016(84)90023-3. [DOI] [PubMed] [Google Scholar]
- 9.Kishi K, Shirai S, Sonomura T, Kawai N, Naya Y, Matsunaka M, Sato M, Yamada R. Lead contact lens for crystalline lens shielding in electron therapy for eyelid tumors. Radiat Med. 1996;14:107–109. [PubMed] [Google Scholar]
- 10.Austin-Seymour MM, Donaldson SS, Egbert PR, McDougall IR, Kriss JP. Radiotherapy of lymphoid diseases of the orbit. Int J Radiat Oncol Biol Phys. 1985;11:371–379. doi: 10.1016/0360-3016(85)90160-9. [DOI] [PubMed] [Google Scholar]
- 11.Bessell EM, Henk JM, Wright JE, Whitelocke RA. Orbital and conjunctival lymphoma treatment and prognosis. Radiother Oncol. 1988;13:237–244. doi: 10.1016/0167-8140(88)90218-6. [DOI] [PubMed] [Google Scholar]
- 12.Minehan KJ, Martenson JA, Garrity JA, Kurtin PJ, Banks PM, Chen MG, Earle JD. Local control and complications after radiation therapy for primary orbital lymphoma: a case for low-dose treatment. Int J Radiat Oncol Biol Phys. 1991;20:791–796. doi: 10.1016/0360-3016(91)90025-y. [DOI] [PubMed] [Google Scholar]
- 13.Letschert JG, Gonzalez D, Oskam J, Koornneef L, van Dijk JD, Boukes R, Bras J, van Heerde P, Bartelink H. Results of radiotherapy in patients with stage I orbital non-Hodgkin's lymphoma. Radiother Oncol. 1991;22:36–44. doi: 10.1016/0167-8140(91)90067-q. [DOI] [PubMed] [Google Scholar]
- 14.Schlienger P, Brunin F, Desjardins L, Laurent M, Haye C, Vilcoq JR. External radiotherapy for carcinoma of the eyelid: Report of 850 cases treated. Int J Radiat Oncol Biol Phys. 1996;34:277–287. doi: 10.1016/0360-3016(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 15.Esik O, Ikeda H, Mukai K, Kaneko A. A retrospective analysis of different modalities for treatment of primary orbital non-Hodgkin's lymphomas. Radiother Oncol. 1996;38:13–18. doi: 10.1016/0167-8140(95)01658-9. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu H, editor. Standard Ophthalmology. ed 7. Tokyo: Igaku-Shoin; 1998. pp. 223–224. [Google Scholar]
- 17.Weaver RD, Gerbi BJ, Dusenbery KE. Evaluation of eye shields made of tungsten and aluminum in high-energy electron beams. Int J Radiat Oncol Biol Phys. 1998;41:233–237. doi: 10.1016/s0360-3016(97)00905-x. [DOI] [PubMed] [Google Scholar]
- 18.Hashizume T, Taniguchi H, Inoue T, Oki M, Takeda M, Shibuya H, Ohyama T. Application of individual lead shield in radiotherapy of eyelid tumor. Maxillofacial Prosthetics. 1999;22:34–40. [Google Scholar]
- 19.Khan FM. The Physics of Radiation Therapy. ed 2. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 20.Merriam GR, Jr, Szechter A, Focht EF. The effects of ionizing radiation on the eye. Front Radiat Ther Oncol. 1972;6:346–385. [Google Scholar]
- 21.Rubin R, Casarett G.W. Clinical Radiation Pathology. New York: W.B. Saunders; 1968. [DOI] [PubMed] [Google Scholar]